Abstract
The powerful and intriguing idea that drives the emerging technology of microneedles—shrinking the standard needle to a micron scale—has fostered an entire field of microneedle study and subsequent exponential growth in research and product development. Originally enabled by microfabrication tools derived from the microelectronic industry, microneedles are now produced through a number of methods in a variety of forms including solid, coated, dissolvable, and hollow microneedles. They are used to deliver a broad spectrum of molecules, including small molecules, biomolecules, and vaccines, as well as various forms of energy into the skin, eye, and other tissues. Microneedles are also being exploited for use in diagnostics, as well as additional medical, cosmetic, and other applications. This review elucidates the relative roles of different aspects of microneedle technology development, as shown through scientific papers, patents, clinical studies, and internet/social media activity. Considering >1000 papers, 750 patents, and almost 80 clinical trials, we analyze different attributes of microneedles such as usage of microneedles, types of microneedles, testing environment, types of patent claims, and phases of clinical trials, as well as which institutions and people in academia and industry from different locations and in different journals are publishing, patenting, and otherwise studying the potential of microneedles. We conclude that there is robust and growing activity in the field of microneedles; the technology is rapidly developing and being used for novel applications to benefit human health and well-being.
Keywords: Microneedle, transdermal drug delivery, skin patch, diagnostic device, clinical trial, patent
1. Introduction
Conventionally, the hypodermic needle has been the most effective way to pass through the skin to deliver drugs and other substances into different tissues, including intramuscular and subcutaneous tissues. Although widely used, hypodermic needles have significant drawbacks: needle injections are often painful; self-administration with them is difficult; and their use entails risks of blood-borne disease transmission from re-used needles or accidental needle sticks [1], [2], [3], [4].
Skin, the largest organ of the body, offers a potential interface for delivery both into and out of the body [4], [5], [6]. Drug can be delivered into the body for systemic administration and/or local effects in the skin. In addition to drugs, other compounds, for example for diagnostic applications, can be administered into the body, as can energy, such as electromagnetic fields and light, for both therapeutic and diagnostic purposes.
Delivery of drug molecules through the skin can be more effective than administration through other routes, such as the oral route, where the drug can be affected by enzymatic degradation in the gastrointestinal fluid and poor absorption across the intestinal epithelium. Although the skin is an attractive route, few therapeutically active molecules can naturally penetrate the outermost layer of the skin (called the stratum corneum). Different approaches including chemical penetration enhancers, electric fields, ultrasound energy, thermal ablation, mechanical abrasion, and other physical interventions have been exploited to overcome the natural barrier of the skin [7], [8]. While these methods can increase drug delivery into skin, they often cause skin irritation and/or involve bulky devices requiring an energy source, which has limited their use in medicine. As a result, though some drugs are given topically or as transdermal patches, delivery across the skin is performed for most drugs using hypodermic needles.
The skin barrier often impedes application of electric fields to the skin, as in transcutaneous electrical nerve stimulation or electroporation [9], [10], [11], or delivery of laser light, as in photodynamic therapy and tattoo removal [12], [13], [14], [15]. Transport out of the body is also impeded by the skin, e.g., when sampling skin tissue and/or fluid to measure glucose concentration [16], diagnosing skin disease [17], or collecting electrical signals for electrocardiograms [18], [19].
Other tissue barriers inhibit transport, such as epithelial barriers in the gastrointestinal tract, oral mucosa and eye, and endothelial barriers, like in the cardiovascular system [20], [21], [22]. In these cases, various formulations and devices have been developed, which sometimes involve hypodermic needles. While most applications seek to avoid tissue damage when crossing a biological barrier, in some cases causing minor injury is intended, such as when inducing collagen production in skin for cosmetic purposes [23] or stimulating the immune system to enhance vaccine responses [24].
Microneedle technology was invented to create a delivery system as robust as hypodermic needles, but without the associated pain and other disadvantages. Microneedles are micron-sized projections that cross biological barriers in a minimally invasive manner. Microneedles can be broadly categorized into four categories: solid microneedles, coated microneedles, dissolvable microneedles, and hollow microneedles. Solid microneedles have no hollow bore and have no drug physically associated with them, and are typically used as piercing structures that create transport pathways or stimulate collagen production in skin, and sometimes used as electrodes. Coated microneedles are also solid but have a drug or other material coated onto their surface, typically for therapeutic or sensing applications. Dissolvable microneedles are made of materials that dissolve in water (i.e., in tissue) and typically have drug encapsulated within the microneedles. Finally, hollow microneedles have typically one or more hollow bores through which fluid can flow during injection.
Microneedle technology and applications have been the subject of many prior reviews that can provide further background and context [25–42]. The goal of this review is to provide an overview of activity and identify trends in the field of microneedles. Broadly, activities were identified and analyzed from four information sources: the scientific literature (i.e., research papers), patents, clinical trials, and internet/social media. For each of the four groups, further sub-classification was made to allow for organization and communication of the data.
2. Methods
For each of the four information sources, we selected search terms to identify activities related to microneedles. Among the microneedle activities, we further classified the activities according to their scope and application. Because of the nature of the activities and, more importantly, the way information was presented and classified in different information sources, classification categories were not always the same for data obtained from each information source, although we tried to make them similar.
2.1. Scientific literature
Research papers in the scientific literature were identified by searching PubMed (www.ncbi.nlm.nih.gov/pubmed/) with keywords “microneedle” or “microprojection”. A search date range was set from 1990 to 2018 (with a cut-off of October 1, 2018, the data the search was conducted). This timeframe was selected, as it provides a roughly 20 year perspective since the first paper was published on microneedles for drug delivery in 1998 . The small number of book chapters that appeared in searches were disregarded. The final count for the published papers was 1190 (including research and review articles). We further narrowed the analysis to just research papers (1027). For these 1027 papers, objective data explicitly presented in the paper (e.g., title, authors, and publication year) and subjective data determined by expert review of the paper (e.g., type of microneedle, study testing environment) were tabulated in Microsoft Office Excel. A classification tree showing all of the types of data collected is provided in Fig. 1 (i).
Figure 1.
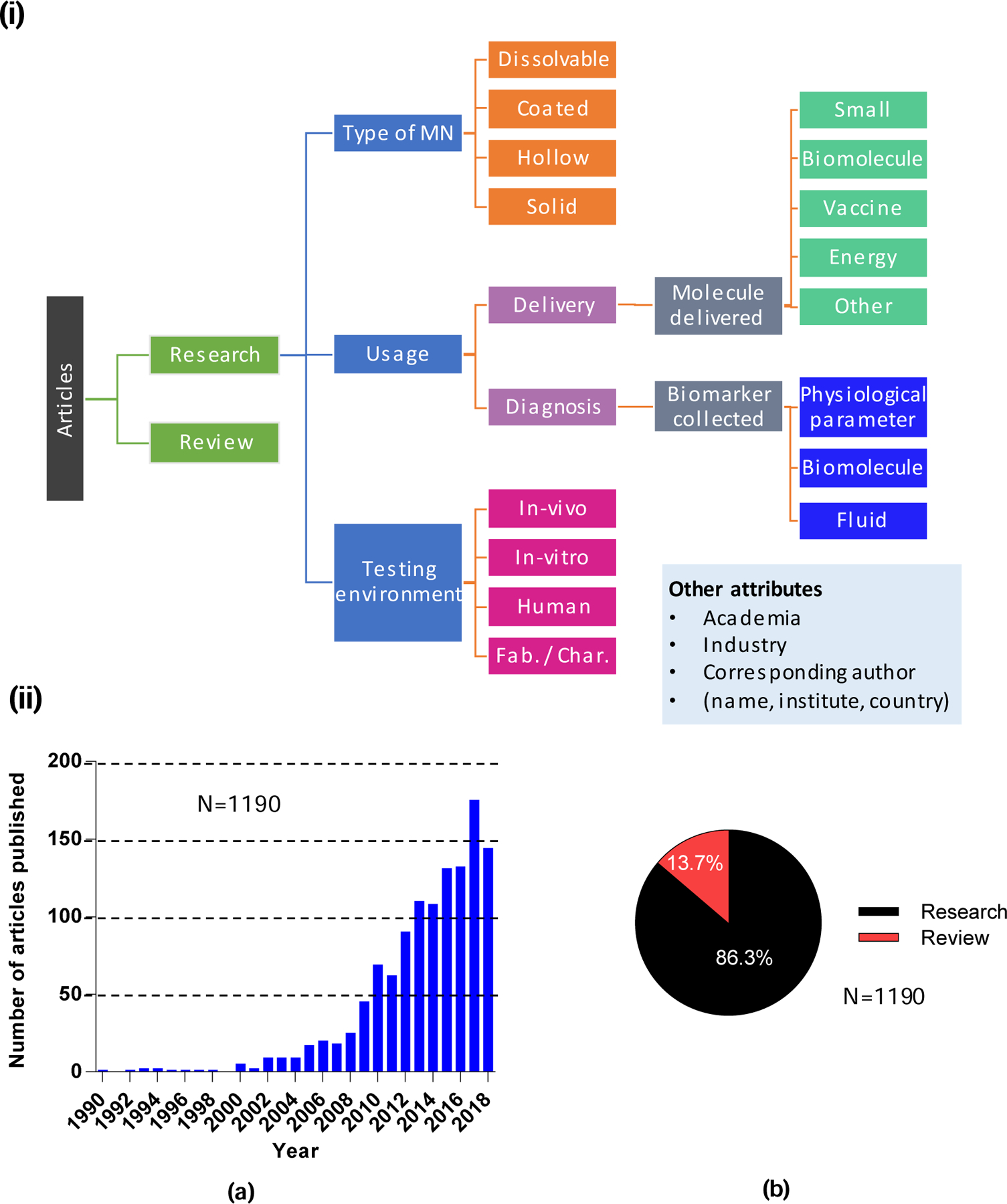
Overview of research papers included in the study. (i). General outline for the categorization of microneedles and their uses. (ii). Total number of research and review articles published on microneedles from 1990 until Oct. 1, 2018: a) number of yearly publications, and b) percentage of research versus review articles.
A code was written in Excel to extract different Boolean combinations of microneedle classification attributes (such as “hollow” and “vaccine”). All graphs were plotted using GraphPad Prism (GraphPad Software, San Diego, CA). Some papers could be classified into multiple categories: for example, some described use of microneedles for both molecule delivery and diagnosis. Further, within the category of molecule delivery, some papers discussed use of microneedles to deliver multiple molecules. As a result, the total count for many categories did not sum to 1027. In some cases, the model compound being delivered in the study might have been used with the objective of understanding how to deliver a different kind of compound (e.g., a model small molecule might have been studied with the objective of eventually delivering a protein). In these cases, the paper was categorized based on the compound actually delivered and not what the investigators were thinking of delivering in the future.
Solid, porous, pocketed (unless coated), skin pre-treatment, and post-treatment microneedles were all categorized as solid microneedles. Biodegradable, swellable, and biocompatible microneedles were all categorized as dissolvable (even though in some cases dissolution first requires biodegradation over an extended period of time and, in some cases, include microneedles that release drug but do not dissolve). Delivery of bacteria, quantum dots, cells, gold nanoparticles, etc. were categorized as “Other” under molecule delivery. In analyzing use of microneedles for diagnosis, collection of biomarkers such as bio-signals; sensing of pH; use of microneedle for intraocular pressure measurement; and electromyography were all categorized under physiological parameters. The term “other” in usage represented other aspects of microneedles such as cost-effectiveness, acceptability, anti-bacterial properties of microneedles, and fabrication/characterization of microneedles.
We also categorized research papers on use of microneedles in different types of testing environments. In vivo referred to use of microneedles (for delivery or diagnosis) in living organisms, such as common laboratory animals where the microneedle patch is applied to different parts of the body depending upon its application. In vitro studies revolved around use of excised tissue to study different aspects of microneedles. Studies of microneedles used in humans for delivery of molecules, diagnosis, or to study microneedle safety were categorized as “human” studies. Fabrication/characterization studies discussed different fabrication techniques for creation of microneedles and characterization of their stability, strength, and safety/acceptability. Cost-effectiveness of microneedles was also included under fabrication/characterization.
Other aspects of the research papers categorized included affiliation of corresponding author with either academic institutions or industry. Other information such as country, author name, and institution was also determined based on association of corresponding authors for research papers (and for multiple corresponding authors on papers with more than one).
2.2. Patents
Inventors use different keywords to define microneedles. These can be general terms like “microprotrusions” or “microstructures”; this lack of specificity can complicate searches. We used the CPC (Cooperative Patent Classification) system developed by the European Patent Office (EPO) and United States Patent and Trademark Office (USPTO) to enable efficient searching. In this system, CPC symbols are used to find patents according to content, regardless of words used for definition.
The EPO database was used as the source for patents in this study. Searching was carried out with two different methods. In this study, we only considered issued US patents. The area for the publication number was filled with “US” and “USB”. US was used as a country code. “B” was used to narrow the results to the issued US patents and exclude patent applications since they may or may not issue into a patent.
The first search was made with keywords to identify the frequently used CPC symbols associated with microneedles. The search form of keyword-based search used the following Boolean logic: “microneedle*” OR “micro needle*” OR “microprojection* array*” OR “microneedle* array*” OR “microstructure* array*” OR “microprotrusion* array*” in the title or abstract AND USB as the publication number. The asterisk was used to include possible plural forms.
After this first search to identify relevant CPC symbols, we performed a second CPC-based search using the CPC symbols shown in Table S1 in Supplementary Information (SI). This analysis focused on frequently used CPC symbols. Some frequently used symbols were not used as search criteria because they were not specific (e.g., “for piercing elements” or “blade, lancet, cannula, needle”). Some CPC symbols were included even though they might include irrelevant patents (e.g., “intradermal administration” or “through microneedle arrays, needleless injectors”). Selected CPC symbols were combined with the final search form USB as the publication number AND a single year, e.g., “1990”, used as the publication date for each search: AND (A61B5/14514 OR A61B5/150984 OR A61B5/685 OR A61K9/0021 OR A61M37/0015/low OR B81B2201/055) were used as the CPC symbols. Espacenet, the database of EPO, displays only the first 500 results per search. Therefore, individual searches were conducted for each year between 1990 and 2018, and the results from all searches were combined. Patent files were then reviewed based on the abstracts, mosaics, description, and claims of the patents to exclude tangentially related or irrelevant patents. The remaining data were processed using conditional formatting formulas and Boolean combinations in Excel. The graphs were plotted using GraphPad Prism.
The data on total issued patents were used to determine the number of yearly issued patents and their general classification based on type of assignee. Data exported from Espacenet were also processed to provide information about the top inventors, assignees, and contributions of countries and continents in this field. A further search was made with the CPC symbols. The symbols were fragmented for a diagram of categories at different levels to get an overview of areas of focus in the microneedle field. The CPC symbols associated with each patent were expressed according to the taxonomy shown in Fig. 2, which follows conventional practice in Espacenet [43]. Microneedle types, application areas, and relationships between these categories were analyzed based on the assigned CPC symbols. A general outline for the analysis is shown in Fig. 3.
Figure 2.
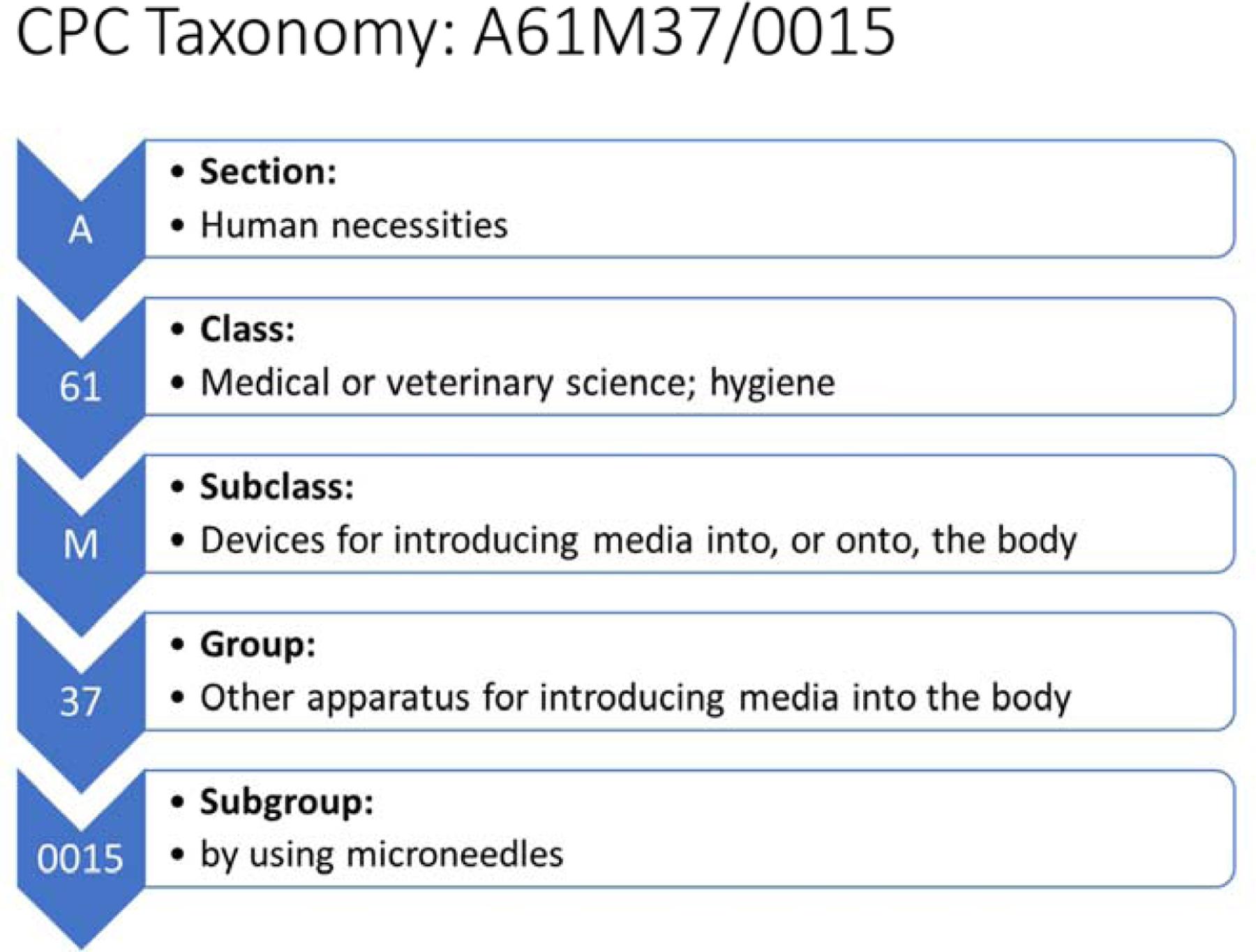
Taxonomy of a CPC symbol with definitions of categories and subcategories.
Figure 3.
