Abstract
Although neutrophils are considered a histologic hallmark of psoriasis, their pathophysiologic role in psoriasis remains unclear. We characterized the effects of neutrophil depletion via injection of monoclonal antibody 1A8 on the development of imiquimod (IMQ)-induced psoriatic lesions in a murine model. Lesions were followed with photographs and histologic analysis, revealing reduced psoriasiform scale and epidermal hyperplasia in neutrophil-depleted. ELISA and flow cytometry were used to determine relative levels of cytokines and immune cells. Compared to controls, IMQ-treated neutropenic mice had significantly lower levels of macrophages in tissue samples (P<0.05) and displayed significantly lower numbers of CD4+ T-cells (P<0.05). Neutropenic animals exhibited lower levels of TNF-α, IFN-γ, and IL-1β than controls (P<0.05). These results show that neutropenia reduces the development of psoriasiform skin lesions and substantially decreases infiltration of pro-inflammatory cytokines and immune cells to IMQ-induced cutaneous lesions, suggesting an active role of neutrophils in maintaining inflammation in psoriasis.
Keywords: cytokines, imiquimod, inflammation, macrophages, neutrophils, psoriasis
INTRODUCTION
Psoriasis is a chronic autoimmune disorder affecting approximately 2% of the global population [1]. Although mainly considered a dermatologic condition, psoriasis is associated with important systemic and psychological comorbidities, including psoriatic arthritis, cardiovascular disease, and inflammatory bowel disease [2–5], and has significant effects on patient morbidity and mortality. The pathophysiology of psoriasis is poorly understood. Historically, evidence has suggested that psoriasis is a T helper 1 (Th1)-driven disease [6–8]. However, recent studies have demonstrated a complex immunologic milieu in psoriatic lesions consisting of interrelated cytokine pathways including TNF-α, IL-17, and IL-23 [9, 10], which have recently become central targets for therapeutic intervention.
Neutrophils play an important role in early control of acute bacterial infections by killing bacteria through powerful oxidative and non-oxidative mechanisms and production of proinflammatory cytokines [11]. However, prolonged presence of neutrophils in tissue may result in collateral damage due to excessive production of reactive oxygen species [12, 13]. In psoriasis, neutrophils are considered a histologic hallmark and marker of disease activity [14, 15]. Though the role of neutrophils in modulating the psoriatic phenotype remains unclear, they are present in early psoriatic lesions [16], specifically recruited in the setting of psoriasis by IL-8 [17]. Furthermore, neutrophils contribute to the psoriatic lesion immunologic milieu by secreting IL-1β, thereby stimulating keratinocyte production of IL-19 [18], a component of the IL-17/IL-23 pathways [19]. Neutrophils are also a major cellular source of IL-17 in psoriatic lesions [20–22], triggering keratinocyte hyperplasia and amplification of the inflammatory response by generating this key inflammatory cytokine in the pathogenesis of psoriasis [21].
Imiquimod (IMQ)-induced psoriasiform lesions in mouse models appear to hinge on the IL-23/IL-17 axis [23], a critical pathway in human patients with psoriasis [10]. Ly-6G is a small protein on the surface of mouse neutrophils that plays a critical role in the neutrophil response to chemotactic stimuli. Blockade of Ly-6G with the monoclonal antibody (mAb) 1A8 depletes neutrophils in mice [24]. Mab 1A8-induced murine neutropenia can be utilized to understand the impact of specific inhibition of the neutrophil response and its role in stimulating/inhibiting the action of other immune cell mediators, as well as their effector molecules such as proinflammatory cytokines. Therefore, we hypothesized that depletion of neutrophils would attenuate the severity of IMQ-induced psoriatic lesions in an experimental murine model. We showed that neutrophil depletion reduces IMQ-mediated psoriasis by altering the host immune response. Our findings provide additional data on the impact of neutrophils in promoting psoriatic disease, rendering these leukocytes an important target, which may lead to the development of more effective therapeutic strategies.
MATERIALS AND METHODS
Psoriatic murine model and treatment.
