ABSTRACT
Background: Adrenocortical carcinoma is a rare malignancy, with poor prognosis and limited treatment options for patients with advanced disease. Chemotherapy is the current standard first-line treatment, providing only a modest survival benefit. There is only limited treatment experience with immunotherapy using single-agent anti-PD-1/PD-L1 therapy. To date no clinical trials have been reported using combination immunotherapy with anti-CTLA-4 and anti-PD-1 blockade in this patient population.
Methods: CA209-538 is a prospective multicentre clinical trial in patients with advanced rare cancers. Participants received the anti-PD-1 antibody nivolumab (3 mg/kg IV) and the anti-CTLA-4 antibody ipilimumab (1 mg/kg IV) every three weeks for four doses, followed by nivolumab (3 mg/kg IV) every two weeks and continued for up to 96 weeks, until disease progression or unacceptable toxicity. Response was assessed every 12 weeks by RECIST version 1.1. Primary endpoint was clinical benefit rate (complete response, partial response, stable disease at 12 weeks).
Results: Six patients with adrenocortical carcinoma were enrolled and received treatment. Two patients (33%) have an ongoing partial response (10 and 25 months +) and two patients (33%) stable disease leading to a disease control rate of 66%. Both responders had tumors with a microsatellite instable phenotype. One patient rapidly progressed shortly after enrollment into the trial and did not undergo restaging. Immunotherapy-related toxicity was reported in all patients, with four patients (67%) experiencing grade 3/4 hepatitis leading to discontinuation of treatment.
Conclusions: This is the first treatment experience using ipilimumab and nivolumab combination immunotherapy in patients with advanced adrenocortical carcinoma. Durable responses have been observed in a subset of patients suggesting that this treatment regimen should be further investigated in this patient population.
KEYWORDS: Adrenocortical carcinoma, anti-pd-1, anti-pd-l1, anti-ctla-4, nivolumab, ipilimumab
Background
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with a reported incidence of 0.5–2 cases per million population per year.1 The overall prognosis of patients with locally advanced or metastatic ACC not amendable to surgery is poor and treatment options are very limited. Expert consensus guidelines have been published to assist with the management of ACC patients.2 The current standard treatment is a polychemotherapy regimen combined with the adrenolytic agent mitotane that has demonstrated a progression free survival without an overall survival benefit and is associated with a high rate of treatment-related sided effects.3 There are no established second-line therapies and several clinical trials using different targeted agents have been unsuccessful.4
Early clinical trials using immunotherapy with single-agent anti-programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) antibodies have demonstrated limited activity,5–8 although a recent trial using the anti-PD-1 antibody pembrolizumab has shown a more substantial benefit in a subset of ACC patients.8 Clinical trials using combined anti-PD-1/anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade have revealed superior efficacy compared to single-agent anti-PD-1 therapy across several malignancies9,10 but so far no treatment experiences have been reported in patients with metastatic ACC.
Patients with ACC were enrolled into CA209-538, a clinical trial treating patients with advanced rare cancers with combination immunotherapy of the anti-PD-1 antibody nivolumab and the anti-CTLA-4 antibody ipilimumab.
Methods
Study design
CA209-538 is a multicentre open label phase 2 study conducted at five Australia sites (Austin Health, Peter McCallum Cancer Center and Monash Health, Melbourne; Blacktown Hospital, Sydney; and Border Medical Oncology, Albury/Wodonga, local sponsor was the Olivia Newton-John Cancer Research Institute, Melbourne). The trial enrolled patients into three tumor cohorts (rare upper gastrointestinal cancers, rare gynecological cancers and neuro-/endocrine neoplasms) with each cohort being limited to 40 patients. Patients with advanced ACC were enrolled into the neuro-/endocrine cohort. Eligible patients were aged 18 years or older and had a histologically confirmed metastatic rare cancer. Central pathology review was not undertaken as part of the trial but the diagnosis of ACC in all six patients presented here has been confirmed by an anatomical pathologist with expertise in this tumor type. Patients had at least one measurable lesion according to Response Evaluation Criteria In Solid Tumor (RECIST) version 1.1 and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Other inclusion criteria were a life expectancy of three months or more and an adequate organ function. Patients could either be treatment naive or had received prior systemic therapy with a minimum washout period of 28 days before initiation of study treatment. Disease progression following prior therapy was not an inclusion requirement. Key exclusion criteria were active brain metastases and a history of autoimmune conditions.
