Abstract
Objective:
Better characterize granular cell tumor of the bladder, with only 20 cases reported to date and unclear management guidelines.
Methods:
We report 5 benign and 1 malignant granular cell tumor of the bladder.
Results:
Patient ages ranged from 33 to 73. Tumor sizes ranged from 0.2 to 6.3 cm. Two benign granular cell tumors were incidental findings with others presenting with painless hematuria. Benign granular cell tumors infiltrated the muscularis propria, and were confirmed by immunohistochemistry for S100 protein with negative stains for keratins. The malignant granular cell tumor involved the entire bladder wall with extension into peri-vesical tissue. Benign granular cell tumors were treated by transurethral resection (TUR) or partial cystectomy; all patients were disease-free at last follow-up. The malignant granular cell tumor was treated by anterior exenteration and bilateral pelvic lymphadenectomy. This patient developed pulmonary and pleural metastases two years after surgery.
Conclusions:
Given the locally infiltrative nature of granular cell tumors and that 50% of reported benign granular cell tumors with sufficient follow-up recurred following initial TUR, it is prudent to recommend partial cystectomy if technically feasible. A later TUR at a time of tumor regrowth could result in obstruction of ureters depending on their location and with greater infiltrative growth, with larger subsequent resections be needed for complete removal. In other cases, immediate repeat TUR after a diagnosis of granular cell tumor would lessen the likelihood of local recurrence. Either partial or radical cystectomy is needed for the rare malignant granular cell tumor.
Keywords: granular cell tumor, bladder neoplasm
INTRODUCTION
Granular cell tumor is a type of peripheral nerve sheath tumor that was initially described by Abrikosoff in 1926 1. It typically involves the head and neck region where it can incite pseudoepitheliomatous hyperplasia in the overlying squamous epithelium mimicking a squamous cell carcinoma. Granular cell tumor is an extremely rare lesion in the bladder with unclear guidelines how to manage them. The vast majority cases have been benign and with only 2 malignant granular cell tumors of the bladder reported 2, 3. We present the largest series of granular cell tumor of the bladder, and also describe one malignant granular cell tumor.
MATERIAL AND METHODS
Six cases of granular cell tumor were identified, including 5 that were benign (Fig. 1) and 1 which was malignant (Fig. 2). All the H&E slides and immunohistochemistry stains were reviewed to study the morphologic features and its immunoprofile. Immunohistochemistry was performed using antibodies against keratin AE1/3 (monoclonal, clone pck-26, predilutes, Ventana, Tucson, AZ), S100 protein (monoclonal, clone 4C4.9, predilutes, Ventana, Tucson, AZ), and ki67 (monoclonal, clone 30–9, predilutes, Ventana, Tucson, AZ. S100 protein immunohistochemistry was performed, as it is the one antibody that it has the most sensitivity for granular cell tumor. Keratin AE1/3 stains were done, which are negative in granular cell tumors, and can rule our urothelial carcinoma. Clinical history and follow-up data was obtained from the treating urologists.
