Abstract
Objective
This study aimed to provide population-level data regarding trends in multimorbidity over 13 years.
Methods
We linked provincial health administrative data in Ontario, Canada, to create 3 cross-sectional panels of residents of any age in 2003, 2009, and 2016 to describe: (i) 13-year trends in multimorbidity prevalence and constellations among residents and across age, sex, and income; and (ii) chronic condition clusters. Multimorbidity was defined as having at least any 2 of 18 selected conditions, and further grouped into levels of 2, 3, 4, or 5 or more conditions. Age-sex standardized multimorbidity prevalence was estimated using the 2009 population as the standard. Clustering was defined using the observed combinations of conditions within levels of multimorbidity.
Results
Standardized prevalence of multimorbidity increased over time (26.5%, 28.8%, and 30.0% across sequential panels), across sex, age, and area-based income. Females, older adults and those living in lower income areas exhibited higher rates in all years. However, multimorbidity increased relatively more among males, younger adults, and those with 4 or 5 or more conditions. We observed numerous and increasing diversity in disease clusters, namely at higher levels of multimorbidity.
Conclusion
Our study provides relevant and needed population-based information on the growing burden of multimorbidity, and related socio-demographic risk factors. Multimorbidity is markedly increasing among younger age cohorts. Also, there is an increasing complexity and lack of common clustering patterns at higher multimorbidity levels.
Supplementary Information
The online version contains supplementary material available at 10.17269/s41997-021-00474-y.
Keywords: Multimorbidity, Trends, Socio-demographics, Disease clusters, Administrative data, Ontario
Résumé
Objectif
Cette étude a pour but d’offrir des données populationnelles sur la multimorbidité et les tendances sur 13 années.
Méthodes
Cette étude transversale utilise des données administratives provinciales, incluant trois panels d’individus de tous âges, en 2003, 2009 et 2016, pour décrire : (i) les tendances de la multimorbidité en Ontario, et les différences entre âge, sexe et niveaux de revenus; ainsi que (ii) les combinaisons de maladies chroniques. La multimorbidité a été définie comme ayant au moins deux des 18 maladies chroniques sélectionnées, et ensuite groupée par niveau de 2, 3, 4 ou 5 maladies ou plus. Les taux de prévalence standardisés ont été estimés à partir de la population de 2009. Les combinaisons fréquentes de maladies chroniques observées par niveau de multimorbidité sont également arborées.
Résultats
La prévalence standardisée de multimorbidité a augmenté au fil des années (26,5 %, 28,8 % et 30,0 %). Elle était plus élevée chez les femmes, les personnes âgées, et celles vivant dans les endroits à faible revenus, peu importe l’année. Toutefois l’augmentation dans le temps était plus importante chez les hommes, les jeunes adultes, et pour les niveaux élevés de multimorbidité (4, 5 ou plus). Nous avons observé un nombre élevé de combinaisons de maladies, et une diversité grandissante spécialement pour les niveaux de multimorbidité élevés.
Conclusion
Cette étude fournit des données épidémiologiques probantes sur le problème grandissant de la multimorbidité, notamment au sein des jeunes cohortes, et les facteurs sociodémographiques associés. Il existe également une complexité grandissante et pas de profils communs dans les combinaisons de maladies, aux niveaux élevés de multimorbidité.
Mots-clés: Multimorbidité, tendances, facteurs sociodémographiques, combinaisons de maladies, données administratives, Ontario
Introduction
The prevalence of multimorbidity (the co-occurrence of 2 or more chronic conditions in an individual) continues to rise, reflecting ongoing population aging and trends in select negative health behaviours (Feely et al. 2017; Fortin et al. 2014; Koné Pefoyo et al. 2015; Lebenbaum et al. 2018; Mokraoui et al. 2016). Multimorbidity increases with age and is more common among women and those from lower socio-economic status (SES) subgroups (Barnett et al. 2012; Forslund et al. 2020), including within Canada (Canizares et al. 2018; Feely et al. 2017; Koné Pefoyo et al. 2015; Roberts et al. 2015). Increasing multimorbidity rates pose significant challenges to healthcare systems and to the health and well-being of affected individuals and their family caregivers (Barnett et al. 2012; Boyd and Fortin 2010; Ploeg et al. 2017; St. John et al. 2014). Early observations from the current COVID-19 pandemic have shown that pre-existing chronic health conditions, including diabetes mellitus, chronic lung disease, and cardiovascular disease (CVD), predispose affected individuals to an increased risk of severe COVID-19 symptoms and death (CDC COVID-19 Response Team 2020; Williamson et al. 2020). While these conditions are considered separately, co-occurrence of multiple conditions, i.e., multimorbidity, may lead to worse outcomes among individuals affected by the virus.
Persons with high multimorbidity often interact with multiple care providers and settings (Boyd and Fortin 2010; Wallace et al. 2015), leading to a heightened risk for fragmented and poor quality of care, high (and potentially inappropriate) medication use, and adverse health outcomes (Gruneir et al. 2016; Nguyen et al. 2019; Petrosyan et al. 2017; Ploeg et al. 2017). It is essential to inform current and future health promotion and chronic disease management (Kernick et al. 2017; Salisbury et al. 2018); thus, providing timely population-level data regarding trends in multimorbidity across age, sex, and SES subgroups is key. These data should also speak to the potential impact of changing multimorbidity patterns on patients’ clinical complexity (Kadam 2012; Koné Pefoyo et al. 2015).
The objectives of this study were therefore (i) to describe trends in multimorbidity prevalence among all Ontario residents and across age, sex, and area-based income, comparing 3 time points (2003, 2009, 2016), and (ii) to contrast chronic condition clusters by multimorbidity levels in 2003 and 2016. These findings may help identify vulnerable population subgroups needing enhanced and/or better-integrated preventive health and therapeutic care strategies and policies.
