Abstract
Alzheimer’s dementia (AD) is the sixth leading cause of death in the U.S., with an estimated $305 billion cost of care in 2020. Currently there are no cures or therapies to ameliorate the disease progression and symptoms. Growing evidence links a diet characterized by high antioxidant components with benefits to cognitive function, which is indicative of the preventative potential of dietary interventions.
The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study is a 3-year, multicenter, randomized controlled trial to test the effects of the MIND diet on cognitive function in 604 individuals at risk for AD.
Men and women ages 65 to 84 years were recruited. Eligible participants were randomized to either the MIND diet with mild caloric restriction or their usual diet with mild caloric restriction. Cognitive assessments, medical history, blood pressure, anthropometric measurements, and blood and urine sample collections will be taken at baseline and follow-up visits. MRI scans will be completed on approximately half of the enrolled participants at the start and end of the study.
Unique features of the MIND study include: 1) a dietary pattern, rather than single nutrient or food, tested in an at-risk population; 2) foods featured as key components of the MIND diet (i.e. extra-virgin olive oil, blueberries, and nuts) provided for participants; and 3) MRI scans of brain structure and volume that may provide potential mechanistic evidence on the effects of the diet. Results from the study will be crucial to the development of dietary guidelines for the prevention of AD.
Keywords: Randomized controlled trial, MIND diet, Nutrition, Cognition, Aging, Study Design, Study protocols
INTRODUCTION
Alzheimer’s dementia (AD) is a devastating disease characterized by the gradual loss of memory and ability to function independently. In the absence of effective therapies, preventive strategies are an urgent public health priority. Behavioral prevention trials have consistently demonstrated reductions in cognitive decline or brain atrophy through exercise [1–5], cognitive training [6–9], and a combination of both [10–13]. Diet, as a behavioral prevention strategy, is gaining increased attention. Investigations of the neuroprotective effects of individual nutrients such as folate, vitamin E, B vitamins, and n-3 polyunsaturated fatty acids, have indicated that the consumption of foods containing these nutrients are related to a lower risk of dementia [14]. Because nutrients exist in a food matrix acting interactively as part of a diet, there has been an emergence of research investigating the potential synergetic effects of dietary patterns on health.
For example, previous studies have shown that greater adherence to the Mediterranean diet demonstrated a reduction in AD risk [15, 16], prevention of mild cognitive impairment [17, 18], and better performance on cognitive function tests [25]. Similarly, the Dietary Approaches to Stop Hypertension (DASH) diet [19], which features high consumption of fruits, vegetables, whole grains, and low-fat dairy products, and reduced intake of sodium, has also been associated with slower cognitive decline [20–22]. Although both dietary patterns show benefits in preventing cognitive decline, they are not tailored specifically for brain health. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet is a hybrid of the Mediterranean and DASH diets with selected modifications based on the most compelling evidence in the diet-dementia field [14, 21, 23, 24]. It uniquely specifies the intake of food components in servings that reflect the findings of scientific studies on nutrition and dementia [25–30], and it also specifically recommends limited intake of foods that are unhealthy for the brain because of high saturated fat content [31].
The MIND diet features the consumption of vegetables (particularly, green leafy vegetables), berries, extra-virgin olive oil, nuts, whole grains, and low-fat sources of protein. Previous observational studies showed that greater adherence to the MIND diet was related to a lower risk of AD [31, 32] and slower cognitive decline [33]. However, evidence from a large-scale, randomized controlled trial to test the causal relationship between the MIND diet and protection from cognitive decline is lacking. This paper describes the MIND intervention trial, which is designed to test the effects of a 3-year MIND dietary counseling intervention (plus mild caloric restriction for weight loss) versus a usual diet (plus mild caloric restriction for weight loss) on cognitive decline among cognitively unimpaired and overweight older adults with suboptimal diets.
The primary aim is to examine the effects of the MIND diet on a global measure of cognition based on 12 individual cognitive tests. A secondary aim is to test the MIND diet on MRI-derived changes to the structural integrity of the brain in a random subset of participants. We hypothesize that the MIND diet will reduce the rate of cognitive decline and total brain volume loss compared with a usual diet.
MATERIALS AND METHODS
Overview of Study Design
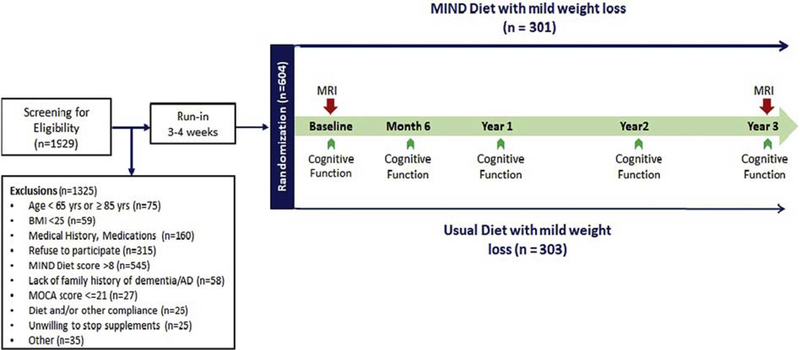
The MIND study is a randomized controlled intervention trial designed to compare the effects of the MIND diet with mild weight loss, versus participants’ usual diet with mild weight loss, on brain health in an overweight population of 604 participants at risk for AD. The protocol consists of screening for eligibility, a 3 to 4 week run-in period, randomization to one of the intervention groups, and a 3-year intervention period (Figure 1). There were 738 participants who started the run-in. The 604 participants who successfully completed their run-in and baseline visit were randomized to interventions. Both interventions contain a mild weight loss component through mild caloric restriction (250 kcal/d). Cognitive function will be assessed at baseline, and Months 6, 12, 24, and 36. A sub-group of participants will have MRI scans at the beginning and end of the intervention period.
