Abstract
Chlorine is a toxic industrial chemical with a history of use as a chemical weapon. Chlorine is also produced, stored, and transported in bulk making it a high-priority pulmonary threat in the USA. Due to the high reactivity of chlorine, few biomarkers exist to identify exposure in clinical and environmental samples. Our laboratory evaluates acute chlorine exposure in clinical samples by measuring 3-chlorotyrosine (Cl-Tyr) and 3,5-dichlorotyrosine (Cl2-Tyr) using liquid chromatography tandem mass spectrometry (LC-MS/MS). Individuals can have elevated biomarker levels due to their environment and chronic health conditions, but levels are significantly lower in individuals exposed to chlorine. Historically these biomarkers have been evaluated in serum, plasma, blood, and bronchoalveolar lavage (BAL) fluid. We report the expansion into hair and lung tissue samples using our newly developed tissue homogenization protocol which fits seamlessly with our current chlorinated tyrosine quantitative assay. Furthermore, we have updated the chlorinated tyrosine assay to improve throughput and ruggedness and reduce sample volume requirements. The improved assay was used to measure chlorinated tyrosine levels in 198 mice exposed to either chlorine gas or air. From this animal study, we compared Cl-Tyr and Cl2-Tyr levels among three matrices (i.e., lung, hair, and blood) and found that hair had the most abundant chlorine exposure biomarkers. Furthermore, we captured the first timeline of each analyte in the lung, hair, and blood samples. In mice exposed to chlorine gas, both Cl-Tyr and Cl2-Tyr were present in blood and lung samples up to 24 h and up to 30 days in hair samples.
Keywords: Chlorine gas; 3-Chlorotyrosine; 3,5-Dichlorotyrosine; In vivo mouse study; LC-MS/MS; Hair and lung tissue
Introduction
Chlorine is a powerful oxidizing agent with many beneficial uses in creating plastics, synthetic rubbers, pesticides, and chlorides [1]. The USA generates over 10 million tons of chlorine each year, making it among the highest volume chemicals produced [2]. However, chlorine has also been used as a chemical weapon, first during World War I [3] and more recently in Syria [4]. In April of 2018, a deliberate attack using chlorine bombs was reported by the OPCW in Douma, Syria, resulting in 40–70 deaths and hundreds of chemical-related injuries [5]. According to the Global Public Policy Institute, 227 of the 336 confirmed chemical weapon attacks during the Syrian civil war involved chlorine [6, 7]. The Organisation for the Prohibition of Chemical Weapons (OPCW) classifies chlorine as a choking agent but has not listed it as a scheduled agent due to its overwhelming history of safe and beneficial industrial use [8]. However, releasing chlorine gas with the intent to kill or harm is in direct violation of the Chemical Weapons Convention (CWC). Due to the recent and potential future use of chlorine gas as a chemical weapon, it is crucial to have reliable environmental and clinical assays to identify violations of the CWC and assist in treatment.
Chlorinating chemicals are used in many household cleaning and hair products which can produce detectable levels of chlorine exposure biomarkers in clinical samples [9]. Individuals who work with higher concentrations of reactive chlorine chemicals (e.g., janitors, pool workers) may have elevated levels [10]. Furthermore, the in vivo production of hypochlorous acid in individuals with chronic inflammatory diseases can result in the same biomarkers as chlorine gas exposure [11–13]. Identifying biomarkers of chlorine gas exposure is difficult since chlorine is highly reactive and non-specific, and individuals can have elevated biomarker levels due to their environment and chronic health conditions.
The most common biomarkers for acute chlorine exposure in humans are chlorinated tyrosine, chlorinated lipids, and phosphatidylglycerol chlorohydrins [12–16]. Sochaski et al. developed a GC-MS method to measure two biomarkers of chlorine stress: 3-chlorotyrosine (Cl-Tyr) and 3,5-dichlorotyrosine (Cl2-Tyr) [12]. Hemström et al. measured chlorine exposure in bronchoalveolar lavage (BAL) fluid by monitoring pulmonary surfactant lipids, unsaturated phosphatidylglycerol, and phosphatidylcholine, which form chlorohydrins upon exposure to chlorine [14]. Ford et al. evaluated three chemically different but related forms of chlorinated lipids in blood: 2-chloroacids, 2-chloroalcohols, and 2-chloroaldehydes [15]. Massa et al. measured chlorinated derivatives of surfactant proteins in lung tissue [16]. To date, each biomarker has only been detected in vivo up to 72 h post-exposure. This paper outlines our efforts to find a long-term biomarker of chlorine gas exposure by evaluating alternate sample types such as hair and lung tissues.
Previously, our laboratory reported the development of a clinical assay for confirming acute chlorine exposures via measurement of Cl-Tyr and Cl2-Tyr in serum, plasma, blood, and BAL fluid samples using LC-MS/MS [13]. Since that initial publication in early 2016, we have adapted our assay to increase sample throughput, reduce sample volume, simplify the preparation, and improve chromatography. In addition, we have developed a homogenization protocol to process hair and lung tissues. Using our updated chlorinated tyrosine assay and new tissue homogenization protocol, we analyzed blood, lung, and hair tissue samples from 198 mice exposed to chlorine gas or air. Figure 1 outlines the three key features described in this paper: (1) mouse in vivo exposure of chlorine gas or air; (2) development of a tissue homogenization protocol; and (3) evaluation of the lung, muzzle, and blood samples harvested from the mouse study using the updated chlorinated tyrosine method. Our goals are to improve sample handling and processing, to allow for additional matrices, and to standardize the chlorinated tyrosine protocol resulting in a robust and easily transferrable method.
Fig. 1.