Methods
Study design and data sources
We conducted a repeated cross-sectional study using linked population-level health administrative databases in Ontario. These data included the Registered Persons Database (RPDB), Canadian Institute for Health Information’s Discharge Abstract Database (DAD), Ontario Health Insurance Plan (OHIP), Ontario Drug Benefits (ODB), Census data, and Ontario Marginalization Index data (ON-Marg). These data are linked using unique encoded identifiers and analyzed at ICES.
The study population included all persons alive in Ontario with a valid health card on April 1 in each study year 2003, 2009, or 2016 (index dates), as identified in the RPDB. We excluded persons having no contact with the healthcare system in the five years preceding each index date as many of these individuals may have migrated out of the province.
Measures
We identified the prevalence of 18 chronic conditions (at each index date), using hospital discharge (DAD), physician billings (OHIP), and prescription dispensing (ODB) health administrative databases. Included were: acute myocardial infarction (AMI), asthma, (any) cancer, cardiac arrhythmia, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), chronic coronary syndrome, dementia, diabetes, hypertension, non-psychotic mood and anxiety disorders, other mental illnesses (including schizophrenia, delusions, and other psychoses; personality disorders; and substance abuse), osteoarthritis, osteoporosis, renal failure, rheumatoid arthritis, stroke (excluding transient ischemic attack), and inflammatory bowel disease (IBD). These conditions were selected for their overall health system burden in terms of population prevalence (Koné Pefoyo et al. 2015) and economic costs (Public Health Agency of Canada 2014; Thavorn et al. 2017), or inclusion in previous multimorbidity research in Ontario (Koné Pefoyo et al. 2015; Mondor et al. 2018; Petrosyan et al. 2017; Rosella et al. 2018; Thavorn et al. 2017) and elsewhere (Goodman et al. 2013). Where applicable, we used validated administrative data algorithms to ascertain cases (specifically for AMI, asthma, CHF, COPD, dementia, diabetes, hypertension, IBD, and rheumatoid arthritis; see Table S1). All others were defined based on the presence of any one inpatient hospital diagnostic code (DAD) or 2 or more outpatient physician billing codes (OHIP) within a 2-year period using relevant ICD-9/ICD-10 codes (Table S1), consistent with the definitions for validated cohorts. Follow-up data were included up to March 2018, according to these case definition algorithms. Each condition was defined with all available health administrative data (looking back to 1998), with the exceptions of AMI (1-year lookback from index), cancers, non-psychotic mood and anxiety disorders, and other mental illnesses (2-year lookback each) due to the nature of these diseases.
Multimorbidity was defined as the co-occurrence of any 2 or more of these 18 conditions, prevalent at index, consistent with past research (Diederichs et al. 2011; Fortin et al. 2012; Koné Pefoyo et al. 2015; Mondor et al. 2018). We also further grouped multimorbidity by levels of 2, 3, 4, or 5 or more conditions. Clustering was defined using the observed combinations of conditions within individuals with 2, with 3, with 4, or with 5 or more conditions.
The RPDB was used to provide basic demographic information, including age and sex, for each index cohort. Age groups were defined as < 18, 18–44, 45–64, and 65+ years. Area-level income quintiles were derived from linked census data.
Analyses
We estimated the unadjusted prevalence of multimorbidity for each index year and by age and sex. The proportion with multimorbidity among those with each of the 18 chronic conditions was also estimated by index year. Age-sex standardized multimorbidity prevalence estimates (using the 2009 population as the standard) were derived for each index year, overall and by income quintiles. The distribution of the most common clusters of conditions (up to 10) within each multimorbidity level (i.e., among those with 2, 3, 4, or 5+ conditions) was examined for 2016 relative to 2003 and by sex and age group.
Results
Trends in prevalence, level of multimorbidity, and associated factors
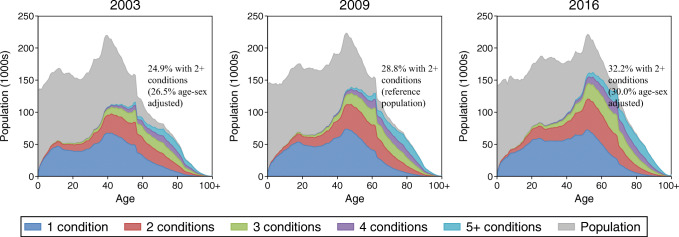
The crude prevalence of multimorbidity (2+ conditions) increased in Ontario, from 24.9% in 2003 to 28.8% in 2009 and 32.2% in 2016 (Fig. 1, Table 1). The respective age-sex standardized estimates using the 2009 population were 26.5%, 28.8%, and 30.0%. This increase in multimorbidity was evident for females and males of all ages. Across all 3 index years, multimorbidity was higher with increasing age and higher among females than among males (with the exception of the youngest age group, 0–17). However, younger males aged 18–44 and 45–64 showed a larger increase in multimorbidity from 2003 (10.8% and 34%) to 2016 (14.8% and 41.5%) than females of the same age (respectively 16.2% and 41.8% in 2003 vs. 17.9% and 46% in 2016). In 2016, 81.1% of females and 78.3% of males aged ≥ 65 years exhibited multimorbidity.
Fig. 1.
Ontario population (gray) and distribution of multimorbidity level by age and index year (2003, 2009, and 2016)
Table 1.
