Abstract
Background:
Sex differences exist in asthma susceptibility and severity. Accumulating evidence has linked airway microbiome dysbiosis to asthma, and airway microbial communities have been found to differ by sex. However, whether sex modifies the link between airway microbiome and asthma has not been investigated.
Objective:
To evaluate sex effects in the association between airway microbiome and asthma.
Methods:
We analyzed induced sputum samples from 47 subjects (n = 23 patients with asthma and n = 24 normal controls) using 16S ribosomal RNA gene sequencing methods. The bacterial composition was analyzed for sex differences. Bacterial associations with asthma were assessed for each sex at the core taxa and genus levels.
Results:
The microbiome in induced sputum differed in women vs men at the community level. A total of 5 core bacterial taxa were found in all samples. No sex-specific core taxa were detected. The most abundant core taxon, Streptococcus salivarius, was significantly enriched in women than in men (P = .02). Within each sex, individuals with relatively lower abundance of S salivarius were more likely to have asthma (P = .006). For both sexes, increased Lactobacillus species were found in sputum samples of patients with patients compared with normal controls (adjusted P = .01). Haemophilus species were associated with asthma in men and not in women.
Conclusion:
The airway microbiome differed by sex, and sex effects exist in the association of airway microbial markers and asthma. Future airway microbiome studies may yield better resolution if the context of specific sex is considered. The airway microbiome is a potential mechanism driving sex differences in asthma.
Introduction
Sex differences have been well established in asthma. Asthma is more prevalent in boys than girls during childhood, whereas asthma is more prevalent in women than men after puberty.1,2 As adults, women are more severely affected by asthma and are more likely to be hospitalized than men for an asthma-related event.3–5 Previous studies have revealed that associations of asthma with major host factors, such as hormones, immune responses, airway anatomy, and genetics, could be influenced by sex.6–10 This indicates that sex effects may exist extensively in the underlying pathways of asthma development and exacerbations.
Over the past 10 years, studies from multiple laboratories have helped highlight the important correlation between dysbiosis of airway microbiome and asthma.11–13 Altered airway microbial composition is proposed to have an essential role in asthma-related immune responses, clinical phenotypes, and medication responsiveness.14–16 Cross-sectional and family-based studies comparing the airway microbes of patients with asthma and those of healthy controls have yielded broad bacterial community differences, which may be largely owing to the heterogeneity of asthma implying that different pathogenic pathways underpinning asthma could involve different microbes.14,17–19 Indeed, a large body of studies over the past decade have emphasized the intimate associations between differential patterns of airway microbial composition and phenotypic features of asthma in adults.12,14,18,20 Because clinical expressions of asthma are intrinsically linked to sex, and the Human Microbiome Project data have revealed marked sex differences in the airway microbial communities, it is of importance to elucidate sex effects modifying the established relationship between airway microbiome and asthma.21,22
Here, we performed a study of the airway microbial communities in 47 subjects (n = 23 patients with asthma and n = 24 controls). We analyzed 16S ribosomal RNAs in induced sputum samples, compared sex-related differences in airway microbiomes, and compared sex-specific associations between airway microbiome and asthma at core taxa and genus levels.
Methods
Study Participants
We recruited subjects and collected induced sputum samples at the Washington University School of Medicine, St. Louis, Missouri. All participants provided written informed consent for sample use in future analyses, and all subjects with adequate sputum samples for microbiome sequencing were included in this analysis. This study was reviewed and approved by the Washington University Human Research Protection Office (IRB# 201810123). Inclusion criteria for patients with asthma were as follows: (1) aged 18 to 60 years and (2) physician-diagnosed asthma. Exclusion criteria for patients with asthma were as follows: (1) pregnant; (2) considerable bronchospasm before starting the sputum-induction procedure; (3) physician-diagnosed emphysema or chronic obstructive pulmonary disease; (4) history of respiratory failure requiring intubation; (5) bronchial or upper respiratory tract infection or evidence of a sinus infection within 1 month before screening; (6) antibiotic use within 1 month before sputum induction; and (7) current smoker or smoked within the previous year. Inclusion criteria for normal controls were as follows: (1) aged 18 to 60 years and (2) no history of physician-diagnosed asthma or asthma symptoms. Exclusion criteria for normal controls were as follows: (1) pregnant; (2) asthma diagnosis or evidence of asthma symptoms; (3) physician-diagnosed emphysema or chronic obstructive pulmonary disease; (4) history of respiratory failure requiring intubation; (5) bronchial or upper respiratory tract infection or evidence of a sinus infection within 1 month before screening; (6) antibiotic use within 1 month before sputum induction; (7) current smoker or smoked within the previous year; and (8) use of any corticosteroid within 1 month before screening.
