Abstract
Across vertebrates, high social status affords preferential access to resources, and is expected to correlate positively with health and longevity. Increasing evidence, however, suggests that although dominant females generally enjoy reduced exposure to physiological and psychosocial stressors, dominant males do not. Here we test the hypothesis that costly mating competition by high-ranking males results in chronic, potentially harmful elevations in glucocorticoid production. We examined urinary glucocorticoids (n = 8029 samples) in a 20-year longitudinal study of wild male chimpanzees (n = 20 adults) in the Kanyawara community of Kibale National Park, Uganda. We tested whether glucocorticoid production was associated with dominance rank in the long term, and with mating competition and dominance instability in the short term. Using mixed models, we found that both male aggression and glucocorticoid excretion increased when the dominance hierarchy was unstable, and when parous females were sexually available. Glucocorticoid excretion was positively associated with male rank in stable and unstable hierarchies, and in mating and non-mating contexts. Glucorticoids increased with both giving and receiving aggression, but giving aggression was the primary mechanism linking elevated glucocorticoids with high rank. Glucocorticoids also increased with age. Together these results show that investment in male-male competition increases cumulative exposure to glucocorticoids, suggesting a long-term tradeoff with health that may constrain the ability to maintain high status across the life course. Our data suggest that the relationship between social rank and glucocorticoid production often differs in males and females owing to sex differences in the operation of sexual selection.
Keywords: cortisol, stress, dominance rank, male-male competition, dominance instability, sex difference
Background
In many group-living animals, including humans, high social status has clear reproductive benefits (Alberts 2012, Clutton-Brock 2016, Ellis 1995, Majolo et al. 2012, von Rueden and Jaeggi 2016). Social status also has effects on health and longevity, but the direction and magnitude of these vary across species and between the sexes (Sapolsky 2004). High-ranking animals typically enjoy preferential access to important resources, such as food, water, safe foraging and sleeping sites, and social support (Krause 1994, Snyder-Mackler et al. 2016, Stockley and Bro-Jørgensen 2011). Consequently, rank is generally expected to show positive correlations with health and survival (Snyder-Mackler et al. 2020).
A growing body of evidence, however, suggests that a positive relationship between health and social status is more common among females than males. Across a wide range of vertebrates, dominant males routinely face greater parasitism than subordinates (Habig and Archie 2015, Habig et al. 2018), and fail to show consistent advantages in longevity (Clutton-Brock 2016, Creel and Creel 2002, Hoogland 1995, McElligott and Hayden 2000, Robinson et al. 2006, Verhulst et al. 2014). Dominant females, by contrast, routinely outlive subordinates (Creel and Creel 2002, Robbins et al. 2011, Snyder-Mackler et al. 2020), and fail to show consistently elevated levels of parasitism (Habig et al. 2018).
One explanation for this sex difference is that, in many species, high-ranking males invest considerably more in mating effort than do subordinates, and this trades off against investments in somatic maintenance (Rolff 2002, Stoehr and Kokko 2006). High-ranking males often maintain energetically expensive armaments, including sexually dimorphic musculature, and engage in energetically expensive behaviors, such as aggressive displays (Clutton-Brock and Huchard 2013, Emery Thompson and Georgiev 2014, Emlen 2008, Key and Ross 1999). They also attract violent challenges from other males, increasing their risk of injury (Clutton-Brock 2016, Knott and Kahlenberg 2007, Koren et al. 2008, MacCormick et al. 2011). Among females, escalated fights are generally less frequent, and weapons less highly developed (Clutton-Brock 2016). Further, fitness gains from high rank are often less pronounced in females, reducing variation in reproductive effort across ranks compared to males (Clutton-Brock and Huchard 2013, Ellis 1995).
An important mechanism linking social status and health is the glucocorticoid stress response (Sapolsky 2004, 2005). Glucocorticoids are steroid hormones produced by the adrenal cortex under stimulation from the pituitary. Glucocorticoids interact with insulin to regulate metabolism, increasing blood glucose concentrations, stimulating the release of free fatty acids from adipose tissue, and inhibiting glucose uptake and glycogen synthesis (Arlt and Stewart 2005, Dallman et al. 1993). Basal glucocorticoid release shows a distinct daily rhythm that is responsive to food consumption (Dallman et al. 1993). In diurnal species a peak generally occurs in the early morning, following the overnight fast, to release stored energy in anticipation of the active period (Oster et al. 2017).
Glucocorticoids are also released at higher concentrations in reaction to noxious or threatening stimuli, as a component of the “classic stress response” (Romero and Wingfield 2016). Such stimuli come in two broad forms. Physiological (or reactive) stressors are direct, external challenges to homeostasis. Psychosocial (or anticipatory) stressors are indications that an external challenge to homeostasis is forthcoming (Boonstra 2013, Sapolsky 2004). Acute increases in glucocorticoid secretion coordinate a range of behavioral and physiological responses that help animals to cope with stressors (Sapolsky et al. 2000). These include changes in metabolism and cardiovascular function that mobilize energy and direct it to skeletal muscle (ibid.). The glucocorticoid stress response is thus critical for promoting short-term survival.
When chronic stressors are present, or acute stressors recur frequently, persistent activation of the hypothalamic-pituitary-adrenal axis can sustain high circulating levels of glucocorticoids. Under these conditions, the same metabolic responses that are protective in the short-term can produce deleterious effects, including atherosclerosis, muscle wasting, and immunosuppression (Romero and Wingfield 2016, Sapolsky 1993a). How frequently this condition, termed “homeostatic overload” (Romero et al. 2009), actually occurs in wild animals, as opposed to animals in the laboratory or humans, is unclear (Beehner and Bergman 2017, Boonstra 2013, Romero and Wingfield 2016).
Even under less extreme circumstances, however, there are energetic costs to mounting a stress response (Sapolsky 1993a). Consequently, frequent exposure to stressors increases the energy needed to preserve homeostasis, curtailing investment in somatic maintenance. Over time this generates wear and tear that reduces an animal’s ability to cope with future stressors (Romero et al. 2009, Romero and Wingfield 2016). This means that even though the stress response is ultimately adaptive (i.e. fitness maximizing), over the long term elevated glucocorticoid production might be associated with increased morbidity or mortality, particularly in long-lived species (Schoenle et al. 2018).
Consistent with the prediction that elevated glucocorticoid levels over time lead to cumulative damage, a recent meta-analysis reported that both baseline and stress-induced glucocorticoid levels showed stronger negative associations with survival in longer-lived species (Schoenle et al. 2021). Experimentally increasing glucocorticoid levels also predictably reduced survival, with stronger effects the longer that survival was monitored (ibid.). Four primate field studies have directly examined the relationship between glucocorticoid exposure and survival, all reporting negative effects. Ring-tailed lemurs (Lemur catta) with high fecal glucocorticoid levels showed elevated mortality over a two-year study (Pride 2005; the only study of these four considered in Schoenle et al. 2021). Vervet monkeys (Chlorocebus pygerythrus) that exhibited high fecal glucocorticoid levels under non-drought conditions showed elevated mortality in response to a drought (Young et al. 2019). Grey mouse lemurs (Microcebus murinus) with high concentrations of hair cortisol (a measure that averages production across weeks or months) showed elevated mortality, both over time and in response to the breeding season (Rakotoniaina et al. 2017). Finally, in a large, long-term study of wild baboons (Papio cynocephalus), females with chronically elevated glucocorticoid levels had shorter lifespans (197 females observed over 1784 female years: Alberts et al. 2019).
Early work on social status and health hypothesized that low-ranking animals would generally experience higher glucocorticoid levels than dominants (Sapolsky 1992). The logic of this stress of subordination hypothesis is straightforward. Suboptimal access to resources is expected to produce physiological stress. Decreased predictability and control in social interactions is expected to produce psychosocial stress. This pattern has indeed been observed in a range of vertebrates (Abbott et al. 2003; Blanchard et al. 2001; Sapolsky 1992, 2005).
In many species, however, dominants maintain higher glucocorticoid levels than subordinates (e.g. cooperative breeders: Creel 2001, 2005). Among primates, the most intensively surveyed group, more than 60 studies have examined the relationship between rank and glucocorticoid production in the wild (Beehner and Bergman 2017). These studies reveal a sex difference in rank effects that mirrors the ones previously discussed for parasitism and longevity. Specifically, where rank-related differences have been detected in primates, among females dominants normally show lower glucocorticoid levels than subordinates, whereas among males dominants normally show higher glucocorticoid levels than subordinates (Beehner and Bergman 2017, Cavigelli and Caruso 2015).
Why are glucocorticoid levels commonly elevated in high-ranking males? The costs of dominance hypothesis posits that rank and glucocorticoid production are positively correlated in contexts where acquiring and maintaining rank are energetically expensive (Creel 2001, Creel et al. 2013, Goymann and Wingfield 2004, Muller and Wrangham 2004b). Aggression is often a salient costly behavior. For example, in species where high-ranking males are habitually more aggressive than other group members, they habitually show elevated glucocorticoid levels (e.g. Arlet et al. 2009, Koren et al. 2008, Muller and Wrangham 2004b). Other species show positive correlations among rank, aggression, and glucocorticoid production only when hierarchies are unstable and the status of dominant animals is threatened (Sapolsky 1992, 1993b, 2005; Setchell et al. 2010). And, in some species, high-ranking males are more aggressive than others primarily in mating contexts, leading to a positive correlation between rank and glucocorticoid production when mating opportunities are contested (Mooring et al. 2006, Setchell et al. 2010, Surbeck et al. 2012).
In other cases the mechanism linking high rank and glucocorticoid production is uncertain. In a study of African wild dogs (Lycaon pictus), for example, dominants of both sexes showed higher rates of aggression than subordinates only during the mating season, yet maintained elevated glucocorticoid levels throughout the year (Creel 2005). And, in gray wolves (Canis lupus), dominants of both sexes routinely had higher glucocorticoid levels than subordinates, but did not show higher rates of aggression (Creel 2005). Cooperative breeders like these may represent a special case in which dominants of both sexes invest much more in reproductive effort than do subordinates, not only through aggression, but in ways that are more difficult to measure (ibid.).
Among primates, Cavigelli and Caruso (2015) argue that the high levels of glucocorticoid production observed in dominant males may not have negative effects on health, because they are often transitory. They note that dominant animals show acute peaks of glucocorticoid production during mating competition, or when hierarchies are unstable, but hypothesize that they quickly return to low baseline levels. Subordinates, by contrast, are hypothesized to regularly maintain high baseline glucocorticoid levels, even if their acute reactions to competition are muted. Such chronic stress is expected to have stronger adverse effects on health. In practice this acute costs of dominance, chronic stress of subordination hypothesis has rarely been tested, as it requires sufficient longitudinal data on individuals to distinguish short-term elevations in glucocorticoid production from long-term baseline levels. Limited evidence suggests that it may apply to female ring-tailed lemurs (Lemur catta), which maintain a short (1–3 week) annual breeding season in the wild (Cavigelli and Caruso 2015, Sauther et al. 1999).
In this paper we use 20 years of longitudinal data on male chimpanzees (Pan troglodytes schweinfurthii) living in the Kanyawara community, Kibale National Park, Uganda, to examine the relationship between glucocorticoid production and social status. Male chimpanzees are interesting because they generally form despotic, linear dominance hierarchies in which rank is frequently contested through aggression (Goodall 1986, Muller 2002), sometimes lethally (Fawcett and Muhumuza 2000, Kaburu et al. 2013, Pruetz et al. 2017, Wilson et al. 2014). Males also fight over access to estrous females, who do not breed seasonally, but are available unpredictably throughout the year (Goodall 1986, Muller 2017, Muller and Mitani 2005). Dominant males gain clear reproductive benefits and enjoy priority of access to food (Boesch et al. 2006, Goodall 1986, Newton-Fisher et al. 2010, Pusey et al. 2005, Wroblewski et al. 2009). However, in Kanyawara they also maintain lower levels of urinary C-peptide (a biomarker of insulin production) than subordinates, suggesting energetic costs to maintaining rank (Emery Thompson et al. 2009).
