Abstract
Introduction
The current diagnostic pathways for cognitive impairment rarely identify babies at risk before 2 years of age. Very early detection and timely targeted intervention has potential to improve outcomes for these children and support them to reach their full life potential. Early Moves aims to identify early biomarkers, including general movements (GMs), for babies at risk of cognitive impairment, allowing early intervention within critical developmental windows to enable these children to have the best possible start to life.
Method and analysis
Early Moves is a double-masked prospective cohort study that will recruit 3000 term and preterm babies from a secondary care setting. Early Moves will determine the diagnostic value of abnormal GMs (at writhing and fidgety age) for mild, moderate and severe cognitive delay at 2 years measured by the Bayley-4. Parents will use the Baby Moves smartphone application to video their babies’ GMs. Trained GMs assessors will be masked to any risk factors and assessors of the primary outcome will be masked to the GMs result. Automated scoring of GMs will be developed through applying machine-based learning to the data and the predictive value for an abnormal GM will be investigated. Screening algorithms for identification of children at risk of cognitive impairment, using the GM assessment (GMA), and routinely collected social and environmental profile data will be developed to allow more accurate prediction of cognitive outcome at 2 years. A cost evaluation for GMA implementation in preparation for national implementation will be undertaken including exploring the relationship between cognitive status and healthcare utilisation, medical costs, health-related quality of life and caregiver burden.
Ethics and dissemination
Ethics approval has been granted by the Medical Research Ethics Committee of Joondalup Health Services and the Health Service Human Research Ethics Committee (1902) of Curtin University (HRE2019-0739).
Trial registration number
ACTRN12619001422112.
Keywords: developmental neurology & neurodisability, community child health, paediatrics
Strengths and limitations of this study.
This is the first population based prospective cohort study investigating the utility of the general movements assessment (GMA) as a biomarker to identify children with cognitive impairment during early infancy.
This is the first study to explore the feasibility of using smartphone application based video collection of writhing and fidgety GMs in a large representative population.
This study will develop automated scoring of the GMs using machine learning making wide scale screening possible in the future.
This study will combine the GMA outcome, with routinely collected demographic and health data to develop a screening algorithm for identification of infants at risk of cognitive impairment.
Introduction
Neurodevelopmental disorders (NDD) result from changes in the brain that lead to an impairment in skill development, including cognitive, language and motor skills.1 The lifelong impact of NDD has enormous personal and financial burden on the individual, their family and the community. Nationally in Australia, the cost of intellectual disability alone, is estimated to be $A14.720 billion annually.2 In Western Australian (WA), 6.6% of children meet the criteria for ‘developmentally vulnerable’ at school entry with regard to language and cognition,3 while the prevalence of diagnosed intellectual disability in WA children is 14.3/1000.4
The first 2 years of life are a critical period for motor and cognitive development due to the timing of corticospinal tract development and the plasticity mechanisms at work in the infant’s brain.5 Thus, the earlier cognitive impairment can be detected, the greater the potential benefits of ensuing early interventions for optimising neuroplasticity, preventing or ameliorating neurodevelopmental disorders and enhancing parental well-being. Early interventions for cognitive development have been explored in preterm and low birth weight infants. Though systematic review of the topic suggests benefits may be restricted to short-term gains,6 7 comprehensive long-term follow-up analysis indicates some biological risk factors significantly affect response to the intervention.8 For example, higher-low birth weight infants stood to gain the more from early intervention with cognitive improvements seen up at 18 years of age compared with lower-low birth weight infants.8 9
It remains difficult to accurately identify infants at risk of cognitive impairment10–13 in the absence of other risk factors such as prematurity or low birth weight, making it impossible to assess interventions for children in the general population at risk of cognitive delay. This lack of identification pathways is highlighted by the considerable delay that is often reported between parents’ first concerns and confirmation of a diagnosis.14 This is more pronounced for those residing outside major centres, with a known health inequality in regional and rural Australia, and in poorly served outer metropolitan areas of large cities.15
General movements (GMs) are a distinct spontaneous movement pattern evident in babies before and after birth.16 Writhing GMs are movement sequences of variable speed, amplitude and intensity which are observed in utero up to 8 weeks post partum, with the most significant abnormality involving sudden and synchronised cramping of the trunk and limbs.17 Fidgety GMs are small movements of moderate speed, with variable accelerations in the neck, trunk and limbs, which are present from 8 to 20 weeks’ post-term18. The absence of these small movements is the most notable abnormality seen at this age.19 20 GMs are now recognised as a sensitive tool for providing information on the integrity of a baby’s brain function.21 22 The absence of fidgety GMs is the best predictor of cerebral palsy in high-risk infants, with pooled estimates of 98% sensitivity and 91% specificity.22
While the GMs are accurate for predicting motor impairment, recent evidence suggests GMs may be a biomarker for identifying cognitive impairment in preterm infants.23 24 In two systematic reviews a higher risk of cognitive impairment was associated with persistence of abnormal writhing GMs until 8 weeks after term and with monotonous movement sequences and postural abnormalities at 12 to 20 weeks. Further, the developmental quotient at 2 to 3 years of children born preterm, with abnormal writhing GMs at 4 weeks post-term, was lower than gestation and age matched infants with normal writhing GMs.24
Abnormal fidgety GMs in preterm infants was also found to be associated with a score on average eight points lower on the Bayley Scales of Infant and Toddler Development-Second Edition at 2 years of age compared with those with normal fidgety GMs.25 This difference in cognition was greater when the children were reassessed at age 4 years on the Differential Ability Scale.25 These findings indicate that abnormal spontaneous movement patterns, at both the writhing and fidgety stages, may presage later cognitive impairment. The majority of this evidence however exists in preterm and high-risk infants; there is a paucity of information for healthy term infants.
