Abstract
The clinical and imaging data of 121 ICU patients with SARS-CoV-2 infection (63 survivors and 58 non-survivors) were retrospectively reviewed. The clinical results and radiographic features were compared between survivors and non-survivors. Compared with survivors, non-survivors were more likely to develop ARDS (53 [91 %] vs. 22 [35 %], P < 0.0001), shock (6 [10 %] vs. 0, P = 0.009), cardiac injury(18 [31 %] vs. 6 [10 %], P = 0.003), acute kidney injury(21 [36 %] vs. 10 [16 %], P = 0.01), and pneumothorax(5 [9%] vs. 0, P = 0.017). There were typical radiographic features for ICU patients with SARS-CoV-2 pneumonia. Extensive air-space opacities could be seen in all patients. Middle and lower lung involvement was significantly more serious than upper lung (score 6.8 ± 1.9, 7.2 ± 2.1, and 5.7 ± 1.7, respectively, P < 0.0001). Based on X-ray involvement score, non-survivors were in a more critical condition than survivors (20.3 ± 4.6 vs. 19.1 ± 3.1, P = 0.038).
Keywords: X-ray, Radiographic features, Critically ill, ICU, COVID
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia is clinically divided into mild, common, severe and critically ill types [[1], [2], [3]]. Critical ill patients need to be treated in intensive care unit (ICU), which has a high mortality rate. High resolution CT could identify typical ground-glass opacities (GGO), multifocal patchy consolidation, and crazy-paving sign in the peripheral area of the lungs of patients with SARS-CoV-2 pneumonia [[4], [5], [6]]. Follow-up CT can be used to determine whether pulmonary infection improves or progresses [7]. However, CT scan is difficult to be performed for patients in ICU, where mobile X-ray system serves as alternative in monitoring SARS-CoV-2 pneumonia. Many previous studies reported the clinical and imaging features [4,[7], [8], [9], [10], [11], [12]] of SARS-CoV-2 pneumonia. However, there are few studies comparing radiographic manifestations between survivors and non-survivors from ICU. We retrospectively investigated 121 ICU patients with SARS-CoV-2 infection (including 63 survivors and 58 non-survivors), and aimed to clarify the difference in X-ray manifestations between the two groups.
2. Materials and methods
This retrospective study was approved by our ethics committee, and written informed consent was waived.
The clinical data of 121 critically ill SARS-CoV-2 patients in ICU were collected and retrieved using the Radiologists Information System (RIS).The diagnosis of SARS-CoV-2 infection was confirmed by positive RT-PCR results, and the critically ill patients were defined as those admitted to ICU who required mechanical ventilation or those had shock [3] or those had a fraction of inspired oxygen (FiO2) of at least 60 % or more [13,14]. The recorded information included: age, gender, underlying diseases, initial symptoms, X-ray imaging signs and scores.
3. Acquisition of chest X-ray radiographs
Chest radiographs were all taken with a mobile X-ray scanner (uDR370i; United Imaging, Shanghai, China) in ICU. Scanning protocol was as follows: 80 kV, 3.2 mAs, 100-cm film-to-focus distance, with a broad tube focus. The radiograph images were reviewed on a picture archiving and communication system (Synapse; Fujifilm).
4. Image analysis
Two experienced thoracic radiologists (with 8 and 10 years of thoracic diagnostic experience, respectively) without knowing the patient’s clinical data assessed the X-rays. The radiograph signs and involvement scores were determined on consensus.
The involvement of lung on X-ray was assessed using a visual scoring method according to previous studies [15,16] as follows: each lung was divided into three equidistant zones (upper, middle, and lower) from the apex to the bottom (diaphragm), adding up to six zones together. For each zone: score 0, no involvement; score 1, 1%–25 % involved; score 2, 26 %–50 % involved; score 3, 51 %–75 % involved; score 4, 76 %–100 % involved. The total score was acquired by summing the scores of all the six zones, with the maximum value of 24. If a patient had multiple X-ray examinations, all X-rays were assessed, and the highest score was recorded.