Female Balb/c mice (6–8 weeks; Charles Rivers) were first injected intraperitoneally (i.p.) with a single dose of 500 μg/mL of mAb 1A8 (Rat anti-mouse IgG2a; (BD) in a 100 μL of sterile phosphate buffer saline (PBS). Control animals were injected with irrelevant IgG2a antibody (control IgG2a; Southern Biotech). Three days after mAb administration, mice were retro-orbitally bled and neutrophil depletion was first determined by differential leukocyte count in all experimental animals using a Hema 3 Stat Pack (Fisher HealthCare) and light microscopy and confirmed using flow cytometry. The next day, mAb 1A8 or IgG2a injected rodents were anesthetized with 100 mg/kg ketamine (Keta-set®) and 10 mg/kg xylazine (Anased®), the hair on their backs removed, and the skin disinfected with 70% ethanol. Each mouse received a daily topical dose (62.5 mg) of IMQ (5%; Aldara; 3M Pharmaceuticals) cream formulation on the hairless area. Animals were euthanized 3, 5, and 7 days post-IMQ administration and tissues were collected to determine changes in inflammation. All animal studies were conducted according to the experimental practices and standards approved by the Institutional Animal Care and Use Committee (IACUC) at NYIT College of Osteopathic Medicine (Protocol #: 2016-LRM-01). The IACUC at NYIT College of Osteopathic Medicine approved this study.
Histological processing and gross examination.
At day 7 post-treatment, cutaneous tissues were excised from euthanized mice; the tissues were fixed in 10% formalin and embedded in paraffin. Four micrometer vertical sections were cut and then fixed to glass slides and subjected to Haematoxylin and Eosin (H&E) to assess morphology. The slides were visualized using an Axiovert 200 M inverted microscope (Carl Zeiss) at a magnification of ×40. Images were collected using an AxioCam MrC digital camera using Zen 2011 digital imaging software (Carl Zeiss).
Flow cytometry.
Flow cytometry was performed on primary cells from cutaneous tissue excised from untreated and IMQ-treated mice on days 3, 5, and 7. Two mm of skin tissue were weighted and homogenized in 5 mL of digestion medium (0.385 mg/mL of Liberase TL; Roche; in DMEM; Gibco) in a 15 mL conical tube. The homogenates were then incubated in a 37°C water bath for 1 h. Cell suspension preparations were centrifuged at 300g for 5 min at 4°C. After centrifugation, the supernatant was discarded, the cell pellet re-suspended in PBS supplemented with 1% bovine serum albumin (Fisher), passed through a 70 μm strainer, and centrifuged as described above and washed twice in PBS (1% BSA). Finally, the cell pellet was re-suspended in buffer and the suspension was filtered through a 30 μm filter. For flow cytometry, primary murine blood and skin cells were washed with PBS (1% BSA) and stained with rat anti-mouse fluorescence-labeled antibodies (BD), including FITC-Ly-6G (neutrophils), PE-F4/80 (macrophages), and APC-CD4 (CD4 T-cells). Samples were processed (10,000 events per sample) on a BD Accuri C6 flow cytometer, and the number of Ly-6G, F4/80, and CD4 positive cells was analyzed using BD FCS Express software.
Cytokine determinations.
Three mice per group were sacrificed 3, 5, and 7 days post-topical treatment with or without IMQ. Tissues were excised and homogenized in PBS with protease inhibitors (Roche). Cell debris was removed from homogenates by centrifugation at 6,000 g for 10 min. Supernatants were stored at –80°C until tested. Samples were tested for IFN-γ, TNF-α, IL-1β, IL-4, IL-6, and IL-10 by ELISA (BD). The limits of detection were 31.3 pg/mL for IFN-γ and IL-10, 15.6 pg/mL for TNF-α, IL-1β, and IL-6, and 7.8 pg/mL for IL-4.
Statistical analysis.
All data were subjected to statistical analysis using Prism 7.0 (GraphPad). P values for individual comparisons were calculated by student’s t-test analysis. P values of <0.05 were considered significant.
RESULTS
Administration of mAb 1A8 decreases the formation of psoriatic lesions.