Sputum Induction Procedures
Study participants were invited into the Washington University School of Medicine to fill out a questionnaire and perform maximum bronchodilator reversibility tests (spirometry before and after 4 puffs of albuterol). If the postalbuterol baseline forced expiratory volume in 1 second is greater than or equal to 50% of the predicted value, the sputum induction can proceed. Similar to the previously described procedure, the subjects inhaled nebulized 3% saline through a mouthpiece with an interruption of every 2 minutes or less for a peak flow measurement.17 If the participant felt a need to cough or swallow at any time during the induction, he or she was instructed to clear saliva by spitting the saliva into the saliva container and to vigorously cough and spit everything into the sputum container. At least 1 mL of induced sputum was collected for each subject. The induced sputum sample was placed on ice immediately after collection.
Sputum Microbiome Extraction and Sequencing Analysis
The sputum sample was mixed with an equivalent volume of dithiothreitol (1:9 v/v, dithiothreitol:phosphate buffered saline) and shaken on ice for 15 minutes. The samples were then stored at −80°C until use. Total genomic DNA was extracted from 250 μL of the stored sputum samples using the ZymoBIOMICS DNA Kit (Zymo Research, Irvine, California) according to the manufacturer’s instructions. A sequencing library was prepared from the sputum genomic DNA extract. Briefly, the V1 to V2 region of the 16S ribosomal RNA gene was amplified and barcoded using the Quick-16S NGS Library Prep Kit (Zymo Research, Irvine, California). Samples were pooled and sequenced using the Illumina MiSeq platform (Washington University Center for Genome Sciences; 2 × 250 standard run). The forward reads from demultiplexed fastq files were used to infer the amplicon sequencing variances (ASVs). The process was performed with the DADA2 R package. Quality visualization and control were performed by trimming out the lengths that fell below a quality threshold of 25. The Ribosomal Database Project’s Training Set 16 was used as reference for taxonomy assignments. The ASV counts, taxonomy assignments, phylogeny, and sample metadata were combined to generate a PhyloSeq object. The Shannon index, Bray-Curtis distance between each pair of samples, and principal components were obtained from the DATA 2 pipeline in R.
Statistical Analysis
The alpha diversity of the microbial community was measured using the Shannon index.23 Principal coordinate analysis of samples according to Bray-Curtis distance was used to evaluate overall differences in beta-diversity between bacterial communities. The analysis of similarities test was performed to analyze the significance of compositional differences. ASV count data were analyzed using a generalized linear model based on the negative binomial distribution. The DESeq2 R package was used to define contrast vectors to assess the significance of differences between sample groups and determine the differential abundance of taxa.24 The resulting P values were adjusted for multiple testing according to the Benjamini-Hochberg procedure. The relative abundance of taxa was evaluated, and Wilcoxon rank-sum test was used to evaluate the statistical significance. We used R version 3.4.2 for statistical analyses and ggplot2 for visualization. The α values of less than .05 were considered as statistically significant.
Results
The Airway Microbial Community Differed in Women and Men
Based on the inclusion and exclusion criteria, all the participants were nonsmokers and not exposed to antibiotics during the past 1 month before sputum sample collection. The participants’ demographic details are listed in Table 1. There is no marked difference in age, race, body mass index, or atopy between sex. Asthma symptoms as measured by the score on the asthma control test are similar in both sexes.