5. Statistical analysis
Continuous variables were expressed as mean ± SD or median (IQR), and categorical variables as number (%). All data statistical analysis was performed with SPSS (22.0, IBM, USA). We assessed differences between survivors and non-survivors using two-sample t-test for normal distribution data or Mann-Whitney U test for non-normal continuous variables, and Chi-square test or Fisher’s exact-test for categorical variables. The Kruskal-Wallis test was used to compare involvement score among upper, middle and lower lung. P value less than 0.05 was considered significant difference.
6. Results
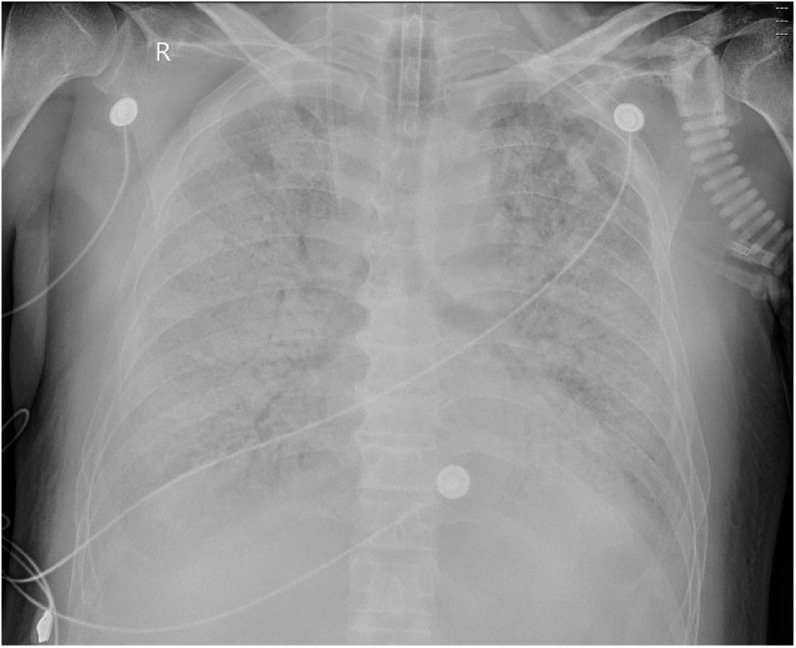
121 ICU patients with SARS-CoV-2 pneumonia were included in this study. The median age was 63 years. 78 (64 %) patients were men. 74 (61 %) patients had underlying diseases. Hypertension (26 %) and chronic cardiac disease (15 %) were most common underlying diseases, followed by malignancy (12 %) and chronic pulmonary disease (12 %). The most common initial symptoms were fever (92 %), cough (68 %), and dyspnea (49 %). 75 (62 %) patients developed acute respiratory distress syndrome (ARDS), 6 (5%) with shock, 31 (26 %) with acute kidney injury, 24 (20 %) with cardiac injury, and 5 (4%) with pneumothorax. 38 (31 %) patients were treated with invasive mechanical ventilation, 4 (3 %) with extracorporeal membrane oxygenation (ECMO), and 12 (10 %) with renal replacement therapy. All patients received antiviral therapy and antibacterial agents, and 48 (40 %) patients received corticosteroids (Table 1). The patients underwent one or more mobile X-rays. 119 (98 %) patients had bilateral infiltrates on chest x-ray. Extensive air-space opacities (Fig. 1, Fig. 2) could be seen in all patients. 24 (20 %) patients had pleural effusion, and 5 (4%) had pneumothorax (Fig. 3). Middle and lower lung involvement was significantly more serious than upper lung (score 6.8 ± 1.9, 7.2 ± 2.1, and 5.7 ± 1.7, respectively, P < 0.01).
Table 1.