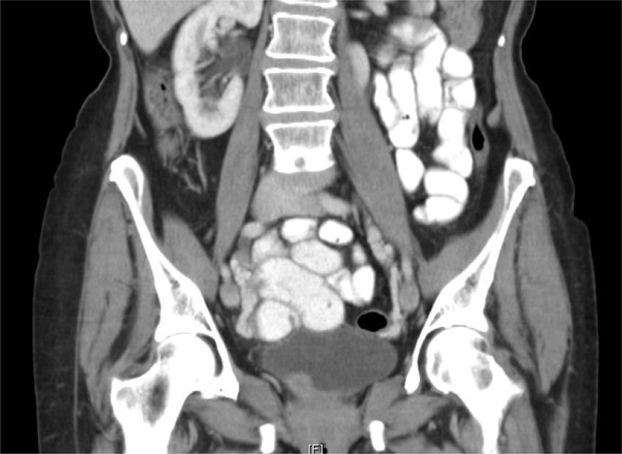
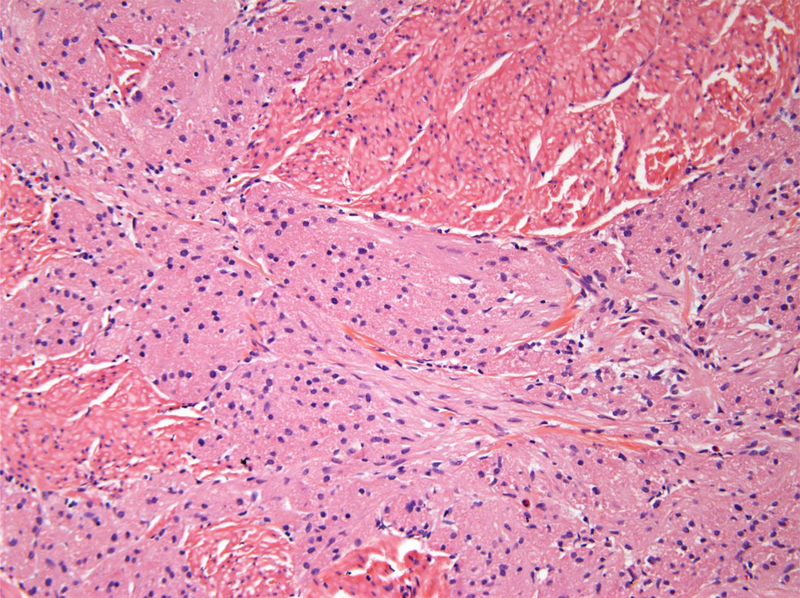
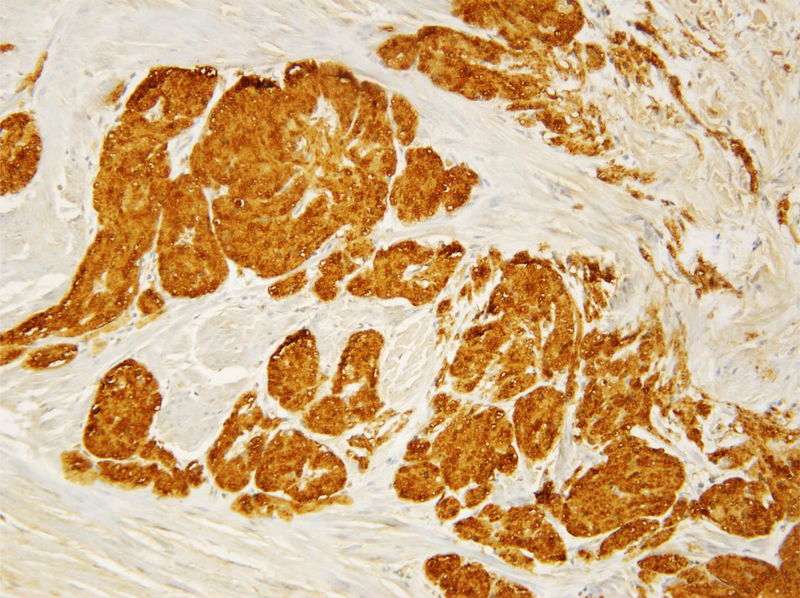
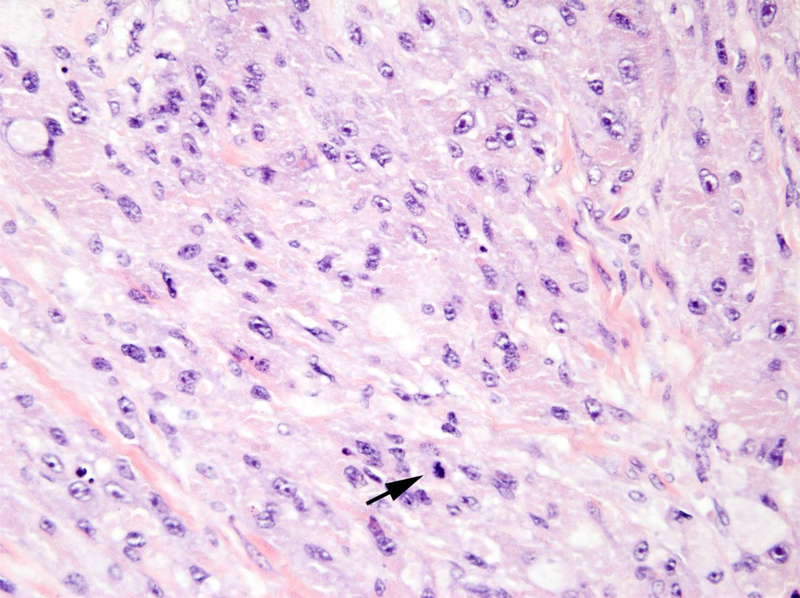
Figure 1.
