Abstract
OBJECTIVE AND METHODS
Worldwide, tuberculosis (TB) is the leading cause of death from a single infectious agent. In many countries, national TB prevalence surveys are the only way to reliably measure the burden of TB disease and can also provide other evidence to inform national efforts to improve TB detection and treatment. Our objective was to synthesise the results and lessons learned from national surveys completed in Africa between 2008 and 2016, to complement a previous review for Asia.
RESULTS
Twelve surveys completed in Africa were identified: Ethiopia (2010–2011), Gambia (2011–2013), Ghana (2013), Kenya (2015–2016), Malawi (2013–2014), Nigeria (2012), Rwanda (2012), Sudan (2013–2014), Tanzania (2011–2012), Uganda (2014–2015), Zambia (2013–2014) and Zimbabwe (2014). The eligible population in all surveys was people aged ≥15 years who met residency criteria. In total 588 105 individuals participated, equivalent to 82% (range 57–96%) of those eligible. The prevalence of bacteriologically confirmed pulmonary TB disease in those ≥15 years varied from 119 (95% CI 79–160) per 100 000 population in Rwanda and 638 (95% CI 502–774) per 100 000 population in Zambia. The male:female ratio was 2.0 overall, ranging from 1.2 (Ethiopia) to 4.1 (Uganda). Prevalence per 100 000 population generally increased with age, but the absolute number of cases was usually highest among those aged 35–44 years. Of identified TB cases, 44% (95% CI 40–49) did not report TB symptoms during screening and were only identified as eligible for diagnostic testing due to an abnormal chest X-ray. The overall ratio of prevalence to case notifications was 2.5 (95% CI 1.8–3.2) and was consistently higher for men than women. Many participants who did report TB symptoms had not sought care; those that had were more likely to seek care in a public health facility. HIV prevalence was systematically lower among prevalent cases than officially notified TB patients with an overall ratio of 0.5 (95% CI 0.3–0.7). The two main study limitations were that none of the surveys included people <15 years, and 5 of 12 surveys did not have data on HIV status.
CONCLUSIONS
National TB prevalence surveys implemented in Africa between 2010 and 2016 have contributed substantial new evidence about the burden of TB disease, its distribution by age and sex, and gaps in TB detection and treatment. Policies and practices to improve access to health services and reduce under-reporting of detected TB cases are needed, especially among men. All surveys provide a valuable baseline for future assessment of trends in TB disease burden.
Keywords: Tuberculosis, prevalence survey, Africa, epidemiology, public health
Introduction
Worldwide in 2018 an estimated 10.0 million people (range, 9.0–11.1 million) developed tuberculosis (TB). It was the leading cause of death from a single infectious agent with an estimated 1.2 million deaths (range, 1.2–1.3 million) among HIV-negative people and 251 000 deaths (range, 223 000–281 000) among HIV-positive people [1]. The African region accounted for 24% of global cases and 32% of deaths. It included countries with some of the severest TB epidemics in the world, especially those with a high prevalence of HIV in the general population.
During the period 2000–2015, global and national efforts to address the TB epidemic had the aim of achieving targets for reductions in the burden of TB disease (incidence, prevalence, mortality) that were set as part of the United Nations Millennium Development Goals (MDGs), WHO’s Stop TB Strategy (2006–2015) and the Stop TB Partnership’s Global Plan to Stop TB (2006–2015) [2-4]. Three targets were set, one of which was to halve TB prevalence by 2015 compared with 1990 levels.
The most accurate method for measuring prevalence in countries with a high burden of TB disease is a national population-based survey. Between 1990 and 2002, only four countries in Asia (Cambodia, China, Philippines, Republic of Korea) conducted such surveys [5]. As a result, in the mid-2000s, country-level TB prevalence could only be estimated indirectly, and with a high degree of uncertainty, as the product of estimated TB incidence and estimated average duration of disease [6].
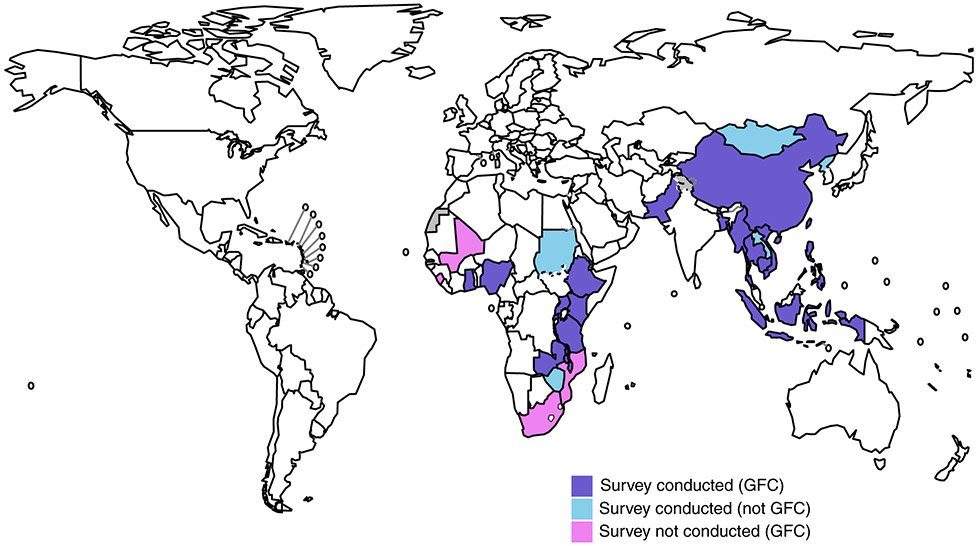
In 2006, WHO established a Global Task Force on TB Impact Measurement, with the goal of ensuring that assessment of whether global TB targets set for 2015 were achieved was robust, rigorous and consensus-based [7]. In December 2007, the Task Force defined three strategic areas of work, one of which was national TB prevalence surveys in 22 global focus countries [7]. These were a prioritised subset of 53 countries considered eligible to implement a national survey, 13 in Africa (Figure 1) and nine in Asia [8]. At this time, no national TB prevalence survey had been conducted in Africa since the 1950s, with the sole exception of a survey in Eritrea in 2005 that was limited by the diagnostic methods used to detect cases (sputum smear microscopy only, without culture) [9, 10]. A few African countries had conducted subnational surveys in the 2000s [11-14].
Figure 1.
a The WHO Global Task Force on TB impact Measurement selected 22 global focus countries (GFC) to undertake a national TB prevalence survey during the period 2008- 2015. Of the 13 GFCs in Africa, nine completed a survey (violet) between 2010-2016. The other four GFCs that did not conduct a survey during 2010-2016 were Mali, Mozambique, Sierra Leone and South Africa (pink). DPR Korea, Gambia, Lao PDR, Mongolia, Sudan and Zimbabwe completed a survey during the period 2010-2016 but were not GFCs (skyblue). Grey, not applicable.
Countries that completed a national TB prevalence survey, 2008–2016a.
In addition to providing more direct measurements of TB prevalence, repeat surveys, when conducted with intervals of about 10 years or more, are able assess trends to assess progress towards national and global targets for reductions in the burden of TB disease [15-17]. These surveys can also help national TB programmes (NTPs) to better understand the epidemiology of TB disease (such as its distribution by age and sex), document healthcare-seeking behaviour in the public and private sectors, identify reasons for why TB cases were not previously diagnosed and/or officially reported to national authorities, and develop targeted strategies and interventions to reach more TB cases.
A previous paper has synthesised results and lessons learned from national TB surveys implemented in Asia [5]. Following national and global efforts to design and implement national TB prevalence surveys that started in 2008, our objective was to provide an overview of and describe lessons learned from national surveys that were completed in Africa between 2008 and 2016.
Methods
Identification of surveys
A literature search for the period January 2008 to April 2020, restricted to the English language, was conducted by one author (I.L.) in PubMed (April 2020) using the following search terms: ‘tuberculosis’ and ‘prevalence’ in the title and ‘survey’ as text word. Reference lists of identified studies were also examined. Studies that were not conducted in Africa, not nationally representative, that were about a subset of TB cases (e.g. drug-resistant TB, women only, healthcare workers, miners, prisons), TB infection rather than TB disease and risk factors for TB (e.g. diabetes, smoking) or bovine TB were excluded. Of 162 published papers, only seven were of relevance [18-24]. Grey literature, such as unpublished survey reports produced by national TB programmes, abstracts and presentations from international meetings, and routine progress updates collated by the WHO Global Task Force on TB Impact Measurement on the status of surveys since 2008, was also systematically reviewed [25-36].
We identified a total of 12 national TB prevalence surveys that were implemented between 2008 and 2016: Ethiopia (2010–2011), Gambia (2011–2013), Ghana (2013), Kenya (2015–2016), Malawi (2013–2014), Nigeria (2012), Rwanda (2012), Sudan (2013–2014), Tanzania (2011–2012), Uganda (2014–2015), Zambia (2013–2014) and Zimbabwe (2014). Of these, nine were global focus countries as defined by the WHO Global Task Force on TB Impact Measurement and the others were Gambia, Sudan and Zimbabwe.
All surveys were of the adult population (defined as aged ≥15 years) and focused on bacteriologically confirmed pulmonary TB (as opposed to clinically diagnosed TB), consistent with WHO guidance [8]. All surveys followed WHO recommendations for survey screening methodology, which is based on symptom screening and chest X-ray for all participants [8]. If participants reported symptoms suggestive of TB and/or had an abnormal chest X-ray, they were invited to submit sputum samples for TB diagnosis, using smear microscopy and at least one of culture, Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) or line probe assay (LPA) (Hain LifeScience GmbH, Nehren, Germany). Bacteriological confirmation was based on either a positive culture for Mycobacterium tuberculosis (MTB) and/or detection by Xpert MTB/RIF or LPA. All national and subpopulation prevalence estimates (such as by age group and sex) were adjusted for sampling design effects, accounting for sampling probabilities, and using multiple imputation of missing data and inverse probability weighting to represent all eligible individuals including those who did not participate, according to previously published methods [37].
Identified surveys were assessed with a 10-criteria tool developed to assess the risk of bias in prevalence surveys [38]. The likelihood of non-response bias for the Nigerian survey was potentially high, and the case definition originally used in the Tanzanian survey was re-defined for this paper; all other surveys were assessed to have low risk of bias for all criteria (Table S1).
Data extraction and analysis
Key results to be identified, analysed and reported were based on a previous publication about national TB prevalence surveys in Asia [5].
Key characteristics of survey design and implementation were summarised for the 12 surveys. These included residency criteria; sampling design including the number of clusters, stratification and planned sample size; screening and diagnostic tests used; and duration of field operations.
