Abstract
Background:
Limited data suggest air pollution exposures may contribute to pediatric high blood pressure (HBP), a known predictor of adult cardiovascular diseases.
Methods:
We investigated this association in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study, a sociodemographically diverse pregnancy cohort in the southern United States with participants enrolled from 2006 to 2011. We included 822 mother–child dyads with available address histories and a valid child blood pressure measurement at 4–6 y. Systolic (SBP) and diastolic blood pressures (DBP) were converted to age-, sex-, and height-specific percentiles for normal-weight U.S. children. HBP was classified based on SBP or DBP percentile. Nitrogen dioxide () and particulate matter in aerodynamic diameter () estimates in both pre- and postnatal windows were obtained from annual national models and spatiotemporal models, respectively. We fit multivariate Linear and Poisson regressions and explored multiplicative joint effects with maternal nutrition, child sex, and maternal race using interaction terms.
Results:
Mean and in the prenatal period were 10.8 [standard deviation (SD): 0.9] and 10.0 (SD: 2.4) ppb, respectively, and 9.9 (SD: 0.6) and 8.8 (SD: 1.9) ppb from birth to the 4-y-old birthday. On average, SBP percentile increased by 14.6 (95% CI: 4.6, 24.6), and DBP percentile increased by 8.7 (95% CI: 1.4, 15.9) with each increase in second-trimester . averaged over the prenatal period was only significantly associated with higher DBP percentiles [ 11.6 (95% CI: 2.9, 20.2)]. Positive associations of second-trimester with SBP and DBP percentiles were stronger in children with maternal folate concentrations in the lowest quartile ( 0.05 and 0.07, respectively) and associations with DBP percentiles were stronger in female children ( 0.05). We did not detect significant association of , road proximity, and postnatal with any outcomes.
Conclusions:
The findings suggest that higher prenatal exposure, particularly in the second trimester, is associated with elevated early childhood blood pressure. This adverse association could be modified by pregnancy folate concentrations. https://doi.org/10.1289/EHP7486
Introduction
High blood pressure (HBP) is a major contributor to cardiovascular disease (CVD) in adults (Benjamin et al. 2017). In U.S. children and adolescents, the HBP prevalence has been shown to be 15–20% based on a single blood pressure measurement (Bell et al. 2019; Jackson et al. 2018), with the highest rates in Hispanic and African American youth (Cheung et al. 2017). Early identification of children with HBP is crucial given than untreated hypertension is associated with target organ damage in childhood, including left ventricular hypertrophy and decreased arterial compliance (Aatola et al. 2013). In addition, HBP in early life may persist over time and progress to clinical hypertension in adulthood (Chen and Wang 2008). Child HBP is multifactorial. Primary HBP has been chiefly attributed to heredity, obesity, and diet, whereas major determinants of secondary HBP include renal abnormalities, medications, and neoplasms (Patel and Walker 2016). However, currently recognized risk factors do not sufficiently explain disease occurrence, and a growing body of literature suggests that environmental risk factors may play a role (Bruno et al. 2017; Sanders et al. 2018; Warembourg et al. 2019).
Air pollution exposures have been linked with several chronic health conditions in children, including asthma and neurodevelopmental disorders, even in regions where the level of exposure is in compliance with regulations (Kim et al. 2018; Loftus et al. 2019; Orellano et al. 2017). There is mounting evidence from animal studies and epidemiologic studies of adult cohorts implicating air pollution as a driver of cardiovascular outcomes, with a particular focus on ambient particulate matter [PM in aerodynamic diameter ()] and nitrogen dioxide () (Brook et al. 2010; Giorgini et al. 2016). However, studies of prenatal or early life air pollution exposure and child cardiovascular health are sparse. To the best of our knowledge, only two population studies—one in Boston and one in Mexico City (Rosa et al. 2020; Zhang et al. 2018) have estimated the associations of child blood pressure with air pollution exposures in both pre- and postnatal windows, and three other studies—two in the United States and one in Europe (Breton et al. 2016; Madhloum et al. 2019; van Rossem et al. 2015)—have assessed the associations with prenatal exposures only. All of them provided evidence of positive relationships. More studies—mainly conducted in China and Europe—investigated adverse effects of postnatal air pollution exposures on child blood pressure (Bilenko et al. 2015a, 2015b; Clark et al. 2012; Dong et al. 2014, 2015; Lawrence et al. 2018; Liu et al. 2014; Ntarladima et al. 2019; Pieters et al. 2015; Poursafa et al. 2014; Sughis et al. 2012; Wang et al. 2019; Zeng et al. 2017; Zhang et al. 2019). These studies varied by population characteristics, study design, pollutant types, and exposure windows, and many of them found significant associations with one or both blood pressures while some reported null results.
This study seeks to build on the emerging hypothesis that both pre- and postnatal air pollution exposures may increase child blood pressure. Using data from the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study, a large sociodemographically diverse pregnancy cohort in the urban southern United States, we investigated associations of child blood pressure with air pollution exposures in different windows, and assessed whether the associations would be modified by child sex and maternal race. Furthermore, we examined pregnancy nutritional factors as potential modifiers, a topic that has not been previously explored despite their known critical roles in fetal programming.
Methods
Study Population
The CANDLE study is an ongoing longitudinal study established to identify risk factors that impact child neurodevelopment and learning. Women were considered eligible if they were Shelby County, Tennessee, residents between 16–40 y of age, had singleton pregnancies without complications, and planned to deliver at a participating study hospital (Sontag-Padilla et al. 2015). From 2006 to 2011, the research staff recruited 1,503 pregnant women in their second trimester in two stages. The first stage of enrollment took place between December 2006 and August 2008 at the University of Tennessee Medical Group clinics, where 343 women were recruited during their regular obstetric appointments. In the second stage, which happened between September 2007 and July 2011, an additional 1,160 women were recruited from the general community via media campaigns. All women provided informed consent upon enrollment. The enrolled CANDLE population was considered representative of the source population in Shelby County, with similar distributions of major sociodemographic characteristics such as race and household income (Sontag-Padilla et al. 2015). The participants recruited from the Medical Group clinics were more likely to have socioeconomic disadvantages compared with those recruited from the general community. From the prenatal period to child age 3 y, families completed eight in-person visits (two prenatal clinic visits, one hospital visit at delivery, three postnatal clinic visits, and two postnatal home visits) and nine phone-based assessments. At the age 4- to 6-y visit, 1,143 mother–child dyads completed self-administered questionnaires and anthropometric measures, achieving a retention rate of 76%. For this analysis, conducted as part of the ECHO PATHWAYS Consortium (https://deohs.washington.edu/echo/), we included 822 mother–child dyads who completed the age 4- to 6-y visit with blood pressure assessment and had valid residential address histories. All CANDLE research activities were approved by the institutional review board of the University of Tennessee Health Sciences Center, and the analyses were approved by the University of Washington Human Subjects Division.
Blood Pressure Assessment
At the age 4- to 6-y visit, child SBP and DBP were measured by clinical researchers using BP Tru Medical Devices Model BPM-100 blood pressure monitor according to a standardized protocol (NHANES 2009–2010 Procedure Manuals). Arm circumference was measured to select the correct cuff size. Following at least a 2-min rest, child blood pressure was measured twice in the right arm at heart level. Up to four measurements were taken if there was a discrepancy . Final blood pressure values were calculated by averaging the measurements within a difference. Using the American Academy of Pediatrics 2017 Clinical Practice Guideline (Flynn et al. 2017), we calculated sex-, age-, and height-specific blood pressure percentiles based on the U.S. pediatric population with normal weight. HBP was defined as SBP and/or DBP at .
Air Pollution Assessments
Residential addresses were collected at enrollment and updated at each subsequent visit. exposures were estimated using regionalized annual average national models—land-use regressions with universal kriging components (Young et al. 2016). Briefly, the models used monitoring data from regulatory networks, further enhanced with satellite data. We used a geographic information system to identify covariates representing land-use characteristics that could reflect spatial variability in air pollution distributions. Final dimension-reduced regression covariates were obtained using partial least squares from more than 400 geographic variables. Daily concentrations obtained from the U.S. Environmental Protection Agency’s Air Quality System network were aggregated into calendar year annual averages and further modeled separately by region (“East,” “Mountain West,” and “West Coast”). We calculated final exposures by applying the year-specific models from 2006 to 2014 to participants’ residential history corresponding to both pre- and postnatal windows (from birth to the 4-y-old birthday).
exposures were estimated from spatiotemporal models predicting point-based estimates on a 2-wk time scale (Keller et al. 2015). The models used monitoring data from regulatory networks supplemented with measurements from intensive research cohort-specific monitors. The model decomposed the space–time field of concentrations into spatially varying long-term averages, spatially varying seasonal and long-term trends, and spatially correlated but temporally independent residuals. Time trends were estimated from observed time series, and spatial smoothing spline methods were used to borrow strength between observations. We estimated by averaging biweekly predictions over the following windows: first, second, and third trimesters; full prenatal period; and postnatal period from birth to the 4-y-old birthday. Three children born before the 27th gestational week were missing the third-trimester predictions. For families who moved between two points of contact and did not report a move date, we used the midpoint of the two contact dates to calculate the time-weighted averages of and in the relevant exposure windows. Postnatal predictions for families who moved out of Shelby County between childbirth and the age 4- to 6-y visit () were further excluded to avoid outliers. No families moved out of the study area before childbirth.
Finally, we estimated residential distance to the nearest roadways as a proxy of exposure to traffic-related air pollutants. Specifically, major roads were classified as A1, A2, or A3 according to the U.S. Census feature class, and “near road” was defined as a distance of from any major roadway. If a family moved during one of the pre- or postnatal time windows, road proximity was assigned based on the single address with the longest residential history in the window.