Unadjusted prevalence of overall (2+) and level of multimorbidity by age, sex, and index year (2003, 2009, and 2016)
| Age group | Sex | Population | Prevalence of MMB (%) | ||||
|---|---|---|---|---|---|---|---|
| 2 conditions | 3 | 4 | 5+ conditions | Overall 2 or more conditions | |||
| 2003 | |||||||
| All | All | 12,143,428 | 12.6 | 6.3 | 3.1 | 2.9 | 24.9 |
| 0–17 | Males | 1,431,701 | 4.0 | 0.5 | 0.04 | < 0.1 | 4.6 |
| Females | 1,359,631 | 2.6 | 0.3 | 0.03 | < 0.1 | 3.0 | |
| 18–44 | Males | 2,421,250 | 8.1 | 2.1 | 0.5 | 0.1 | 10.8 |
| Females | 2,417,777 | 11.6 | 3.5 | 0.9 | 0.3 | 16.2 | |
| 45–64 | Males | 1,466,970 | 18.7 | 9.1 | 3.8 | 2.4 | 34.0 |
| Females | 1,500,558 | 22.2 | 11.7 | 5.0 | 2.9 | 41.8 | |
| 65+ | Males | 667,648 | 22.1 | 19.4 | 13.5 | 16.5 | 71.6 |
| Females | 877,893 | 23.3 | 20.8 | 14.3 | 16.9 | 75.2 | |
| 2009 | |||||||
| All | All | 12,907,659 | 13.7 | 7.4 | 3.9 | 3.8 | 28.8 |
| 0–17 | Males | 1,419,031 | 4.3 | 0.5 | < 0.1 | < 0.1 | 4.9 |
| Females | 1,347,052 | 2.8 | 0.4 | < 0.1 | < 0.1 | 3.2 | |
| 18–44 | Males | 2,376,897 | 9.4 | 2.4 | 0.6 | 0.2 | 12.4 |
| Females | 2,421,432 | 12.0 | 3.6 | 0.9 | 0.3 | 16.9 | |
| 45–64 | Males | 1,766,086 | 20.4 | 10.5 | 4.6 | 2.9 | 38.5 |
| Females | 1,809,702 | 23.2 | 12.9 | 5.7 | 3.4 | 45.3 | |
| 65+ | Males | 780,257 | 21.1 | 20.4 | 15.4 | 20.2 | 77.2 |
| Females | 987,202 | 21.9 | 22.1 | 16.4 | 20.6 | 81.0 | |
| 2016 | |||||||
| All | All | 13,738,113 | 14.7 | 8.3 | 4.5 | 4.6 | 32.2 |
| 0–17 | Males | 1,392,632 | 4.4 | 0.6 | < 0.1 | < 0.1 | 5.0 |
| Females | 1,322,687 | 3.2 | 0.5 | < 0.1 | < 0.1 | 3.8 | |
| 18–44 | Males | 2,400,908 | 11.0 | 2.9 | 0.7 | 0.2 | 14.8 |
| Females | 2,466,206 | 12.7 | 3.9 | 1.0 | 0.3 | 17.9 | |
| 45–64 | Males | 1,913,804 | 21.4 | 11.5 | 5.2 | 3.4 | 41.5 |
| Females | 1,985,374 | 23.2 | 13.1 | 6.0 | 3.8 | 46.0 | |
| 65+ | Males | 1,021,885 | 20.8 | 20.6 | 15.6 | 21.4 | 78.3 |
| Females | 1,234,617 | 21.2 | 21.8 | 16.4 | 21.7 | 81.1 | |
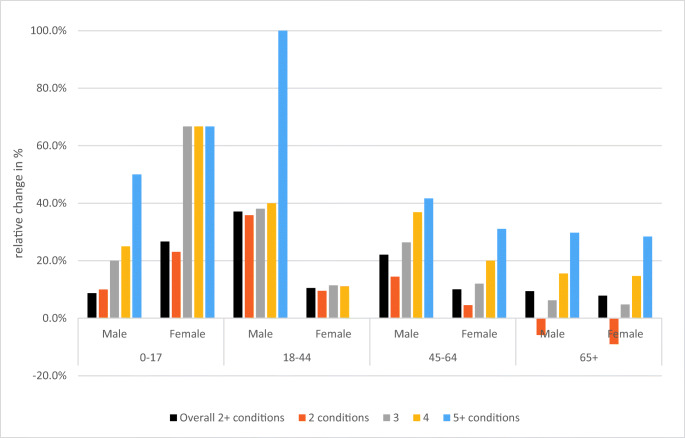
Unadjusted prevalence estimates increased for all multimorbidity levels within the total population (Table 1). However, the direction or magnitude of relative trends varied within multimorbidity levels by age and sex (Fig. 2). The largest relative increase in multimorbidity from 2003 to 2016 was among those with 5 or more conditions. For the oldest age group (65+), there was a decrease in the proportion of those having 2 conditions. The prevalence of each of the 18 chronic conditions and the prevalence of multimorbidity among individuals with each condition, by index year, are presented in Fig. S1.
Fig. 2.
Relative differences from 2003 to 2016 in multimorbidity prevalence, by age, sex, and multimorbidity level
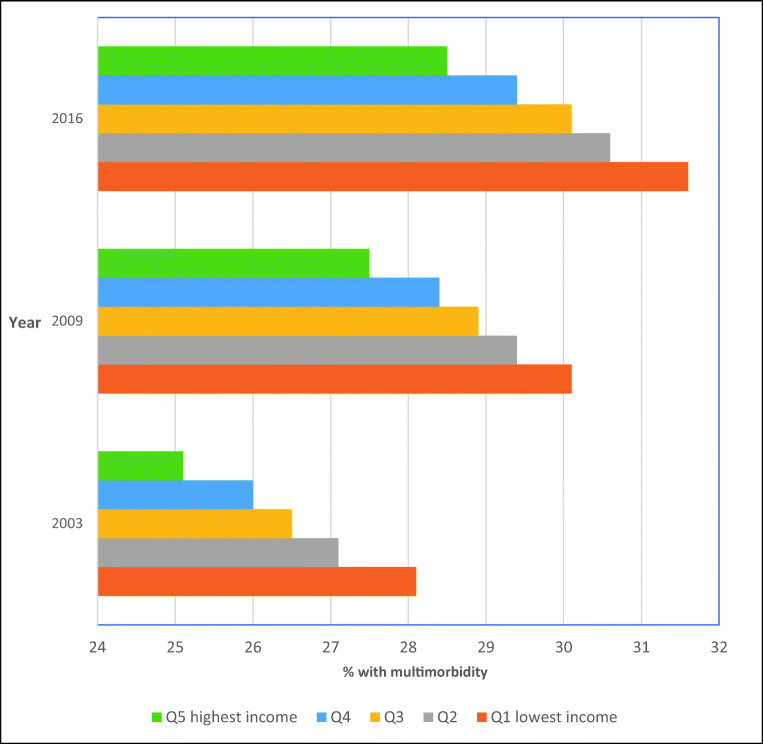
As shown in Fig. 3, across all index years, the age-sex standardized prevalence of multimorbidity was highest for those from the lowest area-based income quintile and was less prevalent as area income levels increased (e.g., 31.6% for the lowest vs. 28.5% for the highest income quintile in 2016). The relative difference in multimorbidity prevalence for those in the lowest vs. highest income quintile was generally consistent across index years.
