Abstract
Biologic scaffolds composed of extracellular matrix (ECM) are frequently used for clinical purposes of tissue regeneration. Different methods have been developed for this purpose. All methods of decellularization including chemical and physical approaches leave some damage on the ECM; however, the effects of these methods are different which make some of these procedures more proper to maintain ECM structure than other methods. This review is aimed to introduce and compare new physical methods for the decellularization of different tissues and organs in tissue engineering. All recent reports and research that have used at least one physical method in the procedure of decellularization, were included and evaluated in this paper. The advantages and drawbacks of each method were examined and compared considering the effectiveness. This review tried to highlight the prospective potentials and benefits of applying physical methods for decellularization protocols in tissue engineering instead of the current chemical methods. These chemical methods are harsh in nature and were shown to be destructive and harmful to essential substances of ECM and scaffold structure. Therefore, using physical methods as a partial or even a whole protocol could save time, costs, and quality of the final acellular tissue in complicated decellularization procedures. Moreover, regarding the control factor that could be achieved easily with physical methods, optimization of different decellularization protocols would be quite satisfactory. Combined methods take advantage of both chemical and physical approaches.
Keywords: Bioscaffold, decellularization, extracellular matrix, physical methods, tissue engineering
Introduction
Biological scaffolds obtained from decellularized tissues and organs have been used successfully in tissue engineering. Natural scaffolds derived from decellularized tissues can be a good basis for progress in reconstructive medicine while preserving the major constituents of the ECM. Removing the limitations and risks of transplantation of vital organs and regeneration of defective or damaged organs are essential goals of reconstructive medicine.[1] Bioscaffolds provide structural support for cell attachment and a suitable environment (with sufficient porosity) for cell growth, proliferation, and ECM secretion.[2] Bioscaffolds are superior to synthetic and polymeric scaffolds, due to the retained components of a native ECM.
Tissue decellularization is a promising method for the preparation of bio-scaffolds for regenerative medicine. Removing cellular components from tissue or organs produces an ECM consisting of active structural proteins that can be used in tissue engineering. The most effective method of tissue and organ decellularization depends on many factors, such as cell (tissue) type, cell density, tissue's thickness, and lipid content.
Due to its important effects on cell migration and proliferation, the preservation of the basic structure and properties of the ECM is vital during the decellularization process.[3] These ECM components, such as collagen, elastin, and proteoglycans prepare the scaffold for tolerant bending and load-bearing, respectively. In addition, decellularized scaffolds also preserve most of the biologically active molecules such as fibronectin, laminin, and glycoprotein that act as growth factor signals.[4]
It should be noted that any decellularization agent and method partially disrupt the composition of the ECM and cause damage to its structure. The goal of optimal decellularization is to minimize these adverse effects.[4,5] A decellularization protocol mainly begins by tearing the cell membrane using physical methods or ionic solutions, then extruding cellular components from the ECM with enzymatic agents, and removing the cytoplasm and cellular components by dissolving in the detergent to remove the remaining waste. To enhance the decellularization effect, it can be immersed or agitated in different agents. Finally, all chemical and toxic agents need to be rinsed off, and the tissue would be sterilized for preservation or subsequent actions such as cell culturing in tissue engineering.[6]
Applied Methods in Tissue Decellularization
Decellularization methods are a combination of physical, chemical, and enzymatic methods in which factors such as detergents, enzymes, and temperatures are used to break down and disrupt the cells. These methods effectively reduce immune responses in the host tissue and create the spaces in which host cells can penetrate and proliferate. Each decellularization method may comprise one or more decellularization agents applied to the tissue. The order and timing of these methods constitute a decellularization protocol. Different protocols are tested depending on the type of tissue by modifying application methods to obtain the desired decellularized scaffold.[7] In this review, different physical methods for tissue decellularization are discussed and the effectiveness of all methods is reported.
Thermal shock
Thermal shock (freeze-thaw cycle) effectively destroys tissue and organ cells; however, the remaining membrane and cellular content would be eliminated by subsequent complementary processes. Frozen water crystals occupy the volume inside the cell and cause the membrane to burst. The freeze-thaw cycle causes a small degradation in the structure of the ECM, due to the geometric shape of the crystals that may damage the scaffold, with little effect on the mechanical properties of ECM.[8] Azuma et al. used a rapid freeze-thaw method to remove cells from tendon fragments for tissue engineering studies.[9] Freezing temperatures can be arbitrary as low as −80°C and thawing temperatures as high as 37°C.[10] The numbers of heat shock cycles in the reports have been variable and arbitrary; for example, two studies have used three cycles to decellularize fibroblast cell sheets[11] and one cycle for lumbar vertebrae cells.[12] Although the mechanical strength and the percentage of collagen and elastin were maintained to a high degree, 88% of the DNA content remained in the fibroblast cells, which may trigger an acute immune response. This result indicates that the heat shock cycle alone is not capable of removing sensitive cellular components.[10] Burk et al. conducted a study to investigate the effect of heat shocks on the decellularization of large tendons along with Triton X-100 and sodium dodecyl sulfate (SDS) as detergents and found that freezing-thaw cycles resulted in a significant reduction (20%) in DNA and nucleic acid content.[13] Roth et al., in a new study to automate the cell freeze-thaw protocol with rapid-thaw cycles, used a nitrogen-controlled liquid-freezer at a controlled temperature to freeze superficial flexor tendons. The decellularization protocol was completed with a nonionic detergent. According to the results, no differences were observed in the efficiency of the methods, and 2% nucleic acid and 13% DNA remained in the decellularized cell, which was higher than the previous Burke's protocol.[14] According to a study by Poornejad et al., heat shock did not affect mechanical properties such as the elastic modulus of the decellularized kidneys without the addition of the cryoprotectants, which prevents the formation of water crystals in the tissue and the destruction of its structure and properties. This observation indicates that, unlike the primary method where the presence of ice crystals destroys the scaffold, thermal cycles solely do not play a role in the destruction of structure and properties of the decellularized scaffold as well as the ECM.[15] In another study, Nonaka et al. to investigate the effects of rapid freeze-thaw heat shock decellularization on the mechanical properties of decellularized lungs under a standard physiological respiratory regimen, decellularized 15 lung specimens of mice by the regular method, and three decellularized specimens were subjected to three additional fast freeze-thaw cycles. Comparison of the results showed that changes in the mechanical properties of the lung tissue after exposure to the respiratory cycle were negligible. In addition, tissue integrity was maintained, and no leakage was reported.[16] In Zhao et al.'s study they used 3–5 freeze-thaw cycles from 37°C to −80°C accompanied by chemical and enzymatic methods to decellularize human adipose tissue. It is stated that the components of cells and lipids were desirably removed, whereas collagens and other ingredients of human adipose tissue were retained.[17] Finally, concluded that thermal shock is a useful method for decellularization which destroys cells and keeps the structural properties for most tissues almost intact.
