Abstract
Background
OH2 is a genetically engineered oncolytic herpes simplex virus type 2 designed to selectively amplify in tumor cells and express granulocyte-macrophage colony-stimulating factor to enhance antitumor immune responses. We investigated the safety, tolerability and antitumor activity of OH2 as single agent or in combination with HX008, an anti-programmed cell death protein 1 antibody, in patients with advanced solid tumors.
Methods
In this multicenter, phase I/II trial, we enrolled patients with standard treatment-refractory advanced solid tumors who have injectable lesions. In phase I, patients received intratumoral injection of OH2 at escalating doses (106, 107 and 108CCID50/mL) as single agent or with fixed-dose HX008. The recommended doses were then expanded in phase II. Primary endpoints were safety and tolerability defined by the maximum-tolerated dose and dose-limiting toxicities (DLTs) in phase I, and antitumor activity assessed per Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) and immune-RECIST in phase II.
Results
Between April 17, 2019 and September 22, 2020, 54 patients with metastatic cancers were enrolled. Forty patients were treated with single agent OH2, and 14 with OH2 plus HX008. No DLTs were reported with single agent OH2 in phase I. Four patients, having metastatic mismatch repair-proficient rectal cancer or metastatic esophageal cancer, achieved immune-partial response, with two from the single agent cohort and two from the combination cohort. The duration of response were 11.25+ and 14.03+ months for the two responders treated with single agent OH2, and 1.38+ and 2.56+ months for the two responders in the combination cohort. The most common treatment-related adverse event (TRAE) with single agent OH2 was fever (n=18, 45.0%). All TRAEs were of grade 1–2, except one case of grade 3 fever in the 108CCID50/mL group. No treatment-related serious AEs occurred. Single agent OH2 induced alterations in the tumor microenvironment, with clear increases in CD3+ and CD8+ cell density and programmed death-ligand 1 expression in the patients’ post-treatment biopsies relative to baseline.
Conclusions
Intratumoral injection of OH2 was well-tolerated, and demonstrated durable antitumor activity in patients with metastatic esophageal and rectal cancer. Further clinical development of OH2 as single agent or with immune checkpoint inhibitors in selected tumor types is warranted.
Keywords: oncolytic viruses, immunotherapy, clinical trials as topic, tumor microenvironment
Background
Oncolytic virotherapy represents a unique antitumor strategy using natural or genetically engineered viruses to infect and replicate in tumor cells. The mechanisms of the tumoricidal effect include direct tumor cell lysis caused by selective infection, and the subsequent release of cell debris and viral antigens, which may overcome immunosuppression in the tumor microenvironment and trigger antitumor immune responses. In addition, the advances in biotechnology have enabled the insertion of therapeutic transgenes into the viral genomes to enhance efficacy.1 2
A diverse range of viruses have been studied as candidates of oncolytic virus, among which the herpes simplex virus type 1 (HSV-1) was the earliest reported oncolytic viruses generated by genetic engineering.3 HSV derived oncolytic viruses may offer a few advantages, including a broad host range allowing clinical application in many different types of cancers, the potential for incorporating a sizeable foreign DNA, and the readily available antiherpetic therapy to control undesired infection and replication.4 The significant benefits observed in the phase III randomized trial of the HSV-based talimogene laherparepvec (T-VEC) in patients with advanced melanoma5 and its approval in the USA and European Union have shown great promise for this class of anticancer agent and inspired further advances in this field.
OH2 is a novel oncolytic virus derived from the wild-type HSV-2 strain HG52. In the construction of this virus, the ICP34.5 neurovirulence gene was deleted to attenuate the toxicity and enhance tumor selectivity. The ICP47 gene was removed to help present tumor associated antigens and promote the oncolytic activity. The gene encoding the human granulocyte-macrophage colony-stimulating factor (GM-CSF) was added to potentiate antitumor immunity.6 Preclinical studies demonstrated that OH2 was genetically and biologically stable,7 and exhibited potent oncolytic activity and safety in animal models.6 8 Moreover, a specific antitumor immune response and a long-term effect to inhibit tumor recurrence and metastasis were observed in a murine colon cancer model treated with OH2.9 These data validated subsequent clinical investigation of the drug.
There have been limited reports concerning the application of HSV-based oncolytic viruses in solid tumors other than glioma and melanoma. We therefore initiated a phase I/II study in patients with advanced solid tumors to evaluate the safety, biodistribution and antitumor activity of OH2 as single agent or in combination with other anticancer drugs. Meanwhile, with tumor samples obtained from baseline and on-therapy biopsies, we also aim to explore the alterations of the tumor microenvironment after OH2 virotherapy. Here we report the results of treatment with single agent OH2, and OH2 in combination with HX008, an anti-programmed cell death protein 1 (PD-1) antibody.