Fig. 3.
Age-sex standardized prevalence of multimorbidity by area-level income quintiles and index year (2003, 2009, and 2016)
Complexity of multimorbidity burden
As shown in Table 2, numerous disease clusters were observed among individuals with multimorbidity, and the total number of unique clusters for those with 4 conditions (2123 clusters in 2016) and particularly those with 5 or more conditions (24,708 clusters in 2016) increased substantially from 2003 to 2016. The top 5 disease clusters, among those with 2 conditions, accounted for a large and similar proportion across years (50% in 2003 vs. 51% in 2016). At higher levels of multimorbidity, concordant with the increasing total number of clusters, none of the top 5 clusters accounted for very much of the population; also, top 10 clusters composition slightly varied over time. Examination of age- and sex-specific clusters exposed additional insights. Across all age groups, there was considerable concordance between the most common clusters for men and women except in the top 5 clusters which varied considerably between sexes across all age groups. Cancer for example appeared in more clusters for women at the highest level of multimorbidity. There were also differences in the prevalence of conditions across ages within the same degree of multimorbidity. For example, at 3 conditions, other mental health (including psychoses) was most common in ages 18–44 and 45–64 but did not appear in the top 5 clusters in the oldest age group where coronary syndromes had greater representation (Table S2).
Table 2.
Top ten clusters of conditions in 2003 and 2016, by multimorbidity level
| Co-existing chronic conditions within level of multimorbidity (MMB) | 2003 | 2016 | ||
|---|---|---|---|---|
| Rank | Prevalence of combination within MMB level | Rank | Prevalence of combination within MMB level | |
| 2 conditions | n = 1,529,784; 152 clusters | n = 2,025,120; 151 clusters | ||
| Hypertension/osteoarthritis | 1 | 17.3 | 1 | 18.6 |
| Mood disorder/osteoarthritis | 2 | 13.6 | 3 | 8.7 |
| Asthma/osteoarthritis | 3 | 9.4 | 2 | 11.8 |
| Cancer/osteoarthritis | 4 | 5.1 | 4 | 6.1 |
| Asthma/mood disorder | 5 | 4.5 | 7 | 4.7 |
| Diabetes/hypertension | 6 | 4.2 | 5 | 5.8 |
| Other mental health problem/mood disorder | 7 | 4.1 | 8 | 3.2 |
| Hypertension/mood disorder | 8 | 3.4 | - | - |
| Diabetes/osteoarthritis | 9 | 3.3 | 6 | 5.0 |
| Coronary syndrome/hypertension | 10 | 3.0 | - | - |
| Cancer/hypertension | - | - | 9 | 2.5 |
| Asthma/other mental health problem | - | - | 10 | 2.4 |
| 3 conditions | n = 768,526; 726 clusters | n = 1,138,626; 726 clusters | ||
| Diabetes/hypertension/osteoarthritis | 1 | 7.7 | 1 | 13.2 |
| Hypertension/mood disorder/osteoarthritis | 2 | 7.3 | 5 | 4.7 |
| Coronary syndrome/hypertension/osteoarthritis | 3 | 7.1 | 3 | 5.2 |
| Asthma/mood disorder/osteoarthritis | 4 | 5.4 | 6 | 4.4 |
| Cancer/hypertension/osteoarthritis | 5 | 5.1 | 2 | 6.3 |
| Other mental health problem/mood disorder/osteoarthritis | 6 | 4.0 | 8 | 2.4 |
| Asthma/hypertension/osteoarthritis | 7 | 3.9 | 4 | 5.0 |
| Cancer/mood disorder/osteoarthritis | 8 | 3.1 | 9 | 2.2 |
| Hypertension/osteoarthritis/osteoporosis | 9 | 2.4 | 7 | 3.1 |
| Coronary syndrome/diabetes/hypertension | 10 | 2.1 | - | - |
| Arrhythmia/hypertension/osteoarthritis | - | - | 10 | 2.1 |
| 4 conditions | n = 379,508; 2012 clusters | n = 622,092; 2123 clusters | ||
| Coronary syndrome/diabetes/hypertension/osteoarthritis | 1 | 5.4 | 1 | 6.9 |
| Coronary syndrome/hypertension/mood disorder/osteoarthritis | 2 | 3.1 | - | - |
| Diabetes/hypertension/mood disorder/osteoarthritis | 3 | 3.0 | 4 | 3.0 |
| Cancer/coronary syndrome/hypertension/osteoarthritis | 4 | 2.9 | 5 | 2.5 |
| Asthma/hypertension/mood disorder/osteoarthritis | 5 | 2.8 | 6 | 2.1 |
| Cancer/hypertension/mood disorder/osteoarthritis | 6 | 2.7 | 8 | 1.9 |
| Arrhythmia/coronary syndrome/hypertension/osteoarthritis | 7 | 2.7 | 7 | 2.1 |
| Cancer/diabetes/hypertension/osteoarthritis | 8 | 2.2 | 2 | 4.3 |
| Asthma/other mental health problem/mood disorder/osteoarthritis | 9 | 2.1 | - | - |
| Asthma/diabetes/hypertension/osteoarthritis | 10 | 2.0 | 3 | 4.2 |
| Diabetes/hypertension/osteoarthritis/renal disease | - | - | 9 | 1.8 |
| Asthma/cancer/hypertension/osteoarthritis | - | - | 10 | 1.7 |
| 5+ conditions | n = 346,826; 19,419 clusters | n = 638,853; 24,708 clusters | ||
| Arrhythmia/chronic heart failure/coronary syndrome/hypertension/osteoarthritis | 1 | 1.3 | 8 | 0.7 |
| Coronary syndrome/diabetes/hypertension/mood disorder/osteoarthritis | 2 | 1.2 | 6 | 0.8 |
| Chronic heart failure/coronary syndrome/diabetes/hypertension/osteoarthritis | 3 | 1.1 | 4 | 1.0 |
| Cancer/coronary syndrome/diabetes/hypertension/osteoarthritis | 4 | 1.0 | 1 | 1.5 |
| Arrhythmia/coronary syndrome/diabetes/hypertension/osteoarthritis | 5 | 0.9 | 2 | 1.2 |
| Cancer/coronary syndrome/hypertension/mood disorder/osteoarthritis | 6 | 0.8 | - | - |
| Arrhythmia/cancer/coronary syndrome/hypertension/osteoarthritis | 7 | 0.8 | 9 | 0.7 |