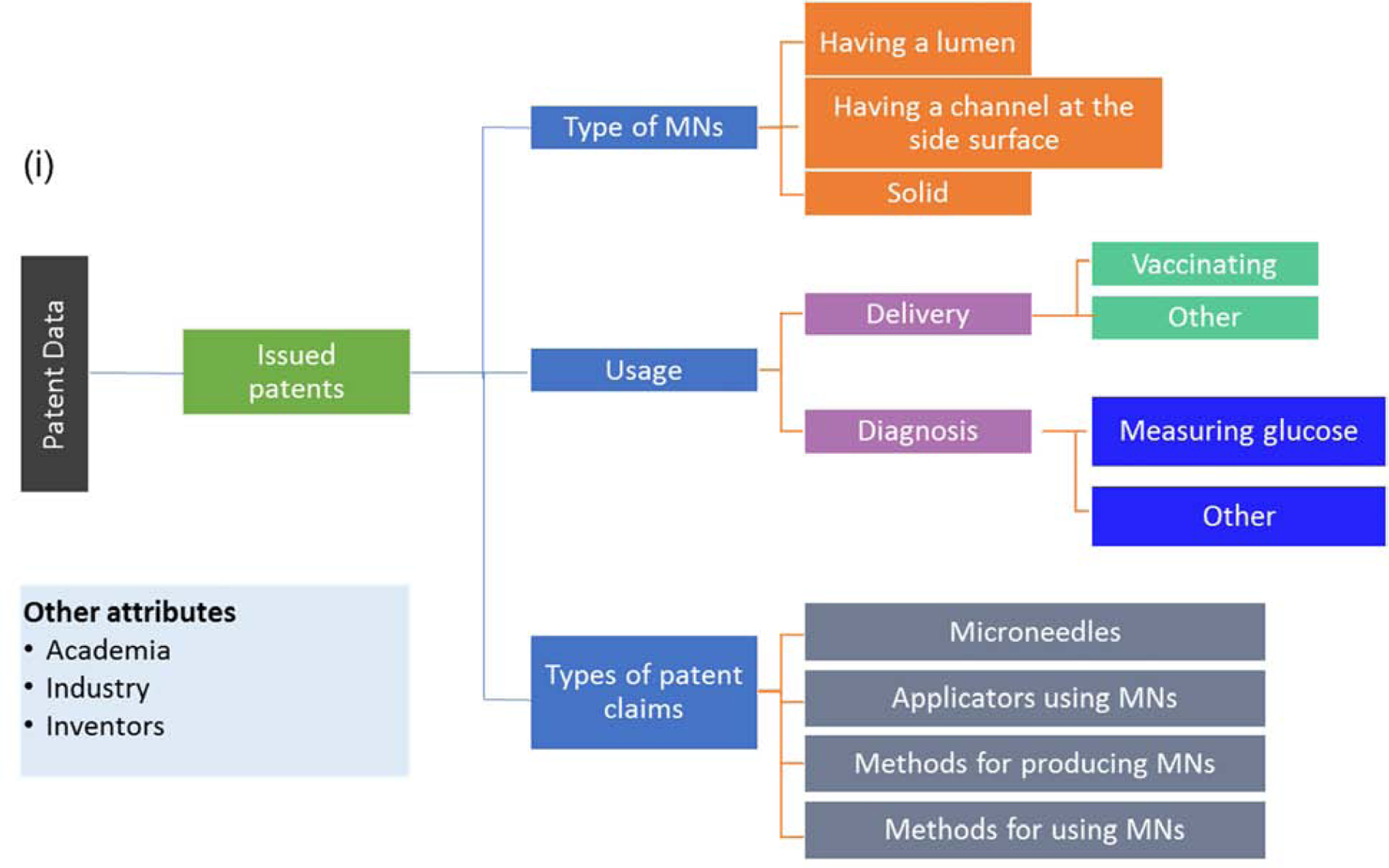
General outline of the categorization of microneedles and their uses.
Patents are commonly issued with multiple CPC symbols, and such patents were double-counted in different areas. For this reason, the total number of some charts is larger than the total number of reviewed patents. It should also be noted that some categories of classification used in patents differ from other sources of information. For example, CPC symbols for microneedle types are defined as microneedles having a lumen (A61M2037/003); microneedles having a channel at the side surface (A61M2037/0038), and solid microneedles (A61M2037/0046), which differs from the microneedle categories used to analyze the scientific literature. Also, related CPC symbols like detecting, measuring, or recording for diagnostic purposes (A61B5) and other methods or instruments for diagnosis (A61B10) were combined within the general diagnosis category to better align with categorization used in other information sources.
2.3. Clinical trials
A search was performed using the ClinicalTrials.gov database using the keywords “microneedle” OR “microprojection” [44]. That search initially identified 83 clinical trials. Two of those studies were disregarded because the study did not actually concern use of microneedles, and a further two studies had been withdrawn by investigators and were therefore not included in our analysis. The final number of clinical trials used in the analyses was 79, where 17 were ongoing and 62 studies had been completed at the time of analyses.
From each of the 79 clinical trials, the following information was abstracted into an Excel spreadsheet: i) year when the clinical trial started, ii) stage (i.e., phase) of clinical trial, iii) status of clinical trial, iv) application of microneedle, i.e., delivery or diagnosis, v) type of microneedle, i.e., coated, dissolvable, hollow, solid, or non-specified, vi) type of material delivered, i.e., biomolecule, energy, placebo, small molecule, vaccine, or other, vii) indications to be treated, viii) sponsor affiliation, i.e., academia and/or hospital versus industry, and ix) study location. Microneedles used for skin pre-treatment were categorized as solid microneedles. Trials without FDA-defined phases, including trials of devices or behavioral interventions, were classified in terms of stage as not applicable, i.e., N/C (Fig. 4 (i)). The analyzed data were plotted using GraphPad Prism.
Figure 4.
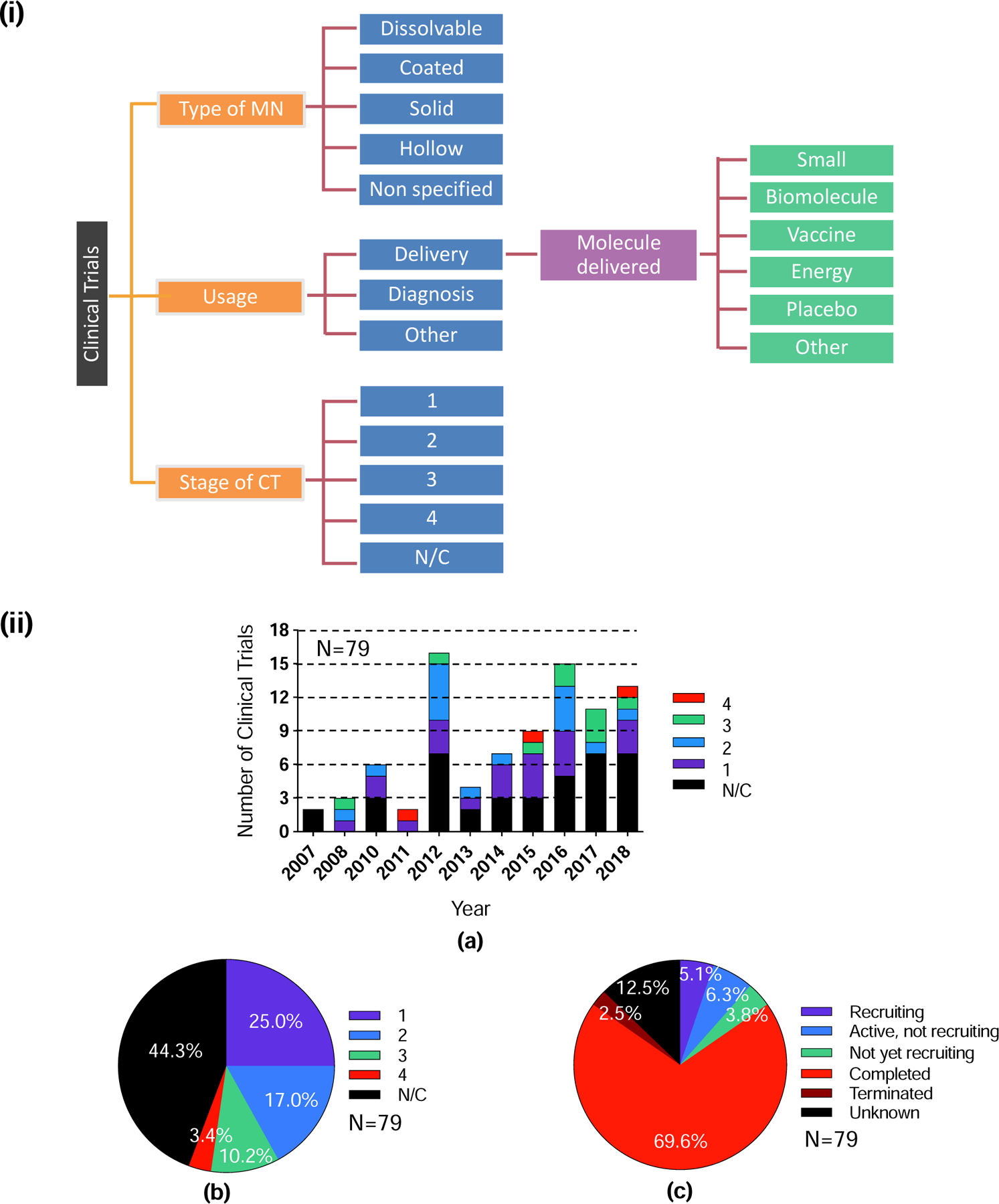
Overview of clinical trials included in the study. (i) General outline of the categorization of microneedles and their uses in clinical trials. (ii) Total number of registered clinical trials involving the use of microneedles listed in ClinicalTrials.gov on Oct. 18, 2018 (N=79): a) classification of clinical trials based on number of clinical trials at various stages each year, b) percentage of clinical trials at each stage, c) percentage of clinical trials having different status. A full listing of clinical trials is shown in Table S2 in SI.
2.4. Internet/social media
The level of internet activity relating to microneedles was determined through analyses of statistics provided from Google Trends (Google, Mountain View, CA) and Altmetric (London, UK). Google Trends provided insight into internet search patterns by analyzing the proportion of all web queries on the Google Search website and other affiliated Google sites [45]. The Google Trends search was performed using the search terms “microneedle” OR “microprojection”; however, only results from the microneedle search contributed to the data, as the term microprojection resulted in a finding of “not enough data.” The data were expressed in terms of interest in the related search term over time, where the numbers represent search interest relative to the highest point on the chart for the given region (worldwide) and time. A value of 100 is the peak popularity for the term. A score of 0 means that there was not enough data for the term. Google Trends provided data from the year 2004 to October 22, 2018. The tool also provided information on related topics or queries, which we searched for alongside the term microneedle. The scoring for top related topics or queries is a relative scale, where a value of 100 is the most commonly search topic or query.
Bibliometrics of research impact within the field were provided by Altmetric, a source of metrics that tracks the attention that research outputs such as scholarly papers and databases receive online [46]. Altmetric searches information on the chosen term from a wide variety of sources such as policy documents, news articles, academic and non-academic blogs, online reference managers (e.g., Mendeley and CiteULike), online journal clubs, and social media (including Twitter, Facebook, Weibo, Google+, Pinterest, and Reddit). Altmetric also analyses online references within Wikipedia, reviews on YouTube, and patent citations. A demographic analysis of the metrics for online mentions (total number of 4410) of microneedle or microprojection were also included. The analyzed data were plotted using GraphPad Prism.
3. Results
Based on analysis of publicly available information sources, we identified and analyzed trends in microneedle technology activities in the scientific literature, patents, clinical trials, and internet/social media.
3.1. Analysis of the scientific literature
We identified trends in the scientific literature by searching PubMed.
3.1.1. Scientific literature: annual publication trend
A total of 1190 papers on microneedles were published in the period analyzed between 1990 and 2018. More than 85% (1027 papers) original research; the remainder were review articles (Fig. 1 (ii) b). Among the research papers, just 1% were published from 1990 to 2000, 20% from 2001 to 2010, and the remaining 79% from 2011 to 2018 (Fig. 5 (i)). The number of papers published per year has increased almost every year, with 148 papers being published in 2017 (only a partial year of publications is shown for 2018).
Figure 5.
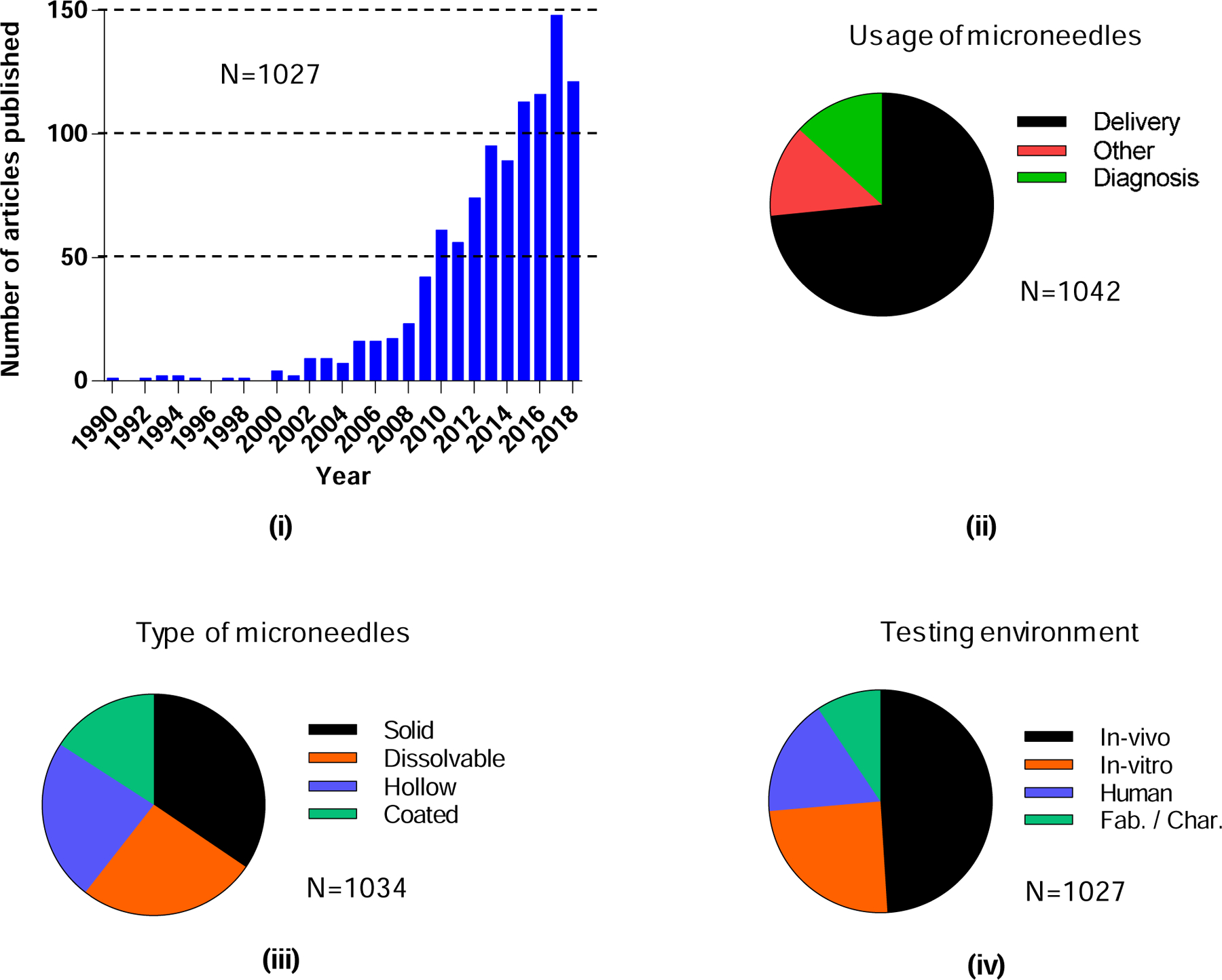
Total research papers published on microneedles. i) Number of yearly publications, and their classification based on ii) usage of microneedles, iii) type of microneedles, and iv) testing environment. “Fab./Char.” means fabrication or characterization of microneedles.
3.1.2. Scientific literature: trends in microneedle types, use, and testing environment
Microneedles were originally conceptualized for delivery of different molecules, primarily into the skin. Of the 1027 published research papers on microneedles, 73% (Fig. 5 (ii)) were related to the delivery of molecules. Use of microneedles for diagnosis was studied to a lesser extent, 13% (Fig. 5 (ii)). There is, however, growing interest in use of microneedles to extract biomarkers from the skin for diagnostic purposes, in addition to ongoing interest in making physiological measurements with microneedles. The remaining 13% of publications were categorized as other: studying aspects of microneedles such as cost-effectiveness and acceptability; anti-microbial properties of microneedles; in vivo imaging of microneedle insertion; kinetics of skin resealing; evaluation of pain; improving piercing ability of microneedles; effect of tissue stiffness on microneedle insertion; and fabrication/characterization of microneedles (Fig. 5 (ii)).
Among the different types of microneedles, the largest fraction of papers (35%) were published about solid microneedles. An almost equal number of papers addressed dissolvable and hollow microneedles (~25% each), and the lowest share belonged to coated microneedles (~16%) (Fig. 5 (iii)). Coated microneedles were heavily represented in the early literature but have been displaced by dissolvable microneedles more recently. With respect to the environment of investigation, almost half (49%) of the papers involved in vivo (excluding humans) studies, 25% were studied in vitro, 17% were studied in humans, and the remaining 9% only addressed fabrication and/or physical characterization of the microneedles (Fig. 5 (iv)).
3.1.3. Scientific literature: trends in delivery using microneedles
We examined various uses of microneedles for delivery to determine trends. Use of microneedles for delivery has been largely via the transdermal route. The choice of microneedle insertion site depends upon the target molecule as well as the targeted indication. It is well-established that microneedles enhance the permeability of molecules into skin, where the molecule is delivered into the viable epidermis and/or dermis. Microneedles can achieve targeted delivery in the suprachoroidal space of the eye as well as in the mouth and tongue for targeted delivery of anti-cancer drugs and vaccines. Solid and dissolvable microneedles together formed the dominant approach of microneedle-based delivery (>30% each). These were followed by almost equal use of hollow and coated microneedles, each of whose use was described in 18–19% of delivery-related research papers (Fig. 6 (i) a).
Figure 6.
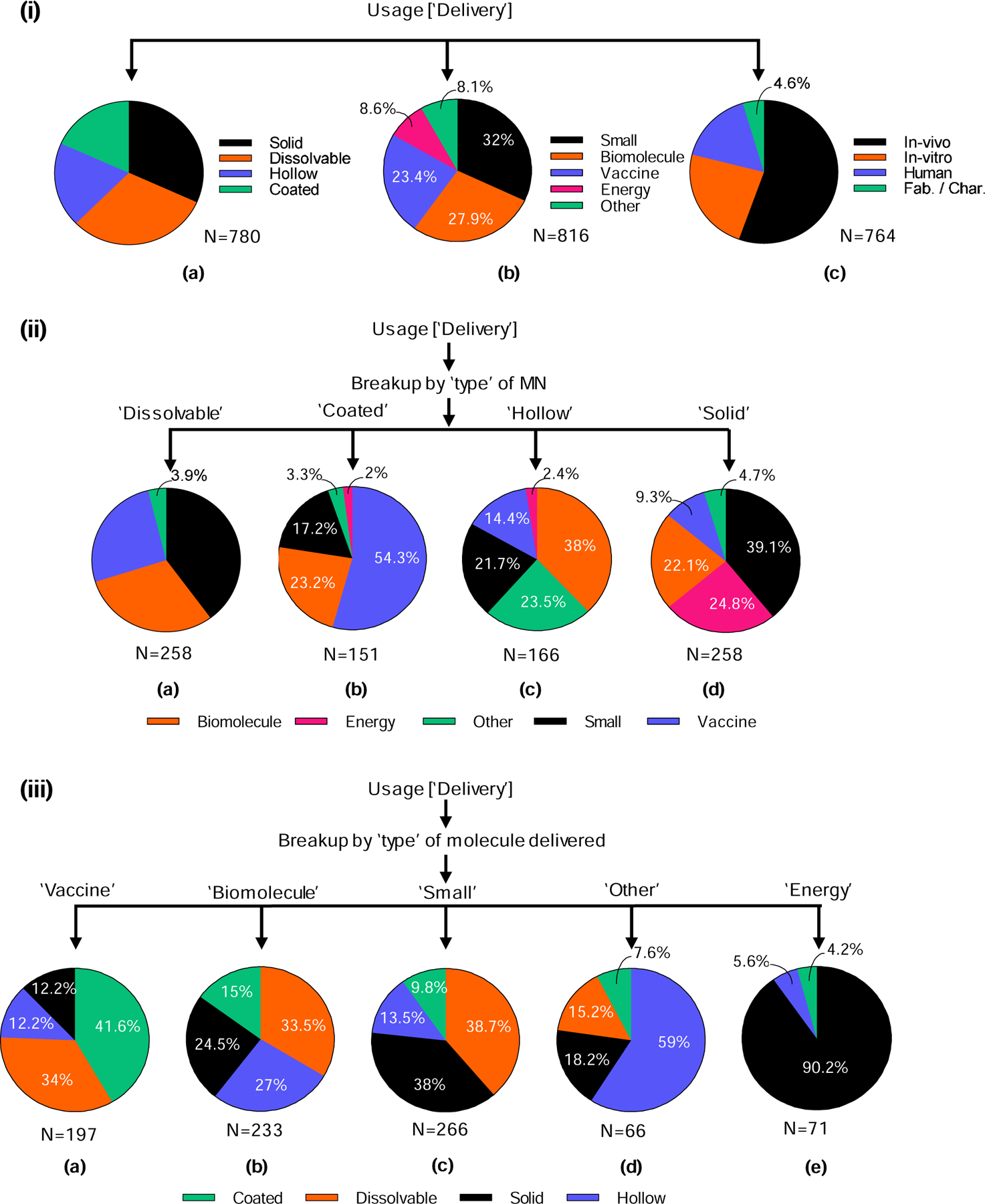
Analysis of microneedles used for delivery in the scientific literature. (i) Classification based on a) type of microneedle used for delivery, b) type of material or energy delivered, and c) testing environment. (ii) The different types of molecules delivered are identified for each type of microneedle: a) dissolvable, b) coated, c) hollow, and d) solid. (iii) The different types of microneedles are identified for each type of molecule delivered: a) vaccine, b) biomolecule, c) small, d) other and e) energy.