Figure 1.
Summary of the MIND diet trial protocol.
The primary end point is change in global cognitive score measured by a battery of tests (over a 3-year period. Secondary end points are 1) 3-year change in the four cognitive domains: executive functioning, perceptual speed, episodic memory, and semantic memory; 2) 3-year change in MRI-derived normalized measures of total brain volume and hippocampal volume; and 3) other measures of brain macro- and micro-structural integrity, including normalized volumes of white/gray matter, segmented gray matter regions, white matter lesions, and thickness of segmented cortical regions.
Study Population
We recruited community-dwelling adults in the Boston and Chicago city areas through mass mailings, and we later amplified the recruitment by advertisements in newspapers, radio ads and through hospital media channels. We enrolled 604 overweight participants between the ages of 65 and 84 with suboptimal diets who are at risk for dementia. Inclusion and exclusion criteria are presented in Table 1. Major exclusion criteria include allergy to the intervention foods; medication for Alzheimer’s disease, Parkinson’s disease, or psychiatric conditions; individuals with mental disorder; excessive alcohol consumption or substance abuse; individuals who had onset of stroke or transient ischemic attack within the previous 3 months; history of brain injury, liver disease, Hepatitis C, or HIV; illnesses and diseases that are associated with weight change; and diagnosis of cancer within the previous 5 years.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
| • Men and women, 65–84 years of age |
| • BMI ≥ 25 kg/m2 |
| • Willing to participate and give informed consent |
| • Family history of dementia, but without personal cognitive impairment (as measured by the Montreal Cognitive Assessment (MoCA) ≥22) [34] |
| • Must agree to not take non-prescribed vitamin supplements, multi-vitamin or individual supplements of vitamin E, folic acid, n-3 fatty acids, or carotenoids |
| • Must not have a member of their household already enrolled in the Mind Diet Trial |
| • Suboptimal diet (MIND screener score 8 or lower out of 14)1 |
| • Successful completion of 3–4 week run-in period |
| Exclusion criteria |
| • Have allergy to more than one type of food (nuts, berries, olive oil, or fish) |
| • Use of medications to treat Alzheimer’s disease or Parkinson’s disease |
| • Psychosis or bipolar disorder |
| • Depression or other psychiatric disorders2 |
| • Psychiatric medicines |
| • Unstable or recent onset of cardiovascular disease, such as myocardial infarction within the previous 6 months or presence of heart failure above Type 1 |
| • Recent onset of stroke or TIA within previous 3 months |
| • Diagnosis of cancer within previous 5 years except non-melanoma skin cancer2 |
| • History of brain injury, liver disease, Hepatitis C, or HIV |
| • Illness and diseases related to weight change (i.e., history of stomach or gastrointestinal conditions, inflammatory bowel disease, Crohn’s disease, malabsorption, colostomy, bowel resection, or gastric bypass surgery)2 |
| • Report of alcohol or substance abuse within previous 6 months or heavy alcohol consumption (> 2 drinks/day for women; > 3 drinks/day for men) |
MIND diet screening instrument and scoring (Supplementary Table 1)
Clinical judgments by PI and the steering committee
Participants’ baseline characteristics are shown in Table 2. The participants’ mean age is 70.4 with a mean BMI of 33.2. The majority of the participants are white (88%), and 65% are female. Half of the study population has obtained a post-graduate degree.
Table 2.
Baseline Characteristics of the MIND Study Participants
| Characteristic | MIND (n=301) | Active Control (n=303) | All (N=604) |
|---|---|---|---|
| Age | 70.4 ± 4.2 | 70.4 ± 4.2 | 70.4 ± 4.2 |
| Weight (kg) | 93.2 ± 17.4 | 94.3 ± 20.2 | 93.7 ± 18.8 |
| BMI (kgm2) | 33.8 ± 5.4 | 34.0 ± 6.5 | 33.9 ± 6.0 |
| Sex | |||
| Female | 196(65) | 197(65) | 393(65) |
| Race | |||
| White | 263(87) | 267(88) | 530(88) |
| Black or African American | 35(12) | 31(10) | 66(11) |
| Other race | 3(1) | 5(1.7) | 8(1.3) |
| Ethnicity | |||
| Non-Hispanic | 296(98) | 298(98) | 594(98) |
| Education | |||
| Some high school | 0 ( 0) | 1(0) | 1(0) |
| High school diploma/GED | 16(5) | 13(4) | 29(5) |
| 1–3 years college, business, or tech school | 57(19) | 47(16) | 104(17) |
| college degree | 72(24) | 87(29) | 159(26) |
| Post-graduate degree | 156(52) | 155(51) | 311(51) |
| Marital status | |||
| Never married | 35(12) | 36(12) | 71(12) |
| Married | 165(55) | 165(54) | 330(55) |
| Divorced/separated | 57(19) | 65(21) | 122(20) |
| Widowed | 44(15) | 37(12) | 81(13) |
| Annual household income | |||
| Less than 25,000 | 27(9) | 26(9) | 53(9) |
| 25,000 to 49,999 | 39(13) | 57(19) | 96(16) |
| 50,000 to 99,999 | 109(36) | 105(35.0) | 214 (35) |
| 100,000 and above | 111(37) | 98(32) | 209(35) |
| Refused or Unknown | 15(5) | 17(6) | 32(5) |
Continuous variables are expressed as Mean ± SD.
