Abstract
Background
Immune activation markers associate with morbidity and mortality in HIV and hepatitis C virus (HCV) infection. We investigated how T-cell and monocyte activation are related over the course of HCV direct-acting antiviral (DAA) therapy during HCV/HIV coinfection.
Methods
Peripheral blood mononuclear cells from AIDS Clinical Trials Group (ACTG) A5329 participants and a single-site separate cohort treated with DAAs were analyzed for central memory (CM)/effector memory (EM) T-cell subsets, monocyte subsets, and cell activation (CD38 and HLA-DR expression) before, during, and after therapy.
Results
Before therapy, classical and inflammatory monocyte subset HLA-DR expression positively correlated with absolute counts and frequencies of CD38+HLA-DR+-expressing CD4+ and CD8 T cells and corresponding CM and EM subsets. After therapy initiation, CD38+HLA-DR+ co-expression on CD4+ and CD8+ memory T cells decreased by 12 weeks and 36 weeks, and plasma sCD14 positively correlated with CD38+HLA-DR+ CD4+ and CD4+CM T-cell frequencies. Monocyte subset activation remained similar over time.
Conclusions
During HCV/HIV coinfection, memory T-cell activation is associated with monocyte subset activation, consistent with related underlying mechanisms. Following therapy initiation, memory T-cell, but not monocyte, activation decreased. Residual CD4+ T-cell activation after therapy completion is associated with sCD14, potentially linking the remaining CD4+ T-cell activation to residual factors driving activation in antiretroviral therapy–controlled HIV.
Keywords: human, immunity, T cell, monocyte, hepatitis C and HIV coinfection, inflammation, DAA therapy
In the United States, ~25% of HIV-infected individuals have chronic hepatitis C virus (HCV) coinfection [1]. Currently, 53% of the 1.1 million HIV-infected individuals in the United States are treated with antiretroviral therapy (ART) and are virally suppressed [2]. Notably, 75% of chronic HCV-infected individuals in the United States were born between 1945 and 1965 [1] and encountered infection in an era when HCV and HIV infection were not routinely treated. Thus, the long-term consequences of chronic active infection are yet to be realized. HCV infection is a primary cause of chronic liver disease in the United States [3], and HIV coinfection can accelerate progression of HCV-related liver disease [4–6]. ART may not remove these risks [7].
Chronic immune activation is associated with morbidity in HIV and HCV infections and may be a key contributor to immune dysfunction [8], yet the impact of HCV DAA treatment on cellular immune activation in HCV/HIV coinfection has not been clearly determined. Previous studies have focused on total CD4+ and CD8+ T-cell activation and provided varied evidence on whether T-cell activation is reduced following DAA treatment completion [9–11]. Whether this change is sustained and/or is extended to the memory T-cell subsets or innate cell compartment is unclear.
METHODS
Study Participants
A5329 (NCT02194998) was a nonrandomized, open-label, phase 2 trial of interferon (IFN)-free HCV therapy for 12 or 24 weeks in 45 sequentially enrolled participants with HIV-1 coinfection taking protocol-defined ART. Trial participants were HCV treatment naïve or experienced (previous unsuccessful IFN-based treatment), with genotype 1a or 1b infection, and were taking ART based on integrase strand transfer inhibitors (INSTIs; raltegravir [RAL] or dolutegravir [DTG]) or HIV-1 protease inhibitors (darunavir [DRV] or atazanavir [ATV]). Inclusion criteria included age 18–70 years, CD4 >200 cells/mm3, and HIV-1 RNA <50 copies/mL for >6 months before study entry. Exclusion criteria included active hepatitis B virus infection and decompensated liver disease. Persons with compensated cirrhosis were eligible. Longitudinal samples of cryopreserved peripheral blood mononuclear cells (PBMCs) at therapy start, week 12 (during therapy), and week 36 (12 weeks post–therapy completion) from 40 participants (where viability permitted) were analyzed here (24-week therapy n = 34 and 12-week therapy n = 6). The sample size for each flow cytometric analysis is included in the respective figure legend.
To evaluate longer-term effects (up to 1.5 years), an additional local single-site cohort of HCV/HIV-coinfected persons (n = 15) with controlled (PCR-negative) HIV infection scheduled for initiation of standard-of-care HCV DAA therapy (12 week therapy separate from ACTG trial) were recruited (2015–2018). The follow-up time points included weeks 0, 4, and 24 and 1–1.5 years after HCV DAA therapy start.
Patient Consent Statement
Study participants were enrolled under institutional review board committee–approved protocols for the ACTG and MetroHealth study sites, and written informed consent was obtained from each participant. The design of this work conforms to the standards currently applied in the United States.
Flow Cytometry
PBMCs were thawed and stained, and viable cells were enumerated using a 5-laser BD LSRFortessa Flow Cytometer and a panel of fluorochrome conjugated antibodies (Supplementary Table 1) using the gating scheme outlined in Supplementary Figure 1. Absolute CD4 T-cell counts obtained through Clinical Laboratory Improvement Amendments (CLIA)–certified clinical laboratories were utilized to calculate CD4+ T-cell subset absolute counts. T-cell and monocyte analysis was performed on ACTG cohort samples, while only T-cell analysis was performed on single-site cohort samples.
Soluble Markers of Immune Activation
As part of a recently published analysis [12], plasma was assessed by enzyme-linked immunosorbent assay at therapy start, week 12, and week 36 for the following inflammatory markers: soluble CD14 (sCD14), interferon-inducible protein-10 (IP10), autotaxin (ATX), sCD163, and interleukin 6 (IL6).
Clinical Laboratory Investigations
CLIA-certified clinical laboratories performed investigations to determine the extent of liver damage index or fibrosis; FibroScan and Fibrosis-4 (Fib-4) index scores and liver function; aspartate transaminase (AST), alanine transaminase (ALT), and albumin levels. Participants were considered cirrhotic if 1 of the following criteria was documented: liver biopsy demonstrating cirrhosis within 24 months of study entry, HCV FibroSURE score of >0.72 and AST to Platelet Ratio Index (APRI) >2 within 6 months of study entry, or FibroScan result of >12.5 kPa within 6 months of study entry.