Mechanical loading and hydrostatic pressure
Superficial cells of a tissue or organ can be effectively eliminated by physical scraping with a sharp tool or abrasive accompanied by enzymes or salt solution. Physical removal of the extra layers initially helps to make the decellularization regimen more efficient. However, the amount of force required must be precise because the underlying structure and membrane attachment are vulnerable to any kind of direct mechanical stress.[8,18]
In the hydrostatic pressure method, water is sprayed with pressure on the target tissue. This method takes less time to apply and works more effectively than detergents or enzymes. However, the formation of ice crystals caused by the presence of water may damage the ECM structure. Increasing the temperature during the decellularization process suppresses the creation of these crystals, but also increases the entropy and thereby leads to the ECM vulnerability.[4,8] In a study by Hashimoto et al. the porcine retinal specimen was decellularized entirely using hydrostatic pressure of 980 MPa for 10 min.[19] A similar study by this group was performed to decellularize the aortic artery from a porcine sample. A 4-week observation, after animal transplantation, confirmed that the decellularized vessel withstands blood pressure and that no blood clot was present in its pathway.[20] In both studies, the physical method alone was not able to remove DNA residues from the tissue, and therefore a chemical agent was used to destroy and remove the remains. In the temperature of 10°C, the structure and flexibility of the scaffolds due to the content of these proteins were maintained better compared to the 30°C condition.[10] comparing the SDS method and hydrostatic pressure in the rat uterine decellularization, they showed that both methods were successful in decellularization, but the DNA content was significantly lower in the hydrostatic pressure method, and the collagen content was well preserved.[21] Kim et al. used high hydrostatic pressure to fabricate a decellularized uterine matrix. Rat specimens were applied to high hydrostatic pressure and followed by a washing process. The chamber where specimens were placed was pressurized up to 50 MPa or 980 MPa at 65.3 MPa/min. As the pressure was kept for 10 min, it decreased to atmospheric pressure at 65.3 MPa/min at 30°C. The washing buffer contained 0.2 mg/ml deoxyribonuclease I (DNase), 0.9% NaCl, 0.05 M magnesium chloride hexahydrate, and 1% penicillin and streptomycin. The washing process was performed on a shaker at 4°C for 7 days. The results represented that 7 days of washing treatment against the tissue pressurized by 50 MPa failed to remove the cells inside the tissue as well as their actin cytoskeleton. The present findings of this study indicated that decellularization under 980 MPa pressure depolymerized the actin cytoskeletons in the native rat uterine tissue. It is also stated that pressure altered the nucleus morphology after applying the high hydrostatic pressure to the uterine tissue.[22] Watanabe et al. investigated the effects of high hydrostatic pressure on the decellularization of the porcine meniscus. In their study, the middle portion of the meniscus was packed in a plastic bag filled with saline and sealed to prevent implosion and leakage during the decellularization process. The pressure of the pack was then increased to 1000 MPa at 30°C for 10 min. After that the meniscus was washed by continuous shaking at 0.5 rpm in saline containing 0.4 U/ml recombinant DNase solution, MgCl2 (50 mM), and antibiotics for 3 or 7 days at 37°C. The histological and biochemical findings showed that high hydrostatic pressure effectively decellularized the meniscus. In addition, it is stated that some cells were observed at 14 days, and more numbers of cells were observed at 28 days after recellularization.[23] Hydrostatic pressure requires relatively little time and results in a good percentage of tissue decellularization while preserving ECM structure and preserving proper recellularization rate.
Electroporation
Nonthermal irreversible electroporation has been investigated as a method of tissue decellularization. In this method, microsecond electrical pulses are applied throughout a tissue, causing micropores in the cell membrane. These pores can lead to loss of cell homeostasis and eventually cell death. In irreversible electroporation, depletion is selective, and the heat control produced during the process preserves the ECM and adjacent tissue. One of the limitations of this method is the relatively small electrodes that limit the size of the tissue for decellularization. However, more importantly, decellularization must be carried out in vivo, in order to prevent the inflammatory response of the immune system.[4] Phillips et al. were the first group to use this method to decellularize carotid arteries of rat specimens in vivo. Their results showed that the cell components were gradually removed from the tissue over a 3-day period.[24]
Sano et al. designed a set-up to decellularize the liver of porcine samples. Intense but short electrical pulses were applied to the tissue while vascular perfusion was conducted with a low-temperature chemical agent. The 99 distinct pulses with the specified amplitude and frequency were able to destroy the cells' membrane within 24 h. Continuous mechanical perfusion contributed to the removal of cell debris that could not be removed from tissue in the initial electroporation study.[25] Zager et al. optimized the method of irreversible electroporation for myocardial muscle tissue engineering. Seven different protocols with different frequencies, wavelengths and iteration were compared to evaluate muscle decellularization. According to the results, the lower the frequency of the pulses, the higher the functional damage to the muscle. Compared to myocardial infarction, electroporation damage was similar in performance, but the ECM remained intact. One of the limitations of this method in cardiac tissue engineering is its adverse effects on cardiac muscle function due to the external electric field.[26] Hence, electroporation has a classified and graded effect on the tissue. The extent of decellularization could be controlled by changing pulse length, frequency, and iteration numbers, as well as electric field density.
