Abstract
Triple negative breast cancer (TNBC) is an aggressive disease with a 5-y relative survival rate of 11% after distant metastasis. To survive the metastatic cascade, tumor cells remodel their signaling pathways by regulating energy production and upregulating survival pathways. AMP-activated protein kinase (AMPK) and Akt regulate energy homeostasis and survival, however, the individual or synergistic role of AMPK and Akt isoforms during lung colonization by TNBC cells is unknown. The purpose of this study was to establish whether targeting Akt, AMPKα or both Akt and AMPKα isoforms in circulating cancer cells can suppress TNBC lung colonization. Transient silencing of Akt1 or Akt2 dramatically decreased metastatic colonization of lungs by inducing apoptosis or inhibiting invasion, respectively. Importantly, transient pharmacologic inhibition of Akt activity with MK-2206 or AZD5363 inhibitors did not prevent colonization of lung tissue by TNBC cells. Knockdown of AMPKα1, AMPKα2, or AMPKα1/2 also had no effect on metastatic colonization of lungs. Taken together, these findings demonstrate that transient decrease in AMPK isoforms expression alone or in combination with Akt1 in circulating tumor cells does not synergistically reduce TNBC metastatic lung colonization. Our results also provide evidence that Akt1 and Akt2 expression serve as a bottleneck that can challenge colonization of lungs by TNBC cells.
Keywords: Triple negative breast cancer, Organ metastasis, Akt, AMPKα
Abbreviations: Akt, Protein kinase B; AMPK, AMP-activated protein kinase; ER+, Estrogen receptor-positive; FBS, Fetal bovine serum; GFP, Green fluorescent protein; IHC, Immunohistochemistry; NSG, NOD-scid IL2Rgammanull; PBS, Phosphate buffered saline; PS, Pencillin-streptomycin; RPMI, Roswell Park Memorial Institute; siRNA, Small interfering RNA; TNBC, Triple negative breast cancer; WB, Western blot
Introduction
Breast cancer is the second leading cause of cancer death among women globally, and projections estimate that there will be over 42,000 deaths in the United States in 2020 [1]. Most deaths are due to metastatic spread of the cancer to the lungs, brain or bone [2]. Triple negative breast cancer (TNBC) is an aggressive subtype of breast cancer that comprises 15% to 20% of overall cases [3], [4], [5], [6], [7]. TNBC, characterized by a lack of expression of the estrogen and progesterone receptors and no overexpression of the HER2 protein, consists of a diverse group of tumors that affect younger women and are found with greater frequency in African Americans [6], [7], [8], [9]. TNBC is resistant to targeted therapy and is more likely than other breast cancer types to recur and metastasize to distant organs [6], [7], [8], [9]. Lungs are the primary site of TNBC colonization with approximately 40% of TNBC metastases occurring in the lungs [10]. The survival rate for TNBC significantly decreases once it has metastasized to systemic organs [11]. The 5-y relative survival rate of localized TNBC is 91%, and falls to 65% with regional metastasis; however, survival precipitously drops to 11% with distant spread [11]. TNBC patients with lung metastases have a median survival of only 15 mo [12]. Understanding the mechanism of TNBC metastatic spread to lungs and preventing the establishment of micrometastases in distant organs will directly improve patient survival.
Metastasis is a multistep process that requires cells to follow five sequential steps: infiltrate surrounding tissue, intravasate into a blood or lymphatic vessel, survive during transit, extravasate into an organ, and proliferate to form colonies [13]. Cells must become motile before infiltrating surrounding tissue, and they undergo the epithelial-to-mesenchymal transition to increase migratory ability [13]. Once in a vessel, cells travel and must survive until they lodge in capillaries or adhere to endothelial cells of the vessel wall [14]. Cells then extravasate into surrounding tissue within a few hours [14]. After arriving at a distant site, metastatic cells rely on oncogenes to promote survival [14]. Only a subset of surviving cells proliferates to form a colony [14]. Metastasis is extremely inefficient with less than 0.1% of cells surviving at distant sites [13]. Survival during transport and extravasation into an organ are key steps in early-stage metastasis that can be targeted to prevent the establishment of colonies at distant sites. Since metastasis to distant organs happens in a short timeframe, we decided to identify the role of key survival or metabolism proteins by regulating their expression in circulating cancer cells. This model focuses specifically on the fate of cancer cells after intravasation but before proliferation of micrometastases.
During organ metastasis, cells rely on increased energy production and oncogene expression to adapt to stressful conditions [14, 15]. Inhibiting pathways that help cells alter metabolism or overcome stresses during early-stage metastasis may reduce their ability to successfully metastasize. Akt and AMP-activated protein kinase (AMPK) are 2 proteins that play crucial roles in cellular survival and metabolism, respectively. Moreover, Akt and AMPK reciprocally regulate each other during matrix attachment and detachment in breast cancer [16]. Akt promotes growth of matrix-attached cells, while AMPK increases energy availability for matrix-detached cells [16]. Concurrently targeting signaling through Akt and AMPK may synergistically reduce the ability of TNBC to metastasize.
Akt, a downstream effector of phosphatidylinositol 3-kinase (PI3K), is composed of three isoforms: Akt1, Akt2, and Akt3 [17], [18], [19], [20], [21], [22]. Akt promotes cellular growth and survival by phosphorylating and regulating many targets, including the mammalian target of rapamycin (mTOR) [17], [18], [19], [20], [21], [22]. The ability of Akt1 and Akt2 to control metastasis of estrogen receptor-positive (ER+) and HER2-amplified breast cancers has previously been studied. Akt1 inhibits metastasis of both breast cancer subtypes, while Akt2 has a prometastatic role in both cases [23], [24], [25]. However, the impact of Akt1 and Akt2 on TNBC metastasis is unknown. AMPK is a heterotrimeric complex composed of an α catalytic subunit and β and γ regulatory subunits [26], [27], [28], [29], [30]. The catalytic domain is comprised of α1 and α2 isoforms [26], [27], [28], [29], [30]. AMPK is a major metabolic regulator that maintains energy homeostasis of cells by increasing ATP production and reducing ATP consumption [26], [27], [28], [29], [30]. In particular, AMPK reduces lipid synthesis and stimulates fatty acid oxidation by phosphorylating acetyl CoA carboxylase [26], [27], [28], [29]. Both fatty acid synthesis and oxidation can promote metastasis [31]. AMPK may play a key role in balancing flux through these pathways during different stages of metastasis. However, the specific steps of TNBC metastasis that are regulated by AMPKα1 and AMPKα2 are unclear. Moreover, combined targeting of individual Akt and AMPKα isoforms to reduce TNBC metastasis has not been previously examined.
Here, we used transient knockdown to determine the roles of Akt and AMPKα isoforms during systemic dissemination and whether synergistic knockdown of both Akt and AMPKα can block TNBC metastasis-initiating cell colonization of lungs. We found that silencing either Akt1 or Akt2 dramatically decreased lung colonization of TNBC cells by inducing apoptosis or inhibiting invasion, respectively. Knockdown of AMPKα1, AMPKα2, AMPKα1/2 or suppression of Akt activity with small molecule drugs did not prevent lung metastasis. Taken together, our findings demonstrate that reduction in Akt1 or Akt2 expression, but not activity, may directly decrease mortality from metastatic cancer in TNBC patients.