Categorical variables are presented as n (%)
Screening, Run-in, and Randomization
Screening included a brief prescreen by telephone followed by an in-person screening visit. Individuals who met the inclusion criteria at the telephone prescreen were invited to an in-person screening visit (SV1). During the screening visit, we examined BMI eligibility, administered a brief health history questionnaire, and conducted a standardized assessment of cognitive impairment, using the validated Montreal Cognitive Assessment (MoCA) [34].
After screening, eligible participants were invited to complete a 3 to 4 week run-in period to assess compliance. Participants were required to complete at least two diet records each week (one day during the week and, one day on the weekend) and to consult weekly by phone with case managers and in person in the final week(s). Only participants who demonstrated excellent adherence were eligible for randomization. To be eligible, participants had to complete 100% of the contacts and at least 67% of the diet records (i.e. 4 of 6 scheduled diet records). A number of measurements, questionnaires, and tests were collected over 2 days of baseline visits. The maximum numbers of days between baseline visit Day 1 and Day 2 was 6 weeks. Upon completion of all baseline measurements and assessments, participants were randomized by the Coordinating Center to one of the two interventions. The two diet group assignments were stratified by site with varying block sizes to ensure a balance at each site.
Intervention
The two diet approaches to dementia prevention were designed to be similar in content and intensity to promote clinical and personal equipoise in the intervention. Nutrition case managers had a background in research and nutrition and were trained to deliver the dietary counseling by certified dieticians. The training covered behavioral self-management strategies based on social cognitive theory concepts (e.g. self-regulation, behavioral rehearsal, and motivational interviewing techniques).Re-certification of all case managers occurs at least annually. After participants were randomized, case managers conducted the first individual visit consisting of an initial diet instruction and counseling session on the assigned diet, and this began the 3-year intervention. The counseling program for the intervention diet group includes instructions on foods to incorporate into the diet, ways to prepare these foods, and behavioral strategies to lose weight. For the active control group, participants are offered equivalent frequency of consultation focusing on calorie tracking and portion control. Participants from both groups are trained in the daily use of self-monitoring strategies to encourage adherence to each assigned diet. Motivational strategies including newsletters and website activities are implemented to enhance participants’ engagement for both groups. Once monthly group sessions are also offered to promote social support from study peers and hands-on application of the assigned diet through interactive learning modules such as cooking demonstrations and diet-specific games.
Participants randomized to the MIND diet (Table 3) are being supplied with nuts, extra virgin olive oil, and blueberries. These specific foods were chosen as participant incentives to reduce the financial burden associated with regular consumption of the highest quality versions of food items rich in important nutrients to promote brain health. Nuts are nutrient dense with unsaturated fat, vitamin E, protein and fibers; blueberries with high antioxidant capacity, and extra virgin olive oil is rich in polyphenols. The antioxidants and anti-inflammatory properties of these foods represent mechanistic evidence for mitigating cognitive decline [35]. Remote coaching consists of a series of educational modules focused on evidence-based recommendations for adopting the MIND diet into the regular eating regimen. These strategies are reviewed by the case-managers to document goal achievements and diet adherence on a weekly basis for the first 6 months of the trial and every other week during the second 6 months. In Years 2 and 3, case managers use various methods to provide individualized attention to participants on their assigned diet at least twice monthly. In-person consultations (individual or group sessions) will occur quarterly in Year 1 and bi-annually in Years 2 and 3.
Table 3.
Intervention targets of the MIND Diet
| Foods to Eat | Recommended Serving | Foods to Limit | Serving Limitation |
|---|---|---|---|
| Green leafy vegetables | 0.5–1.0 cups per day | Red and processed meats | No more than 3 servings (3–5oz) per week |
| Other vegetables | 0.5 cups per day | Butter and stick margarine | No more than 1 pat (tsp) per day |
| Nuts (mixed nuts and/or peanut butter) | 5 oz per week | Cheese (whole fat) | Less than 1 oz per week |
| Berries | 0.5 cups 5 times per week | Pastries, candy bars, sweets | No more than 4 servings per week |
| Beans/legumes | 0.5 cups 3 times per week | Fried foods and fast food | No more than 1 meal per week |
| Whole grains | 3 servings per day | ||
| Fish (not fried) | 3–5 oz per week | ||
| Poultry (not fried, white meat/skinless) | 3–5 oz 2 times per week | ||
| Extra virgin olive oil | 2 Tbsp per day |
The active control diet consists of the participants’ usual diet with a mild calorie restriction (250 kcal/d) and a target goal of 3–5% weight loss. Remote coaching consists of educational modules focused on behavioral strategies towards mild weight loss, such as portion control, calorie counting, mindful eating, and environmental restructuring. Self-monitoring of weight and the option to track all food intake is possible through paper tracking logs and commonly used nutrition tracking apps. Monthly group sessions are being offered with cooking demonstrations centered on how to continue enjoying the usual diet while cutting calories through strategies such as portion reduction and mindful eating techniques.