Methods
Patients
In this open-label, multicenter, phase I/II trial, we enrolled patients aged between 18 and 75 years with a histologically confirmed advanced solid tumor whose disease had progressed despite standard systemic therapies. Inclusion criteria included having tumor(s) deemed safe to inject the virus; at least one measurable lesion as defined in the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; an Eastern Cooperative Oncology Group performance status score of 0 or 1; and adequate organ functions assessed by the complete blood count, blood chemistry and coagulation tests. Patients were excluded if they had symptomatic central nervous system metastases, presentations of active infection or an unexplained fever >38.5°C, or treatment with antiviral drugs within 4 weeks before treatment initiation.
Study design and treatment
The phase I part of the study comprised the OH2 single agent cohort and the OH2 with HX008 combination cohort. Dose-escalation of each cohort followed the traditional “3+3” design. Patients were treated with OH2 at three dose levels (106, 107 and 108CCID50/mL) every 2 weeks. In the combination cohort, HX008 was administered at the fixed dose of 200 mg every 3 weeks. Dose escalation proceeded if no dose-limiting toxicities (DLTs) were observed during the 3-week DLT assessment period in the first three patients, otherwise another three patients would be enrolled. If two or more DLTs were observed at one dose level, the previous lower dose would be defined as the maximum-tolerated dose (MTD). The recommended dose level of single agent OH2 was determined on completion of dose-escalation, taking into consideration the MTD, safety, biodistribution parameters and tumor response. In the phase II dose-expansion part of the study, patients were assigned either to receive single agent OH2 at the recommended dose level, or HX008 plus OH2 at dosages proved to be tolerable in phase I of the combination therapy.
The intratumoral injection was performed either directly for cutaneous or subcutaneous lesions, or with ultrasound guidance for deep-located nodes or organ metastases. The volume injected to each lesion was based on the longest diameter of the tumor (≤1.5 cm, up to 1 mL; >1.5 to ≤2.5 cm, up to 2 mL; >2.5 to ≤5.0 cm, up to 4 mL; >5.0 cm, up to 8 mL). Injection of multiple tumors was allowed. There were no limits on the number of lesions that can be injected per patient, but the maximum volume that can be injected during each visit was 8 mL.
Safety and response evaluation
Adverse events (AEs) and DLTs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). DLT was defined as any of the following events within the first 3 weeks of therapy that was deemed treatment-related by the investigator(s): grade 4 neutropenia lasting over 5 days or febrile neutropenia; grade 3 thrombocytopenia with bleeding; any other grade 4–5 hematological AEs; grade 3 rash over 3 days; grade 3 nausea, vomiting and diarrhea lasting over 3 days despite appropriate supportive care; grade 3 alanine aminotransferase or aspartate aminotransferase level increase lasting over 7 days; grade 2 proteinuria lasting over 7 days; any other grade 3–5 non-hematological AEs. AEs were collected throughout the study treatment and for 60 days after the last dose of OH2.
Tumor assessments were conducted at screening, every 6 weeks during the first 6 months after treatment initiation, every 8 weeks after 6 months and every 12 weeks after 12 months. Radiographic assessments were performed using CT or MRI. Measurement of cutaneous or subcutaneous lesions were conducted with calipers. Evaluation of response was performed by the investigators using both the RECIST version 1.1 and the immune-RECIST (iRECIST) criteria.
Biodistribution and biological activity
In phase I, the biodistribution of single agent OH2 was evaluated by detection of viral loads in the blood, urine and saliva by PCR. The injection sites were swabbed for virus shedding on the next day after each injection. To assess the biological activity of single agent OH2, we detected GM-CSF mRNA in tumor samples by reverse transcription-PCR, and blood GM-CSF protein by ELISA before and after OH2 administration. Serum samples were collected for anti-HSV-2 antibody assays by ELISA until confirmation of HSV-seropositive status or 28 days after the last dose, whichever occurred first. Presence of blood anti-GM-CSF antibodies was assessed using a validated electrochemiluminescence immunoassay on the MesoScale Discovery platform. After the completion of dose-escalation, monitoring for blood OH2 viral loads, HSV serology and anti-GM-CSF antibodies continued in phase II of the trial.
Biomarker analysis
Biopsies of the injected lesions were performed before the first dose of OH2 and subsequently at each response evaluation. Three biomarkers, including programmed death-ligand 1 (PD-L1), CD8 and CD3, using immunohistochemistry (IHC) were stained to characterize the changes in the tumor microenvironment. PD-L1 expression was assessed with the Ventana SP263 assay, and was reported using both the tumor proportion score (TPS) and the combined positive score (CPS). TPS was defined as the percentage of tumor cells with membranous PD-L1 staining, whereas CPS was defined as the number of PD-L1 staining cells (tumor cells, lymphocytes and macrophages) divided by the total number of viable tumor cells, multiplied by 100. For the quantification of CD8+ and CD3+ cells, the anti-CD8 mouse monoclonal antibody clone C8/144B was used for CD8 IHC, while the CD3 IHC analysis was conducted using the anti-CD3 (2GV6) rabbit monoclonal antibody. The density of CD8+ and CD3+ stained cells in the intratumoral area plus the invasive margin, if present in the biopsy, were reported as cells/mm2. The definition of the invasive margin was a region of 360 µm width on the frontier between malignant cells and peritumoral stroma, which was identical to that reported by Pagès et al.10
Endpoints and statistical analysis
The primary endpoints were safety and tolerability defined by MTD and DLT in phase I and antitumor activity in phase II. The analyses were descriptive. The Kaplan-Meier method was used to estimate time-to-event variables. SPSS statistics (Version22) was used for data analyses.