More detailed scoring of the GM, in which every movement criterion is given a score18 is known as the GM Optimality Score (GMOS) at the writhing age,17 and the Motor Optimality Score (MOS) at the fidgety age,26 where a higher score represents more optimal movements. Full explanation of movement criteria is available in previous publications by the GM trust.17 18 In a study of 40 extremely preterm infants, 33 infants showed normal fidgety movements, but of these 33 infants only 6 were found to have the highest MOS score possible,27 highlighting the increased sensitivity of optimality scoring compared with global GM assessment (GMA).
Should GMs be shown to be an early biomarker for cognitive impairment, there are still barriers to implementing GMA as a population level screening tool. These barriers include access to trained assessors in many locations, and the cost involved in video recording of the infant. To overcome these barriers, a smartphone application called Baby Moves has been developed allowing families or health professionals to record and upload GM recordings directly and has been successfully used for fidgety age assessments on high-risk infants,28 removing the need for in-person appointments.
The use of machine based movement recognition, aimed at automatic detection, classification and quality assessments of limb movements, has the potential to further reduce the time and financial costs of GMA. This approach has been explored by a number of researchers aiming to automate reporting of fidgety GMs in small samples of clinical GM videos.29–34 Results suggest automated readings of fidgety movements are feasible, reporting sensitivity and specificity of 79% to 85% and 63% to 71%, respectively.29–31 The machine learning field is relatively young, and is rapidly evolving and advancing. Through adoption of new techniques, and a large training data set, it is expected the sensitivity and specificity can be improved.35 Automated video analysis may provide a low-cost, high sensitivity approach by combining the sensitivity of advanced machine classification (used as a primary screening mechanism), and the specificity of human expert opinion (for any videos classified as high risk by the automation).
It is known that a child’s biological and environmental profile is related to developmental outcomes.36–38 A number of protective and risk factors, particularly birth weight, gender and prematurity, and maternal age are routinely collected and documented. Applying a bioecological model39 to explore developmental vulnerability using routinely collected data, in conjunction with GMA may provide a stronger predictive tool than GMs alone creating a robust and meaningful screening tool.36
Identification of an early biomarker- along with the development and validation of an accessible, affordable and scalable screening tool for the early identification of cognitive impairment - would allow a greater number of infants to receive effective early interventions during the critical window of brain development: an advantage to the child, family and greater society.
‘The economic benefit (of early detection and intervention) could be great, but the benefit to the families is priceless.’ - Kids Rehab WA consumer group member
Aims and hypothesis
Phase one: GMA as a biomarker
The primary aim of phase one is to determine the diagnostic value of GMs for cognitive delay at 2 years.
It is hypothesised that abnormal GMs at either writhing or fidgety age will be predictive of cognitive delay at 2 years. As this is the first study to look at the predictive ability of GMA for cognitive delay or impairment in a large representative birth cohort, we have insufficient data to hypothesise the diagnostic test accuracy.
The secondary aim of phase one is to develop and refine automated assessment for both writhing and fidgety periods, respectively, including optimality scoring, through applying machine based learning to the data. It is hypothesised that automated scoring of GMA will have >90% sensitivity and >85% specificity to detect global GM abnormalities, with lower accuracy for optimality scores.
Phase two: screening algorithm
The primary aim of phase two is to develop screening algorithms for identification of children at risk of cognitive impairment, using the GMA, and routinely collected social and environmental profile data.
It is hypothesised an algorithm of early child, family and societal risk factors, GMA and optimality scores will be a more accurate predictor of cognitive status at 2 years corrected age than GMA or optimality scores alone.
Phase three: cost and economic evaluations
The primary aim of phase three is to conduct a cost evaluation for GMA implementation from the perspective of the funder in preparation for national implementation.
The secondary aim of phase three is to assess the relationship between cognitive status and healthcare utilisation, medical costs, health-related quality of life and caregiver burden.
It is hypothesised cognitive impairment will predict: higher healthcare utilisation and direct medical costs, poorer health-related quality of life and higher caregiver burden.
Methods
Study design
This study is a double-masked, prospective cohort study of 3000 babies. The methodological design of this cohort study has been informed by the 2007 ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) checklist for cohort studies.40 The methodological design of phase one (a study of diagnostic test accuracy), was informed by the 2015 ‘Standard for Reporting of Diagnostic Accuracy Studies’ (STARD) checklist.41
Setting
Early Moves is a multi-site study, recruiting in secondary care settings in metropolitan Perth, WA. This study is a substudy of the ORIGINS project, a major Western Australian cohort study of 10 000 families who birth at Joondalup Health Campus, WA, a public/private secondary hospital in Perth’s northern suburbs.42 43 The ORIGINS project is the largest representative sample of Australian infants in an observational cohort study, and includes a number of optional nested interventional studies. Early Moves will initiate recruitment through the ORIGINS project, before expanding to additional metropolitan secondary hospitals.