Results from each survey were then summarised for the following process and outcome indicators: size of the eligible population; number of people screened and associated participation rate; number of people who screened positive; absolute number of smear-positive and bacteriologically confirmed pulmonary TB cases detected in the survey, both overall and disaggregated by sex and age group; prevalence of smear-positive and bacteriologically confirmed pulmonary TB per 100 000 population aged ≥15 years, both overall and disaggregated by sex and age group, and associated sex (male to female) ratio; the coefficient of variation (k) of the cluster-specific TB prevalence; pre- and post-survey estimates of prevalence (all forms, all ages); proportion of bacteriologically confirmed pulmonary TB cases who did not report TB symptoms; the ratio of prevalent to notified cases (P:N) per 100 000 population; number of bacteriologically confirmed pulmonary TB cases with known HIV status; HIV prevalence in TB survey cases compared with notified TB cases, expressed as a ratio; number of participants with at least one smear-positive sample who were not bacteriologically confirmed for MTB by culture, Xpert MTB/RIF or LPA; and type of healthcare facility used by participants who reported symptoms or were on TB treatment at the time of the survey.
These variables were obtained from survey reports, published papers and available data sets (Text S1) provided by lead survey investigators. The other two sources were the WHO Global TB database for notification data and prevalence of HIV among notified TB cases; [39] and the United Nations Population Division for estimates of the total population in the main year of the survey [40], which were used for calculation of rates per 100 000 population.
For Tanzania, it was only possible to verify the number of smear-positive cases (and not the number of culture-positive, smear-negative cases). Therefore, the prevalence of bacteriologically confirmed TB was estimated using the combined value for the ratio of bacteriologically confirmed to smear-positive TB in the neighbouring countries of Ethiopia, Malawi, Rwanda, Uganda and Zambia (ratio = 2.16:1 (standard deviation [SD]: 0.46)).
Meta-analyses were conducted for four indicators: sex ratio of bacteriologically confirmed prevalence per 100 000 population; proportion of bacteriologically confirmed TB cases that screened negative for symptoms; the ratio of prevalent to notified cases (P:N) per 100 000 population; and HIV prevalence in TB survey cases compared with notified TB cases, expressed as a ratio. These four indicators were summarised using a random-effects model. The metafor package in R (v3.5.2, R Foundation for Statistical Computing, Vienna, Austria) was used to estimate all ratios [41], and heterogeneity was assessed using the I2 statistic [42]. A meta-analysis was not conducted for prevalence estimates because the countries were not considered representative of TB disease burden for the entire African region.
Ethical approval
All 12 prevalence surveys were approved by their respective national ethics committees. Protocols were also reviewed and approved by the WHO Global Task Force on TB Impact Measurement prior to implementation.
Results
Main survey characteristics
The main characteristics of the 12 surveys are shown in Table 1. All surveys were of adults aged ≥15 years who met residency criteria (typically living in the household for a period ranging from the last 2 weeks to the last month). The median duration of field operations was approximately 11 months (range 9–14). The number of clusters per survey ranged from 62 in Tanzania to 109 in Sudan. The sample size was usually in the range 40 000–60 000, with larger surveys in Ghana, Kenya and Sudan. Most surveys used stratified sampling to increase the precision and representativeness of the overall country estimate of TB prevalence; urban and rural were the most common strata defined by the national bureaus of statistics.
Table 1.
Main characteristics of national TB prevalence surveys completed in Africa, 2008-2016
| Country and year |
Residency criteria | Geographical area excluded† |
Number of clusters |
Stratified sampling |
Planned sample size |
Screening strategy¶ |
Diagnostic tests |
Duration of field operations (months) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms interview | Chest X-ray | Smear | Culture | Xpert MTB/RIF | |||||||
| Ethiopia 2010-2011 | Permanent residents who stayed in the household at least one night during the 14 days prior to the census day. Temporary visitors who stayed in the household at least 14 days prior to the census day. | 37/810 woredas excluded from the sampling frame due to security and logistical challenges. | 85 | Urban/ Rural/ Pastoralist | 46 514 | Cough 2 weeks or more | Lung abnormality‡‡ | 2 FM | 1 LJ | No | 9 |
| Gambia 2012 | Residents who spent at least one night in the household in the last 4 weeks before the census. Visitors who arrived in the household 4 weeks or more before the census. | None | 80 | No | 55 281 | (i) Cough 2 weeks or more (ii) Any participant with a cough lasting <2 weeks and 2 or more other symptoms (iii) Any participant without a cough AND 3 or more other symptoms†† | Abnormal and suggestive of TB for any abnormality in lung field or mediastinum‡‡ | 2 FM | 2 MGIT | No | 14 |
| Ghana 2013 | Residents who have not been away for 2 weeks or more. | None | 98 | Urban/Rural | 64 000 | Cough 2 weeks or more | Lung abnormality‡‡ | 2 ZN§§ | 2 MGIT | Smear-positive or contaminated cultures | 10 |
| Kenya 2015-2016 | Residents who lived in the household for a minimum of 30 consecutive days prior to the census. | One cluster excluded due to security issues | 99‡ | Urban/Rural | 72 000 | Cough 2 weeks or more | Lung abnormality‡‡ | 2 FM | 2 LJ | At least one Xpert test for all participants who screened positive | 12 |
| Malawi 2013 | Residents who spent at least 14 days in the household before the census. | None | 74 | Urban/Semi-urban/Rural | 37 200 | Any symptom for 7 days or longer: cough, cough with sputum, blood stained sputum, chest pain, body weight loss, night sweat, fatigue/malaise, fever, shortness of breath | Lung abnormality | 2 FM¶¶ | 2 LJ | Smear-positive or contaminated cultures | 11 |
| Nigeria 2012 | Slept in the household for 14 days or more. | Three clusters replaced due to security issues | 70 | Zonal [6] | 49 000 | Cough 2 weeks or more | Lung abnormality | 2 ZN | 2 LJ | No | 9 |
| Rwanda 2012 | Residents who lived in the household for at least 1 month prior to interview. | None | 73 | No | 44 500 | Cough (any duration) | Lung abnormality | 2 FM | 2 LJ | No | 10 |
| Sudan 2013-2014 | Household resident or visitor for at least 3 weeks. | Four clusters were excluded due to security issues | 109§ | Urban/Rural (Nomadic) | 91 131 | Cough 2 weeks or more; currently on TB treatment | Lung abnormality‡‡ | 2 FM | 2 Ogawa | No | 12 |
| Tanzania 2012 | A person having slept for the last 2 weeks in the household | None | 62 | Urban/Rural/Semi-urban/Zanzibar | 46 792 | Cough 2 weeks or more, haemoptysis, fever for more than 2 weeks, weight loss, and excessive sweating | Any abnormality in the lung fields or mediastinum | 3 FM | 1 LJ | No (Smear-positive slides examined retrospectively) | 11 |
| Uganda 2014-2015 | Individuals who have resided in the household in the survey cluster for at least 14 days before the census day. | None | 70 | Urban/Rural | 40 180 | Cough 2 weeks or more | Lung abnormality‡‡ | 2 ZN | 2 LJ | Smear-positive or contaminated cultures | 10 |
| Zambia 2013-2014 | Individuals who have slept in the household in the previous 24 h prior to census. | None | 66 | Urban/Rural | 54 400 | Cough or fever or chest pains for 2 weeks or more | Lung abnormality or chest X-ray indeterminate‡‡ | 2 ZN | 2 MGIT | Smear-positive or contaminated cultures | 11 |
| Zimbabwe 2014 | Permanent residents who had spent a night at the household. Visitors who were residing in the selected cluster for 14 days or more before the survey. | Two clusters were replaced due to logistical issues. | 75 | Urban/Rural | 44 951 | Cough of any duration, drenching night sweats, and/or haemoptysis | Lung abnormality‡‡ | 2 FM | 2 MGIT | Smear-positive or contaminated cultures | 12 |
C, Conventional radiology; CXR, Chest X-ray; DR, Digital radiology; FM, Fluorescence microscopy; LJ, Löwenstein-Jensen; MGIT, Mycobacterial growth indicator tube; MOH, Ministry of Health; N/A, Not applicable; NTP, National TB Programme; ZN, Ziehl-Neelsen stain.
Although some surveys excluded certain geographical areas from their sampling frames, we included national surveys when most populations were covered.
In Kenya, 1 cluster was excluded from the original 100.
In Sudan, 5 clusters were excluded from the original 114; one for protocol violation and four for security reasons.
Criteria for eligibility of sputum examination.
In Gambia, other symptoms included chest pain, fever, haemoptysis, night sweats, shortness of breath, loss appetite and weight loss.
Other criteria were used especially if a participant was exempt or refused to have a chest X-ray. Please see supplementary file (Text S2) for details.
In Ghana, Zield-Neelsen smears used the concentrated method
In Malawi, FM smears used the concentrated method.
All survey participants were screened using both direct chest X-ray (CXR) and an interview about TB symptoms. The main symptom screening criterion was a cough for two or more weeks, but some countries had a broader definition. Four countries used conventional CXR technologies, and eight used digital systems. Individuals with findings suggestive of TB on CXR and/or symptoms suggestive of TB were eligible for sputum collection and examination. In eight countries, participants who declined or were exempt from having a CXR were also eligible for sputum submission (Table 1).
Sputum samples were tested for the presence of acid-fast bacilli (AFB) using smear microscopy, as well as by culture and/or Xpert MTB/RIF. Most countries examined direct smears (at least two per sputum-eligible participant) using Ziehl-Neelsen or fluorescent microscopy, apart from Ghana and Malawi, which used concentrated smears. Most countries conducted two culture examinations (performed on Löwenstein-Jensen, Ogawa solid media, or BACTEC Mycobacterial Growth Indicator Tube (MGIT) system (Becton Dickinson, Franklin Lakes, NJ, USA)) for each sputum-eligible participant, except for Ethiopia and Tanzania, which only performed one culture test per participant [43, 44].
Testing with Xpert MTB/RIF was typically restricted to participants with sputum smear-positive results or contaminated cultures: this was the case for Ghana, Malawi, Uganda, Zambia and Zimbabwe. Kenya was the first country in Africa to test all submitted sputum samples from positively screened survey participants with Xpert MTB/RIF in addition to culture and smear microscopy. In Tanzania, DNA extracted from smear-positive slides was also tested using Xpert MTB/RIF in the supranational TB reference laboratory in Antwerp, Belgium, due to challenges with culture testing in this survey [45]. Smear-positive samples from Sudan were tested using LPA instead of Xpert MTB/RIF for diagnostic confirmation.
Size of eligible population, survey participation and screening outcomes
For each survey, the number of individuals eligible to participate, the participation rate and screening outcomes are shown in Table 2. The number of eligible individuals who were invited to participate was similar to the planned sample size for most countries. In Nigeria and Tanzania, 59% and 40% more people were invited to participate than planned, respectively, but the total number of participants was similar to the planned sample size.