| Asthma/coronary syndrome/diabetes/hypertension/osteoarthritis | 8 | 0.8 | 3 | 1.2 |
| Asthma/diabetes/hypertension/mood disorder/osteoarthritis | 9 | 0.7 | 5 | 0.9 |
| Arrhythmia/coronary syndrome/hypertension/mood disorder/osteoarthritis | 10 | 0.7 | - | - |
| Coronary syndrome/diabetes/hypertension/osteoarthritis/renal disease | - | - | 7 | 0.8 |
| Asthma/cancer/diabetes/hypertension/osteoarthritis | - | - | 10 | 0.7 |
Discussion
Multimorbidity is increasing, and leading to higher complexity in all ages, sexes, and socio-economic strata
Our study found that multimorbidity is highly prevalent and continues to increase over time in all ages, sexes, and income quintiles. Individuals with multimorbidity may not always receive appropriate quality of care due to their increasing clinical complexity, care fragmentation, and inappropriate care interventions. Thus, it is crucial to provide ongoing evidence on this important issue, considering that multimorbidity is highly associated with negative health outcomes, including mortality (Gruneir et al. 2016; Lane et al. 2015; Rosella et al. 2018), and healthcare costs (Thavorn et al. 2017).
Age and sex constitute important risk factors, with multimorbidity highest in older adults and more prevalent in females than males. Age differences in multimorbidity prevalence have narrowed over time, due in large part to larger increases in prevalence over time among young adults. Multimorbidity is also concentrated among those living in lower income areas. Complexity is also an increasing issue, as we observed numerous and increasing diversity of disease clusters, as well as more individuals exhibiting higher level of multimorbidity (5 or more conditions) over time.
In relative terms, multimorbidity increased by 29% (from 24.9% to 32.2%) over 13 years for the whole population; in other words, there were 1.4 million more individuals with multimorbidity in Ontario in 2016 than there were in 2003. Similar increases have been reported in the literature (Canizares et al. 2018; Feely et al. 2017; Katikireddi et al. 2017; Mokraoui et al. 2016). Feely et al. (2017) reported a Canada-wide increase of 29% among adults over age 40, from 2001–2002 to 2011–2012. Our previous research, using 16 conditions and focusing solely on age differences, found a 40% increase in multimorbidity in the general population of all ages in Ontario, from 17.4% in 2003 to 24.3% in 2009 (Koné Pefoyo et al. 2015). The smaller increase observed in the current study can be explained by differences in the methodology, including the fact that this study examined 18 conditions rather than 16 (in a prior study which did not include other mental health or inflammatory bowel disease) and different lookback periods for case identification were used. The baseline prevalence in the first study was likely underestimated, leading to a higher estimation of the increase. Nevertheless, if the lower increase found in the current study remains constant, we may reach 50% of the population with multimorbidity in less than 20 years (i.e., around year 2035).
Previous studies have reported clusters either within specific age groups or by sex (Sinnige et al. 2013; Valenzuela et al. 2017); the current study, however, compares clusters across both sex and age groups. Though multimorbidity increases with age and has often been considered an issue for older adults, posing considerable clinical and self-care challenges for them, this study shows that it is becoming a greater concern among younger adults in Ontario. Canizares et al. reported that members of younger cohorts (e.g., born 1965–1974) were more likely to report multimorbidity than their predecessors (e.g., born 1925–1934), based on the Canadian National Population Health Survey cycles from 1994 to 2011 (Canizares et al. 2018). Furthermore, in their 20-year cohort study in west Scotland, Katikireddi et al. showed a cohort effect, with higher rates in younger cohorts and greater sex differences over time (Katikireddi et al. 2017).
We found that sex differences in multimorbidity became smaller over time, because adult males appear to be catching up to females. In particular, males aged 18–64 years had larger increases than females in the same age bracket. Also, females age < 18 years initially had a lower rate than males of the same age, but over time, this rate is growing closer to that for males. Regarding socio-economic factors, we found a clear gradient in the impact of income on multimorbidity. Many studies using different populations have found comparable results, with the highest multimorbidity prevalence or number of conditions among lower income levels (Canizares et al. 2018; Katikireddi et al. 2017; Lane et al. 2015; Mondor et al. 2018; Roberts et al. 2015). We observed that differences between the lowest and highest quintiles remain similar over time, indicating a comparable increase within each area-based income level between 2003 and 2016. These findings and previous research confirm the robustness of the associations with income across populations, despite limitations of the area-level measure.