Microneedles have been used to deliver small molecules, biomolecules, vaccines, energy, and other molecule types. In our classification, small molecules include dyes and low molecular weight drugs. Biomolecules include macromolecules such as DNA, RNA, proteins, and peptides. Vaccines include different antigens such as subunit vaccines (e.g., proteins), live and inactivated viruses, and DNA. Delivery of energy is often related to beautification and associated cosmetic applications (e.g., collagen induction therapy). Energy also includes use of microneedles for delivery of electromagnetic energy (such as radiofrequency energy) to the skin for treatment of acne vulgaris, primary axillary hyperhidrosis, and acne scars; to cause electroporation for gene transfer; and to stimulate nerves. Finally, delivery of molecules such as bacteria, quantum dots, cells, gold nanoparticles are categorized as other.
Almost one-third (32%) of papers discussed delivery of small molecules. Similar numbers of papers were published examining delivery of biomolecules or vaccines using microneedles (23–28% each). Delivery of energy and other molecules collectively represented 17% of total usage for delivery (Fig. 6 (i) b). Delivery of molecules in humans was studied in 16% of the research papers. In over 55% of the research papers, use of microneedles involved delivery of molecules in vivo (excluding humans). About 23% of the research papers studied delivery of molecules in vitro, while the remaining 5% focused on fabrication/characterization of microneedles (Fig. 6 (i) c). The fact that almost three-quarters (72%) of papers studied delivery in humans or animals in vivo indicates the translational emphasis of the field from device engineering to medical and other applications.
Considering different types of microneedles, we found that dissolvable microneedles were used most frequently for delivery of small molecules (40%) followed by biomolecules (30%) and vaccines (26%). They were rarely used for other delivery applications (4%), and not used at all for energy delivery (Fig. 6 (ii) a). In contrast, coated microneedles were used for vaccine delivery the majority of the time (54%), followed by biomolecules (23%) and small molecules (17%), with other uses (3%) and energy delivery (2%) accounting for the remaining applications (Fig. 6 (ii) b). Unlike other microneedle types, hollow microneedles were often used for “other” delivery scenarios (24%), but their primary use was for biomolecule delivery (38%). Hollow microneedles were also used for delivery of small molecules (22%) and vaccines (14%), but rarely for energy (2%) (Fig. 6 (ii) c). Finally, solid microneedles differed from the others in that they were often used for delivery of energy (25%). Solid microneedles were used most frequently for small molecules (39%), with biomolecules (22%), vaccines (9%) and others (5%) delivered as well (Fig. 6 (ii) d).
We examined the different molecules delivered to see which types of microneedles were preferred. Vaccines were mostly delivered using coated (42%) and dissolvable (34%) microneedles, with hollow (12%) and solid (12%) microneedles used the rest of the time (Fig. 6 (iii) a). Biomolecule delivery was more evenly split among dissolvable (34%), hollow (27%), solid (25%), and coated (15%) microneedles (Fig. 6 (iii) b). Small molecules were delivered primarily by dissolvable (39%) and solid (38%) microneedles, followed by hollow (14%) and coated (10%) microneedles (Fig. 6 (iii) c). Other compounds were mostly delivered by hollow microneedles (59%), in addition to solid (18%), dissolvable (15%), and coated (8%) microneedles (Fig. 6 (iii) d). Finally, energy was almost exclusively delivered using solid microneedles (90%), with hollow (6%) and coated (4%) microneedles comprising the remaining scenarios (Fig. 6 (iii) e).
Overall, all four types of microneedles were popular for delivery applications, where delivery of molecules (small molecules, biomolecules and vaccines) dominated over other materials and energy and the majority of studies addressed delivery in vivo (Fig. 6 (i)). Dissolvable and coated microneedles were used mostly for small molecules, biomolecules, and vaccines. Hollow microneedles were also used for delivery of other compounds, and solid microneedles were used additionally for energy delivery (Fig. 6 (ii)).
Energy was mostly delivered by solid microneedles; other materials were mostly delivered by hollow microneedles; and small molecules, biomolecules, and vaccines were delivered using all four types of microneedles. Each type of microneedle is useful in different scenarios for delivering small molecules, biomolecules, and vaccines, but the versatility and minimal formulation needs associated with hollow microneedle delivery makes it preferred for other materials, and the electrically conductive pathway enabled by (metal) solid microneedles makes them most attractive for (electrical) energy delivery.
3.1.4. Scientific literature: trends in diagnosis using microneedles
In recent years, microneedle usage for diagnosis has seen an upward trend. Although only 13% of the scientific literature overall deals with use of microneedles for diagnosis (Fig. 5 (ii)), there has been a recent increase in collection of body biomarkers to detect presence of different biomarkers in the body; 71% of papers on diagnosis have been published since 2010. Microneedles have also been coupled to pumps for extraction of interstitial fluid from skin and to microfluidic devices where biomarkers are separated and analyzed in real time. Swellable microneedles have been used to collect bodily fluids, which fill the porous structure of these microneedles, for subsequent analysis. Examples of diagnostic applications include measurement of physiological parameters, biomolecules, and bodily fluids for biomarker measurements. As per our classification, collection of different biomarkers such as bio-signals, sensing of pH, use of microneedles for intraocular pressure measurement, and electromyography are all categorized under physiological parameters. Biomarker encompass detection of glucose, antibodies, cancer biomarkers, DNA, and lactate. “Collection of fluids” refers to use of microneedles to collect different bodily fluids such as blood, sweat, and interstitial fluid. Note that the term diagnosis is used broadly here to include all types of measurements, whether they are actually used, for example, to diagnose a disease or for other applications.
Unlike delivery applications, for diagnosis, hollow microneedles were most widely used (47%), followed by solid microneedles (34%). Coated microneedles (11%) and dissolvable microneedles (8%) were less frequently studied for use in diagnosis (Fig. 7 (i) a). This may be because hollow microneedles provide a conduit for fluid collection, solid microneedles create skin puncture for fluid flow, coated microneedles can have biomarker collection or sensing functionality on their surface, and dissolvable microneedles (when crosslinked) can swell to collect body fluid.
Figure 7.
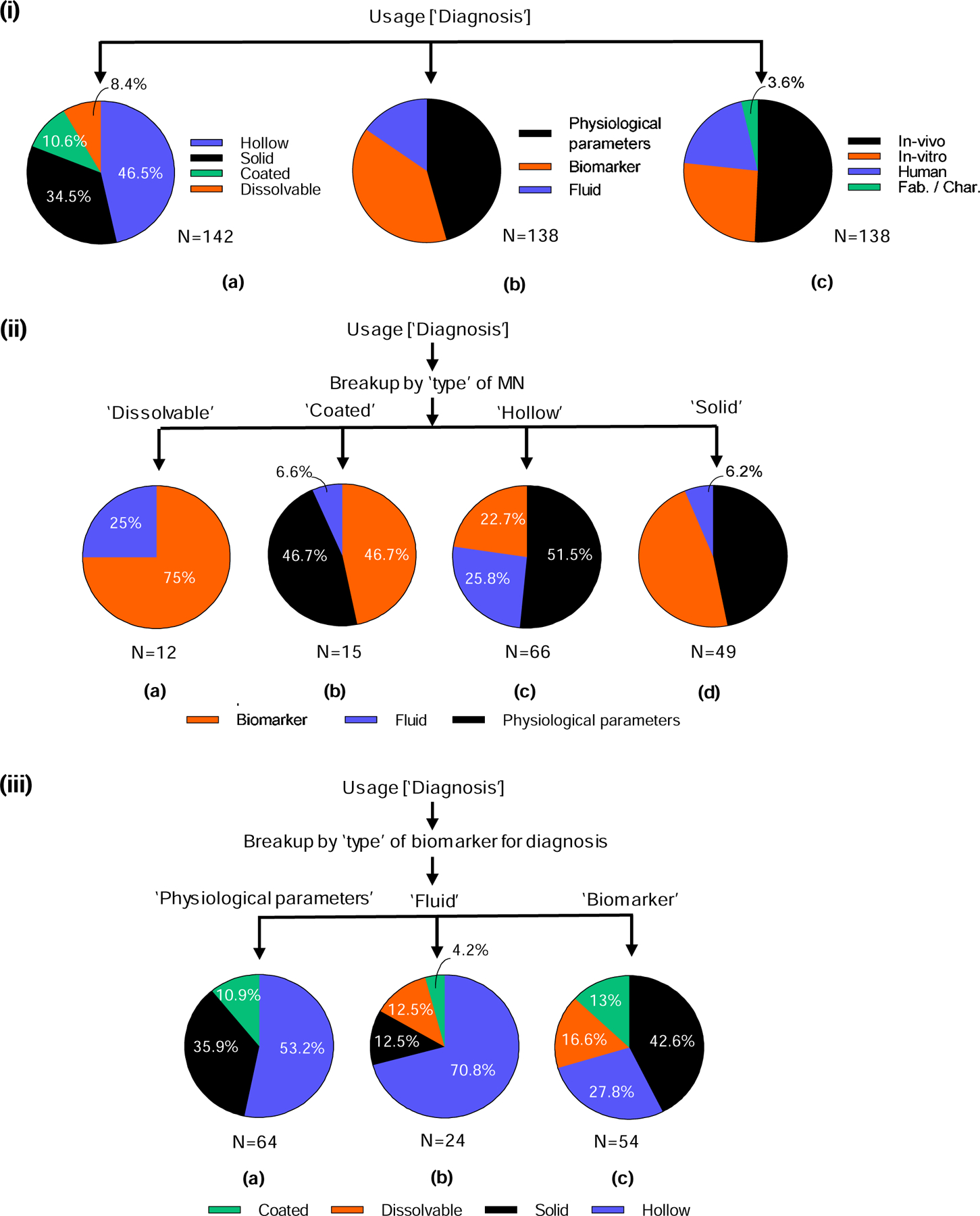
Analysis of microneedles used for diagnosis in the scientific literature. (i) Classification based on a) type of microneedle used for diagnosis, b) type of biomarker for diagnosis, and c) testing environment. (ii) The different types of biomarkers identified for each type of microneedle: a) dissolvable, b) coated, c) hollow and d) solid. (iii) The different types of microneedles identified for each type of biomarker: a) collection of physiological parameters, b) collection of fluid, and c) collection of biomarker.
In diagnostic settings, microneedles were mostly used to directly collect physiological parameters (46%) or biomarkers, (39%). Collection of body fluid for indirect measurement of biomarkers was less popular (15%) (Fig. 7 (i) b). Roughly half (51%) of the research papers involved in vivo studies, 26% in vitro, and 20% studies in humans; the remainder (4%) dealt with characterization/fabrication of microneedles (Fig. 7 (i) c).
Among different types of microneedles, dissolvable microneedles were mostly used to collect biomarkers (75%) and sometimes for fluid collection (25%) (Fig. 7 (ii) a). Coated microneedles were used predominantly for biomarker (47%) and physiological parameter (47%) collection, with limited use for fluid collection (7%) (Fig. 7 (ii) b). Hollow microneedles were used about half of the time for measurement of physiological parameters (52%), with the remaining applications on fluid (26%) and biomarker (23%) collection (Fig. 7 (ii) c). Finally, solid microneedles were evenly split between physiological parameter (47%) and biomarker (47%) applications, with the remaining uses for fluid collection (6%) (Fig. 7 (ii) d).
Considering the types of microneedles used for each diagnostic scenario, there was a greater preference for use of hollow (53%) and solid (36%) microneedles in collection of physiological parameters; the remaining papers used coated microneedles (11%), and none used dissolvable microneedles (Fig. 7 (iii) a). Collection of fluid took place primarily with hollow microneedles (71%), in addition to solid (13%) and dissolvable (13%) microneedles, and occasionally coated microneedles (4%) (Fig. 7 (iii) b). Biomarkers were frequently collected using solid microneedles (43%), followed by hollow (28%), dissolvable (17%), and coated (13%) microneedles (Fig. 7 (iii) c).
Overall, hollow and solid microneedles were most commonly used for diagnostic applications (Fig. 7 (i)). Dissolvable microneedles were used mostly for biomarker measurement, coated and solid microneedles were used mostly for measurement of physiological parameters and biomarkers, and hollow microneedles were used for physiological parameters, biomarkers, and fluid collection (Fig. 7 (ii)). Hollow and solid microneedles may be most popular for all three of the diagnostic applications (Fig. 7 (iii)) due to their ability to provide channels for flow of fluids (hollow microneedles) and electricity (solid microneedles) out of the skin. Also, the amount of fluid that can be collected is generally greater when flowing through hollow microneedles or channels created by puncture with solid microneedles, compared to fluid volumes that can be collected within coated or dissolvable microneedles.
3.1.5. Scientific literature: testing environments used in performing microneedle research
Two-thirds (66%) of microneedle studies were conducted in living organisms, with 49% performed in vivo (animals) and 17% in humans. Most of the remaining studies were performed in vitro (25%), with some addressing fabrication/characterization (9%) (Fig. 5 (iv)). Similar trends were seen when analyzing the use of hollow microneedles (Fig. 8 (i) c), whereas dissolvable and coated microneedles showed greater use for in vivo studies (65% and 72%, respectively) and less use in humans (9% and 4%, respectively), and solid microneedles found greater purpose for in vitro (31%) and human (29%) studies, and relatively less for in vivo studies (38%) (Fig. 8 (i)).
Figure 8.
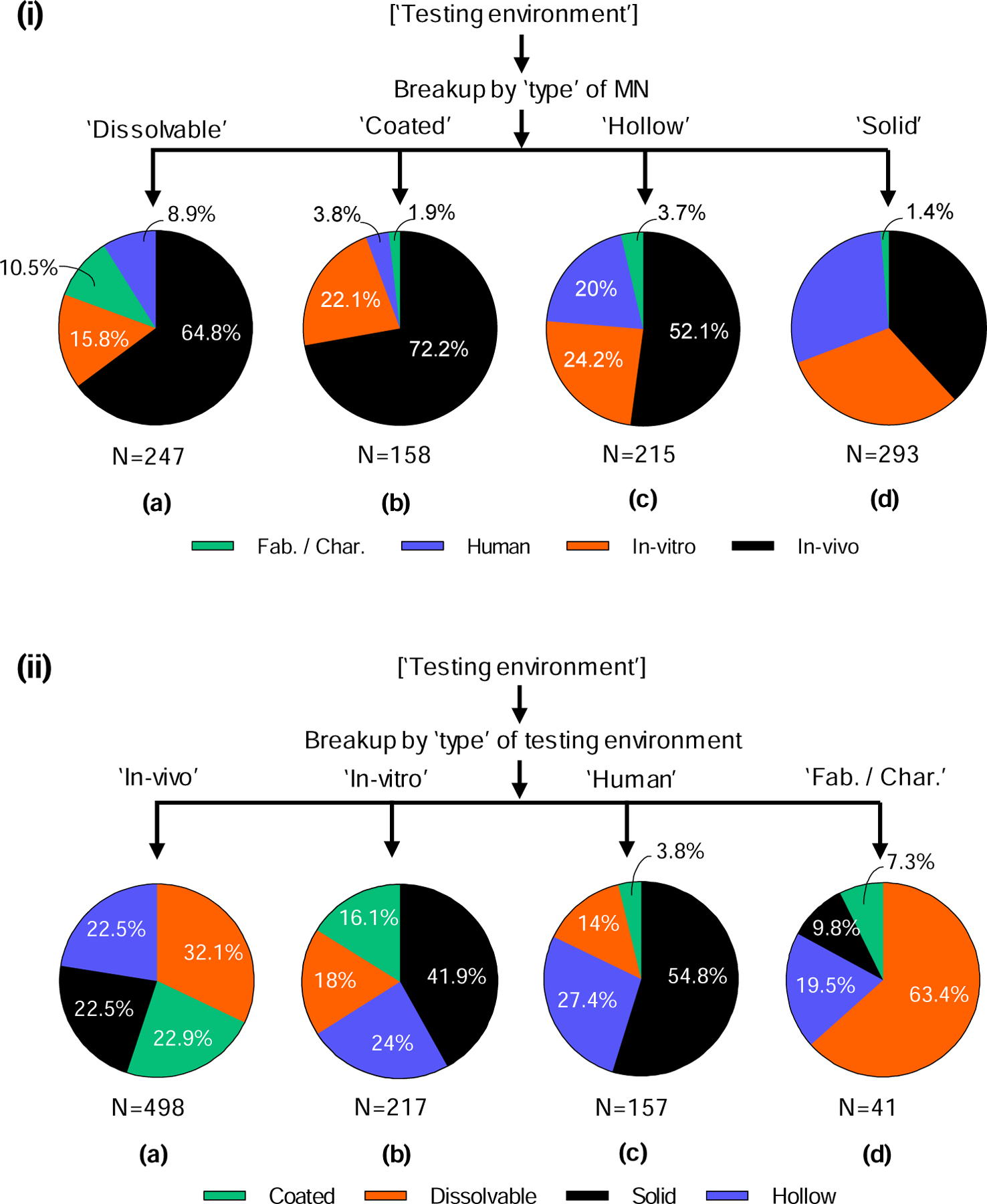
Analysis of testing environment of microneedles in the scientific literature. (i) Different testing environments for each type of microneedle: a) dissolvable, b) coated, c) hollow, and d) solid. (ii) Different types of microneedles in each type of testing environment: a) in vivo, b) in vitro, c) in humans, and d) fabrication/characterization of microneedles.
When analyzing the different testing environments, all four types of microneedles were often used in vivo and in vitro (Fig. 8 (ii) a and b), whereas human studies were dominated by solid microneedles (55%) followed by hollow microneedles (27%) (Fig. 8 (ii) c)., and fabrication/characterization of microneedles was performed mostly on dissolvable microneedles (63%) (Fig. 8 (ii) d).