Materials And methods
Materials
Roswell Park Memorial Institute (RPMI) 1640 medium was from Thermo Fisher (Waltham, MA). DMEM/F12 was from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS), 100x penicillin-streptomycin solution (PS), and Dulbecco's Phosphate Buffered Saline (PBS) were from Sigma-Aldrich. Opti-MEM and Lipofectamine RNAiMAX were from Thermo Fisher. Protein Assay Dye was from Bio-Rad (Hercules, CA). Amersham ECL Prime Western Blotting Detection Reagent was from GE Healthcare Life Sciences (Chicago, IL). Immobilon Western Chemiluminescent HRP Substrate was from Millipore (Burlington, MA). Pooled siRNAs for AMPKα1, AMPKα2, and AMPKα1/2 were from Santa Cruz Biotechnology (Dallas, TX). Pooled siRNAs for Akt1 and Akt2 were from Thermo Fisher. MISSION pLKO.1-puro-CMV-TurboGFP Positive Control Transduction Particles (Sigma-Millipore; #SHC003V) were used to establish MDA-MB-231 green fluorescent protein (GFP) cells. MK-2206 2HCl and AZD5363 were from Cayman Chemical (Ann Arbor, MI). Primary antibodies used in this study include: Abcam: (1) AMPKα1, ab32047 (1:1000 for WB and 1:250 for IHC) and (2) AMPKα2, ab3760 (1:1000 for WB and 1:500 for IHC); Cell Signaling Technology (Danvers, MA): (1) pAkt, #4060 (1:2000 for WB), (2) Akt1, #75692 (1:1000 for WB), (3) Akt2, #3063 (1:1000 for WB), (4) Cleaved PARP, #5625 (1:1000 for WB), (5) ZO-1, #8193 (1:1000 for WB), (6) ZEB1, #3396 (1:1000 for WB), (7) Claudin-1, #13255 (1:1000 for WB), (8) E-cadherin, #3195 (1:1000 for WB), (9) Vimentin, #5741 (1:1000 for WB), (10) β-catenin, #8480 (1:1000 for WB), (11) Snail, #3879 (1:1000 for WB), (12) Bcl-2, #4223 (1:1000 for WB), (13) Bcl-xL, #2764 (1:1000 for WB), (14) Mcl-1, #5453 (1:1000 for WB), (15) Bim, #2933 (1:1000 for WB), (16) Bad, #9239 (1:1000 for WB), and (17) BID, #2202 (1:1000 for WB); and Sigma-Aldrich: β-actin (1:10,000 for WB). Akt1, #sc-5298 (1:500 for IHC), Akt2, #sc-5270 (1:500 for IHC), and secondary antibodies were from Santa Cruz Biotechnology.
Cell culture
TNBC cells (MDA-MB-231 and MDA-MB-468 cells) were from the American Type Culture Collection and cultured in a humidified incubator at 37°C and 5% CO2. MDA-MB-231 cells were cultured in RPMI + 10% FBS + 1% PS. MDA-MB-468 cells were cultured in DMEM/F12 + 10% FBS + 1% PS. To generate MDA-MB-231 GFP cell line, 5000 cells were seeded into a 96-well plate and incubated at standard cell culture conditions overnight. On the following day, cells were transfected with GFP lentiviral particles (MOI = 40) in medium supplemented with 10 μg/mL polybrene. The medium was changed after 24 h, and cells were seeded 24 h later into a 24-well plate. The cells were selected the next day with 1 μg/mL of puromycin.
siRNA transfection
TNBC cells were transfected with 50 nM of siRNA to NTC, Akt1, Akt2, AMPKα1, AMPKα2, or AMPKα1/2. The siRNAs were mixed with Lipofectamine RNAiMAX in Opti-MEM for 20 min. The ratio of Opti-MEM to complete medium was 1:4. Medium was changed every 24 h until cells were injected into mice or lysed for western blotting.
Western blotting
TNBC cells were seeded and then transfected as described above. Medium was changed after 24 h and again after 48 h. After incubation for a total of 72h, medium was removed, and cells were washed with ice cold 1× PBS. Cells were then scraped and lysed in 1× radioimmunoprecipitation assay buffer containing 1 mM phenylmethylsulfonyl fluoride. Cells were incubated on ice for 20 min with 10-s vortexes every 5 min before centrifugation at 14,000 rpm for 20 min at 4°C. Protein concentrations in the lysates were then determined. Equal amounts of protein were reduced and denatured by heating at 80°C for 10 min before being resolved on 4% to 12% Bis-Tris gels. The proteins were then transferred to polyvinylidene fluoride) membranes, blocked with 10% milk for at least 1 h, and incubated in primary antibody solutions overnight at 4°C. On the next day, the membranes were washed twice with 1× Tris-buffered saline with Tween 20 (TBST) for 5 min and 10 min before incubation with secondary antibody solutions (1:10,000 dilutions) for 1h at room temperature. The membranes were then washed twice with TBST for 15 min and 20 min before Amersham ECL or Immobilon was added to the membranes for protein detection. Stripping buffer was used on membranes where required.
Immunohistochemistry
Samples of TNBC lymph node or lung metastases were identified by the Markey Cancer Center Biospecimen Procurement and Translational Pathology shared resource facility. Immunohistochemistry (IHC) was performed as previously described [32]. Briefly, slides were deparaffinized in xylene, rehydrated, incubated for 15 min with fresh 0.3% hydrogen peroxide, washed with PBS, and heated to 95°C in 10 mM citrate buffer (pH 6.0; 30 min) for Akt1 and Akt2 antibody staining. Antigen retrieval for slides stained with AMPKα1 and AMPKα2 antibody was performed in Diva Decloaker, RTU (Biocare Medical; # DV2004G1) buffer. Antigen retrieval for sliders stained with Akt1 and Akt2 were performed in sodium citrate buffer (10 mM Sodium Citrate, 0.05% Tween 20, pH 6.0).
Endogenous peroxidase activity was blocked with Bloxall blocking solution (Vector Laboratories; # SP-6000). Next, sections were blocked for 1 h with 2.5% normal horse serum (Vector Laboratories; #S-2012). AMPKα1, AMPKα2, Akt1, and Akt2 antibodies were diluted in Dako background reducing antibody diluent (Agilent Dako; #s3022). Primary antibody was incubated with slides for 12 h at 4°C in a humidifier chamber, washed with TBST (Tris-Buffered Saline and Tween 20) and incubated with ImmPRESS universal antibody IgG polymer detection kit (Vector Laboratories, #MP-7500) for 1 h, RT. Antibody reaction was visualized with Immpact DAB EqV peroxidase substrate (Vector Laboratories, #SK-4103). All sections were counterstained with hematoxylin (VWR; #95057-844) and observed by light microscopy. For negative controls, primary antibody was omitted from the above protocol.
The number of positive cells was visually evaluated in each core by a pathologist (EL) and the staining intensity was classified using a semi-quantitative seven-tier system developed by Allred et al. [33, 34]. The system assesses the percentage of positive cells (none=0; <10%=1; 10% to 50%, =2; >50%=3) and intensity of staining (none=0; weak=1; intermediate=2; and strong=3).
TNBC lung metastasis model
NOD-scid IL2Rgammanull mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Housing for these animals was maintained in a HEPA-filtrated environment within sterilized cages with 12 h light/12 h dark cycles. All animal procedures were conducted with approval of and in compliance with the University of Kentucky Institutional Animal Care and Use Committee.