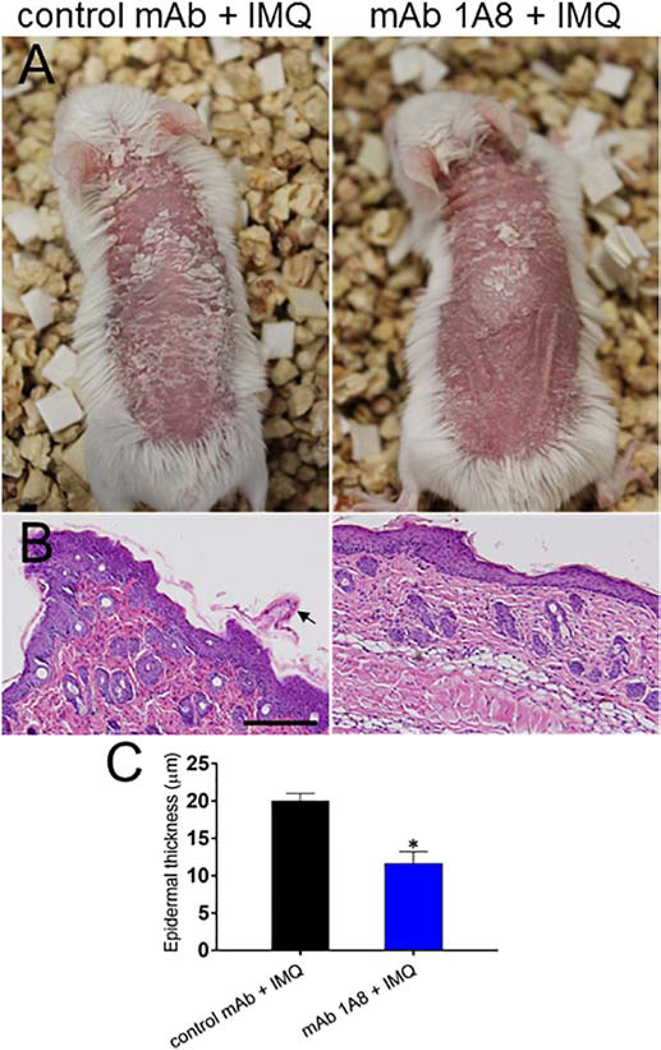
In psoriasis and its IMQ-induced mouse model, infiltration of neutrophils and inflammatory monocytes can be clearly observed in the skin [18]. Therefore, we investigated the role of neutrophils in the generation of psoriatic lesions in Balb/c mice after daily topical treatment with IMQ (Fig. 1). Gross anatomical examination demonstrated that neutropenic mice treated with IMQ (mAb 1A8 + IMQ; Fig. 1A; upper right panel) for 7 days exhibited reduced psoriasiform scale compared to control animals (control mAb + IMQ; Fig. 1A; upper left panel). Histopathological analysis revealed substantial psoriasiform epidermal hyperplasia and parakeratosis with neutrophil infiltration in control mAb + IMQ mice (Fig. 1B; lower left panel). MAb 1A8 + IMQ rodents displayed considerably less inflammation, with mostly normal epidermal and dermal tissue (Fig. 1B; lower right panel). Our results indicate that neutrophils play an important role in the development of psoriatic lesions in the IMQ-induced mouse model.
Fig. 1. Neutrophil depletion attenuates generation of IMQ-induced psoriatic lesions.
Balb/c mice were injected intraperitoneally with a single dose of 500 μg/mL of mAb 1A8 or IgG2a control mAb. Three days later, the backs of each mouse received a daily topical dose (62.5 mg) of imiquimod (IMQ) cream formulation. Mice were treated for 7 days. (A) Gross (upper panels) and (B) histopathological (lower panels; representative 20X H&E stained sections of the skin lesions are shown) examination of mice injected with control IgG2a (left panels) or 1A8 (right panels) mAb and treated with IMQ daily for 7 days. Scale bar: 20 μm.
MAb 1A8 administration reduces inflammatory cell infiltration to IMQ-induced lesions.