Results
Between April 17, 2019 and September 22, 2020, we enrolled 54 patients from seven participating centers, among whom 40 patients were treated with single agent OH2 and 14 received OH2 in combination with HX008. The baseline characteristics are summarized in table 1. Notably, the present study was suspended in two centers in early 2020 due to the impact of the COVID-19 pandemic, causing interruptions of the study treatment and sample collection. Thirteen patients were affected, including three patients from phase I and ten from phase II. As a result, one patient in phase II withdrew consent, and five patients in phase II permanently discontinued treatment with single agent OH2 during the study suspension.
Table 1.
Baseline characteristics and best response to the study treatment
| Single agent OH2 (n=40) |
OH2 with HX008 (n=14) |
|
| Median age at entry, years (range) | 55 (27–73) | 53 (36–64) |
| Male | 30 (75.0%) | 10 (71.4%) |
| Tumor types | ||
| Esophageal cancer | 14 | 3 |
| Colorectal (including appendix) cancer | 12 | 6 |
| Gastric cancer | 5 | 5 |
| Melanoma and other skin cancer | 4 | 0 |
| Head and neck cancer | 2 | 0 |
| Cholangiocarcinoma | 1 | 0 |
| Ovarian cancer | 1 | 0 |
| Breast cancer | 1 | 0 |
| Prior treatment with anti-PD-1 antibodies | 10 (25.0%) | 1 (7.1%) |
| Best response (iRECIST) | ||
| iPR | 2 | 2 |
| iSD | 9 | 4 |
| iUPD | 22 | 6 |
iPR, immune-partial response; iRECIST, immune-Response Evaluation Criteria in Solid Tumors; iSD, immune-stable disease; iUPD, immune-unconfirmed progressive disease; PD-1, programmed cell death protein 1.
All patients were included in the safety analyses, and 45 patients who had completed at least one tumor assessment were included in the efficacy analysis. As of the data cut-off date (December 1, 2020), the median follow-up was 5.41 months (range: 0.46–19.48+). Forty patients had discontinued the study treatment, and the most common reason for treatment discontinuation was progressive disease (PD) (n=27).
OH2 had a favorable safety profile as no DLTs were observed and the MTD was not reached during single agent dose-escalation. Most treatment-related AEs (TRAEs) observed with single agent OH2 were of grade 1 or 2, except that one patient in the 108CCID50/mL group was injected with 8 mL of the study drug and developed grade 3 fever. No grade 4 TRAEs or treatment-related deaths occurred. The most frequent TRAE with single agent OH2 was fever (n=18, 45.0%), which typically occurred within the first 24 hours after OH2 injection, and could be managed with antipyretics. Other common TRAEs were anemia (n=5, 12.5%) and leukopenia (n=3, 7.5%). Dose-escalation of OH2 in combination with HX008 is ongoing, and no DLTs occurred in the 106CCID50/mL and 107CCID50/mL groups. There were no treatment-related serious AEs throughout the study. The TRAEs observed in the trial are summarized in table 2.
Table 2.
Treatment-related adverse events
| Adverse events | Single agent OH2 (n=40) |
OH2 with HX008 (n=14) |
||||
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Fever | 10 | 7 | 1 | 1 | 3 | 0 |
| Leukopenia | 3 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 2 | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 1 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 3 | 2 | 0 | 0 | 0 | 0 |
| Hyperbilirubinemia | 1 | 1 | 0 | 0 | 1 | 0 |
| ALT increased | 2 | 0 | 0 | 0 | 0 | 0 |
| AST increased | 2 | 0 | 0 | 0 | 0 | 0 |
| QTc prolongation | 0 | 1 | 0 | 0 | 0 | 0 |
| Pruritus | 1 | 0 | 0 | 0 | 0 | 0 |
| Rash | 2 | 0 | 0 | 0 | 1 | 0 |
| Complications of injection | 0 | 3 | 0 | 0 | 0 | 0 |
| Abdominal pain | 0 | 2 | 0 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 1 | 0 | 0 |
| Nausea | 0 | 1 | 0 | 0 | 0 | 0 |
| Fatigue | 1 | 0 | 0 | 3 | 0 | 0 |
| Myalgia | 1 | 0 | 0 | 0 | 0 | 0 |
| Arthralgia | 1 | 0 | 0 | 0 | 0 | 0 |
| Proteinuria | 0 | 0 | 0 | 1 | 0 | 0 |
| Hypothyroidism | 0 | 0 | 0 | 0 | 1 | 0 |
No grade 4 or 5 treatment-related adverse events occurred in the study.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In dose-escalation of single agent OH2, four blood samples were positive for OH2 viral loads (range: 1.43×103–4.38×104 copies/μL), between 1 hour and 7 days after injection. These samples were from four different patients, but none reported signs or symptoms of HSV infection. Meanwhile, none of the four patients presented with fever at the timepoints of blood sample collection. The blood viral loads of these four patients and the corresponding timepoints are shown in table 3. Viral copies in the blood did not seem to correlate with clinical benefits, since all the four patients with detectable viral loads did not respond to OH2. Pretreatment and post-treatment saliva, urine and injection site swabs were all negative for OH2 viral DNA. In patients enrolled for single agent dose-expansion, blood OH2 replication was not detected at all timepoints.