A schematic showing the three key components of this study: (1) chlorine gas or air (control) in vivo exposure in mice; (2) the development of a bead-based tissue homogenization protocol; and (3) quantitative analysis using the updated chlorinated tyrosine method
Materials and methods
Chemicals and materials
Native and 13C6-isotopically labeled 3-chloro-l-tyrosine were purchased from Sigma-Aldrich (St. Louis, MO) and Cambridge Isotopic Laboratories (Andover, MA), respectively. Native and 13C9, 15N-isotopically labeled 3,5-dichloro-l-tyrosine were purchased from IsoSciences (King of Prussia, PA). Protease from Streptomyces griseus and sodium hypochlorite solution (active chlorine: 4.00–4.99 wt%) were purchased from Sigma-Aldrich. Hypercarb™ Porous Graphitic Carbon (2.1 mm × 30 mm; 3 μm) analytical columns, Optima™ LC/MS-grade formic acid, ammonium bicarbonate, 2-mL 96-well plates, Pierce™ protein precipitation plates, Thermomixer R, 96-well PCR plates, tweezers, aluminum foil, and disposable scalpels were purchased from Fisher Scientific (Hanover Park, IL). Bead Ruptor Elite homogenizer and hard tissue grinding mix in 2-mL tubes were purchased from Omni International Inc (Kennesaw, GA). A Staticmaster 2U500 static eliminator was purchased from Thomas Scientific (Swedesboro, NJ). High-performance liquid chromatography (HPLC)-grade acetonitrile, HPLC-grade methanol, and HPLC-grade water were purchased from MG Scientific (Pleasant Prairie, WI). PlateLoc™ peelable aluminum heat sealing foil was purchased from Agilent Technologies (Santa Clara, CA). XPE105 analytical balance was purchased from Mettler Toledo (Columbus, OH). Pooled human plasma was purchased from Tennessee Blood Services (Memphis, TN). All purchased plasma was pre-screened by the vendor in accordance with U.S. Food and Drug Administration (FDA) regulations to be free of hepatitis B, syphilis, and HIV. This study used de-identified plasma acquired from commercial sources, and thus, the work did not meet the definition of human subjects as specified in 45 CFR 46.102 (f) [17].
Preparation of the calibration curve, internal standard, and quality control materials
Synthetic 3-chloro-l-tyrosine and 3,5-dichloro-l-tyrosine were used to prepare seven calibrators at concentrations of 2.50, 5.00, 10.0, 25.0, 50.0, 100.0, and 500.0 ng/mL in 0.1% formic acid in HPLC-grade water. An internal standard (ISTD) solution containing 13C6-isotopically labeled 3-chloro-l-tyrosine and 13C9,15N-isotopically labeled 3,5-dichloro-l-tyrosine was also prepared at a concentration of 25.0 ng/mL in 0.1% formic acid in HPLC-grade water. The calibrators and ISTD were determined to be stable for at least 2 years when stored at − 70 °C (data not shown), and there were no isotopic contributions between the native and labeled compounds. A stock quality control (QC) material was prepared by adding 8 μL sodium hypochlorite (4.9–4.99% active chlorine) to 40 mL pooled human plasma (K2-EDTA). The stock QC material was further diluted into pooled human plasma to create a QC low (QCL; 1.10 mL stock QC into 38.9 mL plasma), QC mid (QCM; 3.75 mL stock QC into 36.25 mL plasma), and QC high (QCH; 27.5 mL stock QC into 12.5 mL plasma) materials. The same pool of human plasma (unspiked) served as the matrix blank (MB). The QCs and MB were stable for at least 2 years when stored at − 70 °C (data not shown).
Animal samples
Lung, muzzle, and blood samples from chlorine- and air (control)-exposed mice were gifted to the Centers for Disease Control and Prevention (CDC) as part of a collaborative effort between CDC, Battelle Memorial Institute, and Biomedical Advanced Research and Development Authority (BARDA) aimed at the development of a murine model of pulmonary chlorine exposure injury. The objective was to develop a murine model of pulmonary inhalational chlorine exposure injury with associated biomarkers for monitoring injury progression that could then be employed to evaluate and screen potential medical countermeasures. During the animal study performed by Battelle, male C57BL/6 mice were purchased from Charles River Laboratories (approximately 23 g and 7 weeks of age at the time of challenge) and exposed to 655 ppm chlorine gas (LCt50/7 of chlorine, data not presented) or air for 15 min via the nose-only route. A 1% chlorine cylinder, balance nitrogen, was used to deliver the chlorine to a 32-port Cannon style nose-only inhalation tower. The desired chlorine concentration was achieved by mixing the chlorine gas with dry air (< 3% relative humidity) and controlled by mass flow controllers/meters. Chlorine concentration was monitored in real time using an OMA-300 ultraviolet (UV) spectrometer. For the air-only exposures, a separate air flow control system and exposure tower were used. The air flow rate supplied during the control exposures was similar to the total flow rate during the chlorine exposures. All control animals were handled the same as chlorine-exposed animals.
After the 15-min exposure, the mice were observed through death or euthanasia at their pre-determined endpoint. The endpoints were as follows: 2, 6, 12, and 24 h, and 3, 7, 14, 21, and 30 days. Mice were euthanized using exsanguination or other American Veterinary Medical Association (AVMA)-approved methods following internal Battelle protocols. Once deceased, lung, muzzle, and blood samples were harvested and immediately frozen. Blood was collected into EDTA-containing tubes unless the mice were euthanized before their pre-determined endpoint. In which case, blood was collected without anti-coagulant via cardiac puncture. Samples (59 lung, 115 muzzle, and 118 blood samples) were shipped on dry ice to the CDC for anaslysis. These samples were used to develop a homogenization protocol and to investigate chlorinated tyrosine levels. The CDC was not involved with the design or funding of the chlorine gas animal model; therefore, internal Institutional Animal Care and Use Committee (IACUC) approval was not required by the CDC.
All animals were maintained under the Battelle animal care and use program accredited by the Association for Assessment and Accreditation for Laboratory Animal Care International. This care and use program was in accordance with guidelines set forth in the “Guide for the Care and Use of Laboratory Animals,” National Research Council, and/or the regulations and standards promulgated by the Agricultural Research Service, United States Department of Agriculture (USDA), pursuant to the Laboratory Animal Welfare Act of August 24, 1966, as amended. The Battelle IACUC at Battelle, Columbus, OH, approved the experimental protocol.
Homogenization of tissues
Frozen lung and muzzle samples were brought to room temperature. Hair was collected from the muzzle tissue by placing each muzzle on a piece of aluminum foil (approximately 2″ × 2″) and gently shaving the hair from the muzzle using a disposable scalpel. Approximately 1.0 mg of hair was weighed on an analytical balance (readability 0.01 mg) and then placed into a 2-mL tube containing hard tissue grinding metal beads, and the exact mass of the hair was recorded. A Staticmaster ionizing cartridge was used to eliminate electrostatic charges in hair samples. Lung samples were air-dried and placed on a piece of aluminum foil. A small section of lung (~ 5 mg) was cut off using a disposable scalpel. The lung sample was weighed on the analytical balance and placed into a 2-mL tube containing hard tissue grinding metal beads, and the exact mass of the lung was recorded. Unused portions of the lung and muzzle samples were stored at − 70 °C in the aluminum foil.