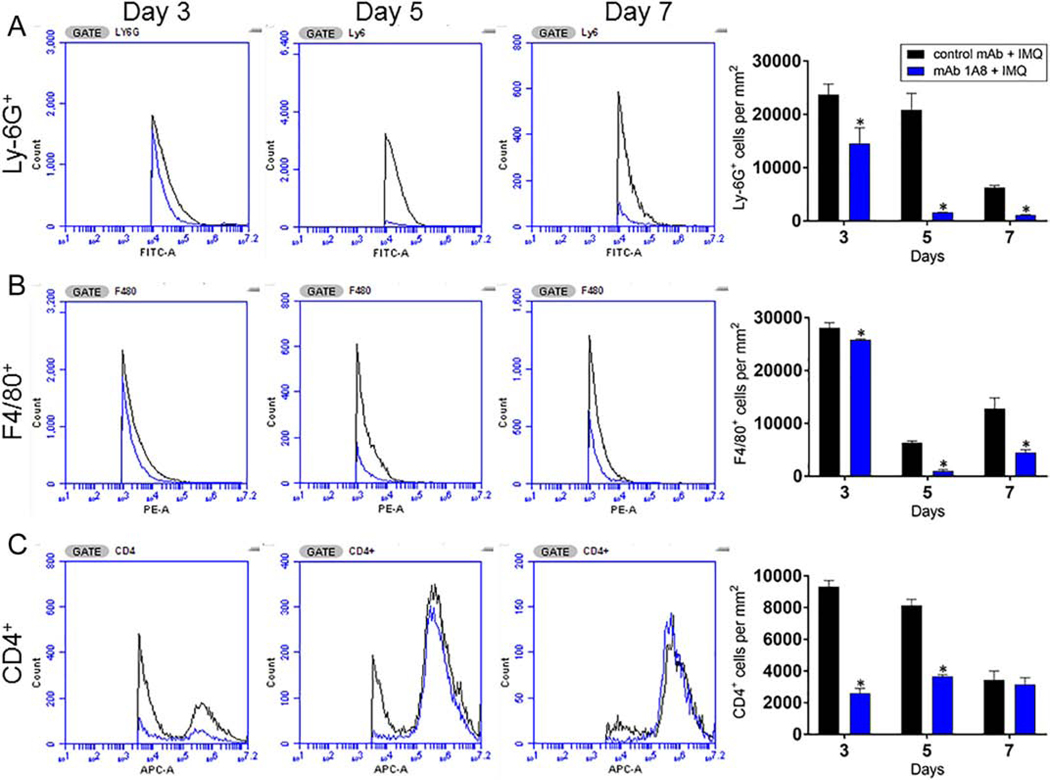
Since we observed that neutrophils are involved in regulatory networks underlying the pathophysiology of psoriasis, we determined the effect of mAb 1A8 administration on the longterm recruitment of neutrophils (Ly6-G+), macrophages (F4/80+), and helper T-cells (CD4+) to IMQ-induced lesional skin using flow cytometry (Fig. 2). As expected, we found reduced numbers of Ly6-G+ cells in mAb 1A8 + IMQ mice on days 3, 5, and 7 (P<0.05) (Fig. 2A). Similarly, we did not observe any difference in the infiltration of F4/80+ in the skin of mAb 1A8 + IMQ or control mAb + IMQ mice excised 3 days post-treatment. However, in tissue samples extracted on days 5 and 7 post-treatment, we found that mAb 1A8 + IMQ mice had significantly lower recruitment of F4/80+ cells relative to tissue in control animals (P<0.05) (Fig. 2B). Finally, mAb 1A8 + IMQ mice displayed significantly lower numbers of CD4+ cells in excised tissue on days 3 and 5 post-treatment compared to control mAb + IMQ mice (P<0.05) (Fig. 2C). These findings indicate that mAb 1A8 administration decreases cellular infiltration to cutaneous tissue, which may help explain the reduced development of psoriaform skin lesions in IMQ-treated animals.
Fig. 2. Administration of mAb 1A8 reduces the infiltration of inflammatory cells to IMQ-induced psoriatic lesions.
One mm2 of cutaneous tissue was excised, weighed, and homogenized at days 3, 5, and 7 following application of daily topical IMQ cream. Samples were stained with fluorescence-labeled mAb for (A) Ly6-G+ (neutrophils), (B) F4/80+ (macrophages), and (C) CD4+ (T-cells) cells. Bars represent the mean values; error bars indicate standard deviations. Asterisks denote P-value significance (P < 0.05) calculated using student’s t-test analysis. The experiments were performed twice with similar results obtained.