Covariates
In the CANDLE study, extensive data collection was conducted on mother, child, and family characteristics, including multilevel social determinants of health. Maternal characteristics included age at delivery, self-identified race (Black vs. non-Black), education (high school and below vs. postsecondary education), pregnancy insurance status (no insurance or Medicaid/Medicare only vs. some or only private insurance), pregnancy smoking measured by urinary cotinine concentrations in the second trimester adjusted by specific gravity (Boeniger et al. 1993), pregnancy maternal psychological distress measured by the Global Severity Index (GSI) from the Brief Symptom Inventory (Derogatis and Melisaratos 1983), maternal hypertensive disorder for the index pregnancy obtained from medical records, gestational age abstracted from birth records, and self-reported breastfeeding practice (never vs. vs. ). Reported family income and household size was collected primarily at enrollment, with missing data supplemented by the information at the following visits. We adjusted the midpoint of each income category by household size, incorporating different weights for adults and children (Burniaux et al. 1998). Prepregnancy height and weight were requested at enrollment. Maternal prepregnancy obesity was defined as a body mass index (BMI) of or higher; overweight was defined as a BMI of ; normal weight was defined as a BMI of ; underweight was defined as a BMI of (Garrow and Webster 1985). Child characteristics included sex, age, height, and BMI at blood pressure assessment and birthweight obtained from birth records. At the age 4- to 6-y visit, parents reported the child’s physical activity level (never or occasionally vs. once or twice per week vs. three or more times per week), secondhand smoking exposures (anyone living in the child’s home smoked vs. no one smoked), and current use of medications. We used the questionnaire data to identify medications that were relatively common in pediatric disease management and may increase blood pressure, such as albuterol, methylphenidate, and prednisone. Child sleep quality was also measured at the same visit by using the Children’s Sleep Habits Questionnaire, the total score of which encompassed the major medical and behavioral sleep disorders (Owens et al. 2000). Neighborhood conditions were estimated using the Childhood Opportunity Index (COI), a spatial measurement of relative childhood neighborhood opportunity (Acevedo-Garcia et al. 2014). We used two of the three “opportunity domains” comprising the COI—educational and economic opportunity—and calculated these indices based on the residual address history in pre- and postnatal windows. Other potential confounders were also incorporated, including recruitment sites (Medical Group clinics vs. general community) that differed in enrollment patterns and socioeconomic status (SES) of participants, and time splines of conception date and visit date.
The Maternal Healthy Eating Index (HEI-2010) was calculated from the Block (2005) Food Frequency Questionnaire during the second trimester (Guenther et al. 2014). In addition, maternal folate concentration was derived from maternal second- and third-trimester blood samples. Plasma folate was assessed using the Lactobacillus casei microbiological assay, with a minimum detection limit of (Roy et al. 2018), and measurements from both trimesters were averaged. We dichotomized HEI at the median, and folate at the first quartile.
Statistical Analysis
We conducted descriptive analyses to summarize the characteristics of the participants. Associations of each outcome measurement with and in each window were examined independently using complete-case analyses, with observations missing data for any model variable excluded. Linear regressions with robust standard error were preformed to quantify the associations between air pollution exposures and continuous blood pressure percentiles, and Poisson regressions with robust standard error were used to estimate the incidence rate ratio (IRR) of binary HBP. To enable comparisons with studies with relatively low levels of air pollutants, effect estimates were rescaled to two-unit increments of and , which were close to interquartile ranges (IQRs) for exposures across different windows in the study area.
We developed directed acyclic graphs (DAGs) to inform our thinking about the role of the various covariates in the associations of interest with pre- and postnatal exposure windows (Textor et al. 2016), based on existing literature on CVD risk factors and biological plausibility (Figure S1), and used a hierarchical adjustment approach of four models to assess the sensitivity of the effect estimates to covariate selection. Model 1 was adjusted for child sex, age, and height at the age 4- to 6-y visit, and recruitment site. In Model 2, we additionally controlled for times splines of both visit date and conception date for and . Time was not adjusted for proximity to major roadway ( vs. ) given its lack of temporal variability in either the pre- or postnatal window. Visit date was used to capture the secular trends of child blood pressure, and time splines for visit date were universally modeled with 1 degree of freedom (df)/y in all models. Conception date was correlated with air pollution concentrations and might be associated with child blood pressure owing to time-varying enrollment patterns. The graphic descriptions of air pollution exposures by conception date showed higher temporal variabilities in than in , and higher temporal variabilities in pollutant aggregations over shorter windows than longer windows (Figure S2). Thus, we modeled conception date with 1 df/y for analyses with , and applied varied df to time splines for in different windows: 8 df/y of conception date for trimester-specific , 4 df/y for prenatal , and 1 df/y for postnatal . Model 3 was considered the full model, and it included other potential confounders: maternal race, maternal age at childbirth, maternal education levels, log-transformed income adjusted by household size, breastfeeding, log-transformed urinary cotinine levels in the second trimester adjusted by specific gravity, BMI class before pregnancy, insurance status, maternal GSI, child sleep scores, child physical activity levels, child use of medication that potentially increased blood pressure, child secondhand smoking exposures, and COI. We further identified and adjusted for several potential confounders that might also be in the causal pathway in Model 4 (the extended model) to estimate their role in the associations of interest—including maternal hypertensive disorder, gestational age, birthweight, and child BMI at the age 4- to 6-y visit in the models with prenatal exposures but only child BMI in the models with postnatal exposures.
Previous research indicated that low SES populations disproportionately exposed to ambient air pollution may have poor nutrition status (Drewnowski et al. 2016; Hajat et al. 2015). It is increasingly recognized that in utero exposure to poor maternal nutrition may predispose to a future cardiometabolic abnormality (Barker 1988, 1999; Whincup 1995). We elected to use maternal HEI because it reflects the adherence to the dietary guideline for general American populations (Guenther et al. 2014). Systematic reviews and large meta-analyses have convincingly shown that higher diet quality as measured by the HEI is associated with lower risks of CVD morbidity and mortality (Schwingshackl et al. 2018; Sotos-Prieto et al. 2015). Sufficient maternal folate intake may protect against elevated blood pressure in children by improving endothelial functions (McRae 2009), and folate biomarkers directly reflect physiological responses to overall intakes after absorptive and metabolic processes (Picó et al. 2019). There is also evidence for sex-specific differences and racial disparities in CVD programming (CDC 2011). As such, we assessed these effect modifications by including cross product terms of maternal HEI ( vs. ), plasma folate (the first quartile vs. the second to fourth quartiles), child sex (male vs. female), or maternal race (non-Black vs. Black) with exposures in each window in separate analyses using the full models, and estimated interaction -values as well as strata-specific associations.
Six sensitivity analyses were performed. a) To disentangle the estimated effects of spatial contrast from temporal variability, we replaced time-varying concentrations from the annual national models and the concentrations from the spatiotemporal models in the pre- and postnatal windows with predictions from 2006 (the first year of recruitment) and 2011 (the first year of age 4- to 6-y visit) fixed-year models respectively, based on residential history during the relevant exposure periods. b) We varied the df for date of conception from zero (no adjustment of conception date) to 12 in fully adjusted models for the second-trimester and prenatal with blood pressure percentiles to evaluate the robustness of results when progressively controlling for temporal trends. c) We simultaneously included estimates in all three trimesters in the fully adjusted model and also adjusted for postnatal exposures in the models with prenatal exposures in each window. d) Based on the significant associations of the second-trimester and prenatal with blood pressure percentiles, we modeled child age and height at the age 4- to 6-y visit flexibly using two-dimensional unpenalized thin-plate regression splines (TPRS) for adjustment in the analyses of two sets of continuous outcomes—blood pressure percentiles and blood pressure raw measurements—to examine their potential combined effect because of multidimensional appearance. TPRS were generated from the MGCV package (Wood 2017) with df varying from 5 to 12. e) To further assess potential nonlinear relationships between the covariates and the outcomes, we adjusted for linear restricted cubic splines of all continuous variables in the fully adjusted models, including child age, child height, maternal age at delivery, income adjusted by household size, maternal GSI, maternal urinary cotinine adjusted by specific gravity, child sleep scores, and COI. f) Considering the left-skewed DBP percentiles, we log transformed this measurement and repeated the primary analysis with fully adjusted models to confirm the patterns of associations. All analyses were conducted in STATA 15 (StataCorp) and R (version 3.6.2; R Development Core Team).
Results
Characteristics of the Study Population
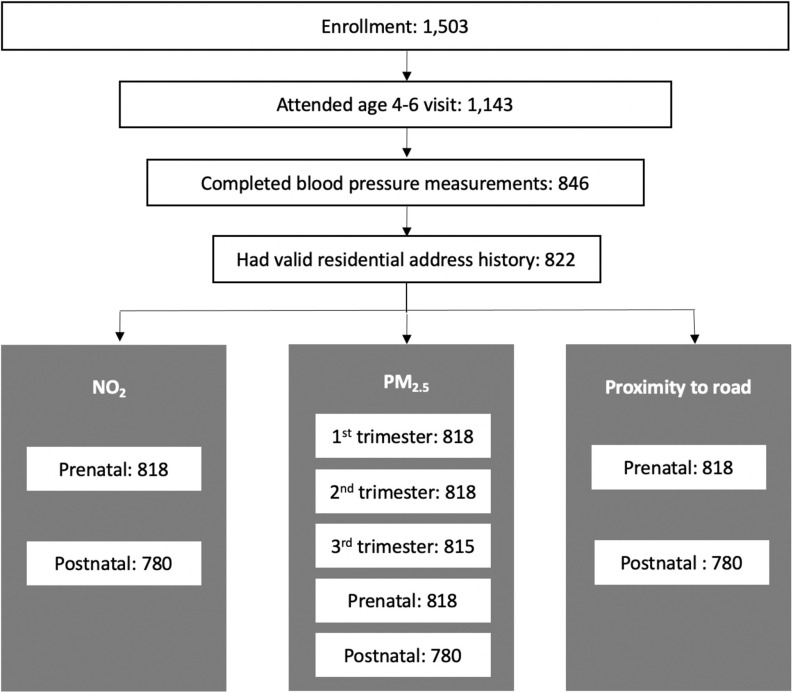
Figure 1 illustrates CANDLE cohort retention between enrollment and the age 4- to 6-y visit as well as sample sizes for primary analysis of each exposure. Mothers included in the analysis were racially diverse, with 67% identifying as Black and 26% identifying as White (Table 1). There were 62% of mothers with a high school education or less, and 45% who had never been married. One-third of the participating families had a household income of per year, and more than half were covered by Medicaid or Medicare only. Many (60%) breastfed their newborn, and 25% breastfed for . The median urinary cotinine concentration in the second trimester was 0.5 (IQR: 4.7) ng/mL, and only 7% of mothers had urinary cotinine concentrations , a common cutoff to define smokers set by medical testing kits manufacturers (Schick et al. 2017). The median maternal HEI was 60.1 (IQR: 16.2) (Table 1 and Table S1). According to a grading system recently proposed by Krebs-Smith et al. (2018), overall HEI scores of 0–59 and 60–69 are the two lowest categories among five in the general population. Hence, we defined maternal HEI below the median in the current analysis as poor adherence to the Dietary Guidelines for Americans. The lowest quartile of plasma folate ranged from 2.6 to . Although universally accepted cutoffs to characterize folate deficiency using plasma samples in pregnancy are undetermined, the folate concentrations within the second to fourth quartiles were considered adequate according to a report from the World Health Organization Technical Consultation and a population-based randomized trial in China (Chen et al. 2019; de Benoist 2008).