Previous studies of social status and glucocorticoid production in chimpanzees have drawn mixed conclusions. Our own early studies in Kanyawara documented the same sex difference observed among primates generally. In a six-year study of females, dominant individuals showed lower glucocorticoid levels than subordinates, an effect that was strongest during the energetically costly period of lactation (Emery Thompson et al. 2010). And, in a one-year study of males, glucocorticoid production, aggression, and rank were positively correlated, with males showing increases in aggression and glucocorticoid production when competing over sexually receptive females (Muller and Wrangham 2004b). We did not, however, quantify stability of the dominance hierarchy, nor address the issue of acute versus chronic increases in glucocorticoid production.
By contrast, two subsequent studies reported no relationship between rank and glucocorticoid production in male chimpanzees. The first, a short 3-month study in the Ngogo community, Kibale National Park, is difficult to interpret. It involved a stable dominance hierarchy, but relied on a small number of samples (1–5 per individual), and did not report whether any of these were collected in the presence of sexually receptive females (Muehlenbein and Watts 2010).
The second, a recent study in Ivory Coast, is more puzzling (Preis et al. 2019). Two chimpanzee communities in Taï National Park were studied for ~17 months, during which 983 urine samples were collected from 10 males. Unsurprisingly, males showed elevated glucocorticoid production during periods of dominance instability, and in the presence of estrous females. However, glucocorticoid levels were associated neither with dominance rank nor with aggression. Oddly, there was no increase in aggression during the periods of dominance instability. This might partly be due to the unusual demographic composition of the Taï communities during the study. Because of recent high mortality, only 2 fully adult males were present in one community, and 3 in the other, for the complete study period. The remaining males were adolescents for all or part of the study. It is plausible that a high-ranking male in a community containing only 1–2 other adult males will experience less uncertainty surrounding dominance interactions than males in a larger group.
However, it is also possible that the Taï study systematically underestimated the glucocorticoid levels of fully adult males, in relation to adolescents, by indexing glucocorticoid measurements to creatinine. Urinary steroids are frequently indexed to creatinine, which is produced at a relatively constant rate by muscle tissue, to correct for variation in fluid intake and urine concentration (Miller et al. 2004). This procedure can mislead, however, when individuals show differences in creatinine production resulting from differences in muscle mass (Emery Thompson et al. 2012). For this reason, among others, a specific gravity correction for urine concentration is preferable when age-sex classes are compared (Anestis et al. 2009, Miller et al. 2004, White et al. 2010). Because adolescent males ages 9–15 years at Taï were still developing musculature, as indicated by increasing creatinine over time (Samuni et al. 2020), the creatinine correction would have inflated their glucocorticoid measures in relation to larger adults. And, if adolescent males tended to be low ranking, this would have obscured any relationship between rank and glucocorticoid excretion.
Here we examine glucocorticoid levels assayed from 8029 urine samples collected from 20 adult males in the Kanyawara community, Kibale National Park, over two decades (December 1997 through May 2017). These long-term endocrine data allowed us to look at the effects of mating opportunity, food availability, aggression, instability in the dominance hierarchy, and other factors on glucocorticoid levels both within and between individuals over time. The primary goal was to identify the major stressors that drive glucocorticoid production in male chimpanzees, and to examine how these interact with rank. We predicted that low food availability would disproportionately increase glucocorticoid production in low-ranking males, as they are likely to be displaced from the best feeding locations. We predicted that instability in the dominance hierarchy and competition over mating opportunities would disproportionately increase glucocorticoid levels in dominant individuals, since their status would be at risk, and they invest more in mating effort. Finally, we predicted that aggression would be a critical mechanism linking glucocorticoid production with rank, instability, and mating opportunity (Muller and Wrangham 2004b).
Our secondary goal was to distinguish among (1) the stress of subordination hypothesis, (2) the costs of dominance hypothesis and (3) the acute costs of dominance/chronic costs of subordinance hypothesis. The first posits that subordinate males show chronically elevated glucocorticoid levels, owing to a lack of social predictability and control, and reduced access to high-quality food. The second posits that high-ranking males show chronically elevated glucocorticoid production, owing to their investments in maintaining rank and competing for mates, and that this represents a cost of mating effort. The third predicts that baseline glucocorticoid levels will be higher in subordinates than in dominants, after controlling for the acute elevations that may occur in response to short-term challenges.
Methods
Chimpanzees in the Kanyawara community, Kibale National Park, southwestern Uganda, were first studied systematically from 1983 to 1985, and have been continuously monitored by the Kibale Chimpanzee Project (KCP) since 1987 (Isabirye-Basuta 1988, Muller and Wrangham 2014). The Kanyawara chimpanzees were habituated without provisioning, and observations were conducted with a minimum observer distance of five meters. Over the main study period, from late 1997 to early 2017, the community comprised 49–54 chimpanzees, including 9–11 adult males and 13–18 adult females. Struhsaker (1997) provides a detailed description of the study site. All research was conducted with the approval of the Institutional Animal Care and Use Committees of Harvard University, Tufts University, and the University of New Mexico.
Behavior was recorded by a team of observers, which normally consisted of 2–3 long-term Ugandan field assistants and 1–2 university-based researchers. Whenever possible, observers followed chimpanzees from the time that they woke in the morning until they constructed their night nests. Chimpanzees were usually located at the site where they had nested the previous evening, but also by following their tracks, listening for calls, and waiting near fruiting trees. Because chimpanzees exhibit fission-fusion grouping, multiple teams sometimes followed different chimpanzee parties simultaneously. A party was defined as all chimpanzees within 50 continuous meters of each other.
This study draws on three sets of long-term data. (1) Dominance ranks were calculated from a combination of ad libitum and all-occurrence sampling data (Altmann 1974) collected between January 1993 and June 2017. (2) Endocrine data came from urine samples collected between November 1997 and May 2017. These were matched with behavioral data, including 15-minute scan sampling of party composition and group-level feeding behavior, collected over 6538 study days and 75,212 hours of observation. (3) Detailed, all-occurrence sampling data on aggression were available from January 2005 to May 2017. These included 4316 unique study days and 55,484 hours of observation. Confidence in the accuracy of the long-term behavioral data comes from tests documenting close agreement between focal data collected by researchers and all-occurrence sampling data collected independently by field assistants, together with routine measures of inter-observer reliability (Muller et al. 2007, Gilby et al. 2010).
Hormone methods
To quantify rates of glucocorticoid excretion, we assayed 8029 urine samples collected non-invasively from 20 individuals (mean: 401.5 samples/male, range: 17–1395) between November 1997 and May 2017 (Table 1). We considered only adult males (those 15 years or older) in our analyses, as they were physically, socially, and sexually mature.
Table 1.
Sample sizes by male ID
| Male | Ages | Urine samples (1997–2017) | Observation hours (2005–2017) | Months (Models 2–3) | Months 2 (Model 4) |
|---|---|---|---|---|---|
| AJ | 23–40 | 529 | 8123 | 69 | 51 |
| AT | 15–17 | 308 | 4516 | 29 | 26 |
| BB | 31–50 | 496 | 8526 | 75 | 54 |
| BF | 31–32 | 17 | 0 | 0 | 0 |
| ES | 15–22 | 477 | 9274 | 70 | 54 |
| KK | 15–27 | 873 | 11391 | 80 | 73 |
| LB | 29–32 | 133 | 0 | 0 | 0 |
| LK | 15–33 | 1395 | 16601 | 122 | 112 |
| MS | 22–35 | 435 | 5916 | 48 | 37 |
| MX | 15–19 | 149 | 3161 | 34 | 30 |
| OG | 15–16 | 110 | 1555 | 12 | 12 |
| PB | 15–22 | 451 | 7525 | 65 | 57 |
| PG | 15–24 | 530 | 4939 | 41 | 36 |
| SL | 26–33 | 73 | 284 | 2 | 2 |
| ST | 42–57 | 355 | 3784 | 38 | 28 |
| SY | 33–35 | 84 | 0 | 0 | 0 |
| TJ | 15–21 | 390 | 6825 | 65 | 59 |
| TT | 15–16 | 136 | 2103 | 16 | 14 |
| TU | 37–53 | 261 | 5425 | 48 | 32 |
| YB | 24–43 | 827 | 11060 | 96 | 66 |
| Total | 8029 | 111,008 | 910 | 743 | |
“Months” indicates the number of months in which a male was observed for at least 15 hours (Models 2–3). “Months 2” indicates the number of months in which a male was observed for at least 15 hours and provided at least 2 urine samples (Model 4).
When a chimpanzee urinated from a tree, observers trapped urine on a disposable plastic bag attached to a two-meter pole. If a bag could not be placed in time, then urine was pipetted from leaves in the ground layer of vegetation. To minimize the risk of sample cross-contamination, urine was collected from vegetation only when it was clear that multiple individuals had not urinated in the same area. Care was also taken to avoid collecting urine contaminated with feces. Immediately after collection, the identity of the chimpanzee, the date, and the time of urination were recorded, and samples were placed in a thermos bottle containing a cold pack. Samples were frozen at approximately −20°C at the end of the daily follow (within 14 hours). Later they were transported on ice to the U.S., in compliance with U.S. Centers for Disease Control and World Health Organization regulations. Muller and Wrangham (2004b) provide additional details on the validation of sample collection procedures.
Immunoreactive glucocorticoids were measured using enzyme-immunoassay reagents and protocols provided by the Clinical Endocrinology Laboratory at the University of California at Davis (polyclonal rabbit anti-cortisol R4866: Munro and Lasley 1988). This assay has been widely validated and used across taxa for the assessment of glucocorticoids in urine and other media (for chimpanzees: Kahlenberg et al. 2008, Muller et al. 2007). Intra-assay CV, calculated as the average CV between duplicate determinations, was 7.1%. Due to the longitudinal nature of our study, samples were assayed at intervals between 2005 and 2018, involving the same assay protocol but two different laboratories at Boston University (2005–2007) and the University of New Mexico (2008–2018). We employed iterative cross-validations, using pooled urine samples as controls, to ensure replicability of the assay over time (for details see Sabbi et al. 2020). Briefly, inter-assay CVs of all assays (i.e. across labs) were 13.4% and 12.5% for low and high controls, respectively, while the CV of mean values between labs was 7.3% for the low control and 5.0% for the high control. While these assays required using two different lots of reagents (A: 2005–2015, B: 2015–2018), these performed nearly identically (inter-low CVs: low control 1.6%, high control 0.1%). While this suggests that our assay performed consistently over time, we entered a random variable for the year of assay to address potential variation due to differences in laboratories, reagents, or equipment. This procedure improved model fit but did not alter overall findings relative to modeling that omitted this random effect.
All samples were assayed within ten years of collection, and 78% were analyzed within 5 years (mean interval = 3.3 years). To control for the possibility of glucocorticoid degradation over time (for discussion see Emery Thompson et al. 2020), we included a variable in our models for time between sample collection and assay (0–4 years, 4–7 years, 7–10 years). To control for the dilution of analytes by water, we corrected all results for specific gravity, measured with a handheld refractometer (Atago PAL-10S). This correction took the original glucocorticoid value and divided it by (SGs-1)/(SGX-1) where SGs was the specific gravity of the sample and SGX was the average sample specific gravity across the population (Buchwald 1964). Corrected values were natural log-transformed prior to statistical analysis.