Injection of mAb 1A8 alters the pro- and anti-inflammatory cytokine levels in the skin.
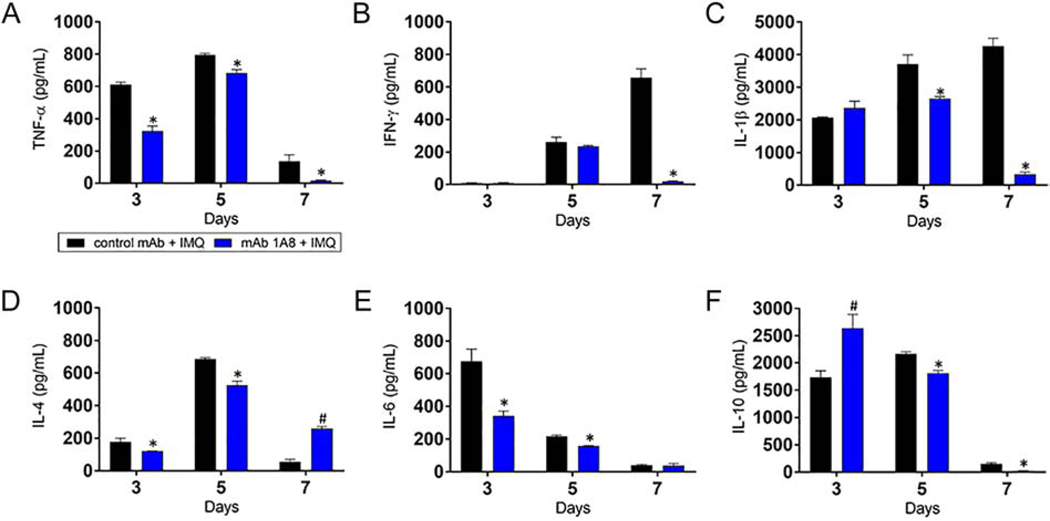
Upon activation, neutrophils release inflammatory mediators that are important in modulating the immune response in psoriatic lesions [21]. We investigated the impact of neutrophil depletion on the progression of pro- and anti-inflammatory cytokine production in IMQ-induced lesions on days 3, 5, and 7 post-treatment (Fig. 3). On day 3, we observed a significant reduction in proinflammatory TNF-α and IL-6 and anti-inflammatory IL-4 in mAb 1A8 + IMQ mice (P<0.05). In contrast, we found a significant increase in anti-inflammatory IL-10 in mAb 1A8 + IMQ mice relative to control animals (P<0.05). Control mAb + IMQ and mAb 1A8 + IMQ mice showed no differences in IFN-γ and IL-1β levels in cutaneous tissue. On day 5, mAb 1A8 + IMQ mice evinced a significant reduction in the production of all the tested cytokines in skin tissue compared to control rodents (P<0.05), with the exception of IFN-γ. On day 7, mAb 1A8injected animals exhibited lower levels of TNF-α, IFN-γ, IL-1β, and IL-10, and higher levels of IL-4 than control mAb + IMQ mice (P<0.05). However, there were no differences in the production of IL-6 in skin tissue of animals in the control mAb + IMQ and mAb 1A8 + IMQ groups. These results show that neutropenia substantially reduces the production of numerous pro-inflammatory cytokines early during IMQ topical application while increasing the production of anti-inflammatory cytokines early (IL-10) and late (IL-4) during IMQ treatment.
Fig. 3. Neutropenia alters cytokine production in skin lesions.
Homogenates of excised cutaneous lesions from control mAb IgG2a and mAb 1A8 mice 3, 5, and 7 days post-IMQ topical application were prepared and the supernatants were analyzed for (A) TNF-α, (B) IFN-γ, (C) IL-1β, (D) IL-4, (E) IL-6, and (F) IL-10 levels. Bars represent the mean values; error bars indicate standard deviations. Symbols (* lower; # higher) denote P-value significance (P < 0.05) calculated using student’s t-test analysis. Cytokine quantification was performed thrice with similar results obtained.