Archival tumor tissue, or a fresh tumor biopsy during screening, were required for predictive biomarker analysis. Archival formalin-fixed paraffin-embedded tumor tissue was tested for PD-L1 expression by immunohistochemistry (IHC). The antibody used was Ventana PD-L1 (SP263) according to the ULTRA VENTANA PD-L1 (SP263) Assay (Roche diagnostics). A tumor was deemed PD-L1 positive if there was at least 1% expression on tumor cells. DNA was extracted from tumor tissue for gene sequencing and the tumor mutational burden (TMB) was determined by the Oncomine tumor mutation load assay (Thermo Fisher Scientific Ltd). The tumor microsatellite status was determined by examining the expression of mismatch repair proteins by immunohistochemistry. Genomic profiling has been performed by using multigene panel assays.11,12
The clinical trial protocol was reviewed and approved by the Institutional Review Board at Austin Health (Melbourne, Australia) HREC/16/Austin/152 and was undertaken in accordance with Declaration of Helsinki and guidelines of Good Clinical Practice. Written informed consent was obtained from all participants prior to enrollment into the study.
Treatment
Nivolumab and ipilimumab were administered intravenously at a dose of 3 mg/kg and 1 mg/kg, respectively, every three weeks for four doses (induction phase), followed by nivolumab monotherapy at a dose of 3 mg/kg every two weeks (maintenance phase) until disease progression, unacceptable toxicity or a maximum of two years after enrollment. Dose reductions were not permitted; however, study treatment could be interrupted to enable recovery from adverse reactions for up to six weeks unless a longer interruption was clinically indicated. If treatment was discontinued patients were followed up until disease progression or initiation of a different treatment, survival analysis was ongoing for up to two years. Tumor assessments were performed by radiological assessment (computer tomography of brain, chest, abdomen, pelvis) at baseline and then every 12 weeks during treatment or follow up. A confirmatory scan was performed six weeks after the first restaging scan at week 18. Tumor response was assessed according to RECIST version 1.1. Patients with evidence of progressive disease at their first restaging scan in week 12 were permitted to continue on study treatment at the discretion of the investigator for another six weeks until radiological confirmation of progression at week 18. Safety analyses were performed on all patients who received at least one dose of study treatment. Laboratory monitoring and safety assessments were performed at baseline and prior to treatment according to the study protocol. Adverse events were graded in accordance with the NCI Common Terminology Criteria for Adverse Events version 4.0 and collected during treatment and for 100 days after the last dose received.
Outcomes
The primary endpoint was the proportion of patients with disease control at week 12 (complete response, partial response or stable disease) according to RECIST 1.1 criteria. The secondary objective was identification of a tumor agonistic predictive biomarker or immune signature.
Statistical analysis
Given the heterogeneous nature of the rare cancer patient population enrolled in the trial, statistics were descriptive and no sample size calculation was undertaken.
Results
Between November 2017 and September 2019, six patients with adrenocortical carcinoma were enrolled into the CA209-538 clinical trial, the median follow up for this cohort is 14.7 months. Demographics and disease characteristics are outlined in Table 1. Four patients were female and the median age was 48 years. Five patients were ECOG 0; three patients were treatment naïve, one patient had received one line and two patients, two lines of prior systemic treatment at the time of enrollment. All pre-treated patients had progressive disease at time of study enrollment. None of the patients received concomitant mitotane treatment during the trial and mitotane has been ceased in two patients 28 days prior to commencement of study treatment. Four of six patients had hormonally nonfunctioning tumors and two a cortisol producing ACC
Table 1.
