Abstract
Objective:
The paper’s primary goal is to report the devastating impact of carbon tetrachloride (CCl4) on rat testicular tissue and the possible protecting function of propolis against CCl4 based on its free radical scavenging and inflammatory relief properties.
Materials and Methods:
A total of 24 adult male albino rats had been classified into four groups (six rats/group). Rats of group 1 served as control, whereas groups 2–4 received propolis (200 mg/kg/day), CCl4 (3 ml/kg/day), and propolis/CCl4, respectively. After 4 weeks, the collected sera were applied for the estimation of lipid profile and sex hormones. Also, histopathological picture, malondialdehyde, and tumor necrosis factor alpha (TNFα) gene profile was measured in collected testicular tissues.
Results:
The present information revealed a noteworthy change (p < 0.05) in lipid profile, decrease in testicular weight, testosterone, antioxidants values along with a prominent increase (p < 0.05) in estradiol, lipid peroxidation values, and expression of TNFα in rats administrated with CCl4 compared to control. Moreover, the histopathological profile showed the degeneration of the epithelium. Interestingly, propolis attenuated the destructive effect of CCl4 on rat testes.
Conclusion:
The examined dose of propolis reduced oxidation, and inflammatory reactions resulted from CCl4 exposure and proved that it might have a helpful part in free radicals interceded diseases.
Keywords: Glutathione, lipid, oxidative stress, testosterone, TNF-α
Introduction
Carbon tetrachloride (CCl4) is a compound that damages the testes, kidneys, liver, brain, and lungs [1]. Testicular toxicity had been reported as a consequence of oxidative stress (OS) state, following CCl4 exposure [2,3]. CCl4 is metabolized within the liver, creating exceedingly reactive and deadly trichloromethyl free radicals like carbon trichloride (CCl3). These free radicals are changed over to trichloromethyl peroxyradical through the cytochrome P450 oxygenase enzyme-producing OS condition. CCl4 starts the autoxidation of lipids through binding to the cytoplasmic membrane polyunsaturated fatty acids, resulting in membrane damage, reducing enzyme activity, disrupting sexual hormones, and finally inducing necrosis [4]. During initial inflammatory processes, OS is associated with elevated cytokines as tumor necrosis factor alpha (TNFα) and Interleukine-1 (IL-1) [5,6]. Inflammation takes place in nearly all disease processes, involving immunological and vascular complications, cancer, sepsis, and metabolic damage. Kupffer cells activated by CCl4 start a pathway of inflammatory mediators through the upregulation of TNF-α, IL-1b, and IL-6 expressions [7]. TNF-α is a pro-inflammatory cytokine that regulates a broad range of physiological events, including apoptosis and inflammatory processes [8] and impacting various diseases as diabetes [9,10].
Testicles include an extensive amplification of antioxidant enzymes and free radical scavengers against OS. This antioxidant shield is of significant importance because peroxidative damage is currently considered the most critical reason behind impaired testicular functioning. It supports the pathological consequences of many conditions, from testicular torsion to diabetes xenobiotic exposure. The body can be protected against various oxidative stresses caused by free radicals through body and food antioxidants [11].
Egyptians, Greeks, and Romans used propolis as a portion of human medication for thousands of years, benefiting from its properties [12]. Propolis is considered a powerful antioxidant used in the preparation of functional foods and food supplements. It may help prevent and in the dietary control of patients with chronic diseases caused by OS [13]. Propolis is regarded as a resinous material harvested by honeybees from distinctive plants. It contains many biochemical constituents, like polyphenols, flavonoids, aglycones, phenolic, and ketones, with several biological and pharmacological properties [14]. It has gastroprotective, hepatoprotective, immunomodulatory, wound healing, antidiabetic, and antineoplastic functions, owing to propolis’ antioxidant, anti-inflammatory, and antimicrobial properties [15,16]. Propolis inhibits the generation of various inflammatory markers, such as nitric oxide, IL-1 and IL, due to its high flavonoid content [12]. Propolis’ defensive capacity is brought about from its modulator impact on antioxidant enzymes, suppressing free radicals initiation, and reducing subsequent damage [17]. Studies on mammals clarified that propolis elevated testosterone levels in rats [18,19] and markedly increased rabbit body weight (B.W.), the relative weight of the testes, and epididymis [20]. Caffeic acid phenyl ester (CAPE), a propolis active compound, has been proven to inhibit nuclear factor kappa B and TNFα [21,22]. Testosterone level, lipid profile, and pathological alterations in testes of male rats induced by different chemicals / pesticides as chlorpyrifos were estimated by ElMazoudy et al. [19]; however, estradiol level, OS, and antioxidants, TNF α were not measured too. The current study aimed to elucidate the defensive impact of propolis on CCl4 testicular damage in male rats based on its antioxidant and anti-inflammatory proprieties reflecting on selected biochemical, histopathological, and molecular indices.
Materials and Methods
Ethical approval
All experimental procedures are maintained and carried out by national guidelines and protocols, authorized by the Alexandria University Institutional Animal Care and Use Committee (3082020).
Chemicals and reagents
CCl4 was purchased from Central Drug House, India. Olive oil and bee propolis extract capsules were obtained from Best Naturals, USA. Kits for Total Cholesterol (TC), and triacylglycerol (TAG) were obtained from Vitro Scient Co, Egypt. High-density lipoprotein cholesterol (HDL-c) kit had been received from Spectrum Co, Egypt. Testosterone and estradiol kits were obtained from Cayman Chemical Co., USA. Malondialdehyde (MDA), catalase (CAT), reduced glutathione (GSH), glutathione peroxidase (GPX), and superoxide dismutase (SOD) kits were obtained from Bio diagnostic Co, Egypt. RNeasy Mini Kit (Catalogue #74104) and Quantitect SYBR green PCR kit (Catalogue # 204141) were obtained from Qiagen, USA.