DISCUSSION
Psoriasis is a chronic inflammatory disorder that often arises in response to environmental triggers such as traumatic injury to the skin or physical stress. Though multiple studies have recognized neutrophils as a major component in the pathophysiology of psoriasis, few studies to date have examined neutrophils as therapeutic targets in the treatment of psoriasis. In one study of 14 patients, selective depletion of myeloid lineage leukocytes through adsorptive granulocyte and monocyte apheresis significantly improved the severity of generalized pustular psoriasis [25]. Depletion of neutrophils with anti-Ly-6G antibody was also found to ameliorate disease severity in IMQ-induced psoriatic skin lesions in mice [18]. We confirmed these results and additionally found that reduced numbers of neutrophils in skin tissue resulted in decreased recruitment of F4/80+ and CD4+ cells. In this regard, blocking of neutrophil localization in vivo with the M1/70 mAb causes considerable reduction in epidermal thickness and neutrophil accumulation in psoriasiform skin lesions [26]. In patients with severe psoriasis, mAb neutralization of TNF-α and IL-12/23p40 decreased neutrophil activity and resulted in improved clinical outcomes [14]. Our study contributes to the mounting evidence suggesting a promising role of neutrophils as therapeutic targets for the treatment of psoriasis, and highlights the need for further research to elucidate the precise mechanism by which neutrophils mediate the development of psoriatic lesions.
Infiltration of neutrophils into the epidermis – a hallmark of psoriasis – is thought to be mediated by pro-inflammatory cytokines [27], underscoring the role of dysregulated cytokine production in psoriatic lesions. However, there is an open question as to whether neutrophils significantly contribute to the maintenance of a pro-inflammatory milieu in established psoriatic plaques. Our study indicates that neutrophil depletion reduces early levels of pro-inflammatory cytokines and increases the early and late production of IL-10 and IL-4, respectively, during IMQ treatment. Within inflamed skin, neutrophils release many pro-inflammatory cytokines, including IL-1β, IL-6, IL-17, and IL-23. These cytokines facilitate hyper-proliferation, activation of keratinocytes, and the mobilization of diverse immune cells [21, 28]. For instance, IL-17A is known to induce keratinocyte production of TNF-α, IL-1β, IL-6, and IL-19 [19, 29–31]. Although we did not measure the levels of IL-17A, our results are consistent with these observations; we found low levels of TNF-α (throughout; days 3 to 7), IL-1β (late; days 5 and 7), and IL-6 (early; days 3 and 5) in skin tissue during IMQ treatment. TNF-α activates neutrophils and enhances their infiltration into the epidermis [32]. Neutrophil secretion of IL-1β in cutaneous tissue promotes the expression of ICAM and VCAM-1 by dermal endothelial cells, leading to increased recruitment of immune cells to the skin [33]. It is conceivable that the reduced late production of IL-1β in neutropenic mice was associated with low numbers of macrophages and T cells. Th1 cells secrete IFN-γ, which may perpetuate the immunologic milieu in psoriasis by enhancing the production of other cytokines, particularly IL-17A [34]. Notably, we demonstrated that CD4+ cells were significantly reduced by day 3 in mAb 1A8 injected mice treated with IMQ, and this was associated with low levels of IFN-γ in cutaneous tissue. This is an important observation given that Th1 cells have traditionally been regarded as the primary mediators of psoriasis, whereas our results suggest that neutrophils modulate the activity of these lymphocytes. Hence, the current data suggest that neutrophils play a key role in a number of positive feedback loops observed in psoriatic epidermal lesions.
In conclusion, our findings suggest that neutrophil depletion modifies immune cell infiltration and the levels of inflammatory cytokines in IMQ-induced cutaneous lesions. These results show that neutrophils may be involved in a number of regulatory networks underlying the pathophysiology of IMQ-induced psoriasis. While neutrophils seem to play a part in the development of the type of inflammation that is the hallmark of psoriasis, it is important to note the many important biological functions of neutrophils in host response. Thus, while systemic depletion of all neutrophils may not be reasonable in human patients, either targeted therapeutics against activated neutrophils (which are under development) or a topical treatment strategy to suppress neutrophils may be advisable. Although further studies are warranted to elucidate the mechanisms associated with biological reduction of neutrophils in the psoriasis phenotype, targeting neutrophils may be a plausible therapeutic strategy for the treatment of psoriasis.