Statistical Analysis
Associations between continuous variables were evaluated using Spearman’s rank correlation coefficient (GraphPad Prism, version 8). All statistical tests were 2-sided and used a nominal P value threshold of .05, without adjustments for multiplicity. Wilcoxon signed rank tests were used to assess intraparticipant changes between time points using SPSS for Windows (version 24).
RESULTS
Study Participant Characteristics
Participant characteristics from the ACTG A5329 trial (n = 40) are described in Table 1. Key characteristics included plasma HIV-1 RNA <40 copies/mL in 39 of 40 and median CD4+ T-cell count of 622 cells/mm3. ART included INSTI-based ART (RAL [22/40] and DTG [4/40]) and PI-based ART (DRV/r [12/40] and ATV/r [2/40]). The number of cirrhotic participants was 6. Participant characteristics from the single-site cohort are summarized in Supplementary Table 2.
Table 1.
Characteristics of Primary Cohort Participants
| Sample Size | n = 40 |
|---|---|
| DAA therapy regimen: paritaprevir/ritonavir/ombitasvir + dasabuvir +/- ribavirin | |
| DAA therapy duration, No. (%) | |
| 12-week | 6 (15) |
| 24-week | 34 (85) |
| ART regimen, No. (%) | |
| Integrase inhibitor based (RAL or DTG) | 22 (55) |
| Protease inhibitor (DRV or ATV) | 18 (45) |
| HCV genotype, No. (%) | |
| 1a | 6 (40) |
| 1b | 4 (27) |
| 2 | 2 (13) |
| 3 | 2 (13) |
| 4 | 1 (7) |
| Plasma HCV RNA level, IU/L | 2 790 000 (1 020 000–6 065 000) |
| Serum albumin level, g/dL | 4.3 (4.1–4.6) |
| Serum ALT level, IU/L | 48 (28–73) |
| Serum AST level, IU/L | 42 (31–58) |
| Platelets, K/μL | 217 (172–238) |
| APRI, AST/PLT index, No. (%) | |
| <0.4 | 31 (84) |
| 0.4–1.5 | 6 (16) |
| >1.5 | 0 (0) |
| FIB-4 index score | 1.4 (0.9–2.2) |
| FibroScan score | 6.4 (5.1–13.6) |
| Cirrhosis state, No. (%) | |
| Cirrhotics | 6 (15) |
| Noncirrhotics | 34 (85) |
| CD4, cells/mm3 | 622 (456–827) |
| Age, y | 53 (45–58) |
| Gender, No. (%) | |
| Male | 29 (73) |
| Female | 11 (27) |
| Race/ethnicity, No. (%) | |
| Black | 16 (40) |
| White | 8 (40) |
| Hispanic | 16 (20) |
Data are presented as median (interquartile range), unless otherwise indicated.
Abbreviations: APRI, AST to Platelet Ratio Index; ART, antiretroviral therapy; AST, aspartate transaminase; ATV, atazanavir; DAA, direct-acting antiviral; DRV, darunavir; DTG, dolutegravir; HCV, hepatitis C virus; PLT, platelet; RAL, raltegravir.
T-cell CD38+HLA-DR+ Co-expression Positively Correlates With Monocyte Subset HLA-DR Expression, and Both Parameters Are Associated With Abnormal Clinical Liver Parameters Before DAA Therapy
Monocytes can sense HCV via Toll-like receptors (TLRs) such as TLR2 (sensing HCV core protein) and become activated [13]. Chronic HCV and HIV infection are associated with monocyte activation, as evidenced by elevated HLA-DR expression [14, 15]. CD4+ and CD8+ T-cell activation, reflected by CD38 and/or HLA-DR expression, is elevated during HCV/HIV coinfection compared with HIV and HCV monoinfections [16, 17]. Here we first determined the frequencies of peripheral blood monocyte subsets as defined by CD14 and CD16 expression: classical (CD14++CD16-), inflammatory (CD14++CD16+) and nonclassical (CD14+CD16+) monocytes (Supplementary Figure 1) [18]. We also determined the frequency and absolute counts (CD4+ only) of CD38+HLA-DR+-co-expressing (n = 31) and CD38+-mono-expressing (n = 40) CD4+ and CD8+ T cells and corresponding subsets: CM (CD27+CD45RA-) and EM (CD27-CD45RA-) (Supplementary Figure 1) [19]. Proportions of CD4+ T cells expressing CD38 (median, 20%) (not shown) at baseline (before therapy) were similar to those in the published literature [17], while proportions of CD38+HLA-DR+-co-expressing CD4+ and CD8+ T cells (median, 10%) (not shown) were somewhat higher than in the published literature [10]. Monocyte subset distribution (CD14++CD16- median, 67%; CD14++CD16+ median, 6%; CD14+CD16+ median, 2%) at baseline (week 0) was comparable to previously published data in ART-controlled HIV infection [20].