Assessment of Adherence
We are employing a multi-pronged strategy to maintain dietary adherence: 1) frequent telephone/email communications with a nutrition case manager, 2) personalized diet plans and strategies, 3) multiple compliance aids (refrigerator charts and computerized tracking/phone apps), 4) group motivation strategies (group cooking classes, buddy systems, family/friend involvement, and social media), and 5) frequent weight monitoring and check-ins. Diet adherence will also be assessed by the MIND diet score [32]. Adherence will be evaluated based on 15 dietary components, with a score ranging from 0 to 15, with higher scores indicating greater adherence. For safety in the trial, we eliminated the recommendation to consume wine; thus, 14 is the highest possible score of adherence. Intake of each dietary component will be categorized into tertiles based on predefined cut-offs reflecting intake ranging from low to high (Supplementary Table 2). A value of 0.0, 0.5, or 1.0 has been assigned to each category. Participants with high intake of olive oil, fish (not fried), whole grains, berries, green leafy vegetables, other vegetables, nuts, beans, and poultry (not fried, skinless) will receive a score of 1. Participants with low intake of foods for which limited consumption is recommended (i.e., butter and margarine, cheese, red meat and meat products, fast and fried foods, and pastries and sweets) will receive a score of 1. Adherence to the intervention diet will also be assessed by objective biomarkers such as tyrosol, tocopherol, folate, vitamin B12 in blood, and polyphenol in urine at baseline and Months 3, 12, 24, and 36. Adherence to calorie restrictions will be assessed by 24-hour dietary recalls at Months 6, 12, 24, and 36 [36–38].
Blinding of data
We are adhering to established procedures to maintain separation between staff who take outcome measurements and those who deliver the intervention. Participants’ treatment assignments are being maintained at the Coordinating Center, which has no direct participant contact. Staff responsible for outcome assessments will be blinded to study treatment assignment of all participants until the conclusion of the study.
Measurements
The specific schedule for measurements for each outcome variable is shown in Table 4. The primary outcome of the study is the change in the global measure of cognitive function measured at baseline and Months 6, 12, 24, and 36. At each of these time points, participants will complete a neuropsychological test battery of 12 performance-based tests (Table 5). The test battery includes multiple tests for each of four cognitive domains. For episodic memory: immediate and delayed recall of the East Boston story [39] and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List learning, recall, and recognition [40] will be used. For semantic memory: category fluency [40] and the Multilingual Naming Test [41] will be used. For executive function: (Trails B) [42] and the NIH toolbox flanker test [43] will be used, and for perceptual speed: (Trails A) [42], the Digit Symbol Substitution Test [44], and the NIH toolbox pattern comparison test [45] will be used. The global measure is a composite measure of all 12 tests including episodic memory, semantic memory, perceptual speed, and executive functioning, and is created by converting raw scores on each test to z scores, and then averaging the z scores [46], a method that has been used in many previous studies [21–23, 32, 46].
Table 4.
Measurements Schedule
| Variable | Pre-Screen | SV1 | Run-in (Week) | Baseline Visits 1 and 2 | Month 3 | Month 6 | Month 12 | Month 24 | Month 36 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||||
| Pre-Screen Eligibility Form | X | |||||||||||
| Family History AD | X | |||||||||||
| MIND Diet Screener | X | |||||||||||
| Consent | X | |||||||||||
| Health/Med History | X | |||||||||||
| Montreal cognitive assessment | X | |||||||||||
| Height | X | X | ||||||||||
| Weight | X | X | X | X | X | X | ||||||
| Blood Pressure | X | X | X | X | X | |||||||
| Waist Circumference | X | X | X | X | X | |||||||
| Food Records | X | X | X | X | ||||||||
| Diet Randomization | X | |||||||||||
| Initial Diet Instruction | X | |||||||||||
| MRI1 | X | X | ||||||||||
| Diet Intervention | X | X | X | X | X | |||||||
| Move Monitor2 | X | X | X | X | ||||||||
| Fasting Blood draw 3 | X | X | X | X | X | |||||||
| Spot Urine4 | X | X | X | X | X | |||||||
| MIND Interview5 | X | X | X | X | X | |||||||
| Medication usage questionnaire | X | X | X | X | X | |||||||
| 24 hr Dietary Recall | X | X | X | X | X | |||||||
| Food Frequency Questionnaire | X | X | X | X | X | |||||||
| Cognitive Battery6 | X | X | X | X | X | |||||||
Sample of 300 participants; 150 at each site; 150 on each diet
Participants from the Rush site will be asked to wear a physical activity sensor (the McRoberts Move Monitor+) on seven consecutive days
Fasting blood collected on participants at baseline and at months 3, 12, 24, and 36. Measures will include abeta40/42, HbA1c, APOE-ε4 genotyping (Baseline ONLY), BDNF, CRP, IL-6, adiponectin, oxidized LDL, total cholesterol, HDL cholesterol, triglycerides, folate, Vitamin B12, tocopherols, carotenoids, and fatty acids.