Main clinical information of 121 critically ill patients with SARS-CoV-2 infection. Comparisons of clinical features were performed between non-survivors and survivors using two-sample t-test for normal distribution data, and Chi-square test or Fisher’s exact-test for categorical variables.
| Clinical features | all patients (n = 121) | non-survivors (n = 58) | survivors (n = 63) | P value |
|---|---|---|---|---|
| Age | ||||
| mean age (IQR) | 63 (50, 74) | 68 (54,80) | 58 (45,73) | <0.0001 |
| Gender | ||||
| male | 78 (64 %) | 39 (67 %) | 39 (62 %) | 0.540 |
| female | 43 (36 %) | 19 (33 %) | 24 (38 %) | |
| Initial symptoms | ||||
| fever | 111(92 %) | 56 (97 %) | 55(87 %) | 0.065 |
| cough | 82(68 %) | 42 (72 %) | 40(63 %) | 0.294 |
| dyspnea | 59(49 %) | 31 (53 %) | 28(44 %) | 0.322 |
| malaise | 33(27 %) | 12 (21 %) | 11(17 %) | 0.651 |
| diarrhea | 8(7 %) | 5 (9 %) | 3(5 %) | 0.393 |
| myalgia | 4(3 %) | 2 (3 %) | 2(3 %) | 0.933 |
| hemoptysis | 3(2 %) | 2 (3 %) | 1(2 %) | 0.511 |
| headache | 1(1 %) | 1(2 %) | 0 | 0.295 |
| Underlying diseases | ||||
| tuberculosis | 8(7 %) | 5 (9 %) | 3(5 %) | 0.393 |
| diabetes | 13(11 %) | 8 (14 %) | 5(8 %) | 0.299 |
| hypertension | 32(26 %) | 20 (34 %) | 12(19 %) | 0.054 |
| cerebrovascular disease | 9(7 %) | 5 (9 %) | 4(6 %) | 0.634 |
| chronic pulmonary disease |
15(12 %) | 8 (14 %) | 7(11 %) | 0.655 |
| chronic cardiac disease | 18(15 %) | 10 (17 %) | 8(13 %) | 0.483 |
| malignancy | 14(12 %) | 8 (14 %) | 6(10 %) | 0.463 |
| Hepatitis C | 3(2%) | 2 (3%) | 1(2%) | 0.511 |
| goiter | 2(2%) | 1 (2%) | 1(2%) | 0.953 |
| Complications | ||||
| acute respiratory distress syndrome |
75(62 %) | 53(91 %) | 22(35 %) | <0.0001 |
| shock | 6(5 %) | 6(10 %) | 0 | 0.009 |
| acute kidney injury | 31(26 %) | 21(36 %) | 10(16 %) | 0.01 |
| renal insufficiency | 6(5 %) | 6(10 %) | 0 | 0.009 |
| cardiac injury | 24(20 %) | 18(31 %) | 6(10 %) | 0.003 |
| gastrointestinal haemorrhage |
3(2%) | 3(5%) | 0 | 0.068 |
| pneumothorax | 5(4%) | 5(9%) | 0 | 0.017 |
| pulmonary embolism | 2(2%) | 2(3%) | 0 | 0.137 |
| hemolytic anemia | 1(1 %) | 1(2 %) | 0 | 0.295 |
| cellulitis | 1(1 %) | 1(2 %) | 0 | 0.295 |
| Treatment | ||||
| non-invasive mechanical ventilation | 86(71 %) | 49(84 %) | 37(59 %) | 0.002 |
| invasive mechanical ventilation | 38(31 %) | 31(53 %) | 7(11 %) | <0.0001 |
| renal replacement therapy |
12(10 %) | 9(16 %) | 3(5%) | 0.048 |
| corticosteroids | 48(40 %) | 21(36 %) | 27(43 %) | 0.455 |
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2; COPD = chronic obstructive pulmonary disease.
Fig. 1.
A “white lung” at a 69-year-old male with laboratory-confirmed SARS-CoV-2 pneumonia. He died of respiratory failure two days after this scan.
Fig. 2.
A 71-year-old male non-survivor with hypertension and coronary heart disease. His initial symptoms were fever and diarrhea. RT-PCR for SARS-CoV-2 was positive. Multiple air space opacities could be seen on first chest X-ray. Significant progress could be seen on second X-ray 4 days later (arrows). Involvement was more serious for the lower lung compared with upper lung.
Fig. 3.
A 57-year-old female non-survivor with fever and diarrhea as the initial symptom. RT-PCR for SARS-CoV-2 was positive. Typical imaging features of SARS-CoV-2 pneumonia could be seen on first chest CT. Pneumothorax could be easily seen (arrows) on chest X-ray 17 days later, as well as extensive air space opacities and pleural effusion.