Patient and tumor characteristics and treatment outcome
| ID | Age | Sex | Time from diagnosis to study enrollment |
Stage at time of diagnosis (AJCC 8th edition) |
Tumor-Mitotic index (mitoses per 50HPF) |
Hormone status | Sites of metastatic disease |
Tumor burden (mm) a |
Prior systemic therapy | MSI status | Somatic mutations | TML | PD-L1 statusb | Best responsec | IrAEsd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MON15 | 55 | male | 4 months | IV | 75 | nonfunctioning | bone; liver; lymph node | 189 | None | MSS | CTNNB1 | 0 | positive | PD | neutropenia (4) |
| AUS02 | 22 | female | 3 years 8 months | IV | >50 | functioning- cortisol | lung; liver; bone | 111 | Mitotane; Cisplatin/Etoposide/Doxorubicin | MSS | RB1 deletion | 170 | negative | SD | hepatitis(3); rash(2) |
| BLA20 | 72 | female | 6 months | III | 29 | nonfunctioning | lung | 43 | Mitotane (adjuvant) | MSI-H | MSH2; CTNNB1; TP53; STK11 mutation KIT/PDGFRA/BCL6 amplification |
5.05 | negative | PR | hepatitis (3); adrenalitis (3) |
| BLA019 | 40 | female | 6 months | III | >50 | nonfunctioning | lung; liver; lymph node | 75 | None | MSS | NF1 deletion MEN1 rearrangement |
2.52 | positive | PD | rash (1) |
| MON18 | 71 | female | 1 year 11 months | II | 9 | nonfunctioning | lung; lymph node | 87 | None | MSS | No clinically significant variant detected | 5 | negative | SD | hepatitis (4) thyroiditis (2) |
| AUS05 | 38 | male | 11 months | III | >50 | functioning-cortisol | lung; liver; adrenal gland | 62 | Mitotane; Cisplatin/Etoposide (adjuvant) | MSI-H | TP53; BAP1; FANCC; KMT2A; DAXX; MSH2 mutation | 145 | negative | PR; CMR | hepatitis (3); adrenalitis (3); thyroiditis (2) |
aBased on sum of target lesions measured as per RECIST 1.1
bPositive: PD-L1 expression on ≥1% of tumor cells
cResponse according to RECIST 1.1: PR (partial response); SD (stable disease); PD (progressive disease); CMR (complete metabolic response as assessed by FDG-PET scan)
dTreatment-related toxicity according to CTCAE, Version 4.03; toxicity grading in brackets
Abbreviations: MSI: microsatellite instability; MSI-H: microsatellite instable; MSS: microsatellite stable; TMB: tumor mutational burden; IrAEs: immune related adverse events
Overall, four patients (66%) were alive at the time of data analysis, one patient did not undergo a post-baseline assessment as he clinically progressed and died prior to the week 12 restaging scan. Two of six patients (33%) completed the induction treatment with four doses of ipilimumab and nivolumab. Three patients (50%) discontinued treatment during the induction phase and one patient (17%) during the maintenance phase due to severe immune related adverse events (irAEs). The objective response rate was 33%, two patients (33%) had stable disease leading overall to a disease control rate of 66% (Figure 1, Table 1). The two responders obtained reductions of target lesions by 70% and 86% with one of the patients having a complete metabolic response on a FDG – PET scan; both responses are ongoing at the time of analysis (10 and 25 months) (Figure 1). Both responding patients received only one and two doses of induction immunotherapy respectively before discontinuation of study treatment due to the development of grade 3/4 hepatitis. Both responders had also the lowest tumor burden based on the sum of target lesions according to RECIST 1.1.
Figure 1.