Behavioral data
Four categories of behavior constituted male aggression. Stationary threats consisted of an arm wave at the victim, without locomotion. Charging displays involved exaggerated locomotion, piloerection, and sometimes branch dragging or shaking. Displays could be targeted toward specific individuals (directed charges), or the group in general (non-directed displays). Chases were recorded when a male pursued a fleeing individual, who was generally screaming. All incidents of contact aggression were recorded as Attacks. These included hits, kicks, or slaps delivered in passing, as well as extended episodes of pounding, dragging, and biting (Goodall 1986, Muller 2002, Muller & Wrangham 2004a). Chases and attacks were classified as high-level aggression, whereas charges and stationary threats were considered low-level aggression (as in Preis et al. 2019). We focused on high-level aggression when testing the effects of received aggression on glucocorticoids, because in some cases it was unclear whether low-level aggression was directed at a specific individual. Chases and attacks were visibly distressing to unambiguous victims.
Chimpanzee aggression involved exaggerated movements by the perpetrators and loud vocalizations (e.g. screams or waa barks) from the victims, rendering it highly conspicuous to observers. Thus, our record of aggression is equivalent to all-occurrence sampling (Altmann 1974). Nevertheless, the long-term data underestimate true rates of aggression, because some interactions are obscured by vegetation, such that the identities of aggressors or victims cannot be confirmed. Muller et al. (2007) compared focal data on aggression collected by a single observer with long-term data, and showed that these underestimates represent an unbiased sample of the behavior.
Rates of individual male aggression were calculated for each month of the study from 2005 to 2017 in which a male was observed for a minimum of 15 hours (monthly average = 121.8 hours, range = 15.25 – 364.75). This resulted in a sample of 910 chimpanzee months from 17 unique males (average = 53.5 months per male, range = 2–122, Table 1). Rates were based on the number of discrete aggressive events that a male participated in during the period of observation, classified by the most extreme form of aggression observed in the event. For example, if a male charged at another individual, and immediately chased that individual and attacked him, this would have been scored as a single event - an attack. If two separate individuals were attacked, this would have been scored as two events. If, after an episode of aggression, a male engaged in some other behaviour for at least one minute, subsequent aggression was assigned to a new event.
Male dominance ranks were determined from 13,415 dyadic interactions among 27 males, spanning January 1993 through June 2017. Wins and losses were assigned based on the directionality of pant-grunts (a formal signal of subordination), and submissive responses to received aggression (e.g. screaming and fleeing). An Elo rating method was used to assign relative ranks on each day of the study period for males 9 and older (Albers and de Vries 2001, Neumann et al. 2011). Males in 1993 were assigned a starting score of zero, and males that reached adolescence (beginning at age 9) during the study period were assigned a starting score one point below the lowest-ranking male in the hierarchy on the day of their first aggressive interaction with an adult male. The k-constant in the Elo rating equation was set to 20. Elo scores were ultimately transformed into ordinal ranks, which were standardized for the number of males in the community (standardized rank = (n-r)/(n-1), where n is the number of adult males in the hierarchy and r is a male’s ordinal rank in the hierarchy). Thus, the alpha male always had the highest rank score of 1, with other adult males falling between 0 and 1. Because dominance data were available from 1993, a burn-in period of almost 5 years ensured that reliable ranks were established for males before the onset of urine sampling in late 1997.
Hierarchy stability was assayed using Elo scores. Whenever there was a rank reversal between two adult males, we considered the 4 weeks prior to the day of the reversal a period of instability in the hierarchy at the group level. (In order to assess stability, our dominance data necessarily extend one month beyond our other datasets.) To test whether direct involvement in a rank reversal was predictive of increased glucocorticoid production, in some analyses the hierarchy was considered unstable specifically for the two individuals involved in the rank reversal (again, for 4 weeks prior to the day of the reversal).
We employed a simple scale to record the degree of tumescence of the sexual swelling for each adult female in a party (e.g. Wallis 1992). Females with sexual skins that were completely flat received scores of (1) no swelling. Females with sexual skins that were partly inflated, but wrinkled and droopy, received scores of (2) partial swelling. Females with sexual skins that were fully expanded (i.e. tense and shiny with no drooping) received scores of (3) maximally tumescent. Previous studies in Kanyawara have shown that adult males show little interest in monopolizing nulliparous females, but are strongly attracted to parous females with maximally tumescent sexual swellings (Muller et al. 2006, Muller et al. 2007, Muller and Wrangham 2004a). Consequently, we considered whether males were in parties containing at least one maximally-tumescent, parous female.
Dietary Quality
Kanyawara chimpanzees eat ripe fruit in proportion to its availability (Wrangham et al. 1998). Because fruit abundance varies temporally, chimpanzees are occasionally forced to fall back on lower quality piths and herbs, which are more widely distributed through the study site (Wrangham et al. 1991, Conklin-Brittain et al. 1998). Thus, when fruit is scarce, the Kanyawara chimpanzees subsist on a diet that is significantly lower in simple sugars, non-structural carbohydrates, and fat, than when fruit is abundant (Conklin-Brittain et al. 1998). These periods of low fruit availability represent times of increased energetic stress (Emery Thompson et al. 2014, Emery Thompson et al. 2009).
Observers noted at 15-min intervals whether chimpanzees in the party under observation were feeding. If they were, both the species and portion of the plant being consumed by the majority were recorded. Dietary quality was estimated by calculating the total percentage of feeding observations in which chimpanzees consumed fruit. This measure has previously been shown to correlate with direct estimates of fruit abundance from phenology transects (Wrangham et al. 1996).
Age estimation
Individuals who were born before the study began were assigned ages by comparing their physical and behavioral characteristics with those of chimpanzees of known ages. Young adult chimpanzees (15–20 years old) exhibit a suite of morphological characteristics that include thick glossy black hair, unbroken teeth, and light facial creasing. Chimpanzees older than 35 years display thinning brown or gray hair with less sheen, worn or broken teeth, and saggy, wrinkled faces. These individuals also move more slowly and deliberately. Female ages were further calibrated based on the apparent ages of their offspring. Hill et al. (2001) provide additional details on estimating the ages of wild chimpanzees in these study sites. Of the males in this sample: 9 were born during the study period and have known ages, 4 were immature when first identified and have narrow age estimates, and 7 were adults when the study began and were at least 29 years old by the time of first sampling.
Data analysis
Urinary glucocorticoid model (1).
The response variable in this model was a single urinary glucocorticoid measurement (natural log-transformed cortisol ng/ml corrected for specific gravity, n=8039, Table 1). We fitted a linear mixed model (LMM) to examine the effects of dominance rank, hierarchy stability, and mating opportunity on urinary glucocorticoids. For each value, key predictors were male rank on the day of sampling (from 0 to 1), hierarchy stability at the group level (stable or unstable), presence or absence of parous estrous females (one or more present in the party when the urine sample was collected or not), and dietary quality over the 14 days prior to sampling (% feeding scans in which chimpanzees were eating ripe fruit). Previous studies of baboons revealed that alpha status was a better predictor than ordinal rank of glucocorticoid production in males (Gesquiere et al. 2011), so we also included this as a variable (alpha or non-alpha). We further assessed dominance stability at the individual level, to test whether males who were directly involved in dominance reversals showed elevated glucocorticoids during periods of instability compared with those who were not. Specifically, when there was a rank reversal between two males, the preceding four weeks were considered unstable for those males, but not for other males in the community.
Because glucocorticoid production shows a diurnal pattern, with the highest levels in the morning and a decrease through the day (Muller and Lipson 2003), we included time of urination as a control predictor. We further controlled for chimpanzee age and years between urine collection and assay. To control for variation in sampling across individuals and over time, we included chimpanzee ID as a random effect. A random slope was also included for dominance rank, when it was used as a fixed effect. Year of assay was included as a random effect (SI Appendix).
We tested whether males of different status varied in their glucocorticoid responses to mating opportunity and dominance instability by introducing six interactions. These combined our two measures of rank (ordinal and alpha/non-alpha) with two measures of instability (group level and individual level) and one of mating opportunity (presence/absence of parous estrous females). Finally, we tested whether high- and low-ranking males differed in their glucocorticoid response to food availability by introducing interactions between our measure of dietary quality and our two measures of rank.
Aggression models (2–3).
The target variable in these models was a monthly count of either aggression given or aggression received for an individual adult male (n=910 male months, 17 unique males, Table 1).
Model 2.
To examine the effects of dominance rank, hierarchy stability, and mating opportunity on the incidence of male aggression, we fitted a generalized linear mixed model (GLMM) using a negative binomial distribution with a log link function. Aggression data were monthly counts, with an offset for the number of 15-minute scans in which a male was observed during the month (logged). Key predictors were average male rank for the month (from 0 to 1), hierarchy stability (percentage of unstable days during the month, at the group level), and mating opportunity (percentage of 15-minute scans during the month in which the male was in a party with one or more estrous females). As in Model 1, we controlled for male age during the month and dietary quality (% feeding scans in which chimpanzees were eating ripe fruit). Chimpanzee ID was included as a random effect, and a random slope was introduced for dominance rank.
Finally, we introduced two interactions to test whether high- and low-ranking males differed in their aggressive response to hierarchy instability (rank * stability) and mating opportunity (rank * percentage scans with parous estrous females).
Model 3.
This was identical to Model 2, except the target variable was high-level aggression received (chases and attacks) instead of aggression given.
Monthly aggression and glucocorticoid model (4).
The response variable in this model was an average monthly glucocorticoid value for an individual male (n=743 male months, 17 unique males, Table 1). Individual values were time-corrected by calculating residuals from the relationship of glucocorticoids against the time of urination (Emery Thompson et al. 2020).
We fitted a linear mixed model (LMM) to examine the effects of dominance rank, hierarchy stability, presence of estrous females, and aggression given and received on urinary glucocorticoid levels. Key predictors were average male rank for the month (from 0 to 1), hierarchy stability (percentage of unstable days during the month, at the group level), mating opportunity (percentage of 15-minute scans during the month in which the male was in a party with one or more parous estrous females), aggression given (rate of male aggression given during the month), high-level aggression received (rate of chases and attacks experienced by a male in the month), and dietary quality (% feeding scans in which chimpanzees were eating ripe fruit). As in Models 1–3, we controlled for male age, chimpanzee ID was included as a random effect, and a random slope was introduced for dominance rank.
All models were constructed in R using the lmer function in the lme4 package. Continuous predictors were centered on the mean, except for age (centered at 15 years) and time of urination (centered at 12pm). Statistical significance of fixed effects was determined using type III Wald Chi-square tests, and significance of random effects was determined with log-likelihood ratio tests (LLRT) by dropping one term at a time. Diagnostic plots of models 1 and 4 (LMMs) were evaluated to verify that residuals were uncorrelated and distributed normally. All variance inflation factors were <2, indicating no concerns with multicollinearity.
Results
Figure 1 shows Elo ratings for males from January 1993 to June 2017, which includes the initial burn-in period. From November 1997, when urine collection began, through June 2017, there were 145 rank reversals between adult males. Out of 7152 days, the hierarchy was classified as stable in 4619 (65%) and unstable in 2533 (35%). Distinct periods of stability are evident in Figure 1; for example, from mid-2001 to early 2003 there were no dominance reversals. There are also visible periods of churn, such as most of 2006.
Figure 1.