Recruitment
Early Moves will recruit a total of 3000 infants between November 2019 and December 2022. It is anticipated that two-thirds of the participants will be recruited through the ORIGINS project. Recruitment can occur at any time, from initial presentation at antenatal clinic, up until discharge from hospital after the birth of the baby. To reduce risk of self-selection bias on the basis of birth experience, antenatal recruitment will be targeted where possible. Timing of consent relative to birth will be recorded. Potential participants will be recruited directly by their maternity or postnatal care provider, or by a member of the ORIGINS or Early Moves research team. Recruitment flyers and posters will also be used at study sites. The first 3000 eligible participants who provide informed consent will be enrolled in Early Moves. As all mothers birthing at each site are invited to participate in the study we anticipate the rates of preterm to be similar to that found in the general population at 8.2%.44
Inclusion criteria
(a) Mother intending on birthing/have recently birthed at a select WA public or private hospital between 2019 and 2023.
Exclusion criteria
(a) Babies enrolled in an ORIGINS interventional research study that aims to promote cognitive and language development.
Masking
The assessors will be masked to baby’s gestation at birth, the birth, medical and social history and the results of any of the ORIGINS or Early Moves outcomes. Abnormalities in serial GMA are known to be predictive of cerebral palsy, so in cases where abnormalities are identified, the participants will be notified and referred to the appropriate clinical services for further investigation and management. Based on rate of cerebral palsy in Australia of 1.4 per 1000 live births,45 we anticipate to identify approximately five cases of cerebral palsy, where parents will be unmasked to GM outcomes as per above protocol.
Bias
As a prospective cohort study selection bias is minimised as participants will enrol prior to or very soon after the birth of the baby. Inhomogeneity of the cohort and exposure to other interventions (interventions that do not meet the exclusion criteria) will be explored as potential biases. Where available, Ages and Stages Questionnaire42 (administered as part of the ORIGINS project), will be used to explore study bias for drop out in the Early Moves study. Exclusion from Early Moves on the basis of enrolment in an intervention study will be reviewed to explore selection bias relating to risk of neurodevelopmental disorder.
Phase one: predictive variables
Time period 1 ‘Writhing’ – videos collected at 1+0 to 2+6 and 3+0 to 4+6 weeks post-term age. Movements will be classified and GMOS calculated.
Time period 2 ‘Fidgety’ – videos collected at 12+0 to 13+6 and 14+0 to 16+6 weeks post-term age. Movements will be classified and MOS calculated.
General Movements will be obtained using the Baby Moves smartphone application. A 3 minute video is taken using the application with the baby lying supine on a plain, flat surface in an awake, settled state, with arms and legs visible. Videos are securely uploaded to the study database for remote assessment by the GM assessors according to Prechtl’s GMA, and calculation of GMOS17 18 and MOS.18 26 The Baby Moves application has been successfully piloted on 446 infants to determine feasibility at the fidgety period,28 46 with 69.9% to 82.7% of the videos taken by families scorable.46 This study seeks to reduce the proportion of unscorable videos to 15% by employing personalised training and parental education, instructional films, the use of e-reminders of upcoming and currently due videos, and phone support. Further, as this study commences with the first video made within 2 weeks post-term rather than 12 weeks post-term, we anticipate families will be more engaged with the study compared with early studies using the Baby Moves application. Videos received will be reviewed within 2 weeks of submission to check for quality, and families will be contacted via phone if the video is unscorable. Collection of two videos within each time period further increases the likelihood of one scorable video being attained within each time period.
Figure 1.
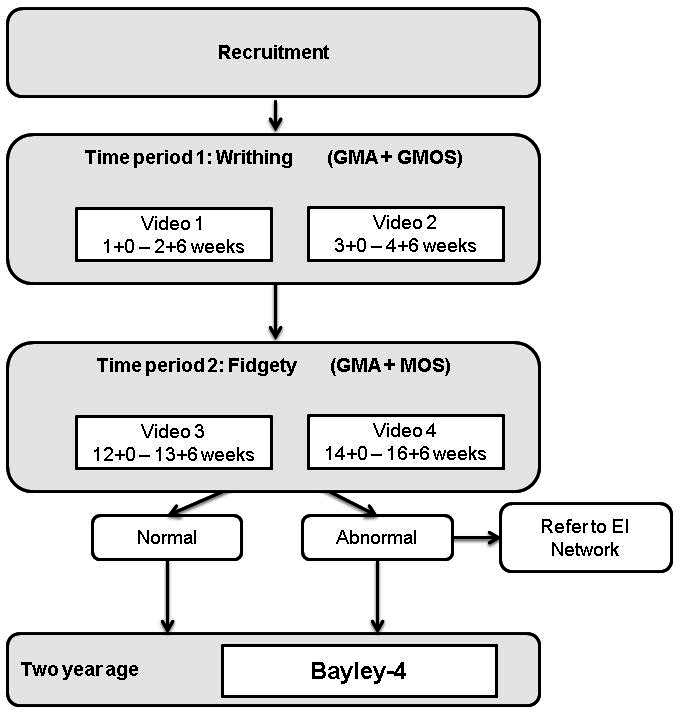
Study assessment timeline. EI, early intervention; GMs, general movements; GMA, GM assessment; GMOS, GM Optimality Score; MOS, Motor Optimality Score.
General movement fidelity
GMAs will be conducted by qualified and registered clinicians who have experience in reporting GMAs and have passed the advanced GMs course by the General Movements Trust. The assessors must have experience in clinical and research application of GM assessment prior to involvement in the study.