Spot urine (Total Polyphenols with creatinine) collected on participants at baseline and at months 3, 12, 24, and 36.
MIND Interview includes: Medical history, CES-D 10-item, Neuroticism Scale 4-item, Yale Physical Activity Scale, Cognitive Activities, Functional Status, Demographics, and Tobacco usage.
Cognitive Battery includes: East Boston Immediate Story recall, Multilingual Naming Test, Word List Memory, Word List Recall, Word List Recognition, Pattern Comparison (NIH toolbox) Verbal Fluency, Logical Memory (East Boston delayed story recall), Trails test A and B, Flanker Inhibitory Control (NIH toolbox), Oral Digit Symbol test (NIH toolbox)
Table 5.
Cognitive Domains and Tests Used in the MIND Diet Trial
| Cognitive Domain | Cognitive Tests |
|---|---|
| Executive functioning | Trails B |
| Flanker inhibitory control | |
| Perceptual speed | Oral Symbol Digit Modalities Test |
| Trails A | |
| Pattern comparison | |
| Episodic memory | Word list memory |
| Word list recall | |
| Word list recognition | |
| Logical memory (East Boston story immediate recall) | |
| Logical memory (East Boston story delayed recall) | |
| Semantic memory | Verbal fluency |
| Multilingual naming test |
We will also perform brain MRIs on a sample of 300 participants (150 within each treatment group) at baseline and at 3 years to assess the effects of the MIND diet on brain macro-and micro-structural integrity. Biomarkers for AD and of cardiovascular risk factors and conditions will be investigated as potential mediators and/or modifiers of the diet intervention. Diabetes, hypertension, BMI, cholesterol, depression, and chronic psychological distress will be measured using standard assessments in the blood and/or through questionnaires. We will measure APOE-ε4 genotype, plasma Abeta 42/Abeta 40, and Brain-derived neurotrophic factor (BDNF) at baseline and Months 3, 12, 24, and 36. Lipid profile, nutrient biomarkers (tyrosol, carotenoid, folate, vitamin B12, tocopherols, carotenoids, and fatty acids) in blood, and total polyphenols and creatinine in urine will be measured at baseline and at Months 3, 12, 24, and 36.
Statistical Power
The study is powered to test the effect of the MIND intervention over the control intervention on the annual rate of change in the global cognitive score. The study power was estimated using a simulation approach in R, which is designed for mixed model analyses. We used data from the Rush Memory and Aging Project to estimate the effect size of the MIND diet score on cognitive change in analyses that used cognitively unimpaired participants in adjusted models. With a sample size of 300 participants the MIND trial has > 85% power to detect a between-group difference of 0.02 in the annual rate of cognitive decline (two-sided p = 0.05) considering 5% drop out at each visit. A treatment effect of this size corresponds to the observed diet effect on cognition in the PREDIMED trial or the effect on cognition seen after 5 years of aging [47]. The power estimates for Aim 1 are conservative for several reasons: 1) MIND participants have a family history of dementia and thus a faster rate of decline; 2) the dropout rate assumes all dropouts occur at the beginning of the trial so that there are many more actual time points of assessment; and 3) the power model assumes four annual visits, but the trial includes a fifth cognitive assessment at 6 months to add precision to slope estimation.
Trial Monitoring
In this multicenter clinical trial, a Data and Safety Monitoring Board (DSMB), appointed by the study PIs, was established to review the protocol; oversee timely recruitment, study progress and data quality; monitor results; and provide recommendations to the investigative team. The DSMB has expertise in the following categories: biostatistics, dementia and AD, clinical trial design and conduct, and cardiovascular disease.
Biostatisticians from the Data Coordinating Center are responsible for preparing the reports to the DSMB. In addition to the board members, meetings are attended by the study PIs, representatives from the Data Coordinating Center, and members of the National Institute on Aging. The DSMB meets twice a year throughout the trial. Meeting minutes are prepared by the Data Coordinating Center, and a summary of the portion of the minutes related to participant safety is distributed to PIs to forward to their individual IRBs.
Analysis Plan
Our primary aim is to test the effects of the MIND diet (with a focus on mild weight loss) versus the usual diet (with a focus on mild weight loss), on cognitive decline. Our primary outcome is the annual rate of change in global cognition measured by a battery of tests at five assessment points over a 3-year period. We will use linear mixed effects models with random effects to characterize individual paths of change in cognitive function, adjusting for differences in baseline level of cognition and rate of cognitive change. Mixed models allow for individual variation in intercepts and slopes while simultaneously accounting for the within-person correlation of multiple measurements [48]. For analyses, models include indicator variables for intervention group and site, a variable for time elapsed from study enrollment, and a term for the product of an indicator of intervention group and time (measuring the treatment effect on cognitive decline).