Human studies may be dominated by hollow and solid microneedles because those types can often be used with little or no drug reformulation, which simplifies the regulatory pathway. Dissolving microneedles may be the greatest subject of fabrication/characterization studies because their fabrication is often the most complex and their characterization involves not only mechanical properties, but also dissolution processes.
3.1.6. Scientific literature: microneedle research in academia and industry
Next, we analyzed the fields of academia and industry, comparing different aspects of microneedle research: usage, type of microneedle, type of molecule delivered, type of biomarker collected, and testing environment. Overall, 88% of papers were published by academics, 7% were published by individuals/groups outside academia and industry (designated as ‘Others’, data not shown), and only 5% were published by industry (Fig. 9). The ‘Others’ included public health institutes, clinics, hospital (not associated with a university) and national labs. Among the academic- and industry-authored papers, microneedles for delivery of molecules were most prevalent (73% and 85%, respectively), while the remaining fraction was equally distributed between diagnosis and other applications (Fig. 9 (i)). In academia, solid (35%) and dissolvable (28%) microneedles were most commonly used, whereas industry studies mostly examined hollow (39%) and coated (30%) microneedles (Fig. 9 (ii)). This difference may reflect industry preference for the more straightforward translational potential of hollow and coated microneedles, which do not generally require direct integration of a drug into the microneedle structure. In contrast, academics may prefer for the more complex but more powerful attributes of dissolvable microneedles, which have a patch format, reduce or eliminate sharps waste, enable enhanced thermostability, etc.
Figure 9.
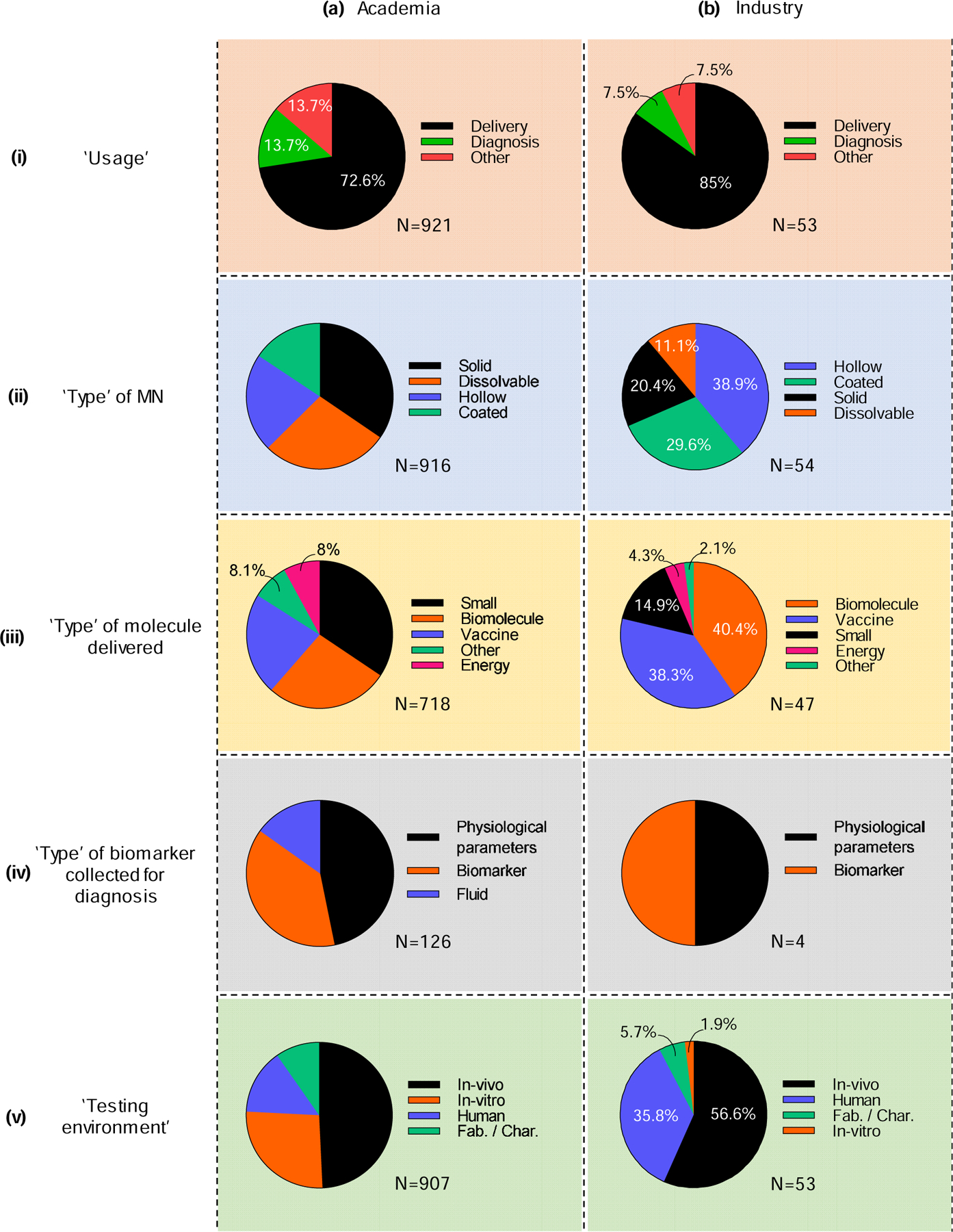
Contributions on microneedles in the scientific literature in terms of (i) usage of microneedles, (ii) type of microneedle, (iii) type of molecule delivered, (iv) type of biomarker collected, and (v) testing environment, as contributed by (a) academia and (b) industry.
Among papers studying delivery, small molecules (34%), biomolecules (27%), and vaccines (22%) each received significant attention in academia (Fig. 9 (iii) a), whereas industry focused more on biomolecules (40%) and vaccines (38%) and less on small molecules (15%) (Fig. 9 (iii) b). Delivery of energy and other materials was least frequently studied in both academic and industry settings (2–8%) (Fig. 9 (iii)). Industry may place a greater emphasis on biomolecules and vaccines because they are often higher added-value products that can justify increased expenses associated with introducing a new delivery technology.
Analyzing papers for diagnosis revealed similar, preferential use of microneedles for collection of physiological parameters and biomarkers by both academia and industry (Fig. 9 (iv)). Studies on collection of fluid using microneedles were only published by those in academia (15%) (Fig. 9 (iv) a). It is also notable that there have only been four papers published by industry authors on microneedle biomarker collection, indicating its very early stage of commercialization.
About half of the papers involved in vivo studies in both academia (49%) and industry (57%) (Fig. 9 (v)). While in vitro studies represented 26% of papers in academia, industry has published very few (2%). Industry instead emphasized studies involving humans (35%), compared to academia where only 14% of papers include human subjects (Fig. 9 (v)). This likely reflects the more-translational nature of industry, in which 92% of studies were in vivo in animals or in humans.
3.1.7. Scientific literature: geographic distribution of microneedle research
Authors from North America (38%) and Asia (36%) each contributed more than one-third of the published literature on microneedles, followed by Europe (22%) and Australia (3%) (Fig. 10 (i)). Among the research papers published globally, 37% were published by authors from the United States (USA), 12% each from South Korea and the United Kingdom (UK), 8% each from China and Japan, and 3.5% from Australia (Fig. 10 (ii)). In total, authors from 33 countries have contributed to the microneedle literature (Table S3 in SI).
Figure 10.
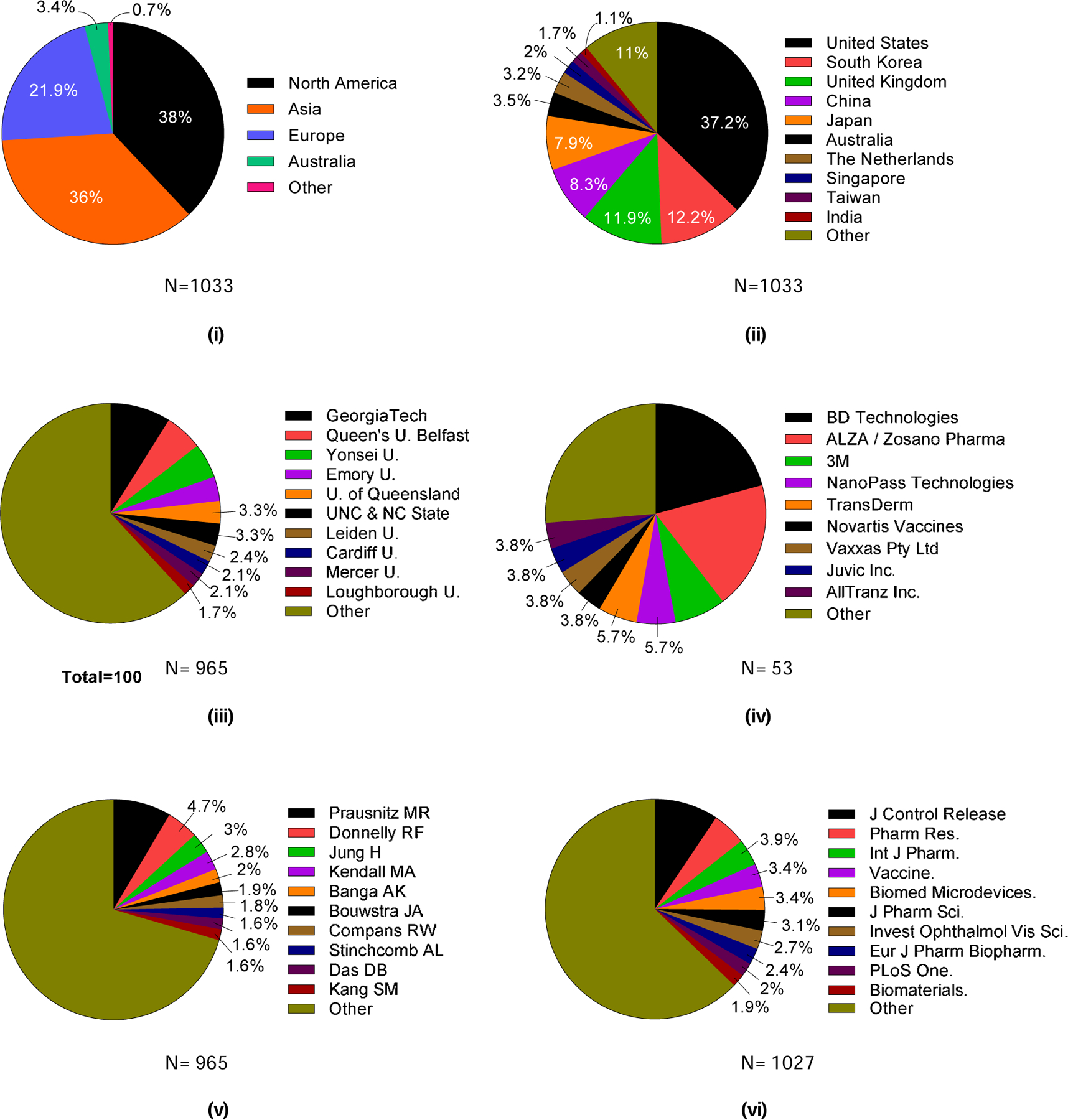
Contributions on microneedles in the scientific literature by location, institution, researcher, and journal. Contributions to the scientific literature on microneedles by location: (i) continents and (ii) top 10 countries. The 10 most prevalent contributors to the scientific literature on microneedles research by institution, researcher, and journal: (iii) universities among academic institutions, (iv) companies among industry institutions, (v) researchers, and (vi) journals. U = University. Georgia Tech = Georgia Institute of Technology. UNC = University of North Carolina. NC State = North Carolina State University.
Papers from different parts of the world sometimes emphasized different topics. Research on delivery represented 64–76% of research in each of the four continents primarily contributing to microneedle research (Fig. S1 (i) in SI). While research in North America was fairly evenly distributed among the four types of microneedles, papers from Europe and Asia somewhat favored solid microneedles and had less emphasis on coated microneedles (Fig. S1 (ii) in SI). In contrast, Australian researchers mostly studied coated and solid microneedles.
The type of molecule delivered was relatively evenly split among small molecules, biomolecules, and vaccines in North America and Europe, with few studies on energy delivery (Fig. S1 (iii) in SI). In Asia, researchers focused more on energy delivery and less on vaccines, whereas Australian researchers conducted more than three-quarters of their work on vaccines. North America and Europe each had roughly equal levels of activity regarding diagnostic systems for measuring physiological parameters and biomarkers, with less work on fluid collection (Fig. S1 (iii) in SI). Asian researchers much more frequently measured physiological parameters, while Australians focused almost exclusively on biomarker measurements. Finally, in vivo research represented at least half of studies in North America, Asia, and Australia, while European researchers’ work was more balanced between in vitro and in vivo studies (Fig. S1 (v) in SI). In general, distribution of research topics in North America and Europe tended to track each other more closely than research in Asia and Australia. Australian research sometimes had a significantly different scope because it was dominated by a single research group.
Of the top ten academic institutions that have published research papers, four were from the United States and three from the United Kingdom. Georgia Institute of Technology has published the most research papers (9%), followed by Queens University Belfast (6%), Yonsei University (5%), Emory University (3.5%), University of Queensland (3.3%), and University of North Carolina and North Carolina State University (3.3%) (Fig. 10 (iii)). While only 10 institutions are shown here, it is worth noting that authors from 258 institutions overall have published papers on microneedles, indicating widespread interest and activity in the field (Table S4 in SI).
Among all papers, 7% were from institutions other than academia or industry. Among these other institutes, 14% of the research papers were published by authors affiliated with the Chinese Academy of Sciences, 9% were published by those with the U.S. Centers for Disease Control and Prevention, and 4% from Sandia National Laboratories (Table S5 in SI).
Among research papers published by industry, authors from BD Technologies published 21% of studies and ALZA/Zosano Pharma published 19%. 3M (7.5%), NanoPass Technologies (6%), and TransDerm (6%) were also notably active in the field (Fig. 10 (iv)). BD Technologies and NanoPass Technologies both emphasized hollow microneedles in their studies, ALZA/Zosano Pharma mostly studied coated microneedles, and 3M utilized three different kinds of microneedles: solid, hollow, and coated. Twenty-two companies published on microneedles, largely comprising companies heavily focused on microneedle technology, in addition to those more broadly focused on pharmaceuticals, medical devices, or other technologies that have an interest in microneedles (Table S6 in SI).
The list of authors publishing on microneedles closely mirrored the list of institutions from which the authors came, indicating that most institutions had just one research group heavily active in microneedles. As such, the largest number of papers came from the laboratory of Mark Prausnitz (9%, Georgia Tech) followed by Ryan Donnelly (5%, Queens University Belfast), Hyungil Jung (3%, Yonsei University), and Mark Kendall (3%, University of Queensland) (Fig. 10(v)). Ajay Banga, Joke Bouwstra, Richard Compans, Audra Stinchcomb, Diganta Das, and Sang Moo Kang have each contributed to ~2% of published research papers.
The top three journals that have published papers related to microneedle research were pharmaceutical journals: The Journal of Controlled Release (9%), Pharmaceutical Research (5%), and International Journal of Pharmaceutics (4%) (Fig. 10(vi)). Papers have been published in journals from other fields, including vaccines (Vaccine, 3.5%), medical devices (Biomedical Microdevices, 3.5%), ophthalmology (Investigative Ophthalmology & Visual Science, 3%) and biomaterials (Biomaterials, 2%). It is worth noting that the journals in which the most publications appear are among the most respected journals in those fields, indicating the high impact of research on microneedles. In total, microneedles research has been published in 259 different journals, indicating the breadth of interest in this interdisciplinary field (Table S7 in SI).
3.2. Analysis of patents
Espacenet was used to gather and evaluate trends of microneedle inventions that appeared in patents.
3.2.1. Patent search: keyword and CPC based searches
Our initial keyword-based search identified 323 US patents, among which 1069 different CPC symbols were used 2941 times. Among the CPC symbols, most were used only once and ~98% were used fewer than 10 times. Our subsequent CPC-based search was based on frequently used and microneedle-related codes identified in the first search. A total of 2342 published and issued US patents were found between 2001–2018. Among these results, 45% were issued patents. Results that were not related to microneedles (e.g., needleless injectors) were removed from the list after our expert review, which left 750 issued patents. Our analysis is based on these patents. We found that all patents were assigned to at least two CPC categories, with a mean of 12 CPC symbols, mode of 5 CPC symbols and a maximum of 66 CPC symbols per patent (Fig. S2).
3.2.2. Patent search: annual US patent issuance trends
Similar to activity among scientific publications (Fig. 5 (i)), the number of issued patents per year has also increased over time (Fig. 11 (i)). In the first decade included in our analysis (2001–2010), 26% of the total patents were issued; in the most recent five years (2014–2018), more than 55% of the total patents were issued. An almost equal number of patents were issued in the first 10 years and the last 2 years, demonstrating greatly increased interest in patenting microneedles. Additional analysis of patenting trends over time are shown in terms of type of microneedles (Fig. S3 in SI) and usage of microneedles (Fig. S4 in SI).
Figure 11.
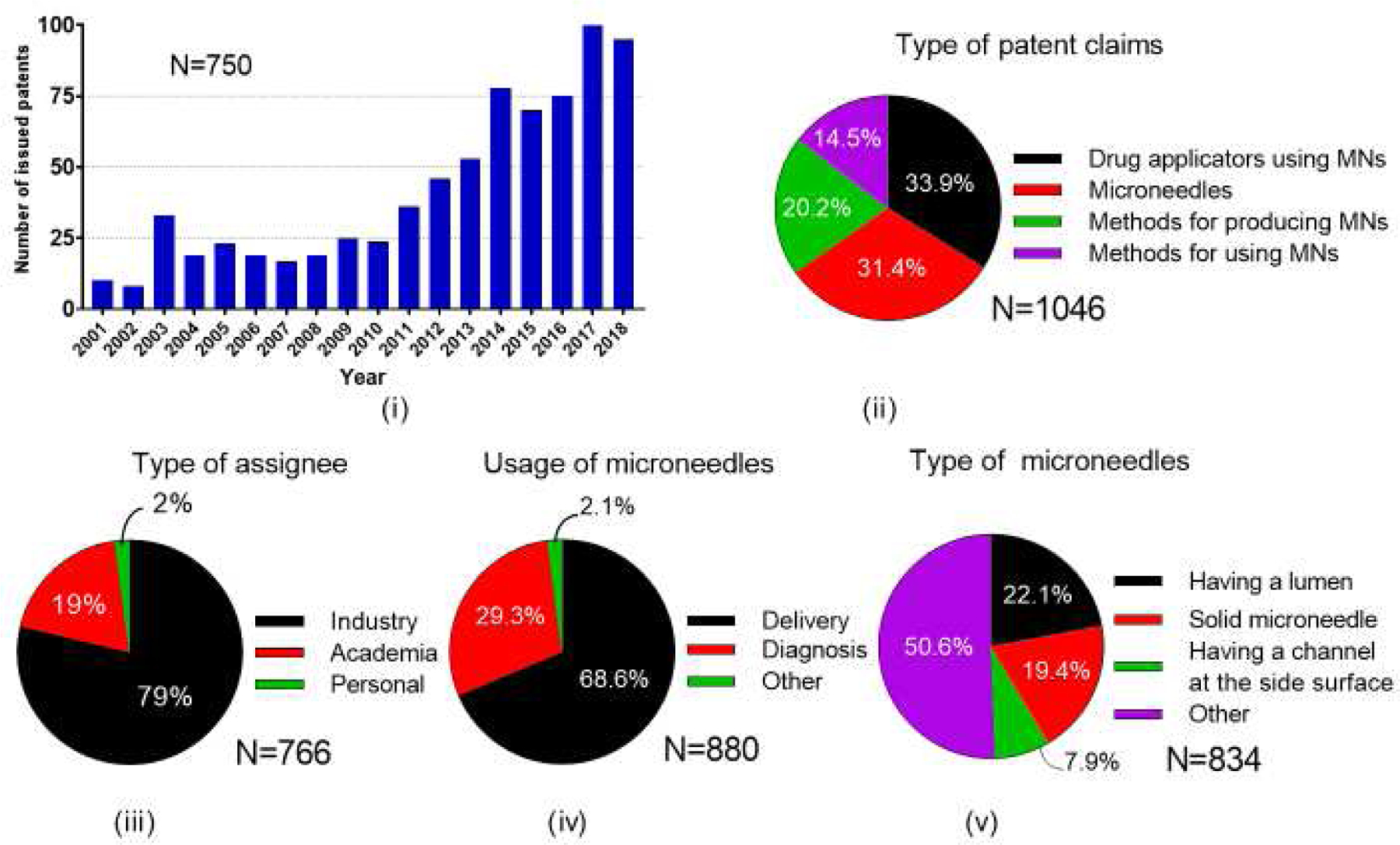
Total number of issued US patents on microneedles. i) Number of yearly issued patents, and their classification based on ii) type of patent claims, iii) type of assignee, iv) usage of microneedles, and v) type of microneedles.