Each GM and GMOS/MOS assessment will be conducted by two individual assessors. If there is disagreement between the two assessors a third blinded, experienced GM Instructor (authors ASp or CM), will make the final decision. Disagreement is defined as difference in GM categorical assessment, or optimality scores of more than five point difference.47
The inter-rater reliability of the three assessors for GMA will be accepted as ‘almost perfect’ (≥82% of data are reliable, with Cohen’s Kappa >0.9). Inter-rater reliability and agreement for GMOS and MOS will be accepted as ‘excellent reliability’ (intraclass correlation coefficient of >0.9 using two-way random effects analysis of variance).48 This will be done by triple scoring the first 10 videos, then 10% (selected at random) of each block of 100 videos until criteria for reliability are met. To ensure reliability is maintained throughout the study, a random selection of 10 videos out of every 300 will be triple scored.
Automated reading of GMA
Advanced machine learning methods have been developed to classify and separate normal versus abnormal videotaped fidgety GM.46 49 Video recordings are processed using a pipeline of computer vision and machine learning techniques to predict GMA. Salient point detection (where the joints related to the GM of the infant are located and tracked in the video frames) is followed by extraction of the local motions of the joints into feature vectors. These feature vectors are automatically classified using anomaly detection algorithms developed during pilot work.49 50 Based on a k—nearest neighbour classification approach on 265 video recordings of babies, and a feature based on the histogram of the optical flow, the accuracy for automated GMA was 72.9%.50
Phase one: primary outcomes
The Bayley Scales of Infant and Toddler Development is the most frequently used test in infant developmental assessments.51 The fourth edition of the scale (Bayley-4) has recently been released and will be used in this study at age 2 years corrected. The Bayley-4 scores across five subdomains: Cognitive, Language, Motor, Social-Emotional and Adaptive Behaviour. In Early Moves the primary outcome will compute a combined cognitive and language score calculated as the average of the cognitive score and the language score.52 53 Cognitive delay will be defined as severe when cognitive and language score is greater than 3 SDs below the Australian mean, moderate when the score is between 2 and 3 SDs below the Australian mean and mild when the score is between 1 and 2 SDs below the Australian mean.54 Children unable to complete psychological testing because of presumed severe cognitive delay will be assigned a score of −4 SDs. Secondary analysis will be conducted on cognitive domain score alone. If babies score <−2 SDs on the Bayley-4 across any domain, they will be referred to the appropriate developmental services for further investigation and management.
Medicare Benefit Schedule data and health resource use questionnaires will be used to identify participants who have received cognitive interventions as part of their standard clinical care and a sensitivity analysis will be performed to assess the extent to which inclusion of these participants identified as high risk of cognitive impairment impacts the primary results.
Phase two: screening algorithms
Screening algorithms for identifying children requiring early intervention for cognitive delay will be developed using data available from the Joondalup Health Campus (JHC) Mothers Health Questionnaire (routinely administered to all mothers intending to birth a JHC), and the Midwives Notification System (table 1). Linked data from the Western Australian Register of Developmental Anomalies at age 1 year will be used.
Table 1.
Source of routinely collected demographic and health factors used for the development of screening algorithms in phase two. Factors are grouped according to levels, employing a bioecological model of child development
| Midwives Notification System | JHC Mother’s Health Questionnaire | |
| Cultural and neighbourhood factors | ||
| Socioeconomic Index (SEIFA) | √ | |
| Ethnicity | √ | |
| Parent/family factors | ||
| Marital status | √ | √ |
| Smoking during pregnancy | √ | √ |
| Alcohol consumption during pregnancy | √ | √ |
| Illicit drug use during pregnancy | √ | |
| Maternal medical conditions | √ | |
| Maternal mental health conditions | √ | |
| Perinatal mental health risk factors | √ | |
| Child/biological factors | ||
| Pregnancy complications | √ | √ |
| Family history of developmental difficulties | √ | |
| Method of birth | √ | |
| Complications of labour and birth | √ | |
| Gender | √ | |
| Infant weight | √ | |
| Resuscitation | √ | |
| Estimated gestation | √ | |
| Birth defects | √ | |
| Birth trauma | √ | |
| Special care number of days | √ | |
| Plurality | √ | |
JHC, Joondalup Health Campus.
Phase three: health economics
Healthcare resources will be measured and standard cost sources will be used to apply unit costs to resources. Costs will be standardised to a reference year and future costs will be discounted according to standard practice. Resources and associated costs will include GMA and Bayley-4. The cost of GMA will include ongoing cost of the application and labour resources required for assessment of the videos. Data collected on health-related resource use will include screening assessments, therapy frequency and duration (traditional/alternate), hospital admissions, general practitioner and medical specialist visits, medications and equipment. Data will be collected via the health resource use questionnaire,55 supplemented by consented access to individual hospital, Medical Benefits Schedule and Pharmaceutical Benefits Scheme records.
The Carer Experience Scale (CES) will be employed as a measure of caregiver burden. This validated measure of care-related quality of life has six domains (activities, support, assistance, fulfilment, control and relationship with the care recipient) and takes approximately 3 min to complete.56 The CES is scored from an algorithm derived from preferences of the general population and can be used to value carer outcomes in economic evaluation using index values.56
Sample size estimation
Early Moves is a double masked prospective cohort study and will recruit 3000 babies. For the primary aim to determine the diagnostic value of abnormal GMs for cognitive delay at 2 years, this sample size will be sufficient to establish >78% sensitivity and >83% specificity (alpha 0.05) This calculation assumes 15% of participants have at least mild cognitive delay, the actual sensitivity and specificity are 82.5% and 85%, respectively, that 15% of participants drop out at 2-year follow-up and that 15% of videos are not scorable. For secondary outcomes, there is sufficiently high power to detect even small associations between early GM results and Bayley-4 interval scores at 2 years. For example if 80% of children have normal GMs as infants, the study is powered to detect between group (GM normal vs abnormal) differences on the Bayley-4 (cognitive and language) at 2 years of 3.5 points or greater with 80% power (assuming alpha=0.05 and SD=15 points).