For 58 non-survivors, the median duration from onset of symptoms to death was 30 days, and the median duration from ICU admission to death was 9 days. The median time from last mobile X-ray to death was 2 days.
Compared with survivors, non-survivors were more likely to develop ARDS (53 [91 %] vs. 22 [35 %], P < 0.01), shock (6 [10 %] vs. 0, P = 0.009), acute kidney injury(21 [36 %] vs. 10 [16 %], P = 0.01), cardiac injury (18 [31 %] vs. 6 [10 %], P = 0.003), pneumothorax(5 [9%] vs. 0, P = 0.017) (Table 1).
Based on X-ray involvement score, non-survivors were in a more critical condition than survivors (20.3 ± 4.6 vs. 19.1 ± 3.1, P = 0.038). As summarized in Table 2, survivors and non-survivors did not differ in involvement score on zone level, but differed significantly in score of whole lung. Non-survivors were more likely to have pneumothorax than survivors (5 [9%] vs. 0, P = 0.017).
Table 2.
Main radiographic features of 121 critically ill patients with SARS-CoV-2 infection. Comparisons of radiographic features were performed between non-survivors and survivors using Mann-Whitney U test for involvement score, and Chi-square test or Fisher’s exact test for categorical variables. The Kruskal-Wallis test was used to compare involvement score among upper, middle and lower lung.
| Radiographic features | Critical ill(n = 121) | Non-survivors (n = 58) | Survivors (n = 63) | P value |
|---|---|---|---|---|
| bilateral pneumonia | 119(98 %) | 57(98 %) | 61(97 %) | 0.608 |
| unilateral pneumonia | 2(2 %) | 1(2 %) | 2(3 %) | |
| mean involvement score (standard deviation) | 19.7 ± 3.8 | 20.3 ± 4.6 | 19.1 ± 3.1 | 0.038 |
| pleural effusion | 24(20 %) | 15 (26 %) | 9(14 %) | 0.111 |
| pleural thickening | 8(7 %) | 4 (7 %) | 4(6 %) | 0.904 |
| pneumothorax | 5(4 %) | 5 (9 %) | 0 | 0.017 |
| Involvement score | ||||
| Six zones | ||||
| right upper | 2.4 ± 0.7 | 2.5 ± 0.8 | 2.3 ± 0.7 | 0.24 |
| right middle | 3.4 ± 0.7 | 3.5 ± 0.9 | 3.3 ± 0.8 | 0.28 |
| right lower | 3.6 ± 1.0 | 3.7 ± 1.1 | 3.5 ± 1.0 | 0.25 |
| left upper | 2.3 ± 0.9 | 2.4 ± 0.9 | 2.2 ± 0.8 | 0.19 |
| left middle | 3.4 ± 0.9 | 3.5 ± 1.0 | 3.3 ± 0.9 | 0.12 |
| left lower | 3.6 ± 1.2 | 3.7 ± 1.2 | 3.5 ± 1.1 | 0.17 |
| Three regions | ||||
| upper lung | 5.7 ± 1.7 | 5.9 ± 1.8 | 5.5 ± 1.7 | 0.09 |
| middle lung | 6.8 ± 1.9 | 7.0 ± 1.9 | 6.6 ± 1.8 | 0.105 |
| lower lung | 7.2 ± 2.1 | 7.4 ± 2.1 | 7.0 ± 2.0 | 0.08 |
| P value | <0.0001 | <0.0001 | <0.0001 | |
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. ARDS = acute respiratory distress syndrome.
As summarized in Table 3, survivors and non-survivors differed significantly in multiple laboratory findings. Fig. 1 shows a “white lung” of a non-survivor with ARDS. Fig. 2 shows the rapid progress of SARS-CoV-2 pneumonia for a non-survivor. Fig. 3 shows pneumothorax in SARS-CoV-2 pneumonia. Fig. 4 shows the evolution of involvement score (with time) in part of patients.
Table 3.