Table 2.
Summary of sampling population, survey participants and screening outcomes
| Country | Timeframe of field operations |
Planned sample size |
Number of people eligible to participate |
Survey participants |
Number and percentage of participants eligible for sputum examination |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Participation rate (%) |
Symptom positive, chest X-ray positive |
% | Symptom positive, chest X-ray negative/ N/A |
% | Symptom negative, chest X-ray positive |
% | Other† | % | Any symptom positive |
% | Any chest X-ray positive |
% | Total eligible |
% | ||||
| Ethiopia | 2010–2011 | 46 514 | 51 667 | 46 697 | 90% | 806 | 1.7% | 2220 | 4.8% | 3013 | 6.5% | 41 | 0.09% | 3026 | 6.5% | 3819 | 8.2% | 6080 | 13% |
| Gambia | 2011–2013 | 55 281 | 55 832 | 43 100 | 77% | 1026 | 2.4% | 2436 | 5.7% | 2384 | 5.5% | 102 | 0.24% | 3462 | 8.0% | 3410 | 7.9% | 5948 | 14% |
| Ghana | 2013 | 63 905 | 67 757 | 61 726 | 91% | 771 | 1.2% | 1198 | 1.9% | 4387 | 7.1% | 1942 | 3.1% | 1969 | 3.2% | 5158 | 8.4% | 8298 | 13% |
| Kenya | 2015–2016 | 72 000 | 76 291 | 63 050 | 83% | 1241 | 2.0% | 2896 | 4.6% | 5184 | 8.2% | 394 | 0.62% | 4137 | 6.6% | 6425 | 10% | 9715 | 15% |
| Malawi | 2013–2014 | 37 200 | 39 026 | 31 579 | 81% | 381 | 1.2% | 2334 | 7.4% | 717 | 2.3% | N/A | N/A | 2715 | 8.6% | 1098 | 3.5% | 3432 | 11% |
| Nigeria | 2012 | 49 000 | 77 797 | 44 186 | 57% | 746 | 1.7% | 1720 | 3.9% | 2222 | 5.0% | N/A | N/A | 2466 | 5.6% | 2968 | 6.7% | 4688 | 11% |
| Rwanda | 2012 | 44 500 | 45 058 | 43 128 | 96% | 545 | 1.3% | 2092 | 4.9% | 2107 | 4.9% | 3 | 0.01% | 2637 | 6.1% | 2652 | 6.1% | 4747 | 11% |
| Sudan | 2013–2014 | 91 131 | 96 979 | 83 202 | 86% | 1823 | 2.2% | 840 | 1.0% | 9838 | 12% | 5040 | 6.1% | 2663 | 3.2% | 11 661 | 14% | 17 541 | 21% |
| Uganda | 2014–2015 | 40 180 | 45 293 | 41 154 | 91% | 552 | 1.3% | 2162 | 5.3% | 2298 | 5.6% | 130 | 0.32% | 2714 | 6.6% | 2850 | 6.9% | 5142 | 12% |
| Tanzania | 2011–2012 | 46 792 | 65 664 | 50 447 | 77% | 804 | 1.6% | 3459 | 6.9% | 2039 | 4.0% | N/A | N/A | 4263 | 8.5% | 2843 | 5.6% | 6302 | 12% |
| Zambia | 2013–2014 | 54 400 | 54 830 | 46 099 | 84% | 1505 | 3.3% | 2948 | 6.4% | 2255 | 4.9% | N/A | N/A | 4453 | 10% | 3760 | 8.2% | 6708 | 15% |
| Zimbabwe | 2014 | 44 951 | 43 478 | 33 736 | 78% | 628 | 1.9% | 1205 | 3.6% | 2803 | 8.3% | 1184 | 3.5% | 1833 | 5.4% | 3431 | 10% | 5820 | 17% |
| Total | 83% | 1.8% | 4.7% | 6.2% | 1.7% | 6.5% | 8.0% | 14% | |||||||||||
N/A, not applicable.
Other refers to criteria used to ascertain if a participant was eligible for sputum collection other than via symptom or chest X-ray screening. See Text S2 for specific details.
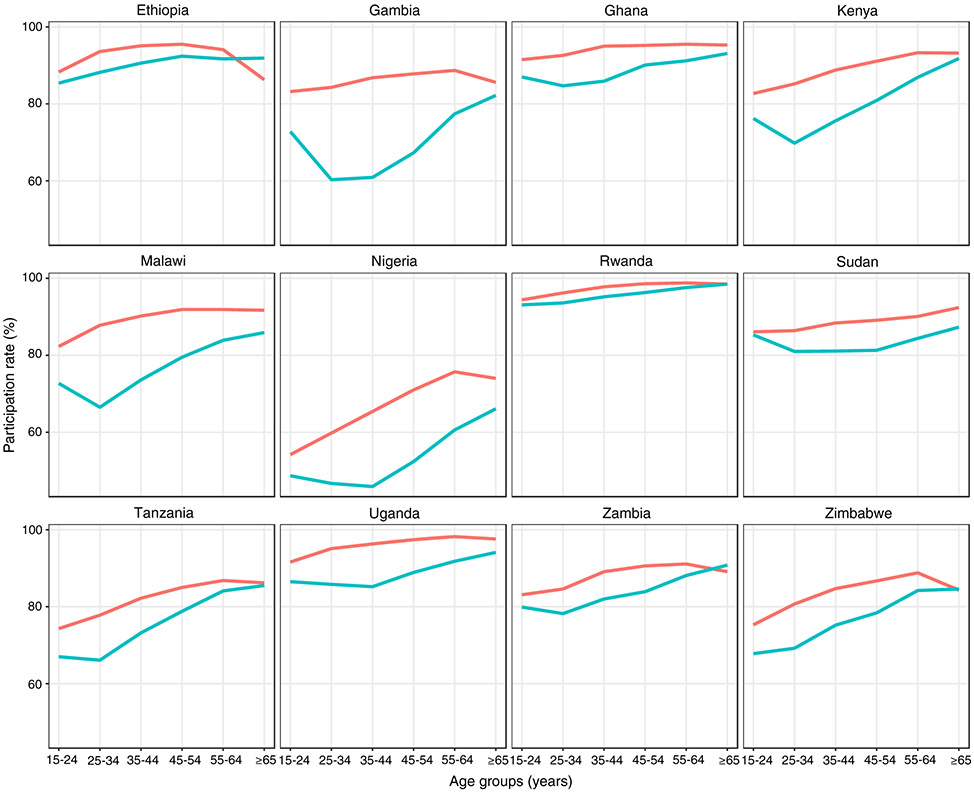
In 12 surveys, 588 105 individuals participated, equivalent to 82% (range 57–96%) of those eligible. The median number of participants enrolled in a survey was 49 000 (range 31 579–83 079). The proportion of the eligible survey population that agreed to participate was ≥80% in eight surveys. The participation rate was lowest in Nigeria (57%) and 77–78% in Gambia, Tanzania and Zimbabwe. In general, participation was higher among females and older age groups than males and younger age groups (Figure 2).
Figure 2.
Participation rate by sex and age group in national TB prevalence surveys implemented in Africa, 2008–2016. Female (red), Male (green).
Overall, the proportion of participants who had a positive TB symptom screen and/or an abnormal CXR was approximately 14% (range 11–21%) in the 12 countries. The proportion of participants that screened positive via CXR (i.e. CXR only, or symptom and CXR; median 8%, range 3.5–14%) was higher than the proportion of participants screened positive via symptoms (i.e. symptom only, or symptom and CXR; median 6.5%, range 3.2–9.7%) especially where the standard criteria of cough for two or more weeks were used. However, countries that used broader symptom screening criteria (i.e. Gambia, Malawi, Tanzania and Zambia) had greater yields from symptom screening than CXR. In Ghana, Sudan and Zimbabwe, more than 3% of participants were eligible to submit sputum samples based on exemption from (or declining) CXR examination.
Prevalence per 100 000 population
Prevalence results overall, and the proportion of bacteriologically confirmed cases that were smear-positive, are shown in Table 3. The prevalence of smear-positive pulmonary TB per 100 000 population aged ≥15 years varied from 74 (95% CI 48–99) in Rwanda to 319 (95% CI 232–406) in Zambia. The prevalence of bacteriologically confirmed pulmonary TB per 100 000 population aged ≥15 years ranged from 119 (95% CI 79–160) in Rwanda to 638 (95% CI 502–774) in Zambia. The proportion of bacteriologically confirmed cases who were smear-positive varied from 21% in Zimbabwe to 74% in Nigeria. Smear-negative, bacteriologically confirmed cases were more common than smear-positive, bacteriologically confirmed cases in all countries except Nigeria, Rwanda, Sudan and Zambia.
Table 3.
Summary of prevalent TB cases and the prevalence of pulmonary TB per 100 000 population aged ≥15 years
| Country | Smear-positive pulmonary TB |
Bacteriologicallyconfirmed pulmonary TB |
Proportion of bacteriologically confirmed cases that were smear-positive |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of cases |
Prevalence per 100 000 population aged ≥ 15 years† |
95% confidence interval |
k‡ | Number of cases |
Prevalence per 100 000 population aged ≥ 15 years† |
95% confidence interval |
k‡ | ||
| Ethiopia | 4 | 108 | 73–143 | 0.7 | 110 | 277 | 208–347 | 0.4 | 39 |
| Gambia | 34 | 90 | 53–127 | 1.3 | 77 | 212 | 152–272 | 0.7 | 42 |
| Ghana | 64 | 111 | 76–145 | 0.9 | 202 | 356 | 288–425 | 0.7 | 31 |
| Kenya | 123 | 230 | 174–286 | 0.7 | 305 | 558 | 455–662 | 0.7 | 41 |
| Malawi | 62 | 220 | 142–297 | 1.1 | 132 | 452 | 312–593 | 1.1 | 49 |
| Nigeria | 107 | 318 | 225–412 | 0.9 | 144 | 524 | 378–670 | 0.7 | 61 |
| Rwanda | 27 | 74 | 48–99 | N/A§ | 40 | 119 | 79–160 | 0.7 | 62 |
| Sudan | 57 | 87 | 52–121 | 1.3 | 112 | 183 | 128–238 | 1.3 | 48 |
| Uganda | 66 | 174 | 111–238 | 0.9 | 160 | 401 | 292–509 | 0.8 | 43 |
| Tanzania¶ | 134 | 275 | 232–326 | 0.6 | N/A | N/A | N/A | N/A | N/A |
| Zambia | 135 | 319 | 232–406 | 0.8 | 265 | 638 | 502–774 | 0.7 | 50 |
| Zimbabwe | 23 | 82 | 47–118 | N/A§ | 107 | 344 | 268–420 | 0.3 | 24 |
N/A, not applicable.