Table 1:
Study Cohort Characteristics
| Characteristic | Women (n = 32) |
Men (n = 15) |
P valuea | ||
|---|---|---|---|---|---|
| Asthma (n = 17) | Normal (n = 15) | Asthma (n = 6) | Normal (n = 9) | ||
| Age | 46.53 (9.18) | 47.47 (11.01) | 42.50 (14.90) | 46.44 (11.73) | .58 |
| Race, AA (%) | 13 (76) | 8 (53) | 5 (83) | 3 (33) | .42 |
| BMI (SD) | 33.26 (8.15) | 30.59 (7.16) | 36.13 (2.97) | 28.52 (2.68) | .81 |
| ACT score (SD) | 17.12 (5.40) | NA | 17.17 (3.97) | NA | >.99 |
| Atopy (%)b | 14 (82) | 8 (53) | 6 (100) | 4 (44) | .89 |
| FEV1/FVC, % (SD) | 70.43 (11.76) | 80.66 (6.95) | 61.46 (13.06) | 78.67 (4.51) | .52 |
Abbreviations: AA, African American; ACT, Asthma Control Test; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NA, not applicable.
t test or χ2 test was used to calculate P values by sex.
Atopy was self-reported.
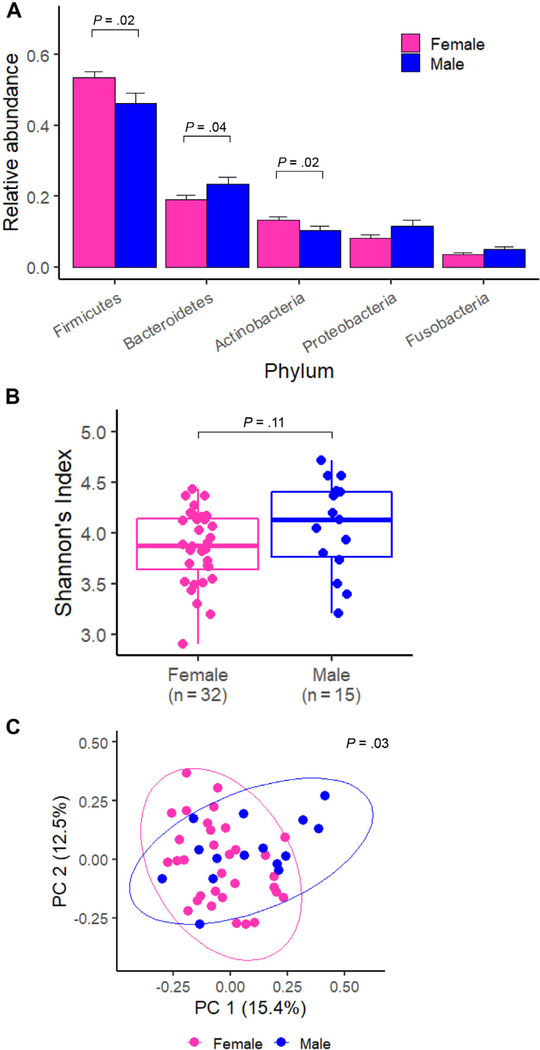
A total of 1759 ASVs were detected in the 47 sputum samples: 693 ASVs appeared in both sexes, 609 ASVs appeared only in women, and 457 ASVs appeared only in men. The 693 ASVs that appeared in both sexes accounted for most of the taxa in sputum samples (mean of 94.2% across all samples), and there were no significant differences between men and women (P = .60, Wilcoxon rank-sum test). The airway bacterial profiles of each subject are presented at the phylum level in eFigure 1. The top 5 most abundant bacterial phyla were Firmicutes (52.0%), Bacteroidetes (20.5%), Actinobacteria (11.9%), Proteobacteria (8.9%), and Fusobacteria (4.1%). We observed sex-based differences at the phylum level (Fig 1A); women had significantly more Firmicutes (P = .02) and Actinobacteria (P = .02) than men, whereas men had significantly more Bacteroidetes than women (P = .04; Fig. 1 and eFig 1).