Knockdown of Akt and AMPK isoforms in circulating cancer cells was achieved by transient knockdown of protein expression in vitro, as described above, and injection of cancer cells intravenously (iv) into mice 48 h after transfection. Briefly, cells were washed with 1× PBS 48 h after transfection, trypsinized, counted and resuspended in PBS at a density of 1 × 106 cells per 100 μL. Cells were kept on ice before intravenous injection. For iv injection of MDA-MB-231 GFP cells, NOD-scid IL2Rgammanull mice were anesthetized with isoflurane (induction 4%, maintenance 2%). The viability of cells used for inoculation was greater than 95% as determined by Vi-CELL™ XR (Beckman Coulter); 1 × 106 cells were injected per animal. Gentle pressure was applied to the inoculation site until there was no visible sign of bleeding. GFP fluorescence imaging was performed using an LT-9500 Illumatool/TLS (Lightools Research, Encinitas, CA), equipped with an excitation source (470 nm) and filter plate (515 nm). Lung metastasis in AZD5363 treatment experiment was also imaged with Lago imaging system (Spectral Instruments, Tucson, AZ) and analyzed in Aura software (Spectral Instruments, Tucson, AZ).
Pharmacologic inhibition of Akt activity with MK-2206 or AZD5363 compounds
GFP-expressing MDA-MB-231 cells were treated with MK-2206 or AZD5363 (0 or 10 μM). After 22 h, medium was replaced with fresh medium containing MK-2206 or AZD5363 (0 or 10 μM) for an additional 2 h. Treatment with chemical inhibitors for 24 h + 2 h was performed to achieve continuous and prolonged Akt inhibition in TNBC cells prior to iv cells injection [35], [36], [37]. Cells were then collected, counted, washed in PBS, resuspended in PBS and injected into tail vein of mice (1 × 106 cells; 100 µL).
Analysis of GFP signal in TNBC lung metastases
ImageJ software was used to evaluate GFP signal in TNBC lung metastases. ImageJ is a Java-based image processing program developed at the National Institutes of Health and the Laboratory for Optical and Computational Instrumentation (LOCI, University of Wisconsin; https://imagej.nih.gov/). Photographs of lung metastases were opened in ImageJ software and split into channels (Image-Color-Split Channels). Blue and red channels were closed, and the green channel was adjusted with threshold function (Image-Adjust-Threshold; select B&W in drop-down menu). Next, a rectangular selection area was drawn over a lung to perform measurement of mean gray values. Analysis properties were set to “area” and “Mean grey value” (Analyze-Set Measurement). Mean grey values in the rectangular selection area drawn over a lung were analyzed and recorded (Analyze-Measure). To keep the area of selection consistent, the rectangular selection area was moved over the next lung and the analysis was repeated (Analyze-Measure).
Statistical analysis
Descriptive statistics, including means and standard deviations (SD), are presented for each experimental group and displayed in bar graphs while frequencies and proportions of IHC score were summarized for Akt and AMPK. Comparisons of green fluorescence area were performed using the one-way analysis of variance (ANOVA) with Holm's adjustment for multiple testing between groups. The χ2 test for goodness of fit was used to test departures from equality of proportions across IHC scores. P < 0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SAS software version 9.4 (SAS Inc., Cary, NC, USA).
Results
Expression of Akt and AMPKα isoforms in TNBC lymph node or lung metastases
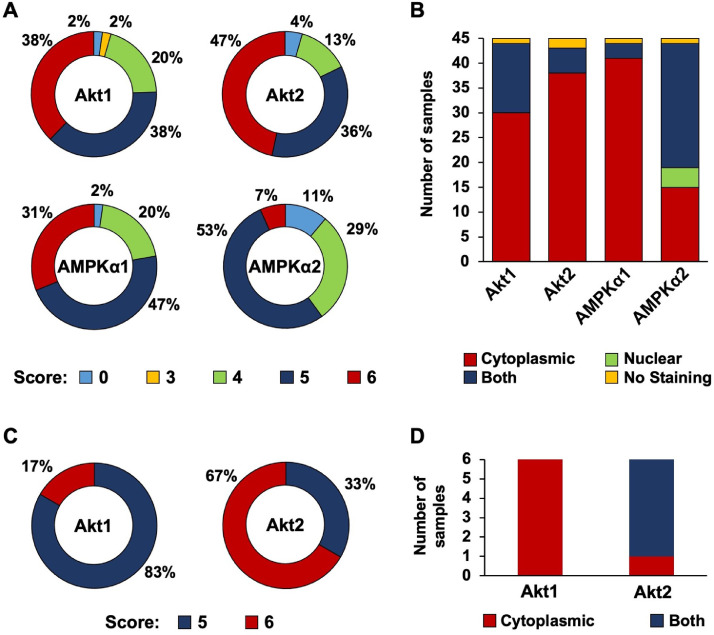
Akt and AMPKα promote cellular survival and energy mobilization and may facilitate organ colonization during metastatic spread [16, 18, [38], [39], [40], [41], [42]]. We have previously shown that AMPKα1 and AMPKα2 are expressed in primary TNBC patient samples [43]. Here, we examined expression and subcellular localization of Akt and AMPKα isoforms in TNBC lymph node metastasis (n = 45 patient samples). Fig 1A indicates the cytoplasmic scoring distribution for each isoform; Akt1, Akt2, and AMPKα1 received scores of 5 or 6 in over 70% of cases, indicating intense and widespread cytoplasmic expression among the lymph node metastases. Chi-square tests indicated statistically significant higher IHC scores for Akt1, Akt2, and AMPKα1. AMPKα2 had slightly lower scores than the other proteins, with over 80% of samples being scored as a 4 or 5; cytoplasmic expression of AMPKα2 is moderately strong and widespread among the patient samples. Supplementary Figs S1-S4 contain representative photographs of Akt1, Akt2, AMPKα1 or AMPKα2 from all patient samples.
Fig. 1.
Analysis of Akt1, Akt2, AMPKα1, and AMPKα2 expression in TNBC lymph node and lung metastases. (A) IHC scoring of Akt1, Akt2, AMPKα1, and AMPKα2 cytoplasmic expression in TNBC lymph node metastases. Samples were scored on scales of 0-3 for both intensity and distribution percentage, and the values were added together (n = 45). (B) Subcellular localization of Akt1, Akt2, AMPKα1, and AMPKα2 in TNBC lymph node metastases (n = 45). (C) IHC scoring of Akt1 and Akt2 cytoplasmic expression in TNBC lung metastases. Samples were scored on scales of 0-3 for both intensity and distribution percentage, and the values were added together (n = 6). (D) Subcellular localization of Akt1 and Akt2 in TNBC lung metastases (n = 6). IHC, Immunohistochemistry; TNBC, triple negative breast cancer.
Fig 1B compares the subcellular localization of Akt1, Akt2, AMPKα1, and AMPKα2 within the TNBC lymph node metastases. Akt1, Akt2, and AMPKα1 were predominantly expressed in the cytoplasm; nuclear and cytoplasmic localization was observed in a minority of cases; nuclear only localization was not observed in any cases. AMPKα2 had a substantially different subcellular localization pattern than Akt1, Akt2, or AMPKα1. AMPKα2 was expressed in both the nucleus and the cytoplasm. This data demonstrates predominant nuclear localization of AMPKα2 compared to predominant cytoplasmic expression of Akt1, Akt2, and AMPKα1 isoforms. Taken together, these results demonstrate strong and widespread expression of Akt and AMPK isoforms in TNBC lymph node metastases.