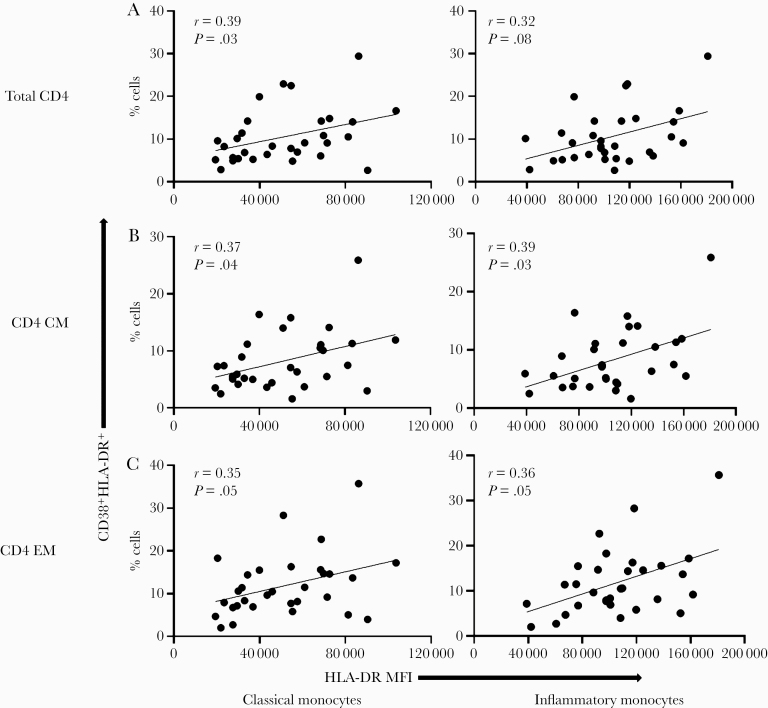
To better understand the interrelationships between pathways of cellular immune activation, we next investigated possible relationships between activated monocyte and T-cell subsets before DAA therapy in HCV/HIV coinfection. Indeed, positive correlations were observed between HLA-DR expression on monocytes (classical and inflammatory subsets) and the frequencies of CD38+HLA-DR+-co-expressing CD4+ T cells (r = 0.39, P = .03, and r = 0.32, P = .08, respectively, for classical and inflammatory), as well as CM (r = 0.37, P = .04, and r = 0.39, P = .03, respectively) and EM (r = 0.35, P = .05, and r = 0.36, P = .05, respectively) T-cell subsets at baseline (Figure 1). Positive correlations were also observed between HLA-DR expression on classical and inflammatory monocytes and the frequencies of CD38+HLA-DR+-co-expressing CD8+ T cells (r = 0.46, P = .009, and r = 0.58, P = .0006, respectively) and their corresponding CM (r = 0.43, P = .02, and r = 0.50, P = .004, respectively) and EM (r = 0.31, P = .09, and r = 0.38, P = .04, respectively) subsets (Supplementary Figure 2), as well as the absolute counts of CD4+ CD38+HLA-DR+ T cells and corresponding subsets (not shown). Together, these data indicate direct or indirect links between T-cell and monocyte activation during chronic active HCV infection and controlled HIV infection.
Figure 1.
Frequencies of CD38 and HLA-DR co-expression on CD4+ T-cell positively correlate with HLA-DR MFI on monocyte subsets during untreated chronic HCV and ART-controlled HIV coinfection. HCV/HIV-coinfected study participants (A5329 cohort) on ART were evaluated before DAA therapy initiation (n = 31). The frequencies of CD4+, CD4+CD27+CD45RA- (CM), and CD4+CD27-CD45RA- (EM) T cells were determined. The HLA-DR MFI was determined on the classical (CD14++CD16-) and inflammatory (CD14++CD16+) monocyte subsets. Correlations were then performed between the total CD4+ (A), CD4 CM (B), CD4 EM (C) T cells co-expressing CD38 and HLA-DR and the HLA-DR MFI on both the classical and inflammatory monocytes. Linear regression line shown. Spearman’s correlation test was used to evaluate correlations; P < .05 considered significant. Abbreviations: ART, antiretroviral therapy; CM, central memory; EM, effector memory; HCV, hepatitis C virus; MFI, mean fluorescence intensity.
The liver is the primary target of HCV infection, and pathogenesis of infection involves local inflammation and fibrosis [21–23]. Hepatic inflammation may partly contribute to systemic inflammation due to release of resident soluble mediators that enter the circulation [24, 25]. We therefore investigated if there was a relationship between monocyte and T-cell activation and the clinical parameters of HCV infection, including plasma HCV level, liver inflammation (ALT level and AST level), synthetic function (albumin [Alb]), and a combination of liver inflammation and fibrosis (FibroScan score and Fib-4 score) before HCV treatment. We observed positive correlations between AST level and the proportions of CD38+HLA-DR+-co-expressing CD4+ (r = 0.4, P = .02) and CD4+ CM (r = 0.5, P = .01) T cells and the counts of CD38+HLA-DR+-co-expressing CD4+ T cells (r = 0.5, P = .005) and corresponding CM (r = 0.6, P = .02) and EM (r = 0.4, P = .04) subsets (not shown). Similar positive correlations were observed between ALT levels and the proportions of CD38+HLA-DR+-co-expressing CD4+ (r = 0.5, P = .02), CD4+CM (r = 0.5, P = .009), CD4+EM (r = 0.4, P = .03), CD8+ (r = 0.4, P = .03), and CD8+ EM (r = 0.4, P = .03) T cells and CD38+HLA-DR+-co-expressing T-cell counts (CD4+ (r = 0.5, P = .01), CD4+ CM (r = 0.5, P = .003), and CD4+ EM (r = 0.5, P = .02) (not shown). We further observed a positive correlation between counts of CD38+HLA-DR+-co-expressing CD4+ T cells and FibroScan score (r = 0.58, P = .01) (not shown). FibroScan score is a reflection of both inflammation and fibrosis [26, 27], and consistent with this, we observed that AST levels positively correlated with FibroScan scores as well (r = 0.7, P = .003) (not shown). We also observed a negative correlation between HCV plasma levels and the proportions of CD38+HLA-DR+-co-expressing CD4+ T cells (r = –0.47, P = .02) (not shown), CD4+ CM T cells (r = –0.4, P = .03), and CD4+ EM T cells (r = –0.4, P = .04) (data not shown). Finally, HLA-DR mean fluorescent intensity (MFI) on inflammatory monocytes negatively correlated with albumin levels (not shown). No significant correlations were observed for other monocyte subsets. These data are consistent with an association between active liver inflammation and both CD4 and CD8 T-cell activation, while CD4 T-cell activation is negatively associated with HCV level.