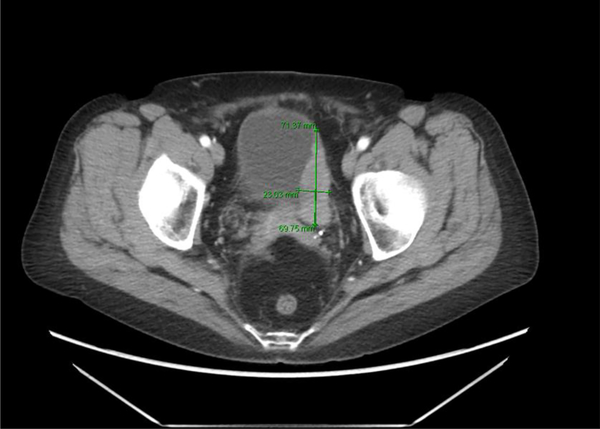
A. Benign granular fell tumors: CT showing 2 cm lesion in the right hemitrigone; B. Low magnification H&E showing cords of benign granular cell tumor infiltrating through the muscularis propria (upper right); C. High magnification H&E showing cords of pale staining benign granular cell tumor (right) infiltrating through the muscularis propria (left); D. Tumor cells are highlighted by diffuse brown positivity for S100 protein.
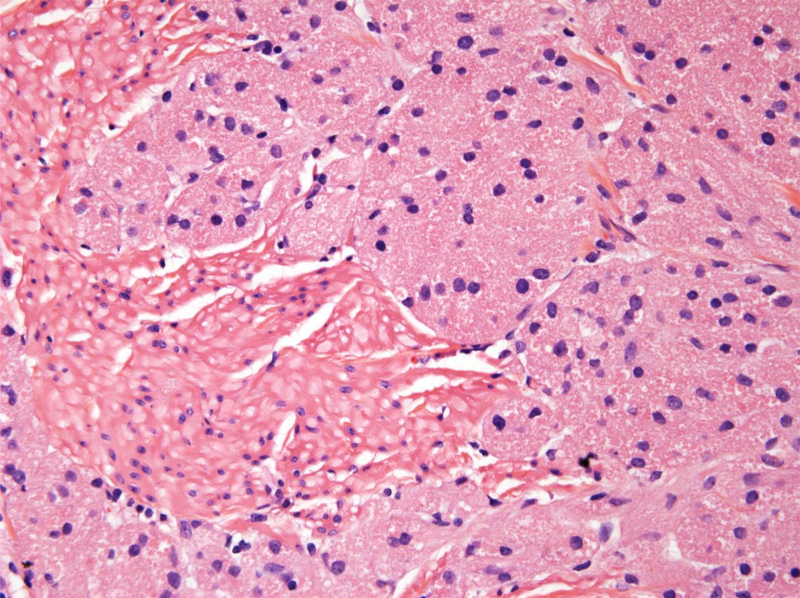
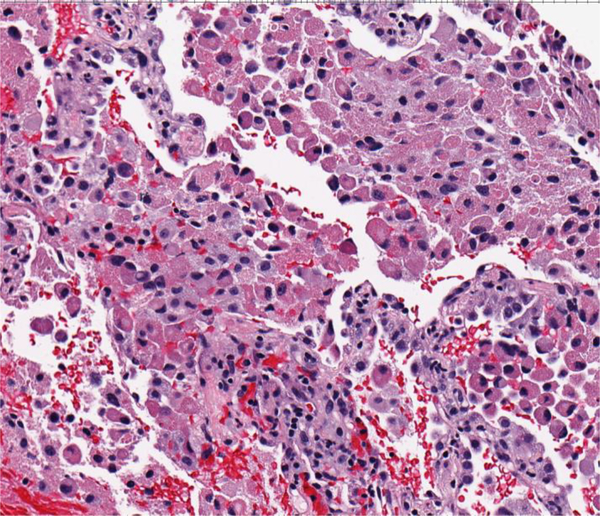
Figure 2.