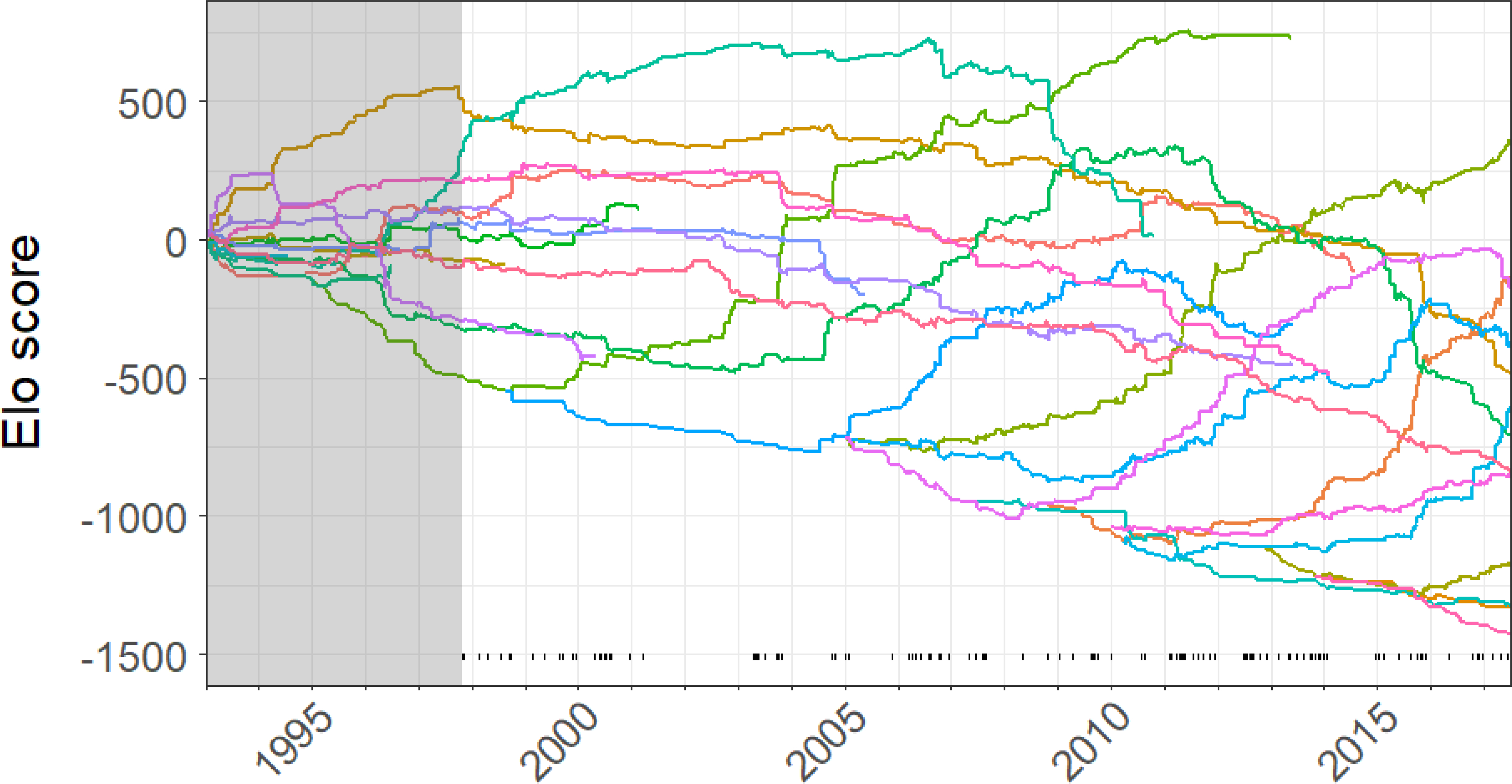
Male chimpanzee dominance trajectories. The colored lines show Elo ratings over time for individual male chimpanzees (n=13,415 dyadic interactions), with scores indicating relative male status. Tick marks at the bottom indicate dates on which rank reversals (n=145) occurred between adult males. The shaded area shows a burn-in period, prior to the initiation of urine sampling in December 1997.
Urinary glucocorticoid model.
Output from the full urinary glucocorticoid model is presented in the supplement (Table S1). Instability (group-level) by rank was the only significant interaction, so all others were omitted from the final model. Because there was no discrete effect of being alpha male, or of dominance instability at the individual level, these predictors were also omitted, to clarify the effects of ordinal rank and dominance instability at the group level. All other predictors and controls were included in the final model (Table 2).
Table 2.
Predictors of male glucocorticoids (Model 1)
| Term | Estimate | Variance | SE | SD | χ2 | P |
|---|---|---|---|---|---|---|
| Intercept | 10.867 | 0.083 | 17008.6 | <0.001 | ||
| TEST | ||||||
| Rank | 0.494 | 0.168 | 8.6 | 0.003 | ||
| Instability | 0.042 | 0.012 | 12.5 | <0.001 | ||
| Estrous females | 0.176 | 0.013 | 171.4 | <0.001 | ||
| Ripe fruit | −0.012 | 0.073 | 0.03 | 0.873 | ||
| Rank*Instability | 0.122 | 0.044 | 7.8 | 0.005 | ||
| CONTROLS | ||||||
| Age | 0.008 | 0.003 | 8.0 | 0.005 | ||
| Time of day | −0.144 | 0.003 | 2202.5 | <0.001 | ||
| Years to assay | 591.6 | <0.001 | ||||
| 4–6 y | −0.650 | 0.030 | ||||
| 7–9 y | −0.736 | 0.042 | ||||
| Random effects | ||||||
| Chimp ID | 0.016 | 0.125 | 64.6 | <0.001 | ||
| Rank*Chimp ID | 0.364 | 0.603 | 24.4 | <0.001 | ||
| Year of assay | 0.030 | 0.172 | 15.6 | <0.001 | ||
n = 8,029 urine samples, 20 males. Significance of fixed effects was determined via type III Wald chi-square tests. Significance of random effects and interactions was determined via LLRTs.
Overall, urinary glucocorticoid levels in male chimpanzees increased with increasing rank (estimate = 0.494, SE = 0.168, χ2 = 8.6, P = 0.003). This was true in both stable and unstable dominance hierarchies, and in both mating and non-mating contexts (Figure 2). Alpha male status had no distinct impact on urinary glucocorticoids.
Figure 2.
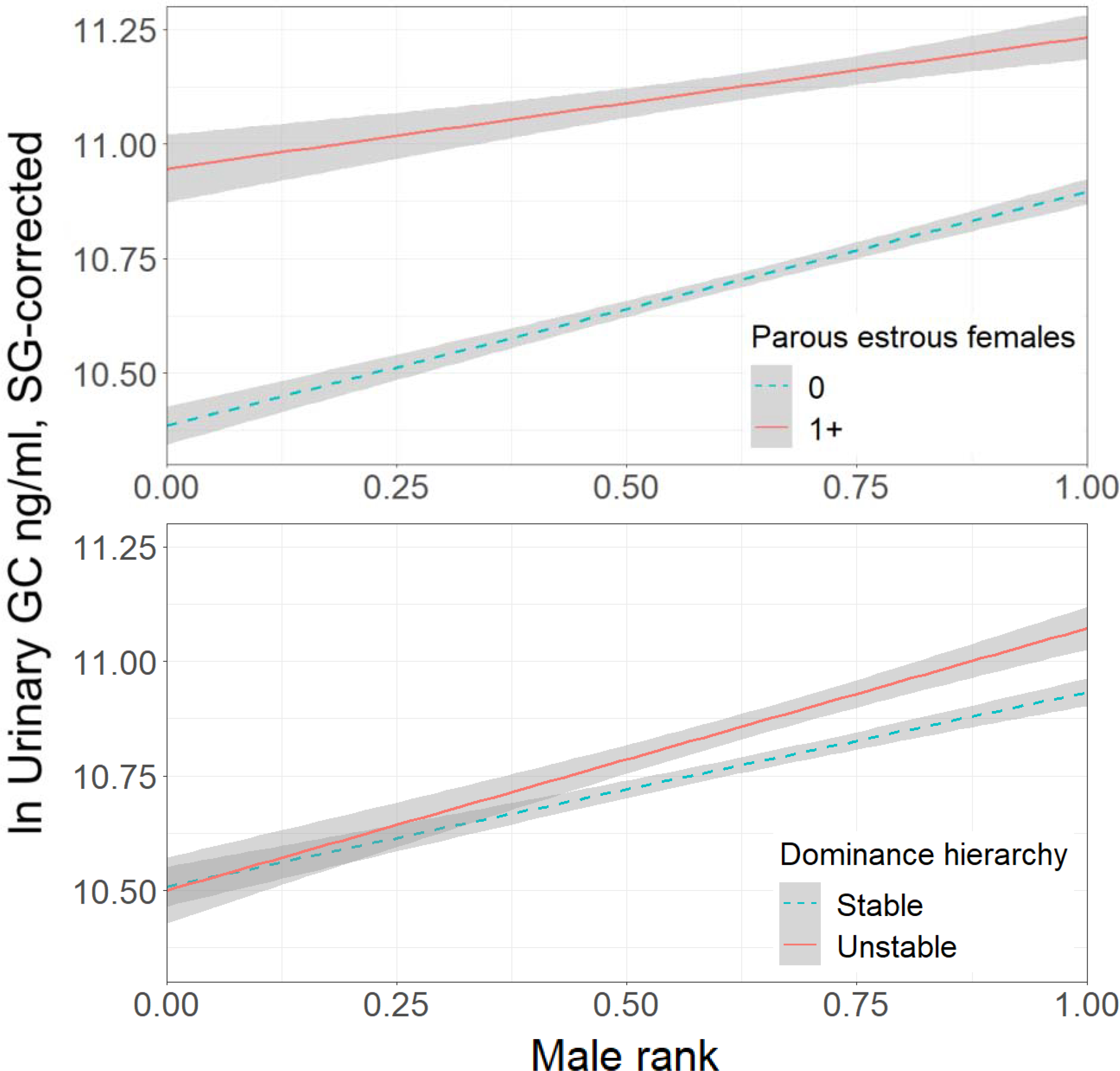
Glucocorticoid (GC) levels by dominance rank in male chimpanzees (n=8029 samples, 20 adults). Both the presence of parous estrous females (top panel) and instability in the dominance hierarchy (bottom panel) were associated with increased glucorticoid production in male chimpanzees. Instability had a greater effect on the glucocorticoids of higher-ranked males. The shaded areas indicate 95% confidence intervals. (Figure S1 plots the same data showing individual points.)
As predicted, mating opportunity had a large effect on male glucocorticoid levels. Across ranks, males exhibited increased glucocorticoid excretion in the presence of parous estrous females (estimate = 0.176, SE = 0.013, χ2 = 171.4, P < 0.001).
Instability in the dominance hierarchy was also associated with male glucocorticoids, but this relationship changed with rank (estimate = 0.122, SE = 0.044, χ2 = 7.8, P = 0.005). The lowest-ranking males showed no difference in glucocorticoid excretion between stable and unstable periods (Figure 2). With increasing rank, however, males showed larger glucocorticoid responses to instability. These effects were all limited to instability at the group level. There was no discrete effect of instability at the individual level.
As previously reported from this population (Emery Thompson et al. 2020), glucocorticoids increased with male age (estimate = 0.008, SE = 0.003, χ2 = 8.0, P = 0.005). Dietary quality, defined as the proportion of ripe fruit in the chimpanzees’ diet in the previous 14 days, did not influence male glucocorticoid concentrations, alone (estimate = −0.012, SE = 0.072, χ2 = 0.03, P = 0.873) or in interaction with rank.
Aggression models.
Output from the full aggression models is presented in Tables 3 and 4. The strongest predictors of male aggression largely mirrored those of glucocorticoids. Overall, high-ranking males were more aggressive than low-ranking males (estimate = 1.353, SE = 0.353, z = 3.83, P < 0.001). This was true in both stable and unstable hierarchies, and in both mating and non-mating contexts (Figure 3).
Table 3.