To homogenize the lung and hair samples, 50 mM ammonium bicarbonate (200 μL) was added to each 2-mL tube containing metal beads. The tubes were loaded onto an Omni Bead Ruptor Elite and homogenized using the following parameters: 5.5 m/s (speed), 50 s (time), and three cycles. These parameters uniformly homogenized the lung and hair samples. At this point, the homogenized samples were ready for chlorinated tyrosine extraction (“Analyte extraction from samples”) or storage in the 2-mL tubes at − 70 °C until analysis.
Analyte extraction from samples
Samples (blood, plasma, and homogenized lung and hair), QCs, MB, and calibrators (10 μL) were added to individual wells of a 2-mL 96-well plate. ISTD (20 μL) and 2 mg/mL pronase in 50 mM ammonium bicarbonate (100 μL) were added to each well. The plate was heat sealed and incubated for 75 min at 60 °C with shaking at 700 rpm. After digestion, each sample was added to a 96-well protein precipitation plate with acetonitrile (360 μL). The plate was mixed for 1 min at 500 rpm and eluted into a new 96-well plate using a vacuum manifold. Samples were dried under a nitrogen stream (60 °C) using an evaporator and reconstituted in 0.1% formic acid in HPLC-grade water (50 μL). The samples were then transferred to a 96-well PCR plate, heat sealed, and stored at − 70 °C until analysis.
LC-MS/MS
Chromatography was performed on an Agilent 1290 Infinity II UHPLC system (Santa Clara, CA) composed of a temperature-controlled sampler, binary pump, and heated column compartment. Analytical separation was performed using a linear gradient on a Hypercarb porous graphitic carbon column (2.1 × 30 mm, 3 μm) maintained at 60 °C. Mobile phase A (MPA) was 0.1% formic acid in HPLC-grade water and mobile phase B (MPB) was 0.1% formic acid in HPLC-grade acetonitrile. The gradient program was the following: 2% MPB hold from 0 to 1 min; gradual increase in MPB from 2 to 50% from 1.01 to 4 min; a step to 98% MPB and held from 4.01 to 4.50 min to clean the column; a step down to 2% MPB at 4.51 min to re-equilibrate the column. The flow rate was maintained at 250 μL/min, and the total analysis time was 4.51 min per sample. The expected retention time was 3.1 ± 0.2 min for Cl-Tyr and 3.8 ± 0.2 min for Cl2-Tyr.
Following chromatographic separation, Cl-Tyr and Cl2-Tyr were detected by MS/MS on an Agilent 6490 triple quadruple mass spectrometer coupled to the UHPLC system. The electrospray source operated in positive ion mode and used the following source parameters for Cl-Tyr and Cl2-Tyr: gas temp (290 °C), gas flow (11 L/min), nebulizer (60 psi), sheath gas temperature (200 °C), sheath gas flow (11.0 L/min), drying gas (14.00 L/min), capillary voltage (1000 V), nozzle voltage (300 V), fragmentor voltage (380 V), and cell accelerator voltage (2 V). The transitions and the corresponding collision energies were the following: Cl-Tyr (quantitation ion) 216.0 m/z → 135.1 m/z, 30 V; Cl-Tyr_C (confirmationion) 216.0 m/z → 170.0 m/z, 10 V; Cl-Tyr_ISTD (internal standard) 222.0 m/z → 205.0 m/z, 5 V; Cl2-Tyr (quantitationion) 250.0 m/z → 169.0 m/z, 30 V; Cl2-Tyr _C (confirmationion) 250.0 m/z → 204.0 m/z, 10 V; Cl2-Tyr _ISTD (internal standard) 260.0 m/z → 178.0 m/z, 30 V.
Agilent MassHunter software was used to process the data. A weighted (1/x) linear regression was established by plotting the native to ISTD peak area ratio against the expected calibrator concentration. The ISTD normalized response was linear over the entire range of 2.50–500.0 ng/mL with a coefficient of determination (R2) of 0.990 or greater for Cl-Tyr and Cl2-Tyr.
Method validation and QC characterization
Method validation assessed recovery of a known spike of analyte into matrix, within and between-day precision, limit of detection (LOD), ruggedness, stability, and evaluation of matrices. The validated method was used to characterize the calibrators and QC materials. The materials were characterized during 22 analytical batches over 4 weeks. Each analytical batch included a calibration curve, matrix blank (MB), and three QCs (QCL, QCM, and QCH). After the limits were established during characterization, the QC materials were evaluated against revised Westgard rules for each [18].
Development of tissue handling protocols
During development of a tissue handling protocol, various homogenization techniques and equipment such as mortar and pestle, sonication, blenders, freeze-thaw cycling, chemical additives, and mechanical homogenization were compared. Wash protocols involving methanol, phosphate buffer, ammonium bicarbonate buffer, or isopropanol were tested. Furthermore, variability within a tissue, ideal sample weight, homogenization buffer type and volume, bead material, and ideal storage conditions for pre- and post-homogenized samples were established. The result yielded a homogenized tissue sample that was compatible with our current chlorinated tyrosine assay.
Results and discussion
Chlorinated tyrosine method improvements
Since our original 2016 publication, we have made minor modifications to our chlorinated tyrosine assay to improve throughput, reproducibility, and ruggedness; reduce sample volume requirements; and expand into other matrices such as lung tissue and hair [13]. The updated protocol uses only 10 μL of sample which is fivefold less than previously reported. The calibration range was modified to 2.50–500.0 to reflect the probable exposure range. The internal standard concentration was reduced to 25.0 ng/mL to reflect the geometric mean of the new calibration curve. The product ion for Cl-Tyr ISTD was modified from 222.0 → 141.0 m/z to 222.0 → 205.0 m/z to address chromatographic interferences observed under the updated chromatographic conditions. The preparation was simplified by replacing the solid-phase extraction step with a simple protein precipitation crash. This change reduced method complexity and cost and resulted in no loss of sensitivity as compared to our previously published assay. In addition, the chromatographic run time was shortened to improve throughput. Finally, a protocol for homogenizing tissue and hair samples was developed (“Tissue handling protocol development”).