Figure 1.
Inclusion flowchart. Shown are the CANDLE cohort retention between enrollment and the age 4- to 6-y visit as well as sample sizes for primary analysis of each exposure. Note: CANDLE, Conditions Affecting Neurocognitive Development and Learning in Early Childhood.
Table 1.
Characteristics of CANDLE study participants included in analysis ().
| Variables | Analytic sample () | |
|---|---|---|
| Mean (SD)/percentage/median (25th, 75th percentile) | ||
| Child characteristics | ||
| Age at age 4–6 visit (y) | 822 | 4.4 (0.6) |
| Height at age 4–6 visit (cm) | 822 | 106.5 (6.1) |
| Sex | ||
| Male | 410 | 49.9 |
| Female | 412 | 50.1 |
| Birth weight (kg) | 817 | 3.2 (0.5) |
| Gestational age at childbirth (wk) | 818 | 38.8 (1.8) |
| BMI at the age 4- to 6-y visit () | 821 | 16.5 (2.3) |
| BMI class at the age 4- to 6-y visita | ||
| Underweight | 17 | 2.1 |
| Normal | 524 | 63.8 |
| Overweight | 115 | 14.0 |
| Obese | 134 | 16.3 |
| Missing | 32 | 3.9 |
| Medication use potentially increasing blood pressureb | ||
| No | 758 | 92.2 |
| Yes | 64 | 7.8 |
| Sleep score at the age 4- to 6-y visit | 816 | 46.9 (7.2) |
| Vigorous activity frequency at the age 4- to 6-y visitc | ||
| Never or occasionally | 119 | 14.5 |
| Once or twice per week | 87 | 10.6 |
| Three or more times per week | 604 | 73.5 |
| Missing | 12 | 1.5 |
| Secondhand smoking exposure age 4- to 6-y visitd | ||
| No | 540 | 65.7 |
| Yes | 276 | 33.6 |
| Missing | 6 | 0.7 |
| Maternal characteristics | ||
| Age at childbirth (y) | 822 | 26 (5.5) |
| Race | ||
| Black | 552 | 67.2 |
| White | 217 | 26.4 |
| Asian | 8 | 1.0 |
| Other | 1 | 0.1 |
| Multiple race | 44 | 5.4 |
| Education at enrollment | ||
| school | 113 | 13.8 |
| High school/GED | 398 | 48.4 |
| Technical school | 84 | 10.2 |
| College degree | 142 | 17.3 |
| Graduate/professional degree | 84 | 10.2 |
| Missing | 1 | 0.1 |
| Marital status at enrollment | ||
| Married/living with partner | 430 | 52.3 |
| Widowed/divorced/separated | 22 | 2.7 |
| Never married | 369 | 44.9 |
| Missing | 1 | 0.1 |
| Insurance status at enrollment | ||
| No insurance | 2 | 0.2 |
| Medicaid or Medicare only | 503 | 61.2 |
| Medicaid/Medicare and private insurance | 28 | 3.4 |
| Private insurance only | 289 | 35.2 |
| Household income at enrollment | ||
| 303 | 36.9 | |
| 197 | 24.0 | |
| 138 | 16.8 | |
| 110 | 13.4 | |
| Missing | 74 | 9.0 |
| Income adjusted by household size (thousand) | 817 | 10.9 (3.6, 23.8) |
| Urinary cotinine adjusted by specific gravity (ng/mL) | 816 | 0.5 (0.1, 4.8) |
| Prepregnancy BMI classe | ||
| Underweight | 38 | 4.6 |
| Normal | 329 | 40.0 |
| Overweight | 180 | 21.9 |
| Obese | 272 | 33.1 |
| Missing | 3 | 0.4 |
| Breastfeeding | ||
| No | 312 | 38.0 |
| Yes () | 295 | 35.9 |
| Yes () | 205 | 24.9 |
| Missing | 10 | 1.2 |
| Pregnancy hypertensive disorder | ||
| No | 773 | 94.0 |
| Yes | 49 | 6.0 |
| Pregnancy BSI Global Severity Indexf | 798 | 46.8 (10.9) |
| Plasma folate in the mid-late trimester (ng/mL) | 822 | 23 (11.1) |
| Healthy eating index in the second trimester | 725 | 60.1 (52.2, 68.4) |
| Other characteristics | ||
| Childhood Opportunity Indexg | ||
| Prenatal educational index | 818 | (0.5) |
| Prenatal economics index | 818 | (0.6) |
| Postnatal educational index | 812 | (0.5) |
| Postnatal economics index | 812 | (0.6) |
| Recruitment site | ||
| General community | 628 | 76.4 |
| Medical Group clinics | 194 | 23.6 |
Note: BMI, body mass index; BSI, Brief Symptom Inventory; CANDLE, Conditions Affecting Neurocognitive Development and Learning in Early Childhood; GED, Graduate Equivalency; SD, standard deviation.
Child obesity was defined as a BMI of for children of the same age and sex; overweight was defined as a BMI from the 85th to ; normal weight was defined as a BMI from the 5th to ; and underweight was defined as a BMI of .
Child current medication use was reported by the parents at the age 4- to 6-y visit, and those who were taking medications that may increase blood pressure—such as albuterol, methylphenidate, and prednisone—were defined as positive.
Child vigorous activity frequency was reported by parents at the age 4- to 6-y visit.
Child secondhand smoking was reported by parents at the age 4- to 6-y visit, and for those with any family members who smoked at home, it was defined as positive.
Maternal prepregnancy obesity was defined as a BMI of or higher; overweight was defined as a BMI of to ; normal weight was defined as a BMI of to ; and underweight was defined as a BMI of .
Pregnancy maternal psychological distress was measured by the Global Severity Index from the BSI via self-report.
Childhood Opportunity Indices were calculated based on the overall address history in pre- and postnatal window.
Children were on average 4.4 years of age (SD: 0.6) at the time of blood pressure measurement, with an approximately equal sex distribution (Table 1). The mean BMI was 16.5 (SD: 2.3), and 16% of the children with a BMI of met the definition of obesity. According to parent reports, 7.8% were taking medications that potentially increased blood pressure, and one-third were exposed to secondhand smoking. Compared with the 1,503 participants who enrolled in the CANDLE study, children in our analysis were more likely to have a mother self-identified as Black and come from low-income families (Table S2). The two groups were similar with respect to other characteristics. Mean SBP was (SD: 10.0) for raw measurement and 48.6 (SD: 25.7) for percentile, and mean DBP was (SD: 9.2) for raw measurement and 75.6 (SD: 19.3) for percentile (Table S3). Both blood pressure raw measurements were normally distributed, whereas the DBP percentile was left skewed (Figure S3). There were 29.2% of the children who had a SBP and/or a DBP percentile and were classified as having HBP, largely driven by isolated elevation in DBP.
Air Pollution Exposures
A summary of air pollution exposures is shown in Table 2, and the distributions are shown as box plots in Figure S4. Trimester-specific levels ranged from 6.2 to . Mean levels were (SD: 0.9) during the prenatal period, and (SD: 0.5) averaged between birth and the 4-y-old birthday. There was little correlation among the measurements in the three trimesters (corr: to 0.15), but there was a moderate correlation between prenatal and postnatal measurements (corr: 0.34) (Table S4). Prenatal and postnatal had average levels of (SD: 2.4) and (SD: 1.9), respectively, and were highly correlated (corr: 0.82). There were 28.0% and 27.2% of the families living from an A1, A2, or A3 main road in the pre- and postnatal window, respectively. We inspected distributions of exposure between strata of effect modifiers. Compared with their counterparts, mothers who self-identified as Black, had HEI lower than the median, or had plasma folate level in the lowest quartile were more likely to live within of any major roadway and to have higher exposures in the pre- and postnatal windows (Table S5). The proportion living within of any major roadway in both windows was 6.9–10.6% greater for Black mothers, 4.0–6.3% greater for mothers with a maternal HEI less than the median, and 2.8–3.4% greater for mothers with folate levels in the lowest quartile. The average exposures in the pre- and postnatal window were higher for Black mothers, higher for mothers with a maternal HEI less than median, and higher for mothers with folate level in the lowest quartile. There were no meaningful differences in by any effect modifiers.
Table 2.