Complexity in disease combinations remains a challenge in all population groups
As previously reported (Koné Pefoyo et al. 2015), patients with multimorbidity in Ontario present a multiplicity of disease clusters. Even though the top clusters have remained similar over time, with a few exceptions, patterns in clustering were different by age groups and sex. Using a population study in the Stockholm region and 40 chronic health conditions, Forslund et al. reported that the largest non-random cluster predominantly included individuals with anxiety, depression, alcohol problems and irritable bowel syndrome (IBS) (Forslund et al. 2020). In their cluster analysis, Islam et al. found that hypertension and arthritis were the most dominant conditions in disease dyads or triads among Australians (Islam et al. 2014). Arthritis and hypertension are often reported to co-occur with depression/mood disorder, asthma, diabetes, or cancer (Lebenbaum et al. 2018; Radner 2016; Roberts et al. 2015). In the present study, arthritis, hypertension, or mood disorders are also present in almost all top 10 clusters within multimorbidity levels due in a large part to the absolute prevalence of these conditions. However, clusters identified would expectedly differ across studies according to the list of conditions used.
The growing burden of multimorbidity has substantial impacts on the health system, as it leads to high health services use and mortality (Gruneir et al. 2016; Gruneir et al. 2020; Lane et al. 2015; Petrosyan et al. 2017; Rosella et al. 2018). For example, the COVID-19 pandemic has demonstrated how individuals with multiple conditions are vulnerable and require special attention. While studies have linked specific pre-existing chronic diseases to COVID-19 infection and outcomes (CDC COVID-19 Response Team 2020; Williamson et al. 2020), multimorbidity may further weaken the immune system and lead to greater susceptibility to infections like COVID-19. Our research highlights the increasing burden and complexity of multimorbidity, suggesting that it is crucial to monitor its trends and impacts on care management. Especially, healthcare professionals need to take special precautions to protect multimorbid high-risk groups during this ongoing pandemic and for future potential health challenges. The situation regarding multimorbidity needs to be continuously monitored and adequately managed, as the prevalence might increase even more and grow faster.
Strengths and limitations
This study provides both a needed update on the burden and trends of multimorbidity in Ontario, and more data on the differences between males and females and socio-economic strata, using population-based data. Study strengths include use of health administrative data, which enabled both robust and population-based evaluation of multimorbidity. Our findings are comparable to other jurisdictions that have health administrative data with population coverage. The health administrative databases (HAD) used have been shown to adequately and rigorously measure chronic disease prevalence and multimorbidity in the population (Muggah et al. 2013), in contrast to self-reported data which is subject to recall bias but is commonly used in multimorbidity research (Mokraoui et al. 2016; Quiñones et al. 2019). Gruneir et al. compared health administrative and survey data, and found that agreement in count and type of conditions was low and decreased with increasing number of conditions; namely, HAD identified more conditions on average and higher prevalence of multimorbidity (Gruneir et al. 2020).
However, this study has limitations requiring comment. Conditions selected for this study are mostly relevant to the general population; thus, a selection of conditions specific to persons aged < 18 years might reveal different and/or more pronounced complexity in this age group. Not all but most chronic conditions were based on validated algorithms for administrative data. This may result in an over- or under-estimate of disease prevalence, but should have minimal impact on multimorbidity, as defined by having two or more conditions (with the exceptions of those exclusively having conditions not identified with validated algorithms). Also, because we are counting conditions and looking at changes over time with a cross-sectional perspective instead of a longitudinal cohort, changes over time may be influenced by changes in the characteristics of the study population, rather than the occurrence of disease itself. However, we are using the whole provincial population, and the design and measurement are consistent across all 3 years; thus, besides the standardization of the estimates, demographic characteristics in the population are comparable between the repeated cross-sectional panels, to allow for a robust estimation of the changes over time.
Finally, income quintiles were measured at the area level and should be interpreted as such to minimize ecological bias. In fact, in their analysis, Buajitti and colleagues found low to moderate agreement between area-based and individual measures of income, leading to larger disparities with individual measures than area-based income (Buajitti et al. 2020). Thus, differences in multimorbidity observed in this study may be more pronounced if we used individual-level income (Rosella et al. 2018). Thus, in addition to using the most adequate measures of income, it will also be important to analyze trends in specific diseases and disease clusters by income level in future work. There was a very small proportion of missing data related to income quintiles (0.4%), which was treated as a separate group, and also showed increasing prevalence of multimorbidity over time. Finally, due to the repeated cross-sectional design, we are unable to infer causality. Nevertheless, the study still provides a basic assessment of differences at the population level, across key characteristics and income, and evaluates changes within these risk factors.
Implications
Multimorbidity is often associated with older age; however, the increase in multimorbidity prevalence and burden among individuals aged 18–64 years constitutes a significant challenge for the health system. The trends observed in those < 18 are also worrying. These age groups represent a large percentage of the population, making the impact even bigger and much longer as complexity starts earlier. Thus, health promotion and chronic disease prevention interventions should start early in the lifespan. Multiple behavioural risk factors, including physical inactivity, smoking, alcohol consumption, and high BMI, are common among children and adolescents and need to be addressed in order to mitigate their impact on the incidence of chronic conditions in older ages (Alamian and Paradis 2009). Also, because multimorbidity is associated with income and other socio-economic factors, efforts must aim to reduce inequities in the whole health system spectrum, from the prevention of preventable diseases to care management in various population groups (Rosella and Kornas 2018).
Though multimorbidity is more common among females overall, our data highlight larger increases among males in recent years. The study illustrates the need to target males with interventions to improve health in order to slow the progression of multimorbidity, while continuing to support interventions for females’ health.