Figure 1.
Differences of the sputum microbiome by sex in major bacterial phyla and at the community level. A, Relative abundance of the top 5 most abundant bacterial phyla for women and men. Statistical significance was assessed by Wilcoxon rank-sum test. B, Alpha diversity (Shannon index) in women and men. Statistical significance was assessed by Wilcoxon rank-sum test. C, PC analysis plot of Bray-Curtis distances. Statistical significance was assessed by the ANOSIM test. ANOSIM, analysis of similarities; PC, principal component.
The alpha diversity (represented by Shannon Diversity Index) of airway microbiomes was numerically higher in men than women, although this difference was not statistically significant (Fig 1B). This trend was consistent with the results from the Human Microbiome Project and was the case in either asthma or normal group22 (eFig 2A and B). Patients with asthma have relatively higher alpha diversity than normal controls, although this difference was not statistically significant (eFig 2C). In addition, we did not detect marked differences of the alpha diversity by race (eFig 2D). The beta-diversity that was evaluated on the basis of Bray-Curtis pair-wise distances was found in the plot of principal component analysis (Fig 1C). The overall microbial populations were significantly different in men and women (P = .03; eFig 2).
The Core Taxa, Streptococcus salivarius, Was Enriched in Women vs Men and Lower Relative Abundance of S salivarius Was Associated With Asthma in Both Sexes
We identified 5 core ASVs that appeared across all 47 sputum samples and accounted for an average of 28.63% of all sputum taxa. No sex-specific or asthma-specific core species were identified. Of the 5 core taxa, 3 were assigned to Streptococcus species (spp.), 1 was assigned to Veillonella spp., and 1 was assigned to Granulicatella spp. We performed comparative analysis of these sequences using the 16S ribosomal RNA database in the National Center for Biotechnology Information and further classified the most abundant taxa to S salivarius with 100% identity (eTable 1).
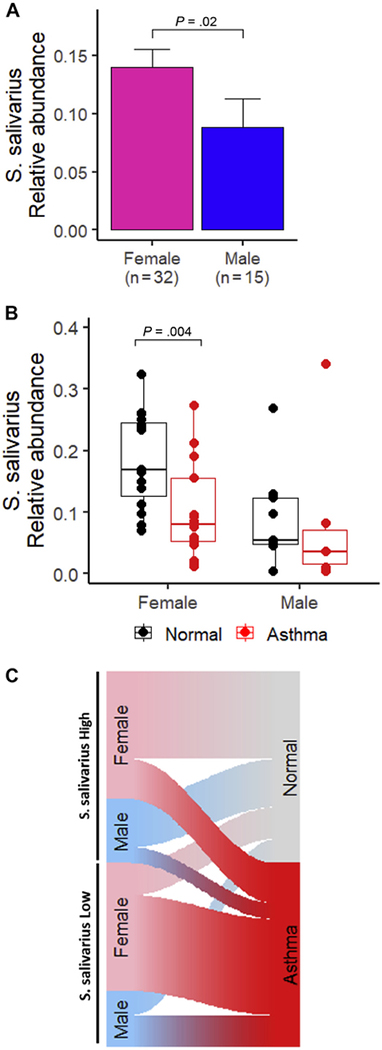
The relative abundance of S salivarius significantly differed in sputum samples of women and men (P = .02, Wilcoxon rank-sum test). The mean relative abundance of S salivarius in women and men were 14.0% and 8.8%, respectively (Fig 2A). Female patients with asthma had significantly lower relative abundance of S salivarius than normal controls in the sputum samples (P = .003, Wilcoxon rank-sum test, Fig 2B). Men exhibited the same trend as women, although the difference was not significant (P = .27). We used the sex-specific median value of S salivarius relative abundance to categorize individuals into the following 2 groups: high S salivarius abundance or low S salivarius abundance. Analysis of these 2 groups indicated that individuals with low S salivarius abundance were more likely to be patients with asthma than individuals with high S salivarius abundance (P = .006, χ2 test, Fig 2C). To determine whether race was a potential confounder in our findings, we evaluated the relative abundance of S salivarius in different race groups. The mean and SD of S salivarius relative abundance in African American (n = 29) and non–African American races (n = 17 White and n = 1 Asian) were 12.73% and 11.74%, respectively. There was no significant association between S salivarius and race (P = .57, Wilcoxon rank-sum test). eFigure 3 displays the relative abundance of S salivarius by race and sex (Fig. 2 and eFig 3).