Table 3.
Biodistribution and biological activity of single agent OH2 in phase I*
| Patient | Dose level (CCID50/mL) |
Tumor types | Blood OH2 viral loads (copies/μL, timepoint) | Tumor GM-CSF mRNA levels (copies/μL, timepoint) | Blood GM-CSF expression (pg/mL, timepoint) | Blood anti-GM-CSF antibody (timepoint) | Best response (RECIST version 1.1/iRECIST) |
| 1 | 106 | Esophageal cancer | BQL | 1.24×104 (C1D3) | BQL | Negative | PD/iPR |
| 2 | 106 | Esophageal cancer | BQL | BQL | BQL | Negative | PD/iUPD |
| 3 | 106 | Breast cancer | BQL | BQL | BQL | Negative | PD/iUPD |
| 4 | 107 | Rectal cancer | BQL | BQL | BQL | Negative | PR/iPR |
| 5 | 107 | Esophageal cancer | BQL | 1.01×104 (C1D3) | BQL | Negative | NA |
| 6 | 107 | Head and neck cancer | 3.38×103 (C1D1, 1 hour) | BQL | BQL | Negative | PD/iUPD |
| 7 | 107 | Appendix cancer | BQL | BQL | BQL | Negative | SD/iSD |
| 8 | 108 | Esophageal cancer | 1.43×103 (C1D7) | 1.41×103 (C3D1) | 17.37 (C2D1) 19.40 (C3D1) |
Negative | PD/iUPD |
| 9 | 108 | Esophageal cancer | BQL | 1.00×104 (C1D3) | BQL | Negative | PD/iUPD |
| 10 | 108 | Gastric cancer | 3.76×103 (C1D2) | 1.72×104 (C1D3) | 30.07 (C1D2) 20.98 (C1D3) |
Negative | NA |
| 11 | 108 | Ovarian cancer | 4.38×104 (C1D7) | BQL | BQL | Transient positivity (C3D1) | SD/iSD |
*The biodistribution studies included OH2 viral DNA in the blood, saliva, urine, and injection site swabs. The results for saliva, urine and injection site swabs were all negative and not shown in the table. The results for blood OH2 viral loads, with values and timepoints of sample collection, are listed.
BQL, below quantifiable limit; C1D1, the first day of cycle 1; 1 hour, one hour (after injection with OH2); iUPD, immune-unconfirmed progressive disease; NA, not available; PD, progressive disease; PR, partial response; SD, stable disease.
In phase I, GM-CSF mRNA was detected in the injected tumors from five patients after treatment with single agent OH2, while two patients had detectable GM-CSF expression in their blood samples. Among the 40 patients treated with single agent OH2, 38 were seronegative and 2 were seropositive for anti-HSV-2 antibodies at baseline; 26 of the seronegative patients received subsequent HSV serology tests and were all converted to seropositive status after the study treatment. One patient in the single agent dose-escalation cohort developed transient antibodies to GM-CSF, as the antibodies were detected only once at the fifth week from the first dose. The results of GM-CSF mRNA in the tumor, GM-CSF expression and anti-GM-CSF antibodies in the blood of each patient are shown in table 3 (single agent OH2 dose-escalation) and online supplemental table 1 (single agent OH2 dose-expansion). No association was noted between clinical responses and any laboratory endpoints, including OH2 biodistribution, GM-CSF mRNA, GM-CSF expression, HSV serology, or anti-GM-CSF antibodies.
jitc-2020-002224supp001.pdf (379.7KB, pdf)
Although the MTD was not reached in dose-escalation of single agent OH2, we found that the incidence of fever seemed dose-related (1/3 with 106CCID50/mL, 1/4 with 107CCID50/mL and 3/4 with 108CCID50/mL), suggesting an increased risk of fever for patients receiving OH2 at 108CCID50/mL. Meanwhile, we did not observe any dose-dependent biodistribution parameters or biological activities induced by OH2. On the other hand, clinical responses were noted in patients from both 106CCID50/mL and 107CCID50/mL groups. Therefore, the recommended dose level for single agent OH2 dose-expansion in phase II was 107CCID50/mL.