Ultrasonic waves
Ultrasonic waves, often performed at frequencies above 20 kHz, are used for cell separation in the bath containing tissue or organ. High-power waves are capable of disrupting intermolecular bonds, disrupting the cell membrane, and removing its internal components. The lower the frequency, the higher the damage of the waves. The practice of applying ultrasound for various applications is called sonication. It is crucial to control the cavitation during the process. The physical phenomenon of cavitation is unavoidable due to the intensity of the fluid pressure changes caused by the waves, but uncontrolled cavitation can severely damage the structure and mechanical properties of the tissue. The intensity of cavitation varies with temperature, viscosity, and dissolved gas in the fluid.[27] Azhim et al. developed a new sonication system to decellularize tissues in the short term. Aortic specimens were decellularized in this system with SDS and controlled amounts of dissolved oxygen gas in it. According to the results, sonication alone was able to achieve desirable decellularization.[27]
Oliveira et al. compared several common methods for small intestine decellularization for use in retinal tissue engineering. In the physical protocol, the specimens were exposed to sonication or ultraviolet irradiation for 5, 10, and 15 min at a distance of 15 cm after removal from the phosphate-buffered saline (PBS). Histological and histochemical analysis showed that collagen fibers were maintained by decellularization with Triton X-100 and sodium chloride and sonication, whereas type III collagen fibers remained intact only by UV irradiation. The results also showed that the sonication method alone did not show enough efficiency, primarily due to the destructive effects of ultrasound radiation on the orientation of fibers in the thin retinal tissue.[28] In Forouzesh et al.'s study, direct and indirect ultrasonic waves were accompanied by SDS with 0.1% and 1% (w/v) concentrations as chemical agents to decellularize cartilage tissue. The decellularization process was investigated by nucleus staining with hematoxylin and eosin (H and E), and by glycosaminoglycans (GAGs) and collagen staining. Results of this study showed that H and E staining indicated that 1% (w/v) SDS, in addition to ultrasonic bath for 5 h, significantly decreased the cell nucleus remnant to the lacuna ratio by 66%. It is declared that ultrasonic bath helps to a better infiltration of decellularization agents, moreover, it is mentioned that the process time has decreased due to this method and no significant defect has been seen on the structure of the tissue.[29]
Yusof et al. used a closed sonication system to decellularize meniscus to obtain scaffolds for orthopedics tissue engineering applications. The decellularization was accomplished using closed sonication treatment for 10 h, and a 5 days washing period. The frequency and temperature of 0.1% SDS solution was set at 40 kHz and 36°C ± 1°C, respectively. In order to assess the decellularization efficiency, a simple immersion treatment was performed. The results showed that there was a 92% decrease of residual DNA content in sonicated scaffolds compared to 68% in immersed scaffolds. Decellularization using immersion showed to be insufficient to achieve whole cell removal in comparison to closed sonication treatment.[30] In another study by Ahim et al. aortic scaffolds were obtained by using the same method. Aorta tissues were placed 10 mm from the ultrasonic transducer and sonicated with 170 kHz frequency in 0.1% and 2% SDS at constant temperature 36°C for 10 h. The tissue was washed in PBS for 5 days to remove the residual SDS after decellularization. The histological analysis through (H and E) staining indicated that the structure of sonicated aortic scaffolds was maintained on the microscopic scale with the intact ECM fibers without the presence of any nucleus. Because the elastic fibers were preserved, decreasing of residual force is most likely due to the removal of cells, resulting in residual force to be released.[31]
Pressure gradient
Induction of a pressure gradient during tissue decellularization can help the enzyme-mediated decellularization method. In the case of hollow tissues such as the vein, a decellularization agent can better penetrate the vessel through a gradient of pressure, as could be used for thicker tissues such as the tendon.[4,8] Sierad et al. developed a new perfusion system that operates with a differential pressure gradient for the removal of thick aortic tissue by preserving its weak valves. The sample was subjected to various chemical protocols with detergents and enzymes in the apparatus. By combining the physical and chemical methods, the anisotropic mechanical properties were well preserved, the thick aortic wall was optimally decellularized, but there was no damage to the valves' wall structure. Although tissue stiffness was increased due to the loss of a large percentage of GAGs during this process, other components of the ECM were highly retained.[32] Montoya and McFetridge developed a perfusion system that worked with fluid displacement to decellularize embryonic veins and similar tissues. The flow pressure was set at three pressures of 5, 50, and 150 mmHg. Phospholipids were eliminated at a higher pressure of 150 mmHg, whereas a pressure of 50 mmHg was more suitable for protein elimination. Fluid outflow occurs due to pressure differences along the transverse wall of the lumen, which along with the dead cells and their remnants, are removed from the tissue by exposure to the chemical agent. The removal of soluble residues in the chemical agent will be more effective by a continuous fluid exchange in the external conduit. The device used a peristaltic pump for constant-rate fluid perfusion and pressure was monitored immediately in the system. A damper was used to damp pulses produced by the pump in the system upstream up to 90% and a one-way valve was used for flow control.[33] Applying pressure gradient as a mechanism of tissue decellularization has been demonstrated to be significantly an effective physical method. Furthermore, the pressure gradient approach reduces processing time, and due to uniform pressure, less aggressive chemical agents are required to achieve the same level of decellularization.
Vacuum assisted
It is hypothesized that use of a vacuum would accelerate and improve the delivery and efficiency of detergents into the target tissue. Lange et al. combined detergent-enzymatic-method together with vacuum technology. Porcine tracheal scaffolds were decellularized through this method and results were compared with the normal decellularization procedure without using vacuum technology. Porcine tracheae specimens were decellularized using exactly the same decellularization protocol but under normal atmospheric pressure. A vacuum was created to <1000 Pa. The results of this study showed a reduction in process time (9 days to prepare scaffold). Moreover, a reduction in residual DNA levels was observed (decellularization no-vac: 137.8 ± 48.82 ng/mg vs. decellularization vac: 36.83 ± 18.45 ng/mg, P < 0.05).[34] In another study by Butler et al. human donor tracheal were decellularized by vacuum-assisted protocol. The results of their study indicated that the vacuum assisted method produces well decellularized bioscaffolds and is comparable with the detergent-enzymatic method. However, the process time in vacuum-assisted decellularization is significantly less than detergent-enzymatic procedure (approximately 9 days vs. 3–8 weeks).[35] Vas et al. studied the vacuum assisted methodology on cartilage tissue. Fresh porcine costal cartilage was obtained from Large-White/Landrace crossbred pigs ranging from 40 to 70 kg. Costal cartilage adjacent to the bone tissue was separated from the rib cage. Any remaining adherent soft tissue was removed using a sterile scalpel and the harvested costal cartilage placed in sterile plastic bags and stored immediately at -20°C. It is described that this methodology allows for the rapid fabrication of nonimmunogenic costal cartilage-derived scaffolds capable of directing cell fate. Based on the finding of this study, the decellularized ECM is capable of improving the chondrogenic differentiation of skeletal cells and provokes a regenerative response in an immunocompetent host after implantation.[36] Removal of detergents from a decellularized tissue is the other application of the vacuum methodology.[37] Alizadeh et al. have investigated vacuum washing (VW) to remove SDS detergents out of a decellularized bovine pericardium. Differences between VW method and normal washing (NW) method (containing distilled water and PBS) were explored. Results indicated that removal of SDS in the VW group was more effective than the NW group. It is also declared that VW for 12 h is the optimum state.