Method validation and material characterization
The updated chlorinated tyrosine assay was validated, and materials were characterized during 22 analytical batches over 4 weeks by two analysts with a maximum of two sample batches per day. Each analytical batch included seven calibrators, three QCs, one MB, and one solvent blank. The characterized values for Cl-Tyr QCL, QCM, and QCH were 10.7 ± 1.4 ng/mL, 33.3 ± 3.1 ng/mL, and 250.9 ± 26.6 ng/mL, respectively. The characterized values for Cl2-Tyr QCL, QCM, and QCH were 6.2 ± 0.9 ng/mL, 19.7 ± 2.4 ng/mL, and 147.0 ± 14.8 ng/mL, respectively.
Accuracy was established by spiking a low-, mid-, and high-level calibrator (5.00, 25.0, and 100.0 ng/mL) into two plasma samples on two different days (n = 3 replicates). Accuracy was calculated as the final concentration divided by the spiking concentration (i.e., 5.00, 25.0, or 100.0 ng/mL). Based on criteria provided by the FDA, accuracies using spiked recovery should be 85–115% [17]. The chlorinated tyrosine method had a mean accuracy of 92.7% for Cl-Tyr and 90.4% for Cl2-Tyr. Precision was established by processing QCL and QCH samples in duplicate over 10 days. The FDA guideline recommends the precision (both within and between batches) to have relative standard deviations (RSD%) within 15% [17]. Within-batch precisions were 10.7% (QCL Cl-Tyr), 12.9% (QCL Cl2-Tyr), 9.4% (QCH Cl-Tyr), and 4.8% (QCH Cl2-Tyr). Between-batch precisions were 7.5% (QCL Cl-Tyr), 5.9% (QCL Cl2-Tyr), 5.3% (QCH Cl-Tyr), and 4.9% (QCH Cl2-Tyr).
The LOD was calculated using a modified Taylor method [19]. The standard deviations from 22 replicates of the four lowest calibrators (2.50, 5.00, 10.0, and 25.0 ng/mL) were plotted against their respective concentrations. The LOD was calculated as three times the y-intercept. The calculated LOD was 0.562 ng/mL for Cl-Tyr and 0.739 ng/mL for Cl2-Tyr. The lowest calibrator, 2.50 ng/mL, was the lower limit of quantitation (LLOQ). At the LLOQ, both Cl-Tyr and Cl2-Tyr consistently confirmed with a signal-to-noise above 5.
Method ruggedness was evaluated by varying digestion time, temperature, volume, enzyme concentration, and enzyme lots. Ruggedness testing focused on digestion parameters since the calibration curve and ISTD are added as the final digested products. Therefore, the largest unaccounted variation was expected to come from the digestion step. To evaluate ruggedness, each QC was measured (n = 5) at ± 20% of the final method parameter and analyzed as unknowns. For instance, when evaluating the digestion temperature, the QCs were digested at 48 °C (− 20% of final method parameter), 60 °C (final method parameter), and 72 °C (+ 20% of final method parameter) and analyzed as unknowns against a curve using the final method parameter. The digestion step was rugged since all five tested digestion conditions were within 85–115% of the characterized QC concentrations.
Stability was evaluated through freeze-thaw cycles, benchtop storage of unprocessed and processed samples, and long-term cold storage using low- and high-level calibrators and QCs. For freeze-thaw experiments, materials were cycled three times between room temperature and − 70 °C. Benchtop storage experiments were conducted by placing the materials at room temperature for 24 h before processing through the method. Processed sample stability was determined by placing the processed sample at room temperature for 24 h prior to LC-MS/MS analysis. Long-term stability was monitored by storing the materials at − 70 °C for over a month. All conditions were compared to the initial material measurement. All calculated concentrations were within expected ± 15% of the nominal concentration.
Matrix comparisons were evaluated by running a calibration curve in each matrix type and comparing the slopes. In brief, a calibration curve was prepared (n = 3) in solvent, plasma, serum, whole blood, and BAL fluid and processed through the method. All matrices were deemed interchangeable since the slopes were within 5% of each other.
Comparison of homogenization techniques
We tested the following homogenization techniques and equipment to determine which performed best at making the solid tissue or hair sample compatible with our updated chlorinated tyrosine assay: mortar and pestle, sonication, blenders, freeze-thaw cycling, and mechanical homogenization. Use of mortar and pestle was eliminated due to the high risk of contamination and incompatibility with a high-throughput format. Sonication, blenders, and freeze-thaw cycling were eliminated since the treatments were not aggressive enough to homogenize the tissue samples examined. Therefore, we focused most of our efforts on mechanical homogenization since it was aggressive enough to homogenize hard tissue samples and showed the most potential for scale-up.
Different mechanical homogenization devices were compared to find a high-throughput device with a low risk of contamination. Two blade-based homogenizers were compared: the hard tissue Omni probe (7 mm × 110 mm) from Omni International (Kennesaw, GA) and the MicroHomogenizer from Claremont BioSolutions, LLC (Upland, CA). Both devices were intended for single use but were unable to completely homogenize hair samples. Bead-based homogenizers were appealing since they simultaneously homogenize four to 96 samples in disposable tubes, making this platform both high throughput and low risk of contamination. The bead-based homogenizer from Omni International offered ceramic, glass, and stainless steel beads for soft and hard tissues. When comparing the bead types, the ceramic and glass beads could homogenize lung tissue but were not adequate for hair. Stainless steel beads (2.4 mm) were selected since they consistently homogenized both hair and lung tissues. We used the Bead Ruptor in a 24-sample format which homogenized each sample in separate cryotubes containing disposable metal beads which eliminated the potential for sample contamination.
Tissue handling protocol development
We selected four common wash protocols used by drug screening labs to test the importance of pre-washing hair for our chlorinated tyrosine assay [20, 21]. Hair from several chlorine gas-exposed mice was harvested and combined to form one pooled sample. The pooled hair was used to establish if excess chlorinated compounds could be washed off. The pooled hair was divided into five batches and washed using either methanol, 10 mM phosphate buffer, 50 mM ammonium bicarbonate buffer, or isopropanol followed by 10 mM phosphate buffer or left unwashed. In the end, the control (no wash) protocol was selected as the differences in Cl-Tyr and Cl2-Tyr levels between the no wash and wash protocols were not significant. Washing the hair only increased margin of error due to sample loss and added a risk of contamination. Furthermore, the wash protocols took over 20 min which unnecessarily slowed down sample processing. It is important to note that these wash protocols were tested on mouse samples; human hair may require a pre-washing step to remove external contamination from hair products or pool chemicals prior to chlorinated tyrosine analysis.