3.2.3. Patent search: general trend in microneedle assignees and topics
Patent assignees were predominantly from industry (79%) with most of the rest from academia (19%) and the remainder filed by personally by individuals (2%) (Fig. 11 (iii)). Classification of patents based on their CPC symbols showed that types of inventions were roughly a third each on drug applicators using microneedles (34%) and on microneedles (31%), with the rest divided among methods for producing microneedles (20% and methods for using microneedles (15%) (Fig. 11 (ii)). The main usage areas were delivery (69%) and diagnosis (29.3%) (Fig. 11 (iv)), and 17% of all reviewed patents were issued using both delivery- and diagnosis-related CPC symbols (data not shown).
Unlike our analysis of the scientific literature and clinical trials, microneedle types were classified in the patent literature as microneedles having a lumen (22%), solid microneedles (19%) and microneedles having a channel at the side surface (8%), where the remainder had other classifications (51%) (Fig. 11 (v)). Looking more closely at how these main three types of microneedles were used, we found that delivery was the usage area at least three times as often as diagnosis for all three microneedle types (Fig. 12 (i)).
Figure 12.
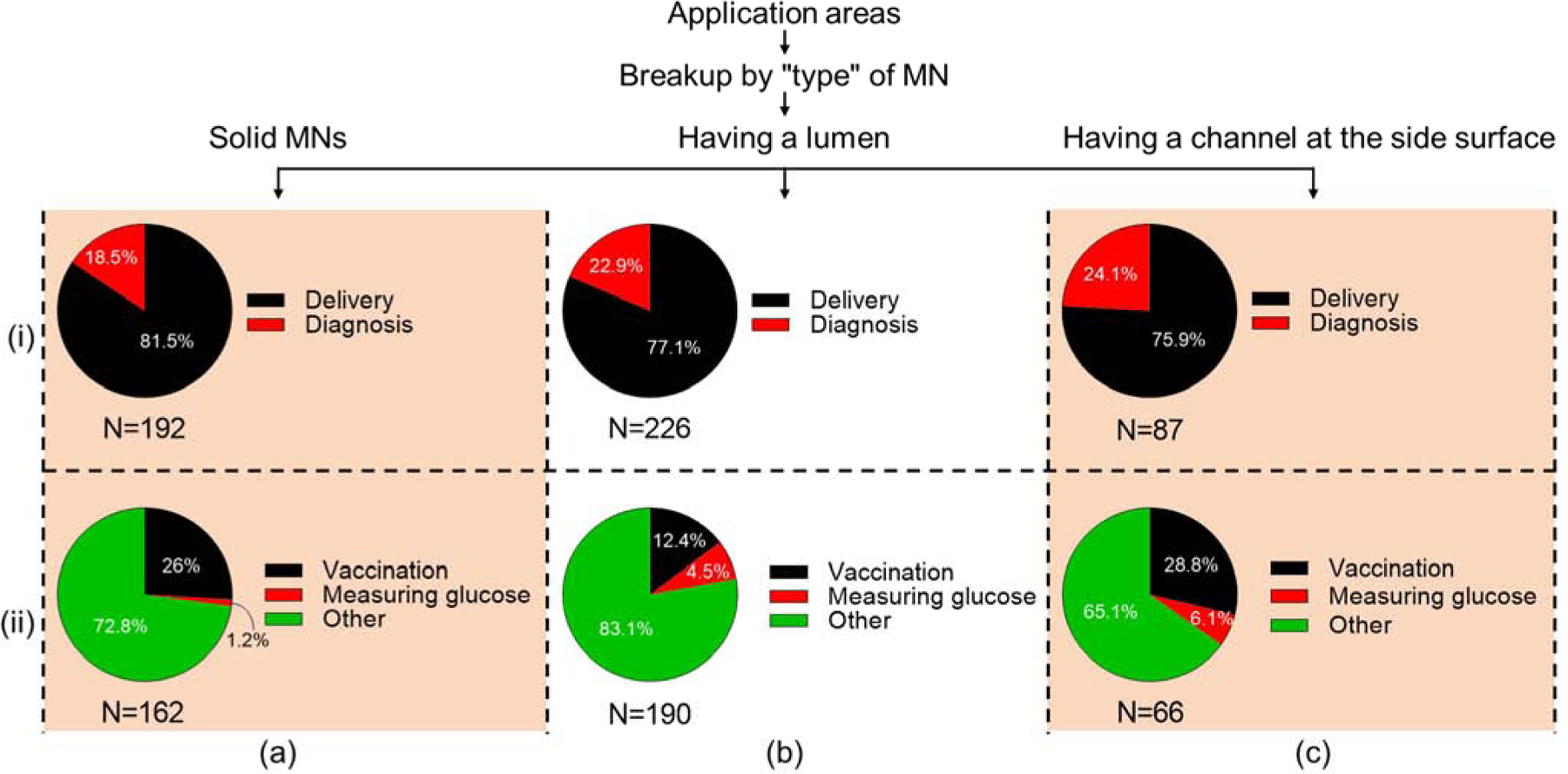
Analysis of application areas of different microneedle types in issued patents. (i) Delivery vs. diagnosis and (ii) vaccination vs. measuring glucose applications made with (a) solid microneedles, (b) microneedles having a lumen, (c) microneedles having a channel at the side surface.
The patents were also examined for vaccination and measuring glucose, which are among the most common application areas (and those for which CPC symbols exist). Vaccination appeared much more frequently as an application for all three microneedle types, and measuring glucose was more often performed with hollow microneedles (microneedles having a channel at the side surface and microneedles having a lumen) (Fig. 12 (ii)). However, the large majority of patents were not identified by a specific category. As such, the other category had the largest area for all three microneedle types. Additional analysis of the most-frequently used CPC symbols and the prevalence in microneedle patents in shown in Figures S5 and S6 in SI.
Among microneedles used for delivery, microneedles having a lumen were often used (27%) which was followed by solid microneedles (24%) and microneedles having a channel at the side (10%) (Fig. 13 (i)). Analysis of the microneedle types used for diagnosis yielded similar ranking. where microneedles having a lumen were more commonly used (15%), followed by solid microneedles (11%) and microneedles having a side channel (8%) (Fig. 13 (ii)). In both scenarios, however, type of microneedle was not specified in the CPC symbols.
Figure 13.
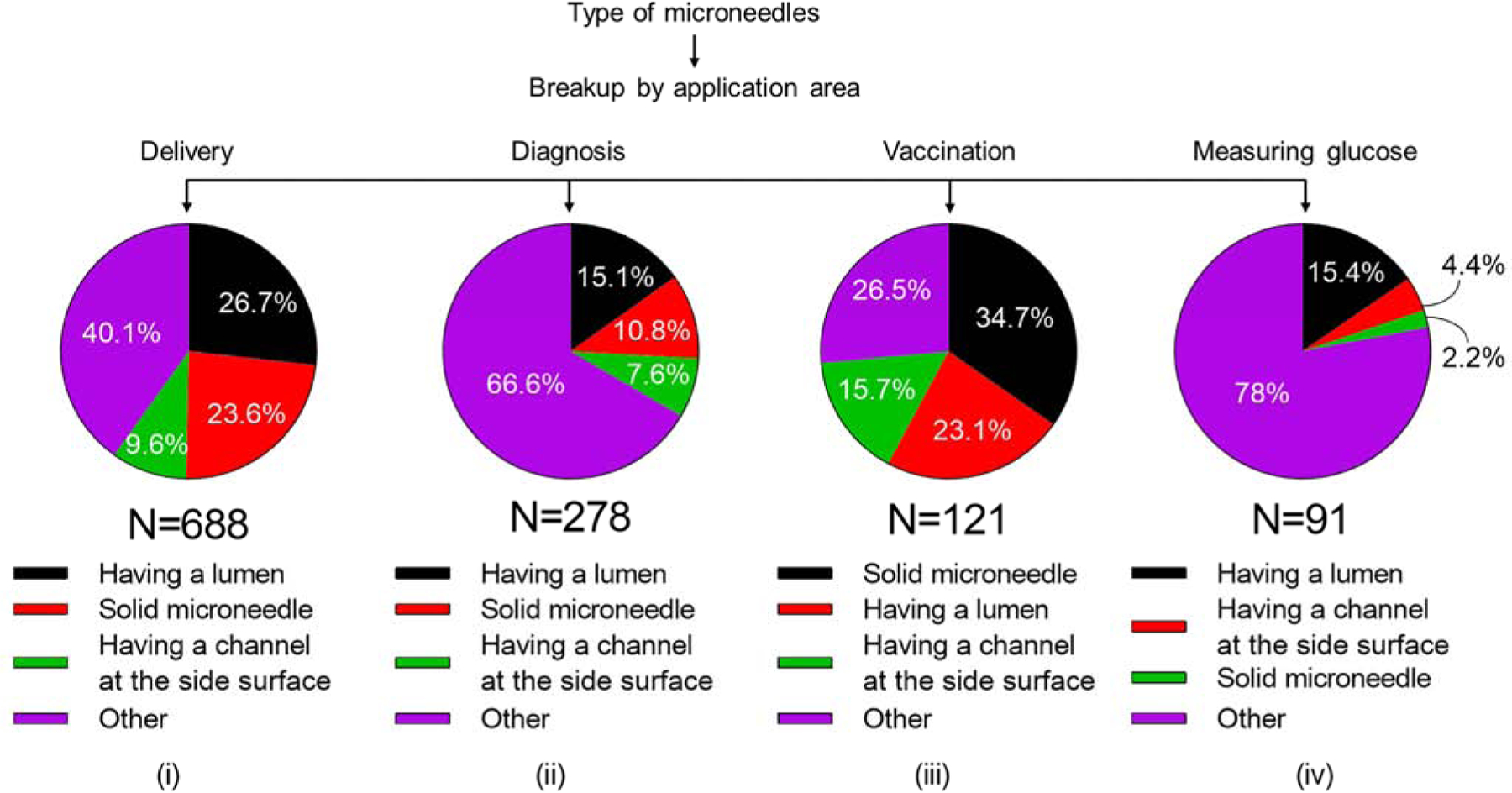
Analysis of microneedle types used in (i) delivery, (ii) diagnosis, (iii) vaccination, and (iv) measuring glucose.
Considering specific applications, microneedles for vaccination were most typically solid microneedles (35%), followed by microneedles having a lumen (23%) and those having a channel on the side (16%) (Fig. 13 (iii)). When used for measuring glucose, microneedles having a lumen (15%) were more popular than microneedles with a side channel (4%) solid microneedles (2%) (Fig. 13 (iv)). Again, however, type of microneedle was often unspecified.
3.2.7. Patent search: microneedle patents in academia and industry; assignees and inventors
Patenting of microneedles was led by industry, with 79% of assignments. Academia had 19%, and personal assignments were only 2% of total issued patents (Fig. 11 (iii)). Among academic institutions, Georgia Tech Research Corporation was at the top of the list with 12% of total patents (17 patents). Next was the University of California (10%) followed by University of Utah Research Foundation (4%). Patents have been issued to 64 institutions in academia (Table S8 in SI), but nearly half of the total patents were assigned to the top 10 universities (Fig. 14 (i)).
Figure 14.
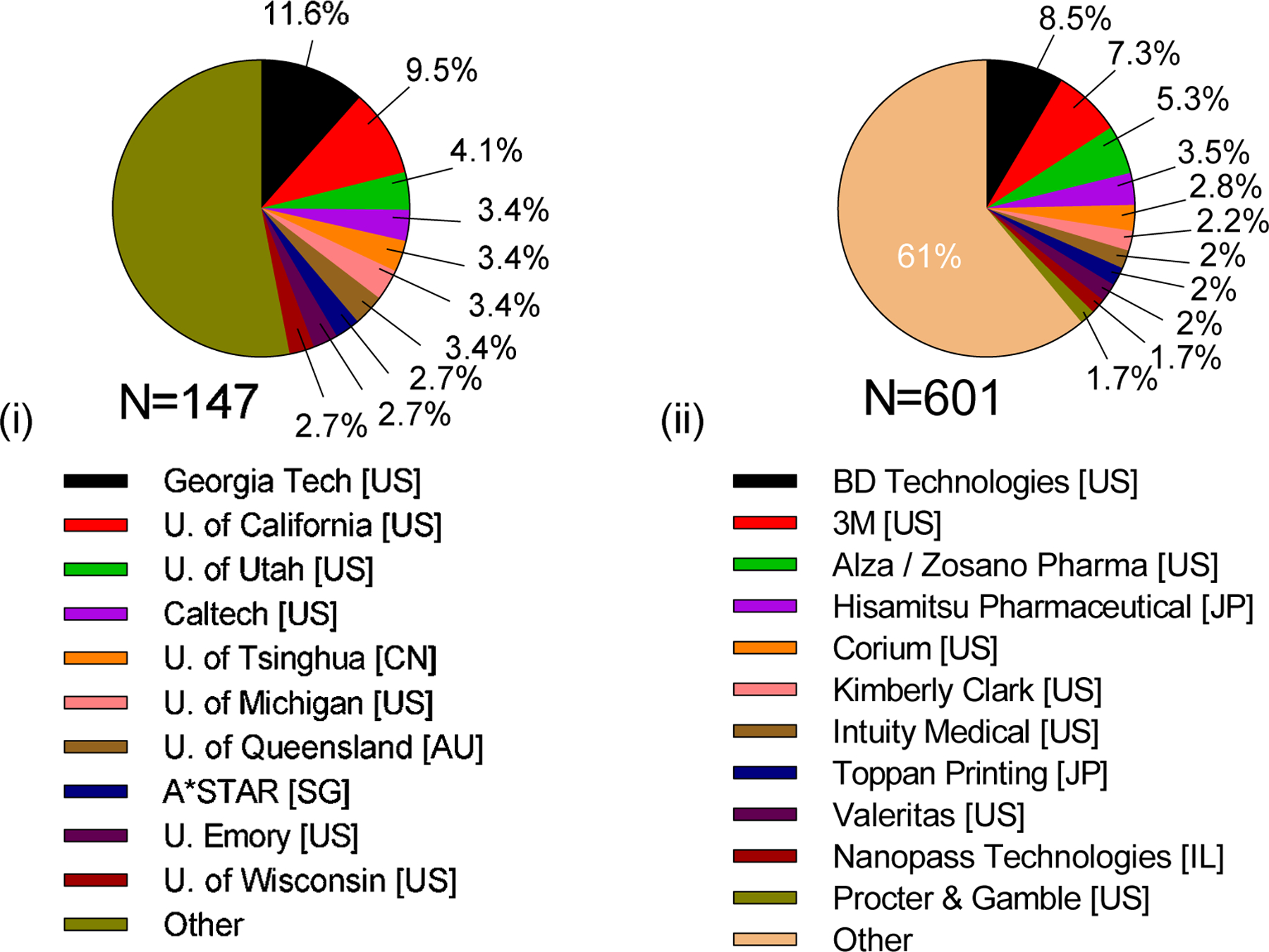
Contributions of microneedle patents invented in academia and industry. (i) Top universities in academia and (ii) top companies in industry patenting inventions on microneedles. U = University. Georgia Tech = Georgia Institute of Technology. Caltech = California Institute of Technology. A*STAR = The Agency for Science, Technology and Research. BD = Becton Dickinson and Company.
In industry, BD Technologies has the largest number of patents (9%, 51 patents). 3M Innovative Properties Company was next (7%), followed by ALZA /Zosano Pharma (5%) and Hisamitsu Pharmaceutical Company (4%). It is notable that the fifth most prolific company at patenting (Corium International) had as many patents as the most prolific university among the academics (Georgia Tech Research Corporation), further demonstrating how much more active industry has been at patenting compared to academia. Among 193 companies that received patents (Table S9 in SI), the top 10 companies had almost 40% of total patents assigned to industry (Fig. 14 (ii)).
Academia and industry patents regarding vaccination were also investigated, since vaccines represent an important application area of microneedles. ALZA/Zosano Pharma had the most patents (17%) followed by BD Technologies (14%). Georgia Tech Research Corporation was the only academic institution among the top 10 (7%), ranking fourth after Corium International (9%) (Fig. S7).
A total of 1197 inventors contributed to the microneedle patent literature and more than 60% of them were inventors of only one patent (Table S10 in SI). Top inventors with 10 or more patents in the microneedle field contributed more than 30% of total patents; they are shown in Fig. 15. Michel Cormier and Ronald Pettis are the most prolific inventors (both 3.1%), followed by Joseph Trautman (2.7%); Mark Prausnitz and Faiz Sherman were in joint fourth place (both 2.1%).
Figure 15.
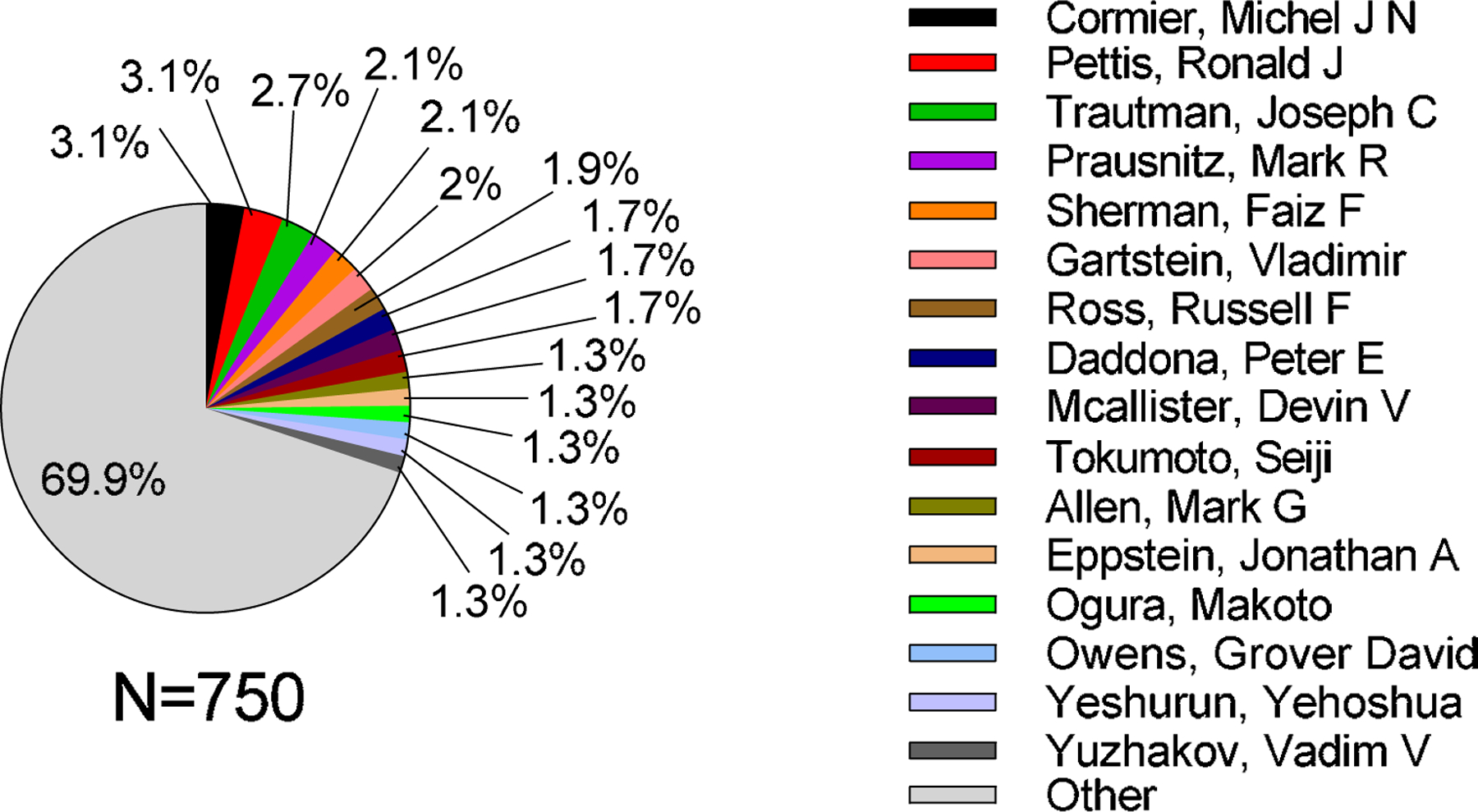
Analysis of microneedle patents by the top inventors. “Other” represents the sum of patents that were not issued to the listed inventors.