Supercritical fluid
Supercritical fluid removes cell debris as it passes through tissue, so it can be used as a neutralizing agent after initial decellularization with a detergent such as alcohol to remove cell debris in the tissue. In addition, this fluid reduces the detrimental effects on the mechanical properties of the ECM. However, the pressure required to apply the supercritical fluid phase can destroy the ECM.[4,8] The supercritical fluid has a density similar to that of liquids but is permeable like gases. The critical temperature and pressure of supercritical carbon dioxide (SC-CO2) are 31.1°C and 7.40 MPa, respectively, which is consistent with 37°C and 15 MPa physiological conditions. Besides, due to its high permeability, carbon dioxide gas will be rapidly removed from the tissue, and the need for additional tissue washing will be eliminated. However, since carbon dioxide gas is nonpolar, adding ethanol can easily remove the polar portion of the membrane which is a phospholipid. Furthermore, all mechanical and structural properties of the tissue remain unchanged.[10]
de Boer et al., decellularized the porcine pericardial tissue with SC-CO2 for manufacturing of aortic prosthesis. By performing mechanical tests, compared to the synthetic prosthesis made of Dacron, the biologically prepared tissue showed favorable properties, and this study demonstrated the high capability of this decellularization method.[38] In one study, Guler et al. used SC-CO2 to decellularize aortic and retinal tissue. Their results showed that high fluid pressure bursts the cells during the process, and rapid pressure reduction contributes significantly to the removal of cells and their remnants from the tissue. Furthermore, the process period is reduced considerably; complete decellularization is achieved while retaining the ECM intact.[39] Wang et al. developed a method based on SC-CO2 that eliminates the need of harsh chemical agents and also reduces the amount of processing time required. The resultant ECM material showed removal of nuclear content while preserving key proteins such as collagen Type I, collagen Type III, collagen Type IV, elastin, fibronectin, and laminin. In addition, biological factors such as GAGs and growth factors such as basic fibroblast growth factor and vascular endothelial growth factor were also retained.[40] The absence of lipids is an important step for decellularization of retina tissue and further recellularization. Gil-Ramirez et al., to obtain a tissue free of undesired cellular components, for the first time developed a set-up using pressurized carbon dioxide fluids for the decellularization of porcine retina tissue. Pressurized CO2-EtOH-H2O (0.87 χCO2) at 300 bar and 37°C for 1 h completely removed the lipid species found in the retina. It is stated that using ethanolic mixture instead of limonene mixture, lipid content decreased by >50% showing good solubility of retina lipids in pressurized CO2-limonene.[41]
Immersion and agitation
Immersion and agitation is one of the applied decellularization techniques that better assists the agent to reach the tissue cells. The desired tissue or organ is immersed in a chamber containing the decellularization agent. The immersion time and intensity of agitation depend on the thickness and density of the tissue. The turbulence in the agent can be induced by a magnetic plate, an ultrasound source, a rotating chamber, or an agitator at the end of the chamber.[4,8] Syed et al., Compared several decellularization protocols for preparation of the submucosal substrate scaffolds for use in laryngeal tissue engineering. The first method consisted of perfusion and immersion of the specimen using a peristaltic pump. The specimens were immersed in SDS, an anionic detergent and Triton X-100, a nonionic detergent. The perfusion outlet from the intestine was recycled back to the pump. The second protocol, involving a mixture of sodium deoxycholate and DNase, was followed by stirring the tissue in the solvent. A shaker was used to agitate the fluid appropriately.[42] The limitation of this process is that cell lysis could start before being exposed to the detergent and release various proteases, which degrade the surrounding ECM and the resultant scaffold could be partially degraded.
Perfusion
Antegrade and retrograde perfusion flow is a method of decellularization of organs in which the organ is completely separated from its main blood vessel and the chemical agents are injected into its vascular system after being washed with detergents. Since the required pressure to drive the agent along the vascular system can cause the capillaries and small vessels to tear, flow rate control is crucial.[8,43] He and Callanan published a detailed report on decellularization techniques in tissue engineering. Different perfusion decellularization protocols have been described and compared with those previously used successfully for the heart, lung, liver, kidney, pancreas, small intestine, and skeletal muscle organs.[44] The first whole-organ decellularization by the perfusion method was performed by Ott et al. for whole rat heart samples. Perfusion was done through the coronary artery. The ECM and vascular network structure were maintained. Moreover, after endothelial and cardiac cells were cultured, the physiological environment of the body restored the heart to 2% of adult heart efficiency by placing the heart in a bioreactor and under electrical stimulation for 28 days.[45] Wainwright et al. proposed a new method of heart decellularization, which heart was decellularized using pulsatile retrograde artery perfusion. The protocol used in this study was time-efficient and could effectively remove DNA content from tissue.[46] Remlinger et al. implemented a retrograde perfusion protocol. Their method was slightly more invasive, leading to a decrease in the DNA remaining in the scaffold.[47] Momtahan et al. stated that there is a need for a balance between invasive cell degradation and maintaining a microscopic environment suitable for cell culture.[48]
Methe et al. proposed another method for decellularization of pig heart: The use of the aqueous solution and ionic and nonionic detergents by means of perfusion and agitation. In this way, the ECM retained its original shape and function, and the heart muscle cells remained unchanged. In addition to cellular diversity, growth factors and nutrients must be provided in the bioreactor environment for cell culture to be effective.[49] Sierad et al., for the decellularization of thick aortic tissue by preserving its weak valves, developed the first perfusion system that operates with a differential pressure gradient. The specimen was subjected to various chemical protocols with detergents and enzymes in the apparatus. By combining the physical and chemical methods the anisotropic mechanical properties were well preserved, the thick aortic wall was optimally decellularized while there was no damage to the valve structure. Although tissue stiffness was increased due to the loss of a large percentage of GAGs during this process, other components of the ECM were highly retained.[32] Petersen et al. showed how a mouses' lungs could be decellularized by perfusion. The decellularized pulmonary scaffold was cultured in a bioreactor that held the airways and sacs open at a negative pressure of 1 breath/min and simultaneously maintained blood pressure in the pulmonary artery at 20 mmHg or less. Results after implantation into the host mice showed that the lung participated in gas exchange. However, blood leakage from the vessels and the formation of edema (interstitial water accumulation) were observed several hours after transplantation, which was a sign of damage caused by the decellularization process.[50] In a follow-up study by Song et al., process optimization, for maintaining scaffold intact and better exchange performance, was able to keep the lung active for up to 7 days after transplantation.[51]