Neutrophil depletion reduces the psoriasis phenotype in a murine model
Neutropenic mice have lower levels of inflammatory cytokines in psoriasis lesions
Neutrophils play an active role in establishing and maintaining psoriasis
Nuanced targeting of neutrophils in psoriasis may be an attractive treatment approach
ACKNOWLEDGEMENTS
L.R.M. was supported by the National Institute of General Medical Sciences of the US National Institutes of Health (NIH) under award number 1R15GM117501-01A1.
Footnotes
Conflict Of Interest
The authors have no conflict of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Raychaudhuri SP, A cutting edge overview: psoriatic disease, Clinical reviews in allergy & immunology 44(2) (2013) 109–13. [DOI] [PubMed] [Google Scholar]
- [2].Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Risk of myocardial infarction in patients with psoriasis, Jama 296(14) (2006) 1735–41. [DOI] [PubMed] [Google Scholar]
- [3].Kimball AB, Robinson D Jr., Wu Y, Guzzo C, Yeilding N, Paramore C, Fraeman K, Bala M, Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001–2002, Dermatology (Basel, Switzerland) 217(1) (2008) 27–37. [DOI] [PubMed] [Google Scholar]
- [4].Makredes M, Robinson D Jr., Bala M, Kimball AB, The burden of autoimmune disease: a comparison of prevalence ratios in patients with psoriatic arthritis and psoriasis, Journal of the American Academy of Dermatology 61(3) (2009) 405–10. [DOI] [PubMed] [Google Scholar]
- [5].Wu JJ, Nguyen TU, Poon KY, Herrinton LJ, The association of psoriasis with autoimmune diseases, Journal of the American Academy of Dermatology 67(5) (2012) 924–30. [DOI] [PubMed] [Google Scholar]
- [6].Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG, The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients, J Invest Dermatol 113(5) (1999) 752–9. [DOI] [PubMed] [Google Scholar]
- [7].Lew W, Bowcock AM, Krueger JG, Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression, Trends Immunol 25(6) (2004) 295–305. [DOI] [PubMed] [Google Scholar]
- [8].Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, Meyer zum Buschenfelde KH, Fleischer B, T cells involved in psoriasis vulgaris belong to the Th1 subset, J Invest Dermatol 102(2) (1994) 145–9. [DOI] [PubMed] [Google Scholar]
- [9].Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C, Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints, International journal of molecular sciences 19(2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Di Cesare A, Di Meglio P, Nestle FO, The IL-23/Th17 axis in the immunopathogenesis of psoriasis, The Journal of investigative dermatology 129(6) (2009) 1339–50. [DOI] [PubMed] [Google Scholar]
- [11].Mantovani A, Cassatella MA, Costantini C, Jaillon S, Neutrophils in the activation and regulation of innate and adaptive immunity, Nature reviews. Immunology 11(8) (2011) 519–31. [DOI] [PubMed] [Google Scholar]
- [12].Wang J, Neutrophils in tissue injury and repair, Cell and tissue research 371(3) (2018) 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wilgus TA, Roy S, McDaniel JC, Neutrophils and Wound Repair: Positive Actions and Negative Reactions, Advances in wound care 2(7) (2013) 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamanaka K, Umezawa Y, Yamagiwa A, Saeki H, Kondo M, Gabazza EC, Nakagawa H, Mizutani H, Biologic therapy improves psoriasis by decreasing the activity of monocytes and neutrophils, The Journal of dermatology 41(8) (2014) 679–85. [DOI] [PubMed] [Google Scholar]
- [15].Zhang L, Wiles C, Martinez LR, Han G, Neutrophil-to-lymphocyte ratio decreases after treatment of psoriasis with therapeutic antibodies, Journal of the European Academy of Dermatology and Venereology : JEADV 31(11) (2017) e491–e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van de Kerkhof PC, Chang A, Migration of polymorphonuclear leukocytes in psoriasis, Skin Pharmacol 2(3) (1989) 138–54. [DOI] [PubMed] [Google Scholar]