3.2.8. Patent search: geographic distribution of microneedle patents
Inventions in the USA contributed about two-thirds of patents on microneedles for industry (69%), academia (63%) and overall (65%). Japan was the second highest producer of patents in industry (12%) and overall (9%), but was not a top contributor among academics. Other important countries for inventions from industry were Israel and Germany (3% each); from academia were Taiwan (8%) and Korea (5%); and overall from Korea (4%) and Germany (3%) (Fig. 16 (i) and Table S11 in SI).
Figure 16.
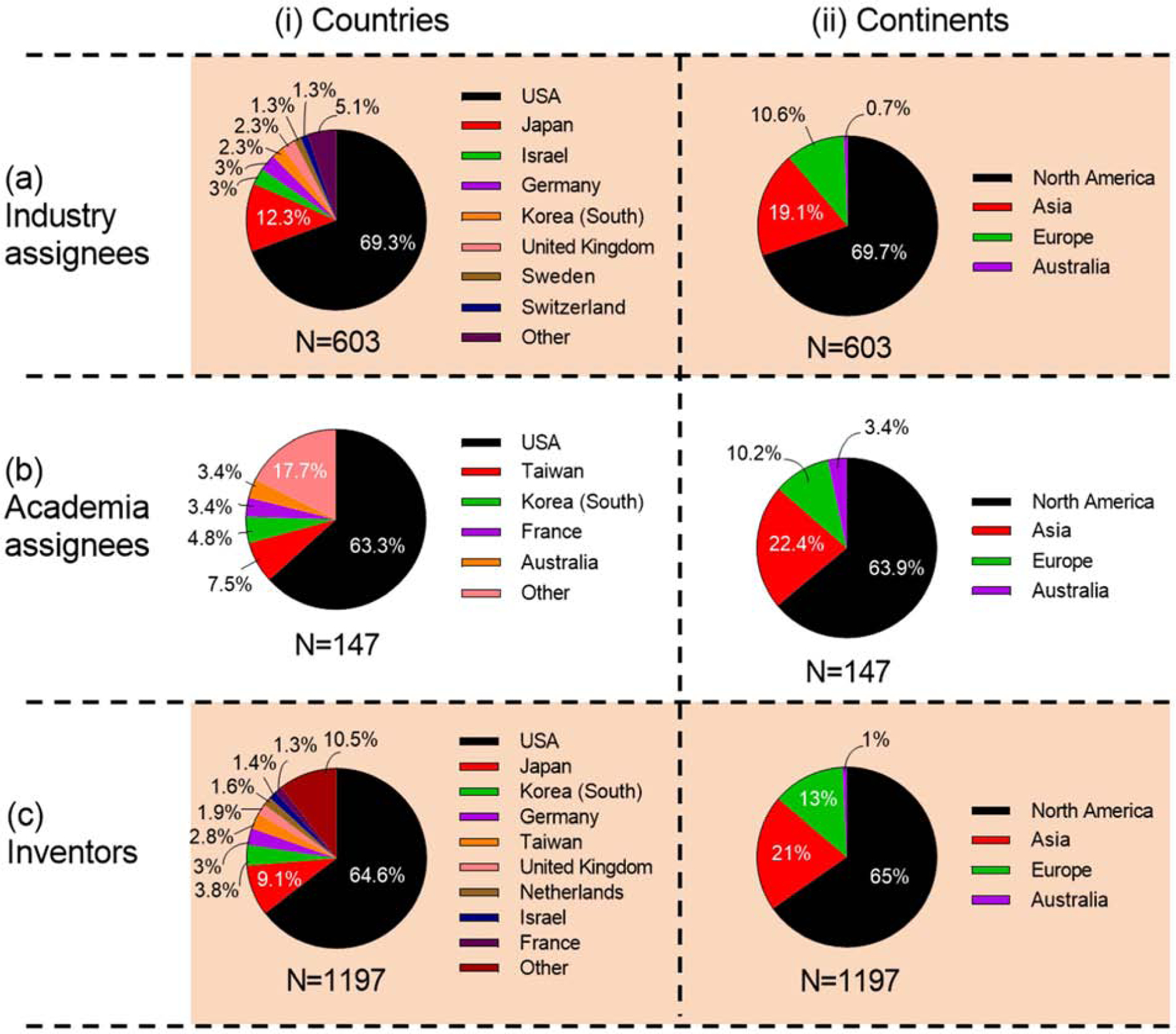
Contributions of microneedle patents by countries and continents of assignees. (i) Countries and (ii) continents of assignees from (a) industry, (b) academia, and (c) overall.
The rank order of continents as a source of patents were the same for industry, academia, and overall. North America had the highest contribution with more than 60% in each category, followed by Asia (~20%), Europe (~10%), and Australia (Fig. 16 (ii)).
3.3. Analysis of clinical trials
An analysis of clinical trials reported at clinicaltrials.gov identified the applications of microneedle technology that had advanced furthest towards clinical exemplification and practice. This database includes clinical trials that are completed, active, recruiting, or not yet recruiting, or have some other status.
3.3.1. Clinical trials: annual publication trend and clinical phase
A total of 79 clinical trials were found based on our search of clinicaltrials.gov (Fig. 4 (ii) a). Among those trials, 25% were designated Phase 1, 17% Phase 2, 10% Phase 3, and 3% Phase 4. A large proportion of the trials (44%) were not formally classified by phase (N/C) (Fig. 4 (ii) b). The data show an increase in clinical trial activity, from the first study reported in 2007 to 10–15 trials per year in recent years (Fig. 4 (ii) a). The most prolific year so far for clinical trial activity was 2012, with 16 clinical trials, 6 in either Phase 2 or 3, representing a wide range of microneedle formats and exploring the treatment of, or protection against, a wide variety of conditions. Unsurprisingly, clinical study phases have tended to transition from early stages (Phase 1 and 2) in earlier years towards later stages more recently: 19% of clinical studies between 2015 and 2018 were classified as Phase 3 or 4. Most of the reported clinical studies have been completed (70%), although some are either recruiting (5%), not yet recruiting (10%), have been terminated (3%), or have an unknown status (13%) (Fig. 4 (ii) c).
3.3.2. Clinical trials: general trend in microneedle types, their use, and medical indication
Almost all (98%) clinical trials focused on delivery of substances to the skin or eye, with just 1% using microneedles for diagnosis and 1% for another use (i.e., assisted hatching in embryo transfer) (Fig. 17 (i)). With respect to delivery, 27% of clinical trials studied the delivery of small molecules, followed by biomolecules (22%) and vaccines (18%) (Fig. 17 (ii)). Considering biomolecules and vaccines together, we noted that more studies explored the potential clinical utility of microneedles for delivery of biologics (i.e., biomolecules and vaccines, at 40%) than small molecule drugs (27%). Energy delivery was the focus of 16% of the trials, and studies using placebos represented 12% of trials.
Figure 17.
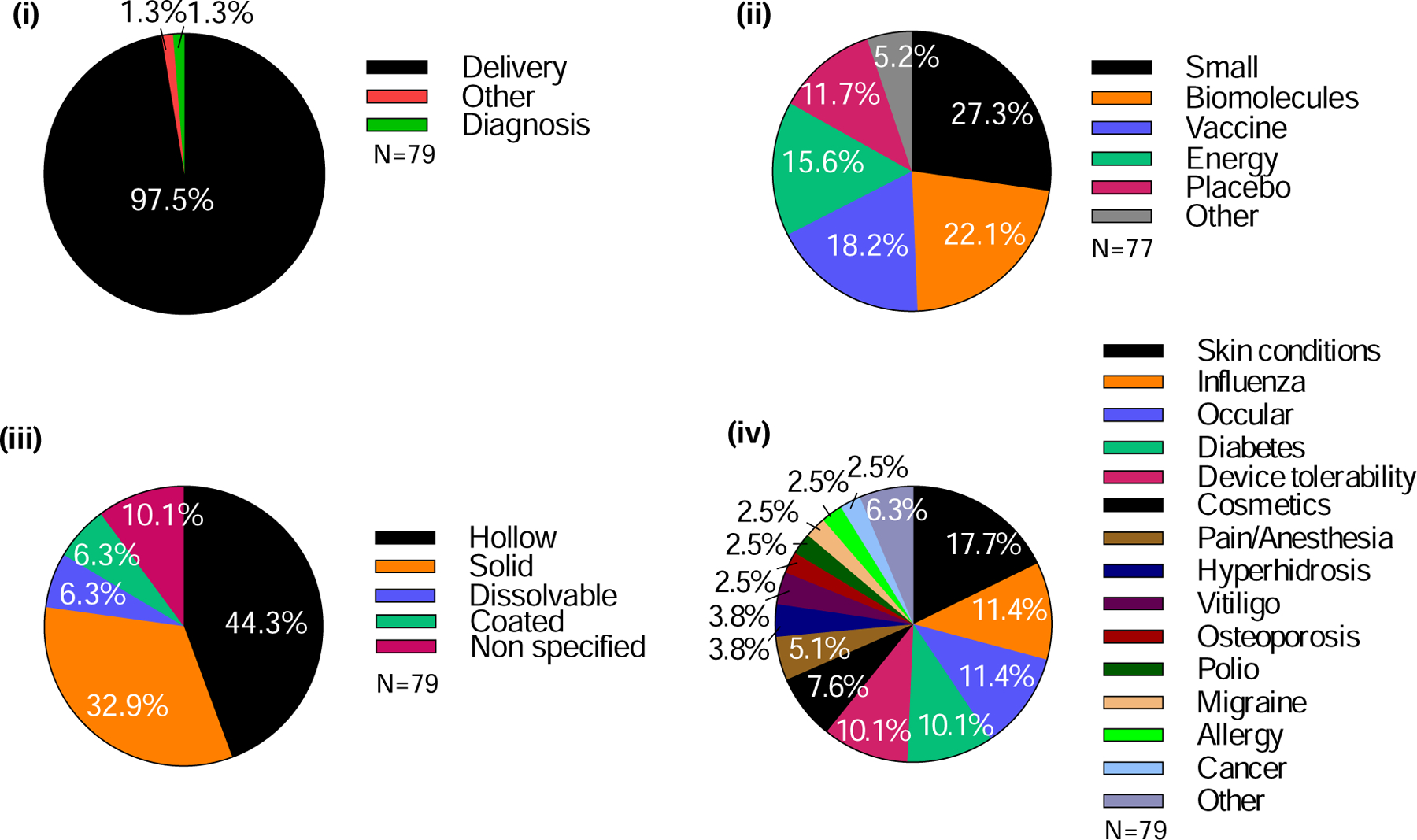
Analysis of attributes of clinical trials involving the use of microneedles. (i) Purpose of study, (ii) type of molecule delivered, (iii) type of microneedle, and (iv) indications to be treated.
Hollow microneedles were used in 44% of the clinical trials and solid microneedles were used in 33% (Fig. 17 (iii)). Dissolvable and coated microneedles have each only been used in 6% of the trials, and only since 2012 (coated) and 2015 (dissolvable).
With respect to the therapeutic purpose of the clinical trials, skin conditions have been studied the most (18%), followed by influenza vaccination (11%), treatment of ocular diseases (11%), diabetes (10%), and general studies of microneedle device tolerability (10%) (Fig. 17 (iv)). Other significant categories include the use of microneedles for cosmetic indications (8%) and for pain/anesthesia (5%).
3.3.3. Clinical trials: microneedle studies in academia and industry; institution and location
Almost two-thirds of the reported microneedle clinical trials (62%) were sponsored by academic institutions, with the remaining 38% of trials sponsored by industry (Fig. 18 (i) a). Notably, among trials conducted in academia, more than three-quarters (79%) were either non-classified (N/C) (57% of academic studies, representing 34% of the total studies) or in Phase 1 (22% of academic; 14% of total); the remaining 21% (13% of total) were in Phases 2, 3, or 4 (Fig. 18 (i) b). For those trials conducted in industry, 54% (22% of total) were either N/C or Phase 1, and 46% (18% of total) were in Phase 2, 3, or 4 (Fig. 18 (i) c).
Figure 18.
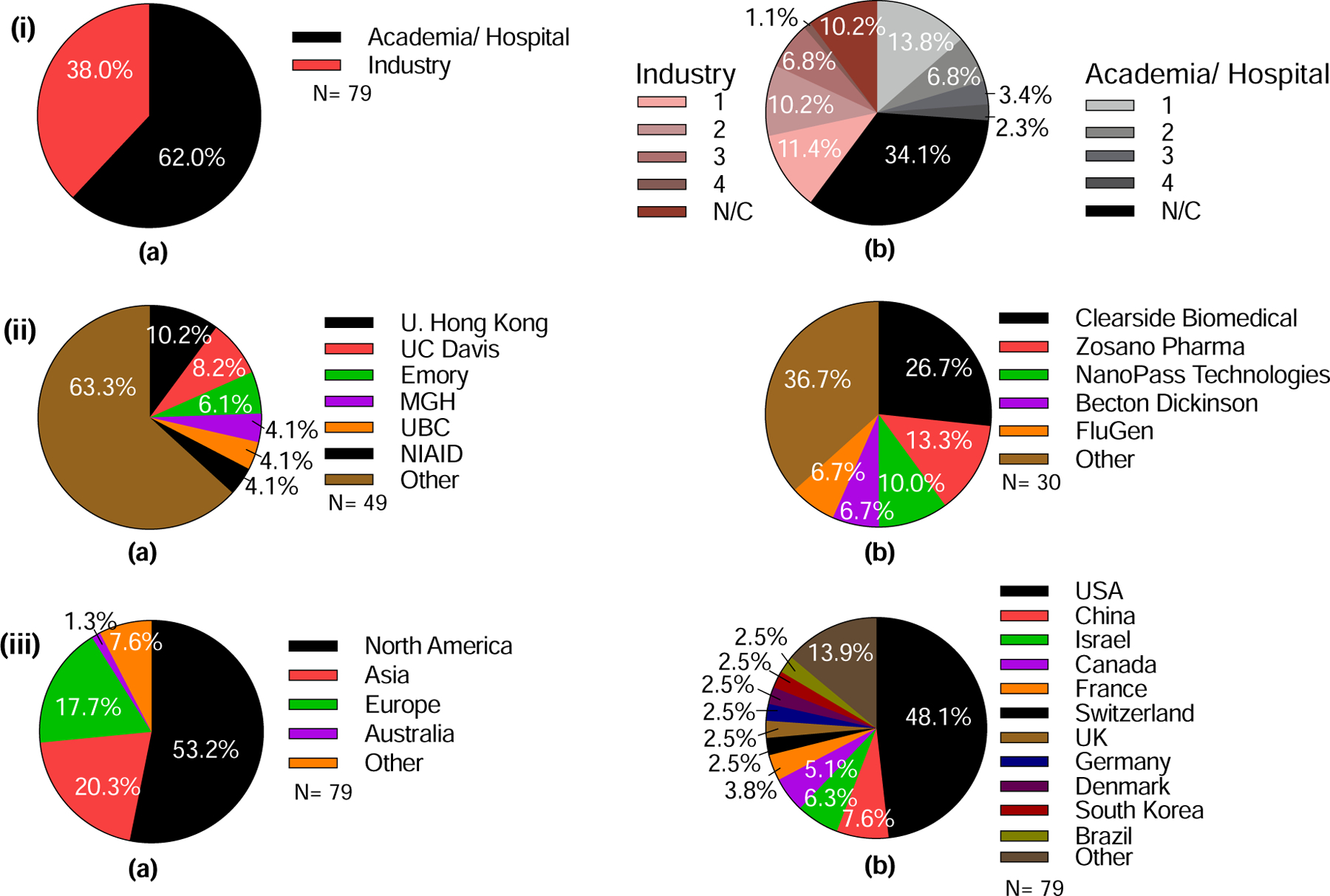
Analysis of clinical trials involving the use of microneedles in terms of sponsor type, organization, and location. (i) Clinical trials sponsored by academia/hospital versus industry. (a) Percentage of clinical trials carried out by academia/hospital or industry. b) Detailed analysis of stages of clinical trials sponsored by academia/hospital or industry. (ii) Institutions sponsoring microneedle clinical trials from (a) academia/hospital or (b) industry that carried out more than one clinical trial. (iii) Location of microneedle clinical trials sponsors, in terms of (a) Continents and (b) Countries. UC Davis = University of California, Davis, MGH = Massachusetts General Hospital, UBC = University of British Columbia, NIAID = National Institute of Allergy and Infectious Diseases. Note, the percentages shown on the charts relate to the total number of studies (n=79) in (i) and (iii) and the number of studies sponsored by academia (n=49) (a) and industry (n=30) (b) in (ii).
Among academic institutions, the University of Hong Kong conducted the largest number of microneedle clinical trials (10%), followed by UC Davis (8%), Emory University (6%), and Massachusetts General Hospital, the University of British Columbia, and National Institute of Allergy and Infectious Diseases (4% each) (Fig. 18 (ii) a). Specifically, the University of Hong Kong has primarily studied the use of hollow microneedles for vaccination; similar studies were carried out by the National Institute of Allergy and Infectious Diseases. The University of California, Davis investigated the use of solid microneedles to assist photodynamic therapy and topical anesthesia. Emory University trials focused on the use of dissolvable, hollow, and solid microneedles in collaboration with the Georgia Institute of Technology. Massachusetts General Hospital and the University of British Columbia used both solid and hollow microneedles for insulin delivery and vaccine delivery, respectively. A total of 37 academic institutions conducted clinical trials using microneedles (Table S12 in SI).
With regard to clinical studies sponsored by industry, Clearside Biomedical sponsored more than a quarter of the reported clinical trials (27%), specifically studying suprachoroidal steroid delivery to the eye (Fig. 18 (ii) b). Other pioneering companies in the field include ALZA/Zosano Pharma (14%), delivering small molecules using coated microneedles, and NanoPass Technologies (10%), who exclusively focused on hollow microneedles for vaccination, local anesthesia, or insulin delivery. Becton Dickinson and FluGen each sponsored 7% of the industry trials, addressing the intradermal infusion of insulin or optimization of the delivery method, respectively. In all, 16 companies had conducted clinical trials (Table S13 in SI).