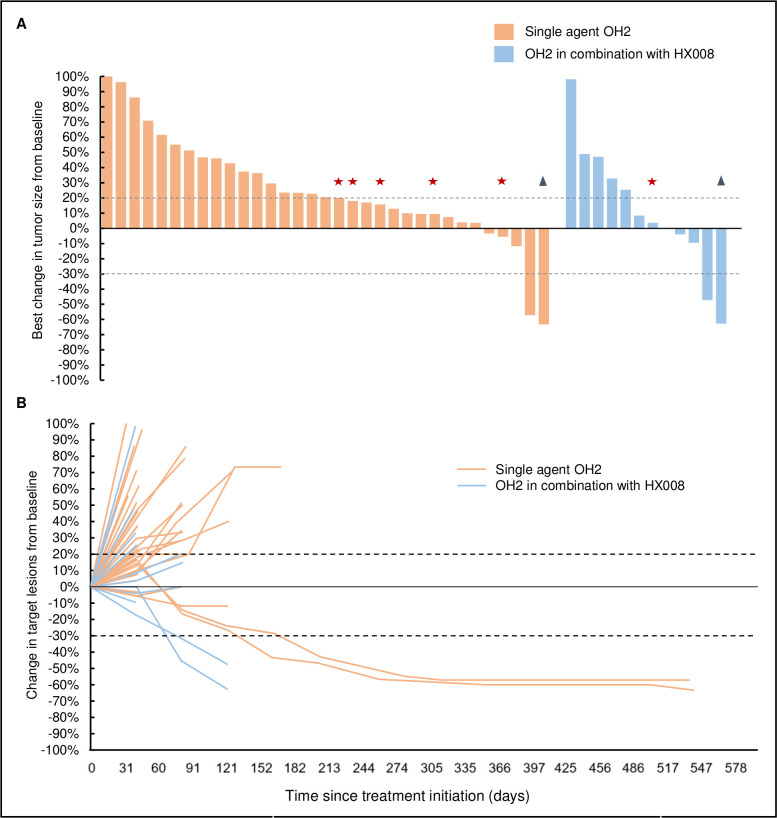
Thirty-three patients receiving single agent OH2 and twelve patients treated with OH2 plus HX008 were assessable for response. According to RECIST version 1.1, one patient in the single agent cohort and one in the combination cohort achieved partial response (PR), nine patients in the single agent cohort and four in the combination cohort had stable disease (SD), while the other 30 patients had PD. Immune-PR (iPR) was observed in four patients by iRECIST, with two in the single agent cohort and two in the combination cohort. The clinical responses as per iRECIST are summarized in table 1. The changes in the size of target lesions from baseline are shown in figure 1.
Figure 1.
Response to the study treatment. (A) Waterfall plot showing best percentage change in target lesions from baseline. The stars indicate patients with new lesions despite stable target lesions; the triangles indicate patients having pseudo-progression before achieving immune-partial response. (B) Chronological change of target lesions from baseline.
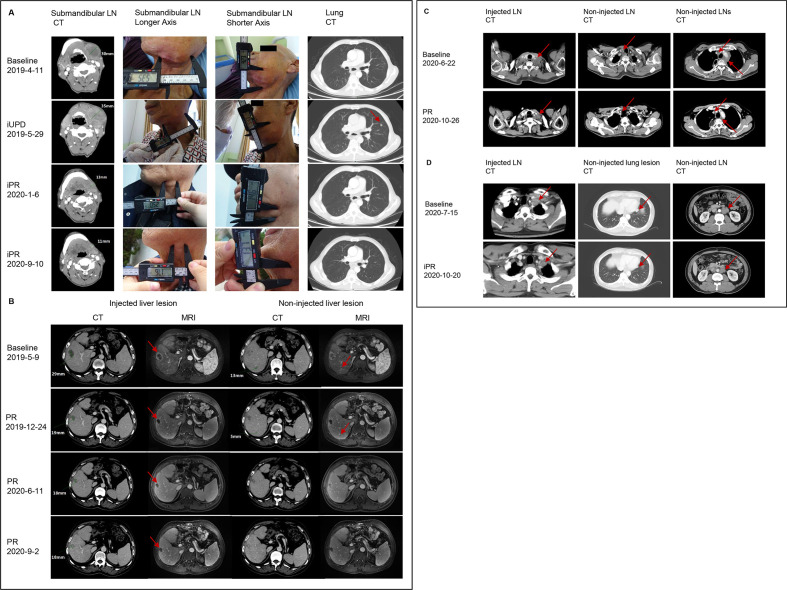
In the single agent cohort, the patient achieving PR per RECIST had multiple liver metastases from rectal cancer and was treated with OH2 in one of the liver lesions. Notably, both injected and non-injected liver metastasis reduced in size. The other responder with metastatic esophageal cancer had iPR after initial enlargement of the injected submandibular node and a suspected new lesion in the lung at the first radiological evaluation. The primary tumors of both patients were mismatch repair-proficient (pMMR) as confirmed by immunochemistry. The time to initial response for the two patients were 6.89 and 5.44 months, respectively. Both patients had discontinued treatment with OH2 due to difficulties of injection with increased intratumoral pressure, and absence of tumor cells in their latest post-treatment biopsies. The responses were ongoing, and the duration of response had reached 11.25+ and 14.03+ months. Moreover, the metastatic lymph node in the patient with esophageal cancer continued to regress despite treatment discontinuation. The representative tumor response images of the two responders in the single agent cohort are shown in figure 2A, B.