To optimize homogenization volumes, tissue samples from several chlorine gas-exposed mice were harvested and pooled together to form one hair sample and one lung sample. Several homogenization solution volumes (100, 200, 500, and 1000 μL) were compared (n = 3) using the pooled lung and hair samples (Fig. 2). While lung tissue did not require solution to homogenize the sample using the bead-based technique, hair did require addition of solution. Too much solution prevented uniform sample break down, especially in hair samples, causing a decrease in Cl-Tyr and Cl2-Tyr levels. As a result, 200 μL volume was selected to homogenize both tissue sample types since it was the most reproducible. Two homogenization solutions were tested: water and 50 mM ammonium bicarbonate (Fig. 2). During these experiments, the pooled lung and hair samples were processed through the homogenization protocol using either water or 50 mM ammonium bicarbonate (n = 3). The results for both biomarkers were indistinguishable between the solutions; therefore, 50 mM ammonium bicarbonate was selected to keep the sample at a stable and neutral pH and to match the conditions used during the pronase digestion step of the chlorinated tyrosine assay. Optimal sample weight was determined by processing different amounts of the pooled tissues through the chlorinated tyrosine method. The following weights were tested in triplicate: 0.5, 1, 5, and 10 mg of hair and 5, 10, and 20 mg of lung tissue (Fig. 2). The exact mass was recorded to normalize the sample to the final calculated concentration. As a result, 1.0 mg of hair was selected since the analytical balance could reliably measure this amount, and the homogenization protocol could uniformly homogenize the tissue. While 0.5 mg of hair tissue yields higher levels of Cl-Tyr and Cl2-Tyr, it was difficult to confidently measure hair samples under 1 mg, particularly on high static days. Five milligrams of lung tissue was selected since amounts greater than 5 mg occasionally clogged the pipette tips or the protein precipitation plates during the chlorinated tyrosine method. Additionally, amounts under 5 mg were difficult to accurately weigh for the lung samples.
Fig. 2.

3-Chlorotyrosine (Cl-Tyr) and 3,5-dichlorotyrosine (Cl2-Tyr) levels in hair shaved from muzzles and lung samples (n = 3 replicates) homogenized while varying three different conditions. The homogenization solvent volumes tested were 100, 200*, 500, and 1000 μL 50 mM ammonium bicarbonate; tissue amounts tested were 0.5, 1*, 5, and 10 mg for hair or 2.5, 5*, 10, and 20 mg for lung tissue; and homogenization solvent types tested were water and 50 mM ammonium bicarbonate*. The final method conditions are indicated by an asterisk. The y-axes are shown on two scales to display the Cl-Tyr (left axis) and Cl2-Tyr (right axis) bar graphs
Sampling variability was tested for potential analyte variation between different regions within a sample. To test sampling variability, a muzzle from a gas-exposed mouse was dissected to separate the whiskers, hair, and nasal skin tissue from each other. Three subsections were taken from each sample region (except for the whiskers due to limited sample [n = 1]), homogenized, and processed through the chlorinated tyrosine method (Fig. 3). The %CV for hair was 23.5% for Cl-Tyr and 21.0% for Cl2-Tyr. The %CV for nasal skin tissue was 13.2% for Cl-Tyr and 30.2% for Cl2-Tyr. The large %CV implies that there is variation between the different regions within the same tissue. The same process was conducted using the lung tissue from the same animal, in which three subsections of the lung were harvested, homogenized, and processed through the method. The CV% for lung was 9.3% for Cl-Tyr and 29.7% for Cl2-Tyr. To prevent large sampling variability, pool multiple regions of the same sample prior to processing. For instance, shave the entire mouse muzzle into one uniform pool before taking a 1.0-mg fraction for weighing.
Fig. 3.
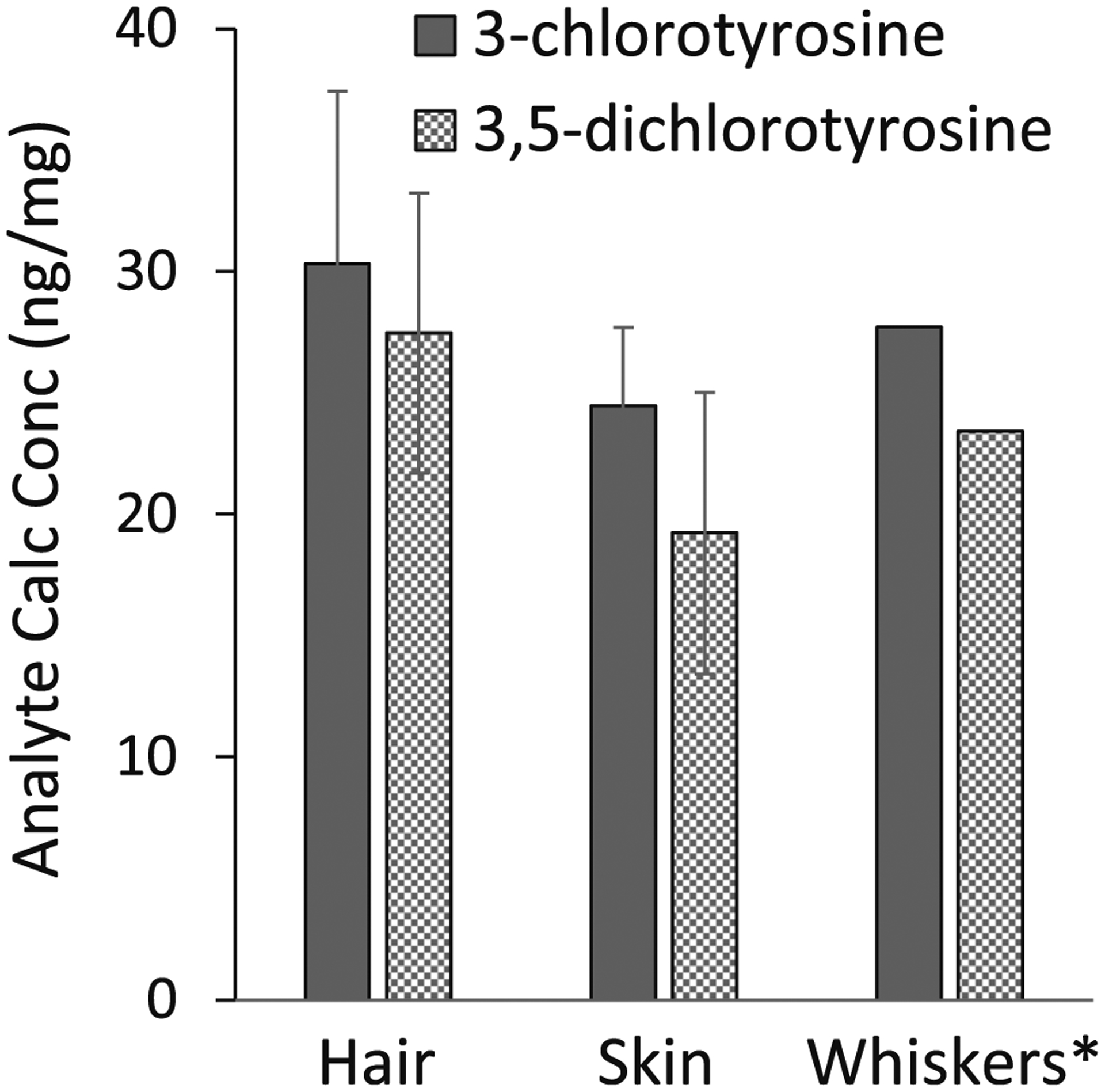
A single muzzle was dissected to separate the whiskers, skin, and hair. Three subsections were harvested within the hair and skin sections and processed through the method to evaluate sample homogeneity. *Whiskers had a single sample collection due to sample amount. The final 3-chlorotyrosine (Cl-Tyr) and 3,5-dichlorotyrosine (Cl2-Tyr) calculated concentrations were normalized to tissue mass (ng/mg)