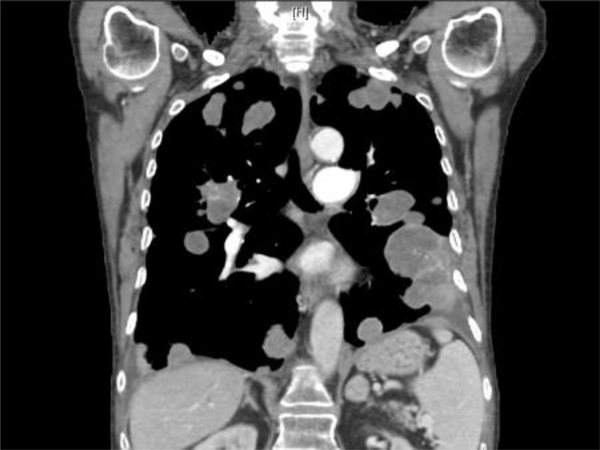
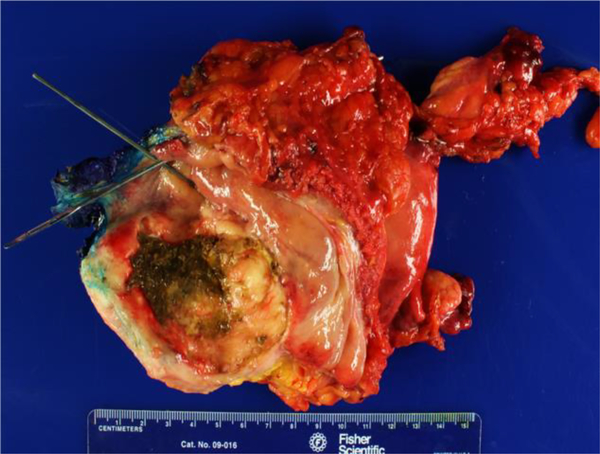
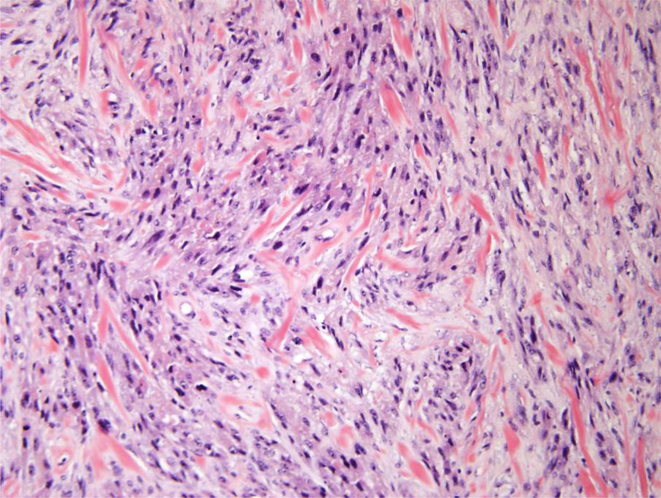
A. Malignant granular cell tumor: CT showing 5.8 cm left-sided bladder lesion; B. Multiple bilateral pulmonary and pleural metastases 2 years after radical cystectomy; C. Gross appearance of malignant granular cell in radical cystectomy consisting of a large fungating mass with necrosis (green); D. Malignant granular cell tumor showing spindling; E. Malignant granular cell tumor with greater nuclear pleomorphism consisting of prominent nucleoli and mitotic figures (arrows); F. Malignant granular cell tumor metastatic to lung.