Distributions of exposure measurements in the CANDLE cohort.
| Measurements | Mean (SD)/percentage | Min | 25th percentile | Median | 75th percentile | Max | IQR | |
|---|---|---|---|---|---|---|---|---|
| Prenatal exposures | ||||||||
| in the first trimester () | 818 | 10.7 (1.5) | 7.8 | 9.8 | 10.4 | 11.2 | 16.8 | 1.4 |
| in the second trimester () | 818 | 10.7 (1.4) | 7.7 | 9.9 | 10.5 | 11.4 | 16.6 | 1.5 |
| in the third trimester () | 815 | 11.0 (1.7) | 6.2 | 9.9 | 10.7 | 11.7 | 17.2 | 1.8 |
| Prenatal () | 818 | 10.8 (0.9) | 8.6 | 10.2 | 10.8 | 11.3 | 13.8 | 1.1 |
| Prenatal (ppb) | 818 | 10.0 (2.4) | 4.0 | 8.3 | 10.0 | 11.7 | 16.4 | 3.4 |
| Distance to A1 roadway (m) | 818 | 2,490 (1,841) | 35 | 1,130 | 2,017 | 3,443 | 11,400 | 2,313 |
| Distance to A2 roadway (m) | 818 | 1,920 (1,631) | 9 | 580 | 1,385 | 2,900 | 8,658 | 2,320 |
| Distance to A3 roadway (m) | 818 | 449 (542) | 9 | 155 | 307 | 559 | 6,220 | 404 |
| Proximity to any major roadwaya | ||||||||
| 230 | 71.5% | |||||||
| 588 | 28.0% | |||||||
| Missing | 4 | 0.5% | ||||||
| Postnatal exposures | ||||||||
| Postnatal () | 780 | 9.9 (0.5) | 8.7 | 9.5 | 9.8 | 10.2 | 11.6 | 0.7 |
| Postnatal (ppb) | 780 | 8.9 (1.9) | 3.7 | 7.6 | 8.9 | 10.2 | 13.5 | 2.6 |
| Distance to A1 roadway (m) | 780 | 2,529 (2,013) | 38 | 1,090 | 1,991 | 3,462 | 16,307 | 2,372 |
| Distance to A2 roadway (m) | 780 | 1,932 (1,609) | 9 | 617 | 1,438 | 2,912 | 8,069 | 2,295 |
| Distance to A3 roadway (m) | 780 | 440 (452) | 9 | 156 | 316 | 557 | 3,090 | 401 |
| Proximity to any major roadwaya | ||||||||
| 212 | 27.2% | |||||||
| 568 | 72.8% | |||||||
Note: IQR, interquartile range; , nitrogen dioxide; , ambient particulate matter (particulate matter in aerodynamic diameter); SD, standard deviation.
Proximity to major roadway was estimated based on the single address with the longest residential history in the pre- and postnatal window and was dichotomized at from any major roadway of A1, A2, or A3.
Associations between Air Pollution Exposures and Child Blood Pressure
The estimated associations between air pollution exposures in each window and child blood pressure are shown in Table 3. When blood pressure was analyzed as continuous percentiles, we found evidence supporting positive associations of in the second trimester with both SBP and DBP. The fully adjusted model estimated a 14.61-percentile increase in SBP [95% confidence interval (CI): 4.62, 24.6] and an 8.65-percentile increase in DBP (95% CI: 1.38, 15.92) for each increase of in the second trimester. Prenatal average was significantly associated with DBP only, with each increase of associated with an 11.58-percentile elevation (95% CI: 2.94, 20.22), although a positive but insignificant association with SBP was also suggested [8.83 (95% CI: , 20.11)]. These detected associations were slightly attenuated after extensively adjusting for potential confounders that might also be in the causal pathway, but the conclusions remained the same. Postnatal was insignificantly associated with higher SBP [9.55 (95% CI: , 27.94)] and DBP percentiles [9.94 (95% CI: , 23.02)]. There was also no evidence that either or road proximity was associated with blood pressure, regardless of exposure timing. When blood pressure was analyzed as a binary outcome, we found increased risks of HBP with both pre- and postnatal , although these associations were insignificant [1.50 (95% CI: 0.71, 3.14); 2.12 (95% CI: 0.73, 6.15)].
Table 3.
Estimated effects of air pollution exposures on blood pressure percentiles and HBP from multivariate linear and Poisson regressions in the CANDLE cohort.
| Measurementsa | b | SBP percentile | DBP percentile | HBP | |
|---|---|---|---|---|---|
| (95% CI)c | (95% CI)c | of HBPd | IRRb (95% CI)c | ||
| Prenatal exposures | |||||
| in the first trimester | |||||
| Model 1 | 818 | (, 0.83) | (, 1.57) | 238 | 0.95 (0.83, 1.1) |
| Model 2 | 817 | 2.52 (, 10.8) | 2.12 (, 8.04) | 237 | 0.79 (0.48, 1.32) |
| Model 3 | 756 | 2.11 (, 11.4) | 3.87 (, 10.63) | 219 | 0.9 (0.5, 1.59) |
| Model 4 | 754 | 1.44 (, 10.52) | 3.66 (, 10.31) | 218 | 0.88 (0.5, 1.58) |
| in the second trimester | |||||
| Model 1 | 818 | 0.03 (, 2.62) | 0.22 (, 2.21) | 238 | 0.88 (0.74, 1.05) |
| Model 2 | 817 | 13.1 (4.54, 21.66) | 6.3 (0.21, 12.39) | 237 | 0.93 (0.54, 1.61) |
| Model 3 | 756 | 14.61 (4.62, 24.6) | 8.65 (1.38, 15.92) | 219 | 0.97 (0.52, 1.82) |
| Model 4 | 754 | 13.42 (3.39, 23.44) | 7.91 (0.66, 15.16) | 218 | 0.9 (0.48, 1.69) |
| in the third trimester | |||||
| Model 1 | 815 | (, 2.19) | (, 1.36) | 238 | 0.94 (0.83, 1.07) |
| Model 2 | 814 | 0.31 (, 6.97) | 4.12 (, 9.49) | 237 | 1.17 (0.79, 1.73) |
| Model 3 | 753 | (, 6.05) | 4.28 (, 10.05) | 219 | 1.11 (0.72, 1.71) |
| Model 4 | 751 | (, 6.95) | 4.83 (, 10.49) | 218 | 1.15 (0.74, 1.78) |
| Prenatal | |||||
| Model 1 | 818 | (, 2.83) | (, 3.36) | 238 | 0.78 (0.59, 1.03) |
| Model 2 | 817 | 9.06 (, 18.28) | 8.81 (1.77, 15.85) | 237 | 1.26 (0.7, 2.28) |
| Model 3 | 756 | 8.83 (, 20.11) | 11.58 (2.94, 20.22) | 219 | 1.5 (0.71, 3.14) |
| Model 4 | 754 | 8.26 (, 19.66) | 11.03 (2.54, 19.52) | 218 | 1.39 (0.66, 2.94) |
| Prenatal | |||||
| Model 1 | 818 | 0.79 (, 2.49) | (, 1.19) | 238 | 0.97 (0.87, 1.08) |
| Model 2 | 817 | 1.13 (, 2.82) | 0.29 (, 1.57) | 237 | 1 (0.9, 1.12) |
| Model 3 | 756 | 1.28 (, 3.31) | 0.47 (, 1.97) | 219 | 1.03 (0.91, 1.18) |
| Model 4 | 754 | 1 (, 3.02) | 0.36 (, 1.81) | 218 | 1.03 (0.9, 1.17) |
| Proximity to major roadway ( vs. )e | |||||
| Model 1 | 818 | 1.02 (, 4.86) | 0.39 (, 3.28) | 238 | 1.05 (0.84, 1.32) |
| Model 3 | 757 | 0.45 (, 4.59) | (, 3.04) | 220 | 1.02 (0.8, 1.3) |
| Model 4 | 755 | (, 3.92) | (, 2.49) | 219 | 0.97 (0.76, 1.25) |
| Postnatal exposures | |||||
| Postnatal | |||||
| Model 1 | 780 | 16.99 (7.46, 26.51) | 6.7 (, 13.65) | 228 | 1.74 (0.98, 3.1) |
| Model 2 | 775 | 15.08 (1.42, 28.73) | 8.98 (, 18.74) | 225 | 1.78 (0.76, 4.17) |
| Model 3 | 715 | 9.55 (, 27.94) | 9.94 (, 23.02) | 208 | 2.12 (0.73, 6.15) |
| Model 4 | 714 | 8.79 (, 27.31) | 9.72 (, 22.81) | 208 | 2.1 (0.71, 6.17) |
| Postnatal | |||||
| Model 1 | 780 | 1.55 (, 3.77) | 1.13 (, 2.77) | 228 | 1.13 (0.98, 1.3) |
| Model 2 | 775 | 1.09 (, 3.32) | 0.86 (, 2.52) | 225 | 1.11 (0.96, 1.28) |
| Model 3 | 715 | 0.25 (, 3.04) | 1 (, 3.07) | 208 | 1.15 (0.96, 1.37) |
| Model 4 | 714 | 0.14 (, 2.88) | 0.94 (, 2.99) | 208 | 1.14 (0.95, 1.36) |
| Proximity to major roadway ( vs. )e | |||||
| Model 1 | 780 | 1.75 (, 5.79) | (, 2.92) | 228 | 1.01 (0.8, 1.28) |
| Model 3 | 719 | 0.2 (, 4.49) | (, 2.05) | 210 | 0.96 (0.74, 1.24) |
| Model 4 | 718 | (, 4.02) | (, 1.83) | 210 | 0.94 (0.73, 1.22) |
Note: BMI, body mass index; CANDLE, Conditions Affecting Neurocognitive Development and Learning in Early Childhood; CI, confidence interval; DBP, diastolic blood pressure; df, degrees of freedom; HBP, high blood pressure; IRR, incidence rate ratio; , nitrogen dioxide; , ambient particulate matter (particulate matter in aerodynamic diameter); SBP, systolic blood pressure.
and in each window were rescaled to 2-unit increments.
is the analytic sample size for each model.
Multivariate linear regressions were used for blood pressure percentiles and Poisson regressions were used for HBP based on complete data. Model 1 was adjusted for child sex, child age and height at the age 4- to 6-y visit, and recruitment site. Model 2 was additionally adjusted for times splines for visit date and conception date for and . Visit date was universally modeled with 1 df/y in all models. Conception date was modeled with 1 df/y for analyses with and was modeled with varied df for in different windows: 8 df/y of conception date for trimester-specific , 4 df/y for prenatal , and 1 df/y for postnatal . There was no time adjustment for proximity to major roadway in all models. Model 3 (the fully adjusted model) was additionally adjusted for maternal race, maternal age at childbirth, maternal education, income adjusted by household size, breastfeeding, maternal Global Severity Index, urinary cotinine adjusted by specific gravity in the second trimester, BMI class before pregnancy, insurance status, child sleep scores, child physical activity levels, child secondhand smoking exposures, child use of medication that potentially increased blood pressure, and Child Opportunity Indices. Model 4 (the extended model) was further controlled for maternal hypertensive disorder, gestational age, birthweight, and child BMI at the age 4- to 6-y visit for prenatal exposures, and was only additionally for child BMI at the age 4- to 6-y visit for postnatal exposures.
of HBP is the number of cases in the analytic sample size for each model.
Proximity to major roadway was estimated based on the single address with the longest residential history in the pre- and postnatal window and was dichotomized at from any major roadway of A1, A2, or A3.