Statistical analysis
Summary statistics will be described using either mean (SD) or median (25th to 75th percentile) for continuous variables, according to distribution or as frequency (percentage) for categorical variables.
For phase one, the primary aim will be assessed using standard diagnostic statistics (eg, sensitivity, specificity, predictive values and likelihood ratios). The predictive validity of the GM categorical classification will be established using logistic regression. The diagnostic value of machine learning for identifying GM writhing and fidgety categorical classification will be evaluated by determining the accuracy, precision, recall and area under the curve of both automated machine assessment and machine–human hybrid assessment using the entire data set of videos (n=6000). An algorithm for early diagnosis of cognitive impairment will be developed using logistic regression modelling, using variables from the GMOS/MOS, GMA categorical classification. Variables will be entered using forward selection based on the Wald statistic. Sensitivity and specificity of the regression model, with 95% CIs will be established.
For phase two, screening algorithms based on the association between measurements recorded at birth or in infancy (eg, GM category) and measurements recorded at 2 years will be assessed using linear regression for interval outcome data, logistic regression for binary outcome data and Poisson regression for count outcome data. Hierarchical mixed-effects models will be used with ‘participant’ included in the model as a random effect in order to account for the non-independence of observations from the same participant. Motor impairment and known risk factors (prematurity, low birth weight, diagnosis of other developmental or genetic disorder) will be tested as a potentially confounding variable for all models. Variables will be selected for potential inclusion in multivariable models based on univariable significance at the p<0.2 level. Multivariable models will be built in a stepwise manner with redundant variables eliminated using Akaike’s and Schwarz’s Bayesian criteria. Interactions will be investigated as appropriate.
For phase three, cost and economic evaluations will consider service use and service costs. We will describe patterns of met and unmet need in the study children, and indirect costs to families will be examined. Associations between costs and all other outcome variables, including those related to cognitive outcome will be assessed, with adjustment for confounders.
Ethics and dissemination
The ORIGINS Project (ref. #1440) and Early Moves (ref. #1902) has been approved by the Human Research Ethics Committee of JHC. Participant information booklets will be provided to all participants prior to entry into the study, and full written and informed consent will be obtained from all participants.
A collaborative (push, pull, exchange) knowledge translation model has been adopted in this study.57 58 Project investigators will champion knowledge translation across five levels of Health: Consumer and Service Providers; Department; Programme; and Health Service Level. Specific knowledge translation strategies and skill building activities will be targeted across the phases of the Early Moves project, and in consultation with our stakeholders. This will include, but are not limited to, dissemination of findings to consumers and stakeholders via peer-reviewed publication of study results, plain language summaries, newsletter feedback and media case studies, as well as presentations at key national and international conferences.
Public/patient involvement
Community engagement is at the core of the ORIGINS and Early Moves projects,42 with the implementation of a collaborative model of involvement.59 Community members of a clinical consumer reference group were involved in the priority setting for the study, specifically parents of children with cognitive impairment. When asked whether they felt the study was important and worthwhile, the response was very positive, for example, ‘Yes, yes, yes. I don’t understand why you wouldn’t do it.’ The group also endorsed time points, methods for collection of data and follow-up protocols for abnormal GMA results. Furthermore, ORIGINS has a dedicated community stakeholder coordinator and 12 parents who form a consumer reference group. This ORIGINS consumer reference group has been involved in development of recruitment, information and consent materials for Early Moves. Consumer and community representation is also incorporated in the ORIGINS and Early Moves governance structure.
Bidirectional, effective and continuous communication with consumers will guide research directions, interpretation of findings and their implications for policy.
Discussion
At present, lack of early biomarkers for cognitive impairment hinders referral to early interventions. Early Moves aims to identify early biomarkers for babies at risk of cognitive impairment, allowing early intervention within critical developmental windows to enable babies to have the best possible start to life.
The study has a number of strengths. It is a well powered study with a population-based sample. Where possible, participants will be recruited during the antenatal period to minimise self-selection bias where it relates to the birth experience (eg, prematurity; late pregnancy or birth complications). The assessment variables used in this study have proven reliability and validity. As a subproject of the ORIGINS project, Early Moves will have access to biobank data to generate a detailed biological, and environmental risk profile data to inform predictive algorithms and investigate links between cognitive impairment and biological and environmental factors. The study has strong consumer involvement and an embedded knowledge translation plan which will help guide and facilitate the translation of research findings in to clinical practice.
A potential limitation is that the final outcome measure is conducted at 2 years. Research in premature babies shows the association between abnormal GMs and cognitive impairment is weaker at 2 years compared with 4 years.25 A subset of the cohort will however be followed until age 5 years as part of the ORIGINS project, with later assessments to include developmental assessments such as Ages and Stages questionnaires and linkage to the Australian Early Development Census (AEDC) providing data on early childhood development at entry to the first year of full-time school. The AEDC is an Australian wide data collection conducted by teachers using the Australian version of the Early Development Instrument. This study is also potentially limited by recruitment at greater metropolitan sites within one Australian city. Demographic data will be available to aid in the interpretation of generalisability to other populations.