We extended our analysis to include expression and subcellular localization of Akt1 and Akt2 isoforms in TNBC lung metastasis (n = 6 patient samples). Fig 1C denotes the cytoplasmic scoring distribution for both isoforms; Akt1 and Akt2 received scores of 5 or 6 in all samples, indicating intense and widespread cytoplasmic staining among the TNBC lung metastases. Supplementary Figs S5 and S6 contain representative photographs of Akt1 or Akt2 from all patient samples. Fig 1D compares the subcellular localization of Akt1 and Akt2 within the TNBC lung metastases. Akt1 was expressed in only the cytoplasm in all samples, while Akt2 was expressed in both the nucleus and the cytoplasm in 5 of the 6 samples. Taken together, these results indicate robust and extensive expression of Akt1 and Akt2 in TNBC lung metastases.
AMPK isoforms expression is not crucial for lung colonization by MDA-MB-231 cells
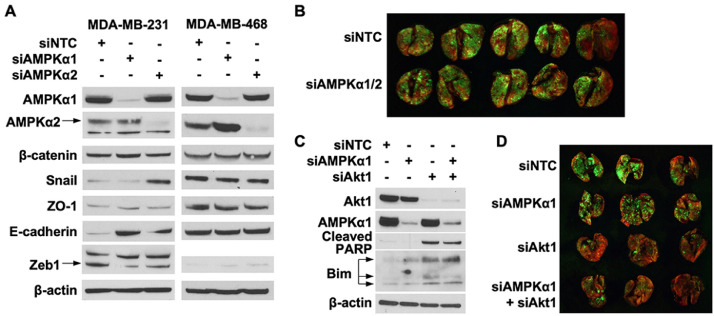
AMPKα is a key regulator of important metabolic pathways that promote metastasis [31], but the role of individual AMPKα isoforms and metabolic pathways in circulating cancer cells survival and lung colonization is unknown. The metastatic cascade includes multiple steps – cell invasion, entry into vasculature, systemic circulation, arrest, and extravasation in secondary organs [44]. Therefore, we used transient knockdown approach to examine role of AMPKα1 or AMPKα2 isoforms in select steps of the metastatic cascade – systemic circulation and lung colonization. The expression of AMPKα1 or AMPKα2 proteins restored to original levels 48 h after intravenous injection of cancer cells [45, 46]. Fig 2A indicates AMPKα1 and AMPKα2 silencing 72 h after siRNA transfection in MDA-MB-231 and MDA-MB-468 cells. MDA-MB-231 GFP cells with transient knockdown of AMPKα1/2 expression were prepared 48 h after transfection with siRNA for injection into mice. As shown in Fig 2B, silencing AMPKα1/2 did not alter the ability of MDA-MB-231 cells to establish lung metastases. Taken together, these findings suggest that energy balance regulation by AMPK during cancer cells systemic circulation is not essential for lung colonization by TNBC cells.
Fig. 2.
AMPKα isoforms expression in TNBC lung colonization. (A) MDA-MB-231 and MDA-MB-468 cells were transfected with 50 nM of siRNA targeting AMPKα1 or AMPKα2. Expression of AMPKα1, AMPKα2, β-catenin, Snail, ZO-1, E-cadherin, and Zeb1 was analyzed by western blot 72 h after transfection. (B) MDA-MB-231 GFP cells were transfected 50 nM siRNA targeting AMPKα1/2 and 100 nM for NTC. Cells were collected 48 h after transfection and injected intravenously into NSG mice. MDA-MB-231 lung metastasis was visualized with fluorescence imaging 36 d later. (C) MDA-MB-231 cells were transfected with siRNA targeting Akt1, AMPKα1, or Akt1/AMPKα1. Expression of Akt1, AMPKα1, cleaved PARP, and Bim was detected 72 h after transfection. (D) MDA-MB-231 GFP cells were transfected with siRNA targeting Akt1, AMPKα1, or Akt1/AMPKα1. Transfection concentrations were: (1) individual siRNA: 50 nM, (2) combination siRNA: 50 nM each (100 nM total), and (3) NTC: 100 nM. Cells were collected 48 h after transfection and injected intravenously into NSG mice. MDA-MB-231 lung metastasis was visualized with fluorescence imaging 40 d later. NTC, Non-targeting control was used as a negative control.
Breast cancer cell survival in suspension is dependent on pAMPKhigh/pAktlow status [16]. Therefore, we next determined whether TNBC cells also depend on AMPK and Akt expression or activity. Analysis of cancer cells survival after silencing AMPKα1 and Akt1 alone or in combination is demonstrated in Fig 2C. Depleting Akt1 levels induced PARP cleavage and increased Bim expression, but combined knockdown with AMPKα1 did not enhance PARP cleavage or Bim expression (Fig 2C). We then transiently knockdown of AMPKα1 and Akt1 expression alone or in combination in MDA-MB-231 GFP cells before injecting into mice. As shown in Fig 2D, silencing AMPKα1 did not alter the ability of MDA-MB-231 cells to establish lung metastases. However, suppressing Akt1 expression dramatically decreased MDA-MB-231 lung metastasis. Combined knockdown of AMPKα1 and Akt1 also did not synergistically suppress TNBC cells lung colonization. Taken together, these findings indicate that AMPKα isoforms play limited role in TNBC cells lung colonization, however, Akt1 appears to play a key role in facilitating TNBC lung colonization.
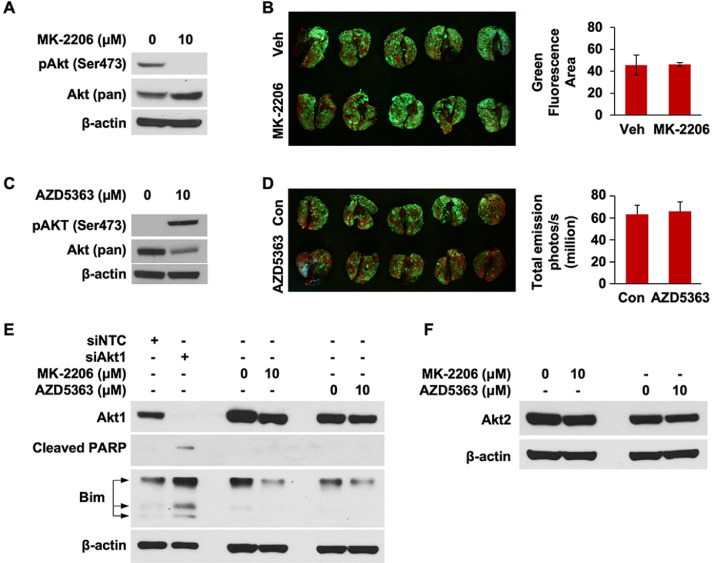
Next, we examined what role Akt activation plays in the dramatic decrease in MDA-MB-231 lung metastasis observed after Akt1 knockdown. First, we suppressed Akt activity in MDA-MB-231 cells with MK-2206, an allosteric Akt inhibitor, prior to iv cell injection. MK-2206 treatment reduced Akt activity in MDA-MB-231 cells (Fig 3A) but had no effect on lung colonization (Fig 3B). We repeated the study with AZD5363, a small molecule inhibitor that causes Akt to enter a hyperphosphorylated, but catalytically inactive state [37]. Administration of AZD5363 induced Akt hyperphosphorylation (Fig 3C) but did not reduce TNBC lung colonization (Fig 3D). We next examined whether MK-2206 or AZD5363 affect expression of Akt1 or Akt2 isoforms in TNBC cells. In Fig 3E, we compared the relative abilities of siAkt, MK-2206, and AZD5363 to alter Akt1 expression. siAkt1 administration robustly reduced Akt1 expression while inducing PARP cleavage and increasing Bim expression. In contrast, treatment with MK-2206 or AZD5363 induced very minimal decreases in Akt1 expression. PARP cleavage was not detected with MK-2206 or AZD5363 treatment, indicating that these compounds are much weaker apoptotic agents than siAkt1. Moreover, neither MK-2206 nor AZD5363 induced a substantial change in Akt2 expression (Fig 3F). Taken together, these findings indicate that TNBC lung colonization can be regulated through Akt1 expression in a manner independent of its phosphorylation status.