Caralt et al. have published research on the optimization of the decellularization process from rat kidney. According to the study, the most effective method of renal decellularization, by preserving the primary structure and major biological properties of the tissue, is the use of Triton X-100 and SDS in perfusion. However, the use of the ureteral route through which urine flows through the kidneys can be another auxiliary route.[52] Pellegata et al. developed a new automatic system for the decellularization of arterial scaffolds. Three separate chambers were provided with chemical decellularization agents; the perfusion pump automatically selected the desired fluid by the system and inserted the fluid into the circuit through three routes. One chamber would collect the used fluid at the end of the route. The tissue fixture clamps inside the chamber were able to extend the tissue longitudinally, thereby increasing the rate of diffusion into the tissue. The perfused flow rate in the system was 40 ml/min.[53] According to Kajbafzadeh et al., whole organ perfusion is the most effective method of liver decellularization. Perfusion is done through the liver or biliary vascular system. Compared with the concentration gradient (diffusion) method, perfusion maintains the internal vascular system of the liver and is more effective.[54] In Verstegen et al.'s study, whole human liver was decellularized to achieve transplantable organ bioscaffold using controlled perfusion. Whole human livers were decellularized by dual machine perfusion through the portal vein and hepatic artery. A perfusion setup was made to enable the decellularization through the hepatic artery and portal vein applying controlled perfusion fluids including 4% Triton X-100 with 1% ammonium hydroxide. The flow rate was increased on day 3 from 60 mL/min to 350 mL/min and was refreshed every 4 h. The remainder of cellular debris was washed out by perfusion with 0.9% NaCl. It is declared that decellularized vascular matrix remained appropriate for normal suturing and no major histocompatibility complex molecules were detected and the achieved scaffold were nontoxic when human mesenchymal stromal or umbilical vein endothelial endothelium cells were reseeded. The authors also assert that their findings of this study support new opportunities for bioengineering of transplantable grafts in the future.[55] Perfusion seems to be one of the most popular methods for tissue decellularization. Because of natural vascular trees to distribute detergents, better access would be provided and tissue deep exposure to decellularization agents would occur. As a result, improved removal of cellular components helps to better decellularize the tissue.[56] Tajima et al. used freeze-thaw and perfusion together to decellularize canine kidney. The renal artery of cadaveric canine was cannulated and the whole organ of the kidney was frozen at −80°C. After the kidney was completely thawed, it was perfused with 5% SDS in physiological saline for 6 h through the cannulated artery to achieve decellularization. Their protocol for decellularization could remove cellular components while preserving the native ECM.[57]
Combined methods
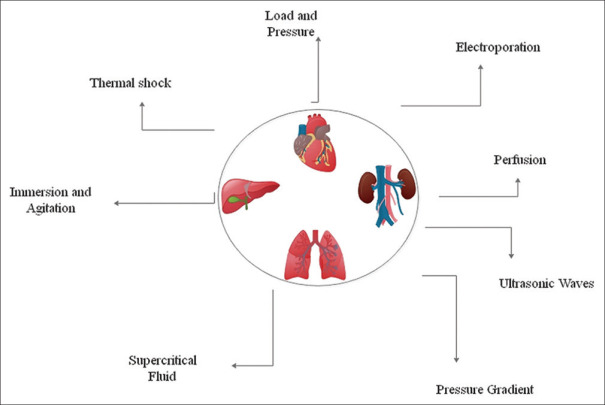
Since each method has its own advantages and disadvantages, several techniques are used to complement each other to produce desirable properties such as specific mechanical properties in engineered tissue. Figure 1 shows different physical methods in the decellularization process that are used separately or in a combined order. Physical methods, for example, cause the least damage to tissue structure, although they cause significant immune responses. On the other hand, chemical agents such as enzymes and detergents alone cannot eliminate cell debris. Although combined methods require more chemical agents and more process time, they work more efficiently and optimally. Thick tissues often require multi-stage decellularization protocols, including mechanical, chemical, and enzymatic methods.[10] For example, fat tissue, despite being simple, requires 13 different stages of mechanical pressure, enzymatic, polar detergent, and sterilization for complete decellularization while maintaining important mechanical and biochemical properties of the tissue.[44] Another combined approach to decellularize fat tissue is the 5-day procedure of several heat shock cycles between two full-fledged enzymatic rinses accompanied by polar solvent rinses.[58] In a combined protocol, two different hypotonic buffers were used to decellularize the cartilage disc of the porcine sample. The tissue was then incubated in the hypertonic buffer containing DNase to increase porosity, followed by freeze-thaw and sonication cycles.[59] The whole murine lung sample was decellularized, using two mechanical methods along with the detergent and enzymatic methods. Four freeze-thaw cycles and then perfusion with a chemical agent for 5 weeks, and finally the tissue was washed with a nuclease agent. A large percentage of the important components of the ECM was retained, while the cellular components were well removed. This was a favorable result of prolonged exposure to extremely low concentrations of chemical agents.[60] Gardin et al. used a combined protocol consisting of several stages of heat shock, eluting, and dehydrating with alcohol to decellularize bovine specimens and showed that this protocol was superior to the other three protocols tested. In the second part of the study, the bovine pericardium was decellularized by heat shock and osmotic shock methods. The results showed the relative superiority of the osmotic shock method in maintaining ECM and host tissue response at the implantation site.[61] In another study by Hung et al. on laryngeal decellularization, two methods of rapid freeze-thaw and sonication were employed together. The result showed that one or more heat shock cycles alone had little effect on the process. However, by adding 3 cycles of sonication during freezing of the laryngeal tissue, the cellular and nucleic acid content were significantly reduced.[62] Casali et al. used a dual method to maintain the decellularized scaffold hydration and mechanical properties. In this method, the tissue was exposed to a detergent for 48 h and then washed with SC-CO2 for 1 h. This procedure completely eliminated cellular remnants of DNA and reduced the processing period significantly from 4 to 7 days to 2 days. At the same time, the mechanical and structural properties of the tissue were well preserved.[63] Manalastas et al. used SDS solution together with a sonication method to obtain a decellularized bioscaffold. The results of their studies indicated that there is a significant decrease in decellularization time compared to the state when SDS is used for decellularization solely. It is also stated that sonicator power had a significant effect on the microarchitecture integrity of the scaffold.[64] Combined methods are mainly used to reduce the decellularization process. Besides, due to the application of combined methods, less exposure to chemical agents could prevent side effects on the ECM.[65] Table 1 indicates different methods used in tissue decellularization, although some of these methods could be used together in order to achieve the optimum decellularized tissue.