RESULTS
Clinical Features
Patient ages ranged from 33 to 73 years of age (Table 1). Two of the benign granular cell tumors were incidental findings (0.2 and 0.4 cm) in cystectomy specimens performed for either endometriosis or neurogenic bladder. Three of the 4 remaining cases presented with painless hematuria. The 4 non-incidental cases were grossly visible bladder lesions including one malignant granular cell tumor (6.3 cm).
Table 1.
Summary of Granular cell tumor of the urinary bladder cases (present series)
| References | Age/Sex | Tumor Size | Histologic Diagnosis | Surgery | Recurrence | Follow up |
|---|---|---|---|---|---|---|
| Present series | 67/F | 63cm | MGCT | Radical cystectomy, hysterectomy, BSO, pelvic node dissection | Yes | Metastases at 2 years |
| Present series | 44/M | 3.1cm | BGCT | TUR | No | NED for 2 years |
| Present series | 33/F | 0.2 cm | BGCT | Partial cystectomy for endometriosis, malakoplakia | No | N/A |
| Present series | 42/F | 0.4 cm | BGCT | Partial cystectomy for neurogenic bladder | No | NED for 9 years |
| Present series | 49/F | 1.9 cm | BGCT | TUR | No | NED for 7 months |
| Present series | 73/F | 1.9 cm | BGCT | TUR | No | NED for 4 months |
BGCT – benign granular cell tumor; MGCT – malignant granu ar cell tumor; TUR – transurethral resection; BSO – bilateral salpingo-oophorectomy; NED – no evidence of disease; NA – not available
Imaging and Cystoscopy Findings
Imaging information was available for two benign granular cell tumors. In one case, CT showed a 1.9 cm. enhancing soft tissue nodule near the right ureteral orifice protruding into the bladder in one case (Fig. 1A). A 2 cm. nodule was palpable at the right hemitrigone on bimanual exam and an erythematous area was visible surrounding the right orifice but with no obvious tumor. In another case, CT showed a 1.9 cm. intraluminal mass along the superior right lateral aspect of the bladder.
CT review of the malignant granular cell tumor showed a 5.8 cm. left-sided bladder mass (Fig. 2A). Cystoscopy in this cases showed a large left sessile tumor engulfing the left lateral wall.
Gross Findings
Tumor sizes ranged from 0.2 to 6.3 cm. The location of the tumors in the bladder was random. Gross examination of the radical cystectomy specimen performed for the malignant granular cell tumor showed an indurated, tan-white tumor measuring 6.3 × 5.1 cm. in the left anterior and posterior bladder walls with tumor extension into the peri-vesical adipose tissue (Fig. 2C).
Histologic and Immunohistochemical Findings
The histological findings in the benign cases were typical of granular cell tumor showing nests of tumor cells with abundant granular cytoplasm. Four out of the five benign granular cell tumors infiltrated deeply into the thick muscle bundles of the muscularis propria (Figs 1B and 1C). The nuclei appeared bland without prominent nucleoli. The tumors lacked spindling with an absence of mitoses or necrosis. The overlying urothelium appeared normal without pseudocarcinomatous hyperplasia. Immunostains confirmed that the tumor cells were strongly and diffusely positive for S100 protein (Fig. 1D), while negative for pankeratin.