Figure 2.
Representative tumor response data of patients who responded. (A) Patient with submandibular lymph node metastasis from esophageal cancer responded to single agent OH2. The red arrow indicates the suspected new lesion in the lung. The injected lymph node continued to regress after discontinuation of the study treatment on January 3, 2020. (B) Patient with liver metastasis from rectal cancer responded to single agent OH2. The red arrows indicate the lesions on MRI. (C) Patient with metastatic esophageal cancer responded to OH2 plus HX008. The red arrows indicate tumor lesions. (D) Patient with metastatic rectal cancer responded to OH2 plus HX008. The red arrows indicate tumor lesions. CT, computed tomography; iPR, immune-partial response; iUPD, immune-unconfirmed progressive disease; LN, lymph node; MRI, magnetic resonance imaging; PR: partial response.
In the combination cohort, one patient with metastatic esophageal cancer achieved PR, and one patient with pMMR metastatic rectal cancer had iPR after initial immune-unconfirmed PD (iUPD). The time to response was 2.72 months for both patients, and the duration ofresponse were 1.38+ and 2.56+ months, respectively. The representative tumor response images of these two patients are shown in figure 2C, D.
CD8 and CD3 staining of baseline and on-treatment biopsies were performed in 14 patients, and PD-L1 expression was assessed in 17 patients. The results of PD-L1 CPS and TPSs as well as densities of CD8+ and CD3+ infiltrating cells for each patient are listed in table 4.
Table 4.
Results of biomarkers analysis
| Patient | Time of biopsy | PD-L1 TPS | PD-L1 CPS | Density of CD8+ cells (cells/mm2) | Density of CD3+ cells (cells/mm2) |
| 1* | Baseline | 3 | 6 | 3.8 | 22.9 |
| Week 6 | 15 | 28 | 412.5 | 575.0 | |
| 2 | Baseline | 0 | 2 | 116.7 | 266.7 |
| Week 6 | 0 | 3 | 106.7 | 208.3 | |
| 3 | Baseline | 0 | 13 | 163.9 | 250.0 |
| Week 7 | 0 | 20 | 265.4 | 371.8 | |
| 4* | Baseline | 3 | 6 | 38.1 | 64.3 |
| Week 3 | 10 | 13 | 62.5 | 208.3 | |
| Week 6 | 10 | 14 | 104.2 | 208.3 | |
| 6 | Baseline | 0 | 0 | 6.7 | 16.3 |
| Week 6 | 0 | 0 | 12.5 | 20.8 | |
| 7 | Baseline | 0 | 1 | 93.8 | 145.8 |
| Week 6 | 0 | 6 | 885.2 | 944.4 | |
| 8 | Baseline | 0 | 1 | 83.3 | 168.8 |
| Week 6 | 0 | 17 | 366.7 | 761.9 | |
| 9 | Baseline | 5 | 5 | NA | NA |
| 11 | Baseline | 0 | 0 | 25.8 | 36.9 |
| Week 12 | 0 | 1 | 81.7 | 115 | |
| 12 | Baseline | 20 | 20 | 66.7 | 84.0 |
| Week 5 | 20 | 20 | 114.9 | 147.1 | |
| 13 | Baseline | 5 | 5 | 62.1 | 131.8 |
| Week 3 | 40 | 46 | 570.4 | 870.4 | |
| 16 | Baseline | 0 | 1 | 32.1 | 73.8 |
| Week 21 | 0 | 47 | 121.4 | 290.5 | |
| 17 | Baseline | 0 | 0 | 98.5 | 50.0 |
| Week 11 | 0 | 15 | 61.4 | 233.3 | |
| 18 | Baseline | 2 | 4 | NA | NA |
| 19 | Baseline | 5 | 6 | NA | NA |
| 21 | Baseline | 1 | 2 | 128.0 | 269.3 |
| Week 10 | 0 | 0 | 61.1 | 147.2 | |
| 24 | Baseline | 0 | 0 | 51.4 | 105.6 |
| Week 7 | 0 | 0 | 78.3 | 200.0 |
*Patients responded to OH2.
CPS, combined positive score; NA, not available; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
We observed an increase in PD-L1 CPS after OH2 injection in 10 out of the 14 patients. Meanwhile, three patients showed an increase in PD-L1 TPS after treatment. We also noted that clinical responses were seen only in patients having a positive PD-L1 TPS with their baseline specimens. Further studies of the potential predictive value of baseline TPS for the treatment with OH2 is justified.