Estimates based on the use of robust standard errors with missing value imputation and inverse probability weighting for all countries except for Tanzania for which a cluster-level analytical model was used.
k is the coefficient of variation of the cluster-specific TB prevalences. When the coefficient of variation (k) of cluster-specific TB prevalence was not reported, it was derived from the reported design effect.
k could not be calculated because the design effect was less than one.
The number of bacteriologically confirmed cases could not be verified for the estimation of prevalence by WHO. The smear-positive and bacteriologically confirmed prevalence reported by the Tanzanian survey team was 249 per 100 000 (95% CI: 192–305) and 293 per 100 000 (95% CI: 228–358) population, respectively [22].
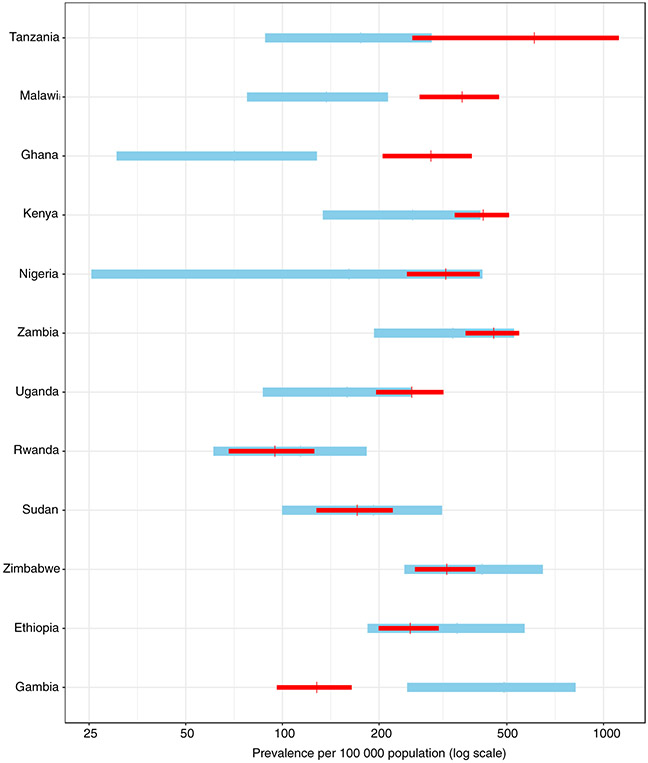
Estimates of TB prevalence for all ages and all forms of TB (i.e. including children and extrapulmonary TB) published by WHO before the survey compared with updated estimates based on survey findings are shown in Figure 3. Post-survey, TB prevalence estimates were higher in Ghana, Kenya, Malawi, Nigeria, Tanzania, Uganda and Zambia, but lower in the other five countries. The post-survey range of uncertainty was outside the pre-survey bounds for Ghana, Gambia and Malawi. With the exception of Tanzania, post-survey estimates were much more precise (i.e. narrow uncertainty intervals).
Figure 3.
a Countries are listed in decreasing order according to the before-after difference. The vertical line denotes the best estimate of prevalence and its range (depicted as a 95% uncertainty interval). These prevalence estimates were indirectly derived from estimates of incidence and the duration of disease previously published by WHO, adjusted to the year of the prevalence survey using previously published trends in incidence.
Estimates of TB prevalence (all ages, all forms of TB) for 12 surveys, before (in blue) and after (in red) results from national TB prevalence surveys implemented in Africa, 2008–2016a.
Sex and age distribution of TB cases
Overall, men were more likely to have TB than women (sex ratio 2.0; 95% C.I. 1.6–2.5) (Figure 4). The male:female sex ratio of bacteriologically confirmed TB prevalence ranged from 1.2 in Ethiopia to 4.1 in Uganda.
Figure 4.
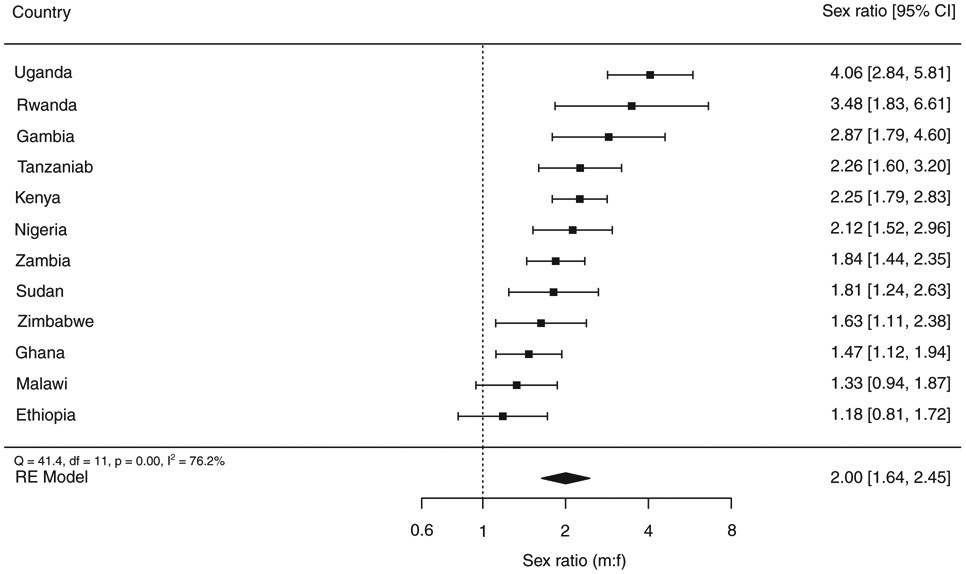
a The size of the best estimate (black square) is proportional to the model’s weights (inverse variance).
b The sex ratio of smear-positive TB prevalence is shown for Tanzania.
The sex ratio (male to female) of bacteriologicallyconfirmed pulmonary TB cases detected in national TB prevalence surveys implemented in Africa, 2008–2016a.
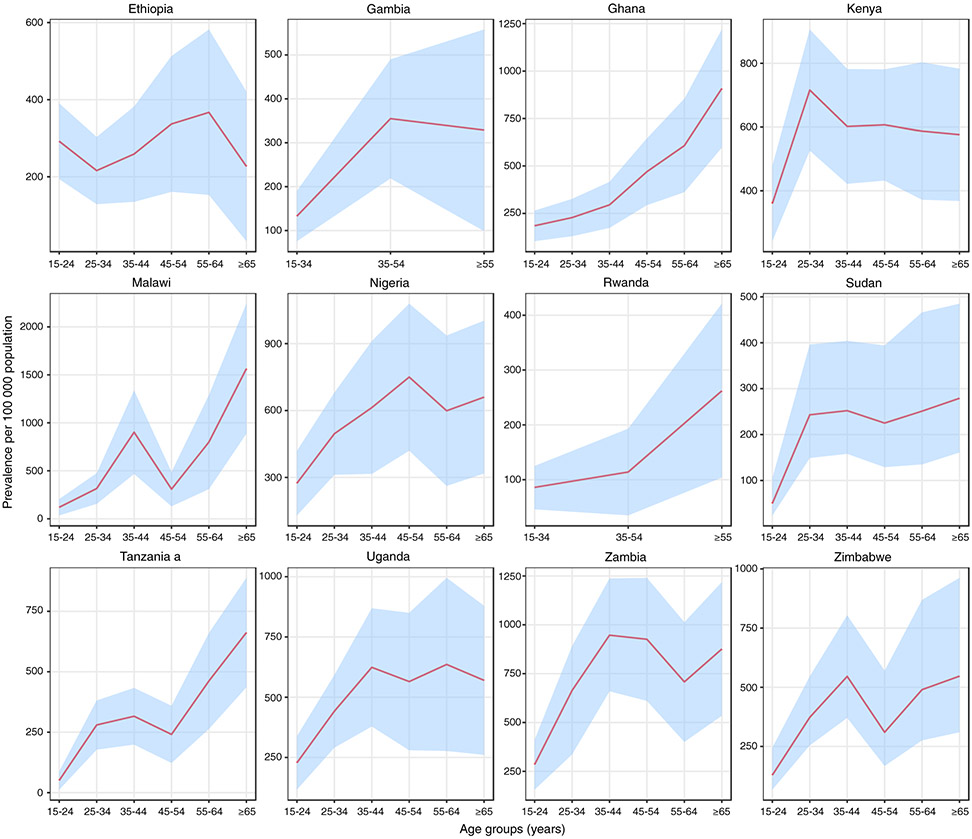
The age distribution of cases varied considerably (Figure 5. In Ghana and Rwanda, the prevalence per 100 000 population increased steadily with age; in Malawi, Tanzania and Zimbabwe there was a general increase up to the age group 35–44 years, followed by a decrease in the next age group and then an increase to a peak in those aged ≥65 years; in other countries, there was an increase to a peak in young or middle-aged adults, with similar levels in older age groups. The absolute number of TB cases was highest among participants aged 35–44 years (Table S2) in most countries; the exceptions were Ghana, Malawi and Tanzania, where the absolute number of cases was highest among those aged ≥65 years.
Figure 5.
a Bacteriologically-confirmed TB cases could not be verified, so the value for smear-positive TB is shown instead.
Estimated age-specific prevalence of bacteriologically-confirmed pulmonary TB in national TB prevalence surveys implemented in Africa, 2008–2016. The pink line denotes the best estimate and the blue shaded areas are the 95% confidence intervals.
Proportion of TB cases that screened negative for TB symptoms or had no symptoms
The mean proportion of bacteriologically confirmed TB cases that were eligible for sputum examination based on CXR results alone (i.e. they did not report any TB screening symptoms) was 44% (range 30–60%) (Figure 6 and Table S3). Of note, Malawi, Gambia, Tanzania, Zambia and Zimbabwe had more sensitive screening criteria than the other countries, which typically used a cough of two or more weeks and/or haemoptysis as screening criteria (Table 1). There was no difference in the proportion of symptom screening negative bacteriologically confirmed TB cases by HIV status in Kenya, Malawi and Uganda, where case-based data on HIV status were available.
Figure 6.