CD38+HLA-DR+ Co-expression on CD4+ and CD8+ T-Cell Subsets Declines 12 Weeks After DAA Initiation
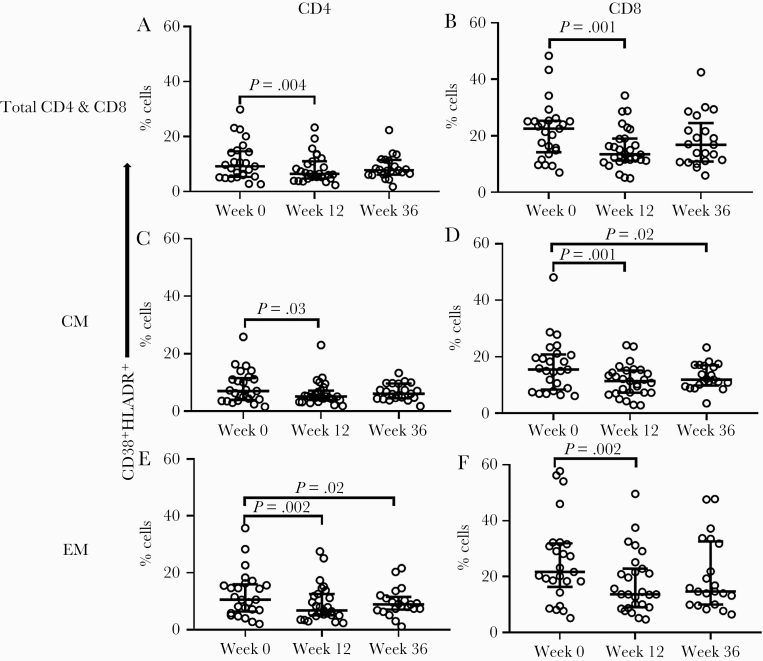
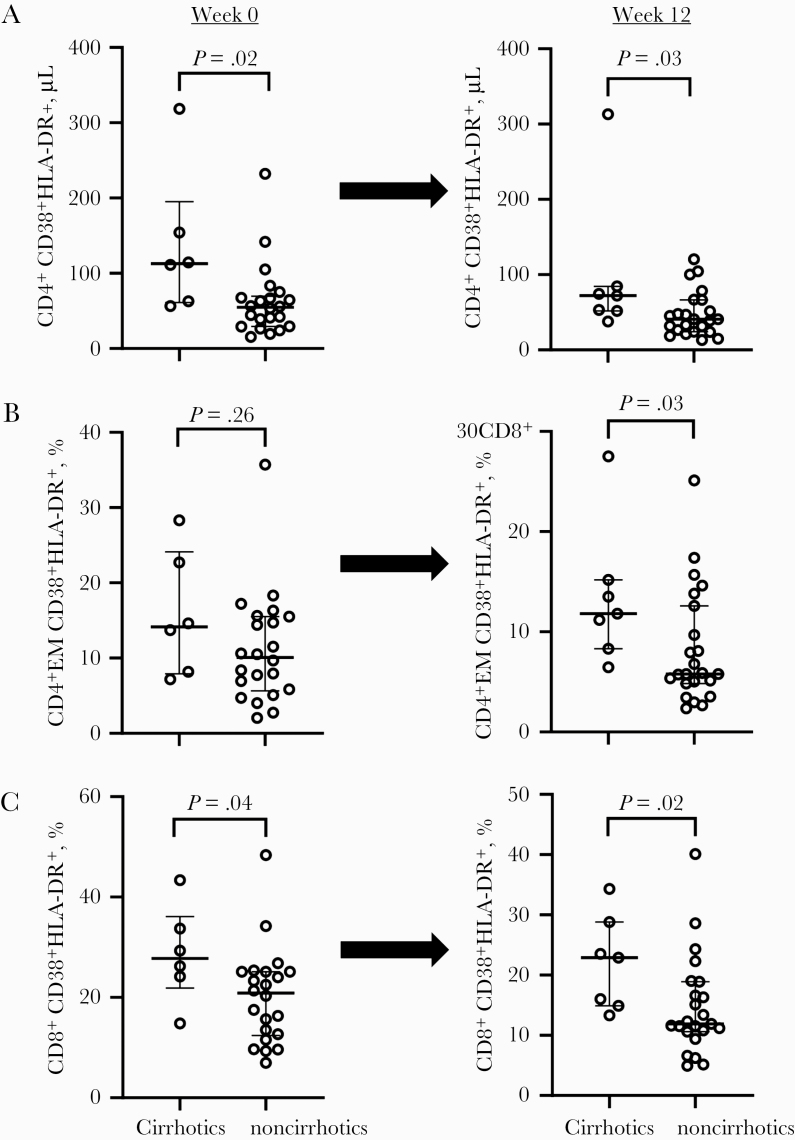
To understand whether T-cell, monocyte, and/or coordinated T-cell/monocyte activation depends on continued presence of HCV, we next examined the change and timing of change in the frequency of CD38+HLA-DR+-co-expressing CD4+ and CD8+ T cells and corresponding subsets at baseline (week 0), week 12, and week 36 after DAA therapy initiation (24-week therapy; n = 28). Compared with baseline, we observed decreased frequency of the CD38+HLA-DR+-co-expressing CD4+ (P = .004), CD4+ CM (P = .03), CD4+ EM (P = .002) (Figure 2A, C and E), CD8+ (P = .001), CD8+ CM (P = .001), and CD8+ EM (P = .002) T cells (Figure 2B, D and F) 12 weeks after DAA therapy initiation. Similar results were observed when the 12-week therapy participants (n = 3) were added to the analysis (not shown). Decreased CD38+HLA-DR+ co-expression appeared to be sustained in the CD4+ EM (P = .02) and CD8+ CM T-cell subsets (P = .02) 36 weeks after DAA initiation compared with baseline, also indicating that this phenotype is preserved at least 12 weeks after therapy discontinuation. Furthermore, we observed a reduction in absolute counts of CD38+HLA-DR+-co-expressing CD4+ (P = .003) and CD4+ EM (P = .001) T cells within 12 weeks of DAA initiation compared with baseline (not shown). We further evaluated the differences in T-cell activation by cirrhosis state and observed greater CD38+HLA-DR+ co-expression on T cells in cirrhotic participants before (week 0) and 12 weeks after DAA initiation compared with noncirrhotic participants: CD4+ (P = .02 and P = .03), CD4+ EM (P = .26 and P = .03), and CD8+ (P = .04 and P = .02) at weeks 0 and 12, respectively (Figure 3A–C).