- [17].Gearing AJ, Fincham NJ, Bird CR, Wadhwa M, Meager A, Cartwright JE, Camp RD, Cytokines in skin lesions of psoriasis, Cytokine 2(1) (1990) 68–75. [DOI] [PubMed] [Google Scholar]
- [18].Sumida H, Yanagida K, Kita Y, Abe J, Matsushima K, Nakamura M, Ishii S, Sato S, Shimizu T, Interplay between CXCR2 and BLT1 facilitates neutrophil infiltration and resultant keratinocyte activation in a murine model of imiquimod-induced psoriasis, J Immunol 192(9) (2014) 4361–9. [DOI] [PubMed] [Google Scholar]
- [19].Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, Babel N, Wittig BM, Warszawska K, Kurek A, Erdmann-Keding M, Kunz S, Asadullah K, Kadin ME, Volk HD, Sterry W, Wolk K, Sabat R, IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis, The Journal of investigative dermatology 134(11) (2014) 2757–2767. [DOI] [PubMed] [Google Scholar]
- [20].Keijsers R, Hendriks AGM, van Erp PEJ, van Cranenbroek B, van de Kerkhof PCM, Koenen H, Joosten I, In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing RORgammat and IL-17, The Journal of investigative dermatology 134(5) (2014) 1276–1284. [DOI] [PubMed] [Google Scholar]
- [21].Keijsers RR, Joosten I, van Erp PE, Koenen HJ, van de Kerkhof PC, Cellular sources of IL-17 in psoriasis: a paradigm shift?, Experimental dermatology 23(11) (2014) 799–803. [DOI] [PubMed] [Google Scholar]
- [22].Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT, Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis, J Immunol 187(1) (2011) 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E, Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis, J Immunol 182(9) (2009) 5836–45. [DOI] [PubMed] [Google Scholar]
- [24].Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE, Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice, Journal of leukocyte biology 83(1) (2008) 64–70. [DOI] [PubMed] [Google Scholar]
- [25].Ikeda S, Takahashi H, Suga Y, Eto H, Etoh T, Okuma K, Takahashi K, Kanbara T, Seishima M, Morita A, Imai Y, Kanekura T, Therapeutic depletion of myeloid lineage leukocytes in patients with generalized pustular psoriasis indicates a major role for neutrophils in the immunopathogenesis of psoriasis, Journal of the American Academy of Dermatology 68(4) (2013) 609–617. [DOI] [PubMed] [Google Scholar]
- [26].Schon M, Denzer D, Kubitza RC, Ruzicka T, Schon MP, Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice, The Journal of investigative dermatology 114(5) (2000) 976–83. [DOI] [PubMed] [Google Scholar]
- [27].Heidenreich R, Rocken M, Ghoreschi K, Angiogenesis drives psoriasis pathogenesis, Int J Exp Pathol 90(3) (2009) 232–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nestle FO, Kaplan DH, Barker J, Psoriasis, The New England journal of medicine 361(5) (2009) 496–509. [DOI] [PubMed] [Google Scholar]
- [29].Albanesi C, Scarponi C, Cavani A, Federici M, Nasorri F, Girolomoni G, Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes, The Journal of investigative dermatology 115(1) (2000) 81–7. [DOI] [PubMed] [Google Scholar]
- [30].Cho KA, Suh JW, Lee KH, Kang JL, Woo SY, IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1beta by keratinocytes via the ROS-NLRP3caspase-1 pathway, International immunology 24(3) (2012) 147–58. [DOI] [PubMed] [Google Scholar]
- [31].Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y, Possible pathogenic role of Th17 cells for atopic dermatitis, The Journal of investigative dermatology 128(11) (2008) 2625–30. [DOI] [PubMed] [Google Scholar]
- [32].Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG, TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques, J Immunol 175(4) (2005) 2721–9. [DOI] [PubMed] [Google Scholar]
- [33].Baliwag J, Barnes DH, Johnston A, Cytokines in psoriasis, Cytokine 73(2) (2015) 342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, Elder JT, Zou W, Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis, J Immunol 181(7) (2008) 4733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]