Fig. 3.
TNBC lung colonization is not dependent on Akt activation. (A) MDA-MB-231 GFP cells were treated with 10 μM MK-2206 for 22 h. Fresh medium with 10 μM MK-2206 was replaced 2 h before cells were collected and intravenously injected into NSG mice. Expression of pAkt (Ser473) was analyzed by western blot. (B) MDA-MB-231 lung metastasis was visualized with fluorescence imaging 46 d after cell injection. (C) MDA-MB-231 GFP cells were treated with 10 μM AZD5363 for 22 h. Fresh medium with 10 μM AZD5363 was replaced 2 h before cells were collected and intravenously injected into NSG mice. Expression of pAkt (Ser473) was analyzed by western blot. (D) MDA-MB-231 lung metastasis was visualized with fluorescence im aging 45 d after cell injection. (E) Analysis of Akt1, cleaved PARP, and Bim expression in MDA-MB-231 cells treated with siAkt1 or MDA-MB-231 GFP cells treated with 10 μM MK-2206 or AZD5363 from (A) and (C), respectively, by western blot. (F) Analysis of Akt2 expression in MDA-MB-231 GFP cells treated with 10 μM MK-2206 or AZD5363 from (A) and (C), respectively, by western blot.
Akt1 and Akt2 isoforms regulate MDA-MB-231 lung colonization
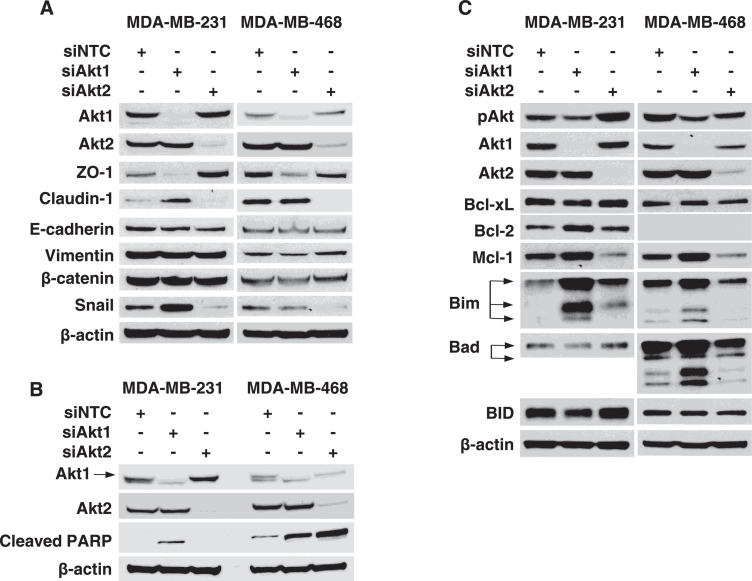
Survival during systemic circulation and extravasation are essential steps for cells to establish distant metastases [13, 14]. Therefore, proteins that prevent apoptosis or facilitate invasion may play a major role in promoting TNBC lung colonization. We first determined the differential expression of proteins essential for invasive properties of TNBC cells after knockdown of Akt isoforms. Knockdown of Akt2 decreased expression of Snail and Claudin-1 in MDA-MB-231 and MDA-MB-468 cells. However, silencing Akt1 had minor effect on Snail and Claudin-1 expression in MDA-MB-231 and MDA-MB-468 cells (Fig 4A). Taken together, these findings indicate that Akt2 regulates expression of proteins that may promote lung colonization of circulating TNBC cells.
Fig. 4.
Akt isoforms promote invasion and suppress apoptosis in TNBC cells. (A) MDA-MB-231 and MDA-MB-468 cells were transfected with 50 nM of siRNA targeting Akt1 or Akt2. Expression of Akt1, Akt2, ZO-1, Claudin-1, E-cadherin, Vimentin, β-catenin, and Snail was analyzed by western blot 72 h after transfection. (B) MDA-MB-231 and MDA-MB-468 cells were transfected with 50 nM siRNA targeting Akt1 or Akt2. Expression of Akt1, Akt2, and cleaved PARP was detected with western blot 72 h after transfection. (C) MDA-MB-231 and MDA-MB-468 cells were transfected with 50 nM siRNA targeting Akt1 or Akt2. Expression of Akt1, Akt2, and Bcl-2 family proteins was detected with western blot 72 h after transfection. NTC, Nontargeting control was used as a negative control.
We next determined role of Akt isoforms in TNBC apoptosis control. Akt1 knockdown induced PARP cleavage in both MDA-MB-231 and MDA-MB-468 cells, while silencing Akt2 led to robust PARP cleavage in MDA-MB-468 cells (Fig 4B). These findings suggest that either Akt1 or Akt2 knockdown promotes apoptosis induction in TNBC cells. Finally, we determined how silencing Akt1 or Akt2 impacted expression of Bcl2 family proteins, which regulate apoptosis induction. Akt1 knockdown increased the expression of Bim, a promoter of apoptosis, in MDA-MB-231 and MDA-MB-468 cells (Fig 4C). Silencing Akt2 reduced levels of Mcl-1, an antiapoptotic protein, in both MDA-MB-231 and MDA-MB-468 cells (Fig 4C). Taken together, these findings indicate that Akt1 and Akt2 prevent apoptosis in TNBC cells by reducing Bim expression or upregulating Mcl-1 levels, respectively.