We investigated four potential effect modifiers of associations between air pollution and child blood pressure: maternal HEI, maternal plasma folate, child sex, and maternal race (Figure 2 and Tables S6 and S7). The associations between the second-trimester and SBP percentile were positive and significant in strata of all potential effect modifiers. Except for maternal plasma folate [18.53 (95% CI: 7.85, 29.2) in the lowest folate quartile; 12.89 (95% CI: 2.68, 23.09) in the second to fourth folate quartiles; 0.05], there was no appreciable divergence in association by other potential modifiers. For DBP percentile, the estimated effects of in the second trimester were more pronounced in female children [10.64 (95% CI: 3.03, 18.25) in females; 6.39 (95% CI: , 13.93) in males; 0.05]. There was a potential but insignificant modification by maternal plasma folate concentrations [11.38 (95% CI: 3.66, 19.09) in the lowest folate quartile; 7.43 (95% CI: , 14.89) in the second to fourth folate quartiles; 0.07], but we observed no modification of associations by maternal HEI during pregnancy [8.92 (95% CI: 0.89, 16.94) for HEI below median; 7.80 (95% CI: , 16.48) for HEI median and above; 0.66] or maternal race [9.35 (95% CI: 2.00, 16.70) in Black; 7.68 (95% CI: , 15.62) in non-Black; 0.47]. Associations between prenatal and DBP percentile were positive and similar across strata of all potential modifiers (0.18–0.59). The results also suggested a possible modification by maternal race for the associations between prenatal proximity to major roadway and SBP (0.03) and a modification by maternal plasma folate for the associations between postnatal proximity to major roadway and SBP (0.03), but these findings need to be interpreted with caution owing to the null results from the primary analysis and multiple comparisons in the effect modifier analysis.
Figure 2.
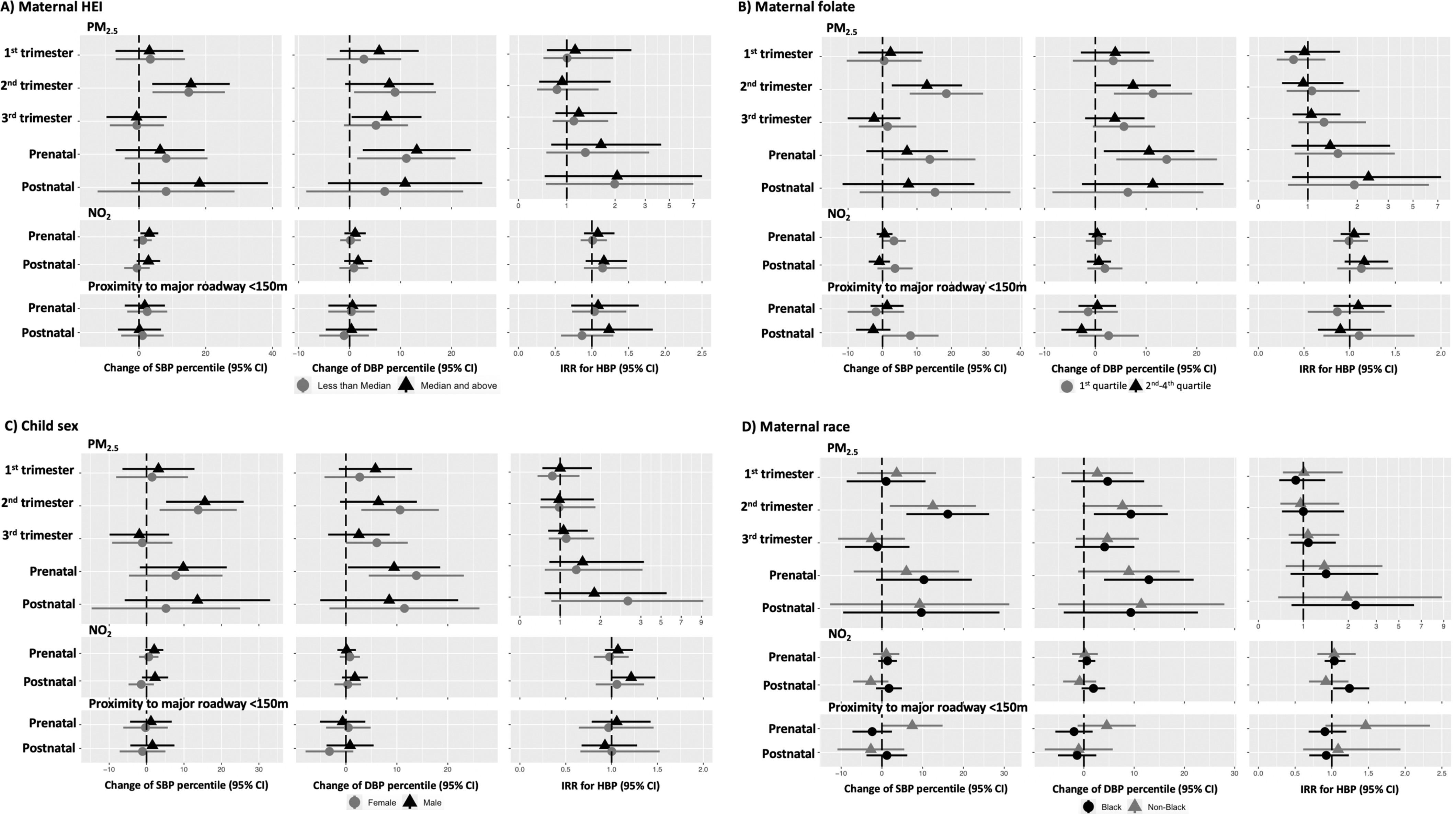
Shown are estimated effects of air pollution exposures on BP percentiles and HBP by maternal HEI levels (), maternal plasma folate (first quartile vs. second to fourth quartile), child sex (female vs. male), and maternal race (Black vs. non-Black) from the interaction models. In addition to effect modifiers and interaction terms, the models (linear regressions for blood pressure percentiles and Poisson regressions for HBP) were controlled for child sex, child age and height at the age 4- to 6-y visit, study site, time splines of both visit date and date of conception (only for and ), maternal age at childbirth, maternal race, maternal education, income adjusted by household size, breastfeeding, urinary cotinine adjusted by specific gravity in the second trimester, BMI class before pregnancy, insurance status, maternal Global Severity Index, child sleep scores, child physical activity levels, child secondhand smoking exposures, child use of medication that potentially increased blood pressure, and Child Opportunity Indices. Visit date was universally modeled with 1 df/y in all models. Conception date was modeled with 1 df/y for analyses with and was modeled with varied df for in different windows: 8 df/y of conception date for trimester-specific , 4 df/y for prenatal , and 1 df/y for postnatal . There was no time adjustment for proximity to major roadway in all models. The symbols of triangles and circles indicate the effect estimate, the error bars show 95% confidence intervals, and the dotted lines show null values. Numeric data is shown in Table S6 and S7. Note: BMI, body mass index; BP, blood pressure; df, degrees of freedom; HBP, high blood pressure; HEI, Healthy Eating Index; , nitrogen dioxide; , ambient particulate matter (particulate matter in aerodynamic diameter).
Sensitivity Analyses
Replacing and assessments with predictions from the fixed-year 2006 and 2011 models did not produce meaningful changes in the effect estimates derived from the models that incorporated time spline adjustment (Models 2–4) (Table S8 and S9). In the primary analysis, we modeled conception date with 8 df/y for the second-trimester and 4 df/y for averaged over the pregnancy, according to the relatively high temporal variabilities in shorter windows. With varied df from 0 to 4 for conception date, the estimated effects of the second-trimester and prenatal on both blood pressure percentiles became stronger, and the result gained significance. Both the point estimates and precisions remained steady with time splines of (Figure S5 and Table S10). For prenatal , similar patterns were observed in the associations with DBP percentile, with 3 df/y as the turning point. Varying the df/y had a smaller impact on the precision of the associations between prenatal and SBP percentile. In addition, the partial autocorrelations of were low (0.09, , and 0.14 for in the second trimester, third trimester, and postnatal period with in the first trimester). Neither the inclusion of all three trimester-specific exposures in the full models (Table S11) nor further adjustments for postnatal exposures in the models of prenatal exposures produced appreciable changes (Table S12). When controlling for child age and height using unpenalized TPRS with varied degrees of freedom, we obtained similar effect estimates with those from primary analysis of the second-trimester and prenatal with blood pressure percentiles and equivalent estimates of raw blood pressure () (Figure S6 and Table S13). When all the continuous covariates were modeled flexibly using linear restricted cubic splines, the magnitude of exposure–outcome associations did not change noticeably (Table S14). We did not find discrepancy in the patterns of associations after replacing DBP percentiles with the log-transformed measurements (Table S15).
Discussion
In this community-based pregnancy cohort in the southern United States, higher in utero exposures were associated with higher age-, sex-, and height-specific blood pressure percentiles in children 4–6 years of age (Flynn et al. 2017). Specifically, in the second trimester and averaged over the full pregnancy period was associated with increased child SBP and DBP percentiles, whereas the association between in the pregnancy period and DBP percentile was insignificant. These findings were robust to extensive adjustment for confounders that might also be in the causal pathway and to exposures in other pre- and postnatal windows. The conclusions remained unchanged in sensitivity analyses as well. Notably, our derived associations occurred in a setting with levels that fall within current regulatory guidelines. The estimated effects of the second-trimester on both blood pressure percentiles were stronger in children whose mother had plasma folate levels in the lowest quartile, and the associations between the second-trimester and DBP percentile were more evident in female children. Associations of SBP and DBP percentile with postnatal were similar in magnitude to associations with prenatal , but not statistically significant. We did not find clear or consistent evidence of relationships of child blood pressure with or with living within of a major roadway in either the pre- or postnatal window.