Figure 2.
Analysis of relative Streptococcus salivarius abundance based on sex and asthma status. A, Comparative analysis of S salivarius in female and male sputum samples. Statistical significance was assessed by Wilcoxon rank-sum test. B, Comparative analysis of S salivarius in normal controls and women and men with asthma. Statistical significance was assessed by Wilcoxon rank-sum test. C, Sankey plot revealing the relationship between S salivarius abundance and asthma.
Lactobacillus spp. Were Associated With Asthma in Both Sexes and Haemophilus spp. Were Detected To Be With Asthma in Men and Not Women
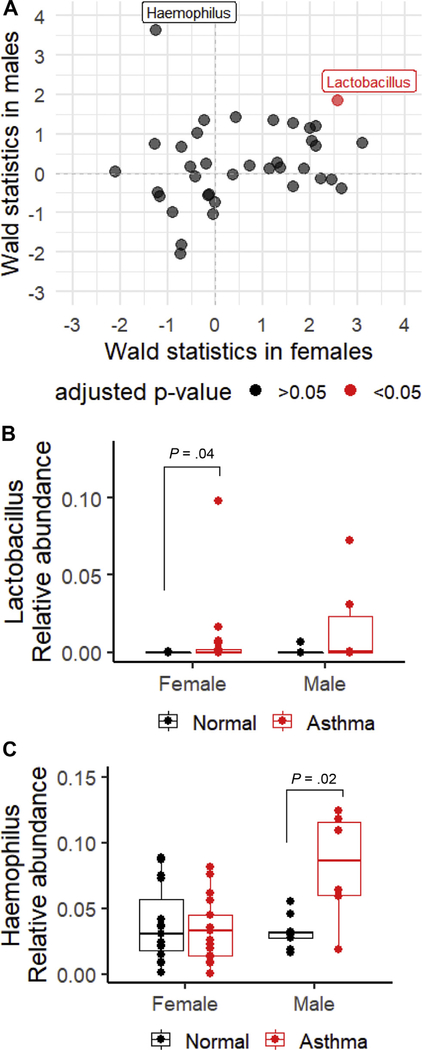
To evaluate sex differences in the association between bacterial genera in the sputum and asthma, we performed comparative analyses of taxonomic profiles in women, men, and both women and men using DESeq2 R package. We displayed results for all the genera with prevalence greater than 20% and mean relative abundance greater than 0.1% in Figure 3A. Each data point represents a bacterial genus. The x-axis revealed statistics of the Wald’s test calculated from a linear regression model only including female subjects.24 The y-axis revealed statistics of the same test but only including male subjects. The significance of the association indicated by color was based on adjusted P values calculated from a linear regression model, including both sexes. Most associations did not reach significant level, but we did identify that Lactobacillus spp. were significantly enriched in patients with asthma than in normal controls (adjusted P = .01), with same association directions and similar effect sizes for men and women. Many bacterial genera have different effect sizes in their associations with asthma between men and women. As pointed in Figure 3A, Haemophilus spp. were identified in association with asthma, but this relationship was detected only in men (Fig. 3).
Figure 3.