Figure 1.
Different physical methods used in tissue decellularization
Table 1.
Different physical methods used for animal tissue decellularization
| Agent/method | Application | Effect on the ECM | Reference |
|---|---|---|---|
| Thermal shock | The formation of water crystals inside the cell destroys the cell membrane | Water crystals can destroy the ECM | [8,9,10,11,12,13,14,15,16,17] |
| Mechanical pressure | Pressure can disrupt tissue and cells | Pressure can damage the ECM components | [4,8,10,19,20,21,22,23] |
| Electroporation | The pulsed electric fields destroy the cell membrane | The pulsed electric field can destroy the ECM. The electrodes are relatively small and cover a limited area | [4,24,25,26] |
| Perfusion | It facilitates the distribution of the chemical agent and the removal of cellular substances | Pressure induced by perfusion can destroy the ECM | [32,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] |
| Pressure gradient | It facilitates the distribution of the chemical agent and the removal of cellular substances. Pressure can disrupt the cell | The pressure gradient can destroy the ECM | [4,8,32,33] |
| Supercritical fluids | It facilitates the distribution of the chemical agent and the removal of cellular substances | The pressure required to apply the supercritical fluid phase can destroy the ECM | [10,38,39,40] |
| Ultrasonic waves | High-power waves are capable of disrupting intermolecular bonds, disrupting the cell membrane, and removing its internal components | Uncontrolled cavitation can damage the structure and mechanical properties of the tissue. Structural fibers may have transverse connections | [27,28,29,30,31] |
| Immersion and agitation | It causes cell death and often facilitates the distribution of chemical agents and the removal of cellular substances | Severe stirring or the use of ultrasound to cause turbulence can damage the ECM | [42] |
| Vacuum-assisted | This method facilitates decellularization by allowing more agents to reach cells | A high negative pressure could have adverse effects on the ECM | [34,35,36,37] |
ECM – Extracellular matrix
Conclusion
In general, physical methods of decellularization can disrupt the cell membrane, release cellular contents, and facilitate the removal of cellular contents from the ECM. Physical methods of decellularization include rapid freeze-thaw (heat shock), chemical agent perfusion, application of supercritical fluid, vacuum assisted, electroporation, immersion and agitation, and mechanical forces such as hydrostatic pressure and sound waves application. Since physical methods are unable to remove cellular debris in the decellularization process in cases where there is no fluid flow such as ultrasound or electroporation, it is necessary to combine a tissue washing step with a suitable detergent. Although each physical method can work individually and with suitable effect, physical methods are usually insufficient to achieve complete decellularization for all tissues and must be accompanied by chemical methods.
Based on the kind of the tissue, each method could have its own benefits, however, the perfusion method seems to attract more attention than other methods. Perfusion decellularization allows tissue regeneration at a clinically relevant scale with an intact structure by meeting metabolic demands through intact vasculature and maintenance of native ECM-contained cues. In addition, in freeze-thaw procedure, the collagen and GAGs content, as well as the mechanical strength, were similar to those of the native specimen, although, this method could lead to immunogenic response due to removal of genetic materials.
The application of physical methods for tissue and organ decellularization has a short but significant history. These methods have shortened and optimized decellularization processes and protocols by increasing efficiency and diminishing damage to the structure and components of the tissue scaffold and ECM. The cost and time of chemical agents for tissue decellularization along with the destructive effects on the integrity of tissue and critical components of the ECM have made them difficult and undesirable to use. Depending on the tissue type and texture, it is necessary to select the most optimal protocol; the effects and benefits of physical methods could be exploited to enhance process efficiency.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
BIOGRAPHIES

Mohsen Rabbani received the B.Sc. in Mechanical Engineering in 2000 from Sharif University of Technology. He received M.Sc. and Ph.D. in Biomedical Engineering (Biomechanics) from Amirkabir University of Technology (Tehran polytechnic) in 2004 and 2011, respectively. He is currently a faculty member of biomedical engineering department at University of Isfahan. His research focuses are on “Biomechanics role in tissue engineering” and “Tissue Decellularization”. Furthermore, He is working on biosensors based on electrochemical, chemical and biological activities.
Email: m.rabbani@eng.ui.ac.ir

Nasrin Zakian graduated from University of Isfahan in 2019 with a master's degree in Biomedical Engineering. She received her B.Sc. degree in Mechanical Engineering from Iran University of Science and Technology in 2013. Her research interests include Biomechanics and Tissue Engineering.
Email: nasrin.zakian@gmail.com

Nima Alimoradi recieved his B.Sc in Biomedical Engineering form University of Isfahan. He has also earned his M.Sc degree in Biomedical Engineering-Biomechanics from Amirkabir University of Technology (Tehran Polytechnic). His interests are in Cell and Tissue Engineering and BioMicrofluidics.