This study has several important strengths. The cohort was well characterized, allowing control for key confounders of concern. Missing data was limited, and the large sample size enabled us to conduct analyses of effect modification with a better statistical power than many other cohort studies with a similar research topic. In addition, we estimated spatiotemporally resolved and spatially resolved using well-validated advanced modeling approaches that predict exposures at individually geocoded participant home locations, allowing us to exploit small-scale spatial variability in the pollutant surfaces. Moving is common in families with young parents and low SES (Phinney 2013), and we were able to leverage detailed address history data collected by the CANDLE study and further improve the accuracy of the exposure assessment. The biweekly resolution of the model also enabled averaging the exposures over multiple pre- and postnatal windows, chosen a priori. Last, two nutritional measurements—the HEI (a validated and standardized tool) and plasma folate (a biomarker), were used to assess potential effect modification by maternal diet during pregnancy.
The effect estimates derived from our study were generally larger than those from previous studies conducted in low-polluted areas. We estimated positive associations between prenatal and both blood pressure percentiles, in common with the study of Madhloum et al. (2019) in Belgium, although the results were statistically insignificant with DBP. Similar to the two studies conducted in Massachusetts (van Rossem et al. 2015; Zhang et al. 2018) and the one in Mexico City (Rosa et al. 2020), we found adverse relationships of both blood pressure percentiles with trimester-specific , but with the second instead of the third trimester. However, no association of averaged over the pregnancy period with child blood pressure was found in the CANDLE cohort, whereas the Child Health Study in California (Breton et al. 2016) detected effects of exposure in the third trimester on SBP. Because residential distance to the nearest roadways was used as a proxy for exposure to traffic-related air pollutants, which predominantly consist of nitrogen oxides (: NO and ), we detected null associations for the population as whole, consistent with findings for . We also did not estimate significant associations between postnatal exposures and the outcomes of interest, contrary to most recent well-powered studies conducted in medium to highly polluted areas. Five studies in China have reported positive relationships between air pollution exposures and both SBP and DBP (Dong et al. 2014, 2015; Lawrence et al. 2018; Wang et al. 2019; Zeng et al. 2017), whereas five other studies (Bilenko et al. 2015a, 2015b; Pieters et al. 2015; Poursafa et al. 2014; Zhang et al. 2019), mainly in Europe, detected positive associations with either measurement but not both. Several factors could potentially explain the different findings between our study and the others: First, the blood pressure was assessed earlier in life in our participants than in most of the other studies. In particular, the studies conducted outside of the United States primarily targeted schoolchildren or adolescents. Second, our population was diverse, with a substantial number of low-SES families, a high proportion having at least one relative with family history of hypertension (75%) and diabetes (55%) and an elevated child obesity rate, likely contributing to the high incidence of HBP. The outcomes of interest were standardized using the 2017 guidelines with the normal-weight pediatric population as the reference. Compared with previous studies with blood pressure percentiles determined by the 2004 guidelines with the general pediatric population as the reference, our outcome definition performed better in identifying children with adverse cardiometabolic profiles (Du et al. 2019). Third, we defined the postnatal window as 4 y after childbirth, which was the longest postnatal period in common for all CANDLE study children. Compared with the previous studies with exposures in shorter periods, our aggregated air pollution measurements may have a lower variability. Fourth, all air pollution exposure prediction models, including ours, may induce complex forms of exposure measurement error that can introduce bias in either direction and lead to excess variability in estimating effect sizes (Spiegelman 2010; Szpiro and Paciorek 2013). Last, but not least, differences may also reflect varieties in residual confounding among studies.
In hypertensive youth, elevated peripheral DBP is superior to SBP in predicting future CVD, but with advancing age, SBP gradually overtakes DBP as a more powerful predictor (Franklin et al. 2001). We elected to examine both SBP and DBP and found positive associations for both. The existing science suggests that potential mechanisms for associations of prenatal air pollution exposures with child blood pressure may involve induction of placenta and fetal systematic inflammation, oxidative stress or endocrine disruption, subsequent changes of placental vascularization, and restricted fetal programming (Gold and Zanobetti 2018; Nachman et al. 2016). Recent research has also indicated that complex layers of epigenetic regulation in placental tissue and cord blood may play a role through methylation changes and telomere length shortening (Breton et al. 2016; Maghbooli et al. 2018; Rosa et al. 2019). Three major pathways linking postnatal air pollution exposures and cardiovascular health have been substantiated from previous studies, including spillover of pulmonary inflammation, which further induces systemic inflammation, modulation of autonomic influences, and direct target organ effects of pollutants or their products (Brook 2007; Brook et al. 2010; Franklin et al. 2015; Rajagopalan et al. 2018). The differences in routes of exposure and biological mechanism of disease progression between the two windows may partially explain why we detected significant associations in only the prenatal period. In addition, although the cardiovascular and renal systems develop throughout pregnancy, there is an exponential increase in nephrons between 18 and 32 wk of gestation, which may explain the trimester-specific associations detected in this study (Hinchliffe et al. 1991; Potter 1972; Rosenblum et al. 2017). Animal and human data also support the theory that maternal nutritional deficiencies may cause intrauterine growth restriction (Barker 1995; Lewis et al. 2002). When exposed to an adequate nutrient supply after birth, an undernourished infant might experience catch-up growth, which has been associated with amplified risks of hypertension, diabetes, and CVD later in life (Kelishadi et al. 2015; Kerkhof et al. 2012; Vuguin 2007). Folate may protect against elevated blood pressure by increasing nitric oxide synthesis in endothelial cells and subsequently counteracting oxidative stress caused by prenatal air pollution exposure and promoting resilience (Krikke et al. 2016; Wang et al. 2017). A limited number of studies reported no sex-specific associations between prenatal air pollution exposures and child blood pressure (van Rossem et al. 2015; Zhang et al. 2018), or reported associations only in boys (Rosa et al. 2020), which are inconsistent with our findings. The biomechanisms for sex-specific cardiovascular effects of air pollution exposures are unclear. A recent natural twin study has shown that male infants had higher levels of oxidative stress than their female counterparts, suggesting that male fetus may be more susceptible to in utero exposure to environmental challenge and have higher risks of subsequent adverse health outcomes (Minghetti et al. 2013). Continued investigations on potential mechanisms underlying our observed associations are required.
Our study has some limitations. One is the potential misclassification of HBP. Although blood pressure was measured repeatedly during assessment, the examination was performed on a single occasion. As such, the definition of HBP in our analysis does not meet the clinical definition (Du et al. 2019; Gillman and Cook 1995; Pickering et al. 2005). The distribution of DBP percentiles in CANDLE study children was left skewed. Young children may have a high vascular compliance but also premature maturation of the vascular system, and overweight/obese children are more likely to have cardiovascular dysfunction compared with lean children (Cote et al. 2013). The different distributions in DBP and SBP percentile could be explained by the fact that DBP may be more sensitive to capturing adverse cardiometabolic profiles such as obesity in early childhood. Beyond that, previous pediatric studies reported upward bias in both SBP and DBP and overestimation of hypertension when comparing oscillometric measurements to auscultatory measurements (Flynn et al. 2012; Park et al. 2001), and a greater difference in DBP than in SBP was observed in obese children (Fonseca-Reyes et al. 2018). Therefore, we cannot rule out the possibility of higher measurement errors in DBP. In addition, we used the mother’s address at enrollment (16–28 gestational wk) to estimate exposures during the first and second trimesters, which could reduce the accuracy of exposure assessment for families who moved prior to enrollment. In addition, the annual national model was suboptimal for capturing temporal variability of , resulting in longer aggregated periods and relatively high correlations across windows. Future implementation of spatiotemporal models on this exposure may remedy this deficiency. Moreover, given that a high proportion of disadvantaged young mothers are extremely mobile and difficult to contact, it is a significant accomplishment for the CANDLE study to achieve a high retention rate of 76% for 8–9 y of follow-up. Nevertheless, approximately half of the study population at enrollment was excluded from the current analysis largely because participants did not complete the age 4–6 years visit. Although the characteristics of mothers and children included in the present study were similar to the population at enrollment, selection bias remains a concern. The CANDLE population may represent only the source population in Shelby county, and thus the external generalizability of our results is restricted. In addition, as plasma folate is an indicator for recent folate exposure, it is an imperfect measure of chronic deficiency in the prenatal period (WHO 2015). Another limitation is the lack of data regarding composition as well as information on indoor air pollution exposures. Furthermore, even if we averaged the pollution measurements over a 4-y period, which reflected relatively long-term exposure levels before blood pressure measurements at the age 4- to 6-y visit, reverse causality could still exist. Finally, some other potential limitations, such as residual confounding and missing data in covariates, need to be acknowledged as well.
Despite the limitations, the primary contribution of this study is 2-fold. It highlights the potential harmful associations of prenatal , even at low levels, with an important feature of child cardiovascular health. Such considerations can inform regulatory policy on acceptable air pollution levels and appropriate controls, and further improve the health of urban high-risk pediatric populations, who have been underrepresented in research. We also identified that maternal folate concentrations in pregnancy could ameliorate the adverse associations between air pollution and child health. Continuing investigations are needed to confirm these modified associations in other study populations to inform future intervention.
Supplementary Material
Acknowledgments
This research is supported by the ECHO PATHWAYS consortium [National Institutes of Health (NIH) 1UG3OD023271 and 4UH3OD023271]. The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study is funded by the Urban Child Institute (NIH 1R01HL109977). Air pollution models were developed under STAR research assistance agreements RD831697 [Multi-Ethnic Study of Atherosclerosis Air Study (MESA Air)] and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency (EPA). This research has not been formally reviewed by the U.S. EPA. The views expressed in this document are solely those of the authors and the U.S. EPA does not endorse any products or commercial services mentioned in this publication.
The data from the CANDLE study can be requested from the study website (https://candlestudy.uthsc.edu/research/guidelines-collaboration). The computing code in STATA and R can be obtained from the corresponding author via email request.