Association of bacterial genera (prevalence is greater than 20% and mean abundance is greater than 0.1%) with asthma. A, Plot of the Wald’s statistics to indicate associations of bacterial genera and asthma among women (x-axis) and men (y-axis). Each data point represents a bacterial genus. The analysis was performed using the GLM in the DESeq2 package in R. B, Relative abundance of Lactobacillus spp. in normal controls and patients with asthma based on sex. Statistical significance was assessed by Wilcoxon rank-sum test. C, Relative abundance of Haemophilus spp. in normal controls and patients with asthma based on sex. Statistical significance was assessed by Wilcoxon rank-sum test. GLM, generalized linear model; spp., species.
The identified associations were evaluated by plotting the relative abundances. Figure 3B revealed that the relative abundance of Lactobacillus spp. was higher in patients with asthma than in normal controls in both sexes, and this trend was significant in women (P = .04). In addition, Lactobacillus spp. were found with greater frequencies in the patients with asthma. A total of 11 of 23 patients with asthma had Lactobacillus spp., whereas only 4 of 24 control subjects had Lactobacillus spp. in sputum samples. Figure 3C shows that the relative abundance of Haemophilus spp. was significantly higher in men with asthma than in male normal controls (P = .02), but this was not the case in women. To evaluate whether race is a confounder in our analysis results, we evaluated the relative abundance of Lactobacillus spp. and Haemophilus spp. by race and asthma. Each race group exhibited the same trends (eTable 2 and eTable 3).
Discussion
Sex-based asthma susceptibility or mortality differences could be largely attributable to innate and adaptive immunity.25 The airway microbiome plays an important role in modulating the respiratory immune responses and is postulated to be a mechanism underlying immunity-mediated sex disparities in asthma. However, currently, there is little research to support this important hypothesis. We performed a cross-sectional study using sputum samples from patients with asthma and normal individuals as controls to reveal sex effects behind the associations between airway microflora and asthma for the first time. Our study confirmed a previous finding that the composition of airway bacteria in men and women differed. We further reported the sex differences at phylum level and in beta-diversity. Men and women shared core taxa in the sputum samples. S salivarius was the most abundant taxon and it was more abundant in women than men. Individuals of either sex with relatively lower abundance of S salivarius were more likely to have asthma. In addition, we detected that Lactobacillus spp. were associated with asthma in both sexes, whereas Haemophilus spp. was associated with asthma only in men.
To our knowledge, this study is the first to characterize airway core taxa and their correlation with asthma. Our results revealed that S salivarius was 100% prevalent in sputum samples with different abundances in men and women. In women, patients with asthma had significantly lower relative abundance of S salivarius than normal controls in the sputum samples (P = .004). In men, the same trend was observed, but it was not statistically significant. We then applied a sex-specific normalization (median value of the relative S salivarius abundance in each sex) to individuals within each sex and identified a strong correlation between this core taxon and asthma: individuals with relatively lower S salivarius abundance were more likely to have asthma than those with relatively higher S salivarius abundance. S salivarius is an α-hemolytic streptococci and an early colonizer in the human airway.26–28 The presence of S salivarius is suggestive of healthy airway flora with desirable characteristics, such as production of bacteriocin-like peptides and disruption of pathogenic biofilm formation.28,29 The S salivarius abundance in the oropharyngeal microbiome of patients with severe asthma increased approximately 5 times after a 6-month treatment with azithromycin.30 Azithromycin is a macrolide antibiotic with broad-spectrum activity against many microorganisms, suggesting the observed changes in S salivarius abundance in their study may result directly from antibacterial activity. However, macrolides also exert immunomodulatory and anti-inflammatory effects, and they provide benefits for patients with a variety of inflammatory airway diseases.31–33 Therefore, azithromycin therapy may mitigate airway inflammation and thereby favor the growth of specific taxa, such as S salivarius. All our subjects had no exposure to antibiotics for at least 1 month before sputum induction. Our results indicated that a typically abundant airway taxon, S salivarius, could have essential roles in shaping and indicating the local immunity and disease status. Further studies are needed to better understand the mechanism and its role in sex differences of asthma prevalence.