The large number of randomized participants should eliminate the need for model adjustment for potential confounders, but in the unlikely event of risk factor imbalance between the treatment groups, we will analyze adjusted models according to the methods of Tsiatis et al. [49]. Analyses identical to those performed for the global measure will be performed on each of the composite measures of the individual cognitive domains. Transformation or generalized linear mixed effects models will be used when distribution normality does not apply [50]. We will test these aims with individual significance level at 0.05 and will emphasize exploratory aspects of analyses that will require follow-up in independent research. Model assumptions will be explored both analytically and graphically.
The primary analysis is an intent-to-treat analysis including all randomized participants. A secondary analysis will be performed on participants who complete the study and achieve the adherence goals. For these analyses, we will adjust for potential confounding variables. All available assessments for cognitive function will be used in the analysis for individuals who discontinue the intervention. Sample size computations will accommodate a dropout fraction of 20%, but in the event of dropout fraction exceeding 5%, intent-to-treat analysis will incorporate appropriate methods for valid inference, including multiple imputation.
For the secondary aim, we will examine the effects of the MIND diet on the 3-year change in MRI-derived normalized measures of brain structure. We will also explore the effects on other measures of brain MRI macro- and micro-structural integrity, including normalized volumes of white/gray matter, segmented gray matter regions, white matter lesions, and thickness of segmented cortical regions. Diffusion tensor imaging (DTI) information from the white matter will be projected onto a white matter skeleton using tract-based spatial statistics and will be analyzed voxel-wise (considering only voxels of the white matter skeleton) [51]. We will first compute the pre- to post-treatment difference in each measure and then perform t-tests of the differences between the treatment groups. Variable distributions will be checked for normality, and the appropriate variable transformations will be performed, with nonparametric inference employed in case approximate normality cannot be achieved. For the DTI voxel-wise analysis, the null distribution will be built using the randomize tool in the FMRIB Software Library (FSL), with 5000 permutations of the data. Family-wise error correction and the threshold-free cluster enhancement method will be used to define clusters with significant effects.
DISCUSSION
An unhealthy diet is a modifiable risk factor for dementia. Early evidence demonstrates that individual nutrients (i.e., omega-3 fatty acids, vitamins B6 and B12, folate, and vitamin D) are associated with a lower risk of dementia [14]. As opposed to single nutrients, the balance of dietary patterns is more relevant to public health because nutrients have cumulative and synergetic effects in dietary patterns [52]. Emerging epidemiologic evidence on dietary patterns suggests that several diets, including the Mediterranean dietary pattern, the DASH diet, the MIND diet, and the Nordic diet, are all preventive of cognitive decline [53]. Findings from observational studies need to be verified by randomized controlled trials, which are considered the gold standard to establish the causal relationship between diet and dementia.
Previous trials that have investigated the effects of diet/lifestyle intervention on dementia have been predominantly conducted in Europe. A randomized intervention trial showed that the Mediterranean diet supplemented with extra virgin olive oil or nuts significantly increased global cognition and/or specific domains of cognition in a Spanish population with cardiovascular disease risk factors after 6.5 years of follow-up [54]. Another randomized controlled trial demonstrated that the DASH diet plus weight management significantly improved executive function and memory/learning, and the DASH diet alone also improved psychomotor speed among hypertensive, overweight adults in the U.S. over 4 months [55]. The FINGER trial showed a significant positive effect of a multidomain lifestyle intervention including dietary counseling on cognitive performance [56, 57].
The MIND dietary pattern is tailored for brain health by targeting the intake of food components that reflect scientific findings linking nutrition and dementia [25–30]; it also specifically recommends limiting the intake of foods that are unhealthy for the brain. [31]. To date, there has been no long-term randomized intervention trial in the US to establish the causal relationship between the MIND diet and cognition. Therefore, we are conducting the MIND study to examine the effects of the MIND diet on change in global cognitive function and specific domains of function over 3 years in a U.S. population of older adults at risk for dementia.
The study has limitations. First, the 3-year intervention period may present challenges in observing change in cognition due to the influence of practice effects. However, the FINGER trial demonstrated significant effects of a multidomain intervention on cognitive function over just 2 years. The relatively large sample size of the present study and 3-year duration will provide statistical power to detect small treatment effects. Second, participants in the control arm may consume components of the MIND diet due to the widespread interest in diet as a preventive strategy for dementia. We have instituted a number of safeguards to monitor this possibility, including 24-hour diet recalls, weekly counseling, and measurement of nutrients and biomarkers in the blood.
The study also has a number of strengths. First, we are including an active control arm that receives an equal frequency of interpersonal interaction and the same elements of the intervention as the MIND group, but without the intervention foods [58]. That is, participants from the MIND group and the control group are receiving an equal intensity of attention for working on calorie reduction to achieve weight loss. This active control approach minimizes the benefits of attention that may come from interpersonal interaction in the intervention arm, and therefore maximizes the chance to assess the true effects of the intervention. Second, we are addressing multiple risk factors for dementia simultaneously in a single trial (e.g., hypertension and diabetes), allowing for covariate adjustment of factors that may confound the association of diet with the primary outcome. Third, we are employing a novel methodological approach of randomizing only those participants with sub-optimal diets. This will help ensure a contrast in nutritional status between the intervention and control groups, and will thus potentiate the protective benefit of the intervention. Lastly, we will use both the MIND diet score, a cost-effective quantified approach, and objective nutrient biomarkers to assess study adherence.