3.3.4. Clinical trials: geographic distribution of microneedle studies
Consistent with the scientific literature, North America was the site of most clinical research activity (53%), followed by Asia and Europe, each conducting 18–20% of clinical trials (Fig. 18 (iii) a). The remainder were carried out in Australia, Brazil, Egypt, Morocco, and Turkey. More specifically, almost half (48%) of the clinical trials took place in the United States, followed by China (8%), Israel (6%), Canada (5%), and France (4%) (Fig. 18 (iii) b). Clinical trials have been conducted in a total of 21 countries around the world (Table S14 in SI).
3.4. Analysis of internet/social media
Microneedle technology has received significant attention beyond the conventional scientific arena. The degree and foci of this attention were captured by i) reviewing the timeline of relative interest and tabulating topics and queries identified by Google Trends, and ii) conducting Altmetric analyses of interest across social media platforms.
3.4.1. Internet/social media: general trends of interest in microneedles
As shown in Fig. 19 (i), the level of interest in searching for online content relating to microneedles has steadily increased since 2008 from a relative interest score around 15 until a recent peak value of 100 (as measured by the number of online searches through the Google search engine). A notable spike in interest was observed in the first quarter of 2013, which may be attributed to the introduction of a new cosmetic “dermaroller” device to the market. Dermaroller devices puncture solid microneedles into the skin to cause focal micro-injuries in the dermis. The resulting healing process produces collagen, which makes the skin fuller and reduces the appearance of wrinkles. This treatment is called collagen induction therapy [47].
Figure 19.
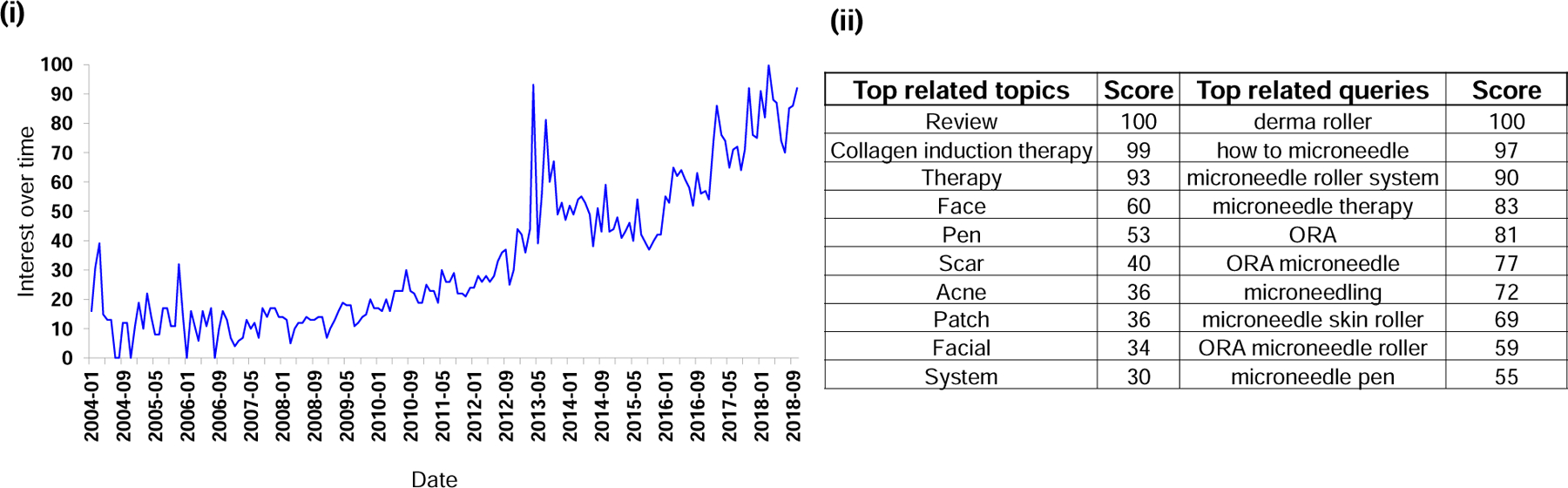
Interest in microneedles as determined by searches on Google. (i) Interest in microneedles via searches over time. The value for search interest over time is relative to the highest point on the chart. (ii) Top topics and queries related to the microneedle search, i.e., users searching for microneedles also searched for these topics or queries.
We tracked Google search engine activity to determine related “queries”, which are searches that are also searched for alongside the entered search term (i.e., microneedle), and related “topics”, which are broader topics that users searching for microneedles are searching for. Most topics searched in Google relate to the use of microneedles in cosmetic skincare; such search terms included collagen induction therapy, face, pen, scars, acne, patch, facial, etc. (Fig. 19 (ii)). Although outside of the top ten most-searched topics, there were also other search topics related to the use of microneedles for medical purposes, including pharmaceutical drug, vaccine, and drug delivery, which all had scores (a relative scale based on the absolute search term for the volume) below 22. Top queries (searches that are most frequently searched alongside the entered term) related to microneedles include dermaroller, how to microneedle, and other aspects related to use of microneedles for beautification, such as microneedle roller system, microneedle therapy, microneedling, microneedle skin roller, microneedle roller, ORA microneedle roller (ORA is a brand name of microneedles), microneedle pen, etc. (Fig. 19 (ii)). It is clear that the general public tended to be much more interested in the use of microneedles for cosmetic purposes than for medical applications.
3.4.2. Internet/social media: geographic distribution of interest in microneedles
Data obtained through Altmetric demonstrate the distribution of global interest in microneedles, based on 4410 specific online mentions of microneedle or microprojection in various media and social media outputs in 93 countries around the world. Almost one-third of these mentions were in the United States (32%), followed by the United Kingdom (12%), Spain (3%), India (2%), and Australia (2%), among a total of 93 countries, although the country of origin in the case of 30% of the mentions is unknown (Fig. 20 (i)). With regard to the actual platforms that produce these mentions, 69% of mentions were on Twitter, 6% on Facebook, and 25% in news media outlets (Fig. 20 (ii)–(iv)). Again, microneedle mentions on these specific social media outlets are dominated by the United States, followed by the United Kingdom and, depending on the platform, Spain, Italy, and India. Given the widespread use of microneedles for cosmetics in Asia, it is perhaps notable that there are not more online mentions of microneedles in that part of the world.
Figure 20.
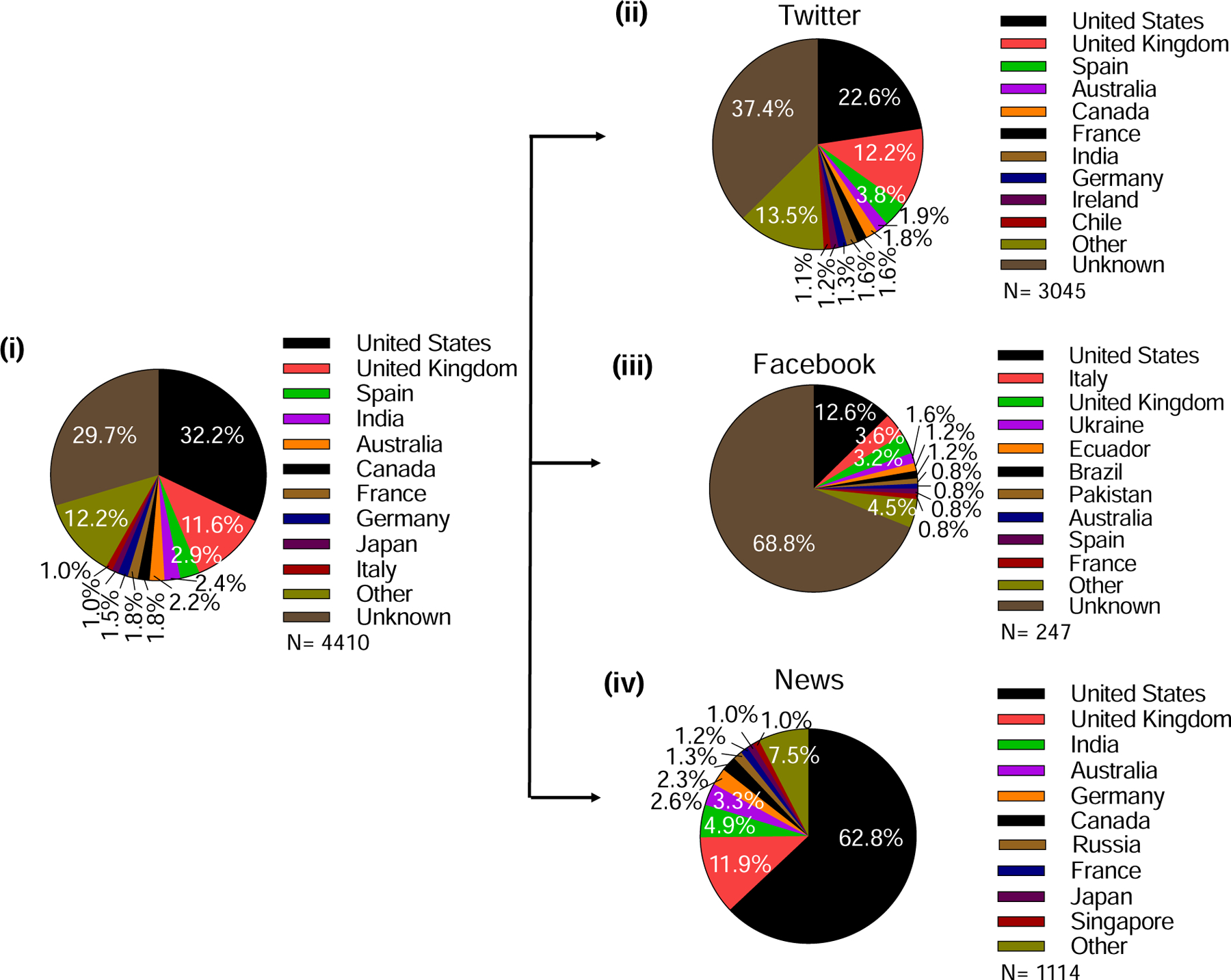
Demographics of metrics showing online mentions of microneedle or microprojection by various media. (i) Analysis of all mentions. Analysis of mentions posted on (ii) Twitter, (iii) Facebook, and (iv) in the news media.
3.5. Commercially available microneedle products
A number of products based on microneedle technology have been introduced into the market for use on patients by health care personnel for medical purposes or, more commonly, for use by people on themselves for cosmetic applications. While there are many products that could be mentioned, here we present a few representative examples as an illustration.
Although larger than a typical microneedle, measuring ~2 mm in length, an array of solid, metal microneedles has been approved for many years for use in a number of countries as a skin pretreatment before application of bacille Calmette-Guerin (BCG) vaccine to increase vaccine uptake into the skin (Fig. 21 (i)). A similar type of device containing an array of sub-millimeter, plastic microneedles received FDA clearance to create microchannels in the skin (Fig. 21 (ii)). A hollow microneedle mounted into a syringe was also developed for targeted skin injections, and has been used as part of a prefilled microinjection system for intradermal delivery of influenza vaccine, which was approved for use in many countries (Fluzone, Sanofi Pasteur, Swiftwater, PA) (Fig. 21 (iii)). Other microneedle-based drug products are under development, but do not yet have FDA approval.
Figure 21.
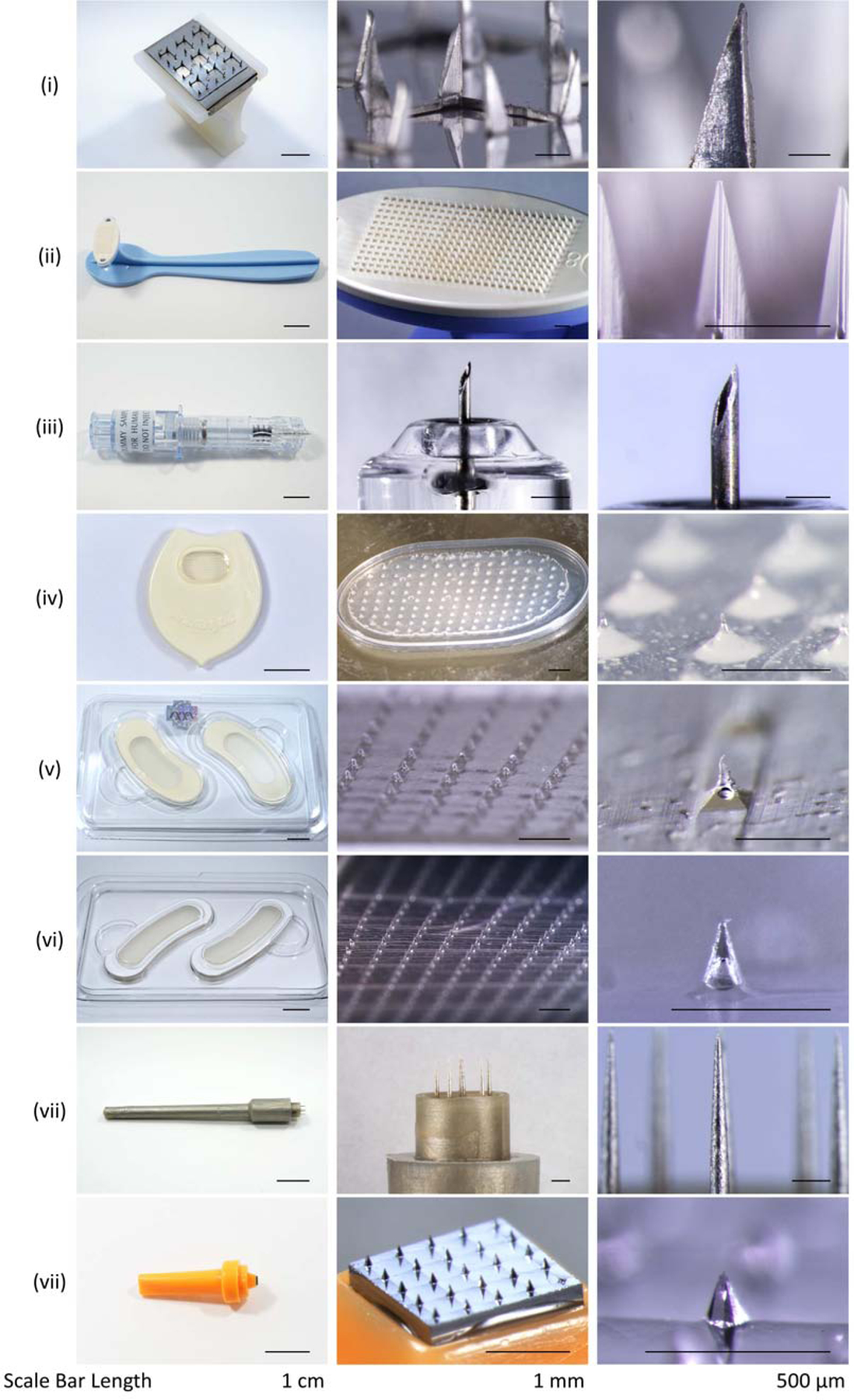
Representative examples of microneedle products approved for medical use or sold as cosmetics. (i) Sterile Multipuncture Device, Organon Teknika Corporation (Durham NC, USA). (ii) Microchannel Skin System, 3M (St. Paul, MN, USA). (iii) Soluvia microinjection system, BD (Franklin Lakes, NJ, USA). (iv) MicroHyala, CosMED Pharmaceutical (Kyoto, Japan). (v) Wellage Hyaluronic Acid Micro Needle Patch, Hugel (Chuncheon, South Korea). (vi) Reviewcell Snow White Hyaluronic Sheet, Soya Greentech (Seoul, South Korea). (vii) Dermastamp, Dermaroller (Wolfenbüttel, Germany). (viii) Liteclear Acne Treatment System, Nanomed Skincare (Cupertino, CA, USA).
A variety of different microneedle patches have been developed for cosmetic application, especially to combat changes in skin appearance due to aging (Figs. 23 (iv) – (vi)). These microneedles are often made of hyaluronic acid, which is the same material commonly injected into the skin as a filler to reduce the appearance of wrinkles. Microneedle arrays have also been used to have mechanical effects on the skin for collagen induction therapy, where the microneedle penetration into skin stimulates collagen production that reduces the appearance of wrinkles and scars (Fig. 21 (vii)). This technique is often called ‘microneedling’. Microneedle patches have also been paired with topical formulations containing active ingredients (e.g., anti-acne) applied to the skin after microneedle pre-treatment (Fig. 21 (viii)).
4. Discussion
4.1. Increasing interest in microneedles
Activity in microneedles research and patenting has increased, especially over the past 10–15 years. Annual publications in 2017 increased by ~10-fold in the ten years since 2007, reaching a total of more than 1000 papers. The number of clinical trials per year increased ~6-fold from 2007 to 2018, totaling 79 as of the time of this analysis. Annual patent approvals in 2017 increased ~10-fold from 2001, with a total of 750 issued patents. There is, of course, a lag between research being published (e.g., ~1 year) and research leading to an issued patent (e.g., many years). Internet/social media focus on microneedles has increased as well, growing 5–10 fold from a steady baseline in 2004–2008, followed by an increasing rate after that. Online interest in microneedles, however, was fueled predominantly by cosmetic applications, which play a relatively small role in the number of papers, patents, or clinical trials.
4.2. Topics of interest for microneedle applications
Among the research papers and patents, the large majority addressed delivery, and the remaining materials were evenly split between addressing diagnosis and other topics. Essentially all clinical trials were on delivery. This may be driven by a compelling need to simplify administration of drugs and vaccines that would otherwise require injection, which is greater than the perceived need for minimally invasive diagnostic methods. It may also reflect that fact that research funding for pharmaceuticals is much greater than for diagnostic devices.
The research papers had a fairly evenly distributed focus on each of the four types of microneedles, which indicates a recognition that each type has strengths and weaknesses that make microneedle design selection highly application dependent. Hollow microneedles may be regarded as smaller versions of conventional hypodermic needles, which can make manufacturing, drug formulation, and regulatory approval more straightforward. Solid microneedles similarly do not have drug directly associated with them, which can simplify product development. Dissolvable and coated microneedles represent a greater departure from existing products, but accordingly offer more potential advantages because of their simple-to-use patch-based format. These microneedles must incorporate drug, which complicates formulation and manufacturing, especially for dissolvable microneedles, where the microneedle itself is made of drug, but offers the advantage of generating no sharps waste.
Drug delivery studies were approximately evenly divided among small molecule, biomolecule, and vaccine delivery systems in papers and clinical trials. Small molecules were preferentially administered using solid and dissolvable microneedles, biomolecules were delivered using all four types of microneedles, and vaccines were mostly given by dissolvable and coated microneedle patches. This further supports the assertion that choice of microneedle type depends on application. Energy is administered almost exclusively using solid microneedles, which provide the simplest means of conducting electricity into the body.
Studies addressing diagnostic applications tended to utilize hollow and solid microneedles, since the added functionality—and associated design complexity—of dissolvable and coated microneedles were often not needed. Uses were roughly evenly split between measuring physiological parameters (i.e., mostly physical measurements) and biomarkers (i.e., mostly biochemical measurements). Many fewer studies addressed general methods of collection of fluid from the body for extracorporeal analysis, instead preferring to make specific measurements directly on the body.
Most delivery and diagnostic studies were performed in animals or humans, indicating that although engineering and formulation challenges remain in the design of microneedle systems, much emphasis is being placed on using the microneedle systems. While in vitro and in vivo (animal) studies included all four types of microneedles, human studies papers and registered clinical trials strongly emphasized use of solid and hollow microneedles, probably because their design and regulation are simpler, as discussed above, which provides an easier pathway for use in humans.