Figure 2.
CD38 and HLA-DR co-expression on CD4+ and CD8+ T cells and their corresponding subsets declines over the course of DAA HCV therapy during ART-controlled HCV/HIV coinfection. HCV/HIV-coinfected study participants (A5329 cohort) on ART were treated with a 24-week course of DAA therapy and longitudinally followed before (week 0; n = 28), during (week 12; n = 27), and after (week 36; n = 21) therapy. The proportion of CD38 and HLA-DR co-expression on total CD4+ and CD8+ (A and B) and the corresponding CD27+CD45RA- (CM) (C and D) and CD27-CD45RA- (EM) (E and F) T-cell subsets were evaluated at all time points. The Wilcoxon signed rank test was used for paired comparisons between 2 time points; P < .05 considered significant. Abbreviations: ART, antiretroviral therapy; CM, central memory; DAA, direct-acting antiviral; EM, effector memory; HCV, hepatitis C virus.
Figure 3.
T-cell activation is greater in cirrhotics before and after DAA therapy initiation when compared with noncirrhotics during ART-controlled HCV/HIV coinfection. HCV/HIV-coinfected study participants (A5329 cohort) on ART were stratified according to cirrhosis status before (week 0; cirrhotics n = 6 and noncirrhotics n = 23) and after (week 12; cirrhotics n = 7 and noncirrhotics n = 23) DAA therapy. The absolute counts of CD38 and HLA-DR-co-expressing CD4+ T cells were calculated using the CD4+ T cells (A). The proportions of CD38 and HLA-DR co-expression on total CD8+ T cells and CD4+ CD27-CD45RA- (EM) subsets (B and C) were evaluated at both time points. The Mann-Whitney U test was used for unpaired comparisons between 2 time points; P < .05 considered significant. Abbreviations: ART, antiretroviral therapy; DAA, direct-acting antiviral; EM, effector memory; HCV, hepatitis C virus.
In an additional cohort (n = 15) where HCV/HIV-coinfected participants were followed for a longer duration, we observed a progressive decline in CD38 expression that was sustained up to 1.5 years after DAA initiation on the CD4+ CM (P = .02) and EM T cells (P = .02) and the CD8+ CM (P = .02) and EM T cells (P = .03) (Supplementary Figure 3). CD38 expression also declined within 12 weeks of DAA initiation in the ACTG cohort: CD8+ CM (P = .0001), CD4+ EM (P = .02), and CD8+ EM T cells (P = .001) (not shown). Together, these data indicate sustained reduction in CD4+ and CD8+ T-cell activation as soon as 12 weeks after the start of DAA therapy, and this effect is likely sustained at least 1 year after therapy initiation.
Inflammatory Monocyte Subset Frequency Positively Correlates With CD38 and HLA-DR Co-expression on CD8+ Central Memory T Cells 12 Weeks After DAA Initiation
We next evaluated changes in the frequency and activation phenotype of monocyte subsets following DAA initiation. There was a trend for decreased frequency of inflammatory monocytes (P = .05) within 12 weeks of DAA therapy start, while the total monocyte and other monocyte subset frequencies remained unchanged (not shown). Further, there was a trend toward decreased HLA-DR expression on monocyte subsets 12 weeks after DAA initiation when compared with baseline (P = .06) (Supplementary Figure 4).
To understand if the presence of HCV was related to coordinated monocyte and T-cell activation, we evaluated relationships between monocyte and T-cell activation after the start of DAA therapy. After 12 weeks of therapy, we observed no significant correlations between monocyte and T-cell activation (not shown). However, there was a positive correlation between the frequencies of inflammatory monocytes and CD38+HLA-DR+-co-expressing CD8+ CM T cells 12 weeks after the start of DAA therapy (r = 0.4, P = .03), an association that was not present before DAA initiation (not shown).
Classical Monocyte Frequency Negatively Correlates With Plasma IL6 Levels Before DAA Therapy, and CD4+ CD38+HLA-DR+ T-cell Frequency Positively Correlates With Plasma sCD14 After DAA Therapy
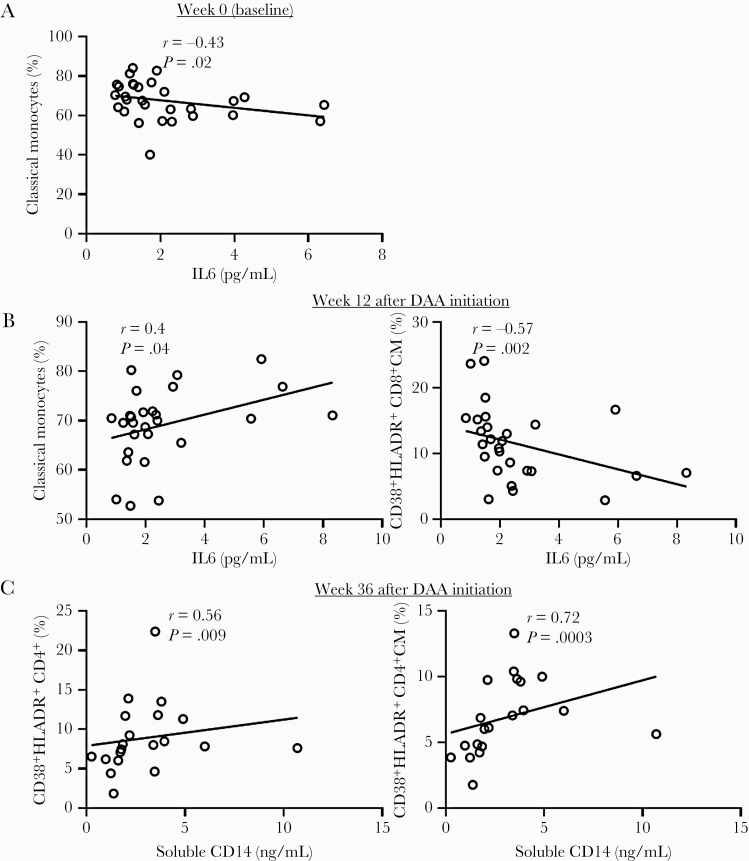
In addition to cellular markers of immune activation, chronic HCV infection is also characterized by systemic elevation of soluble markers of inflammation, including sCD14, sCD163, autotaxin, IP10, and IL6 [28]. Some of these markers may directly reflect monocyte or Kupffer cell activation (sCD14 and sCD163), while others may reflect activation of other cellular compartments. Autotaxin, sCD163, and IP10 levels diminished within 12 weeks of DAA treatment, changes were sustained up to 36 weeks [12]. IL6 and sCD14 levels remained unchanged [12]. To better understand pathways related to monocyte and T-cell activation, we evaluated how peripheral levels of soluble inflammatory biomarkers related to absolute numbers and frequencies of CD38 and/or HLA-DR-expressing monocytes and T cells in HCV/HIV-coinfected participants. Before DAA, IP-10 levels positively correlated with nonclassical monocyte frequency (r = 0.4, P = .04) (not shown), and IL6 levels negatively correlated with classical monocyte frequency (r = –0.43, P = .02) (Figure 4A).