Results from this trial will add precision to our understanding of the role of a dietary pattern intervention as a preventative strategy for AD and will add to the evidence base for development of dietary guidelines for brain health. This dietary pattern intervention approach will have broad implications at a population level. Further, MRI studies assembled in this trial will contribute to the development of mechanistic evidence of the role of diet in AD etiology.
Supplementary Material
Acknowledgements
The MIND diet trial is supported by grant R01 AG052583, which includes support from the National Institute on Aging. Members of the Data Safety and Monitoring Board include Lawrence J. Appel (chair), Deborah E. Barnes, Claudia H. Kawas, and David M. Reboussin, with Kristina McLinden and Alvin McKelvy as ex-officio members.
The sponsor of the MIND diet trial is the National Institute on Aging. Additionally, several corporations are generously donating mixed nuts (International Tree Nut Council Nutrition Research and Education Foundation), peanut butter (The Peanut Institute), extra virgin olive oil (Innoliva-ADM Capital Europe LLP), and blueberries (U.S. Highbush Blueberry Council). These items are being distributed to those participants who are randomized to the MIND diet arm. The MIND diet trial is registered at clinicaltrials.gov (NCT02817074), and the study website is www.mind-diet-trial.org
Footnotes
This paper is dedicated to the memory of Dr. Martha Clare Morris, who designed and obtained funding for the MIND intervention before her untimely passing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration
Name of Registry: MIND Diet Intervention and Cognitive Decline (MIND) ClinicalTrials.gov Identifier: NCT02817074 Date of Registration: June 29 2016
REFERENCES
- 1.Baker LD, et al. , Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis, 2010. 22(2): p. 569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker LD, et al. , Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol, 2010. 67(1): p. 71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu-Ambrose T, et al. , Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med, 2010. 170(2): p. 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maki Y, et al. , Effects of intervention using a community-based walking program for prevention of mental decline: a randomized controlled trial. J Am Geriatr Soc, 2012. 60(3): p. 505–10. [DOI] [PubMed] [Google Scholar]
- 5.van de Rest O, et al. , Effect of resistance-type exercise training with or without protein supplementation on cognitive functioning in frail and pre-frail elderly: secondary analysis of a randomized, double-blind, placebo-controlled trial. Mech Ageing Dev, 2014. 136–137: p.85–93. [DOI] [PubMed] [Google Scholar]
- 6.Forster S,, et al. , Effects of a 6-month cognitive intervention program on brain metabolism in amnestic mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis, 2011. 25(4): p. 695–706. [DOI] [PubMed] [Google Scholar]
- 7.Miller KJ, et al. , Effect of a computerized brain exercise program on cognitive performance in older adults. Am J Geriatr Psychiatry, 2013. 21(7): p. 655–63. [DOI] [PubMed] [Google Scholar]
- 8.Mowszowski L, et al. , Cognitive training enhances pre-attentive neurophysiological responses in older adults ‘at risk’ of dementia. J Alzheimers Dis, 2014. 41(4): p. 1095–108. [DOI] [PubMed] [Google Scholar]
- 9.Wolinsky FD, et al. , A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One, 2013. 8(5): p. e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson-Hanley C, et al. , Exergaming and older adult cognition: a cluster randomized clinical trial. Am J Prev Med, 2012. 42(2): p. 109–19. [DOI] [PubMed] [Google Scholar]
- 11.Barnes DE, et al. , The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med, 2013. 173(9): p. 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klusmann V, et al. , Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci, 2010. 65(6): p. 680–8. [DOI] [PubMed] [Google Scholar]
- 13.Lam LC, et al. , A 1-year randomized controlled trial comparing mind body exercise (Tai Chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc, 2012. 13(6): p. 568 e15-20. [DOI] [PubMed] [Google Scholar]
- 14.Morris MC, Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc, 2012. 71(1): p. 1–13. [DOI] [PubMed] [Google Scholar]
- 15.Scarmeas N, et al. , Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol, 2006. 59(6): p.912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarmeas N, et al. , Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol, 2006. 63(12): p. 1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarmeas N, et al. , Mediterranean diet and mild cognitive impairment. Arch Neurol, 2009. 66(2): p. 216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tangney CC, et al. , Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr, 2011. 93(3): p. 601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacks FM, et al. , A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin Cardiol, 1999. 22(7 Suppl): p. III6–10. [DOI] [PubMed] [Google Scholar]
- 20.Tangney CC, et al. , Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology, 2014. 83(16): p. 1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris MC, et al. , MIND diet score more predictive than DASH or Mediterranean diet scores Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 2014. 10(4): p. P166. [Google Scholar]
- 22.Tangney C, et al. , Accordance to Dietary Approaches to Stop Hypertension (DASH) is associated with slower cognitive decline. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 2013. 9(4): p. P135. [Google Scholar]
- 23.Morris MC and Tangney CC, Dietary fat composition and dementia risk. Neurobiol Aging, 2014. 35 Suppl 2: p. S59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillette Guyonnet S, et al. , IANAtask force on nutrition and cognitive decline with aging. J Nutr Health Aging, 2007. 11(2): p. 132–52. [PubMed] [Google Scholar]