The malignant granular cell tumor showed marked pleomorphism, abundant mitotic figures, including atypical forms, and necrosis (Figs 2D and 2E). The tumor involved the entire bladder wall thickness, extending into peri-vesical soft tissue. The overlying urothelium showed polypoid cystitis. Immunohistochemistry showed that the tumor cells were positive for S100 protein, while negative for keratin AE1/3. The Ki67 proliferation index was approximately 10%.
Treatment and Follow-up
The five benign granular cell tumors were treated by transurethral resection (TUR) (n=3) or partial cystectomy (n=2). Margins were negative in the benign granular cell tumors treated by partial cystectomy. The margins could not be assessed histologically in the cases treated by TUR. Follow-up information was available in four out of our five benign granular cell tumors. All four cases were disease-free at last follow-up (4 months, 7 months, 2 years, and 9 years). The incidental benign granular cell tumor case treated by partial cystectomy for endometriosis was lost during follow-up. The malignant granular cell tumor case was treated by radical cystectomy with radical hysterectomy, bilateral salpingo-oophorectomy, anterior vaginectomy and bilateral pelvic lymphadenectomy in 10/2013. Surgical margins were negative for tumor. A left lung biopsy in 1/2015 showed metastatic granular cell tumor (Fig. 2F). The patient started chemotherapy with Cisplatin and Etoposide in 2/2015. CT of chest, abdomen, and pelvis showed multiple bilateral pulmonary and pleural metastases, some slightly increased on 11/14/2017 (Fig. 2B). The patient received multiple chemotherapy regimens last with Gemcitabine on 11/27/2017.
COMMENT
Granular cell tumors are rare lesions of the bladder with a spectrum from benign to atypical to malignant. Review of the literature reveals only 20 prior reported cases of granular cell tumor of the bladder (Table 2). The vast majority cases have been benign and with only 2 malignant granular cell tumors of the bladder reported 2,3. Atypical histological features include spindling, increased mitotic figures (>2/10 high power fields), nuclear pleomorphism, prominent nucleoli, high nuclear to cytoplasmic ratio, and necrosis 4. Malignant granular cell tumor can be diagnosed in the presence of three or more of these features. Benign granular cell tumors demonstrate ≤1% ki67 proliferation rate, whereas a little over 50% of malignant granular cell tumors have ki67 rates of 10% or higher.4 In the current series, the one case of malignant granular cell tumor had a lesion is diagnosed as an “atypical granular cell tumor”. Pleomorphism by itself in a granular cell tumor is still consistent with a benign lesion. Adipose tissue can be seen at all levels of the bladder from the lamina propria to the peri-vesicle soft tissue, such that invasion of fat on TUR is not diagnostic of peri-vesicle invasion and should not be mentioned in the report. Similarly, muscularis propria invasion is common in granular cell tumor and not an indication of malignancy. Either partial or radical cystectomy is needed for the rare malignant granular cell tumor.
Table 2.