Early Moves will provide novel care models in rural and remote communities through the use of smartphone technology and machine based learning, facilitating the feasibility of application-based GM assessments as a population wide assessment tool. Through combining automated application-based GM assessments with routinely collected risk and protective factors, employing a bioecological model of development, Early Moves recognises the complex interplays of risk and protective factors to create a robust screening tool for cognitive impairment in infants. Inclusion of health economics evaluation will further enhance the potential for this technology to be translated into clinical care.
Supplementary Material
Acknowledgments
The authors would like to thank Svetha Venkatesh from Deakin University for her contribution to this study, and The ORIGINS Project Team, including, but not limited to Erika Haggeman, Jackie Davis and Lucy Giggs for their contribution and support.
Footnotes
Correction notice: This article has been corrected since it first published. The provenance and peer review statement has been included.
Collaborators: Jane Valentine, Alison Salt, Desiree Silva, Caroline Alexander, Natasha Amery, Arlette Coenen, Rose Morie, Jennifer Moore, Madeleine OConnor, Ravisha Srinivasjois, Jason Tan, Brad Jongeling, Elayne Downie, Ruth Last, Mary Sharp, John Wray, Sue-Anne Davidson, Ashleigh Thornton.
Contributors: CE and JV are the chief investigators and together with CA, ASa, ASp, RNB, NB, CM, DS, EG and RSW designed established and achieved funding for the research study. AA, AM, DB, MS, RW, SB, SP, SW, VL, SD, AT, LJ, NA and the Early Moves Clinical Working Party also contributed to study design. CE, JV and CA are responsible for ethics applications and reporting. CA, DS and SP are responsible for recruitment. CE, JV, CA and ASa supervise the data collection and implementation of training. CE, JV, ASa, ASp, CM, MS, NA and the Early Moves Clinical Working Party are responsible for design and implementation of GM outcomes. NB, CM and VL are responsible for design and implementation of machine learning outcomes. AT, AM, RW, SD and AA are responsible for design and implementation of consumer engagement outcomes. EG and AF-J are responsible for design and implementation of health economics outcomes. SW, DS and CA are responsible for design and implementation of routinely collected screening outcomes. JV, ASa and SB are responsible for design and implementation of developmental outcomes. CE, JV, CA, ASa, ASp, RNB, NB, CM, DS, EG and RSW will take lead roles in preparation for publications on the clinical outcomes of the study. RSW will take on a lead role of statistical analysis for the study. All authors contributed to the preparation of this manuscript and have approved the final version.
Funding: Supported by a Clinical Trials and Cohort Study Grant awarded by the National Health and Medical Research Council, a Project Grant awarded by the Research Foundation, Cerebral Palsy Alliance, a Project Grant awarded by the WA Child Research Foundation and a Project Grant awarded by the Perth Children’s Hospital Foundation.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Early Moves Clinical Working Group, Jane Valentine, Alison Salt, Desiree Silva, Caroline Alexander, Natasha Amery, Arlette Coenen, Rose Morie, Jennifer Moore, Madeleine OConnor, Ravisha Srinivasjois, Jason Tan, Brad Jongeling, Elayne Downie, Ruth Last, Mary Sharp, John Wray, Sue-Anne Davidson, and Ashleigh Thornton
Ethics statements
Patient consent for publication
Not required.
References
- 1.Association, A.P . Neurodevelopmental disorders: DSM-5® selections. Philadelphia: American psychiatric PUB, 2015. [Google Scholar]
- 2.Doran CM, Einfeld SL, Madden RH, et al. How much does intellectual disability really cost? first estimates for Australia. J Intellect Dev Disabil 2012;37:42–9. 10.3109/13668250.2011.648609 [DOI] [PubMed] [Google Scholar]
- 3.Commonwealth of Australia . Australian early development census national report 2018. Canberra ACT: Department of Education and Training, 2019. [Google Scholar]
- 4.Leonard H, Petterson B, Bower C, et al. Prevalence of intellectual disability in Western Australia. Paediatr Perinat Epidemiol 2003;17:58–67. 10.1046/j.1365-3016.2003.00469.x [DOI] [PubMed] [Google Scholar]
- 5.Williams PTJA, Martin JH. Motor cortex activity organizes the developing rubrospinal system. J Neurosci 2015;35:13363–74. 10.1523/JNEUROSCI.1719-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orton J, Spittle A, Doyle L, et al. Do early intervention programmes improve cognitive and motor outcomes for preterm infants after discharge? A systematic review. Dev Med Child Neurol 2009;51:851–9. 10.1111/j.1469-8749.2009.03414.x [DOI] [PubMed] [Google Scholar]
- 7.Spittle A, Orton J, Anderson PJ, et al. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev 2015;11:CD005495. 10.1002/14651858.CD005495.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks-Gunn J, Gross RT, Kraemer HC, et al. Enhancing the cognitive outcomes of low birth weight, premature infants: for whom is the intervention most effective? Pediatrics 1992;89:1209–15. [PubMed] [Google Scholar]