Main laboratory findings of 121 critically ill patients with SARS-CoV-2 infection. Comparisons of laboratory findings were performed between non-survivors and survivors using two-sample t-test for normal distribution data, or Mann-Whitney U test for non-normal data. Medians and IQR were provided in the table.
| All patient (n = 121) | Non-survivors (n = 58) | Survivors(n = 63) | P value | |
|---|---|---|---|---|
| Leucocyte (109/L) | 9.5 [7.1, 13.61] | 11.64 [9.37, 15.61] | 7.22 [6.1, 8.79] | <0.0001 |
| Platelet (109/L) | 130.4 [83, 188] | 118 [63, 179] | 144.5 [113.5, 227.75] | 0.004 |
| Erythrocyte (1012/L) | 3.61 [3.01, 4.06] | 3.48 [2.71, 3.89] | 3.76 [3.59, 4.17] | 0.001 |
| Neutrophils (109/L) | 8.72 [6.5, 11.12] | 9.44 [7.36, 12.71] | 7.8 [5.45, 9.37] | <0.0001 |
| Lymphocyte (109/L) | 0.6 [0.41, 0.81] | 0.5 [0.32, 0.74] | 0.70 [0.47, 0.93] | <0.0001 |
| Hemoglobin (g/L) | 117 [102.2, 128] | 115.5 [91, 127] | 120 [112.5, 129] | 0.01 |
| Glucose (mmol/L) | 8.33 [7.24, 9.76] | 9.11 [7.62, 13.66] | 7.44 [6.46, 9.48] | 0.019 |
| Total protein (g/L) | 60.6 [58.9, 65.8] | 59.9 [56.8, 65.4] | 61.9 [59.8, 67.9] | 0.18 |
| Globulin (g/L) | 35.1 [31.25, 38.7] | 37.9 [33.3, 40.7] | 32.2 [29.25, 33.85] | <0.0001 |
| Albumin (g/L) | 30.1 [27.28, 33.5] | 28 [25.08, 30.95] | 32.3 [28.2, 37.2] | 0.588 |
| Creatinine (μmol/L) | 84 [63, 109.5] | 86 [66, 179.5] | 82 [59, 103.5] | 0.008 |
| Uric acid (μmol/L) | 189 [136.4, 330.9] | 190.5 [114.5, 309] | 188 [148, 362.4] | 0.537 |
| Total bilirubin (μmol/L) | 14.7 [10.4, 18.5] | 17.4 [11.5, 20.4] | 11.4 [8.6, 13.4] | <0.0001 |
| Direct bilirubin (μmol/L) | 8.35 [6.15, 11.4] | 9.75 [6.4, 14.4] | 6.8 [5.35, 9.93] | <0.0001 |
| Indirect bilirubin (μmol/L) | 8.24 [5.72, 9.65] | 9.15 [5.45, 11] | 7.6 [5.8, 9.35] | 0.083 |
| Urea (mmol/L) | 11.35 [7.28, 15.68] | 14.55 [10.23, 20.08] | 7.85 [6.98, 10.17] | <0.0001 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 75.7 [62.35, 89.2] | 69.3 [41.35, 89.4] | 82 [73.4,88.6] | 0.008 |
| Lactate (mmol/L) | 1.98[1.09, 2.95] | 2.7 [1.94, 3.48] | 1.15 [0.95, 1.33] | <0.0001 |
| Alanine aminotransferase (U/L) | 40 [23, 68] | 43 [24, 104] | 37 [21, 57] | 0.054 |
| Aspartate aminotransferase (U/L) | 38.4 [22.75, 67.15] | 39 [17, 77.25] | 38 [24.25, 61.75] | 0.700 |
| Myoglobin (ng/mL) | 214.35 [127.45, 336.6] | 280.6 [152.15, 736.8] | 147 [84.45, 229.55] | 0.002 |
| High sensitive cardiac troponin I (pg/mL) | 127.53 [48.68, 290.5] | 202.2 [68.95, 460.18] | 50.55 [44.5, 77.6] | <0.0001 |
| MB isoenzyme of creatine kinase (ng/mL) | 3.57 [1.1, 6.45] | 5.85 [2.63, 11.93] | 1.2 [0.7, 1.9] | 0.014 |
| Lactate dehydrogenase (U/L) | 398.75 [351.4, 504.4] | 490 [358.5, 591] | 306.5 [281.5, 368.25] | <0.0001 |
| Creatine kinase (U/L) | 179 [82, 303] | 180 [43, 503] | 178 [84, 230.5] | 0.241 |
| Prothrombin time (second) | 15.7 [13.79, 17.23] | 16.5 [15.3, 19.4] | 14.3 [13.25, 15.83] | <0.0001 |