Figure 4.
IL6 and soluble CD14 levels correlate with the frequency of classical monocytes and CD38 and HLA-DR co-expression on CD4+ and CD8+ T cells over the course of DAA therapy during ART-controlled HCV/HIV coinfection. HCV/HIV-coinfected study participants (A5329 cohort) on ART were treated with a 24-week course of DAA therapy (n = 28) and longitudinally followed up to 36 weeks. The plasma levels of IL6 and soluble CD14 were determined by ELISA at baseline (week 0), week 12, and 36 weeks after DAA initiation. Thereafter, correlations were determined between the IL6 levels and classical monocyte frequency at week 0 (A), IL6 levels and frequencies of classical monocytes and CD38 and HLA-DR-co-expressing CD8+ CM T cells at 12 weeks (B), and soluble CD14 levels and frequencies of CD38 and HLA-DR-co-expressing CD4+ and CD4+ CM T cells at 36 weeks (C). Linear regression line shown. Spearman’s correlation test was used to evaluate correlations; P < .05 considered significant. Abbreviations: ART, antiretroviral therapy; CM, central memory; DAA, direct-acting antiviral; ELISA, enzyme-linked immunosorbent assay; HCV, hepatitis C virus; IL6, interleukin 6.
Twelve weeks after DAA initiation, IL6 levels positively correlated with classical monocyte frequency (r = 0.4 P = .04) and negatively correlated with CD38+HLA-DR+-co-expressing CD8 CM T-cell frequency (r = –0.57, P = .002) (Figure 4B) and CD4 EM T-cell (r = –0.39, P = .04) counts (not shown). Notably, we observed a positive correlation between sCD14 levels and CD38+HLA-DR+-co-expressing CD4+ (r = 0.56, P = .009) and CD4+ CM (r = 0.72, P = .0003) T cells 36 weeks after the start of DAA therapy (Figure 4C), relationships that were absent before DAA therapy.
DISCUSSION
We investigated the impact of HCV DAA therapy on the differentiation and activation phenotype of peripheral monocytes and T cells and how this related to clinical laboratory parameters and soluble inflammatory markers in chronic ART-controlled HCV/HIV coinfection. Before DAA initiation, monocyte subset HLA-DR expression positively correlated with CD38+HLA-DR+-co-expressing CD4+ and CD8+ T cells (absolute counts and proportions) and corresponding subsets. Additionally, CD38+HLA-DR+ co-expression on CD4+ T cells positively correlated with liver inflammation (AST and ALT levels) and liver stiffness (FibroScan score). Following DAA initiation, CD38+HLA-DR+ co-expression on CD4+ and CD8+ T cells declined within 12 weeks, and this change was sustained up to 36 weeks. The correlations between T-cell and monocyte activation did not remain after DAA initiation. Furthermore, CD38+HLA-DR+ co-expression on CD4+ T cells positively correlated with sCD14 levels 36 weeks after the start of DAA therapy. Collectively, these data support the concept of coordinated monocyte and T-cell immune activation before DAA therapy and reduction in T-cell activation following DAA HCV treatment, resulting in loss of coordinated monocyte and T-cell activation. Following the DAA-mediated clearance of HCV, the state of residual immune activation appears to be associated with factors yet to normalize in controlled HIV infection.
T-cell activation persists during ART-controlled HIV monoinfection [29–31]. T-cell activation also exists during untreated HCV monoinfection and in HCV/HIV coinfection [32]. Monocyte activation is also elevated in HIV and HCV monoinfections despite effective ART [28, 33, 34]. Proportions of CD38+HLA-DR+-co-expressing CD4+ and CD8+ T cells were modestly higher here than in the published literature in HIV/HCV coinfection, while monocyte subset distribution was comparable to prior literature [10, 20]. We found a positive correlation between T-cell and monocyte subset activation, suggesting a potential causal relationship (direct or indirect mechanism). This relationship was observed before but not after DAA, inferring that active HCV viremia was a key driver of the relationship. Notably, correlations with monocyte activation slightly differed between CD4+ T-cell activation proportions and counts, as these reflect different aspects of T-cell homeostasis, with the former in part representing different T-cell subsets and the latter derived from calculations of CD4+ T cells. Mechanisms underlying HCV-mediated monocyte activation may include detection of HCV proteins by TLRs [13, 35, 36]. Alternatively, monocyte activation may be mediated by inflammatory cytokines including IL6 [37]. Certainly, T-cell activation can potentially occur as a byproduct of monocyte activation due to direct APC–T-cell interactions.
Previous studies have reported accelerated liver fibrosis in HCV/HIV coinfection compared with HCV monoinfection [4, 6]. Immune activation is one of the mechanisms postulated to drive liver damage and vice versa. Here, we found a positive association between CD4+ T-cell activation and liver inflammation (AST, ALT) and liver stiffness (FibroScan score likely reflective of liver fibrosis and inflammation) and a negative association between inflammatory monocyte activation and liver synthetic function (albumin level). Our findings are consistent with previous reports of positive correlations between FIB-4 and T-cell or monocyte immune activation in HIV infection [38], here extending to HCV/HIV coinfection.