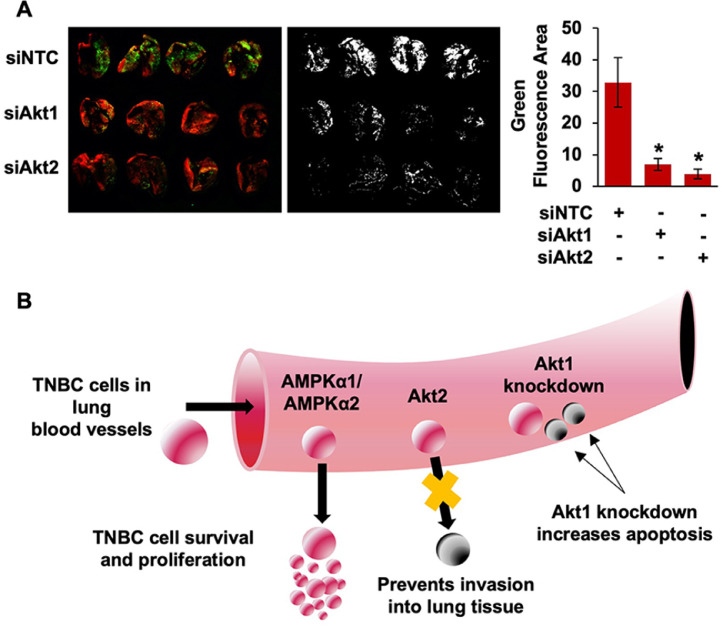
To conclude, we used siRNA directed toward Akt1 or Akt2 to evaluate the ability of each isoform to regulate TNBC lung metastasis. As shown in Fig 5A, knockdown of either Akt1 or Akt2 dramatically decreased lung colonization of MDA-MB-231 cells. Silencing Akt1 or Akt2 blocked metastasis to a similar degree. Quantification of green fluorescence intensity with ImageJ indicated that both reductions were statistically significant (Fig 5A). Taken together, these findings suggest that reducing metastatic potential of TNBC by suppressing Akt1 and/or Akt2 expression is a potentially promising therapeutic strategy that may directly improve patient survival.
Fig. 5.
Akt1 and Akt2 expression promote lung colonization of TNBC cells. (A) MDA-MB-231 GFP cells were transfected with 50 nM of siRNA targeting Akt1 or Akt2. Cells were collected 48 h after transfection and injected intravenously into NSG mice. MDA-MB-231 lung metastasis was visualized with fluorescence imaging 28 d later. (B) Summary diagram for the role of AMPK and Akt isoform expression in TNBC lung colonization.
Discussion
Preventing metastatic spread of TNBC is crucial to improving patient survival. Akt and AMPK are two key proteins that serve vital functions for cancer cells during stress. The Akt signaling cascade promotes cellular survival by inhibiting apoptosis and facilitates cellular growth by stimulating cell cycle progression. AMPK is an important regulator of metabolism and helps cells maintain energy homeostasis in times of stress. Moreover, Akt and AMPK reciprocally regulate each other during matrix attachment and detachment in breast cancer [16]. The existence of this reciprocal regulatory loop prompted our interest in targeting both pathways together to block metastasis. We hypothesized that combined inhibition of Akt and AMPK would prevent TNBC lung colonization more than targeting either pathway alone, thereby improving patient survival.
Mutations that activate the PI3K/Akt/mTOR pathway are frequently found in breast cancer [47]. However, the ability of individual Akt isoforms to mediate metastasis of TNBC cells is not well understood. We found that silencing Akt1 dramatically decreased lung colonization of circulating TNBC cells and induced PARP cleavage and Bim expression. Prior work has found that Akt1 maintains the viability of tumor-initiating cells by reducing Bim expression [39]. Therefore, our results indicate that Akt1 may support lung metastasis of circulating TNBC cells by preventing apoptosis induction. To our knowledge, this is the first study to establish that Akt1 expression promotes the in vivo metastatic potential of TNBC cells. Previous work has found that Akt1 blocks metastasis of other breast cancer subtypes [23], [24], [25]. Akt1 inhibits invasion of ER+ breast cancer cells, and Akt1 overexpression attenuates HER2-mediated metastasis of murine mammary tumors [23], [24], [25]. TNBC is a very aggressive disease that is 4× more likely to metastasize to viscera than non-TNBCs within the first 5 y [48]. Therefore, TNBC metastasis may be promoted by proteins that do not facilitate spread of other cancers. Our findings suggest that Akt1 is a key regulator of lung colonization in TNBC cells.
Akt2 is more frequently amplified in cancer cells than Akt1, but its ability to regulate TNBC lung colonization is also unknown [18]. We found that silencing Akt2 significantly reduced TNBC lung colonization and suppressed expression of Snail, Claudin-1, and the anti-apoptotic protein Mcl-1. Claudin-1 promotes the invasive potential of nasopharyngeal carcinoma cells, and Snail is required for TNBC cells to invade into lymph nodes [49, 50]. Therefore, our findings suggest that Akt2 may promote lung metastasis of circulating TNBC cells 2 ways: (1) facilitating extravasation through the vessel wall and into lung parenchyma and (2) preventing apoptosis induction. To our knowledge, this is the first study to identify Akt2 as a key mediator of lung colonization in circulating TNBC cells. Prior work has indicated that Akt2 facilitates metastasis of other breast cancer subtypes [23, 25]. Akt2 promotes the migration and invasion of ER+ breast cancer cells, and Akt2 overexpression increases HER2-mediated metastasis of murine mammary tumors [23, 25]. Our findings suggest that Akt2 is also an important regulator of metastasis in TNBC cells.
Akt1 and Akt2 facilitate TNBC lung colonization independent of Akt activity. We showed that silencing Akt1 or Akt2 does not correlate with a substantial reduction in pAkt expression. This is most likely due to compensatory phosphorylation of the isoform that is still present within the cells. Despite a corresponding reduction in pAkt levels, knockdown of Akt1 or Akt2 significantly suppressed TNBC metastasis. Other studies have also found a non-kinase function for Akt [51, 52]. Akt1 has been shown to reduce toll-like receptor signaling in a kinase-independent fashion [51]. Moreover, Akt promotes cell survival in a kinase-independent manner and instead relies on its pleckstrin homology domain [52]. Akt1 and Akt2 may facilitate survival of circulating TNBC cells in a similar manner, but further work is needed to establish the underlying mechanisms. A prior study has found that overexpression of pAkt is correlated with an increased rate of brain metastasis in lung cancer patients [53]. Our findings suggest that the role of pAkt may vary between cancer types or that pAkt mainly stimulates growth of cells that have already colonized distant organs.
Suppressing Akt1 and/or Akt2 expression is a novel therapeutic strategy that could be implemented for TNBC patients who are resistant to current therapeutic regimens. Our findings indicate that pharmacological reduction of Akt1 and/or Akt2 expression in TNBC patients with refractory tumors may prevent metastatic spread, thereby directly improving survival. Current clinical trials are focused on developing Akt inhibitors as therapeutic agents for breast cancer patients. However, we suggest that future studies should identify agents that can decrease Akt1 and/or Akt2 expression and evaluate their ability to limit metastasis of nonresponsive TNBC.
In addition to Akt1 and Akt2, we also studied how AMPKα isoforms impact lung colonization. AMPKα is a major metabolic regulator that may balance energy demand during different stages of metastasis. When cells detach from a matrix, they are unable to import glucose and instead must increase ATP synthesis through other pathways [54]. AMPKα’s ability to mobilize energy via different metabolic pathways suggests that it may have an important role in restoring energy homeostasis for circulating cells. Moreover, fatty acid synthesis and oxidation—both of which are regulated by AMPKα—have been reported to promote metastasis [31]. Prior studies have found that AMPKα affects TNBC metastasis in an isoform-dependent fashion. AMPKα1 prevents metastatic spread of TNBC cells, while AMPKα2 promotes metastasis of TNBC cells [55, 56]. However, the specific stages of metastasis that are controlled by AMPKα1 and AMPKα2 in TNBC cells is unclear. In addition, combined inhibition of Akt and AMPKα isoforms has not been studied. We found that transiently silencing AMPKα1, AMPKα2, or AMPKα1/2 alone did not affect lung colonization of circulating MDA-MB-231 cells. Moreover, AMPKα knockdown did not synergistically block TNBC lung metastasis when combined with Akt isoform knockdown. These findings suggest that AMPKα1, AMPKα2, and AMPKα1/2 do not impact lung colonization of circulating MDA-MB-231 cells.