A programmed homogenization protocol was developed on the Bead Ruptor to maximize ruggedness and throughput. Multiple parameters were tested including speed (0–7 m/s), number of cycles (1–3), and cycle time (30–60 s) to develop the automated program. The final protocol used 2 mL cryovial with metal beads with the following parameters: speed (5.5 m/s), cycles (3), and cycle time (50 s). Aggressive homogenization settings resulted in samples heating up at extended cycle time or increased speeds. Without knowing the full effect on our biomarker at higher speeds and temperatures, we aimed for parameters that uniformly homogenized hair and lung tissues without overheating the sample.
Matrix effects
Matrix comparisons studies were conducted to establish if matrices were interchangeable. To perform these studies, calibration curves (n = 8) were generated in solvent, homogenized lung, and homogenized hair from air-exposed (control) mice and processed through the chlorinated tyrosine method. In this study, the average slopes were within 3% regardless of matrix type and were therefore deemed interchangeable (< 5% difference). For simplicity, all experiments were performed and calculated from a solvent-based calibration curve that was treated through all steps of the method including digestion and precipitation.
Validation of homogenization protocol using samples collected from in vivo mouse study
Using the developed tissue homogenization protocol, we measured chlorinated tyrosine levels in blood, lung, and muzzle samples from mice exposed to chlorine gas. Battelle Memorial Institute exposed mice to chlorine gas or air (control) and returned them to air until their pre-determined endpoint as part of a natural history study. The endpoints ranged from 2 h to 30 days post-exposure. Out of the 198 mouse samples that were shipped to the CDC, 54 mice (27%) were exposed to air and 144 mice (73%) were exposed to chlorine gas. Of the chlorine-exposed mice, 80 out of the 144 mice (56%) died before their pre-determined endpoint. Of those 80 mice, 53 (66%) died within 1 h, 64 (80%) died within 6 h, and all 80 (100%) died before 24 h. Upon termination, the lung, muzzle, and blood samples were harvested from the mice. These harvested tissues were used to develop the reported tissue homogenization protocol and then analyzed using the updated chlorinated tyrosine assay. Representative chromatograms are shown in Fig. 4 for the chlorinated tyrosine method controls (MB and QCH) and from the samples (lung, muzzle, and blood) collected from mice exposed to chlorine gas and air. Chlorinated tyrosine levels from the 198 mice samples are shown in the Supplementary Information (ESM).
Fig. 4.

Overlay of extracted ion chromatograms from representative samples from (A) method control (pooled human plasma), (B) muzzle hair, (C) lung, and (D) blood samples collected from mice exposed to chlorine gas or air. The positive method control (QCH) and chlorine-exposed samples are indicated with a blue line and corresponding blue axis (left). The negative method control (MB) and air-exposed samples are indicated with a black line and corresponding black axis (right). The chromatograms show either the Cl-Tyr transition 216.0 m/z → 135.1 m/z (A1–D1) or the Cl2-Tyr transition 250.0 m/z → 169.0 m/z (A2–D2). Arrows emphasize the Cl-Tyr and Cl2-Tyr peaks which have retention times at 3.1 min and 3.9 min, respectively. The y-axes are shown on two scales to display the differences in response between chlorine- and air-exposed samples
The chlorine animal model provided a glimpse of chlorinated tyrosine levels in each matrix over time. Figure 5 shows an overview of Cl-Tyr and Cl2-Tyr levels over 30 days post-exposure in lung, hair, and blood samples. Interestingly, both analytes are only detected in blood up to 24 h post-chlorine gas exposure. This supports observations from researchers at the University of Alabama at Birmingham noted during their full-body chlorine gas exposure study in mice [15]. Both analytes were detected in lung samples up to 24 h; however, no lung samples were provided beyond the 24-h endpoint and only one air-exposed (control) sample was available for lung samples. Both Cl-Tyr and Cl2-Tyr were detected in hair from shaved muzzles up to 30 days post-exposure. The gradual decrease of biomarker levels in hair could be contributed to the shedding and growth of new fur coats over the 30-day timespan [22, 23]. This suggests that hair is the best long-term biomarker of exposure compared to blood and lung samples. Several of the animals in the first 24 h were animals that succumbed to their deaths prior to their pre-determined endpoint. For both Cl-Tyr and Cl2-Tyr, air-exposed (control) samples were below the LOD in blood and near the LOD in lung and hair samples. Note that the reported units between blood and tissue are different since the tissue samples were normalized to sample mass.
Fig. 5.