Prior reports of diminished T-cell activation in the setting of HCV IFN-based and DAA treatment in HCV/HIV-coinfected individuals are contradictory and limited to total CD4+ and CD8+ T cells [9–11, 39]. Here we longitudinally followed HCV/HIV-coinfected participants on DAA over a 36-week to 1.5-year time window. We demonstrated that CD38+HLA-DR+ co-expression on CD4+ and CD8+ T cells and corresponding memory subsets diminished within 12 weeks after DAA therapy start, consistent with previous findings [10] and extending here to T-cell memory subsets. The diminished T-cell activation state was sustained at 36 weeks (12 weeks post-DAA completion), indicating that this effect appears to be durable, a finding further supported here with an additional cohort analysis of CD38+ T cells over a longer time window (1.5 years). Interestingly, we observed that before DAA therapy, higher CD4+ T-cell activation was associated with lower HCV levels. This may be attributable to a role for activated CD4+ T cells in control of HCV viremia. However, our observation that T-cell activation (proportions and counts) diminished once DAA-mediated HCV clearance was initiated would be most consistent with a role for HCV level in determining levels of activated CD4+ T-cell presence (antigen-specific and/or bystander) in the peripheral blood. We did not observe significant changes in the monocyte subsets (HLA-DR expression or proportions) after DAA therapy start. Notably, frequencies of inflammatory monocytes positively correlated with CD38+HLA-DR+-co-expressing CD8+ CM but not CD4+ T cells 12 weeks after DAA therapy, an observation not present before DAA initiation. While this does not necessarily reflect antigen specificity of the activated T-cell subset, it may reflect a residual link between monocyte and T-cell activation after removal of HCV. Overall, our data suggest diminution in T-cell immune activation after DAA therapy initiation during chronic HCV/HIV infection. Whether activated T cells participate in the control of HCV or are reflective of the inflammatory liver environment is yet to be determined.
Although we report less T-cell activation after DAA therapy, we also see evidence of new relationships between T-cell and monocyte activation that did not exist before therapy, perhaps reflecting either restored liver function or residual immune activation. At 12 weeks, inflammatory monocyte frequency positively correlated with CD8+ CM T-cell activation. After therapy, CD4+ CM T-cell activation positively correlated with monocyte activation (as reflected by sCD14 levels). We have previously reported that sCD14 levels do not normalize over a period of weeks despite effective DAA therapy in chronic HCV infection [28].
Potentially, clearance of HCV from the liver may result in restoration of some immune homeostatic function. Despite effective DAA, complete resolution of immune activation is unlikely in cases of cirrhosis where liver damage is less reversible. Here cirrhotic participants exhibited significantly higher T-cell activation before and after DAA. At 12 weeks, HCV viremia is undetectable in DAA-treated individuals [40–42], consistent with our cohort [12, 43]. It remains to be determined whether other coinfections including cytomegalovirus or Epstein-Barr virus may contribute to this persistent activated state.
The current study has a number of limitations. Intended immunologic analysis was limited by sample viability. Investigation of sustainability of changes could have been boosted by longer follow-up periods. However, this was mitigated by our additional local cohort with longer follow-up longer duration. Analysis of HLA-DR expression on T cells in our local cohort and additional monocyte activation markers would have enhanced characterization of the T-cell and monocyte activation data, respectively. Peripheral blood–based measurements may not completely reflect events within the liver. Clinical liver parameters were only available at baseline, thereby limiting investigation of correlations after DAA therapy initiation. Finally, this is an exploratory data set that is limited by multiple comparisons.
In summary, we have described an association between CD4+ and CD8+ T-cell and monocyte activation before HCV DAA therapy that abates over the course of therapy, where only memory T-cell activation is diminished, a change that was sustained through 12 weeks following therapy in the ACTG cohort and to over a year in a local cohort. Residual low-level cellular activation persisted after SVR, consistent with the low-level persistent immune activation during ART-controlled HIV infection that was previously reported [17].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the study participants for their time and dedication to this effort.
Financial support. This study was supported by AbbVie and the AIDS Clinical Trials Group (ACTG) under award numbers UM1 AI106701, UM1 AI068634; Statistical and Data Management Center (SDMC) for ACTG, Case CFAR AI 36219, VA CDA IK2CX001471 (Shive), VA Merit BX001894 (Anthony), VA Merit CX001791 (Anthony), K24DA034621 (Reddy), D43TW010319 (Auma).
Potential conflicts of interest. Dr. Cohen is employed by AbbVie. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. This work was presented as a poster at the 2020 CROI conference in Boston, Massachusetts.
References
- 1. The Centers for Disease Control and Prevention. HIV and viral hepatitis: fast facts.2017. https://www.cdc.gov/hiv/pdf/library/factsheets/hiv-viral-hepatitis.pdf. Accessed 15 March 2020.
- 2.The Centers for Disease Control and Prevention. HIV in the United States and dependent areas.2019. https://www.cdc.gov/hiv/pdf/statistics/overview/cdc-hiv-us-ataglance.pdf. Accessed 15 March 2020.