Predictors of monthly aggression given (Model 2)
| Term | Estimate | Variance | SE | SD | z | P |
|---|---|---|---|---|---|---|
| Intercept | −1.356 | 0.105 | −12.93 | <0.001 | ||
| TEST | ||||||
| Rank | 1.353 | 0.353 | 3.83 | <0.001 | ||
| Instability | 0.185 | 0.047 | 3.93 | <0.001 | ||
| Estrous females | −0.028 | 0.060 | 0.47 | 0.636 | ||
| Rank*Estrous females | 1.083 | 0.266 | 4.08 | <0.001 | ||
| Rank*Instability | 0.288 | 0.201 | 1.43 | 0.152 | ||
| CONTROLS | ||||||
| Age | −0.028 | 0.005 | −5.49 | <0.001 | ||
| Ripe fruit | 0.070 | 0.114 | 0.61 | 0.540 | ||
| Random effects | ||||||
| Chimp ID | 0.068 | 0.260 | 300.41 | <0.001 | ||
| Rank*Chimp ID | 1.384 | 1.176 | 21.75 | <0.001 | ||
n = 910 male months, 17 unique males. Significance of fixed effects was determined via type III Wald chi-square tests. Significance of random effects and interactions was determined via LLRTs.
Table 4.
Predictors of monthly aggression received (Model 3)
| Term | Estimate | Variance | SE | SD | z | P |
|---|---|---|---|---|---|---|
| Intercept | −3.910 | 0.101 | −38.67 | <0.001 | ||
| TEST | ||||||
| Rank | −1.470 | 0.253 | −5.80 | <0.001 | ||
| Instability | 0.375 | 0.082 | 4.56 | <0.001 | ||
| Estrous females | 0.728 | 0.099 | 7.34 | <0.001 | ||
| Rank*Estrous females | 1.636 | 0.411 | 3.98 | <0.001 | ||
| Rank*Instability | 0.626 | 0.334 | 1.87 | 0.061 | ||
| CONTROLS | ||||||
| Age | 0.004 | 0.005 | 0.80 | 0.422 | ||
| Ripe fruit | 0.021 | 0.202 | 0.10 | 0.918 | ||
| Random effects | ||||||
| Chimp ID | 0.044 | 0.211 | 29.92 | <0.001 | ||
| Rank*Chimp ID | 0.223 | 0.472 | 1.40 | 0.237 | ||
n = 910 male months, 17 unique males. Significance of fixed effects was determined via type III Wald chi-square tests. Significance of random effects and interactions was determined via LLRTs.
Figure 3.
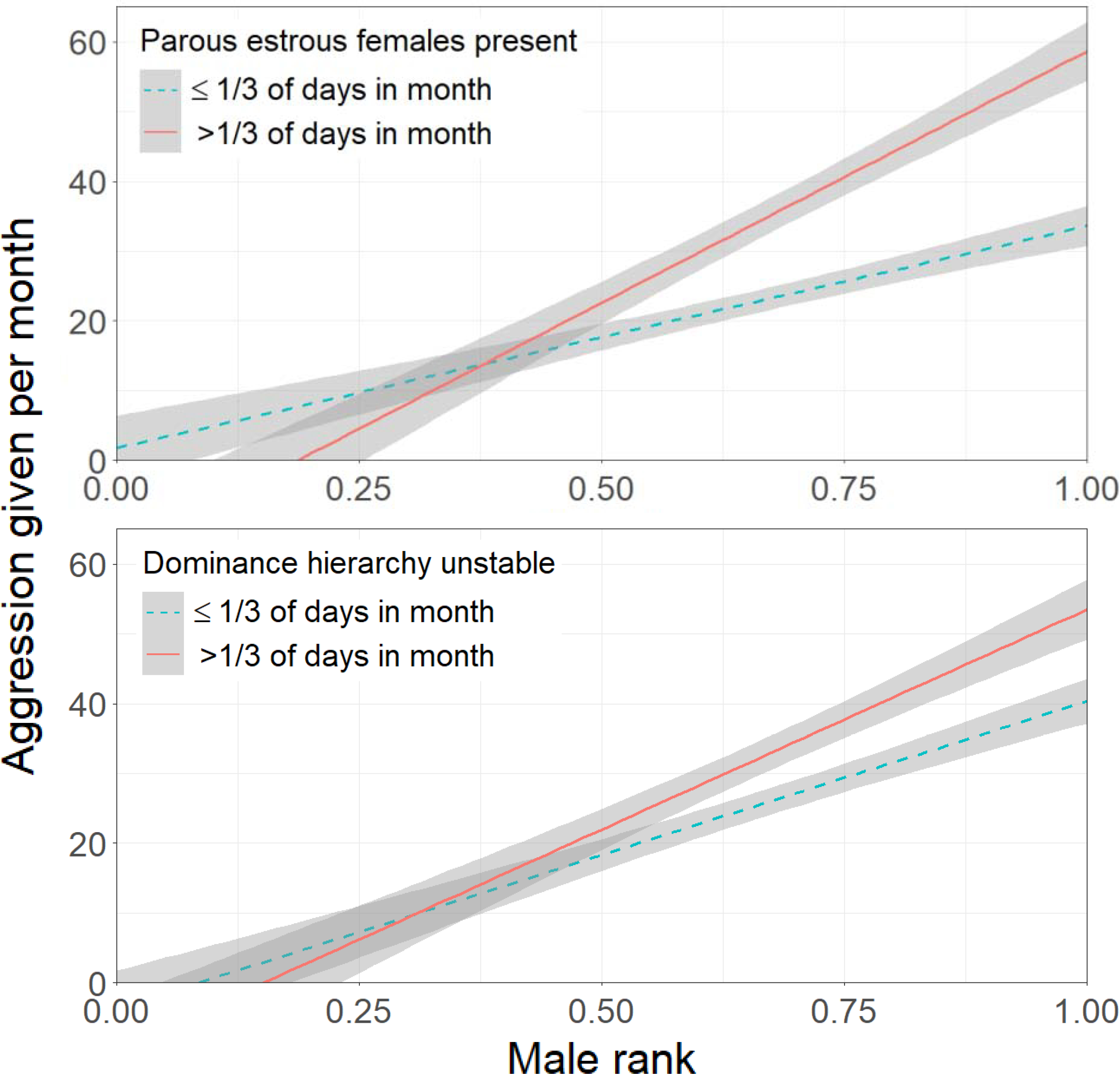
Monthly aggression given by male chimpanzees by rank (n=910 months, 17 adults). Across contexts, high-ranking males were more aggressive than low-ranking males. Males were more aggressive in months when they associated more with parous estrous females (top panel) and in months with more days of instability in the dominance hierarchy (bottom panel). Parous estrous females had a greater effect on the amount of aggression given by higher-ranked males. Counts were based on a mean monthly observation time of 122 hours. Although instability and presence of estrous females were continuous variables in the models, for visualization purposes they are shown here as categorical variables. The shaded areas indicate 95% confidence intervals. (Figure S2 plots the same data showing individual points.)
Mating opportunity had a substantial effect on male aggression rates that was structured by rank (estimate = 1.083, SE = 0.266, z = 4.08, P < 0.001). The lowest-ranking males did not show increased aggression during months in which they spent more time with parous estrous females (Figure 3). With increasing rank, however, males showed increasing aggression in months with more mating days. Males were more aggressive during months containing more days with an unstable hierarchy (estimate = 0.185, SE = 0.047, z = 3.93, P < 0.001).
Adult males became less aggressive with increasing age (estimate = −0.028, SE = 0.005, z = −5.49, P < 0.001). Dietary quality was not associated with aggression given, when other predictors were accounted for (estimate = 0.070, SE = 0.114, z = 0.61, P = 0.540).
Predictors of received aggression, in the form of chases and attacks, followed the same pattern as aggression given, but the effect of dominance rank was reversed. Specifically, high-ranking males received less aggression than low-ranking males, in both stable and unstable hierarchies, and in both mating and non-mating contexts (estimate = −1.470, SE = 0.253, z = −5.80, P < 0.001; Figure 4). Across ranks, males received more aggression in months containing more days with an unstable hierarchy (estimate = 0.375, SE = 0.082, z = 4.56, P < 0.001).
Figure 4.
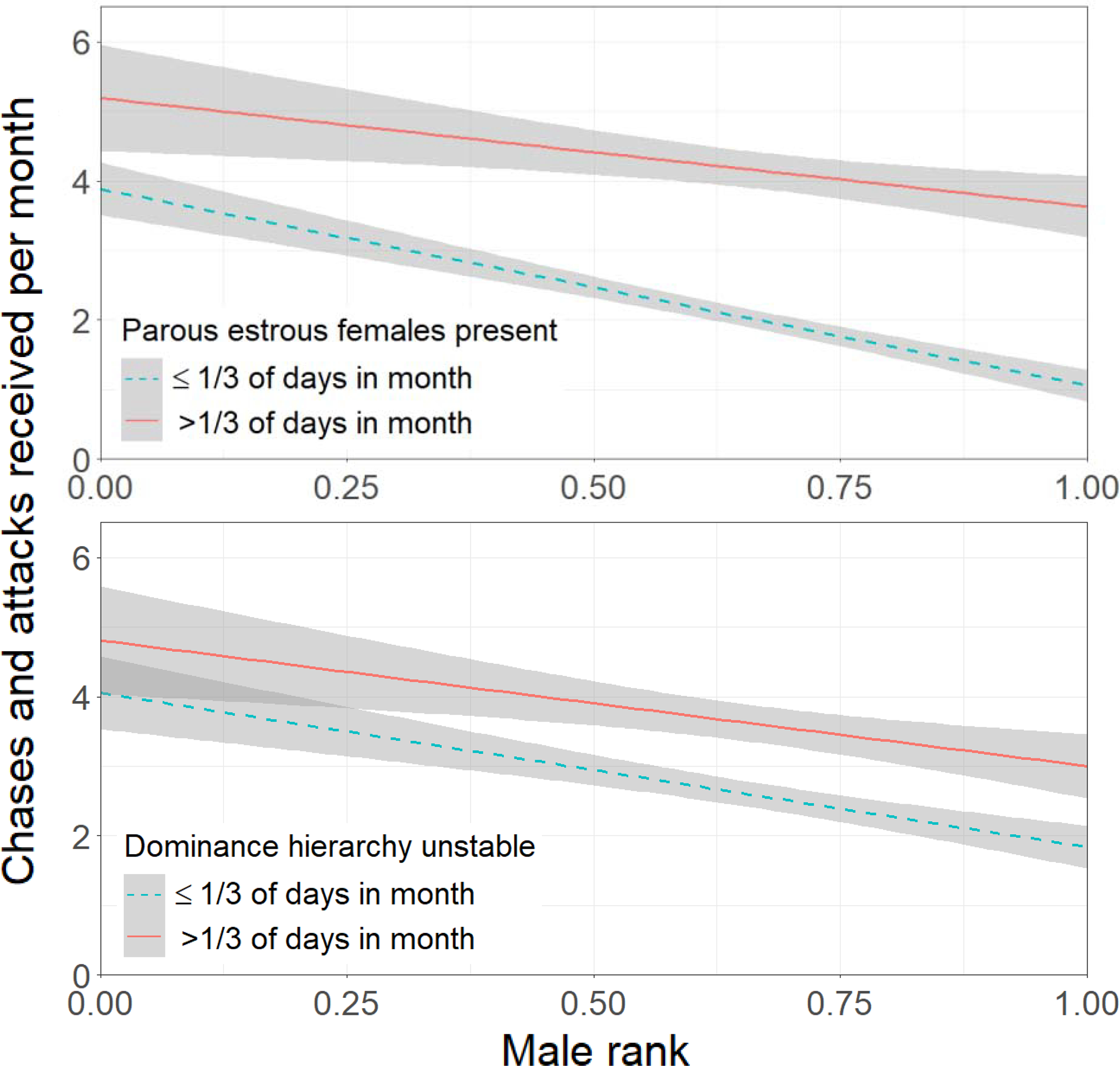
Monthly aggression received by male chimpanzees by rank (n=743 months, 17 adults). Across contexts, high-ranking males received less aggression than low-ranking males. Males received more aggression in months when they associated more with parous estrous females (top panel) and in months with more days of instability in the dominance hierarchy (bottom panel). Parous estrous females had a greater effect on the amount of aggression received by higher-ranked males. Counts were based on a mean monthly observation time of 126 hours. Although instability and presence of estrous females were continuous variables in the models, for visualization purposes they are shown here as categorical variables. The shaded areas indicate 95% confidence intervals. (Figure S3 plots the same data showing individual points.)