Consolidation of 3-chlorotyrosine (Cl-Tyr) and 3,5-dichlorotyrosine (Cl2-Tyr) data collected from 198 mice exposed to chlorine gas or air (control) and arranged by sample collection timepoint: 0–2 h, 2–24 h, and 1–30 days post-exposure. The mean is represented by a blue diamond, outliers (defined as 1.5x inter-quartile range) by an open circle and extreme outliers (defined as 3x inter-quartile range) by an x. The units are different between the tissue (i.e., lung and hair) and blood samples since tissue specimens were normalized to sample mass
A unique feature of this animal study is the ability to compare chlorinated tyrosine levels between different matrices. Among the 198 animals that were involved in the study, we received all three matrices (lung, muzzle, and blood) from a subset of 19 animals, and we were able to compare Cl-Tyr and Cl2-Tyr levels in these samples. As shown in Table 1, Cl-Tyr was detected in 12 blood, 11 lung, and 19 hair from muzzle samples, and Cl2-Tyr was detected in five blood, 11 lung, and 19 hair from muzzle samples. We found that chlorinated tyrosine biomarkers were more abundant in hair compared to lung and blood samples within this subset. Additionally, this data supports previous observations from our lab in which higher Cl-Tyr levels were observed as compared to Cl2-Tyr for both artificially exposed samples and samples from patients with chronic inflammatory diseases [13]. Furthermore, it supports previous observations that Cl2-Tyr is a more sensitive and selective biomarker for chlorine exposure compared to Cl-Tyr since it is rarely seen in background levels but is observed in acute exposures [13].
Table 1.
Matrix-matched comparison of 3-chlorotyrosine (Cl-Tyr) and 3,5-dichlorotyrosine (Cl2-Tyr) data collected from all mice (n = 19) that had matched-set blood, muzzle (hair), and lung samples. The units are different between the tissue (i.e., lung and hair) and blood samples since tissue specimens were normalized to sample mass. ND, not detected. Analytes in all samples were quantified within the validated calibration range (2.50–500.0 ng/mL) prior to normalization to sample mass
| Collection timepoint (hh:mm) | 3-Chlorotyrosine | 3,5-Dichlorotyrosine | ||||
|---|---|---|---|---|---|---|
| Blood (ng/mL) | Lung (ng/mg) | Hair (ng/mg) | Blood (ng/mL) | Lung (ng/mg) | Hair (ng/mg) | |
| 0:10 | 3.6 | 0.2 | 8.0 | ND | 0.2 | 0.6 |
| 0:12 | 7.3 | ND | 5.5 | ND | ND | 2.2 |
| 0:12 | ND | ND | 5.4 | ND | ND | 9.2 |
| 0:13 | 15.5 | ND | 12.9 | ND | ND | 4.9 |
| 0:13 | 9.2 | ND | 2.4 | ND | ND | 1.3 |
| 0:14 | 491 | 11.6 | 69.2 | 80.3 | 3.6 | 56.8 |
| 0:16 | ND | ND | 5.7 | ND | ND | 23.6 |
| 0:26 | 41.1 | 7.9 | 123.3 | 11.7 | 3.1 | 66.1 |
| 0:54 | 35.2 | 7.5 | 28.8 | 9.1 | 2.8 | 6.9 |
| 5:57 | 9.6 | 5.1 | 97.5 | ND | 1.2 | 82.7 |
| 6:00 | 9.7 | 2.9 | 102.0 | ND | 0.8 | 44.8 |
| 6:45 | ND | 10.3 | 74.4 | ND | 2.9 | 21.3 |
| 7:17 | 26.5 | 5.2 | 144.9 | 4.4 | 1.3 | 53.1 |
| 22:33 | 23.3 | 3.4 | 104.5 | 5.4 | 1.0 | 52.1 |
| 23:00 | 14.4 | 2.3 | 59.4 | ND | 0.6 | 36.2 |
| 24:00 | ND | 20.2 | 39.5 | ND | 7.6 | 14.7 |
Conclusions
Using specimens from mice exposed to chlorine gas, we developed a tissue homogenization protocol which seamlessly fits into our chlorinated tyrosine clinical assay. The updates reported for our chlorinated tyrosine method reduce the sample volume to 10 μL, increase throughput, improve chromatography, and simplify the method [13]. During development, we evaluated several homogenization techniques, wash protocols, and tissue amounts, and we developed an automated homogenization protocol. This resulted in a rugged method to convert tissues such as lung and hair into a liquid format that was compatible with our chlorinated tyrosine assay.
Using the tissue homogenization protocol and updated chlorinated tyrosine assay, we measured chlorinated tyrosine levels in 198 mice that were exposed to either chlorine gas or air. This work resulted in a better understanding of the chlorinated tyrosine biomarkers’ compatibility and longevity among three matrices: lung, hair collected from the muzzle, and blood. Mice exposed to chlorine gas had Cl-Tyr and Cl2-Tyr present up to 24 h in blood and 30 days in hair. The Cl-Tyr and Cl2-Tyr levels at these timepoints were significantly higher than levels in air-exposed (control) animals. A clear duration was not available for lung since the longest endpoint received was 24 h. Analysis of samples from the in vivo animal model confirmed that the Cl-Tyr biomarker was more abundant than the Cl2-Tyr biomarker, but the Cl2-Tyr biomarker was more specific. These experiments showed the importance of having matrix-matched air-exposed (control) tissues to compare exposed and non-exposed samples, due to varying endogenous concentrations between tissues and individuals. Finally, we were able to report the first data of chlorinated tyrosine levels between various matrices, revealing that hair that was collected from muzzles gave the highest chlorinated tyrosine levels compared to lung and blood.
Supplementary Material
Acknowledgments
We want to thank Tonya Tuberville and Stephanie Boles for their assistance in testing different homogenization techniques. Furthermore, we want to thank Samantha Isenberg for reviewing all the chromatography as a quality control officer and for providing insightful feedback.
Funding
This work was supported by the Centers for Disease Control and Prevention and the Center for Preparedness and Response (PID 14782). Development of a Murine Model of Pulmonary Chlorine Exposure Injury was funded by the Task Order contract HHSO100201500004I to Battelle Memorial Institute by Biomedical Advanced Research and Development Authority (BARDA).
Abbreviations
- Cl-Tyr
3-chlorotyrosine
- 3,5 Cl2-Tyr
3,5-dichlorotyrosine
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- BAL
bronchoalveolar lavage
- CWC
Chemical Weapons Convention
- OPCW
Organisation for the Prohibition of Chemical Weapons
- HPLC
high-performance liquid chromatography
- FDA
U.S. Food and Drug Administration
- ISTD
internal standard
- QC
quality control
- QCL
QC low
- QCM
QC mid
- QCH
QC high
- MB
matrix blank
- CDC
Centers for Disease Control and Prevention
- BARDA
Biomedical Advanced Research and Development Authority
- UV
ultraviolet
- AVMA
American Veterinary Medical Association
- IACUC
Institutional Animal Care and Use Committee
- MPA
mobile phase A
- MPB
mobile phase B
- R2
coefficient of determination
- LOD
limit of detection
- LLOQ
lower limit of quantitation
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00216-020-03146-x.