Summary of granular cell tumor of the urinary bladder cases
| References | Age/Sex | Tumor Size | Histologic Diagnosis | Surgery | Recurrence | Follow up |
|---|---|---|---|---|---|---|
| Ravich A, 1945 [2] | 31/M | 12 cm | MGCT | Partial cystectomy | Yes | Metastases at 17 month |
| Abbas F, 2007 [3] | 47/F | 4 cm | MGCT | Radical cystectomy, hysterectomy, BSO, pelvic node dissection | No | NED for 4 years |
| Anderson R, 1961 [5] | 61/M | N/A | BGCT | TUR | No | NED for 2 years |
| Seery WH, 1968 [6] | 31/M | 7 cm | BGCT | Partial cystectomy | No | NED for 18 years |
| Okuda N, 1969 [7] | 49/F | N/A | BGCT | Partial cystectomy | No | NED for 1 year |
| Christ ML, 1971 [8] | 23/F | 9 cm | BGCT | Treatment not specified | N/A | N/A |
| Mouradian JA, 1974 [9] | 26/F | 5 cm | BGCT | TUR x 3 | Yes | Recurrence at 10 and 17 months, NED for 2.5 years after 3rd TUR |
| Flecher MS, 1985 [10] | 48/M | 2 cm | BGCT | TUR | No | N/A |
| Kontani K, 1999 [11] | 59/F | 5 cm | BGCT | TUR x 2 | Yes | Recurrence at 7 months, NED for 1.5 years after 2nd TUR |
| Yoshida T, 2001 [12] | 48/M | 2 cm | BGCT | TUR x 2 | Yes | Recurrence at 6 months, NED for 3 years after 2nd TUR |
| Kondo T, 2006 [13] | 55/M | 1.5 cm | BGCT | Partial cystectomy | No | NED for 3 years |
| Mogorovich A, 2007 [14] | 44/F | N/A | GCT | TUR | No | NED for 6 months |
| Eandi JA, 2007 [15] | 43/M | 1.5 cm | BGCT | Radical cystectomy, pelvic node dissection for adenocarcinoma | No | NED for 2 years |
| Abbas F, 2007 [3] | 14/F | 10 cm | BGCT | TUR | No | NED for 4 years |
| Kang H, 2010 [16] | 67/M | 1 cm | BGCT | TUR | No | NED for 6 months |
| Wang A, 2011 [17] | 23/F | 3 cm | BGCT | TUR | No | NED for 6 months |
| Knief J, 2012 [18] | 68/M | 1 cm | BGCT | TUR | No | NED for 2 months |
| Olaya M, 2013 [19] | 54/F | N/A | GCT | TUR | N/A | N/A |
| Bedir R, 2017 [20] | 35/F | 1.7 cm | BGCT | TUR | N/A | N/A |
| Yarlagadda VK, 2017 [21] | 37/M | N/A | BGCT | Partial cystectomy | No | NED for 1 year |
BGCT – benign granular cell tumor; MGCT – malignant granular cell tumor; TUR – transurethral resection; BSO – bilateral salpingo-oophorectomy; NED – no evidence of disease; N/A – not available
The pitfall is to misdiagnose granular cell tumors of the bladder as nested variant of invasive urothelial carcinoma, in part because of the rarity of granular cell tumor in the bladder and not considering it in the differential diagnosis. Positive immunohistochemistry for S100 protein with negative stains for keratins can confirm the diagnosis. Malignant granular cell tumor could also be confused with melanoma due to the S100 protein and Sox10 immunoreactivity. Negative stains for HMB45 are helpful for excluding melanoma. Partial cystectomy or radical TUR with clear clinical margins is recommended for benign granular cell tumor. Three cases in the literature showed local recurrences of benign granular cell tumor, one at 6 months, one at 7 months, and one with two recurrences at 10 and 17 months. All three cases had no evidence of disease following their last TUR at 3 years, 1.5 years, and 2.5 years, respectively. The other 3 cases with no recurrences following initial TUR and a minimal 6 month follow-up were followed for 2 years, 2 years, and 4 years.