Email: nima.alimoradi@aut.ac.ir
References
- 1.Rana D, Zreiqat H, Benkirane-Jessel N, Ramakrishna S, Ramalingam M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J Tissue Eng Regen Med. 2017;11:942–65. doi: 10.1002/term.2061. [DOI] [PubMed] [Google Scholar]
- 2.Chan BP, Leong KW. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur Spine J. 2008;17(Suppl 4):467–79. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–3. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: Factors that influence the host response. Ann Biomed Eng. 2014;42:1517–27. doi: 10.1007/s10439-013-0963-7. [DOI] [PubMed] [Google Scholar]
- 6.Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: Mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577–92. doi: 10.1007/s10439-014-1103-8. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Azuma C, Tohyama H, Nakamura H, Kanaya F, Yasuda K. Antibody neutralization of TGF-beta enhances the deterioration of collagen fascicles in a tissue-cultured tendon matrix with ex vivo fibroblast infiltration. J Biomech. 2007;40:2184–90. doi: 10.1016/j.jbiomech.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Gilpin A, Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int. 2017;2017:9831534. doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing Q, Yates K, Tahtinen M, Shearier E, Qian Z, Zhao F. Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. Tissue Eng Part C Methods. 2015;21:77–87. doi: 10.1089/ten.tec.2013.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elder BD, Kim DH, Athanasiou KA. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery. 2010;66:722–7. doi: 10.1227/01.NEU.0000367616.49291.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burk J, Erbe I, Berner D, Kacza J, Kasper C, Pfeiffer B, et al. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng Part C Methods. 2014;20:276–84. doi: 10.1089/ten.tec.2012.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth SP, Glauche SM, Plenge A, Erbe I, Heller S, Burk J. Automated freeze-thaw cycles for decellularization of tendon tissue – A pilot study. BMC Biotechnol. 2017;17:13. doi: 10.1186/s12896-017-0329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poornejad N, Frost TS, Scott DR, Elton BB, Reynolds PR, Roeder BL, et al. Freezing/thawing without cryoprotectant damages native but not decellularized porcine renal tissue. Organogenesis. 2015;11:30–45. doi: 10.1080/15476278.2015.1022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonaka PN, Campillo N, Uriarte JJ, Garreta E, Melo E, de Oliveira LV, et al. Effects of freezing/thawing on the mechanical properties of decellularized lungs. J Biomed Mater Res A. 2014;102:413–9. doi: 10.1002/jbm.a.34708. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Fan J, Bai S. Biocompatibility of injectable hydrogel from decellularized human adipose tissue in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2019;107:1684–94. doi: 10.1002/jbm.b.34261. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem. 2012;113:2217–22. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto Y, Funamoto S, Sasaki S, Honda T, Hattori S, Nam K, et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941–8. doi: 10.1016/j.biomaterials.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 20.Funamoto S, Nam K, Kimura T, Murakoshi A, Hashimoto Y, Niwaya K, et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials. 2010;31:3590–5. doi: 10.1016/j.biomaterials.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 21.Santoso EG, Yoshida K, Hirota Y, Aizawa M, Yoshino O, Kishida A, et al. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS One. 2014;9:e103201. doi: 10.1371/journal.pone.0103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Takeda S, Charoensombut N, Kawabata K, Kishimoto Y, Kimura T, et al. Fabrication of uterine decellularized matrix using high hydrostatic pressure through depolymerization of actin filaments. J Biomech Sci Eng. 2019;14:19–7. [Google Scholar]
- 23.Watanabe N, Mizuno M, Matsuda J, Nakamura N, Otabe K, Katano H, et al. Comparison of high-hydrostatic-pressure decellularized versus Freeze-Thawed porcine menisci. J Orthop Res. 2019;37:2466–75. doi: 10.1002/jor.24350. [DOI] [PubMed] [Google Scholar]
- 24.Phillips M, Maor E, Rubinsky B. Nonthermal irreversible electroporation for tissue decellularization. J Biomech Eng. 2010;132:091003. doi: 10.1115/1.4001882. [DOI] [PubMed] [Google Scholar]
- 25.Sano MB, Neal RE, 2nd, Garcia PA, Gerber D, Robertson J, Davalos RV. Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed Eng Online. 2010;9:83. doi: 10.1186/1475-925X-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zager Y, Kain D, Landa N, Leor J, Maor E. Optimization of irreversible electroporation protocols for in vivo myocardial decellularization. PLoS One. 2016;11:e0165475. doi: 10.1371/journal.pone.0165475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azhim A, Yamagami K, Muramatsu K, Morimoto Y, Tanaka M. The use of sonication treatment to completely decellularize blood arteries: A pilot study. Conf Proc IEEE Eng Med Biol Soc 2011. 2011:2468–71. doi: 10.1109/IEMBS.2011.6090685. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira AC, Garzón I, Ionescu AM, Carriel V, Cardona Jde L, González-Andrades M, et al. Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS One. 2013;8:e66538. doi: 10.1371/journal.pone.0066538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forouzesh F, Rabbani M, Bonakdar S. A comparison between ultrasonic bath and direct sonicator on osteochondral tissue decellularization. J Med Signals Sens. 2019;9:227–33. doi: 10.4103/jmss.JMSS_64_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yusof F, Sha'ban M, Azhim A. Development of decellularized meniscus using closed sonication treatment system: Potential scaffolds for orthopedics tissue engineering applications. Int J Nanomedicine. 2019;14:5491–502. doi: 10.2147/IJN.S207270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahim A, Hazwani A, Shaban M. Biomechanical and structural properties of aortic scaffolds decellularized by sonication decellularization system. J Cardiovasc Med Ther 2019. 2019;2:1–9. [Google Scholar]
- 32.Sierad LN, Shaw EL, Bina A, Brazile B, Rierson N, Patnaik SS, et al. Functional heart valve scaffolds obtained by complete decellularization of porcine aortic roots in a novel differential pressure gradient perfusion system. Tissue Eng Part C Methods. 2015;21:1284–96. doi: 10.1089/ten.tec.2015.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montoya CV, McFetridge PS. Preparation of ex vivo-based biomaterials using convective flow decellularization. Tissue Eng Part C Methods. 2009;15:191–200. doi: 10.1089/ten.tec.2008.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange P, Greco K, Partington L, Carvalho C, Oliani S, Birchall MA, et al. Pilot study of a novel vacuum-assisted method for decellularization of tracheae for clinical tissue engineering applications. J Tissue Eng Regen Med. 2017;11:800–11. doi: 10.1002/term.1979. [DOI] [PubMed] [Google Scholar]