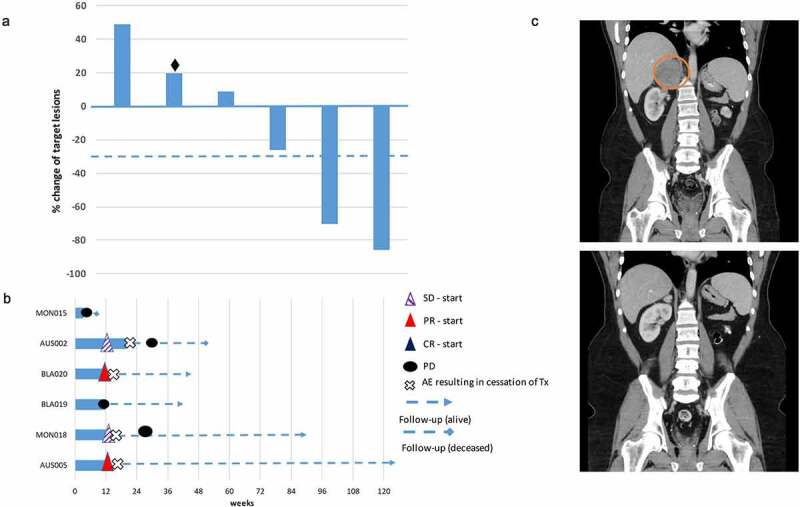
A) Waterfall plot of the best objective response measured as the maximum change from baseline in the sum of the longest diameter of each target lesion. (♦ Patient clinically progressed prior to first restaging. B) Swimmer plot demonstrating time to response and duration of study treatment. C) Computer tomography (CT) obtained from a responding patient with metastases involving lung, liver and right adrenal gland. The patient received two doses of nivolumab and ipilimumab with the treatment being discontinued due to severe autoimmune hepatitis. Restaging at week 12 revealed a partial remission and three monthly follow-up CT scans demonstrated a further reduction in size of his target lesions (Circle encloses the right adrenal gland metastasis at baseline)
All six patients experienced irAEs of any grade. Grade 3/4 irAEs occurred in five out of six patients (83%), most commonly hepatitis in four patients. Other high-grade irAEs included adrenalitis and neutropenia. Both responding patients developed concomitant severe immune-related hepatitis and adrenal gland insufficiency due to adrenalitis. There were no treatment-related deaths (Table 1).
Two of six patients had tumors with PD-L1 expression on at least 1% of tumor cells, both patients did not respond to study treatment. The two patients obtaining an objective response had tumors with an MSH2 mutation in keeping with a microsatellite instable phenotype (MSI-H), one patient on the background of a MSH2 germline mutation. The tumor of this patient demonstrated a high TMB of 145 mutations per megabase (MB) whilst the second MSI-H tumor demonstrated only a TMB of 5.05/MB. The TMB range of the remaining four patients has been 0–170/MB; the patient with the tumor demonstrating the highest TMB had stable disease as her best response to study treatment with a reduction of target lesions that has just fallen short of an objective response, it carried a biallelic RB1 deletion. Genomic profiling of tumor tissue of all six patients by multigene panel testing revealed several known ACC associated gene aberrations (Table 1). The tumors of both responders harbored next to a MSH-2 mutation, an inactivating TP53 mutation.
Discussion
Current systemic treatment options for patients with advanced ACC include mitotane which is associated with a response rate of 23%, median time to progression of 5.5 months and high toxicity.13 Similarly, platinum-based chemotherapy (EDP – etoposide, doxorubicin, cisplatin) in combination with mitotane, has modest efficacy in ACC patients, with a progression free survival of 5.6 months, an overall survival of 14.8 months, and is associated with a high rate (58%) of serious adverse events as reported in the FIRM-ACT study.3 There are no established second-line therapies and there is an urgent need for new treatment approaches to improve the outcome of these patients.
We report here on the first treatment experience with anti-PD-1/CTLA-4 combination immunotherapy in patients with ACC. Our observations confirm that immunotherapy using checkpoint inhibition has significant clinical activity in a subset of patients with advanced ACC, which is in keeping with a recently published clinical trial with the anti-PD-1 antibody pembrolizumab.8 Raj et al. reported on 39 patients who received pembrolizumab with an objective response rate of 23% and a disease control rate of 52%. We observed in our patient cohort an objective response rate of 33% and a disease control rate of 66%, with both responding patients having ongoing responses at 10 and 25 months. Although the response rate in our patient cohort using combined checkpoint blockade is numerically higher to published data using single-agent anti-PD-1/PD-L1 therapy,5–8 the limited number of ACC patients enrolled in our trial precludes any firm conclusions. Further clinical trials will be required to assess if combination immunotherapy demonstrates superior efficacy to single-agent anti-PD-1 therapy in ACC patients as demonstrated in other malignancies.9,10
Efficacy of anti-PD-1/PD-L1 therapy in the so far published clinical trials in patients with advanced ACC differs significantly, with response rates varying between 6% and 23%.5–8 This may be explained by the small number of patients enrolled in these studies or the number of study participants with an MSI-H tumor phenotype.