- 25.Morris MC, et al. , Associations of vegetable and fruit consumption with age-related cognitive change. Neurology, 2006. 67(8): p. 1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JH, Ascherio A, and Grodstein F, Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol, 2005. 57(5): p. 713–20. [DOI] [PubMed] [Google Scholar]
- 27.Devore EE, et al. , Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol, 2012. 72(1): p. 135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larrieu S,, et al. , Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J Nutr Health Aging, 2004. 8(3): p. 150–4. [PubMed] [Google Scholar]
- 29.Schaefer EJ, et al. , Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol, 2006. 63(11): p. 1545–50. [DOI] [PubMed] [Google Scholar]
- 30.Willis LM, Shukitt-Hale B, and Joseph JA, Recent advances in berry supplementation and age-related cognitive decline. Curr Opin Clin Nutr Metab Care, 2009. 12(1): p. 91–4. [DOI] [PubMed] [Google Scholar]
- 31.Morris MC, et al. , MIND diet slows cognitive decline with aging. Alzheimers Dement, 2015. 11(9): p. 1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris MC, et al. , MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement, 2015. 11(9): p. 1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosking DE, et al. , MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement, 2019. 15(4): p. 581–589. [DOI] [PubMed] [Google Scholar]
- 34.Nasreddine ZS, et al. , The Montreal Cognitive Assessment, MoCA a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 2005. 53(4): p. 695–9. [DOI] [PubMed] [Google Scholar]
- 35.Vauzour D, et al. , Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res Rev, 2017. 35: p. 222–240. [DOI] [PubMed] [Google Scholar]
- 36.Anton SD, et al. , Use of a computerized tracking system to monitor and provide feedback on dietary goals for calorie-restricted diets: the POUNDS LOST study. J Diabetes Sci Technol, 2012. 6(5): p. 1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson DA, et al. , Adherence is a multi-dimensional construct in the POUNDS LOST trial. J Behav Med, 2010. 33(1): p. 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson DA, et al. , Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. J Behav Med, 2010. 33(4): p. 305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gfeller JD and Horn GJ, The East Boston Memory Test: a clinical screening measure for memory impairment in the elderly. J Clin Psychol, 1996. 52(2): p. 191–6. [DOI] [PubMed] [Google Scholar]
- 40.Morris JC, et al. , The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 1989. 39(9): p. 1159–65. [DOI] [PubMed] [Google Scholar]
- 41.Ivanova I, Salmon DP, and Gollan TH, The multilingual naming test in Alzheimer’s disease: clues to the origin of naming impairments. J Int Neuropsychol Soc, 2013. 19(3): p. 272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reitan RM, Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Perceptual and Motor Skills, 1958. 8(3): p. 271–276. [Google Scholar]
- 43.Weintraub S, et al. , The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. Journal of the International Neuropsychological Society: JINS, 2014. 20(6): p. 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaeger J, Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. Journal of clinical psychopharmacology, 2018. 38(5): p. 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlozzi NE, et al. , NIH Toolbox Cognitive Battery (NIHTB-CB): the NIHTB Pattern Comparison Processing Speed Test. Journal of the International Neuropsychological Society: JINS, 2014. 20(6): p. 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson RS, et al. , Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging, 2002. 17(2): p. 179–93. [PubMed] [Google Scholar]
- 47.Martinez-Lapiscina EH, et al. , Mediterranean diet improves cognition: the PREDIMED-NAVARRArandomised trial. J Neurol Neurosurg Psychiatry, 2013. 84(12): p. 1318–25. [DOI] [PubMed] [Google Scholar]
- 48.Laird NM and Ware JH, Random-effects models for longitudinal data. Biometrics, 1982. 38(4): p. 963–74. [PubMed] [Google Scholar]
- 49.Tsiatis AA, et al. , Covariate adjustment for two-sample treatment comparisons in randomized clinical trials: a principled yet flexible approach. Stat Med, 2008. 27(23): p. 4658–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breslow NE and Clayton DG, Approximate Inference in Generalized Linear Mixed Models. Journal of the American Statistical Association, 1993. 88(421): p. 9–25. [Google Scholar]
- 51.Smith SM, et al. , Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 2006. 31(4): p. 1487–505. [DOI] [PubMed] [Google Scholar]
- 52.Hu FB, Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol, 2002. 13(1): p. 3–9. [DOI] [PubMed] [Google Scholar]
- 53.van den Brink AC, et al. , The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv Nutr, 2019. 10(6): p. 1040–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valls-Pedret C, et al. , Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Internal Medicine, 2015. 175(7): p. 1094–1103. [DOI] [PubMed] [Google Scholar]
- 55.Smith PJ, et al. , Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension, 2010. 55(6): p. 1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ngandu T, et al. , A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet, 2015. 385(9984): p. 2255–63. [DOI] [PubMed] [Google Scholar]
- 57.Kivipelto M, et al. , The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement, 2013. 9(6): p. 657–65. [DOI] [PubMed] [Google Scholar]
- 58.LaFave SE, et al. , Attention control group activities and perceived benefit in a trial of a behavioral intervention for older adults. Res Nurs Health, 2019. 42(6): p. 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.