All males received more aggression during months in which they spent more time in parties with parous estrous females (estimate = 0.728, SE = 0.099, z = 7.34, P < 0.001). This interacted with rank, however, such that the increase was greater for higher-ranked males (estimate = 1.636, SE = 0.411, z = 3.98, P < 0.001, Figure 4).
Male age was not associated with high-level aggression received (estimate = 0.004, SE = 0.005, z = 0.80, P = 0.422), nor was dietary quality (estimate = 0.021, SE = 0.202, z = 0.10, P = 0.918).
Monthly aggression and glucocorticoids model.
Output from the full model is presented in Table 5. Neither dominance rank (estimate = 0.132, SE = 0.262, χ2 = 0.25, P = 0.615) nor the number of days with an unstable hierarchy (estimate = −0.053, SE = 0.061, χ2 = 0.75, P = 0.386) was a significant predictor of mean monthly glucocorticoid values when aggression rates were included as predictors in the model. By contrast, the proportion of observations in association with parous estrous females continued to have a strong effect on mean monthly glucocorticoid values (estimate = 0.644, SE = 0.079, χ2 = 66.2, P < 0.001). Males showed elevated glucocorticoids during months in which they were more aggressive (estimate = 0.417, SE = 0.177, χ2 = 5.56, P = 0.018), and also during months in which they received more aggression (estimate = 1.972, SE = 0.961, χ2 = 4.21, P = 0.040). Glucocorticoids increased with male age (estimate = 0.008, SE = 0.004, χ2 = 3.86, P = 0.049), but were not influenced by dietary quality (estimate = −0.170, SE = 0.174, χ2 = 0.96, P = 0.328).
Table 5.
Predictors of monthly mean glucocorticoids in male chimpanzees (Model 4)
| Term | Estímate | Variance | SE | SD | χ2 | P |
|---|---|---|---|---|---|---|
| Intercept | 6.261 | 0.072 | 7465.5 | <0.001 | ||
| TEST | ||||||
| Rank | 0.132 | 0.262 | 0.3 | 0.615 | ||
| Instability | −0.053 | 0.061 | 0.8 | 0.386 | ||
| Estrous females | 0.644 | 0.079 | 66.2 | <0.001 | ||
| Ripe Fruit | −0.170 | 0.174 | 1.0 | 0.328 | ||
| Aggression given | 0.417 | 0.177 | 5.6 | 0.018 | ||
| Aggression received | 1.972 | 0.961 | 4.2 | 0.040 | ||
| CONTROLS | ||||||
| Age | 0.008 | 0.004 | 3.9 | 0.049 | ||
| Years to assay | 55.8 | <0.001 | ||||
| 4–6 y | −0.399 | 0.057 | ||||
| 7–9 y | −0.449 | 0.133 | ||||
| Random effects | ||||||
| Chimp ID | 0.019 | 0.138 | 16.3 | <0.001 | ||
| Rank*Chimp ID | 0.603 | 0.776 | 10.7 | 0.001 | ||
n = 743 male months, 17 males. Significance of fixed effects was determined via type III Wald chi-square tests. Significance of random effects and interactions was determined via LLRTs.
In a final model, we looked at the same predictors and controls, but collapsed aggression given and high-level aggression received into a single variable - monthly rate of involvement in aggression. The results mirror those just discussed (Table S2).
Discussion
Across two decades in Kanyawara, dominant males showed chronically elevated glucocorticoid levels. In contrast with yellow baboons (Gesquiere et al. 2011), this effect was not restricted to the alpha male. Rather, glucocorticoids were positively associated with ordinal rank in both stable and unstable hierarchies, and in the presence and absence of estrous females. These findings support the costs of dominance hypothesis over the stress of subordination hypothesis. For chimpanzee males, acquiring and maintaining rank was energetically expensive, imposing persistent physiological costs that subordinates were able to avoid. The fact that a positive association between glucocorticoids and rank persisted even when controlling for prominent short-term stressors, such as rank instability and the availability of estrous females, is evidence against the acute costs of dominance/chronic costs of subordinance hypothesis.
Both giving and receiving aggression were associated with increased glucocorticoid production. Per incident, receiving high-level aggression was a more potent stressor, corresponding to a five-fold increase in glucocorticoids compared to giving aggression (Table 5). However, males gave aggression, primarily in the form of costly dominance displays, around ten times as often as they were chased or attacked. Consequently, aggression given explained more of the total variance in glucocorticoid excretion.
Giving aggression was also the primary mechanism linking glucocorticoids with rank. Dominant males received less high-level aggression than subordinates, but the absolute difference across ranks was modest. For aggression given, by contrast, dominants routinely showed markedly higher rates than subordinates, regardless of hierarchy stability or mating opportunity. When aggression given, aggression received, and rank were included in the same model (Table 5), only the aggression terms were predictive of mean monthly glucocorticoid levels.
Why is aggression so stressful? In part, this likely reflects direct metabolic costs. The most frequent form of chimpanzee male aggression, the charging display, involved a mix of running upright, dragging or throwing branches, swaying vegetation, stomping or slapping the ground, and banging on the buttresses of large trees. Displays were often protracted and elaborate, and males were sometimes observed panting after performing them. Initiating aggression is also likely to be intrinsically stressful psychologically. Although high-ranking chimpanzee males can normally expect to win their fights, the widespread use of coalitions means that the outcome of any particular interaction cannot be guaranteed. A male might commence an ostensibly safe attack on a lower-ranked victim, yet end up fleeing from a group of cooperating males. The distinction between physiological and psychological stressors is tenuous, however, as psychosocial factors would not be expected to induce a glucocorticoid response if they did not reliably anticipate genuine metabolic need.
The largest single factor affecting male glucocorticoid levels was the presence of parous estrous females. Males of all ranks showed substantial glucocorticoid increases in the presence of such females. This response was partly driven by aggression, both given and received, as high-ranking males were more likely to be perpetrators, and low-ranking males victims, in reproductive contexts. However, even controlling for involvement in aggression, males showed elevated glucocorticoids in response to parous estrous females. This likely reveals opportunity costs of male mating effort. Males exhibited heightened vigilance in the presence of estrous mothers, closely monitoring their movements and those of male rivals. Dominant males frequently attempted to prevent others from mating, while subordinates sought opportunities to mate surreptitiously. These pursuits interfered with feeding. For example, Georgiev et al. (2014) reported that Kanyawara males of all ranks reduced their feeding time by an average of 25.5% (about one hour per day) during periods when parous estrous females were available. Males did not compensate for lost feeding time by eating higher quality foods, resulting in a trade-off between mating effort and energy intake.
Instability in the dominance hierarchy was also associated with elevated glucocorticoids in males, but this effect was stronger in higher-ranked individuals, and mediated by aggression. Males who were directly involved in rank changes failed to show disproportionate glucocorticoid increases during periods of instability. It may seem surprising that a high-ranking male was more affected by a reversal at the bottom of the hierarchy than were the males whose ranks actually changed. Young males, however, initiate their careers by challenging individuals at the bottom of the hierarchy, who are often elderly (Goodall 1986). If successful, they predictably move on to provoke males of higher status. Consequently, any threat to the status quo differentially imperils males at the top of the hierarchy, who have further to fall.
Why were high-ranking males more aggressive than low-ranking males, even in stable dominance hierarchies and non-mating contexts? Muller (2002) proposed that because chimpanzee grouping patterns are so variable, dominant males can never know what political maneuvering has occurred in their absence. Consequently, they must continually be alert to the possibility of shifting coalitions and status challenge, and habitually reassert their dominance through costly displays. This explains the large proportion of chimpanzee aggression that takes place in the context of reunions, when two parties meet after a period of separation (Bygott 1979, Goodall 1986). The same dynamic is not expected in species with stable groups, or less pervasive status competition.
Our measure of dietary quality was not associated with male glucocorticoid levels. This finding differs from a previous study in Kanyawara, in which we reported that males showed elevated glucocorticoids during a 4-month period of low food availability in 1998 (Muller and Wrangham 2004b). This inconsistency is likely explained by the fact that, over that 4-month period, chimpanzees ate ripe fruit in only 20% of feeding observations. That was the lowest level of fruit consumption recorded in 25 years of study (mean=64%, SD=15.8, n=300 months). This suggests that glucocorticoids did rise when fruit availability fell below a critical threshold, but that under most conditions, Kanyawara males adequately compensated for a lack of fruit by falling back on abundant piths and herbs (Wrangham et al. 1996). It is notable that even during the worst period of fruit availability, there was no evidence that chimpanzees rapidly lost condition. For example, out of 926 urine samples collected from community members in 1998, none tested positive for ketones, an indicator of fat mobilization (Muller and Wrangham 2005). This contrasts with orangutans in Gunung Palung National Park, who routinely tested positive for urinary ketones during lean periods (Knott 1998).
Finally, glucocorticoids increased with male age, despite older males being less involved in aggression, and less likely to be high ranking. We have previously shown that this increase is probably due to impairments of hypothalamic–pituitary–adrenal regulation that are intrinsic to the aging process (Emery Thompson et al. 2020). Given that both aging and status competition are associated with increased glucocorticoid production, physiological stress may be an important mechanism that constrains a male’s ability to maintain high status across the life course.
The fact that acquiring and maintaining high rank entailed chronically elevated glucocorticoids in male chimpanzees is consonant with previous studies from Kanyawara documenting physiological costs to male status striving. For example, dominant males had lower C-peptide levels than subordinates, reflecting less favorable energy balance (Emery Thompson et al. 2009). They also maintained higher testosterone levels than subordinates (Muller and Wrangham 2004a), indicating elevated energetic costs and potential immunosuppression (Foo et al. 2017, Muehlenbein and Bribiescas 2005, Wingfield et al. 1997).
Were glucocorticoid levels in dominant males high enough to cause reductions in fitness (e.g. Breuner et al. 2008)? Beehner and Bergman (2017: 78) argue that animals in their natural environments are unlikely to experience such reductions, insisting “the starting point for research inquiry should be the assumption that the measured endocrine profile is adaptive.” There is no reason to suspect a priori that elevated glucocorticoids in dominant male chimpanzees should reduce fitness, as high-ranking males have repeatedly been shown to sire more offspring than low-ranking males (Boesch et al. 2006, Newton-Fisher et al. 2010, Surbeck et al. 2017, Wroblewski et al. 2009). However, these reproductive gains potentially come at the expense of health and longevity. As previously noted, repeatedly activating the glucocorticoid stress response uses energy that could otherwise be put into somatic maintenance, increasing wear and tear on an organism (Romero et al. 2009, Sapolsky 1993a). Consequently, in male chimpanzees, glucocorticoids may serve as a proxy for reproductive investment that trades off against long-term survival (cf. Boonstra et al. 2001). Testosterone is also thought to be involved in this tradeoff, increasing male fitness by steering investment toward mating effort, at the expense of maintenance and longevity (Brooks and Garratt 2017, Muller 2017).
We have previously shown that high rank is a risk factor for respiratory disease (the leading cause of chimpanzee mortality) in older, but not younger, males in Kanyawara (Emery Thompson et al. 2018). This finding suggests a long-term tradeoff between dominance striving and health and longevity. However, direct links among glucocorticoids, testosterone, and health are the subject of ongoing investigation at the site.