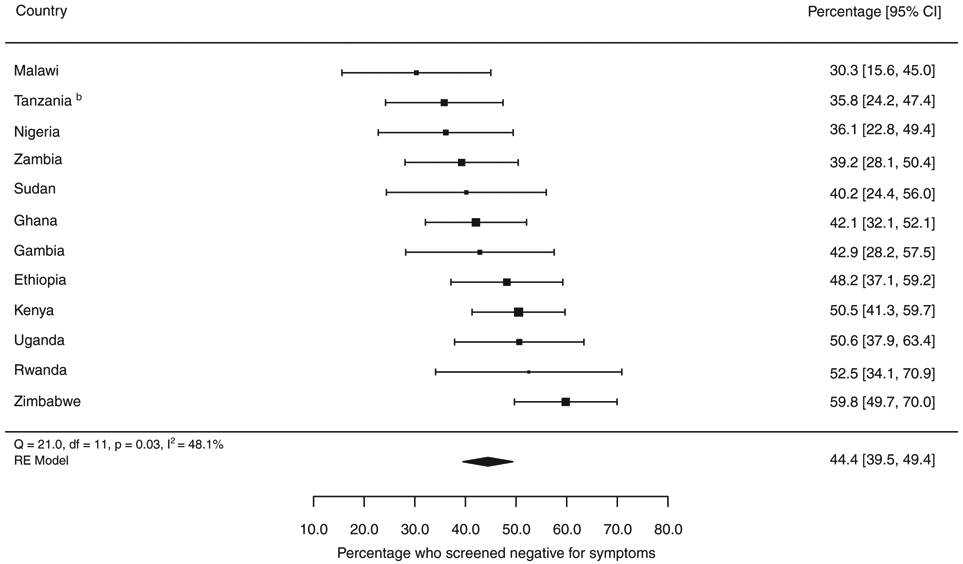
a The size of the best estimate (black square) is proportional to the model’s weights (inverse variance).
b Bacteriologically-confirmed TB cases could not be verified for Tanzania, so the value for smear-positive TB is shown instead.
Percentage of bacteriologically-confirmed pulmonary TB cases who screened symptom negative in national TB prevalence surveys completed in Africa, 2008–2016a.
Ratio of prevalence to notification
The mean prevalence to notification (P:N) ratio among smear-positive TB cases was 2.5, ranging from 0.62 in Gambia to 5.8 in Nigeria (Figure 7). Apart from Ethiopia, Gambia and Rwanda, all surveys had a P:N ratio of at least 2. Across all surveys, the P:N ratio was higher in men than women; the most noticeable difference was in Nigeria, where the P:N ratio was 7.3 for men and 4.6 for women (Figure S1a,b).
Figure 7.
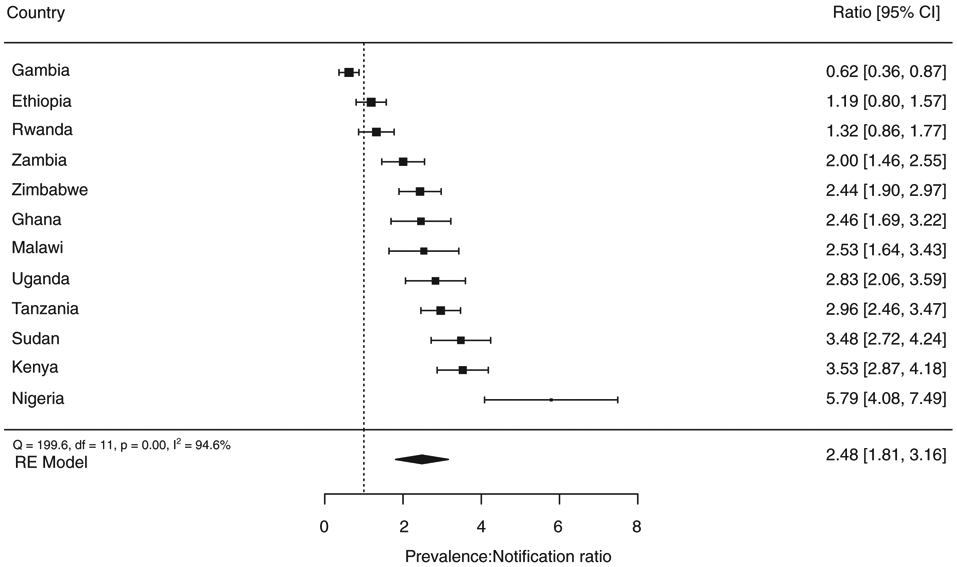
a The comparison is for smear-positive pulmonary TB for all countries except Kenya, Uganda and Zimbabwe, for which the comparison is for bacteriologically confirmed pulmonary TB. The size of the best estimate (black square) is proportional to the model’s weights (inverse variance).
Prevalence to notification (P:N) ratio for TB cases in national TB surveys implemented in Africa, 2008–2016a.
HIV status of participants and bacteriologically confirmed TB cases
HIV testing results from the survey, and/or the self-reported or previously documented HIV status of survey participants, were available for Kenya, Malawi, Rwanda, Tanzania, Uganda, Zambia and Zimbabwe.
HIV testing during field operations was only conducted in four countries: Zambia offered HIV testing (Alere Determine™ HIV-1/2 then Uni-Gold™ HIV) to all participants, whereas Rwanda (Alere Determine™ HIV-1/2 then Uni-Gold™ HIV), Tanzania (SD BIOLINE HIV-1/2 then Alere Determine™ HIV-1/2, changing to Alere Determine™ HIV-1/2 then Uni-Gold™ HIV) and Uganda (Alere Determine™ HIV-1/2, HIV 1/2 STAT-PAK® and Uni-Gold™ HIV) offered to test only those who were eligible for sputum examination. In Zambia, among participants who were tested for HIV infection, 6.7% (2062/30 584) were HIV-positive. In Rwanda, Uganda and Tanzania, of those eligible for sputum examination and tested for HIV infection, 4.9% (218/4445), 9.6% (422/4386) and 5.0% (318/6302) were HIV-positive, respectively.
In Malawi, all participants were asked if they had ever been tested for HIV and were invited to disclose their status; verbal acknowledgement of HIV status was known in 19 703 (62.4%) participants and 1840 (9.3%) of them reported to be HIV-positive. In Kenya and Zimbabwe, the HIV status of TB cases was obtained from records that were routinely linked as part of HIV treatment and care programmes; 17% (41/245) and 51% (42/83) of TB cases were HIV-positive, respectively.
Data on HIV status were available (tested or self-reported) for 51–100% of bacteriologically confirmed TB cases, and of these, the median HIV-positive proportion was 27%, ranging from 3% in Rwanda to 51% in Zimbabwe. For these seven surveys, the proportion of prevalent TB cases co-infected with HIV was found to be systematically lower than the proportion of newly notified TB cases with HIV. The HIV prevalence ratio (i.e. TB/HIV prevalent survey cases over notified TB cases with HIV for the main year of the survey) was 0.47 (95% CI 0.34–0.65) (Figure 8).
Figure 8.
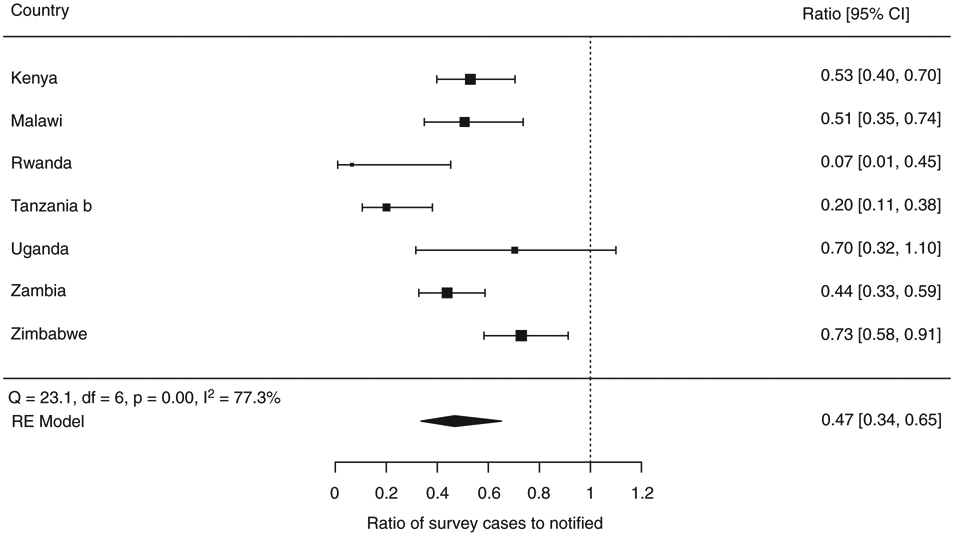
aThe proportions of TB survey cases with known HIV status and notified TB cases that were HIV-positive, respectively, were as follows: Kenya (0.17, 0.31), Malawi (0.28, 0.55), Rwanda (0.03, 0.26), Tanzania (0.08, 0.37), Uganda (0.27, 0.44), Zambia (0.27, 0.61) and Zimbabwe (0.51, 0.69). The size of the best estimate (black square) is proportional to the model’s weights (inverse variance).
b Bacteriologically-confirmed TB cases could not be verified for Tanzania, so the value for smear-positive TB is shown instead.
HIV prevalence in TB survey cases compared with notified TB cases, expressed as a ratio, in national TB prevalence surveys implemented in Africa, 2008–2016a.
Smear-positive results not classified as TB
A positive sputum smear microscopy result was found to be poorly predictive of TB in several surveys. A large proportion of participants with smear-positive results were not confirmed to have TB by Xpert MTB/RIF (or LPA) or culture (Table 4). A high proportion of smear-positive samples tested in Ghana (70%), Malawi (62%), Zambia (62%) and Zimbabwe (89%) were negative and thus not bacteriologically confirmed as MTB.
Table 4.
Percentage of survey participants with smear-positive results that were not confirmed TB. Results shown for surveys in which specimens were tested using smear microscopy, rapid molecular tests and culture†
| Country | Number of participants with at least one smear-positive specimen |
Participants with smear-positive specimens excluded as a TB case |
|
|---|---|---|---|
| Number | % | ||
| Ghana | 198 | 138 | 70% |
| Kenya | 141 | 18 | 13% |
| Malawi | 163 | 101 | 62% |
| Sudan | 61 | 4 | 6.6% |
| Uganda | 91 | 25 | 27% |
| Zambia | 356 | 221 | 62% |
| Zimbabwe | 206 | 183 | 89% |
Results are shown for surveys in which specimens were systematically tested using smear microscopy and rapid molecular tests. All surveys used Xpert MTB/RIF except Sudan which used line probe assays (LPAs). Kenya used both culture and Xpert MTB/RIF whereas other surveys used Xpert (or LPA) to confirm smear-positive specimens only.