Among the patents, delivery inventions strongly outweighed diagnostic inventions. Patents addressing drug applicators were prevalent, which is not a topic extensively addressed in the scientific literature. While solid and hollow microneedles were commonly specified in patents, the category of microneedles with side channels was specifically included among the CPC symbols; this is also not a topic commonly included in scientific research papers. Among delivery patents, vaccination was the most common use identified, and among diagnostic patents, measuring glucose was most prevalent.
Among the clinical trials, almost half appeared to be basic studies that were not designated a particular phase, but more than one-quarter were Phase 2, 3, or 4 trials (24 trials total). Two-thirds of the trials had been completed (55 trials) and at least 12 trials were planned or on-going. Whilst this indicates robust activity in the clinical translation of microneedles, more clinical trial data may be required to provide the pharmaceutical industry with a level of confidence that will stimulate further investment into the development of microneedle products.
Not surprisingly, the indication most frequently addressed in clinical trials was skin conditions. Tied in second place were influenza vaccine studies, which have also been the focus of many preclinical studies and for which there is an approved product using a hollow microneedle design, and ocular drug delivery studies, which have led to a product that also uses a hollow microneedle design and has passed through Phase 3 clinical trials. Whilst almost all available microneedle products are currently for cosmetic applications, there have been relatively few clinical trials in this area.
Internet/social media interest in microneedles has been focused largely on cosmetic devices for collagen induction therapy, scar and acne treatment, and other applications, although interest in the use of microneedles for medical applications was also observed. Overall data suggest an increasing public interest in microneedle technology.
4.3. Where microneedle research takes place
Research papers were dominated by academia, while patents are dominated by industry, which reflects the inherent priorities of these two communities. Academics showed a greater interest in solid and dissolvable microneedles and to investigate delivery of small molecules, biomolecules, and vaccines. Industry research favored studies using hollow and coated microneedles and focused more narrowly on biomolecules and vaccines.
Papers, patents, and clinical trials were dominated by institutions in the United States. Asia played an almost as large a role in publishing papers, but was a lesser player in patenting, which suggests that microneedle commercialization is most heavily focused in the United States. Europe also played a significant role in producing papers and patents. There were a few universities and companies that are especially active in publishing and patenting, but there were (i) thousands of authors from >300 institutions in >30 countries who had published microneedle papers in >250 journals, (ii) >1100 inventors from >250 institutions who had issued patents on microneedles, and (iii) >50 institutions that had conducted clinical trials in >20 countries. It is remarkable how widespread the interest in microneedles is, extending well beyond the core research community.
Social media interest in microneedles is most active in the United States, followed by the United Kingdom, but microneedles have appeared in social media as 4,410 mentions in 93 countries around the world. Twitter was the most common platform mentioning microneedles.
4.4. Value of microneedles reported in the scientific literature
Microneedles were initially conceptualized as an alternative to conventional hypodermic needles for delivery of molecules into the body. Hypodermic needles, although effective, pose several disadvantages: i) pain associated with injections causes many people distress and can even induce phobias; ii) in most cases, self-administration of drug via hypodermic needles is difficult, and usually requires assistance from a healthcare practitioner; iii) use of hypodermic needles generates biohazardous sharps waste that necessitates its safe disposal and can lead to pricking/accidental needle sticks that can transmit bloodborne diseases.
Apart from being minimally invasive, microneedles offer numerous advantages over hypodermic needles. Microneedles are micron-size projections that typically protrude from a flat base to deliver their cargo, by providing a conduit for drug delivery through hollow microneedles or through residual micropores in the tissue created by solids microneedles, or by directly carrying drug into tissue by coating on or encapsulating within solid coated or dissolvable microneedles, respectively. Creating a conduit into tissue enables delivery of larger amounts of drug limited by how long the delivery process can last. For example, injections using hollow microneedles are often on the order of 100 µl in volume, whereas microneedles have also been used for slow infusion of up to 2 ml.
In contrast, coated and dissolvable microneedles are generally limited in the amount of drug they can carry due to their small size, but provide an advantage of delivering their cargo in dry form. Dissolvable microneedles offer the additional advantage of generating no sharps waste: once inserted, the microneedles dissolve away in the tissue. Due to their micron-scale size, once inside a tissue, the microneedles minimize possible interaction with nerves, thereby avoiding stimulation of pain sensors in the skin and other parts of the body. Other advantages of microneedles include dose sparing effects, especially in the case of skin vaccination, where a small dose of vaccine can be delivered by microneedles to induce strong antigen-specific antibody responses. Drugs delivered into skin using microneedles can also have faster uptake in the systemic circulation, which is advantageous for many drugs. Microneedle patches are simple to self-administer by patients at home. Microneedles can be manufactured at low cost that is competitive with hypodermic needles or with pre-filled syringes.
In addition to drug delivery applications, microneedles can facilitate access to tissue interstitial fluid, especially in skin, and thereby facilitate detection of biomarkers (such as glucose) or physiological parameters (such bio-signals or sensing of pH). Such diagnostic or monitoring applications can involve incorporating sensors onto microneedles for in situ measurements or selective capture of biomarkers, for example by coating microneedles with antibodies, that can be removed from the body for analysis. Microliter quantities of interstitial fluid can also be extracted from the body through micropores made by microneedles. Measuring biomarkers in interstitial fluid can be advantageous as certain biomarkers are not found in the systemic circulation and because interstitial fluid does not clot, which simplifies making continuous or repeated measurements.
4.5. Additional observations about microneedle patents and products
The patent literature shows examples of microneedle attributes found in products being developed for use in medicine and cosmetics. Microneedle structures have typically been presented in three ways: an individual microneedle (often hollow), a group of microneedles on a base substrate (usually solid, and possibly incorporating drug), and microneedles incorporated into a complete device or product. Considering marketed products, those for cosmetics have involved dissolvable microneedles with relatively small microneedles. Products containing solid microneedles for skin pre-treatment or collagen induction therapy have been made of polymeric and metallic materials with a broad range of microneedle length (Fig. 21).
Interest in patenting microneedles has increased in parallel with scientific studies. We found 750 patents in the CPC-based search, which is many more than we were able to find with a keyword-based search. This result shows the efficiency of CPC-based searches and the diversity of topics relevant to microneedle inventions. We also found that each patent was usually associated with many CPC symbols (Fig. S2 in SI), perhaps due to the nature of patents trying to cover many areas, as well as the ability of microneedles to be multifunctional.
We observed that drug applicators for microneedles had a very important role in the patent area (Fig. 11 (iii)). Many microneedle designs need an applicator for efficient microneedle tissue penetration due to the application speed, reproducibility of force, and homogenous distribution of pressure that can be provided by an applicator. Patents for solid microneedles have increased in recent years (Fig. S3 in SI), both for delivery and diagnosis (Fig. S4 in SI). This may be associated with solid microneedles’ relatively simpler manufacturing techniques and ease of use.
5. Conclusions
The purpose of this review was to analyze the maturation of microneedle technology over the last 30 years. Beginning as just one possible option for enhanced transdermal delivery, microneedles have become the dominant advanced technology in the field, gaining popularity in both academic and industry settings. The exponential growth in the field of microneedles has led to >1000 scientific papers, 750 patents, almost 80 clinical trials, and extensive activity on the internet and in social media.
The scientific and patent literature is focused largely on use of microneedles for delivery of molecules, especially to skin; clinical trials almost exclusively focused on delivery, primarily for skin indications, influenza vaccination, and ocular drug delivery. All four types of microneedles— solid, coated, dissolvable, and hollow—and all three types of molecules—small molecules, biomolecules, and vaccines—have been studied in scientific papers. Dissolvable microneedles have been used for delivery of all three types of molecules. Small molecules are also delivered using solid microneedles, vaccines are also delivered using hollow microneedles, and biomolecules are delivered using all four types of microneedles.
Papers on diagnostic applications focused on hollow and solid microneedles for measuring physiological parameters and biomarkers, and to a lesser extent for collecting interstitial fluid from tissues.
Most studies have been performed in vivo, either in animals or humans. While animal studies included all four types of microneedles, human studies favored solid and hollow microneedles in both scientific papers and clinical trials. About half of clinical trials address basic science questions, while the rest are roughly evenly split between Phase 1 and Phase 2, 3, and 4 clinical trials. Most microneedle products, however, are for cosmetic applications, with little representation in the scientific literature and essentially none among the clinical trials.
Scientific papers came mostly from academia and patents came mostly from industry. Academic researchers favored use of solid and dissolving microneedles for delivery of small molecules, biomolecules, and vaccines. Industry researchers focused on hollow and coated microneedles for delivery of biomolecules and vaccines.
The United States is the dominant player in terms of scientific papers, patents, clinical trials, and internet/social media activity. Asia and Europe are also significantly active. While there are a few people and organizations who are especially active in microneedles papers and patents, there are a huge number of additional players in the field, including papers published by thousands of authors from >300 institutions in >30 countries in >250 journals, patents by >1100 inventors from >250 institutions, and clinical trials at >50 institutions in >20 countries.
Analysis of internet activity indicates that there is an increased social interest in microneedles, mainly for cosmetic purposes. Content referring to microneedles is also shared via various media and social media outputs, which increases public awareness about this emerging technology.
With the ever-increasing public, academic, and industry interest in research, development, and translation of microneedles, and the growing pipeline of microneedle technologies, applications, and clinical trials, the field of microneedles has excellent potential to deliver innovative new products to improve health, appearance, and quality of life.
Supplementary Material
6. Acknowledgements
This publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number [R01AI135197] (H.S.G.) and [R01AI121322] (H.S.G.). M.D.’s contribution to this paper was supported by PATH’s Microarray Patch Center of Excellence through funding from the United Kingdom government. We thank the sponsors for financial support. The sponsors played no direct role in study design; in the collection, analysis and interpretation of data; in the writing of the article; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. Competing interest
M.R.P. serves as a consultant to companies, is a founding shareholder of companies, and is an inventor on patents licensed to companies developing microneedle-based products (Micron Biomedical, Clearside Biomedical). These potential conflicts of interest have been disclosed and are managed by Georgia Tech. H.S.G. is a co-inventor on a patent related to coated microneedles and has stock ownership in a startup company called Moonlight Therapeutics that is developing microneedles for food allergy immunotherapy. This conflict of interest has been disclosed and is being managed by Texas Tech University. No financial support was provided by Moonlight Therapeutics for this work.
⊠ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
9. Data availability
The processed data required to reproduce these findings are available in the Supplementary Information (SI) section. The raw data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
10. References
- [1].Ma G, Wu C, Microneedle, bio-microneedle and bio-inspired microneedle: A review, Journal of Controlled Release 251 (2017) 11–23. [DOI] [PubMed] [Google Scholar]
- [2].Prausnitz MR, Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin, Annual review of chemical and biomolecular engineering 8(1) (2017) 177–200. [DOI] [PubMed] [Google Scholar]
- [3].van der Maaden K, Luttge R, Vos PJ, Bouwstra J, Kersten G, Ploemen I, Microneedle-based drug and vaccine delivery via nanoporous microneedle arrays, Drug delivery and translational research 5(4) (2015) 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pastore MN, Kalia YN, Horstmann M, Roberts MS, Transdermal patches: history, development and pharmacology, British journal of pharmacology 172(9) (2015) 2179–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prausnitz MR, Langer R, Transdermal drug delivery, Nature biotechnology 26(11) (2008) 1261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].B.e. al., Dermatology, 4 edition ed., Elsevier, USA, 2018. [Google Scholar]
- [7].Munch S, Wohlrab J, Neubert RHH, Dermal and transdermal delivery of pharmaceutically relevant macromolecules, European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 119 (2017) 235–242. [DOI] [PubMed] [Google Scholar]
- [8].Wong TW, Electrical, magnetic, photomechanical and cavitational waves to overcome skin barrier for transdermal drug delivery, J Control Release 193 (2014) 257–69. [DOI] [PubMed] [Google Scholar]
- [9].Denet AR, Vanbever R, Preat V, Skin electroporation for transdermal and topical delivery, Adv Drug Deliv Rev 56(5) (2004) 659–74. [DOI] [PubMed] [Google Scholar]
- [10].Teoli D, An J, Transcutaneous Electrical Nerve Stimulation (TENS), StatPearls, StatPearls Publishing StatPearls Publishing LLC., Treasure Island (FL), 2019. [PubMed] [Google Scholar]
- [11].Prausnitz MR, A practical assessment of transdermal drug delivery by skin electroporation, Adv Drug Deliv Rev 35(1) (1999) 61–76. [DOI] [PubMed] [Google Scholar]
- [12].Mitragotri S, Devices for overcoming biological barriers: the use of physical forces to disrupt the barriers, Adv Drug Deliv Rev 65(1) (2013) 100–3. [DOI] [PubMed] [Google Scholar]
- [13].Baumler W, Weiss KT, Laser assisted tattoo removal - state of the art and new developments, Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 18(2) (2019) 349–358. [DOI] [PubMed] [Google Scholar]
- [14].Bernstein EF, Laser treatment of tattoos, Clinics in dermatology 24(1) (2006) 43–55. [DOI] [PubMed] [Google Scholar]
- [15].Rkein AM, Ozog DM, Photodynamic therapy, Dermatologic clinics 32(3) (2014) 415–25, x. [DOI] [PubMed] [Google Scholar]
- [16].Bruen D, Delaney C, Florea L, Diamond D, Glucose Sensing for Diabetes Monitoring: Recent Developments, Sensors (Basel, Switzerland) 17(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bakos RM, Blumetti TP, Roldan-Marin R, Salerni G, Noninvasive Imaging Tools in the Diagnosis and Treatment of Skin Cancers, American journal of clinical dermatology 19(Suppl 1) (2018) 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yamamoto Y, Yamamoto D, Takada M, Naito H, Arie T, Akita S, Takei K, Efficient Skin Temperature Sensor and Stable Gel-Less Sticky ECG Sensor for a Wearable Flexible Healthcare Patch, Advanced healthcare materials 6(17) (2017). [DOI] [PubMed] [Google Scholar]
- [19].Huang H, Su S, Wu N, Wan H, Wan S, Bi H, Sun L, Graphene-Based Sensors for Human Health Monitoring, Frontiers in chemistry 7 (2019) 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shakya AK, Chowdhury MYE, Tao W, Gill HS, Mucosal vaccine delivery: Current state and a pediatric perspective, J Control Release 240 (2016) 394–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim SM, Faix PH, Schnitzer JE, Overcoming key biological barriers to cancer drug delivery and efficacy, J Control Release 267 (2017) 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reinholz J, Landfester K, Mailander V, The challenges of oral drug delivery via nanocarriers, Drug delivery 25(1) (2018) 1694–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ramaut L, Hoeksema H, Pirayesh A, Stillaert F, Monstrey S, Microneedling: Where do we stand now? A systematic review of the literature, Journal of plastic, reconstructive & aesthetic surgery : JPRAS 71(1) (2018) 1–14. [DOI] [PubMed] [Google Scholar]
- [24].Depelsenaire ACI, Meliga SC, McNeilly CL, Pearson FE, Coffey JW, Haigh OL, Flaim CJ, Frazer IH, Kendall MAF, Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity, The Journal of investigative dermatology 134(9) (2014) 2361–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arya J, Prausnitz MR, Microneedle patches for vaccination in developing countries, J Control Release 240 (2016) 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bhatnagar S, Dave K, Venuganti VVK, Microneedles in the clinic, J Control Release 260 (2017) 164–182. [DOI] [PubMed] [Google Scholar]
- [27].Bragazzi NL, Orsi A, Ansaldi F, Gasparini R, Icardi G, Fluzone(R) intra-dermal (Intanza(R)/Istivac(R) Intra-dermal): An updated overview, Human vaccines & immunotherapeutics 12(10) (2016) 2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen G, Yu J, Gu Z, Glucose-Responsive Microneedle Patches for Diabetes Treatment, Journal of diabetes science and technology 13(1) (2019) 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chiang B, Jung JH, Prausnitz MR, The suprachoroidal space as a route of administration to the posterior segment of the eye, Adv Drug Deliv Rev 126 (2018) 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Creighton RL, Woodrow KA, Microneedle-Mediated Vaccine Delivery to the Oral Mucosa, Advanced healthcare materials 8(4) (2019) e1801180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Duarah S, Sharma M, Wen J, Recent advances in microneedle-based drug delivery: Special emphasis on its use in paediatric population, European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 136 (2019) 48–69. [DOI] [PubMed] [Google Scholar]
- [32].Leone M, Monkare J, Bouwstra JA, Kersten G, Dissolving Microneedle Patches for Dermal Vaccination, Pharmaceutical research 34(11) (2017) 2223–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Levin Y, Kochba E, Hung I, Kenney R, Intradermal vaccination using the novel microneedle device MicronJet600: Past, present, and future, Human vaccines & immunotherapeutics 11(4) (2015) 991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marshall S, Sahm LJ, Moore AC, The success of microneedle-mediated vaccine delivery into skin, Human vaccines & immunotherapeutics 12(11) (2016) 2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McCrudden MT, McAlister E, Courtenay AJ, Gonzalez-Vazquez P, Singh TR, Donnelly RF, Microneedle applications in improving skin appearance, Experimental dermatology 24(8) (2015) 561–6. [DOI] [PubMed] [Google Scholar]
- [36].Moffatt K, Wang Y, Raj Singh TR, Donnelly RF, Microneedles for enhanced transdermal and intraocular drug delivery, Current opinion in pharmacology 36 (2017) 14–21. [DOI] [PubMed] [Google Scholar]
- [37].Nguyen TT, Park JH, Human studies with microneedles for evaluation of their efficacy and safety, Expert opinion on drug delivery 15(3) (2018) 235–245. [DOI] [PubMed] [Google Scholar]
- [38].Prausnitz MR, Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin, Annual review of chemical and biomolecular engineering 8 (2017) 177–200. [DOI] [PubMed] [Google Scholar]
- [39].Richter-Johnson J, Kumar P, Choonara YE, du Toit LC, Pillay V, Therapeutic applications and pharmacoeconomics of microneedle technology, Expert review of pharmacoeconomics & outcomes research 18(4) (2018) 359–369. [DOI] [PubMed] [Google Scholar]
- [40].Rzhevskiy AS, Singh TRR, Donnelly RF, Anissimov YG, Microneedles as the technique of drug delivery enhancement in diverse organs and tissues, J Control Release 270 (2018) 184–202. [DOI] [PubMed] [Google Scholar]
- [41].Sabri AH, Ogilvie J, Abdulhamid K, Shpadaruk V, McKenna J, Segal J, Scurr DJ, Marlow M, Expanding the applications of microneedles in dermatology, European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 140 (2019) 121–140. [DOI] [PubMed] [Google Scholar]
- [42].Zaric M, Ibarzo Yus B, Kalcheva PP, Klavinskis LS, Microneedle-mediated delivery of viral vectored vaccines, Expert opinion on drug delivery 14(10) (2017) 1177–1187. [DOI] [PubMed] [Google Scholar]
- [43].Espacenet. https://worldwide.espacenet.com/. Accessed 23 October 2018.
- [44].ClinicalTrials.gov. https://clinicaltrials.gov. Accessed 18 October 2018.
- [45].Google Trends. http://www.google.com/trends. Accessed 22 October 2018.
- [46].Altmetrics. https://www.altmetric.com. Accessed 23 October 2018.
- [47].Doddaballapur S, Microneedling with dermaroller, J Cutan Aesthet Surg 2(2) (2009) 110–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The processed data required to reproduce these findings are available in the Supplementary Information (SI) section. The raw data required to reproduce these findings cannot be shared at this time due to technical or time limitations.