- 9.McCormick MC, Brooks-Gunn J, Buka SL, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the infant health and development program. Pediatrics 2006;117:771–80. 10.1542/peds.2005-1316 [DOI] [PubMed] [Google Scholar]
- 10.Peyton C, Schreiber MD, Msall ME. The Test of Infant Motor Performance at 3 months predicts language, cognitive, and motor outcomes in infants born preterm at 2 years of age. Dev Med Child Neurol 2018;60:1239–43. 10.1111/dmcn.13736 [DOI] [PubMed] [Google Scholar]
- 11.Morgan C. Towards more accurate prognostication after preterm birth. Dev Med Child Neurol 2018;60:1194–5. 10.1111/dmcn.13765 [DOI] [PubMed] [Google Scholar]
- 12.Wong HS, Santhakumaran S, Cowan FM, et al. Developmental assessments in preterm children: a meta-analysis. Pediatrics 2016;138:e20160251. 10.1542/peds.2016-0251 [DOI] [PubMed] [Google Scholar]
- 13.Van't Hooft J, van der Lee JH, Opmeer BC, et al. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst Rev 2015;4:71. 10.1186/s13643-015-0058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherzer AL, Chhagan M, Kauchali S, et al. Global perspective on early diagnosis and intervention for children with developmental delays and disabilities. Dev Med Child Neurol 2012;54:1079–84. 10.1111/j.1469-8749.2012.04348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Australian Institute of Health and Welfare . A picture of Australia’s children 2012, AIHW, Editor. Canberra: AIHW, 2012. [Google Scholar]
- 16.Prechtl HF, Einspieler C, Cioni G, et al. An early marker for neurological deficits after perinatal brain lesions. Lancet 1997;349:1361–3. 10.1016/S0140-6736(96)10182-3 [DOI] [PubMed] [Google Scholar]
- 17.Einspieler C, Marschik PB, Pansy J, et al. The general movement optimality score: a detailed assessment of general movements during preterm and term age. Dev Med Child Neurol 2016;58:361–8. 10.1111/dmcn.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Einspieler C. Prechtl’s method on the qualitative assessment of general movements in preterm, term and young infants. Clinics in developmental medicine. Vol 167. London: Mackeith Press, 2004. [Google Scholar]
- 19.Ferrari F. Prechtl’s method on the qualitative assessment of general movements in preterm, term and young infants. London: Mackeith Press, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari F, Frassoldati R, Berardi A, et al. The ontogeny of fidgety movements from 4 to 20weeks post-term age in healthy full-term infants. Early Hum Dev 2016;103:219–24. 10.1016/j.earlhumdev.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Burger M, Louw QA. The predictive validity of general movements-a systematic review. Eur J Paediatr Neurol 2009;13:408–20. 10.1016/j.ejpn.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 22.Bosanquet M, Copeland L, Ware R, et al. A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol 2013;55:418–26. 10.1111/dmcn.12140 [DOI] [PubMed] [Google Scholar]
- 23.Peyton C, Einspieler C. General movements: a behavioral biomarker of later motor and cognitive dysfunction in NICU graduates. Pediatr Ann 2018;47:e159–64. 10.3928/19382359-20180325-01 [DOI] [PubMed] [Google Scholar]
- 24.Einspieler C, Bos AF, Libertus ME, et al. The general movement assessment helps us to identify preterm infants at risk for cognitive dysfunction. Front Psychol 2016;7:406. 10.3389/fpsyg.2016.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spittle AJ, Spencer-Smith MM, Cheong JLY, et al. General movements in very preterm children and neurodevelopment at 2 and 4 years. Pediatrics 2013;132:e452–8. 10.1542/peds.2013-0177 [DOI] [PubMed] [Google Scholar]
- 26.Einspieler C, Bos AF, Krieber-Tomantschger M, et al. Cerebral palsy: early markers of clinical phenotype and functional outcome. J Clin Med 2019;8. 10.3390/jcm8101616. [Epub ahead of print: 04 10 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp M, Coenen A, Amery N. General movement assessment and motor optimality score in extremely preterm infants. Early Hum Dev 2018;124:38–41. 10.1016/j.earlhumdev.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 28.Spittle AJ, Olsen J, Kwong A, et al. The Baby Moves prospective cohort study protocol: using a smartphone application with the General Movements Assessment to predict neurodevelopmental outcomes at age 2 years for extremely preterm or extremely low birthweight infants. BMJ Open 2016;6:e013446. 10.1136/bmjopen-2016-013446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adde L, Helbostad JL, Jensenius AR, et al. Early prediction of cerebral palsy by computer-based video analysis of general movements: a feasibility study. Dev Med Child Neurol 2010;52:773–8. 10.1111/j.1469-8749.2010.03629.x [DOI] [PubMed] [Google Scholar]
- 30.Adde L, Helbostad JL, Jensenius AR, et al. Using computer-based video analysis in the study of fidgety movements. Early Hum Dev 2009;85:541–7. 10.1016/j.earlhumdev.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 31.Raghuram K, Orlandi S, Shah V, et al. Automated movement analysis to predict motor impairment in preterm infants: a retrospective study. J Perinatol 2019;39:1362–9. 10.1038/s41372-019-0464-0 [DOI] [PubMed] [Google Scholar]
- 32.Karch D, Kim K-S, Wochner K, et al. Quantification of the segmental kinematics of spontaneous infant movements. J Biomech 2008;41:2860–7. 10.1016/j.jbiomech.2008.06.033 [DOI] [PubMed] [Google Scholar]