MSI-H cancers have shown to be particular sensitive to anti-PD-1 checkpoint blockade in clinical trials using pembrolizumab or nivolumab demonstrating objective responses in around 35% of patients.14,15 The Keynote-158 trial, a basket trial for patients with MSI-H cancers included five patients with ACC however did not report specifically on their outcome.14 A significant proportion of patients with ACC have an MSI-H tumor phenotype16 with a subgroup of patients having a mismatch match repair (MMR) protein germline mutation as part of Lynch syndrome.17 One of our patients who experiences a durable treatment response was diagnosed during his trial participation with an MSH 2 germline mutation.18 Despite the fairly high response rate of MSI-H cancers to single-agent anti-PD-1 therapy, the majority of patients demonstrate either primary or develop secondary resistance.14,15 In keeping with this, Raj et al enrolled six patients with MSI-H ACC in their trial with pembrolizumab and observed only two objective responses in this patient group.8 It is currently unknown if the response to anti-PD-1 therapy in MSI-H cancers can be further improved by using combined anti-PD-1/CTLA-4 blockade as it has already been shown in patients with advanced MSI-H colorectal carcinoma.19 Both patients with MSI-H phenotype in our study cohort obtained durable responses.
Apart from the microsatellite status of the tumor, no biomarkers have so far emerged that can predict for response to checkpoint inhibition in patients with advanced ACC. The frequency of ACC expressing PD-L1 on tumor cells is low20 and PD-L1 expression has not been associated with response in the so far published clinical trials.6,8 In keeping with these observations only two patients in our cohort had a PD-L1 positive tumor, both did not respond to study treatment.
TMB has been associated with treatment response to checkpoint inhibition across a range of malignancies.21 The majority of tumors in our cohort had a low TMB in keeping with previous reports demonstrating that ACC is one of the malignancies with the lowest TMB.22 The tumor with the highest TMB next to an MSI-H tumor was mismatch repair protein proficient and harbored a biallelic RB1 deletion. Preclinical studies have shown that RB1 deletions can lead to increased DNA damage by impaired homologous recombination repair potentially explaining the high TMB in this patient.23
We identified in the tumor tissue of our patient cohort several oncogenic mutations that are well recognized as oncogenic aberrations in ACC.22 Of interest the tumor of one responder had an activating CTNNB1 and an inactivating STK11 mutation both of which have been associated with primary resistance to anti-PD-1 therapy in patients with melanoma and non-small cell lung cancer respectively suggesting that combination immunotherapy may overcome this resistance.24,25
In addition, both responders had the lowest tumor burden which is in keeping with data from anti-PD-1 treated metastatic melanoma patients in which a lower baseline tumor burden correlated with improved clinical outcome.26
Five out of six ACC patients in our trial experienced high-grade immune-related toxicity, including four patients (67%) with severe autoimmune hepatitis. It is well recognized that there is a significantly higher rate of high-grade irAEs with combined anti-PD-1/CTLA-4 blockade compared to single-agent anti-PD-1 therapy. The rate of high-grade irAEs with combination treatment depends on the dose of ipilimumab with low-dose regimens leading to high-grade immune related toxicity in around one third of patients.9,19 Our trial used a low dose ipilimumab regimen so that the high rate of severe irAEs, particularly the high rate of hepatitis in our ACC cohort was unexpected. Frequency, severity and type of immune-related toxicity can differ between tumor types, e.g. a high frequency of severe irAEs and unusual autoimmune toxicity has been observed in patients with thymic carcinoma treated with pembrolizumab.27 In keeping with our observations, Raj et al also detected a high rate of immune related hepatitis in their ACC study population including in all responding patients.8 Both of our responders developed next to severe autoimmune hepatitis primary adrenal gland insufficiency due to presumed adrenalitis leading to study discontinuation after only one and two treatment doses; nevertheless, both patients have durable responses of 10 and 25 months. This may suggest that checkpoint inhibition in ACC patients reinvigorates anti-tumor immune responses that are directed against shared antigens expressed by liver and adrenal gland. Despite the high rate of severe irAEs, toxicity in our patient population could be well managed and resolved completely.