| Fibrinogen (g/L) | 5.87 [4.69, 6.13] | 5.92 [4.82, 6.3] | 5.22 [4.59, 5.78] | 0.069 |
| Activated partial thromboplastin time (second) | 45.3 [41.6, 48.45] | 46.4 [42.5, 56] | 43.5 [40.65, 46.8] | 0.002 |
| Thrombin time (second) | 16.1 [14.3, 17.8] | 17.5 [15.7, 20.6] | 14.55 [13.3, 15.6] | 0.446 |
| D-dimer (μg/mL) | 3.99 [2.16, 5.97] | 5.47 [2.73, 12.52] | 2.22 [1.82, 2.92] | <0.0001 |
| Prothrombin activity | 72 % [62 %, 82 %] | 68 % [55 %, 75 %] | 79 % [64 %, 91 %] | <0.0001 |
| International normalized ratio | 1.19 [1.03, 1.42] | 1.3 [1.22, 1.56] | 1.08 [0.99, 1.21] | <0.0001 |
| Fibrinogen degradation products (μg/mL) | 19.55 [9.6, 35.75] | 29.65 [17.13, 62.65] | 9.9 [9, 11.5] | <0.0001 |
| Procalcitonin (ng/mL) | 0.52 [0.24, 1.25] | 0.97 [0.27, 2.58] | 0.27 [0.13, 0.41] | <0.0001 |
| N-terminal pro-brain natriuretic peptide (pg/mL) | 2425.6 [1145.25, 3489.5] | 3375.5 [1491.75, 8102.75] | 1263 [522, 1483.5] | <0.0001 |
| Ferritin (μg/L) | 945.45 [803.5, 1632.56] | 1064.5 [814.25, 2658.5] | 826.8 [616.75, 1481.5] | 0.137 |
| Hypersensitive C-reactive protein (mg/L) | 105.8 [68.94, 155.43] | 142.7 [80.9, 209] | 73.1 [42.3, 127.1] | <0.0001 |
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Fig. 4.
Seven survivors and nine non-survivors from ICU exactly underwent three X-rays. X-ray images were given a involvement score according to the following rules: each lung was divided into upper, middle, and lower zones, adding up to six zones together; for each zone, score 0 = no involvement, 1 = 1 %–25 % involved, 2 = 26 %–50 % involved, 3 = 51 %–75 % involved, 4 = 76 %–100 % involved; the total score was acquired by summing the scores of six zones. The involvement score varied during disease course. Improvement could be seen in follow-up X-rays of survivors, In contrast, progress could be seen for non-survivors.
7. Discussions
We retrospectively reviewed clinical data and imaging data of 121 ICU patients with confirmed SARS-CoV-2 infection. Compared with survivors, non-survivors were more likely to develop ARDS, to have underlying diseases, and had higher X-ray involvement score, higher incidence of pneumothorax.
Refractory hypoxemia occurred one week after the onset of COVID and then deteriorated into ARDS in part of cases. ARDS is the fundamental pathophysiology of severe viral pneumonia [17,18], with a mortality at 28 days near 50 % [19]. ARDS was pathologically related to diffuse alveolar damage with cellular fibromyxoid exudates [[20], [21], [22], [23], [24]]. Previous studies reported that ACE2, the receptor for SARS-CoV-2, was expressed on myocytes and vascular endothelial cells, as well as on tubular cells, glomerular epithelial cells [25], resulting in potential direct cardiac and renal attack. Acute kidney or cardiac injury was observed in our cohort, which could have been related to direct effects of the virus, hypoxia, or shock. Non-survivors were more likely to develop acute kidney injury and cardiac injury.