References
- Aatola H, Magnussen CG, Koivistoinen T, Hutri-Kähönen N, Juonala M, Viikari JSA, et al. 2013. Simplified definitions of elevated pediatric blood pressure and high adult arterial stiffness. Pediatrics 132(1):e70–e76, PMID: 23753088, 10.1542/peds.2012-3426. [DOI] [PubMed] [Google Scholar]
- Acevedo-Garcia D, McArdle N, Hardy EF, Crisan UI, Romano B, Norris D, et al. 2014. The Child Opportunity Index: improving collaboration between community development and public health. Health Aff (Millwood) 33(11):1948–1957, PMID: 25367989, 10.1377/hlthaff.2014.0679. [DOI] [PubMed] [Google Scholar]
- Barker DJP. 1988. Childhood causes of adult diseases. Arch Dis Child 63(7):867–869, PMID: 3415312, 10.1136/adc.63.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. 1995. Fetal origins of coronary heart disease. BMJ 311(6998):171–174, PMID: 7613432, 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. 1999. Early growth and cardiovascular disease. Arch Dis Child 80(4):305–307, PMID: 10086930, 10.1136/adc.80.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CS, Samuel JP, Samuels JA. 2019. Prevalence of hypertension in children. Hypertension 73(1):148–152, PMID: 30571555, 10.1161/HYPERTENSIONAHA.118.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. 2017. Heart Disease and Stroke Statistics—2017 Update: a report from the American Heart Association. Circulation 135(10):e146–e603, PMID: 28122885, 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilenko N, Brunekreef B, Beelen R, Eeftens M, de Hoogh K, Hoek G, et al. 2015a. Associations between particulate matter composition and childhood blood pressure—the PIAMA study. Environ Int 84:1–6, PMID: 26186643, 10.1016/j.envint.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Bilenko N, van Rossem L, Brunekreef B, Beelen R, Eeftens M, Hoek G, et al. 2015b. Traffic-related air pollution and noise and children’s blood pressure: results from the PIAMA birth cohort study. Eur J Prev Cardiol 22(1):4–12, PMID: 24047569, 10.1177/2047487313505821. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54(10):615–627, PMID: 8237794, 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Breton CV, Yao J, Millstein J, Gao L, Siegmund KD, Mack W, et al. 2016. Prenatal air pollution exposures, DNA methyl transferase genotypes, and associations with newborn LINE1 and Alu methylation and childhood blood pressure and carotid intima-media thickness in the Children’s Health Study. Environ Health Perspect 124(12):1905–1912, PMID: 27219456, 10.1289/EHP181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD. 2007. Is air pollution a cause of cardiovascular disease? Updated review and controversies. Rev Environ Health 22(2):115–137, PMID: 17894203, 10.1515/reveh.2007.22.2.115. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Pilla MD, Ancona C, Sørensen M, Gesi M, Taddei S, et al. 2017. Environmental factors and hypertension. Curr Pharm Des 23(22):3239–3246, PMID: 28356035, 10.2174/1381612823666170321162233. [DOI] [PubMed] [Google Scholar]
- Burniaux JM, Dang TT, Fore D, Förster M, d’Ercole MM, Oxley H. 1998. Income distribution and poverty in selected OECD countries. OECD Economics Department Working Papers. No. 189. Paris, France: OECD Publishing. 10.1787/730801800603. [DOI] [Google Scholar]
- CDC (Centers for Disease Control and Prevention. 2011). Prevalence of coronary heart disease—United States, 2006–2010. MMWR Morb Mortal Wkly Rep 60:1377–1381, PMID: 21993341. [PubMed] [Google Scholar]
- Chen MY, Rose CE, Qi YP, Williams JL, Yeung LF, Berry RJ, et al. 2019. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr 109(5):1452–1461, PMID: 31005964, 10.1093/ajcn/nqz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang Y. 2008. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 117(25):3171–3180, PMID: 18559702, 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EL, Bell CS, Samuel JP, Poffenbarger T, Redwine KM, Samuels JA. 2017. Race and obesity in adolescent hypertension. Pediatrics 139(5):e20161433, PMID: 28557717, 10.1542/peds.2016-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Crombie R, Head J, van Kamp I, van Kempen E, Stansfeld SA. 2012. Does traffic-related air pollution explain associations of aircraft and road traffic noise exposure on children’s health and cognition? A secondary analysis of the United Kingdom sample from the RANCH project. Am J Epidemiol 176(4):327–337, PMID: 22842719, 10.1093/aje/kws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote AT, Harris KC, Panagiotopoulos C, Sandor GGS, Devlin AM. 2013. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol 62(15):1309–1319, PMID: 23954339, 10.1016/j.jacc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- de Benoist B. 2008. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull 29(suppl 2):S238–S244, PMID: 18709899, 10.1177/15648265080292S129. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. 1983. The Brief Symptom Inventory: an introductory report. Psychol Med 13(3):595–605, PMID: 6622612, 10.1017/S0033291700048017. [DOI] [PubMed] [Google Scholar]
- Dong GH, Qian ZM, Trevathan E, Zeng XW, Vaughn MG, Wang J, et al. 2014. Air pollution associated hypertension and increased blood pressure may be reduced by breastfeeding in Chinese children: the Seven Northeastern Cities Chinese Children’s Study. Int J Cardiol 176(3):956–961, PMID: 25186732, 10.1016/j.ijcard.2014.08.099. [DOI] [PubMed] [Google Scholar]
- Dong GH, Wang J, Zeng XW, Chen L, Qin XD, Zhou Y, et al. 2015. Interactions between air pollution and obesity on blood pressure and hypertension in Chinese children. Epidemiology 26(5):740–747, PMID: 26133026, 10.1097/EDE.0000000000000336. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Aggarwal A, Cook A, Stewart O, Moudon AV. 2016. Geographic disparities in Healthy Eating Index scores (HEI-2005 and 2010) by residential property values: findings from Seattle Obesity Study (SOS). Prev Med 83:46–55, PMID: 26657348, 10.1016/j.ypmed.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Fernandez C, Barshop R, Chen W, Urbina EM, Bazzano LA. 2019. 2017 Pediatric hypertension guidelines improve prediction of adult cardiovascular outcomes. Hypertension 73(6):1217–1223, PMID: 31006329, 10.1161/HYPERTENSIONAHA.118.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. 2017. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140(3):e20171904, PMID: 28827377, 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- Flynn JT, Pierce CB, Miller ER III, Charleston J, Samuels JA, Kupferman J, et al. 2012. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr 160(3):434–440.e1, PMID: 22048052, 10.1016/j.jpeds.2011.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca-Reyes S, Romero-Velarde E, Torres-Gudiño E, Illescas-Zarate D, Forsyth-MacQuarrie AM. 2018. Comparison of auscultatory and oscillometric BP measurements in children with obesity and their effect on the diagnosis of arterial hypertension. Arch Cardiol Mex 88(1):16–24, PMID: 28238543, 10.1016/j.acmx.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Brook R, Pope CA III.. 2015. Air pollution and cardiovascular disease. Curr Probl Cardiol 40(5):207–238, PMID: 25882781, 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, et al. 2001. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 103(9):1245–1249, PMID: 11238268, 10.1161/01.CIR.103.9.1245. [DOI] [PubMed] [Google Scholar]
- Garrow JS, Webster J. 1985. Quetelet’s index (W/H2) as a measure of fatness. Int J Obes 9:147–153, PMID: 4030199. [PubMed] [Google Scholar]
- Gillman MW, Cook NR. 1995. Blood pressure measurement in childhood epidemiological studies. Circulation 92(4):1049–1057, PMID: 7641339, 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. 2016. Air pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Des 22(1):28–51, PMID: 26548310, 10.2174/1381612822666151109111712. [DOI] [PubMed] [Google Scholar]
- Gold DR, Zanobetti A. 2018. Do maternal air pollution exposures have long-lasting influences on child blood pressure? Hypertension 72(1):56–58, PMID: 29760153, 10.1161/HYPERTENSIONAHA.118.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, et al. 2014. The Healthy Eating Index–2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr 144(3):399–407, PMID: 24453128, 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Hsia C, O’Neill MS. 2015. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep 2(4):440–450, PMID: 26381684, 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. 1991. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64(6):777–784, PMID: 2046329. [PubMed] [Google Scholar]
- Jackson SL, Zhang Z, Wiltz JL, Loustalot F, Ritchey MD, Goodman AB, et al. 2018. Hypertension among youths—United States, 2001–2016. MMWR Morb Mortal Wkly Rep 67(27):758–762, PMID: 30001558, 10.15585/mmwr.mm6727a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi R, Haghdoost AA, Jamshidi F, Aliramezany M, Moosazadeh M. 2015. Low birthweight or rapid catch-up growth: which is more associated with cardiovascular disease and its risk factors in later life? A systematic review and cryptanalysis. Paediatr Int Child Health 35(2):110–123, PMID: 25034799, 10.1179/2046905514Y.0000000136. [DOI] [PubMed] [Google Scholar]
- Keller JP, Olives C, Kim SY, Sheppard L, Sampson PD, Szpiro AA, et al. 2015. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ Health Perspect 123(4):301–309, PMID: 25398188, 10.1289/ehp.1408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof GF, Willemsen RH, Leunissen RWJ, Breukhoven PE, Hokken-Koelega ACS. 2012. Health profile of young adults born preterm: negative effects of rapid weight gain in early life. J Clin Endocrinol Metab 97(12):4498–4506, PMID: 22993033, 10.1210/jc.2012-1716. [DOI] [PubMed] [Google Scholar]
- Kim JS, Alderete TL, Chen Z, Lurmann F, Rappaport E, Habre R, et al. 2018. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ Health 17(1):64, PMID: 30213262, 10.1186/s12940-018-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. 2018. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet 118(9):1591–1602, PMID: 30146071, 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikke GG, Grooten IJ, Vrijkotte TGM, van Eijsden M, Roseboom TJ, Painter RC. 2016. Vitamin B12 and folate status in early pregnancy and cardiometabolic risk factors in the offspring at age 5–6 years: findings from the ABCD multi-ethnic birth cohort. BJOG 123(3):384–392, PMID: 26810674, 10.1111/1471-0528.13574. [DOI] [PubMed] [Google Scholar]
- Lawrence WR, Yang M, Lin S, Wang SQ, Liu Y, Ma H, et al. 2018. Pet exposure in utero and postnatal decreases the effects of air pollutants on hypertension in children: a large population based cohort study. Environ Pollut 238:177–185, PMID: 29554565, 10.1016/j.envpol.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RM, Forhead AJ, Petry CJ, Ozanne SE, Hales CN. 2002. Long-term programming of blood pressure by maternal dietary iron restriction in the rat. Br J Nutr 88(3):283–290, PMID: 12207838, 10.1079/BJN2002656. [DOI] [PubMed] [Google Scholar]
- Liu C, Fuertes E, Tiesler CMT, Birk M, Babisch W, Bauer CP, et al. 2014. The associations between traffic-related air pollution and noise with blood pressure in children: results from the GINIplus and LISAplus studies. Int J Hyg Environ Health 217(4–5):499–505, PMID: 24183515, 10.1016/j.ijheh.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Loftus CT, Hazlehurst MF, Szpiro AA, Ni Y, Tylavsky FA, Bush NR, et al. 2019. Prenatal air pollution and childhood IQ: preliminary evidence of effect modification by folate. Environ Res 176:108505, PMID: 31229778, 10.1016/j.envres.2019.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhloum N, Nawrot TS, Gyselaers W, Roels HA, Bijnens E, Vanpoucke C, et al. 2019. Neonatal blood pressure in association with prenatal air pollution exposure, traffic, and land use indicators: an ENVIRONAGE birth cohort study. Environ Int 130:104853, PMID: 31226559, 10.1016/j.envint.2019.05.047. [DOI] [PubMed] [Google Scholar]
- Maghbooli Z, Hossein-Nezhad A, Adabi E, Asadollah-Pour E, Sadeghi M, Mohammad-Nabi S, et al. 2018. Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS One 13(7):e0199772, PMID: 29979694, 10.1371/journal.pone.0199772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae MP. 2009. High-dose folic acid supplementation effects on endothelial function and blood pressure in hypertensive patients: a meta-analysis of randomized controlled clinical trials. J Chiropr Med 8(1):15–24, PMID: 19646382, 10.1016/j.jcm.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Zanardo V, Suppiej A. 2013. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med 26(3):259–262, PMID: 23020682, 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- Nachman RM, Mao G, Zhang X, Hong X, Chen Z, Soria CS, et al. 2016. Intrauterine inflammation and maternal exposure to ambient PM2.5 during preconception and specific periods of pregnancy: the Boston Birth Cohort. Environ Health Perspect 124(10):1608–1615, PMID: 27120296, 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHANES (National Health and Nutrition Examination Survey). 2009–2010. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=2009 [accessed 29 March 2021].