- 33.Philippi H, Karch D, Kang K-S, et al. Computer-based analysis of general movements reveals stereotypies predicting cerebral palsy. Dev Med Child Neurol 2014;56:960–7. 10.1111/dmcn.12477 [DOI] [PubMed] [Google Scholar]
- 34.Kanemaru N, Watanabe H, Kihara H, et al. Specific characteristics of spontaneous movements in preterm infants at term age are associated with developmental delays at age 3 years. Dev Med Child Neurol 2013;55:713–721. 10.1111/dmcn.12156 [DOI] [PubMed] [Google Scholar]
- 35.Jordan MI, Mitchell TM. Machine learning:trends, perspectives, and prospects. Science 2015;349:255–60. 10.1126/science.aaa8415 [DOI] [PubMed] [Google Scholar]
- 36.Woolfenden S, Williams K, Eapen V, et al. Developmental vulnerability--don't investigate without a model in mind. Child Care Health Dev 2015;41:337–45. 10.1111/cch.12181 [DOI] [PubMed] [Google Scholar]
- 37.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet 2011;378:1325–38. 10.1016/S0140-6736(11)60555-2 [DOI] [PubMed] [Google Scholar]
- 38.Maggi S, Irwin LJ, Siddiqi A, et al. The social determinants of early child development: an overview. J Paediatr Child Health 2010;46:627–35. 10.1111/j.1440-1754.2010.01817.x [DOI] [PubMed] [Google Scholar]
- 39.Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychol Rev 1994;101:568–86. 10.1037/0033-295X.101.4.568 [DOI] [PubMed] [Google Scholar]
- 40.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 41.Cohen JF, Korevaar DA, Altman DG, et al. Stard 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6:e012799. 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagemann E. The ORIGINS project, in pre-emptive medicine: public health aspects of developmental origins of health and disease. In: Sata F, Fukuoka H, Mark H, eds. Current topics in environmental health and preventive medicine. Singapore: Springer Nature, 2019: 99–116. [Google Scholar]
- 43.Silva DT, Hagemann E, Davis JA, et al. Introducing the origins project: a community-based interventional birth cohort. Rev Environ Health 2020;35:281–93. 10.1515/reveh-2020-0057 [DOI] [PubMed] [Google Scholar]
- 44.Cheong JLY, Doyle LW. Increasing rates of prematurity and epidemiology of late preterm birth. J Paediatr Child Health 2012;48:784–8. 10.1111/j.1440-1754.2012.02536.x [DOI] [PubMed] [Google Scholar]
- 45.The Australian Cerebral Palsy Register, G . Australian cerebral palsy register report 2016. Sydney, Australia: Cerebral Palsy Alliance, 2016. [Google Scholar]
- 46.Kwong AK, Eeles AL, Olsen JE, et al. The baby moves smartphone APP for general movements assessment: engagement amongst extremely preterm and term-born infants in a state-wide geographical study. J Paediatr Child Health 2019;55:548–54. 10.1111/jpc.14240 [DOI] [PubMed] [Google Scholar]
- 47.Ustad T, Evensen KAI, Bertoncelli N, et al. Validity of the general movement optimality list in infants born preterm. Pediatr Phys Ther 2017;29:315–20. 10.1097/PEP.0000000000000445 [DOI] [PubMed] [Google Scholar]
- 48.LeBreton JM, Senter JL. Answers to 20 questions about interrater reliability and interrater agreement. Organ Res Methods 2008;11:815–52. 10.1177/1094428106296642 [DOI] [Google Scholar]
- 49.et alMorais R, Le V, Tran T. Learning regularity in skeletal trajectories for anamoly detection in videos. Computer Vision and Pattern Recognition; 16 - 20 June 2019, Long Beach, California, 2019:11988–96. [Google Scholar]
- 50.Venkatesh S, Vellank P, Le V. Accuracy of machine scoring of fidelity movements from high risk infant populations. New South Wales: Cerebral Palsy Alliance; 2019. [Google Scholar]
- 51.Bayley N, Aylwayd GP. Bayley scales of infant and toddler sevelopment. 4th ed. San Antonio: Harcourt, 2019. [Google Scholar]
- 52.Johnson S, Moore T, Marlow N. Using the bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014;75:670–4. 10.1038/pr.2014.10 [DOI] [PubMed] [Google Scholar]
- 53.Moore T, Johnson S, Haider S, et al. Relationship between test scores using the second and third editions of the Bayley scales in extremely preterm children. J Pediatr 2012;160:553–8. 10.1016/j.jpeds.2011.09.047 [DOI] [PubMed] [Google Scholar]
- 54.Anderson PJ, De Luca CR, Hutchinson E, et al. Underestimation of developmental delay by the new Bayley-III scale. Arch Pediatr Adolesc Med 2010;164:352–6. 10.1001/archpediatrics.2010.20 [DOI] [PubMed] [Google Scholar]
- 55.Smaldone A, Tsimicalis A, Stone PW. Measuring resource utilization in patient-oriented comparative effectiveness research: a psychometric study of the resource utilization questionnaire. Res Theory Nurs Pract 2011;25:80–106. 10.1891/1541-6577.25.2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Making 2011;31:458–68. 10.1177/0272989X10381280 [DOI] [PubMed] [Google Scholar]
- 57.Lavis JN, Robertson D, Woodside JM, et al. How can research organizations more effectively transfer research knowledge to decision makers? Milbank Q 2003;81:221–48. 10.1111/1468-0009.t01-1-00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellen ME, Lavis JN, Ouimet M, et al. Determining research knowledge infrastructure for healthcare systems: a qualitative study. Implement Sci 2011;6:60. 10.1186/1748-5908-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy 2002;61:213–36. 10.1016/S0168-8510(01)00214-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