Additionally, the research highlights the increasing complexity of multimorbid patients, particularly in older age groups. Despite the diversity (thousands of observed clusters), conditions are mostly treated in silos, leading to disconnected and often uncoordinated care. This may further complicate care management (Wallace et al. 2015) and create challenges to patients’ self-management and poor compliance with the recommendations of multiple providers (Duguay et al. 2014; Radner 2016). In fact, co-occurring conditions add to patient complexity and can limit the success of clinical management including treatments, thereby increasing the likelihood of negative outcomes (Petrosyan et al. 2017; Radner 2016). While it is challenging to design individual care management approaches for all of the possible clusters of diseases, our results show that it is important for the health system and care providers to recognize that diseases occur most often in combination rather than in isolation, and certain conditions tend to cluster with each other. As reported by Déruaz-Luyet et al. (2017), the way chronic conditions tend to cluster (e.g., cardiovascular disease risk factors and cardiovascular conditions vs. general pain, musculoskeletal and psychological conditions) could provide indications for care management and prevention. We also found that a few chronic conditions commonly occur in most disease clusters. Such common conditions may differ by age and sex groups. With the growing awareness about the importance of disease clustering, it remains key to consider individuals’ characteristics and specific needs when assessing or treating patients.
Supplementary information
(DOCX 37.9 kb)
Authors’ contributions
APK, LM, CM, and WPW conceptualized the study. LM analyzed and interpreted the data and contributed to the manuscript writing. APK interpreted the findings and wrote the preliminary manuscript draft. CM interpreted the findings and was a major contributor in writing the manuscript. USK performed the literature review and assisted with the analysis and data presentation. WPW and LR contributed to the interpretation of the findings and manuscript writing. All authors read and approved the final manuscript.
Funding
This study received funding from the Ontario Ministry of Health (MOH) to the Health System Performance Network (HSPN). This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Data availability
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
Consent to participate
Not applicable (authorized access to health administrative data).
Consent for publication
Not applicable.
Disclaimer
Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors, and not necessarily those of CIHI. No endorsement by ICES or MOH is intended or should be inferred.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/24/2021
This article was updated to include a revised version of Supplemental Table 2.
Change history
5/5/2021
A Correction to this paper has been published: 10.17269/s41997-021-00533-4
References
- Alamian A, Paradis G. Clustering of chronic disease behavioral risk factors in Canadian children and adolescents. Preventive Medicine. 2009;48(5):493–499. doi: 10.1016/j.ypmed.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Reviews. 2010;32(2):451–474. doi: 10.1007/BF03391611. [DOI] [Google Scholar]
- Buajitti E, Chiodo S, Rosella LC. Agreement between area- and individual-level income measures in a population-based cohort: implications for population health research. SSM - Population Health. 2020;10:100553. doi: 10.1016/j.ssmph.2020.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canizares M, Hogg-Johnson S, Gignac MAM, Glazier RH, Badley EM. Increasing trajectories of multimorbidity over time: birth cohort differences and the role of changes in obesity and income. The Journals of Gerontology: Series B. 2018;73(7):1303–1314. doi: 10.1093/geronb/gbx004. [DOI] [PubMed] [Google Scholar]
- CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12–March 28, 2020. MMWR. Morbidity and Mortality Weekly Report. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déruaz-Luyet, A., N'Goran, A. A., Senn, N., Bodenmann, P., Pasquier, J., Widmer, D., Tandjung, R., Rosemann, T., Frey, P., Streit, S., Zeller, A., Haller, D. M., Excoffier, S., Burnand, B., & Herzig, L. (2017). Multimorbidity and patterns of chronic conditions in a primary care population in Switzerland: A cross-sectional study. BMJ Open, 7(6), e013664. 10.1136/bmjopen-2016-013664. [DOI] [PMC free article] [PubMed]
- Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A(3):301–311. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- Duguay C, Gallagher F, Fortin M. The experience of adults with multimorbidity: a qualitative study. Journal Comorbidity. 2014;4:11–21. doi: 10.15256/joc.2014.4.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feely A, Lix LM, Reimer K. Estimating multimorbidity prevalence with the Canadian Chronic Disease Surveillance System. Health Promotion and Chronic Disease Prevention in Canada. 2017;37(7):215–222. doi: 10.24095/hpcdp.37.7.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund, T., Carlsson, A., Ljunggren, G., Ärnlöv, J., & Wachtler, C. (2020). Patterns of multimorbidity and pharmacotherapy: a total population cross-sectional study. Family Practice. 10.1093/fampra/cmaa056. [DOI] [PMC free article] [PubMed]
- Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Annals of Family Medicine. 2012;10(2):142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Haggerty J, Almirall J, Bouhali T, Sasseville M, Lemieux M. Lifestyle factors and multimorbidity: a cross sectional study. BMC Public Health. 2014;14:686. doi: 10.1186/1471-2458-14-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R. A., Posner, S. F., Huang, E. S., Parekh, A. K., & Koh, H. K. (2013). Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Preventing Chronic Disease, 10 %U. http://www.cdc.gov/pcd/issues/2013/12_0239.htm. [DOI] [PMC free article] [PubMed]
- Gruneir, A., Bronskill, S. E., Maxwell, C. J., Bai, Y. Q., Kone, A. J., Thavorn, K., Petrosyan, Y., Calzavara, A., & Wodchis, W. P. (2016). The association between multimorbidity and hospitalization is modified by individual demographics and physician continuity of care: a retrospective cohort study. BMC Health Services Research, 16, 154 (1 %U http://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-016-1415-5). [DOI] [PMC free article] [PubMed]