- Ntarladima AM, Vaartjes I, Grobbee DE, Dijst M, Schmitz O, Uiterwaal C, et al. 2019. Relations between air pollution and vascular development in 5-year old children: a cross-sectional study in the Netherlands. Environ Health 18(1):50, PMID: 31096974, 10.1186/s12940-019-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. 2017. Effect of outdoor air pollution on asthma exacerbations in children and adults: systematic review and multilevel meta-analysis. PLoS One 12(3):e0174050, PMID: 28319180, 10.1371/journal.pone.0174050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. 2000. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 23(8):1043–1051, PMID: 11145319, 10.1093/sleep/23.8.1d. [DOI] [PubMed] [Google Scholar]
- Park MK, Menard SW, Yuan C. 2001. Comparison of auscultatory and oscillometric blood pressures. Arch Pediatr Adolesc Med 155(1):50–53, PMID: 11177062, 10.1001/archpedi.155.1.50. [DOI] [PubMed] [Google Scholar]
- Patel N, Walker N. 2016. Clinical assessment of hypertension in children. Clin Hypertens 22:15, PMID: 27190633, 10.1186/s40885-016-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney R. 2013. Exploring residential mobility among low-income families. Soc Serv Rev 87(4):780–815, 10.1086/673963. [DOI] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. 2005. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111(5):697–716, PMID: 15699287, 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- Picó C, Serra F, Rodríguez AM, Keijer J, Palou A. 2019. Biomarkers of nutrition and health: new tools for new approaches. Nutrients 11(5):1092, PMID: 31100942, 10.3390/nu11051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters N, Koppen G, Van Poppel M, De Prins S, Cox B, Dons E, et al. 2015. Blood pressure and same-day exposure to air pollution at school: associations with nano-sized to coarse PM in children. Environ Health Perspect 123(7):737–742, PMID: 25756964, 10.1289/ehp.1408121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter EL. 1972. Normal and Abnormal Development of the Kidney. Chicago, IL: Year Book Medical Publishers. [Google Scholar]
- Poursafa P, Mansourian M, Motlagh ME, Ardalan G, Kelishadi R. 2014. Is air quality index associated with cardiometabolic risk factors in adolescents? The CASPIAN-III study. Environ Res 134:105–109, PMID: 25127520, 10.1016/j.envres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Al-Kindi SG, Brook RD. 2018. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 72(17):2054–2070, PMID: 30336830, 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- Rosa MJ, Hair GM, Just AC, Kloog I, Svensson K, Pizano-Zárate ML, et al. 2020. Identifying critical windows of prenatal particulate matter (PM2.5) exposure and early childhood blood pressure. Environ Res 182:109073, PMID: 31881529, 10.1016/j.envres.2019.109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MJ, Hsu HHL, Just AC, Brennan KJ, Bloomquist T, Kloog I, et al. 2019. Association between prenatal particulate air pollution exposure and telomere length in cord blood: effect modification by fetal sex. Environ Res 172:495–501, PMID: 30852452, 10.1016/j.envres.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum S, Pal A, Reidy K. 2017. Renal development in the fetus and premature infant. Semin Fetal Neonatal Med 22(2):58–66, PMID: 28161315, 10.1016/j.siny.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kocak M, Hartman TJ, Vereen S, Adgent M, Piyathilake C, et al. 2018. Association of prenatal folate status with early childhood wheeze and atopic dermatitis. Pediatr Allergy Immunol 29(2):144–150, PMID: 29168294, 10.1111/pai.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, Saland JM, Wright RO, Satlin L. 2018. Perinatal and childhood exposure to environmental chemicals and blood pressure in children: a review of literature 2007–2017. Pediatr Res 84(2):165–180, PMID: 29884847, 10.1038/s41390-018-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick SF, Blount BC, Jacob P III, Saliba NA, Bernert JT, El Hellani A, et al. 2017. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol 313(3):L425–L452, PMID: 28522563, 10.1152/ajplung.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L, Bogensberger B, Hoffmann G. 2018. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 118(1):74–100.e11, PMID: 29111090, 10.1016/j.jand.2017.08.024. [DOI] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, et al. 2015. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description. Santa Monica, CA: Rand Corporation. https://www.rand.org/pubs/research_reports/RR1336.html [accessed 18 February 2020]. [Google Scholar]
- Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. 2015. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation 132(23):2212–2219, PMID: 26644246, 10.1161/CIRCULATIONAHA.115.017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman D. 2010. Approaches to uncertainty in exposure assessment in environmental epidemiology. Annu Rev Public Health 31:149–163, PMID: 20070202, 10.1146/annurev.publhealth.012809.103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sughis M, Nawrot TS, Ihsan-Ul-Haque S, Amjad A, Nemery B. 2012. Blood pressure and particulate air pollution in schoolchildren of Lahore, Pakistan. BMC Public Health 12:378, PMID: 22632576, 10.1186/1471-2458-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpiro AA, Paciorek CJ. 2013. Measurement error in two-stage analyses, with application to air pollution epidemiology. Environmetrics 24(8):501–517, PMID: 24764691, 10.1002/env.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol 45(6):1887–1894, PMID: 28089956, 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- van Rossem L, Rifas-Shiman SL, Melly SJ, Kloog I, Luttmann-Gibson H, Zanobetti A, et al. 2015. Prenatal air pollution exposure and newborn blood pressure. Environ Health Perspect 123(4):353–359, PMID: 25625652, 10.1289/ehp.1307419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuguin PM. 2007. Animal models for small for gestational age and fetal programming of adult disease. Horm Res 68(3):113–123, PMID: 17351325, 10.1159/000100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu BP, Xu RB, Walker SO, Wang G. 2017. Joint effect of maternal plasma homocysteine and prepregnancy obesity on child blood pressure: a prospective birth cohort study. Int J Obes (Lond) 41(9):1447–1453, PMID: 28465603, 10.1038/ijo.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zou Z, Dong B, Dong Y, Ma Y, Gao D, et al. 2019. Association of school residential PM2.5 with childhood high blood pressure: results from an observational study in 6 cities in China. Int J Environ Res Public Health 16(14):2515, PMID: 31337125, 10.3390/ijerph16142515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warembourg C, Maitre L, Tamayo-Uria I, Fossati S, Roumeliotaki T, Aasvang GM, et al. 2019. Early-life environmental exposures and blood pressure in children. J Am Coll Cardiol 74(10):1317–1328, PMID: 31488269, 10.1016/j.jacc.2019.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whincup PH. 1995. Mothers, babies and disease in later life. [Book review.] J R Soc Med 88(8):458. [Google Scholar]
- WHO (World Health Organization). 2015. Serum and red blood cell folate concentrations for assessing folate status in populations. Updated 2015. WHO/NMH/NHD/EPG/15.01. https://apps.who.int/iris/bitstream/handle/10665/162114/WHO_NMH_NHD_EPG_15.01.pdf?ua=1 [accessed 14 February 2021].
- Wood SN. 2017. Generalized Additive Models: An Introduction with R, Second Edition. Philadelphia, PA: CRC Press LLC. [Google Scholar]
- Young MT, Bechle MJ, Sampson PD, Szpiro AA, Marshall JD, Sheppard L, et al. 2016. Satellite-based NO2 and model validation in a national prediction model based on universal kriging and land-use regression. Environ Sci Technol 50(7):3686–3694, PMID: 26927327, 10.1021/acs.est.5b05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XW, Qian ZM, Vaughn MG, Nelson EJ, Dharmage SC, Bowatte G, et al. 2017. Positive association between short-term ambient air pollution exposure and children blood pressure in China—result from the Seven Northeast Cities (SNEC) study. Environ Pollut 224:698–705, PMID: 28259583, 10.1016/j.envpol.2017.02.054. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mueller NT, Wang H, Hong X, Appel LJ, Wang X. 2018. Maternal exposure to ambient particulate matter ≤2.5 μm during pregnancy and the risk for high blood pressure in childhood. Hypertension 72(1):194–201, PMID: 29760154, 10.1161/HYPERTENSIONAHA.117.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dong B, Li S, Chen G, Yang Z, Dong Y, et al. 2019. Exposure to ambient particulate matter air pollution, blood pressure and hypertension in children and adolescents: a national cross-sectional study in China. Environ Int 128:103–108, PMID: 31035113, 10.1016/j.envint.2019.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.