Our findings expand on these prior studies, which used stable knockdown or overexpression cells instead of transiently transfected cells [55, 56]. Unlike our experimental design, stable cell lines will not lose knockdown or overexpression after extravasation into tissue. As a result, previous work has not delineated whether AMPKα isoforms regulate metastasis of circulating TNBC cells or regulate proliferation of established microcolonies. Our findings indicate that neither AMPKα1 nor AMPKα2 impact survival or extravasation of circulating TNBC cells. However, AMPKα has been shown to affect TNBC cell growth [41], [42], [43]. We have previously reported that AMPKα1 and AMPKα2 promote cell cycle progression and proliferation in TNBC cells [43]. Moreover, pharmacological activators of AMPKα have also reduced growth and cell cycle progression of TNBC cells [57], [58], [59]. Therefore, the major effect of AMPKα isoforms on TNBC metastasis may involve growth regulation of cells that have already survived during systemic circulation and lung colonization.
In summary (Fig 5B), we have shown that reducing Akt1 or Akt2 expression prevents lung colonization of TNBC cells. Akt1 facilitates lung metastasis by inhibiting apoptosis induction, while Akt2 promotes lung metastasis by enabling extravasation through the vessel wall and into surrounding tissue. Silencing AMPKα1, AMPKα2, or AMPKα1/2 does not reduce lung colonization or synergistically block lung metastasis when combined with Akt isoform knockdown. Importantly, suppressing Akt1 and/or Akt2 expression is a potentially promising therapeutic strategy that could reduce lung colonization and thus decrease mortality among TNBC patients.
Author Contributions
JJ: Conceptualization, Investigation, Writing – Original Draft, Writing – Review & Editing; ZC: Conceptualization, Writing – Review & Editing; EL: Formal Analysis; HLW: Formal Analysis; BME: Funding Acquisition, Supervision, Writing – Review & Editing; PR: Conceptualization, Investigation, Supervision, Writing – Review & Editing
Competing interest
The authors declare no conflicts of interest.
Funding
This research was funded by National Institutes of Health grant R01 CA195573 (to BME), P30 CA177558, T32 CA160003 (for ZC), and Daphne's Legacy Breast Cancer Research Funds.
Acknowledgments
We acknowledge and thank the Research Communication Office of the Markey Cancer Center for assistance in preparing the figures and the manuscript. The Biostatistics and Bioinformatics Shared Resource Facility of the Markey Cancer Center conducted formal statistical analyses, and the Biospecimen Procurement & Translational Pathology Shared Resource Facility of the Markey Cancer Center acquired tissue sections (both supported by P30 CA177558).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2021.03.005.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Elia I., Schmieder R., Christen S., Fendt S.M. Organ-specific cancer metabolism and its potential for therapy. Handb Exp Pharmacol. 2016;233:321–353. doi: 10.1007/164_2015_10. [DOI] [PubMed] [Google Scholar]
- 3.Andreopoulou E., Schweber S.J., Sparano J.A., McDaid H.M. Therapies for triple negative breast cancer. Expert Opin Pharmacother. 2015;16:983–998. doi: 10.1517/14656566.2015.1032246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao H., He G., Yan S., Chen C., Song L., Rosol T.J., Deng X. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 2017;8:1913–1924. doi: 10.18632/oncotarget.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collignon J., Lousberg L., Schroeder H., Jerusalem G. (2016). Triple-negative breast cancer: treatment challenges and solutions Breast Cancer (Dove Med Press) 8, 93-107. doi: 10.2147/BCTT.S69488 [DOI] [PMC free article] [PubMed]
- 6.Sporikova Z., Koudelakova V., Trojanec R., Hajduch M. (2018). Genetic markers in triple-negative breast cancer clin breast cancer 18, e841-e850. doi: 10.1016/j.clbc.2018.07.023 [DOI] [PubMed]
- 7.Hurvitz S., Mead M. Triple-negative breast cancer: advancements in characterization and treatment approach. Curr Opin Obstet Gynecol. 2016;28:59–69. doi: 10.1097/GCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 8.Pal S.K., Childs B.H., Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–636. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bareche Y., Venet D., Ignatiadis M., Aftimos P., Piccart M., Rothe F., Sotiriou C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. 2018;29:895–902. doi: 10.1093/annonc/mdy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 11.Society A.C. Editor (ed) American Cancer Society; 2019. Triple-Negative Breast Cancer. [Google Scholar]
- 12.Yao Y., Chu Y., Xu B., Hu Q., Song Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci Rep. 2019;39 doi: 10.1042/BSR20190288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zijl F., Krupitza G., Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–749. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 15.Elia I., Doglioni G., Fendt S.M. Metabolic hallmarks of metastasis formation. Trends Cell Biol. 2018;28:673–684. doi: 10.1016/j.tcb.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Saha M., Kumar S., Bukhari S., Balaji S.A., Kumar P., Hindupur S.K., Rangarajan A. AMPK-Akt double-negative feedback loop in breast cancer cells regulates their adaptation to matrix deprivation. Cancer Res. 2018;78:1497–1510. doi: 10.1158/0008-5472.CAN-17-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q., Chen X., Hay N. Akt as a target for cancer therapy: more is not always better (lessons from studies in mice. Br J Cancer. 2017;117:159–163. doi: 10.1038/bjc.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen M.M., Jr. The AKT genes and their roles in various disorders. Am J Med Genet A. 2013;161A:2931–2937. doi: 10.1002/ajmg.a.36101. [DOI] [PubMed] [Google Scholar]
- 19.Jean S., Kiger A.A. Classes of phosphoinositide 3-kinases at a glance. J Cell Sci. 2014;127:923–928. doi: 10.1242/jcs.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. (2017). The PI3K pathway in human disease cell 170, 605-635. doi: 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed]
- 21.Martini M., De Santis M.C., Braccini L., Gulluni F., Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi H., Hsu J.L., Hung M.C. Regulation of ubiquitination-mediated protein degradation by survival kinases in cancer. Front Oncol. 2012;2:15. doi: 10.3389/fonc.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggio M., Perrone M.C., Polo M.L., Rodriguez M.J., May M., Abba M., Lanari C., Novaro V. AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci Rep. 2017;7:44244. doi: 10.1038/srep44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchinson J.N., Jin J., Cardiff R.D., Woodgett J.R., Muller W.J. Activation of Akt-1 (PKB-α) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- 25.Dillon R.L., Marcotte R., Hennessy B.T., Woodgett J.R., Mills G.B., Muller W.J. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faubert B., Vincent E.E., Poffenberger M.C., Jones R.G. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–170. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543–559. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardie D.G., Ross F.A., Hawley S.A. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol. 2012;19:1222–1236. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia D., Shaw R.J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic. Balance Mol Cell. 2017;66:789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross F.A., MacKintosh C., Hardie D.G. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 2016;283:2987–3001. doi: 10.1111/febs.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porporato P.E., Payen V.L., Baselet B., Sonveaux P. Metabolic changes associated with tumor metastasis, part 2: Mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci. 2016;73:1349–1363. doi: 10.1007/s00018-015-2100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rychahou P.G., Jackson L.N., Silva S.R., Rajaraman S., Evers B.M. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 843-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allred D.C., Clark G.M., Elledge R., Fuqua S.A., Brown R.W., Chamness G.C., Osborne C.K., McGuire W.L. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Institute. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 34.Allred D.C., Harvey J.M., Berardo M., Clark G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. https://pubmed.ncbi.nlm.nih.gov/9504686/ DOI: see: [PubMed] [Google Scholar]