Health care-seeking behaviour of participants
All surveys collected data on healthcare-seeking behaviour related to participants who reported symptoms (Table 5). Among participants who reported symptoms and had sought care, the majority (median 82%, range 70–93%) initially sought care in a public health facility. Outside the public sector, private health facilities were more commonly visited in Ethiopia and Malawi, whereas pharmacy visits were more common in Ghana, Nigeria and Uganda. In Rwanda, Tanzania and Zambia, symptomatic participants who sought care were asked which diagnostic tests were performed: 48%, 37% and 12% had sputum collected for examination, respectively, and 28% and 14% had a CXR performed in Tanzania and Zambia [46-48]. A large proportion (median 46%, 13–68%) of symptomatic participants had not sought care prior to the survey, and the main reasons given included their perception that their symptoms were not serious enough and, to a lesser extent, the costs related to seeking care.
Table 5.
Healthcare-seeking behaviour among participants who were symptom-screen positive
| Country | Participants who were symptom- screen positive |
No action taken |
% | Location of care sought | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consulted medical facility |
% | Type of facility | Phar macy |
% | Tradi tional |
% | Other | % | Unspec ified |
% | Self- treated |
% | Un known |
% | |||||||||
| Public facility |
% | Private facility |
% | Other facility |
% | ||||||||||||||||||
| Ethiopia | 3026 | 1932 | 64% | 848 | 28% | 628 | 74% | 199 | 23% | 21 | 2.5% | 40 | 1.3% | 3 | 0.10% | N/A | N/A | 55 | 1.8% | N/A | N/A | 148 | 4.8% |
| Gambia | 3462 | 1424 | 41% | 1706 | 49% | 1398 | 82% | 220 | 13% | 88 | 5.2% | 17 | 0.49% | 14 | 0.40% | 24 | 0.69% | N/A | N/A | N/A | N/A | 277 | 8.0% |
| Ghana | 1969 | 264 | 13% | 793 | 40% | 695 | 88% | 61 | 7.7% | 37 | 4.7% | 324 | 17% | 20 | 1.0% | N/A | N/A | N/A | N/A | 567 | 29% | 1 | 0.10% |
| Kenya† | 4137 | 2763 | 67% | 1257 | 30% | 1047 | N/A | 198 | N/A | 3 | N/A | 56 | N/A | 9 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 117 | 2.8% |
| Malawi | 2715 | 1096 | 40% | 1280 | 47% | 901 | 70% | 379 | 30% | N/A | N/A | 32 | 1.2% | 41 | 1.5% | 4 | 0.15% | N/A | N/A | 236 | 8.7% | 26 | 0.96% |
| Nigeria | 2466 | 604 | 24% | 800 | 32% | 628 | 79% | 172 | 21% | N/A | N/A | 319 | 13% | 11 | 0.45% | 9 | 0.36% | 3 | 0.12% | 680 | 28% | 40 | 1.6% |
| Rwanda† | 2855 | 1934 | 68% | 921 | 32% | 941 | N/A | 48 | N/A | 38 | N/A | 101 | N/A | 54 | N/A | N/A | N/A | N/A | N/A | 0 | 0 | N/A | N/A |
| Sudan | 2663 | 575 | 22% | 1308 | 49% | 1077 | 82% | 90 | 6.9% | 141 | 11% | 52 | 2.0% | 49 | 1.8% | N/A | N/A | 69 | 2.6% | N/A | N/A | 610 | 23% |
| Uganda | 2714 | 1059 | 39% | 1201 | 44% | 1038 | 86% | 146 | 12% | 17 | 1.4% | 421 | 16% | 11 | 0.41% | N/A | N/A | N/A | N/A | 22 | 0.81% | 0 | 0% |
| Tanzania | 3388 | 1688 | 50% | 481 | 14% | 445 | 93% | 36 | 7.5% | N/A | N/A | 147 | 4.3% | 11 | 0.32% | 257 | 7.6% | 155 | 4.6% | N/A | N/A | 649 | 19% |
| Zambia | 4453 | 2534 | 57% | 1829 | 41% | 1680 | 92% | 75 | 4.1% | 74 | 4.0% | 16 | 0.36% | 1 | 0.02% | N/A | N/A | N/A | N/A | N/A | N/A | 73 | 1.6% |
| Zimbabwe† | 1833 | 1130 | 62% | 486 | 26% | 438 | N/A | 45 | N/A | N/A | N/A | 17 | N/A | 13 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 217 | 12% |
N/A, not applicable.
In Kenya, Rwanda and Zimbabwe participants could select more than one category.
Nine surveys collected data about the current place of treatment for participants who self-reported to be on TB treatment (Table S4). The proportion of participants on TB treatment at the time of the survey varied from 0.04% to 0.25%. The majority of those on treatment at the time of the survey were being treated in the public sector, although place of treatment was unknown for at least a quarter of those on treatment in Kenya, Sudan and Zambia. Overall, the proportion of TB patients on treatment in the private sector at the time of the survey was low except for Ghana (10%) and Nigeria (6.1%).
Discussion
During 2008–2016, 12 nationally representative TB prevalence surveys were completed in Africa in countries that collectively accounted for 48% of the regional burden of TB [39]. The prevalence of bacteriologically confirmed pulmonary TB among individuals aged ≥15 years ranged from 119 per 100 000 population in Rwanda to 638 per 100 000 population in Zambia. Surveys conducted in Ghana, Kenya, Malawi, Nigeria, Tanzania, Uganda and Zambia found a higher burden of TB disease than previously estimated, whereas it was similar to or lower than pre-survey estimates in Ethiopia, Gambia, Rwanda, Sudan and Zimbabwe. Apart from Gambia, Ghana and Malawi, estimates were within the uncertainty bounds of previous estimates, and with the exception of Tanzania, all post-survey estimates were more precise (i.e. narrower uncertainty bounds).
Consistent with routine notification data and with the results of prevalence surveys from Asia, the prevalence of TB was higher in men than women across all African surveys [5]. Men of 35 years and above accounted for a significant proportion of all prevalent cases in Africa, highlighting the need to target this group for intervention. The highest absolute number of prevalent TB cases and the prevalence per 100 000 population were found in older age groups in Ghana, Malawi, Rwanda and Tanzania showing that the epidemic is a progressively ageing one in these countries, similar to previous findings from surveys in Asia [5]. The age distribution of cases also indicates that transmission is declining in these four countries, since more cases in older people suggest an increasing contribution of reactivation of past (rather than recent) TB infection to total TB cases. However, in most other African countries, prevalence plateaued across the middle to older age groups and peaked in those aged 25–54 years, suggesting transmission in the community is still widespread despite many years of implementing first the DOTS and then Stop TB strategies recommended by WHO [4, 49]. In Malawi, Zambia and Zimbabwe, where more than 50% of routinely notified TB patients are co-infected with HIV, the significant peaking of TB prevalence in younger age groups likely reflects the effect of the HIV epidemic in these communities [50].
The acceptance of reporting HIV status and/or testing was high in countries that reported data; presumably, because HIV testing is an acceptable and widespread part of routine health care. Five surveys did not have data on HIV status (this included Ethiopia and Nigeria which are high TB/HIV burden countries). Nonetheless, the pattern was still quite striking: HIV infection among TB cases identified in these surveys was consistently lower than in routinely notified TB cases. This finding probably reflects three factors: (i) the duration of illness with TB among people living with HIV is shortened by higher case fatality ratios, compared with people with TB who are HIV-negative; (ii) the severity of disease among people with TB who are HIV-positive may prompt them to seek health care more rapidly; (iii) HIV programmes are improving the detection of TB/HIV co-infected individuals relative to those who are HIV-negative. Scaling-up collaborative TB/HIV activities and joint TB and HIV programming can promote synergies between TB and HIV programmes [51]. At the same time, it is important to give attention to targeted interventions and approaches for people with TB who are HIV-negative, especially given that the overall burden of TB in this population is much higher.
All surveys revealed limitations in the TB screening and diagnostic algorithms used in routine health care settings. A large proportion of survey TB cases were not identified by symptom screening, even when the range of symptoms considered was expanded beyond those typically used in routine healthcare settings (e.g. cough for ≥2 weeks, haemoptysis, weight loss, fever, night sweats). Approximately one-third of smear-positive and half of bacteriologically confirmed TB cases were symptom-screen negative and were detected via CXR only.
CXR is not a routine part of TB diagnosis in most African countries, and therefore, survey findings indicate that many people with TB remain in the community for a long time unless their disease progresses or it is opportunistically detected while accessing the health system for other reasons (e.g. during an examination for another illness, antenatal services, HIV care). Expanding the use of CXR may improve case finding, but at the cost of increased diagnostic testing [52].
Many survey TB cases (and participants) who reported symptoms suggestive of TB did seek health care for their symptoms, but were not initially diagnosed. This indicates a need to strengthen health systems by improving access to TB diagnostic services (especially in the healthcare facilities where the population is most likely to initially seek care) and raising awareness of TB symptoms among healthcare workers. The high proportion of participants who reported symptoms but had not gone to a health facility also suggests geographical and financial barriers to accessing care that need to be addressed. In addition, as previously observed in the context of national TB prevalence surveys in Asia, it is possible that people with TB in older age groups tolerate their symptoms for longer if they already have a chronic health condition [5].
Although a sizeable proportion of symptomatic participants and cases did not seek care, most of those who did sought care in the public sector. Unlike in Asia, the private sector currently plays a small role in TB case management in Africa [5]. However, some health providers, such as pharmacies (which are often the first point of contact), could play an important role in healthcare referrals especially in Ghana, Nigeria and Uganda [53, 54]. Engaging with and raising awareness in the community in general and among all healthcare providers could help to increase case detection in Africa.
Smear microscopy remains the backbone of TB diagnosis in many African countries. In some surveys, close to two-thirds of smear-positive samples did not have MTB detected by culture or Xpert MTB/RIF (or LPA); of these, most were culture or Xpert-negative, and non-tuberculous mycobacteria (NTM) was not uncommon [55]. The lower positive predictive value of smear microscopy in the context of prevalence surveys highlights its limitations as a diagnostic tool in the context of active case finding in the general population.
The P:N ratio is an approximate indicator (expressed in years) of case detection by the NTP [56]. The higher the ratio, the longer the time taken for a prevalent case to be notified to the NTP. Some cases may exit the pool of prevalent cases without being notified, for example because they self-cure or die, or because they are detected and treated by providers not linked to official reporting systems. The overall P:N ratio for smear-positive TB was greater than two, highlighting major gaps in detection and/or official reporting (to national authorities) of TB cases. These ratios were systematically higher in men, suggesting that women may be accessing available diagnostic and treatment services more effectively [57]. Strategies to reduce this gender gap, informed by operational research to understand the reasons for it in different contexts, are required. More positively, it is likely that these gaps could be reduced by improving reporting mechanisms. Inventory studies can be used to assess the degree to which detected TB cases are under-reported [58]. The ratio of <1 in Gambia may reflect a high level of case detection and a relatively short duration of disease prior to diagnosis (if the duration of disease is less than one year and notifications are a good proxy for incidence, the P.N ratio will be less than one).