The diagnostic value of chest CT for COVID-19 has been validated by many studies [[26], [27], [28]]. However, CT is not easily performed for ICU patients, especially when ICU is far from CT rooms. This study found mobile X-ray provided adequate image quality. Follow-up X-ray could also be used to determine whether pneumonia improves or progresses. Extensive lung involvement (or “white lung”) is the key radiographic feature of SARS-CoV-2 ARDS. The middle and lower lung involvement was more serious than upper lung. Lung involvement was more serious in non-survivors versus survivors. For survivors, significant improvement generally occurred in the follow-up X-ray. In contrast, progress of pneumonia occurred in most of non-survivors.
As for laboratory tests, lymphocytes were less in non-survivor compared to survivor, indicating that excessive immune response played an important role on pathogenesis of fatal SARS-CoV-2, and that the degree of lymphocytopenia was related to the severity of the disease. In our study, leukocytes and neutrophils were both high in non-survivors. It suggested that in critically ill patients, perhaps neutrophils were activated to induce the immune response, causing cytokine storms. LDH was a predictor in many diseases related to inflammatory reaction and tissue damage. CRP was a widely used biochemical indicator for inflammation, such as microbial invasion or tissue damage. We found that LDH and CRP were also significantly higher in non-survivors. We speculated that the virus may trigger a series of immune responses and induces cytokine storm in vivo. The level of inflammation indicators may correlate with the severity of the disease and prognosis.
This study has some limitations. First, this study was conducted at a single-center for severe SARS-CoV-2 patients; therefore, there may be selection bias. Second, most patients didn’t have CT images at ICU, which was a more accurate imaging method in monitoring the disease course. A larger cohort study of SARS-CoV-2 pneumonia from multiple centers would help further explore the disease.
In conclusion, there were typical clinical and radiographic features of ICU patients with SARS-CoV-2 pneumonia. Extensive air space opacities or “white lung” was the key radiographic sign for critical ill patients. Compared to survivors, non-survivors had more serious lung involvement, as well as a higher incidence of pneumothorax.
Ethical statement
This study was approved by the ethics committee of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology (approval number: HUST-TJ-20200168). Written informed consent was waived.
Funding
This study was funded by NSFC81801663.
CRediT authorship contribution statement
Gang Wu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Shuchang Zhou: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- 1.World Health Organization. Corona-virus disease (COVID-19) outbreak (https://www.who.int). In.
- 2.World Health Organization.https://www.who.int/emergencies/diseases/novel-coronavirus-2019. In.
- 3.Chinese National Health Committee. Diagnosis and treatment of COVID-19 pneumonia (trial seventh edition) (2020-03-04). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. In.
- 4.Pan F., Ye T., Sun P. Time course of lung changes on chest ct during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am. J. Roentgenol. 2020:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 7.Zu Z.Y., Jiang M.D., Xu P.P. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan Y.N., Qin J. Pre- and posttreatment chest CT findings: 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020:200323. doi: 10.1148/radiol.2020200323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez-Cherit G., Lapinsky S.E., Macias A.E. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;(302):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 14.Fowler R.A., Lapinsky S.E., Hallett D. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 15.Antonio G.E., Ooi C.G., Wong K.T. Radiographic-clinical correlation in severe acute respiratory syndrome: study of 1373 patients in Hong Kong. Radiology. 2005;(237):1081–1090. doi: 10.1148/radiol.2373041919. [DOI] [PubMed] [Google Scholar]
- 16.Ko S.F., Lee T.Y., Huang C.C. Severe acute respiratory syndrome: prognostic implications of chest radiographic findings in 52 patients. Radiology. 2004;233:173–181. doi: 10.1148/radiol.2323031547. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsaad K.O., Hajeer A.H., Al B.M. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellani G., Laffey J.G., Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 20.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y., Wang H., Shen H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng D.L., Al H.F., Keating M.K. Clinicopathologic, immunohistochemical, and ultrastructural H.F.iNdings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am. J. Pathol. 2016;(186):652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza-Torres E., Oyarzun A., Mondaca-Ruff D. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther. Adv. Cardiovasc. Dis. 2015;9:217–237. doi: 10.1177/1753944715597623. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 27.Li K., Wu J., Wu F. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol. 2020 doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020 doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]