- 35.Chorner P.M., Moorehead R.A. A-674563, a putative AKT1 inhibitor that also suppresses CDK2 activity, inhibits human NSCLC cell growth more effectively than the pan-AKT inhibitor, MK-2206. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y., Savage R.E., Eathiraj S., Meade J., Wick M.J., Hall T., Abbadessa G., Schwartz B. Targeting AKT1-E17K and the PI3K/AKT pathway with an allosteric AKT inhibitor, ARQ 092. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies B.R., Greenwood H., Dudley P., Crafter C., Yu D.H., Zhang J., Li J., Gao B., Ji Q., Maynard J. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 38.Green B.D., Jabbour A.M., Sandow J.J., Riffkin C.D., Masouras D., Daunt C.P., Salmanidis M., Brumatti G., Hemmings B.A., Guthridge M.A. Akt1 is the principal Akt isoform regulating apoptosis in limiting cytokine concentrations. Cell Death Differ. 2013;20:1341–1349. doi: 10.1038/cdd.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gargini R., Cerliani J.P., Escoll M., Anton I.M., Wandosell F. Cancer stem cell-like phenotype and survival are coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells. 2015;33:646–660. doi: 10.1002/stem.1904. [DOI] [PubMed] [Google Scholar]
- 40.Hinz N., Jucker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal. 2019;17:154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laderoute K.R., Calaoagan J.M., Chao W.R., Dinh D., Denko N., Duellman S., Kalra J., Liu X., Papandreou I., Sambucetti L. 5′-AMP-activated protein kinase (AMPK) supports the growth of aggressive experimental human breast cancer tumors. J Biol Chem. 2014;289:22850–22864. doi: 10.1074/jbc.M114.576371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu P., Ye F., Xie X., X. L., Tang H., Li S., Huang X., Song C., Wei W., Xie X. mir-101-3p is a key regulator of tumor metabolism in triple negative breast cancer targeting AMPK. Oncotarget. 2016;7:35188–35198. doi: 10.18632/oncotarget.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson J., Chow Z., Napier D., Lee E., Weiss H.L., Evers B.M., Rychahou P. Targeting PI3K and AMPKalpha signaling alone or in combination to enhance radiosensitivity of triple negative breast. Cancer Cells. 2020;9 doi: 10.3390/cells9051253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obenauf A.C., Massagué J. Surviving at a Distance: Organ-Specific Metastasis. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carthew R.W., Sontheimer E.J. (2009). Origins and mechanisms of miRNAs and siRNAs cell 136, 642-655. doi: 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed]
- 46.Dana H., Chalbatani G.M., Mahmoodzadeh H., Karimloo R., Rezaiean O., Moradzadeh A., Mehmandoost N., Moazzen F., Mazraeh A., Marmari V. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13:48–57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5542916/ DOI: see. [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Knowles E., O'Toole S.A., McNeil C.M., Millar E.K., Qiu M.R., Crea P., Daly R.J., Musgrove E.A., Sutherland R.L. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer. 2010;126:1121–1131. doi: 10.1002/ijc.24831. [DOI] [PubMed] [Google Scholar]
- 48.Dent R., Hanna W.M., Trudeau M., Rawlinson E., Sun P., Narod S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 49.Wu X., Xiao J., Zhao C., Zhao C., Han Z., Wang F., Yang Y., Jiang Y., Fang F. Claudin1 promotes the proliferation, invasion and migration of nasopharyngeal carcinoma cells by upregulating the expression and nuclear entry of beta-catenin. Exp Ther Med. 2018;16:3445–3451. doi: 10.3892/etm.2018.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olmeda D., Moreno-Bueno G., Flores J.M., Fabra A., Portillo F., Cano A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 2007;67:11721–11731. doi: 10.1158/0008-5472.CAN-07-2318. [DOI] [PubMed] [Google Scholar]
- 51.Zenke K., Muroi M., Tanamoto K.I. (2018). AKT1 distinctively suppresses MyD88-depenedent and TRIF-dependent toll-like receptor signaling in a kinase activity-independent manner Cell Signal 43, 32-39. doi: 10.1016/j.cellsig.2017.12.002 [DOI] [PubMed]
- 52.Vivanco I., Chen Z.C., Tanos B., Oldrini B., Hsieh W.Y., Yannuzzi N., Campos C., Mellinghoff I.K. (2014). A kinase-independent function of AKT promotes cancer cell survival Elife 3. doi: 10.7554/eLife.03751 [DOI] [PMC free article] [PubMed]
- 53.Jin Y., Yuan Y., Yi M., Han H., Liu B., Li Q. Phosphorylated-Akt overexpression is associated with a higher risk of brain metastasis in patients with non-small cell lung cancer. Biochem Biophys Rep. 2019;18 doi: 10.1016/j.bbrep.2019.100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber G.F. Metabolism in cancer metastasis. Int J Cancer. 2016;138:2061–2066. doi: 10.1002/ijc.29839. [DOI] [PubMed] [Google Scholar]
- 55.Yi Y., Chen D., Ao J., Zhang W., Yi J., Ren X., Fei J., Li F., Niu M., Chen H. Transcriptional suppression of AMPKalpha1 promotes breast cancer metastasis upon oncogene activation. Proc Natl Acad Sci U S A. 2020;117:8013–8021. doi: 10.1073/pnas.1914786117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saxena M., Balaji S.A., Deshpande N., Ranganathan S., Pillai D.M., Hindupur S.K., Rangarajan A. AMP-activated protein kinase promotes epithelial-mesenchymal transition in cancer cells through Twist1 upregulation. J Cell Sci. 2018;131 doi: 10.1242/jcs.208314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson J., Rychahou P., Sviripa V.M., Weiss H.L., Liu C., Watt D.S., Evers B.M. (2019). Induction of AMPK activation by N,N'-diarylurea FND-4b decreases growth and increases apoptosis in triple negative and estrogen-receptor positive breast cancers PLoS One 14, e0209392. doi: 10.1371/journal.pone.0209392 [DOI] [PMC free article] [PubMed]
- 58.Lee K.H., Hsu E.C., Guh J.H., Yang H.C., Wang D., Kulp S.K., Shapiro C.L., Chen C.S. Targeting energy metabolic and oncogenic signaling pathways in triple-negative breast cancer by a novel adenosine monophosphate-activated protein kinase (AMPK) activator. J Biol Chem. 2011;286:39247–39258. doi: 10.1074/jbc.M111.264598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu B., Fan Z., Edgerton S.M., Deng X.-S., Alimova I.N., Lind S.E., Thor A.D. (2014). Metformin induces unique biological and molecular responses in triple negative breast cancer cells cell cycle 8, 2031-2040. doi: 10.4161/cc.8.13.8814 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.