We deciphered the sex-specific relationships between bacterial genera in the sputum and asthma. We identified that Lactobacillus spp. was robustly associated with asthma in both men and women. This finding is consistent with previous reports that Lactobacillus spp. were enriched in patients with asthma than healthy controls from analysis using either the upper or the lower airway sampling methods.34–36 Of note, we observed that the relative Haemophilus spp. abundance was higher in male patients with asthma than healthy controls, but this significant association was not observed in women in our cohort. Haemophilus spp. have been found to be positively associated with different phenotypes of asthma, but no studies have specifically evaluated a sex effect.11,14,37,38 However, 1 study did notice that among patients with asthma, the Haemophilus-dominant group was biased toward men.38 Our finding is in agreement with this previous observation and suggests that this association could be primarily in men.
We recognized several limitations in this study. First, this study is limited by the relatively small number of subjects, especially men. This generally reduced the power and increased bias in comparing sex-based relationships between asthma and airway microbiome. In addition, the male patients with asthma are mainly African American (83%), not well representing the diverse asthma population; hence, the results should be interpreted and generalized with caution. More investigations in larger cohorts are required to further evaluate the sex effects. Second, recruitment of patients with asthma was not limited to specific clinical phenotypes or treatment. Therefore, some previously reported microbe-asthma associations in different clinical phenotypes of asthma were not being detected and evaluated regarding sex effects. As we revealed sex effects on the airway microbiome and a general asthma population, more studies targeted at specific asthma phenotypes are clearly needed to further elucidate the sex influences. Third, the microbial community from induced sputum has been suggested to be superior to that from nasal brush or from oral wash to represent lower airway microbiota composition.39 However, although compositionally similar to the bronchial microbiota, induced sputum samples usually have enriched oral bacteria owing to the nature of collection methods. Therefore, findings from our study should be assessed in more specific sampling methods, such as bronchial brushing, to confirm the associations of microbiome and asthma in the lower airway.
In summary, we evaluated sex differences in the airway microbiome and highlighted that sex affected the associations between airway microbiome and asthma. Our results suggest that future airway microbiome studies may yield better resolution if the context of specific sex is considered. Furthermore, the airway microbiome is a potentially important mechanism driving sex differences in asthma.
Supplementary Material
Acknowledgments
The authors appreciate the scientific advice from Dr Gregory Storch (Washington University in St. Louis, Missouri) and Dr Andrew Kau (Washington University).
Funding Sources: This work was supported by the National Institutes of Health grant 5R21AI139649-02 (to Drs Leyao Wang and Liang Shan).
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2020.09.007.
References
- 1.Shah R, Newcomb DC. Sex bias in asthma prevalence and pathogenesis. Front Immunol. 2018;9:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pignataro FS, Bonini M, Forgione A, Melandri S, Usmani OS. Asthma and gender: the female lung. Pharmacol Res. 2017;119:384–390. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Stewart P, Johansen H, McRae L, Taylor G. Sex difference in hospitalization due to asthma in relation to age. J Clin Epidemiol. 2003;56(2):180–187. [DOI] [PubMed] [Google Scholar]
- 4.Hyndman SJ, Williams DR, Merrill SL, Lipscombe JM, Palmer CR. Rates of admission to hospital for asthma. BMJ. 1994;308(6944):1596–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992;268(24):3437–3440. [PubMed] [Google Scholar]
- 6.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laffont S, Guéry JC. Deconstructing the sex bias in allergy and autoimmunity: from sex hormones and beyond. Adv Immunol. 2019;142:35–64. [DOI] [PubMed] [Google Scholar]
- 8.Chang JC, Liu CA, Chuang H, et al. Gender-limited association of cytotoxic Tlymphocyte antigen-4 (CTLA-4) polymorphism with cord blood IgE levels. Pediatr Allergy Immunol. 2004;15(6):506–512. [DOI] [PubMed] [Google Scholar]