Conflict of interest The authors declare that they have no conflicts of interest.
Ethics approval All animals were maintained under the Battelle animal care and use program accredited by the Association for Assessment and Accreditation for Laboratory Animal Care International. This care and use program was in accordance with guidelines set forth in the “Guide for the Care and Use of Laboratory Animals,” National Research Council, and/or the regulations and standards promulgated by the Agricultural Research Service, United States Department of Agriculture (USDA), pursuant to the Laboratory Animal Welfare Act of August 24, 1966, as amended.
Source of biological materials All biological materials were either purchased from vendors or gifted to the CDC as part of a collaborative effort between CDC, Battelle Memorial Institute, and BARDA aimed at the development of a murine model of pulmonary chlorine exposure injury.
Publisher's Disclaimer: Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Biomedical Advanced Research and Development Authority. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the US Department of Health and Human Services.
References
- 1.Toxicology profile for chlorine. U.S Department of Health and Human Services. 2010. https://www.atsdr.cdc.gov/ToxProfiles/tp172.pdf. Assessed 27 May 2020. [Google Scholar]
- 2.Pamphlet 1: Chlorine basics, edition 8. The Chlorine Institute. 2014. https://bookstore.chlorineinstitute.org/pamphlet-1-chlorine-basics.html?Session_ID=616aaf0d8f2aa0b6171e3e544421f560. Assessed 18 June 2020. [Google Scholar]
- 3.Fitzgerald GJ. Chemical warfare and medical response during World War I. Am J Public Health. 2008. 10.2105/AJPH.2007.11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OPCW Fact finding mission: “compelling confirmation” that chlorine gas used as weapon in Syria. Organisation for the Prohibition of Chemical Weapons. 2014. http://www.opcw.org/news/article/opcw-fact-finding-mission-compelling-confirmation-that-chlorine-gas-used-as-weapon-in-syria. Accessed 27 May 2020. [Google Scholar]
- 5.Report of the fact-finding mission regarding the incident of alleged use of toxic chemicals as a weapon in Douma, Syrian Arab Republic, on 7 April 2018. Organisation for the Prohibition of Chemical Weapons. 2019. https://www.opcw.org/sites/default/files/documents/2019/03/s-1731-2019%28e%29.pdf. Accessed 26 May 2020. [Google Scholar]
- 6.Nowhere to hide: the logic of chemical weapons use in Syria. Global Public Policy Institute. 2019. https://www.gppi.net/media/GPPi_Schneider_Luetkefend_2019_Nowhere_to_Hide_Web.pdf. Accessed 6 August 2020. [Google Scholar]
- 7.More than 300 chemical attacks launched during Syrian civil war, study says. National Public Radio. 2019. https://www.npr.org/2019/02/17/695545252/more-than-300-chemical-attacks-launched-during-syrian-civil-war-study-says. Accessed 6 August 2020. [Google Scholar]
- 8.Convention on the prohibition of the development, production, stockpiling and use of chemical weapons and on their destruction. Organisation for the Prohibition of Chemical Weapons. 2020. https://www.opcw.org/chemical-weapons-convention. Accessed 26 May 2020. [Google Scholar]
- 9.Garlantezéc R, Multigner L, Labat L, Bonvallot N, Pulkkinen J, Dananché B, et al. Urinary biomarkers of exposure to glycol ethers and chlorinated solvents during pregnancy: determinants of exposure and comparison with indirect methods of exposure assessment. Occup Environ Med. 2012. 10.1136/oem.2010.062315. [DOI] [PubMed] [Google Scholar]
- 10.National Response Center 2010–2019 Reports. United States Coast Guard. https://nrc.uscg.mil. Accessed 6 August 2020. [Google Scholar]
- 11.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003. 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Sochaski MA, Jarabek AM, Murphy J, Andersen ME. 3-Chlorotyrosine and 3,5-dichlorotyrosine as biomarkers of respiratory tract exposure to chlorine gas. J Anal Toxicol. 2008. 10.1093/jat/32.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Crow BS, Quiñones-González J, Pantazides BG, Perez JW, Winkeljohn WR, Garton JW, et al. Simultaneous measurement of 3-chlorotyrosine and 3,5-dichlorotyrosine in whole blood, serum and plasma by isotope dilution HPLC-MS-MS. J Anal Toxicol. 2016. 10.1093/jat/bkw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemström P, Larsson A, Elfsmark L, Åstot C. l-Alpha-phosphatidylglycerol chlorohydrins as potential biomarkers for chlorine gas exposure. Anal Chem. 2016. 10.1021/acs.analchem.6b01896. [DOI] [PubMed] [Google Scholar]
- 15.Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, et al. Formation of chlorinated lipids post-chlorine gas exposure. J Lipid Res. 2016. 10.1194/jlr.M069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massa CB, Scott P, Abramova E, Gardner C, Laskin DL, Gow AJ. Acute chlorine gas exposure produces transient inflammation and a progressive alteration in surfactant composition with accompanying mechanical dysfunction. Toxicol Appl Pharmacol. 2014. 10.1016/j.taap.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bioanalytical method validation: guidance for industry. Food and Drug Administration. 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry. Assessed 26 May 2020. [Google Scholar]
- 18.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008. 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 19.Taylor JK. Quality assurance of chemical measurements: Lewis Publishers, Inc.; 1987. [Google Scholar]
- 20.Schaffer MI, Wang W, Irving J. An evaluation of two wash procedures for the differentiation of external contamination versus ingestion in the analysis of human hair samples for cocaine. J Anal Toxicol. 2002. 10.1093/jat/26.7.485. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen RB, Wilkins DG, Slawson MH, Shaw K, Rollins DE. Effect of four laboratory decontamination procedures on the quantitative determination of cocaine and metabolites in hair by HPLC-MS. J Anal Toxicol. 2001. 10.1093/jat/25.7.490. [DOI] [PubMed] [Google Scholar]
- 22.Milner Y, Sudnik J, Filippi M, Kizoulis M, Kashgarian M, Stenn K. Exogen, shedding phase of the hair growth cycle: characterization of a mouse model. J Invest Dermatol. 2002. 10.1046/j.1523-1747.2002.01842.x. [DOI] [PubMed] [Google Scholar]
- 23.Plikus MV, Chuong CM. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J Invest Dematol. 2008. 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