One patient rapidly progressed after study enrollment and received only one treatment dose; the patient’s tumor had a very high proliferation index in keeping with an aggressive tumor biology that likely accounted for his rapid clinical deterioration although we cannot fully exclude a negative impact of checkpoint inhibition leading to an accelerated tumor growth as it has demonstrated in other malignancies.28
Overall, our data set adds to the evidence that immunotherapy using checkpoint inhibition leads to significant clinical efficacy in a subset of patients with advanced adrenocortical carcinoma, which contrasts with the short-lived responses obtained with chemotherapy. Ongoing clinical research is required to identify biomarkers for improved patient selection,29 and to address if combination immunotherapy provides additional benefit compared to single-agent anti-PD-1 therapy.
Funding Statement
The study received funding and drug support from Bristol–Myers Squibb, funding support was also provided in part by a grant from the Commonwealth of Australia, Dept. Health Accelerated Research Program. A Behren is supported by a fellowship from the Department of Health and Human Services acting through the Victorian Cancer Agency; Bristol-Myers Squibb; Commonwealth of Australia, Dept. Health Accelerated Research Program; Victorian Cancer Agency.
Disclosures
Dr. Cebon reports honoraria/Advisory Board fees from Bristol-Myers Squibb, Amgen, Novartis, MSD and speaker fees from Roche. Dr. Klein reports speaker fees from Bristol-Myers Squibb and MSD, travel support from Bristol-Myers Squibb. Dr. Kee reports honoraria/advisory board fees from Novartis, travel support from Bristol-Myers Squibb. Dr. Carlino reports honoraria/advisory board fees from Bristol-Myers Squibb, Amgen, Novartis, MSD, Roche, Pierre Fabre, Ideaya, Sanofi, Merck, Eisai and Nektar. Dr. Markman reports honoraria/advisory board fees from Novartis and Amgen.
References
- 1.Gatta G, Capocaccia R, Botta L, et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18(8):1022–7. [published Online First: 2017/07/09]. doi: 10.1016/s1470-2045(17)30445-x. [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M, Assie G, Baudin E, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1476–1490. [published Online First: 2020/08/31]. doi: 10.1016/j.annonc.2020.08.2099. [DOI] [PubMed] [Google Scholar]
- 3.Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. [published Online First: 2012/05/04]. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 4.Altieri B, Ronchi CL, Kroiss M, et al. Next-generation therapies for adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2020;34(3):101434. [published Online First: 2020/07/06]. doi: 10.1016/j.beem.2020.101434. [DOI] [PubMed] [Google Scholar]
- 5.Carneiro BA, Konda B, Costa RB, et al. Nivolumab in Metastatic Adrenocortical Carcinoma: results of a Phase 2 Trial. J Clin Endocrinol Metab. 2019;104(12):6193–6200. [published Online First: 2019/07/06]. doi: 10.1210/jc.2019-00600. [DOI] [PubMed] [Google Scholar]
- 6.Habra MA, Stephen B, Campbell M, et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J Immunother Cancer. 2019;7(1):253. [published Online First: 2019/09/20]. doi: 10.1186/s40425-019-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Tourneau C, Hoimes C, Zarwan C, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2018;6(1):111. [published Online First: 2018/10/24]. doi: 10.1186/s40425-018-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raj N, Zheng Y, Kelly V, et al. PD-1 Blockade in Advanced Adrenocortical Carcinoma. J Clin Oncol. 2020;38(1):71–80. [published Online First: 2019/10/24]. doi: 10.1200/jco.19.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–1546. [published Online First: 2019/09/29]. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–1385. [published Online First: 2019/08/21]. doi: 10.1016/s1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thavaneswaran S, Ballinger ML, Grady J, et al. The Cancer Molecular Screening and Therapeutics Program (MoST): actionable mutation frequencies in a population with rare and less common cancers. J Clin Oncology. 2019;37(15):3136. doi: 10.1200/JCO.2019.37.15_suppl.3136. [DOI] [Google Scholar]
- 12.Kee D, Kondrashova O, Ananda S, et al. NOMINATOR: feasibility of genomic testing of rare cancers to match cancer to treatment. J Clin Oncology. 2020;38(15_suppl):15. doi: 10.1200/JCO.2020.38.15_suppl.103. [DOI] [Google Scholar]
- 13.Megerle F, Herrmann W, Schloetelburg W, et al. Mitotane Monotherapy in Patients With Advanced Adrenocortical Carcinoma. J Clin Endocrinol Metab. 2018;103(4):1686–1695. doi: 10.1210/jc.2017-02591. [DOI] [PubMed] [Google Scholar]
- 14.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38(1):1–10. [published Online First: 2019/11/05]. doi: 10.1200/jco.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azad NS, Gray RJ, Overman MJ, et al. Nivolumab Is Effective in Mismatch Repair-Deficient Noncolorectal Cancers: results From Arm Z1D-A Subprotocol of the NCI-MATCH (EAY131) Study. J Clin Oncol. 2020;38(3):214–222. [published Online First: 2019/11/26]. doi: 10.1200/jco.19.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latham A, Srinivasan P, Kemel Y, et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J Clin Oncol. 2019;37(4):286–295. [published Online First: 2018/10/31]. doi: 10.1200/jco.18.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymond VM, Everett JN, Furtado LV, et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol. 2013;31(24):3012–3018. [published Online First: 2013/06/12]. doi: 10.1200/jco.2012.48.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevgi A, Klein O, Cheung AS.. Sustained remission of Lynch syndrome-associated metastatic adrenocortical carcinoma following checkpoint inhibitor therapy-associated multiorgan autoimmunity. Clin Endocrinol (Oxf). 2020;93(2):214–216. doi: 10.1111/cen.14258. [published Online First: 2020/05/26] [DOI] [PubMed] [Google Scholar]
- 19.Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36(8):773–779. [published Online First: 2018/01/23]. doi: 10.1200/jco.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 20.Fay AP, Signoretti S, Callea M, et al. Programmed death ligand-1 expression in adrenocortical carcinoma: an exploratory biomarker study. J Immunother Cancer. 2015;3(3). [published Online First: 2015/03/15]. doi: 10.1186/s40425-015-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [published Online First: 2017/12/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng S, Cherniack AD, Dewal N, et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell. 2016;30(2):363. [published Online First: 2016/08/10]. doi: 10.1016/j.ccell.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Marshall AE, Roes MV, Passos DT, et al. RB1 Deletion in Retinoblastoma Protein Pathway-Disrupted Cells Results in DNA Damage and Cancer Progression. Mol Cell Biol. 2019;39(16):e00105–19. doi: 10.1128/MCB.00105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018;8(7):822–835. [published Online First: 2018/05/19]. doi: 10.1158/2159-8290.Cd-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trujillo JA, Luke JJ, Zha Y, et al. Secondary resistance to immunotherapy associated with β-catenin pathway activation or PTEN loss in metastatic melanoma. J Immunother Cancer. 2019;7(1):295. [published Online First: 2019/11/11]. doi: 10.1186/s40425-019-0780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph RW, Elassaiss-Schaap J, Kefford R, et al. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin Cancer Res. 2018;24(20):4960–4967. [published Online First: 2018/04/25]. doi: 10.1158/1078-0432.Ccr-17-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19(3):347–355. [published Online First: 2018/02/06]. doi: 10.1016/s1470-2045(18)30062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15(12):748–762. [published Online First: 2018/10/27]. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 29.Fujii T, Naing A, Rolfo C, et al. Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2018;130:108–120. [published Online First: 2018/09/11]. doi: 10.1016/j.critrevonc.2018.07.010. [DOI] [PubMed] [Google Scholar]