In the immune infiltrates, clear increases in the density of CD8+ and CD3+ cells after OH2 injection were observed in 11 and 12 out of the 14 patients, respectively. Responses to OH2 observed in this trial seemed independent of baseline and subsequent changes in CD8+ and CD3+ cell density. The two patients who responded to OH2 both had increases in PD-L1 CPS, TPS, CD8+ and CD3+ cell density relative to baseline after treatment. The representative changes in the tumor microenvironment induced by OH2 are shown in online supplemental figure 1.
Discussion
Although the ability of viruses to kill cancer cells has been investigated for over a century, the therapeutic benefit of oncolytic virotherapy has been demonstrated only recently in clinical trials.11 To date, several genetically modified mutants of HSV-1 have been applied for the treatment of malignant brain tumors, melanoma and other solid tumors.12–18 Despite their favorable toxicity profiles, convincing evidence of efficacy was noted in a small number of cases in these early trials,15 16 and successful clinical development into phase III stage was achieved only with T-VEC.5 In the present phase I/II trial, the oncolytic HSV-2 virus OH2 was well-tolerated with no DLTs observed and thus the MTD was not identified in the prespecified three dose levels. More importantly, durable objective responses in patients with metastatic esophageal cancer and rectal cancer were noted, showing great promise for future clinical studies. To the best of our knowledge, this is the first report on the safety, biodistribution and efficacy of an HSV-2 based oncolytic virus.
The most frequent OH2-associated AE was transient fever, which was also reported with most other oncolytic viruses administered intratumorally, including the HSV-1 based T-VEC,13 adenovirus,19 poxvirus20 and reovirus,21 and might be a class effect. The fever observed in the present trial was associated with higher volumes of the injected OH2, and we suggest the use of preventive antipyretics in these patients. The biodistribution study revealed no shedding of OH2 in swabbing, urine and saliva collections from any patients. Moreover, the transient replication of OH2 detected in the blood in a limited number of patients was not associated with any AEs of clinical significance. Therefore, we may infer that OH2 was generally retained in the injected tumor, and the risk of spreading the virus to the environment or people in close contact were extremely low. The GM-CSF mRNA detected in tumor samples was not associated with clinical response in our present study. The interpretation of the finding is difficult, since we were unable to distinguish whether the GM-CSF mRNA was derived from OH2 or secreted by immune cells with our current method, and the precise role of GM-CSF expressed by OH2 in altering the tumor microenvironment warrants further investigation.
The antitumor activity was encouraging for this first-in-human study. In the single agent OH2 cohorts, marked tumor regression was observed in patients who were heavily pretreated and had documented disease progression immediately before entry to the present trial. OH2 was effective in both injected and non-injected lesions. The bystander effect proved the mechanism of oncolytic virotherapy as OH2 eradicated distant malignancies through tumor-selective infection or immune responses. The time to response as wells as duration of response in patients treated with single agent OH2 were considerably longer than those for cytotoxic drugs. Furthermore, the pattern of pseudo-progression observed in the present trial was consistent with that seen in other immunotherapy trials. The iRECIST criteria is hence helpful in clinical decision making for patients treated with oncolytic viruses. However, it remains a challenging task to define the optimal timing as to confirm true PD and cease the treatment, and still requires further investigation.
We did not observe objective responses in dose-expansion of single agent OH2. In addition to the limited sample size, the suspension of the trial during the peak of COVID-19 pandemic could also have affected the outcomes. The response rate was possibly underestimated, as some patients permanently discontinued the study treatment and might have missed the opportunity of further clinical benefit.
Biopsies taken following single agent OH2 injection showed increases in CD8+ and CD3+ cell density and PD-L1 expression levels in most patients relative to baseline regardless of clinical responses, suggesting the potential immune-modulating effect of OH2 to enhance the intratumoral infiltration of T cells. The attraction of cytotoxic T cells by injection of OH2 may partly contribute to clinical responses. Specifically, the ability of OH2 to inflame “cold” tumors is especially useful in the setting of combination therapy with immune checkpoint inhibitors. It has been proved that higher numbers of CD8, PD-1 and PD-L1 expressing cells at the invasive tumor margin and inside tumors are associated with responses to anti-PD-1 antibodies in patients with metastatic melanoma.22 Therefore, the addition of PD-1 blockade after priming therapy with an oncolytic virus is of potential synergistic effect. Indeed, the combination of T-VEC with either pembrolizumab or ipilimumab have yielded high response rates in patients with advanced melanoma.23 24 In our present study, although preliminary, we have observed a higher proportion of patients achieving an objective response in the OH2 and HX008 combination cohort, and the time to response seemed shorter, suggesting a trend of improved efficacy. We will seek to confirm the advantage of the combination strategy in specific tumor types with larger sample sizes.
In conclusion, intratumoral injection of the HSV-2 based oncolytic virus OH2 was safe in patients with advanced solid tumors. Encouraging antitumor activities were observed in patients with metastatic rectal cancer and esophageal cancer. The changes in the tumor microenvironment induced by OH2 implied the underlying mechanisms of response, and supported further development of the drug as single agent or in combination with immune checkpoint inhibitors in the future.