Female chimpanzees do not persistently fight over status in the male manner (Foerster et al. 2016), and current evidence suggests that their tradeoffs between reproductive effort and maintenance are less stark. In a previous study at Kanyawara, for example, dominant females maintained lower glucocorticoid levels than subordinates, especially during the energetically challenging period of lactation (Emery Thompson et al. 2010). A new longitudinal analysis found a negligible association between rank and female glucocorticoids, but this only considered rank as a control, and did not explore interactions with factors such as reproductive state (Emery Thompson et al. 2020). Distinct from males, rank was not predictive of respiratory disease in Kanyawara females (Emery Thompson et al. 2018).
Snyder-Mackler et al. (2020) recently proposed that social mammals generally show positive relationships among status, health, and survival. This review was biased toward data on females, however, who are the focus of most demographic research, and often show positive correlations between rank and longevity. Data from a range of vertebrates suggests that high rank in males frequently incurs costs, including elevated parasitism (Habig and Archie 2015, Habig et al. 2018) and glucocorticoid production (Beehner and Bergman 2017; Cavigelli and Caruso 2015; Creel 2001, 2005). In some species only high-quality males, who can afford such costs, become dominant, resulting in a positive correlation between reproductive effort and survival (Cram et al. 2018, Lloyd et al. 2020, von Holst et al. 1999). In many species, however, males who invest heavily in mating effort show either reduced longevity compared to other males (Beirne et al. 2015, Lemaître et al. 2018, Robinson et al. 2006, Verhulst et al. 2014) or no association between rank and longevity (Creel and Creel 2002, Hoogland 1995, McElligott and Hayden 2000). Thus, the data from Kanyawara may reflect a broader sex difference in the effects of status striving and mating effort on health that deserves further attention.
Supplementary Material
Highlights.
Male chimpanzees had elevated glucocorticoids when competing for status.
Male chimpanzees had elevated glucocorticoids when competing over mates.
Rank correlated positively with glucocorticoids in both mating and non-mating contexts.
Rank correlated positively with glucocorticoids in both stable and unstable hierarchies.
Giving aggression was the main behavior linking glucocorticoids with rank.
Acknowledgements
Work at Kanyawara was supported by the U.S. National Science Foundation (grants NCS-FO-1926737, BCS-0849380, BCS-1355014, IOS-LTREB 1052693, and DGE-0237002), NIH grants AI058715 and R01AG049395, the Leakey Foundation, the National Geographic Society, and the Wenner-Gren Foundation. The Uganda Wildlife Authority, Uganda National Council of Science and Technology, and Makerere University Biological Field Station granted research permission. We thank the Kibale Chimpanzee Project field staff for data collection, and Emily Otali for field management. We thank Kristin Sabbi and Andreas Berghänel for assistance with laboratory assays and Lindsey Hagberg and Ashley Menante for data management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers P, De Vries H 2001. Elo-rating as a tool in the sequential estimation of dominance strength. Anim. Behav 61, 489–495. [Google Scholar]
- Alberts SC 2012. Magnitude and sources of variation in male reproductive performance. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB (Eds.), The Evolution of Primate Societies. University of Chicago Press, Chicago, pp. 412–431. [Google Scholar]
- Alberts SC, Campos FA, Altmann J, Gesquiere L, Archie EA 2019. Glucocorticoid levels predict lifespan in wild female baboons Am. J. Phys. Anth S68, 4. [Google Scholar]
- Altmann J 1974. Observational study of behavior: Sampling methods. Behav. 49, 227–267. [DOI] [PubMed] [Google Scholar]
- Anestis SF, Breakey AA, Beuerlein MM, Bribiescas RG 2009. Specific gravity as an alternative to creatinine for estimating urine concentration in captive and wild chimpanzee (Pan troglodytes) samples. Am. J. Primatol 71, 130–135. [DOI] [PubMed] [Google Scholar]
- Arlet ME, Grote MN, Molleman F, Isbell LA, Carey JR, 2009. Reproductive tactics influence cortisol levels in individual male gray-cheeked mangabeys (Lophocebus albigena). Horm. Behav 55, 210–216. [DOI] [PubMed] [Google Scholar]
- Arlt W, Stewart PM 2005. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol. Metab. Clin 34, 293–313. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ 2017. The next step for stress research in primates: To identify relationships between glucocorticoid secretion and fitness. Horm. Behav 91, 68–83. [DOI] [PubMed] [Google Scholar]
- Beirne C, Delahay R, Young A 2015. Sex differences in senescence: The role of intra-sexual competition in early adulthood. Proc Biol Sci 282, 20151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC 2001. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav 73, 261–271. [DOI] [PubMed] [Google Scholar]
- Boesch C, Kohou G, Néné H, Vigilant L 2006. Male competition and paternity in wild chimpanzees of the Taï Forest. Am. J. Phys. Anth 130, 103–115. [DOI] [PubMed] [Google Scholar]
- Boonstra R, McColl CJ, Karels TJ 2001. Reproduction at all costs: the adaptive stress response of male arctic ground squirrels. Ecology 82, 1930–1946. [Google Scholar]
- Boonstra R 2013. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol 27, 11–23. [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP 2008. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol 157, 288–295. [DOI] [PubMed] [Google Scholar]
- Brooks RC, Garratt MG 2017. Life history evolution, reproduction, and the origins of sex- dependent aging and longevity. Ann. N.Y. Acad. Sci 1389, 92–107. [DOI] [PubMed] [Google Scholar]
- Buchwald H 1964. The expression of urine analysis results - Observations on the use of a specific gravity correction. Ann. Occup. Hyg 7, 125–136. [DOI] [PubMed] [Google Scholar]
- Bygott JD 1979. Agonistic behavior, dominance, and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg D, McCown E (Eds.) The Great Apes. Benjamin/Cummings, Menlo Park, CA, pp. 405–427. [Google Scholar]
- Cavigelli SA, Caruso MJ 2015. Sex, social status and physiological stress in primates: The importance of social and glucocorticoid dynamics. Phil. Trans. R. Soc. B 370, 20140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T 2016. Mammal Societies. John Wiley & Sons; Chichester, West Sussex, UK. [Google Scholar]
- Conklin-Brittain NL, Wrangham RW, Hunt KD 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance: II. Macronutrients. Int. J. Primatol 19, 971–987. [Google Scholar]
- Cram DL, Monaghan P, Gillespie R, Dantzer B, Duncan C, Spence-Jones H, Clutton-Brock T 2018. Rank-related contrasts in longevity arise from extra-group excursions not delayed senescence in a cooperative mammal. Curr. Biol 28, 2934–2939. [DOI] [PubMed] [Google Scholar]
- Creel S 2001. Social dominance and stress hormones. TREE 16, 491–497. [Google Scholar]
- Creel S 2005. Dominance, aggression, and glucocorticoid levels in social carnivores. J. Mammal 86, 255–264. [Google Scholar]
- Creel S, Creel NM 2002. The African Wild Dog: Behavior, Ecology, and Conservation. Princeton University Press, Princeton, NJ. [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR 2013. The ecology of stress: Effects of the social environment. Funct. Ecol 27, 66–80. [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M 1993. Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Front. Neuroendocrinol 14, 303–47. [DOI] [PubMed] [Google Scholar]
- Ellis L 1995. Dominance and reproductive success among nonhuman animals: A cross-species comparison. Ethol. Sociobiol 16, 257–333. [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW, Lwanga JS, Potts KB 2009. Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm. Behav 55, 299–305. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Kahlenberg SM, Wrangham RW 2010. Dynamics of social and energetic stress in wild female chimpanzees. Horm. Behav 58, 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW 2012. Technical note: Variation in muscle mass in wild chimpanzees: Application of a modified urinary creatinine method. Am. J. Phys. Anthropol 149, 622–627. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN & Wrangham RW. 2014. Male chimpanzees compromise the foraging success of their mates in Kibale National Park, Uganda. Behav. Ecol. Sociobiol 68, 1973–1983. [Google Scholar]
- Emery Thompson M, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TL, Chapman CA, Wrangham RW 2018. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii). R. Soc. Open Sci 5, 180840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Fox SA, Berghänel A, Sabbi K, Phillips-Garcia S, Enigk D, Otali E, Machanda ZP, Wrangham RW & Muller MN. 2020. Aging of the glucocorticoid stress response in wild chimpanzees. PNAS. 117, 8424–8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Georgiev AV 2014. The high price of success: Costs of mating effort in male primates. Int. J. Primatol 35, 609–627. [Google Scholar]
- Emlen DJ 2008. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst 39, 387–413. [Google Scholar]
- Fawcett K, Muhumuza G 2000. Death of a wild chimpanzee community member: Possible outcome of intense sexual competition. Am. J. Primatol 51, 243–247. [DOI] [PubMed] [Google Scholar]
- Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE 2016. Chimpanzee females queue but males compete for social status. Sci. Rep 6, 35404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo YZ, Nakagawa S, Rhodes G, Simmons LW 2017. The effects of sex hormones on immune function: a meta-analysis. Biol. Rev 92, 551–571. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MC, Onyango PO, Alberts SC, Altmann J 2011. Life at the top: rank and stress in wild male baboons. Science 333, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Pokempner AA, Wrangham RW 2010. A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behaviour. Folia Primatol. 81, 254–264. [DOI] [PubMed] [Google Scholar]
- Goodall J 1986. The Chimpanzees of Gombe: Patterns of Behavior. Harvard University Press, Cambridge, MA. [Google Scholar]
- Goymann W, Wingfield JC 2004. Allostatic load, social status and stress hormones: The costs of social status matter. Anim. Behav 67, 591–602. [Google Scholar]
- Habig B, Archie EA 2015. Social status, immune response and parasitism in males: A meta-analysis. Philos. T. Roy. Soc. B 370, 20140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig B, Doellman MM, Woods K, Olansen J, Archie EA 2018. Social status and parasitism in male and female vertebrates: A meta-analysis. Sci. Rep 8, 3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R 2001. Chimpanzee mortality in the wild. J. Hum. Evol 40, 437–450. [DOI] [PubMed] [Google Scholar]
- Hoogland JL 1995. The Black-Tailed Prairie Dog: Social Life of a Burrowing Mammal. University of Chicago Press, Chicago. [Google Scholar]
- Isabirye-Basuta G 1988. Food competition among individuals in a free-ranging chimpanzee community in Kibale Forest, Uganda. Behav 105, 135–147. [Google Scholar]
- Kaburu SSK, Inoue S, Newton-Fisher NE 2013. Death of the alpha: Within-community lethal violence among chimpanzees of the Mahale Mountains National Park. Am. J. Primatol 75, 789–797. [DOI] [PubMed] [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Muller MN, Wrangham RW 2008. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim. Behav 76, 1497–1509. [Google Scholar]
- Key C, Ross C 1999. Sex differences in energy expenditure in non–human primates. Proc. R. Soc. Lond. B 266, 2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott CD 1998. Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int. J. Primatol 19, 1061–1079. [Google Scholar]
- Knott CD, Kahlenberg S 2007. Orangutans in perspective: Forced copulations and female mating resistance. In: Campbell CJ, Fuentes A, Mackinnon KC, Panger M, Bearder S (Eds.), Primates in Perspective. Oxford University Press, Oxford, pp. 290–305. [Google Scholar]