The survey data have several limitations: (i) children <15 years were excluded from all surveys as per WHO recommendations [59], meaning that extrapolation of estimates to all ages relied on country-specific child TB surveillance or research data; (ii) low culture confirmation among smear-positive cases (<80%) in a few countries (notably Ethiopia which only used one culture, plus Gambia, Nigeria and Rwanda) suggests there was some under-diagnosis of smear-negative TB cases and that reported overall prevalence estimates may be conservative; (iii) although all surveys followed the essential elements of the screening and diagnostic algorithm recommended by WHO, there was some variation in the symptoms used for screening and in the number and specific type of diagnostic tests; (iv) while estimates of prevalence were adjusted for non-participation in the analysis, it was necessary to assume that those who did not participate were the same as those that did for a given age and sex; (v) none of the surveys covered key affected populations such as migrants and prisoners; and (vi) standardisation of healthcare-seeking behaviour data was insufficient and it was necessary to recategorize healthcare facilities to make the data as consistent and comparable as possible. More generally, it was not possible to identify the exact reasons for some of the differences in survey results among countries (e.g. the absolute level of TB prevalence, sex ratio, P:N ratio). Plausible explanations include the past history of efforts in TB control, the overall performance, governance and financing of the health system, and broader determinants of TB (e.g. levels of undernutrition, smoking, HIV, diabetes, poverty, social protection, income per capita). Quantifying and disentangling the relative contribution of these factors is, however, difficult to do and was not the primary purpose of our study. Further cross-country analyses as well as new quantitative and qualitative research at country level are necessary to further unpick the differences observed among surveys.
A study strength is that it is the largest analysis of national TB prevalence survey data from Africa known to date and provides important knowledge about the epidemiology of TB in the region. It also highlights the benefits of these surveys beyond the primary objective of estimating prevalence. Results from surveys completed in 2019–2020 in four high TB/HIV burden countries of southern Africa (Eswatini, Lesotho, Mozambique and South Africa) will provide further evidence in the near future.
Besides the survey results themselves and their implications for policy for programmatic action, the experience of implementing the first wave of surveys in Africa for more than 50 years generated important lessons for future surveys in terms of both successes and challenges. Successes included use of prevalence survey data to inform national strategic plans and associated goals, targets, advocacy and resource mobilisation; building capacity within NTPs and survey implementing agencies, for example, in terms of staff skills and availability of equipment that could be used after the survey was finished; and fostering country–country collaboration. The major challenges were data management, achieving high participation especially in urban areas, ensuring the quality of culture testing, and delays in reporting and disseminating results (Tables S5 and S6).
In 2016, the MDGs (2000–2015) and WHO’s Stop TB Strategy (2006–2015) were succeeded by a new era of Sustainable Development Goals (2016–2030) and the WHO End TB Strategy, which include the overall goal of ending the TB epidemic and targets to reduce incidence (per 100 000 population) and the number of TB deaths by 80% and 90%, respectively, by 2030, compared with 2015 [60, 61]. The prevalence surveys completed in Africa between 2008 and 2016 show the scale of the diagnostic and treatment gaps that need to be closed to make progress towards these targets, and the nature of the interventions required. These include policies and practices to find new TB cases by improving access to health and diagnostic services, and reduce under-reporting of detected TB cases, especially among men. The surveys also provide important national baselines, which were not previously available in any African country, for monitoring of progress in reducing the burden of TB disease in the next decade.
Supplementary Material
Figure S1. Prevalence to notification (P:N) ratio of TB cases disaggregated by sex of national TB prevalence surveys implemented in Africa, 2008–2016.
Table S1. Assessment of bias.
Table S2. Bacteriologically confirmed TB survey cases by age group in national TB prevalence surveys implemented in Africa 2008–2016: prevalence and number.
Table S3. Prevalent TB cases by screening outcome in national TB prevalence surveys implemented in Africa 2008–2016.
Table S4. Location of treatment for participants who were on treatment at the time the survey was implemented, 2008–2016.
Table S5. Major successes of national TB prevalence surveys implemented in Africa 2008–2016.
Table S6. Major challenges faced in national TB prevalence surveys implemented in Africa 2008–2016.
Text S1.
Text S2.
Box 1. Members of the African TB Prevalence Survey Group, in alphabetical order.
Egbal Ahmed Basheir Abukaraig2, Kennedy Kwasi Addo3, Ifedayo Adetifa4, Zeleke Alebachew5, Rhoda Banda6, Adedapo Bashorun7, Emily Bloss8, Frank Adae Bonsu5, Pascalina Chanda-Kapata9, Edward Demba7, Asrar M. Abdel Salam Elegail10, Mai Eltigany11, Julia Ershova8, Michel Gasana12, Belaineh Girma6, Philippe Glaziou1, Nico Kalisvaart13, Hiba Kamal Hamadelneel2, Deus Kamara14, Nathan Kapata9, Samuel Kasozi15, Amha Kebede16, Lindsay Kendall7, Hillary Kipruto17, Bruce J. Kirenga15, Eveline Klinkenberg13,18, Sayori Kobayashi1, Christopher Linda7, Enos Masini19, Ronnie Matambo20, Sayoki Mfinanga21, Patrick Migambi12, Patrick Moonan8, James Mpunga6, Frank Mugabe22, Alister Munthali23, Junior Mutsvangwa20, Wilfred Nkhoma24, Joshua Obasanya25, Semeeh Omoleke7, Ikushi Onozaki26, Philip Patrobas27, Jane Rahedi Ong’ang’o28, Elizeus Rutebemberwa15, Charles Sandy29, Mbazi Senkoro21, Charalambos Sismanidis1, Joseph Sitienei19, Marina Tadolini30, Hazim Timimi1, Fasil Tsegaye31, Claude Bernard Uwizeye32, Norio Yamada33
1 Global Tuberculosis Programme, World Health Organization, Geneva, Switzerland
2 Federal Ministry of Health, Khartoum, Sudan
3 Bacteriology Department, Noguchi Memorial Institute for Medical Research, University of Ghana, Legon-Accra, Ghana
4 Department of Infectious Diseases Epidemiology, London School of Hygiene and Tropical Medicine, UK
5 National Tuberculosis Programme, Ghana Health Service, Accra, Ghana
6 National Tuberculosis Programme, Ministry of Health, Lilongwe, Malawi
7 Medical Research Council Unit, The Gambia at the London School of Hygiene and Tropical Medicine, Banjul, The Gambia
8 Division of Global HIV and TB, Centers for Disease Control and Prevention, Atlanta, USA
9 Department of Public Health, Ministry of Health, Lusaka, Zambia
10 National tuberculosis reference laboratory, Federal Ministry of Health, Khartoum, Sudan
11 World Health Organization, Khartoum, Sudan
12 Ministry of Health, Rwanda Biomedical Centre, Kigali, Rwanda
13 KNCV Tuberculosis Foundation, The Hague, the Netherlands
14 National Tuberculosis and Leprosy Programme, Ministry of Health and Social Welfare, Dar es Salaam, United Republic of Tanzania
15 School of Public Health, Makerere University, Kampala, Uganda
16 African Society of Laboratory Medicine, Addis Ababa, Ethiopia
17 World Health Organization, Nairobi, Kenya
18 Department of Global Health and Amsterdam Institute for Global Health and Development, Amsterdam University Medical Centers, Amsterdam, The Netherlands.
19 National Tuberculosis Programme, Ministry of Health, Nairobi, Kenya
20 Biomedical Research and Training Institute, Harare, Zimbabwe
21 National Institute for Medical Research, Muhimbili Centre, United Republic of Tanzania
22 National Tuberculosis Control Programme, Ministry of Health, Kampala, Uganda
23 Centre for Social Research, University of Malawi, Zomba, Malawi
24 World Health Organization for the African region, Brazzaville, Republic of the Congo
25 Nigeria Center for Disease Control, Abuja, Nigeria
26 World Health Organization, Yangon, Myanmar
27 World Health Organization, Abuja, Nigeria
28 Kenya Medical Research Institute, Nairobi, Kenya
29 Ministry of Health and Child Care, Harare, Zimbabwe
30 Infectious Diseases Unit, Department of Medical and Surgical Sciences, Alma Mater Studiorum University of Bologna, Bologna, Italy
31 Infectious diseases department, Nitsuh Ethiopia, Addis Ababa, Ethiopia
32 Family Health International, Tuberculosis Unit, TRAC Plus, Kigali, Rwanda
33 Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association, Tokyo, Japan
Acknowledgements
We acknowledge all survey participants, all the principal investigators and survey team members from each of the 12 countries that conducted a survey. Furthermore, we thank all those who provided technical support for these surveys, specifically KNCV Tuberculosis Foundation, the London School of Hygiene and Tropical Medicine, the Research Institute of Tuberculosis/Japan Anti-Tuberculosis Association (RIT/JATA), U.S. Centers for Disease Control and Prevention, WHO, and independent consultants. This paper is also written in memory of Dr Amal Bassili, who worked for Tropical Disease Research in the WHO Eastern Mediterranean Regional Office (EMRO) 2000–2013 and who was the surveillance officer within EMRO’s TB unit 2007–2013. She supported the national TB prevalence surveys of Pakistan and Sudan.
Footnotes
Disclaimer
The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of their organisations.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Sustainable Development Goals (SDGs): SDG 3 (good health and well-being)
African TB Prevalence Survey Group members are listed in Box 1
References
- 1.World Health Organization. Global Tuberculosis Report. World Health Organization: Geneva, Switzerland, 2019. https://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.United Nations. Millennium development goals. (Available from: http://www.un.org/millenniumgoals/) [10 December 2018].
- 3.World Health Organization. The Global Plan to Stop TB, 2006–2015: Actions for Life Towards a World Free of Tuberculosis. World Health Organization: Geneva, Switzerland, 2006. https://www.who.int/tb/features_archive/global_plan_to_stop_tb/en/ [Google Scholar]
- 4.World Health Organization. The Stop TB Strategy. World Health Organization: Geneva, Switzerland, 2006. Contract No.: WHO/HTM/TB/2006.368. https://apps.who.int/iris/bitstream/handle/10665/69241/WHO_HTM_STB_2006.368_eng.pdf?sequence=1 [Google Scholar]
- 5.Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Health 2015: 20: 1128–1145. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Technical appendix on methods used to estimate the global burden of disease caused by TB 2017. (Available from: http://www.who.int/tb/publications/global_report/en/) [10 December 2018].