Acknowledgments
We thank the patients, their families and the study personnel involved in this trial.
Footnotes
Contributors: JH, BL and XG conceived and designed the study. All authors contributed in the acquisition, analysis and interpretation of data. BZ drafted the manuscript. All authors participated in the review and final approval of the manuscript.
Funding: This work was supported by Binhui Biopharmaceutical Co., Ltd.
Competing interests: BL is the founder of Binhui Biopharmaceutical Co., Ltd. XG is a salaried employee of Binhui Biopharmaceutical Co., Ltd.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data supporting the results reported in the article are not publicly available and but are accessible from the corresponding author on reasonable request and approval from study sponsor according to available guidelines at time of request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. The study protocol was reviewed and approved by the institutional review board of the Cancer Hospital, Chinese Academy of Medical Sciences. All patients provided written informed consent before the study treatment.
References
- 1.Lichty BD, Breitbach CJ, Stojdl DF, et al. Going viral with cancer immunotherapy. Nat Rev Cancer 2014;14:559–67. 10.1038/nrc3770 [DOI] [PubMed] [Google Scholar]
- 2.Lawler SE, Speranza M-C, Cho C-F, et al. Oncolytic viruses in cancer treatment: a review. JAMA Oncol 2017;3:841–9. 10.1001/jamaoncol.2016.2064 [DOI] [PubMed] [Google Scholar]
- 3.Martuza RL, Malick A, Markert JM, et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991;252:854–6. 10.1126/science.1851332 [DOI] [PubMed] [Google Scholar]
- 4.Ma W, He H, Wang H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol 2018;19:40. 10.1186/s12865-018-0281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780–8. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 6.Zhao Q, Zhang W, Ning Z, et al. A novel oncolytic herpes simplex virus type 2 has potent anti-tumor activity. PLoS One 2014;9:e93103. 10.1371/journal.pone.0093103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Jin J, Wu Z, et al. Stability and anti-tumor effect of oncolytic herpes simplex virus type 2. Oncotarget 2018;9:24672–83. 10.18632/oncotarget.25122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhou X, Wu Z, et al. Preclinical safety evaluation of oncolytic herpes simplex virus type 2. Hum Gene Ther 2019;30:651–60. 10.1089/hum.2018.170 [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Hu X, Liang J, et al. oHSV2 can target murine colon carcinoma by altering the immune status of the tumor microenvironment and inducing antitumor immunity. Mol Ther Oncolytics 2020;16:158–71. 10.1016/j.omto.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagès F, André T, Taieb J, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol 2020;31:921–9. 10.1016/j.annonc.2020.03.310 [DOI] [PubMed] [Google Scholar]
- 11.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 2015;14:642–62. 10.1038/nrd4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000;7:867–74. 10.1038/sj.gt.3301205 [DOI] [PubMed] [Google Scholar]
- 13.Hu JCC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006;12:6737–47. 10.1158/1078-0432.CCR-06-0759 [DOI] [PubMed] [Google Scholar]
- 14.Kimata H, Imai T, Kikumori T, et al. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann Surg Oncol 2006;13:1078–84. 10.1245/ASO.2006.08.035 [DOI] [PubMed] [Google Scholar]
- 15.Nakao A, Kasuya H, Sahin TT, et al. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther 2011;18:167–75. 10.1038/cgt.2010.65 [DOI] [PubMed] [Google Scholar]
- 16.Fong Y, Kim T, Bhargava A, et al. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol Ther 2009;17:389–94. 10.1038/mt.2008.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geevarghese SK, Geller DA, de Haan HA, et al. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum Gene Ther 2010;21:1119–28. 10.1089/hum.2010.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streby KA, Geller JI, Currier MA, et al. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin Cancer Res 2017;23:3566–74. 10.1158/1078-0432.CCR-16-2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J-L, Liu H-L, Zhang X-R, et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing Hsp70 in advanced solid tumor patients. Gene Ther 2009;16:376–82. 10.1038/gt.2008.179 [DOI] [PubMed] [Google Scholar]
- 20.Park B-H, Hwang T, Liu T-C, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol 2008;9:533–42. 10.1016/S1470-2045(08)70107-4 [DOI] [PubMed] [Google Scholar]
- 21.Morris DG, Feng X, DiFrancesco LM, et al. REO-001: a phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs 2013;31:696–706. 10.1007/s10637-012-9865-z [DOI] [PubMed] [Google Scholar]
- 22.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017;170:e1110:1109–19. 10.1016/j.cell.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the efficacy and safety of Talimogene Laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol 2018;36:1658–67. 10.1200/JCO.2017.73.7379 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-002224supp001.pdf (379.7KB, pdf)
Data Availability Statement
Data supporting the results reported in the article are not publicly available and but are accessible from the corresponding author on reasonable request and approval from study sponsor according to available guidelines at time of request.