- 9.Yang KD, Liu CA, Chang JC, et al. Polymorphism of the immune-braking gene CTLA-4 (+49) involved in gender discrepancy of serum total IgE levels and allergic diseases. Clin Exp Allergy. 2004;34(1):32–37. [DOI] [PubMed] [Google Scholar]
- 10.Munakata M, Ohe M, Homma Y, Kawakami Y. Pulmonary dysanapsis, methacholine airway responsiveness and sensitization to airborne antigen. Respirology. 1997;2(2):113–118. [DOI] [PubMed] [Google Scholar]
- 11.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung KF. Potential role of the lung microbiome in shaping asthma phenotypes. Ann Am Thorac Soc. 2017;14(suppl 5):S326–S331. [DOI] [PubMed] [Google Scholar]
- 14.Durack J, Lynch SV, Nariya S, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2017;140(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goleva E, Jackson LP, Harris JK, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med. 2013; 188(10):1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caverly LJ, Huang YJ, Sze MA. Past, present, and future research on the lung microbiome in inflammatory airway disease. Chest. 2019;156(2):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, de Ángel Solá D, Mao Y, et al. Family-based study reveals decreased abundance of sputum Granulicatella in asthmatics. Allergy. 2018;73(9): 1918–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, Qiu R, Yang Z, et al. Sputum microbiota in severe asthma patients: relationship to eosinophilic inflammation. Respir Med. 2017;131: 192–198. [DOI] [PubMed] [Google Scholar]
- 20.Green BJ, Wiriyachaiporn S, Grainge C, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6):e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemanske RF Jr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125(2 suppl 2):S95–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma ZS, Li W. How and why men and women differ in their microbiomes: medical ecology and network analyses of the microgenderome. Adv Sci (Weinh). 2019;6(23):1902054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BR, Shin J, Guevarra R, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27(12): 2089–2093. [DOI] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56(3):308–321. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson J, Grahnén H, Jonsson G, Wikner S. Early establishment of Streptococcus salivarius in the mouth of infants. J Dent Res. 1970;49(2): 415–418. [DOI] [PubMed] [Google Scholar]
- 27.Whiteson K, Agrawal S, Agrawal A. Differential responses of human dendritic cells to metabolites from the oral/airway microbiome. Clin Exp Immunol. 2017; 188(3):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santagati M, Scillato M, Stefani S. Genetic organization of Streptococcus salivarius 24SMBc blp-like bacteriocin locus. Front Biosci (Schol Ed). 2018;10: 238–247. [DOI] [PubMed] [Google Scholar]
- 29.Bidossi A, De Grandi R, Toscano M, et al. Probiotics Streptococcus salivarius 24SMB and Streptococcus oralis 89a interfere with biofilm formation of pathogens of the upper respiratory tract. BMC Infect Dis. 2018; 18(1):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes Dos Santos Santiago G, Brusselle G, Dauwe K, et al. Influence of chronic azithromycin treatment on the composition of the oropharyngeal microbial community in patients with severe asthma. BMC Microbiol. 2017;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD [published correction appears in N Engl J Med. 2012;366(14): 1356]. N Engl J Med. 2011;365(8):689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68(4):322–329. [DOI] [PubMed] [Google Scholar]
- 33.Crosbie PA, Woodhead MA. Long-term macrolide therapy in chronic inflammatory airway diseases. Eur Respir J. 2009;33(1):171–181. [DOI] [PubMed] [Google Scholar]
- 34.Park H, Shin JW, Park SG, Kim W. Microbial communities in the upper respiratory tract of patients with asthma and chronic obstructive pulmonary disease. PLoS One. 2014;9(10):e109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung JW, Choi JC, Shin JW, et al. Lung microbiome analysis in steroid-natïve asthma patients by using whole sputum. Tuberc Respir Dis (Seoul). 2016;79(3): 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denner DR, Sangwan N, Becker JB, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2016; 137(5):1398–1405.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2):346–352.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson JL, Daly J, Baines KJ, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47(3): 792–800. [DOI] [PubMed] [Google Scholar]
- 39.Durack J, Huang YJ, Nariya S, et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome. 2018;6(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.