- 7.World Health Organization. WHO Global Task Force on TB Impact Measurement Geneva, Switzerland. (Available from: https://www.who.int/tb/areas-of-work/monitoring-evaluation/impact_measurement_taskforce/en/) [12 June 2019]. [Google Scholar]
- 8.World Health Organization. Tuberculosis Prevalence Surveys: A Handbook. World Health Organization: Geneva, Switzerland, 2011. https://www.who.int/tb/advisory_bodies/impact_measurement_taskforce/resources_documents/thelimebook/en/ [Google Scholar]
- 9.Roelsgaard E, Iversen E, Blocher C. Tuberculosis in tropical Africa. An Epidemiological Study. Bull World Health Organ 1964: 30: 459–518. [PMC free article] [PubMed] [Google Scholar]
- 10.Sebhatu M, Kiflom B, Seyoum M et al. Determining the burden of tuberculosis in Eritrea: a new approach. Bull World Health Organ 2007: 85: 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayles H, Schaap A, Nota A et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One 2009: 4: e5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claassens M, van Schalkwyk C, den Haan L et al. High prevalence of tuberculosis and insufficient case detection in two communities in the Western Cape, South Africa. PLoS One 2013: 8: e58689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbett EL, Bandason T, Cheung YB et al. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int J Tuberc Lung Dis 2009: 13: 1231–1237. [PMC free article] [PubMed] [Google Scholar]
- 14.van’t Hoog AH, Laserson KF, Githui WA et al. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011: 183: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 15.Mao TE, Okada K, Yamada N et al. Cross-sectional studies of tuberculosis prevalence in Cambodia between 2002 and 2011. Bull World Health Organ 2014: 92: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tupasi TE, Radhakrishna S, Chua JA et al. Significant decline in the tuberculosis burden in the Philippines ten years after initiating DOTS. Int J Tuberc Lung Dis 2009: 13: 1224–1230. [PubMed] [Google Scholar]
- 17.Wang L, Zhang H, Ruan Y et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet 2014: 383: 2057–2064. [DOI] [PubMed] [Google Scholar]
- 18.Adetifa IM, Kendall L, Bashorun A et al. A tuberculosis nationwide prevalence survey in Gambia, 2012. Bull World Health Organ 2016: 94: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enos M, Sitienei J, Ong’ang’o J et al. Kenya tuberculosis prevalence survey 2016: challenges and opportunities of ending TB in Kenya. PLoS One 2018: 13: e0209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapata N, Chanda-Kapata P, Ngosa W et al. The prevalence of tuberculosis in Zambia: results from the First National TB Prevalence Survey, 2013–2014. PLoS One 2016: 11: e0146392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kebede AH, Alebachew Z, Tsegaye F et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010–2011. Int J Tuberc Lung Dis 2014: 18: 635–639. [DOI] [PubMed] [Google Scholar]
- 22.Senkoro M, Mfinanga S, Egwaga S et al. Prevalence of pulmonary tuberculosis in adult population of Tanzania: a national survey, 2012. Int J Tuberc Lung Dis 2016: 20:1014–1021. [DOI] [PubMed] [Google Scholar]
- 23.Bonsu F, Addo KK, Alebachew Z et al. National population-based tuberculosis prevalence survey in Ghana, 2013. Int J Tuberc Lung Dis 2020: 24: 321–328. [DOI] [PubMed] [Google Scholar]
- 24.Migambi P, Gasana M, Uwizeye CB et al. Prevalence of tuberculosis in Rwanda: results of the first nationwide survey in 2012 yielded important lessons for TB control. PLoS One 2020: 15: e0231372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Public Health Federal Republic of Nigeria. Report of the first national TB prevalence survey 2012, Nigeria, 2014. [Google Scholar]
- 26.Ministry of Health. Technical report of the Zambia national tuberculosis prevalence survey (2013–2014). 2015. [Google Scholar]
- 27.Ministry of Health – Federal Democratic Republic of Ethiopia. First Ethiopian National Population Based Tuberculosis Prevalence Survey. Ethiopian Health and Nutrition Research Institute: Addis Ababa, Ethiopia, 2011. https://www.ephi.gov.et/images/downloads/TuberculosisPrevalenceSurvey.pdf [Google Scholar]
- 28.Ministry of Health (national tuberculosis leprosy and lung disease program). National tuberculosis prevalence survey of Kenya 2015–2016. 2016. [Google Scholar]
- 29.Ministry of Health (national tuberculosis program). National tuberculosis prevalence survey of Sudan 2013–2014 (report). 2018. [Google Scholar]
- 30.Ministry of Health and Child Care. The Zimbabwe National Population based Tuberculosis Prevalence Survey. 2014. [Google Scholar]
- 31.Ministry of Health and Social Welfare. The first national tuberculosis prevalence survey in the United Republic of Tanzania. 2013. [Google Scholar]
- 32.Ministry of Health and Social Welfare. The Gambian Survey of Tuberculosis Prevalence Report. Medical Research Council Unit: The Gambia, 2014. https://drive.google.com/file/d/0BxHfv47XsxqBUXM0ZDFhMkhzUUE/view?usp=sharing [Google Scholar]
- 33.Ministry of Health National TB Control Programme. Technical report: Malawi tuberculosis prevalence survey (2013–2014). 2016. [Google Scholar]
- 34.Makerere University School of Public Health. Ministry of Health Uganda and Makerere University School of Public Health. Population-based survey of prevalence of tuberculosis disease in Uganda 2014–15 (report). Makerere University School of Public Health: Kampala, Uganda, 2016. https://www.health.go.ug/cause/the-uganda-national-tuberculosis-prevalence-survey-2014-2015-survey-report/ [Google Scholar]
- 35.National TB Control Programme (Ghana Health Service). Ghanaian national population based tuberculosis prevalence survey in 2013 (report). 2016. [Google Scholar]
- 36.Rwanda Ministry of Health. The first national tuberculosis prevalence survey in Rwanda (2012). 2015. [Google Scholar]
- 37.Floyd S, Sismanidis C, Yamada N et al. Analysis of tuberculosis prevalence surveys: new guidance on best-practice methods. Emerg Themes Epidemiol 2013: 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoy D, Brooks P, Woolf A et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012: 65: 934–939. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Global Tuberculosis Database 2018. (Available from: http://www.who.int/tb/country/en/) [10 December 2018].
- 40.United Nations Population Division. World Population Prospects 2015. (Available from: http://esa.un.org/unpd/wpp/) [10 December 2018].
- 41.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw 2010: 36: 1–48. [Google Scholar]
- 42.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003:327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Laboratory Services in Tuberculosis Control. 1999. [Google Scholar]
- 44.World Health Organization. Policy Statement: Liquid Media for Culture and DST. World Health Organization: Geneva, Switzerland, 2007. https://www.who.int/tb/laboratory/policy_liquid_medium_for_culture_dst/en/ [Google Scholar]
- 45.Van Der Zanden AG, Te Koppele-Vije EM, Vijaya Bhanu N, Van Soolingen D, Schouls LM. Use of DNA extracts from Ziehl-Neelsen-stained slides for molecular detection of rifampin resistance and spoligotyping of Mycobacterium tuberculosis. J Clin Microbiol 2003: 41: 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chanda-Kapata P, Kapata N, Masiye F et al. Health seeking behaviour among individuals with presumptive tuberculosis in Zambia. PLoS One 2016: 11: e0163975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senkoro M, Hinderaker SG, Mfinanga SG et al. Health care-seeking behaviour among people with cough in Tanzania: findings from a tuberculosis prevalence survey. Int J Tuberc Lung Dis 2015: 19: 640–646. [DOI] [PubMed] [Google Scholar]
- 48.Rwanda Ministry of Health. The first national tuberculosis prevalence survey in Rwanda (2012). 2015. [Google Scholar]
- 49.Raviglione MC, Uplekar MW. WHO’s new Stop TB strategy. Lancet 2006: 367: 952–955. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. Global Tuberculosis Report. World Health Organization: Geneva, Switzerland, 2017. https://apps.who.int/iris/bitstream/handle/10665/259366/9789241565516-eng.pdf;jsessionid=8D5E15C89AFC32EA748B6D9A9C0496B1?sequence=1 [Google Scholar]
- 51.World Health Organization. WHO Policy on Collaborative TB/HIV Activities Guidelines for National Programmes and Other Stakeholders. World Health Organization: Geneva, Switzerland, 2012. https://www.who.int/tb/publications/2012/tb_hiv_policy_9789241503006/en/ [PubMed] [Google Scholar]
- 52.World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations. 2013. [PubMed] [Google Scholar]
- 53.Bell CA, Ilomaki J, Pichenda K, Duncan GJ, Saini B. Referral of tuberculosis symptomatic clients from private pharmacies to public sector clinics for diagnosis and treatment in Cambodia. J Eval Clin Pract 2015: 21: 285–291. [DOI] [PubMed] [Google Scholar]
- 54.Satyanarayana S, Kwan A, Daniels B et al. Use of standardised patients to assess antibiotic dispensing for tuberculosis by pharmacies in urban India: a cross-sectional study. Lancet Infect Dis 2016: 16: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chanda-Kapata P, Kapata N, Klinkenberg E et al. Non-tuberculous mycobacteria (NTM) in Zambia: prevalence, clinical, radiological and microbiological characteristics. BMC Infect Dis 2015: 15: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borgdorff MW. New measurable indicator for tuberculosis case detection. Emerg Infect Dis 2004: 10: 1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horton KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex Differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 2016: 13: e1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization. Assessing Tuberculosis Under-Reporting Through Inventory Studies. World Health Organization: Geneva, Switzerland, 2014. https://www.who.int/tb/publications/inventory_studies/en/ [Google Scholar]
- 59.Starke JR, Donald PR. Handbook of Child and Adolescent Tuberculosis. Oxford University Press: Oxford, UK,2016. https://global.oup.com/academic/product/handbook-of-child-and-adolescent-tuberculosis-9780190220891?cc=ch&lang=en& [Google Scholar]
- 60.UnitedNations.SustainableDevelopmentGoals.(Available from: https://www.un.org/sustainabledevelopment/) [10December2018].
- 61.Uplekar M, Weil D, Lonnroth K et al. WHO’s new end TB strategy. Lancet 2015: 385: 1799–1801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Prevalence to notification (P:N) ratio of TB cases disaggregated by sex of national TB prevalence surveys implemented in Africa, 2008–2016.
Table S1. Assessment of bias.
Table S2. Bacteriologically confirmed TB survey cases by age group in national TB prevalence surveys implemented in Africa 2008–2016: prevalence and number.
Table S3. Prevalent TB cases by screening outcome in national TB prevalence surveys implemented in Africa 2008–2016.
Table S4. Location of treatment for participants who were on treatment at the time the survey was implemented, 2008–2016.
Table S5. Major successes of national TB prevalence surveys implemented in Africa 2008–2016.
Table S6. Major challenges faced in national TB prevalence surveys implemented in Africa 2008–2016.
Text S1.
Text S2.