- 35.Butler CR, Hynds RE, Crowley C, Gowers KH, Partington L, Hamilton NJ, et al. Vacuum-assisted decellularization: An accelerated protocol to generate tissue-engineered human tracheal scaffolds. Biomaterials. 2017;124:95–105. doi: 10.1016/j.biomaterials.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vas WJ, Shah M, Blacker TS, Duchen MR, Sibbons P, Roberts SJ. Decellularized cartilage directs chondrogenic differentiation: Creation of a fracture callus mimetic. Tissue Eng Part A. 2018;24:1364–76. doi: 10.1089/ten.TEA.2017.0450. [DOI] [PubMed] [Google Scholar]
- 37.Alizadeh M, Rezakhani L, Soleimannejad M, Sharifi E, Anjomshoa M, Alizadeh A. Evaluation of vacuum washing in the removal of SDS from decellularized bovine pericardium: Method and device description. Heliyon. 2019;5:e02253. doi: 10.1016/j.heliyon.2019.e02253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Boer EC, Geldof F, Gerritse TJ, Winkelhorst EW. Supercritical CO2 decellularized porcine pericardium is a promising material for an ascending aortic prosthesis. University of TWENTE. 2015:23. [Google Scholar]
- 39.Guler S, Aslan B, Hosseinian P, Aydin HM. Supercritical carbon dioxide-assisted decellularization of aorta and cornea. Tissue Eng Part C Methods. 2017;23:540–7. doi: 10.1089/ten.TEC.2017.0090. [DOI] [PubMed] [Google Scholar]
- 40.Wang JK, Luo B, Guneta V, Li L, Foo SEM, Dai Y, et al. Supercritical carbon dioxide extracted extracellular matrix material from adipose tissue. Mater Sci Eng C Mater Biol Appl. 2017;75:349–58. doi: 10.1016/j.msec.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Gil-Ramirez A, Spangenberg A, Spégel P, Rodríguez-Meizoso I. Pressurized carbon dioxide combined with aqueous ethanol as cosolvent induces efficient delipidation of porcine retina for their use as bioscaffolds. J CO2 Util. 2019;34:700–8. [Google Scholar]
- 42.Syed O, Walters NJ, Day RM, Kim HW, Knowles JC. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014;10:5043–54. doi: 10.1016/j.actbio.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization-a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526–9. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 44.He M, Callanan A. Comparison of methods for whole-organ decellularization in tissue engineering of bioartificial organs. Tissue Eng Part B Rev. 2013;19:194–208. doi: 10.1089/ten.teb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: Using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 46.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525–32. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remlinger NT, Wearden PD, Gilbert TW. Procedure for decellularization of porcine heart by retrograde coronary perfusion. J Vis Exp. 2012;70:e50059. doi: 10.3791/50059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Momtahan N, Sukavaneshvar S, Roeder BL, Cook AD. Strategies and processes to decellularize and recellularize hearts to generate functional organs and reduce the risk of thrombosis. Tissue Eng Part B Rev. 2015;21:115–32. doi: 10.1089/ten.TEB.2014.0192. [DOI] [PubMed] [Google Scholar]
- 49.Methe K, Bäckdahl H, Johansson BR, Nayakawde N, Dellgren G, Sumitran-Holgersson S. An alternative approach to decellularize whole porcine heart. Biores Open Access. 2014;3:327–38. doi: 10.1089/biores.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–51. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caralt M, Uzarski JS, Iacob S, Obergfell KP, Berg N, Bijonowski BM, et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am J Transplant. 2015;15:64–75. doi: 10.1111/ajt.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pellegata AF, Asnaghi MA, Zonta S, Zerbini G, Mantero S. A novel device for the automatic decellularization of biological tissues. Int J Artif Organs. 2012;35:191–8. doi: 10.5301/ijao.5000079. [DOI] [PubMed] [Google Scholar]
- 54.Kajbafzadeh AM, Javan-Farazmand N, Monajemzadeh M, Baghayee A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue. Tissue Eng Part C Methods. 2013;19:642–51. doi: 10.1089/ten.TEC.2012.0334. [DOI] [PubMed] [Google Scholar]
- 55.Verstegen MM, Willemse J, van den Hoek S, Kremers GJ, Luider TM, van Huizen NA, et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev. 2017;26:1304–15. doi: 10.1089/scd.2017.0095. [DOI] [PubMed] [Google Scholar]
- 56.Gerli MFM, Guyette JP, Evangelista-Leite D, Ghoshhajra BB, Ott HC. Perfusion decellularization of a human limb: A novel platform for composite tissue engineering and reconstructive surgery. PLoS One. 2018;13:e0191497. doi: 10.1371/journal.pone.0191497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tajima K, Kuroda K, Otaka Y, Kinoshita R, Kita M, Oyamada T, et al. Decellularization of canine kidney for three-dimensional organ regeneration. Vet World. 2020;13:452–7. doi: 10.14202/vetworld.2020.452-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–24. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 59.Luo L, Eswaramoorthy R, Mulhall KJ, Kelly DJ. Decellularization of porcine articular cartilage explants and their subsequent repopulation with human chondroprogenitor cells. J Mech Behav Biomed Mater. 2015;55:21–31. doi: 10.1016/j.jmbbm.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–80. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 61.Gardin C, Ricci S, Ferroni L, Guazzo R, Sbricoli L, De Benedictis G, et al. Decellularization and delipidation protocols of bovine bone and pericardium for bone grafting and guided bone regeneration procedures. PLoS One. 2015;10:e0132344. doi: 10.1371/journal.pone.0132344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hung SH, Su CH, Lee FP, Tseng H. Larynx decellularization: Combining freeze-drying and sonication as an effective method. J Voice. 2013;27:289–94. doi: 10.1016/j.jvoice.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 63.Casali DM, Handleton RM, Shazly T, Matthews MA. A novel supercritical CO2-based decellularization method for maintaining scaffold hydration and mechanical properties. J Supercrit Fluid. 2018;131:72–81. [Google Scholar]
- 64.Manalastas TM, Dugos N, Ramos G, Mondragon JM. Effect of decellularization parameters on the efficient production of kidney bioscaffolds. Appl Biochem Biotechnol. 2020 doi: 10.1007/s12010-020-03338-2. 10.1007/s12010- 020-03338-2. doi:10.1007/s12010-020-03338-2. [DOI] [PubMed] [Google Scholar]
- 65.Batioglu-Karaaltin A, Ovali E, Karaaltin MV, Yener M, Yılmaz M, Eyüpoğlu F, et al. Decellularization of trachea with combined techniques for tissue-engineered trachea transplantation. Clin Exp Otorhinolaryngol. 2019;12:86–94. doi: 10.21053/ceo.2018.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]