- 3. Kim WR, Terrault NA, Pedersen RA, et al. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology 2009; 137:1680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soto B, Sánchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 1997; 26:1–5. [DOI] [PubMed] [Google Scholar]
- 5. Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30:1054–8. [DOI] [PubMed] [Google Scholar]
- 6. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001; 33:562–9. [DOI] [PubMed] [Google Scholar]
- 7. Lo Re V 3rd, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med 2014; 160:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taiwo B, Barcena L, Tressler R. Understanding and controlling chronic immune activation in the HIV-infected patients suppressed on combination antiretroviral therapy. Curr HIV/AIDS Rep 2013; 10:21–32. [DOI] [PubMed] [Google Scholar]
- 9. Najafi Fard S, Schietroma I, Corano Scheri G, et al. Direct-acting antiviral therapy enhances total CD4+ and CD8+ T-cells responses, but does not alter T-cells activation among HCV mono-infected, and HCV/HIV-1 co-infected patients. Clin Res Hepatol Gastroenterol 2018; 42:319–29. [DOI] [PubMed] [Google Scholar]
- 10. López-Cortés LF, Trujillo-Rodríguez M, Báez-Palomo A, et al. Eradication of hepatitis C virus (HCV) reduces immune activation, microbial translocation, and the HIV DNA level in HIV/HCV-coinfected patients. J Infect Dis 2018; 218:624–32. [DOI] [PubMed] [Google Scholar]
- 11. Shrivastava S, Bhatta M, Ward H, et al. Multitarget direct-acting antiviral therapy is associated with superior immunologic recovery in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatol Commun 2018; 2:1451–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anthony DD, Sulkowski MS, Smeaton LM, et al. Hepatitis C virus (HCV) direct-acting antiviral therapy in persons with human immunodeficiency virus-HCV genotype 1 coinfection resulting in high rate of sustained virologic response and variable in normalization of soluble markers of immune activation. J Infect Dis 2020; 222:1334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung H, Watanabe T, Kudo M, Chiba T. Hepatitis C virus core protein induces homotolerance and cross-tolerance to Toll-like receptor ligands by activation of Toll-like receptor 2. J Infect Dis 2010; 202:853–61. [DOI] [PubMed] [Google Scholar]
- 14. Zheng J, Liang H, Xu C, et al. An unbalanced PD-L1/CD86 ratio in CD14(++)CD16(+) monocytes is correlated with HCV viremia during chronic HCV infection. Cell Mol Immunol 2014; 11:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rempel H, Sun B, Calosing C, et al. Monocyte activation in HIV/HCV coinfection correlates with cognitive impairment. PLoS One 2013; 8:e55776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodowanec AC, Brady KE, Gao W, et al. Characterization of CD4+ T-cell immune activation and interleukin 10 levels among HIV, hepatitis C virus, and HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr 2013; 64:232–40. [DOI] [PubMed] [Google Scholar]
- 18. Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120:4599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 1997; 186:1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kulkarni M, Bowman E, Gabriel J, et al. Altered monocyte and endothelial cell adhesion molecule expression is linked to vascular inflammation in human immunodeficiency virus infection. Open Forum Infect Dis 2016; 3:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoofnagle JH. Course and outcome of hepatitis C. Hepatology 2002; 36:S21–9. [DOI] [PubMed] [Google Scholar]
- 22. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut 2004; 53:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Negash AA, Ramos HJ, Crochet N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog 2013; 9:e1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. You CR, Park SH, Jeong SW, et al. Serum IP-10 levels correlate with the severity of liver histopathology in patients infected with genotype-1 HCV. Gut Liver 2011; 5:506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kazankov K, Barrera F, Møller HJ, et al. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology 2014; 60:521–30. [DOI] [PubMed] [Google Scholar]
- 26. Ganne-Carrié N, Ziol M, de Ledinghen V, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology 2006; 44:1511–7. [DOI] [PubMed] [Google Scholar]
- 27. Afdhal NH, Bacon BR, Patel K, et al. Accuracy of FibroScan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: a United States multicenter study. Clin Gastroenterol Hepatol 2015; 13:772–9.e1–3. [DOI] [PubMed] [Google Scholar]
- 28. Kostadinova L, Shive CL, Zebrowski E, et al. Soluble markers of immune activation differentially normalize and selectively associate with improvement in AST, ALT, albumin, and transient elastography during IFN-free HCV therapy. Pathog Immun 2018; 3:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Funderburg NT, Andrade A, Chan ES, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naïve patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One 2013; 8:e83514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis 2006; 6:280–7. [DOI] [PubMed] [Google Scholar]
- 31. Steel A, John L, Shamji MH, et al. CD38 expression on CD8 T cells has a weak association with CD4 T-cell recovery and is a poor marker of viral replication in HIV-1-infected patients on antiretroviral therapy. HIV Med 2008; 9:118–25. [DOI] [PubMed] [Google Scholar]
- 32. Feuth T, Arends JE, Fransen JH, et al. Complementary role of HCV and HIV in T-cell activation and exhaustion in HIV/HCV coinfection. PLoS One 2013; 8:e59302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burdo TH, Weiffenbach A, Woods SP, et al. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013; 27:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee J, Wu CC, Lee KJ, et al. Activation of anti-hepatitis C virus responses via Toll-like receptor 7. Proc Natl Acad Sci U S A 2006; 103:1828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee J, Tian Y, Chan ST, et al. TNF-α induced by hepatitis C virus via TLR7 and TLR8 in hepatocytes supports interferon signaling via an autocrine mechanism. PLoS Pathog 2015; 11:e1004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29:1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kooij KW, Wit FW, van Zoest RA, et al. ; AGEhIV Cohort Study Group . Liver fibrosis in HIV-infected individuals on long-term antiretroviral therapy: associated with immune activation, immunodeficiency and prior use of didanosine. AIDS 2016; 30:1771–80. [DOI] [PubMed] [Google Scholar]
- 39. Gonzalez VD, Falconer K, Blom KG, et al. High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol 2009; 83:11407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawitz E, Makara M, Akarca US, et al. Efficacy and safety of ombitasvir, paritaprevir, and ritonavir in an open-label study of patients with genotype 1b chronic hepatitis C virus infection with and without cirrhosis. Gastroenterology 2015; 149:971–80.e1. [DOI] [PubMed] [Google Scholar]
- 41. Reddy KR, Bourlière M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 2015; 62:79–86. [DOI] [PubMed] [Google Scholar]
- 42.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66:153–94. [DOI] [PubMed] [Google Scholar]
- 43. Balagopal A, Smeaton LM, Quinn J, et al. Intrahepatic viral kinetics during direct-acting antivirals for hepatitis c in human immunodeficiency virus coinfection: the AIDS Clinical Trials Group A5335S Substudy. J Infect Dis 2020; 222:601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.