CONCLUSIONS
Given the locally infiltrative nature of granular cell tumors and that 50% of benign granular cell tumors with sufficient follow-up recurred following initial TUR, it would seem prudent to recommend partial cystectomy if technically feasible. As granular cell tumors of the urinary bladder are rare, they are typically reported with short follow-up, such that their long-term growth is not known. However, these lesions in other organs are characterized by persistent growth if incompletely excised. In other cases, immediate repeat TUR after a diagnosis of granular cell tumor would lessen the likelihood of local recurrence. A later TUR at a time of tumor regrowth could result in increased morbidity depending on the location within the bladder. Recurrent tumors could lead to obstruction of ureters depending on their location and with greater infiltrative growth, larger subsequent resections would be needed for complete removal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abrikossoff A. Uber Myome ausgehend von der quergestreiften Willkurlichen muskulatur. Virchows Arch Pathol Anal 1926; 260: 215–33. [Google Scholar]
- 2.Ravich A, Stout AP, Ravich RA. Malignant granular cell myoblastoma involving the urinarybladder. Ann Surg 1945; 121: 361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas F, Memon A, Siddiqui T, et al. Granular cell tumors of the urinary bladder. World J Surg Oncol 2007; 5: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanburg-Smith JC, Meis-Kindblom JM, Fante R, et al. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol 1998; 22: 779–94. [DOI] [PubMed] [Google Scholar]
- 5.Andersen R, Hoeg K. Myoblastoma of the bladder neck: report of a case. Br J Urol 1961; 33: 76–9. [DOI] [PubMed] [Google Scholar]
- 6.Seery WH. Granular cell myoblastoma of the bladder: report of a case. J Urol 1968; 100: 735–7. [DOI] [PubMed] [Google Scholar]
- 7.Okuda N, Okawa T, Nakamura T, et al. Granular cell myoblastoma of the urinary bladder: report of a case. Hinyokika Kiyo 1969; 15: 505–13. [PubMed] [Google Scholar]
- 8.Christ ML, Ozzello L. Myogenous origin of a granular cell tumor of the urinary bladder. Am J Clin Pathol 1971; 56: 736–49. [DOI] [PubMed] [Google Scholar]
- 9.Mouradian JA, Coleman JW, McGovern JH, et al. Granular cell tumor (myoblastoma) of the bladder. J Urol 1974; 112: 343–5. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher MS, Aker M, Hill JT, et al. Granular cell myoblastoma of the bladder. Br J Urol 1985; 57: 109–10. [DOI] [PubMed] [Google Scholar]
- 11.Kontani K, Okaneya T, Takezaki T. Recurrent granular cell tumour of the bladder in a patient with von Recklinghausen’s disease. BJU Int 1999; 84: 871–2. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Hirai S, Horii Y, et al. Granular cell tumor of the urinary bladder. Int J Urol 2001; 8: 29–31. [DOI] [PubMed] [Google Scholar]
- 13.Kondo T, Kajimoto S, Okuda H, et al. A case of granular cell tumor of the bladder successfully managed with extraperitoneal laparoscopic surgery. Int J Urol 2006; 13: 827–8. [DOI] [PubMed] [Google Scholar]
- 14.Mogorovich A, Giannarini G, De Maria M, et al. Granular cell tumour of the urinary bladder: a case report and review of the literature. Arch Ital Urol Androl 2007; 79: 43–4. [PubMed] [Google Scholar]
- 15.Eandi JA, Asuncion A, Vandewalker KN, et al. Granular cell tumor of the urinary bladder with pseudoepitheliomatous hyperplasia and colocalization with adenocarcinoma. Int J Urol 2007; 14: 862–4. [DOI] [PubMed] [Google Scholar]
- 16.Kang H, Kim Y, Ha Y, et al. Granular cell tumor of the urinary bladder. Korean J Urol 2010; 51: 291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Xu Y, Huang T. Granular cell tumor of the urinary bladder: A case report. Chin -Ger J Clin Oncol 2011; 10: 737–8. [Google Scholar]
- 18.Knief J, Becker JU, Kreipe H, et al. Granular cell tumour of the urinary bladder. Rare Tumors 2012; 4: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olaya M, Vicioso L, Hierro I, et al. Granular cell tumor of the bladder: a case report. Anal Quant Cytopathol Histpathol 2013; 35: 289–93. [PubMed] [Google Scholar]
- 20.Bedir R, Yilmaz R, Özdemir O, et al. Granular cell tumor of the urinarybladder. Turk J Urol 2017; 43: 383–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarlagadda VK, Benson DG, Gordetsky JB, et al. Granular cell tumor of the bladder: A rare neoplasm managed with robotic partial cystectomy using near-infrared filter guidance. Urology 2017; 103: 7–11. [DOI] [PubMed] [Google Scholar]