- Koren L, Mokady O, Geffen E, 2008. Social status and cortisol levels in singing rock hyraxes. Horm. Behav 54, 212–216. [DOI] [PubMed] [Google Scholar]
- Krause J 1994. Differential fitness returns in relation to spatial position in groups. Biol. Rev 69, 187–206. [DOI] [PubMed] [Google Scholar]
- Lemaître JF, Cheynel C, Duhard F, Bourgoin G, Debias F, Ferté H, Gilot-Fromont E, Pardonnet S, Pellerin M, Rey B, Vanpé C, Mark Hewison AJ, Gaillard JM 2018. The influence of early-life allocation to antlers on male performance during adulthood: Evidence from contrasted populations of a large herbivore. J. Anim. Ecol 87, 921–932. [DOI] [PubMed] [Google Scholar]
- Lloyd KJ, Oosthuizen WC, Bester MN, de Bruyn PJN 2020. Trade-offs between age-related breeding improvement and survival senescence in highly polygynous elephant seals: Dominant males always do better. J Anim Ecol 89, 897–909. [DOI] [PubMed] [Google Scholar]
- MacCormick HA, MacNulty DR, Bosacker AL, Lehman C, Bailey A, Collins DA, Packer C 2011. Male and female aggression: lessons from sex, age, and injury in olive baboons. Behav. Ecol 23, 684–691. [Google Scholar]
- Majolo B, Lehmann J, de Bortoli Vizioli A and Schino G 2012. Fitness- related benefits of dominance in primates. Am. J. Phys. Anthropol 147, 652–660. [DOI] [PubMed] [Google Scholar]
- McElligott AG, Hayden TJ 2000. Lifetime mating success, sexual selection and life history of fallow bucks (Dama dama). Behav. Ecol. Sociobiol 48, 203–210. [Google Scholar]
- Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, O’Connor KA 2004. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin. Chem 50, 924–932. [DOI] [PubMed] [Google Scholar]
- Mooring MS, Patton ML, Lance VA, Hall BM, Schaad EW, Fetter GA, Fortin SS, McPeak KM, 2006. Glucocorticoids of bison bulls in relation to social status. Horm. Behav 49, 369–375. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Watts DP 2010. The costs of dominance: testosterone, cortisol and intestinal parasites in wild male chimpanzees. Biopsychosoc. Med 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN 2002. Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant L (Eds.), Behavioral Diversity in Chimpanzees and Bonobos. Cambridge University Press, Cambridge, pp. 112–124. [Google Scholar]
- Muller MN 2017. Testosterone and reproductive effort in male primates. Horm. Behav 91, 36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN 2017. Sexual coercion in chimpanzees and humans. In: Muller MN, Wrangham RW, Pilbeam DR (Eds.), Chimpanzees and Human Evolution. Harvard University Press, Cambridge, MA, pp. 572–601. [Google Scholar]
- Muller MN, Lipson SF 2003. Diurnal patterns of urinary steroid excretion in wild chimpanzees. Am. J. Primatol 60, 161–166. [DOI] [PubMed] [Google Scholar]
- Muller MN, Mitani JC 2005. Conflict and cooperation in wild chimpanzees. Adv. Study Behav 35, 275–331 [Google Scholar]
- Muller MN, Wrangham RW 2004a. Testosterone, dominance and aggression in wild chimpanzees: a test of the challenge hypothesis. Anim. Behav 67, 113–123. [Google Scholar]
- Muller MN, Wrangham RW 2004b. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol 55, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW 2005. Testosterone and energetics in wild chimpanzees. Am. J. Primatol 66, 119–130. [DOI] [PubMed] [Google Scholar]
- Muller MN, Emery Thompson M, Wrangham RW 2006. Male chimpanzees prefer mating with old females. Curr. Biol 16, 2234–2238. [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW 2007. Male coercion and the costs of promiscuous mating for female chimpanzees. Proc. R. Soc. Lond. B 274, 1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW 2014. Mortality rates among Kanyawara chimpanzees. J. Hum. Evol. 66, 107–114. [DOI] [PubMed] [Google Scholar]
- Munro CJ, Lasley BL 1988. Non-radiometric methods for immunoassay of steroid hormones. In: Albertson BD, Haseltine FP (Eds.), Non-Radiometric Assays: Technology and Application in Polypeptide and Steroid Hormone Detection. Alan R. Liss Inc., New York, pp. 289–329. [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Maulana A, Agil M, Widdig A, Engelhardt A 2011. Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Anim. Behav 82, 911–921. [Google Scholar]
- Newton-Fisher NE, Emery Thompson M, Reynolds V, Boesch C, Vigilant L 2010. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am. J. Phys. Anth 142, 417–428. [DOI] [PubMed] [Google Scholar]
- Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E 2017. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev 38, 3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride ER 2005. High faecal glucocorticoid levels predict mortality in ring-tailed lemurs (Lemur catta). Biol. Lett 1, 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruetz JD, Boyer Ontl K, Cleaveland E, Lindshield S, Marshack J, Wessling EG 2017. Intragroup lethal aggression in west African chimpanzees (Pan troglodytes verus): Inferred killing of a former alpha male at Fongoli, Senegal. Int. J.Primatol 38, 31–57. [Google Scholar]
- Pusey AE, Oehlert GW, Williams JM, Goodall J 2005. Influence of ecological and social factors on body mass of wild chimpanzees. Int. J.Primatol. 26, 3–31. [Google Scholar]
- Rakotoniaina JH, Kappeler PM, Kaesler E, Hämäläinen AM, Kirschbaum C, Kraus C 2017. Hair cortisol concentrations correlate negatively with survival in a wild primate population. BMC Ecol. 17, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins AM, Stoinski T, Fawcett K and Robbins MM 2011. Lifetime reproductive success of female mountain gorillas. Am. J. Phys. Anthropol 146, 582–593. [DOI] [PubMed] [Google Scholar]
- Robinson MR, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LE 2006. Live fast, die young: Trade-offs between fitness components and sexually antagonistic selection on weaponry in Soay sheep. Evol. 60, 2168–2181. [PubMed] [Google Scholar]
- Rolff J 2002. Bateman’s principle and immunity. Proc. R. Soc. Lond. B 269, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE 2009. The reactive scope model - A new model integrating homeostasis, allostasis, and stress. Horm. Behav. 55, 375–389. [DOI] [PubMed] [Google Scholar]
- Romero LM, Wingfield JC 2016. Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. Oxford University Press, Oxford. [Google Scholar]
- Sabbi KH, Muller MN, Fox SA, Machanda ZP, Otali E, Wrangham RW, Emery Thompson M 2020. Human-like adrenal development in wild chimpanzees: A longitudinal study of cortisol and dehydroepiandrosterone-sulfate. Am. J. Primatol e23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni L, Tkaczynski P, Deschner T, Löhrrich T, Wittig RM, Crockford C 2020. Maternal effects on offspring growth indicate post-weaning juvenile dependence in chimpanzees (Pan troglodytes verus). Front. Zool 17, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM 1993a. Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D (Eds.), Behavioral Endocrinology. MIT Press, Cambridge, MA, pp. 287–324. [Google Scholar]
- Sapolsky RM 1993b. The physiology of dominance in stable versus unstable social hierarchies. In: Mason WA, Mendoza SP (Eds.), Primate Social Conflict. SUNY Press, Albany, pp. 171–204. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM 2004. Social status and health in humans and other animals. Ann. Rev. Anthropol 33, 393–418. [Google Scholar]
- Sapolsky RM 2005. The influence of social hierarchy on primate health. Science 5722, 648–652. [DOI] [PubMed] [Google Scholar]
- Sauther ML, Sussman RW, Gould L 1999. The socioecology of the ringtailed lemur: Thirty-five years of research. Evol. Anthropol 8, 120–32. [Google Scholar]
- Schoenle LA, Zimmer C, Vitousek MN 2018. Understanding context dependence in glucocorticoid–fitness relationships: the role of the nature of the challenge, the intensity and frequency of stressors, and life history. Integr. Comp. Biol 58, 777–789. [DOI] [PubMed] [Google Scholar]
- Schoenle LA, Zimmer C, Miller ET, Vitousek MN 2021. Does variation in glucocorticoid concentrations predict fitness? A phylogenetic meta-analysis. Gen. Comp. Endocrinol 300, 113611. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Smith T, Wickings EJ, Knapp LA 2010. Stress, social behaviour, and secondary sexual traits in a male primate. Horm. Behav 58, 720–728. [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler N, Kohn JN, Barreiro LB, Johnson ZP, Wilson ME, Tung J 2016. Social status drives social relationships in groups of unrelated female rhesus macaques. Anim. Behav 111, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, Bartolomucci A, Yang YC, Aiello AE, O’Rand A, Harris KM, Shively CA, Alberts SC, Tung J 2020. Social determinants of health and survival in humans and other animals. Science 368, eaax9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P, Bro-Jørgensen J 2011. Female competition and its evolutionary consequences in mammals. Biol. Rev 86, 341–366. [DOI] [PubMed] [Google Scholar]
- Stoehr AM, Kokko H 2006. Sexual dimorphism in immunocompetence: What does life-history theory predict? Behav. Ecol 17, 751–756. [Google Scholar]
- Struhsaker TT 1997. Ecology of an African Rain Forest. University Press of Florida, Gainesville, FL. [Google Scholar]
- Surbeck M, Deschner T, Weltring A, Hohmann G 2012. Social correlates of variation in urinary cortisol in wild male bonobos (Pan paniscus). Horm. Behav 62, 27–35. [DOI] [PubMed] [Google Scholar]
- Surbeck M, Langergraber KE, Fruth B, Vigilant L, Hohmann G 2017. Male reproductive skew is higher in bonobos than chimpanzees. Curr. Biol 27, R640–R641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Geerdink M, Salomons HM, Boonekamp JJ 2014. Social life histories: Jackdaw dominance increases with age, terminally declines and shortens lifespan. Proc. R. Soc. B 281, 20141045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst D, Hutzelmeyer H, Kaetzke P, Khaschei M, Schönheiter R 1999. Social rank, stress, fitness, and life expectancy in wild rabbits. Naturwissenschaften 86, 388–393. [DOI] [PubMed] [Google Scholar]
- von Rueden CR, Jaeggi AV 2016. Men’s status and reproductive success. PNAS 113, 10824–10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J 1992. Chimpanzee genital swelling and its role in the pattern of sociosexual behavior. Am. J. Primatol 28, 101–113. [DOI] [PubMed] [Google Scholar]
- White BC, Jamison KM, Grieb C, Lally D, Luckett C, Kramer KS, Phillips J 2010. Specific gravity and creatinine as corrections for variation in urine concentration in humans, gorillas, and woolly monkeys. Am. J. Primatol 72, 1082–1091. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Boesch C, Furuichi T, Gilby IC, Hashimoto C, Hobaiter CL, Hohmann G, Itoh N, Koops K, Lloyd JN, Matsuzawa T, Mitani JC, Mjungu DC, Morgan D, Mundry R, Muller MN, Nakamura M, Pruetz J, Pusey AE, Riedel J, Sanz C, Schel AM, Simmons N, Waller M, Watts DP, White F, Wittig R, Zuberbühler K, Wrangham RW 2014. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Jacobs J, Hillgarth N 1997. Ecological constraints and the evolution of hormone-behavior interrelationships. Ann. N. Y. Acad. Sci 807, 22e41. [DOI] [PubMed] [Google Scholar]
- Wrangham RW, Conklin NL, Chapman CA, Hunt KD 1991. The significance of fibrous foods for Kibale Forest chimpanzees. Phil. Trans. R. Soc. B 334, 171–178. [DOI] [PubMed] [Google Scholar]
- Wrangham RW, Chapman CA, Clark AP, Isabirye-Basuta G 1996. Social ecology of Kanyawara chimpanzees: Implications for understanding the costs of great ape groups. In: McGrew WC, Marchant LF, Nishida T (Eds.), Great Ape Societies. Cambridge University Press, Cambridge, pp. 45–57. [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav 77, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Bonnell TR, Brown LR, Dostie MJ, Ganswindt A, Kienzle S, McFarland R, Henzi SP, Barrett L 2019. Climate induced stress and mortality in vervet monkeys. R. Soc. Open Sci 6, 191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