- Gruneir A, Griffith LE, Fisher K, Perez R, Favotto L, Patterson C, Markle-Reid M, Ploeg J, Upshur R. Measuring multimorbidity series. An overlooked complexity - comparison of self-report vs. administrative data in community-living adults: paper 3. Agreement across data sources and implications for estimating associations with health service use. Journal of Clinical Epidemiology. 2020;124:173–182. doi: 10.1016/j.jclinepi.2020.04.018. [DOI] [PubMed] [Google Scholar]
- Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, McRae IS. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS One. 2014;9(1):e83783. doi: 10.1371/journal.pone.0083783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam, U. (2012). Redesigning the general practice consultation to improve care for patients with multimorbidity. BMJ, 345(sep17 1), e6202–e6202. http://www.bmj.com/cgi/doi/6210.1136/bmj.e6202. [DOI] [PubMed]
- Katikireddi SV, Skivington K, Leyland AH, Hunt K, Mercer SW. The contribution of risk factors to socioeconomic inequalities in multimorbidity across the lifecourse: a longitudinal analysis of the Twenty-07 cohort. BMC Medicine. 2017;15:152. doi: 10.1186/s12916-017-0913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernick D, Chew-Graham CA, O’Flynn N. Clinical assessment and management of multimorbidity: NICE guideline. The British Journal of General Practice. 2017;67(658):235–236. doi: 10.3399/bjgp17X690857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koné Pefoyo AJ, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, Maxwell CJ, Bai Y, Wodchis WP. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi: 10.1186/s12889-015-1733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NE, Maxwell CJ, Gruneir A, Bronskill SE, Wodchis WP. Absence of a socioeconomic gradient in older adults’ survival with multiple chronic conditions. EBioMedicine. 2015;2(12):2094–2100. doi: 10.1016/j.ebiom.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebenbaum M, Zaric GS, Thind A, Sarma S. Trends in obesity and multimorbidity in Canada. Preventive Medicine. 2018;116:173–179. doi: 10.1016/j.ypmed.2018.08.025. [DOI] [PubMed] [Google Scholar]
- Mokraoui N-M, Haggerty J, Almirall J, Fortin M. Prevalence of self-reported multimorbidity in the general population and in primary care practices: a cross-sectional study. BMC Research Notes. 2016;9:314. doi: 10.1186/s13104-016-2121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondor L, Cohen D, Khan AI, Wodchis WP. Income inequalities in multimorbidity prevalence in Ontario, Canada: a decomposition analysis of linked survey and health administrative data. International Journal for Equity in Health. 2018;17:90. doi: 10.1186/s12939-018-0800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggah E, Graves E, Bennett C, Manuel DG. Ascertainment of chronic diseases using population health data: a comparison of health administrative data and patient self-report. BMC Public Health. 2013;13(1):16. doi: 10.1186/1471-2458-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TN, Ngangue P, Haggerty J, Bouhali T, Fortin M. Multimorbidity, polypharmacy and primary prevention in community-dwelling adults in Quebec: a cross-sectional study. Family Practice. 2019;36(6):706–712. doi: 10.1093/fampra/cmz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosyan Y, Bai YQ, Koné Pefoyo AJ, Gruneir A, Thavorn K, Maxwell CJ, Bronskill SE, Wodchis WP. The relationship between diabetes care quality and diabetes-related hospitalizations and the modifying role of comorbidity. Canadian Journal of Diabetes. 2017;41(1):17–25. doi: 10.1016/j.jcjd.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Ploeg J, Matthew-Maich N, Fraser K, Dufour S, McAiney C, Kaasalainen S, Markle-Reid M, Upshur R, Cleghorn L, Emili A. Managing multiple chronic conditions in the community: a Canadian qualitative study of the experiences of older adults, family caregivers and healthcare providers. BMC Geriatrics. 2017;17:90. doi: 10.1186/s12877-017-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada. (2014). Economic burden of illness in Canada, 2005–2008. %@ 978-1-100-22519-7.
- Quiñones AR, Botoseneanu A, Markwardt S, Nagel CL, Newsom JT, Dorr DA, Allore HG. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS One. 2019;14(6):e0218462. doi: 10.1371/journal.pone.0218462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner H. Multimorbidity in rheumatic conditions. Wiener Klinische Wochenschrift. 2016;128(21–22):786–790. doi: 10.1007/s00508-016-1090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KC, Rao DP, Bennett TL, Loukine L, Jayaraman GC. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promotion and Chronic Disease Prevention in Canada. 2015;35(6):87–94. doi: 10.24095/hpcdp.35.6.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosella L, Kornas K. Putting a population health lens to multimorbidity in Ontario. Healthcare Quarterly. 2018;21(3):8–11. doi: 10.12927/hcq.2018.25709. [DOI] [PubMed] [Google Scholar]
- Rosella L, Kornas K, Huang A, Bornbaum C, Henry D, Wodchis WP. Accumulation of chronic conditions at the time of death increased in Ontario from 1994 to 2013. Health Aff (Millwood) 2018;37(3):464–472. doi: 10.1377/hlthaff.2017.1150. [DOI] [PubMed] [Google Scholar]
- Salisbury C, Man M-S, Bower P, Guthrie B, Chaplin K, Gaunt DM, Brookes S, Fitzpatrick B, Gardner C, Hollinghurst S, Lee V, McLeod J, Mann C, Moffat KR, Mercer SW. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet. 2018;392(10141):41–50. doi: 10.1016/S0140-6736(18)31308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnige J, Braspenning J, Schellevis F, Stirbu-Wagner I, Westert G, Korevaar J. The prevalence of disease clusters in older adults with multiple chronic diseases – a systematic literature review. PLoS One. 2013;8(11):e79641. doi: 10.1371/journal.pone.0079641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John, P. D., Tyas, S. L., Menec, V., & Tate, R. (2014). Multimorbidity, disability, and mortality in community-dwelling older adults. Can Family Physician, 60(5), e272–e280. [PMC free article] [PubMed]
- Thavorn K, Maxwell CJ, Gruneir A, Bronskill SE, Bai Y, Koné Pefoyo AJ, Petrosyan Y, Wodchis WP. Effect of socio-demographic factors on the association between multimorbidity and healthcare costs: a population-based, retrospective cohort study. BMJ Open. 2017;7(10):e017264. doi: 10.1136/bmjopen-2017-017264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JF, Monterola C, Tong VJC, Ng TP, Larbi A. Health and disease phenotyping in old age using a cluster network analysis. Scientific Reports. 2017;7(1):15608. doi: 10.1038/s41598-017-15753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176. doi: 10.1136/bmj.h176. [DOI] [PubMed] [Google Scholar]
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 37.9 kb)
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS.