ABSTRACT
Phenotypic switching is the main cause of the abnormal proliferation and migration of vascular smooth muscle cells (VSMCs). We previously showed that Daxx exerted negative regulatory effect on AngII-induced VSMC proliferation and migration. However, the function of Daxx in VSMC phenotype switching remained unknown. Nicotinate-curcumin (NC) is an esterification derivative of niacin and curcumin that can prevent the formation of atherosclerosis. We found that NC significantly decreased AngII-induced VSMC phenotype switching. Furthermore, NC significantly inhibited AngII-induced cell proliferation and migration. Moreover, NC upregulated Daxx expression and regulated the PTEN/Akt signaling pathway. We concluded that NC inhibited AngII-induced VSMC phenotype switching by regulating the PTEN/Akt pathway, and through a mechanism that might be associated with the upregulation of Daxx expression.
KEYWORDS: Nicotinate-curcumin, vascular smooth muscle cells, phenotype switching, Daxx
Introduction
The constituents of blood vessels mainly include vascular smooth muscle cells (VSMCs), which are the main cell components that maintain vascular elasticity. The two main phenotypes of VSMCs are contractile and synthetic. Fewer VSMCs possess the synthetic phenotype under normal physiological conditions, but they can switch from a contractile to a synthetic phenotype in response to vascular injury or other extracellular stimuli (such as platelet-derived growth factor (PDGF) and angiotensin II [AngII]). Upon infliction of vascular injury, VSMCs proliferate abnormally, migrate, and thicken blood vessel walls. Eventually, they play detrimental roles in the development of cardiovascular diseases such as atherosclerosis and intimal hyperplasia [1,2]. Therefore, inhibition of the VSMC phenotype switching positively affects cardiovascular disease pathophysiology [3]. The mechanism of VSMC phenotype switching is complex and comprises multiple steps, and is subjected to regulation at several levels by transcriptional regulators (such as serum response factor [SRF], myocardin, and Krüppel-like factor 4 [KLF4]), epigenetic level c regulation (microRNA-mediated post-transcriptional regulation), and signaling pathways such as transforming growth factor beta (TGFβ), and phosphatidylinositol- 3-kinase (PI3K)/protein kinase B (Akt) [3]. Among these regulators, PI3K/Akt is a critical pathway that positively modulates phenotype switching [4].
Multifunctional death domain-associated protein (Daxx) is regulated by transcription, cell survival, and several events and it exerts powerful cardioprotective effects by preventing the occurrence ischemia-reperfusion injury [5,6]. However, the effects and mechanism of action of Daxx on VSMCs are unclear. Our recent findings indicate that Daxx exerts a negative regulatory effect on the PI3K/Akt pathway, which inhibits the proliferation and migration of VSMCs [7]. Therefore, the present study further investigated whether Daxx affected the phenotypic transformation of VSMCs.
Curcumin (Cur), the main component of Curcuma longa L., demonstrates anti-inflammatory, antioxidant, and anti-cancer properties [8]. Additionally, curcumin exhibits protective properties in cardiovascular diseases by modulating multiple signaling pathways [9]. However, its poor water solubility, rapid metabolism, and low bioavailability limit its clinical use. Therefore, various methods have been developed to overcome these disadvantages. Among them, the most popular method is the application of drug delivery systems and synthetic derivatives [10]. Nicotinate-curcumin is a novel derivative of nicotinate and curcumin, with improved water solubility and bioavailability that regulates lipid metabolism and inhibits atherosclerosis in ApoE−/- mice [11,12]. However, the precise mechanisms underlying these protective effects remain unclear.
Here, we aimed to determine the effects of Daxx on VSMC phenotype switching, the mechanism by which NC affected VSMC phenotype switching, and whether it was associated with an upregulated Daxx expression.
Materials and methods
Materials
NC was synthesized by the pharmacology laboratory of Hunan University of Chinese Medicine (purity = 98%). Valsartan(B2214) was purchased from Apexbio biotechnology. Angiotensin II (AngII, A9525-1 mg) and MTT(M-2128) was purchased from Sigma-Aldrich. BRDU Cell Proliferation Assay(2750) was purchased from Millipore biotechnology. Daxx Antibody (sc-7152) was purchased from Santa Cruz biotechnology. PTEN Rabbit mAb(9188S), Phospho-Akt (Ser473) Rabbit mAb(4060S), Akt Rabbit mAb(4691S), and α-SMA(D4K9N) were purchased from Cell Signaling technology. OPN(ab8448) and SM22α(ab14106) were purchased from abcam technology. β-actin antibody(010–858), Goat-anti-rabbit IgG(H + L)(SA00001-2) and Goat anti Mouse IgG(H + L)(SA00001-1) were purchased from proteintech biotechnology. Lamin B antibody(15,082,820) was purchased from Wannlei biotechnology. HSP90 antibody(126–1026) were purchased from Shanghai Dianyin biotechnology. Nuclear Protein Extraction Kit(R0050) was purchased from Solarbio life sciences. BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 647(C0081S) was purchased from Beyotime biotechnology. pHIV-EGFP-HA-Daxx lentiviral liquid was purchased from Beijing Kainuo Saisi biotechnology. VSMCs was purchased from central south university.
Methods
Cell culture and treatment
We cultured VSMCs in DMEM containing 10% fetal bovine serum and 100 µg/mL penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere. Before commencement of the experiments, cells were subjected to starvation for 12 h with incubation in 0.1% FBS. We incubated VSMCs with NC for 2 h, and then the cells were stimulated with 1 μmol/L AngII to investigate the effects of NC on VSMC proliferation, migration, and phenotype switching.
MTT assay
We seeded VSMCs (5 × 103 cells/well) in 96-well plates and incubated them overnight. Thereafter, the cells were incubated with Ang II (1 µmol/L) for 24 h, followed by the addition of 100 µL of 0.5 mg/mL MTT solution and further incubation for 4 h at 37°C. The absorbance in each well at 490 nm was measured using a microplate reader.
BRDU assay
We seeded VSMCs (5 × 103 cells/well) in 96-well plates and cultured them overnight. Thereafter, the cells were incubated with AngII (1 µmol/L) for 24 h, followed by the addition of BRDU solution (final concentration: 1:500) and further incubation for 12 h. The medium was removed, and the cells were incubated with 200 μL of fixing solution at room temperature for 30 min, followed by the addition of diluted BRDU monoclonal antibody (100 μL) for 60 min and 100 μL of sheep anti-mouse IgG (1:2,000) and further incubation for 30 min. The cells were subjected to washing steps three times between these incubations. Thereafter, 5,5′-tetramethylbenzidine (TMB) peroxide substrate (100 μL) was added to each well and incubated for 30 min. The reaction was stopped by adding 100 μL of the termination solution, and then the optical density (OD) was measured at 450 nm.
EDU assay
We seeded VSMCs (5 × 103 cells/well) in 96-well plates as described above, followed by incubation with 100 µL of 1:500 diluted 5-ethynyl-2´-deoxyuridine (EDU) for 2 h. Thereafter, the cells were subjected to fixation for 15 min at room temperature in 4% paraformaldehyde, followed by immersion in 100 μL of 0.3% TritonX-100 for 15 min, and addition of 50 μL of click reaction solution to each well. Thereafter, the nuclei were stained with Hoechst 33,342 (diluted 1:1,000) and the cells were assessed using fluorescence microscopy.
Wound healing assay
We seeded VSMCs into 6-well plates, and inflicted wounds in 70%–80% confluent cells by scraping monolayers using a sterile pipette tip. The cells were subjected to washing steps three times with PBS and photographed at 0 and 24 h. Cell migration was quantified using Image-Pro plus.
Immunofluorescence
We seeded VSMCs onto microscope slides in 24-well plates, followed by fixation in 2 mL of 4% paraformaldehyde for 15 min. Thereafter, the cells were incubated with the antibody blocking solution containing 0.3% TritonX-100 for 1 h to block nonspecific antibody binding, followed by incubation with anti-PTEN, anti-α-SMA, anti-SM22α, and anti-OPN primary antibodies overnight, and with the anti-rabbit IgG Alexa Fluor 594 secondary antibody for 2 h. Nuclei were stained using DAPI, and then the slides were removed from the plates and assessed by fluorescence microscopy.
Western blotting
Cytoplasmic/nuclear protein extraction: Cells were scraped and pelleted by centrifugation for 3 min, and were suspended in 200 μL of the cytoplasmic protein extraction reagent. The suspension was vortex-mixed, placed on ice for 10 min, and then centrifuged at 13,000 × g, 4°C for 15 min. The supernatant was considered as the cytoplasmic protein. The remaining precipitate was extracted using 50 μL of a nuclear protein extraction reagent, completely dispersed, and then shaken at 4°C for 30 min and centrifuged at 13,000 × g for 15 min. This supernatant was considered as the nuclear protein.
Total protein was extracted using RIPA buffer supplemented with a protease/phosphatase inhibitor cocktail. The supernatant was collected after centrifugation at 13,000 × g for 15 min. All proteins were subjected to boiling for 10 min in SDS-PAGE sample buffer and resolved by performing 8%–10% SDS-PAGE. The proteins were transferred onto PVDF membranes, and then nonspecific protein binding was blocked by incubation with 5% skim milk for 1 h. Thereafter, the membranes were incubated overnight with anti-Daxx (1:300), anti-p-Akt, anti-Akt, anti-β-actin, anti-LaminB, anti-HSP90 anti-PTEN, anti-α-SMA, anti-SM22α, anti-OPN, followed by incubation with secondary antibodies for 1 h. Images were acquired using the ImageQuant LAS4000 gel imager (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Cell transfection
We seeded VSMCs (1 × 105 cells/mL) in 6-well plates, and then the cells were subjected to transfection for 24 h with the pHIV-EGFP-HA-Daxx lentivirus when they reached 70%–80% confluence. The expression of Daxx protein was detected by performing western blotting for 48 h.
Data analysis
All data are shown as mean ± SEM and were statistically analyzed by using one-way or two-way ANOVA include a simple effect tests using SPSS 17.0. (SPSS Inc., Chicago, IL, USA). Differences were considered significant at P < 0.05.
Results
Daxx inhibits phenotype switching of AngII-induced VSMCs
We transfected cells with pHIV-EGFP-HA-Daxx to obtain cell lines overexpressing Daxx and examined the effects of Daxx on AngII–induced VSMC phenotype switching. Western blotting results confirmed increased Daxx expression (Figure 1(a)). Thereafter, VSMCs expressing baseline or overexpressing Daxx were stimulated with AngII for 24 h. We found decreased expression of the contractile phenotype markers α-SMA and SM22α protein, and increased expression of the synthetic phenotype marker OPN in these cells. The overexpression of Daxx was not altered by AngII (Figure 1(b)).
Figure 1.
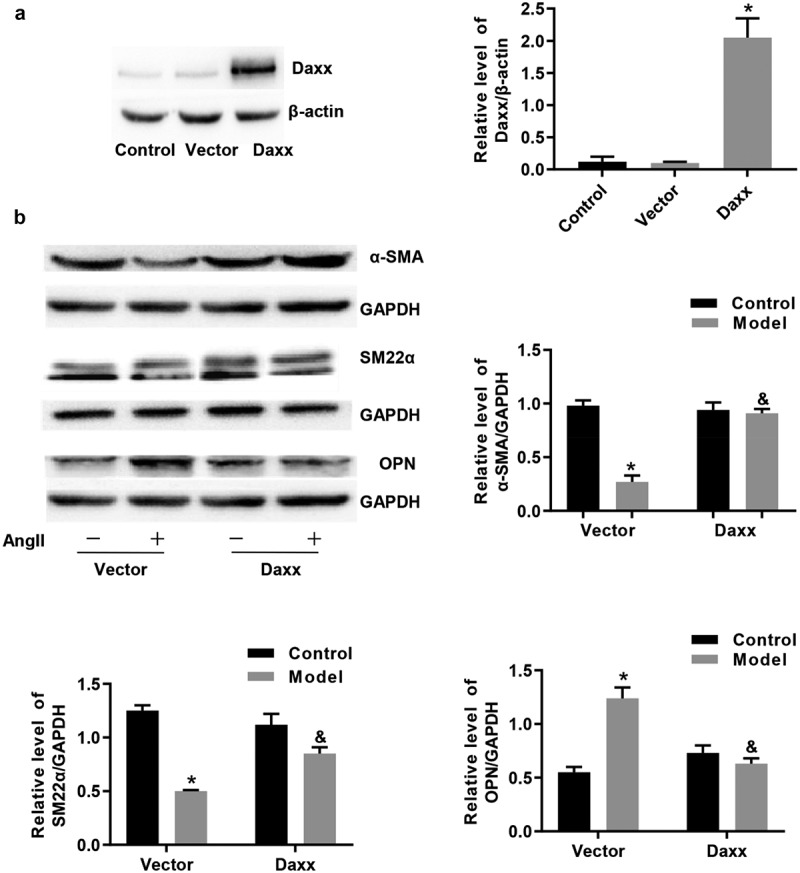
Phenotype switching of VSMCs induced by AngII is inhibited by Daxx
Western blots of (a) Daxx overexpression in VSMCs and (b) effects of Daxx on AngII–induced VSMC phenotype switching. *P < 0.05 vs Vector control, &P < 0.05 vs Vector model.
Proliferation and migration of VSMCs induced by AngII is inhibited by Daxx
The transition from the contractile to the synthetic phenotype causes abnormal VSMC proliferation [1]. Therefore, we evaluated the effects of Daxx on AngII–induced VSMC proliferation and migration. Figure 2(a,b) depict that Daxx overexpression remarkably inhibits Ang II–induced cell proliferation at 24 h and markedly inhibits Ang II-induced VSMC migration compared with the vector group in scratch wound assays (Figure 2(c)).
Figure 2.
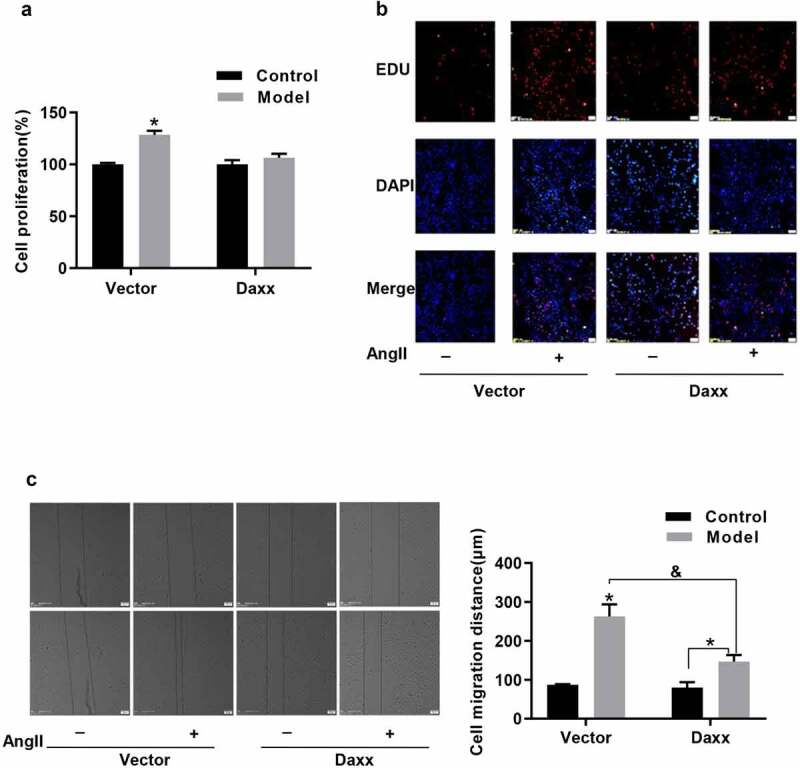
Proliferation and migration of VSMCs induced by AngII is inhibited by Daxx
(a) BRDU and (b) EDU assay findings of Daxx effects exerted on AngII-induced VSMC proliferation. (c) Effects of Daxx exerted on AngII-induced VSMC migration. *P < 0.05 vs Vector control, &P < 0.05 vs Vector model.
Cytoplasmic PTEN protein expression is promoted by Daxx-mediated inhibition of the PI3K/Akt signaling pathway
Although Daxx inhibits AngII-induced VSMC proliferation and migration by inhibiting p-Akt activation, but Daxx how to regulate the activation of p-Akt have not research further [7]. Phosphatase and tensin homolog (PTEN) plays an important role in inhibiting the phenotype transformation, as well as the proliferation and migration of VSNCs via the PI3K/Akt pathway [13]. We found that Daxx and PTEN expression was inhibited and Akt phosphorylation was activated in VSMCs stimulated with AngII for 24 h (supplement Figure S1). Valsartan is an AT1 receptor antagonist that can eliminate the effects of AngII on VSMCs [14]. We found that valsartan reversed the reduced Daxx and PTEN protein expression in VSMCs stimulated with AngII (supplement Figure S1). These results suggested that Daxx and PTEN effects might be correlated in AngII-stimulated VSMCs.
In general, the PI3K/Akt signal pathway activation inhibitor PTEN is mainly found in the cytoplasm, and this localization is regulated via a PML-DAXX-HAUSP molecular network [15,16]. Therefore, we examined the effects of Daxx on PTEN localization and the PTEN/Akt signaling pathway. Western blotting and immunofluorescence assays revealed significantly decreased cytoplasmic PTEN protein expression in cells stimulated with AngII; however, AngII could not alter Daxx overexpression (Figure 3(a,b)). Overexpressed Daxx notably increased PTEN protein expression and subsequently inhibited Akt phosphorylation (Figure 3(c)).
Figure 3.

Cytoplasmic PTEN protein expression is promoted by Daxx-mediated inhibition of the PI3K/Akt signaling pathway
Effects of Daxx on the cytoplasmic expression of PTEN protein (a) visualized using immunofluorescence assays (b). Effects of Daxx on the PI3K/Akt signaling pathway (c). *P < 0.05 vs Vector control, &P < 0.05 vs Vector model.
Nicotinate-curcumin suppresses VSMC phenotype switching induced by AngII
We evaluated whether NC and Cur could reverse the transition from the contractile to the synthetic phenotype and prevent abnormal VSMC proliferation. The findings of MTT assays showed that NC (1 μmol/L) and Cur (10 μmol/L) significantly inhibited the viability of VSMC cells stimulated with AngII (supplement Figure S2). Immunofluorescence and western blotting assay results showed decreased expression of AngII-induced contractile phenotype markers α-SMA and SM22α, and an increase in the expression of the synthetic phenotype marker OPN. Angiotensin II did not cause this transition in VSMCs that were incubated with NC (Figure 4(a–d)). These results indicated that NC suppressed the AngII-induced VSMC phenotype switching.
Figure 4.

Nicotinate-curcumin suppresses the phenotype switching induced in VSMCs by AngII
Immunofluorescence assays results highlighting the effects of NC and Cur on VSMC contractile phenotype markers (a) α-SMA, (b) SM22α, and (c) VSMC synthetic phenotype marker OPN. D. Western blotting results showing the effects of NC and Cur on VSMC phenotype switching. *P < 0.05 vs Control, &P < 0.05 vs Model.
Nicotinate-curcumin inhibits AngII-induced VSMC proliferation and migration
We incubated VSMCs with NC for 2 h, followed by incubation with AngII (1 µmol/L) for 24 h, to evaluate whether NC affected AngII-induced VSMC proliferation and migration. The results of EDU assay results confirmed that NC (1 μmol/L) inhibited AngII-induced VSMC proliferation (Figure 5(a)). We then investigated the effects of NC on AngII-induced VSMC migration in scratch wound assays and found that Ang II-induced migration was significantly inhibited by NC compared with the model group (Figure 5(b)).
Figure 5.

Proliferation and migration of VSMCs induced by AngII is inhibited by NC
(a) Results of EDU assays highlight the effects of NC on AngII-induced VSMC proliferation. (b) Effects of NC on AngII-induced VSMC migration. *P < 0.05 vs Control, &P < 0.05 vs Model.
Nicotinate-curcumin inhibits AngII-induced VSMC phenotype switching via upregulation of Daxx expression
The above-mentioned findings suggested that the Daxx/PTEN/Akt signaling pathway was important for inhibiting the VSMC phenotype switching associated with proliferation and migration. We analyzed the protein expression of Daxx and cytoplasmic PTEN to determine whether NC and Cur regulated the Daxx/PTEN/Akt signaling pathway by performing western blotting. Figure 6(a,b) depict that NC and Cur increases Daxx and cytoplasmic PTEN expression in VSMCs and reduces p-Akt activation.
Figure 6.

Nicotinate-curcumin inhibits AngII-induced VSMC phenotype switching by targeting Daxx expression
Western blotting results highlight the effects of NC and Cur on (a) Daxx, PTEN, p-Akt, and (b) cytoplasmic PTEN. *P < 0.05 vs Control, &P < 0.05 vs Model.
Discussion
The association between VSMC phenotype switching and abnormal proliferation and migration is evident in the development of atherosclerotic cardiovascular diseases. However, therapeutic approaches that can specifically prevent VSMC phenotypic switching toward proatherosclerotic activation are not presently available [17]. The present study explored the roles of Daxx and NC in regulating phenotype switching and the underlying mechanisms when VSMCs are exposed to AngII. We found that Daxx overexpression promoted the expression of the cytoplasmic PTEN protein, blocked the activation of phosphorylated Akt, and consequently affected the expression of phenotype switching markers in VSMCs. Our findings also suggested a novel mechanism of NC involving VSMC phenotype switching and abnormal proliferation and migration achieved by upregulation of Daxx expression. These results indicate that NC can be considered a potential pharmacological agent that may be used in the treatment of atherosclerosis, which is a long-term vascular disease caused by the existence of multifactorial associations among various risk factors [18].
Vascular smooth muscle cells comprise a major cell type that is involved at all stages of atherosclerotic plaque formation, the plasticity of which changes along with the occurrence of pathological intimal thickening. The phenotype switching of VSMCs is characterized by increased and decreased expression of synthetic and contractile proteins, respectively [19]. Hemodynamic forces, oxidized low density lipoprotein (oxLDL), AngII expression, and proinflammatory stimuli promote VSMC phenotype switching associated with proliferation and migration [17]. Accumulating evidence has recently suggested that herbal medicines exert anti-atherosclerotic effects by modulating the phenotype switching of VSMCs. Among such herbal medicinal agents, Curcuma longa L. and its main active component curcumin regulate the phenotype of VSMCs [20]. However, its poor water solubility, rapid metabolism, and low bioavailability limit its clinical application. Synthetic derivatives are chemical agents used for improving the shortcomings of curcumin usage, and demonstrate appreciable pharmacological effects in diabetes and cancer [21,22]. Nicotinate-curcumin is a novel derivative of nicotinate and curcumin, with improved water solubility and bioavailability [11]. Recent studies have reported that NC not only induces HCT116, MCF-7, and CNE2 cell apoptosis and cycle arrest through a P53-mediated mechanism in cancer, but also ameliorates cognitive impairment in diabetic rats [23,24]. Furthermore, NC regulates lipid metabolism, impedes foam cell formation, and inhibits atherosclerosis formation in apoE−/- mice with vascular diseases [11,12]. Our results showed that NC significantly increased the expression of contractile phenotype markers and effectively reversed AngII-induced cell proliferation and migration. Importantly, NC (1 μmol/L) inhibited the VSMC phenotypic switching. However, Cur showed no effects at this concentration. These results suggested that atherosclerosis could be treated more effectively by NC than Cur.
Although Daxx can inhibit Ang II-induced proliferation and migration, much remains unknown about the causality and impact of the phenotype switching [5]. The present findings showed that Daxx overexpression reversed the increase in expression of the synthetic phenotype marker OPN induced by AngII. Moulton et al. have reported that a deficiency of PTEN (an essential regulator of differentiated VSMCs) promotes VSMC dedifferentiation and pathological vascular remodeling in human coronary arteries [25]. The highly proliferative growth phenotype expressed in VSMCs is driven by constitutive Akt expression and actively repressed by PTEN [26]. Therefore, the PTEN/Akt signaling pathway is a key factor regulating the phenotypic transition of VSMCs. The fact that Daxx can promote the expression of PTEN is consistent with the notion [17] that Daxx inhibits the expression of the cytoplasmic PTEN protein in VSMCs. Furthermore, contractile protein expression was increased and accompanied by increased PTEN protein expression. These findings imply that Daxx/PTEN/Akt mainly affects the stages of atherosclerosis development by regulating VSMC phenotypic switching, proliferation, and migration. Importantly, Daxx may be a major target in the treatment of atherosclerosis.
Based on the information in the existing literature, we assessed whether anti-atherosclerotic NC regulated Daxx expression and found that NC upregulated Daxx expression, promoted PTEN expression, and inhibited p-Akt activation.
In conclusion, we showed that Daxx inhibited the AngII-induced VSMC phenotype switching by regulating the PTEN/Akt pathway, and that NC inhibited AngII-induced VSMC phenotype switching, via a mechanism that might be associated with upregulated Daxx expression.
Supplementary Material
Funding Statement
The work was supported by National Natural Science Foundation of China [81673722, 81600291, 81670268], The National Natural Science Foundation of Hunan [2019JJ50426] The Education Department of Hunan Province [20A379, 19B401].
Abbreviations
- VSMCs
vascular smooth muscle cells
- Daxx
Death domain-associated protein
- AngII
angiotensin II
- PTEN
Phosphatase and Tensin homolog deleted on chromosome Ten
- Akt
protein kinase B
- NC
Nicotinate-curcumin
- α-SMA
alpha-smooth muscle actin
- SMA22α
smooth muscle protein 22alpha
- OPN
Osteopontin
- SRF
serum response factor
- KLF4
Krüppel-like factor 4
- TGFβ
transforming growth factor-β
- PI3K
phosphatidylinositol-3-kinase
- PDGF
Platelet derived growth factor
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Zhang X, Huang T, Zhai H, et al. Inhibition of lysine-specific demethylase 1A suppresses neointimal hyperplasia by targeting bone morphogenetic protein 2 and mediating vascular smooth muscle cell phenotype. Cell Prolif. 2020;53(1):e12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jaminon A, Reesink K, Kroon A, et al. The role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. 2019;20(22):5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Frismantiene A, Philippova M, Erne P, et al. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018;52:48–64. [DOI] [PubMed] [Google Scholar]
- [4].Zhang M, Li F, Wang X, et al. MiR-145 alleviates Hcy-induced VSMC proliferation, migration, and phenotypic switch through repression of the PI3K/Akt/mTOR pathway. Histochem Cell Biol. 2020;153(5):357–366. [DOI] [PubMed] [Google Scholar]
- [5].Bogolyubova I, Bogolyubov D.. DAXX is a crucial factor for proper development of mammalian oocytes and early embryos. Int J Mol Sci. 2021;22(3):1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boisguérin P, Covinhes A, Gallot L, et al. A novel therapeutic peptide targeting myocardial reperfusion injury. Cardiovasc Res. 2020;116(3):633–644. [DOI] [PubMed] [Google Scholar]
- [7].Cao Y, Sun S, Yang D, et al. Daxx overexpression inhibits AngII-induced proliferation and migration in vascular smooth muscle cells. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39(10):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharifi-Rad J, Rayess YE, Rizk AA, et al. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020;11:01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li H, Sureda A, Devkota HP, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2020;38:107343. [DOI] [PubMed] [Google Scholar]
- [10].Hassanzadeh K, Buccarello L, Dragotto J, et al. Obstacles against the marketing of curcumin as a drug. Int J Mol Sci. 2020;21(18):6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gu HF, Li HZ, Tang YL, et al. Nicotinate-curcumin impedes foam cell formation from THP-1 cells through restoring autophagy flux. PLoS One. 2016;11(4):e0154820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gong YZ, Yao HL, Sun SW, et al. SREBP-1/caveolin-1 mediate the anti-atherosclerotic effect of curcumin nicotinate in apolipoprotein E-deficient mice. Chin J Arteriosclerosis. 2014;22(12). [Google Scholar]
- [13].Dong X, Yu LG, Sun R, et al. Inhibition of PTEN expression and activity by angiotensin II induces proliferation and migration of vascular smooth muscle cells. J Cell Biochem. 2013;114(1):174–182. [DOI] [PubMed] [Google Scholar]
- [14].Sironi L, Calvio AM, Arnaboldi L, et al. Effect of valsartan on angiotensin II-induced plasminogen activator inhibitor-1 biosynthesis in arterial smooth muscle cells [published correction appears in Hypertension 2001 May;37(5):1350]. Hypertension. 2001;37(3):961–966. [DOI] [PubMed] [Google Scholar]
- [15].Song MS, Salmena L, Carracedo A, et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature. 2008;455(7214):813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Malaney P, Uversky VN, Davé V. PTEN proteoforms in biology and disease. Cell Mol Life Sci. 2017;74(15):2783–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). 2015;214(1):33–50. [DOI] [PubMed] [Google Scholar]
- [18].Bruikman CS, Stoekenbroek RM, Hovingh GK, et al. New drugs for atherosclerosis. Can J Cardiol. 2017;33(3):350–357. [DOI] [PubMed] [Google Scholar]
- [19].Basatemur GL, Jørgensen HF, Clarke MCH, et al. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–744. [DOI] [PubMed] [Google Scholar]
- [20].Saleh Al-Shehabi T, Iratni R, Eid AH. Anti-atherosclerotic plants which modulate the phenotype of vascular smooth muscle cells. Phytomedicine. 2016;23(11):1068–1081. [DOI] [PubMed] [Google Scholar]
- [21].Oliveira S, Monteiro-Alfredo T, Silva S, et al. Curcumin derivatives for type 2 diabetes management and prevention of complications. Arch Pharm Res. 2020;43(6):567–581. [DOI] [PubMed] [Google Scholar]
- [22].Nirgude S, Mahadeva R, Koroth J, et al. ST09, a novel curcumin derivative, blocks cell migration by inhibiting matrix metalloproteases in breast cancer cells and inhibits tumor progression in EAC mouse tumor models. Molecules. 2020;25(19):4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].He YC, He L, Khoshaba R, et al. Curcumin nicotinate selectively induces cancer cell apoptosis and cycle arrest through a P53-mediated mechanism. Molecules. 2019;24(22):4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gu HF, Li N, Tang YL, et al. Nicotinate-curcumin ameliorates cognitive impairment in diabetic rats by rescuing autophagic flux in CA1 hippocampus. CNS Neurosci Ther. 2019;25(4):430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moulton KS, Li M, Strand K, et al. PTEN deficiency promotes pathological vascular remodeling of human coronary arteries. JCI Insight. 2018;3(4):e97228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mourani PM, Garl PJ, Wenzlau JM, et al. Unique, highly proliferative growth phenotype expressed by embryonic and neointimal smooth muscle cells is driven by constitutive Akt, mTOR, and p70S6K signaling and is actively repressed by PTEN. Circulation. 2004;109(10):1299–1306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