Experimental animals
This investigation was carried out with strict rules to maintain and defend animal welfare without subjecting them to any degree of endurance or stress. Twenty-four male rats weighing 150 ± 10 gm were purchased from an Egyptian company to produce vaccine, sera and drugs. Animals were harbored in clean metal cages with a 12 h day-night cycle, the temperature of 22 ± 2.0 and humidity of 45% ± 1. The rats were fed commercial diet pellets and allowed food and water ad libitum for an adaptation period of 2 weeks. To investigate the damage of CCl4, animals were distributed randomly into four groups (six rats/group) during the 4-week experiment period. Group I (control): injected i.p. with olive oil as a vehicle (3 ml/kg B.W./twice/week). Group II (Propolis) injected i.p., with olive oil and treated orally with propolis (200 mg/kg B.W./daily) [23]. Group III (CCl4) injected i.p. with CCl4 (3 ml/ kg B.W./ twice/week) 1:1 diluted with olive oil [24]. Group IV (propolis/ CCl4) was treated with propolis and CCl4, as mentioned before.
Blood collection
After 4 weeks, rats fasted for 12 h, anesthetized, and blood samples were drawn from the eye’s retro-orbital sinus. The blood samples were let to coagulate, centrifuged at 3,000 rpm for 10 min, and kept frozen at −20°C until used to determine serum lipid profile and sexual hormones (testosterone and estradiol).
Tissue preparation
Animals were anesthetized and sacrificed by cervical dislocation directly after gathering blood samples. Testes were rapidly removed, washed by the ice-cold saline buffer, blotted in filter papers, weighted, and split into three parts. The first part was directly soaked up in 10% formalin solution for histological studies. Expression of TNFα was determined using the second portion which was frozen quickly in liquid nitrogen and kept at −80°C. The third part was weighed and homogenized, using tissue lyser with ice-cold saline preparing 25% w/v homogenate and kept frozen at −20°C to measure the MDA level antioxidant enzyme activities.
Determination of biochemical parameters
Enzyme-linked immunosorbent assay kits were used for the determination of testosterone and estradiol concentrations [25]. The concentrations of TC, TAG, and HDL-c have been estimated according to the procedures reported previously [26–28]. The concentrations of very low-density lipoprotein cholesterol (VLDL-c) and low-density lipoprotein cholesterol (LDL-c) were calculated as mentioned previously [29]. The level of MDA was evaluated in testicular tissues according to Satoh [30]. GSH, GPX, CAT, and SOD activities were determined in testicular tissue by methods described earlier [31–34].
Gene expression analysis of TNF-α using quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from testicular tissues using the RNeasy mini kit (Qiagen, USA) according to the manufacture’s guides. qRT-PCR using 2× QuantiTect SYBR Green PCR Master Mix was carried out to estimate relative quantitative determination of the gene expression TNFα mRNA level using a real-time PCR machine (Stratagene MX3005P). Amplification was carried out using the following protocol: 94°C for 15 sec for both β-actin and TNF-α; 55°C for 30 sec for B-actin and 60°C for 30 sec for TNF-α ;and 72°C for 30 sec for both genes. Relative changes in mRNA levels were determined by the “ΔΔCt” method, as stated previously [35]. Table 1 shows the sequence of the used primer.
Table 1. Primer sequences of the amplified genes.
| Gene | Primer sequence(5’-3’) | Reference |
|---|---|---|
| Rat ß-actin | TCCTCCTGAGCGCAAGTACTCT | [69] |
| GCTCAGTAACAGTCCGCCTAGAA | ||
| TNF-α | ACT GAA CTT GGG GGT GATTG | [70] |
| GCT TGG TGG TTT GCT ACG AC |
TNF-α: Tumor necrosis factor alpha
Histopathological examination
Immediately after sacrificing, testes were quickly immersed in 10% buffered formalin solution for 48 h. After that, the samples were transformed through the paraffin embedding method and were stained with hematoxyline and eosin (H&E) according to the procedures mentioned previously [36].
Statistical analysis
The analysis was carried out using a one-way analysis of variance. Data were expressed as means ± standard error of the mean. p < 0.05 was set as statistically significant [37].
Results
The data concluded in Table 2 illustrate that testes weight and testes/B.W. (%) were markedly increased (p < 0.05) in CCl4-treated rats (group III) in comparison with group I. On the other hand, rats protected with propolis (200 mg/kg/B.W.) successfully reduced (p < 0.05) the increased testes weight and testes/B.W. (%) over group III. Rats in group II showed no significant impact on previous values as compared to rats in group I. CCl4 administration induced a prominent decrease (p < 0.05) in serum testosterone and considerable elevation in estradiol concentration (10.13 ± 0.21a) over group I. Propolis reversed alterations of CCl4 in both serum level of testosterone and estradiol. Moreover, it boosted the testosterone level over control rats.
Table 2. Effect of propolis on testes weight, testis/ B.W. (%), testosterone, and estradiol in rats treated with CCl4.
| Groups | Estradiol(ng/ml) | Testosterone(ng/ml) | Testis/body weight (%) | Testis weight (gm) | Final body weight (gm) |
|---|---|---|---|---|---|
| Group I | 3.23 ± 0.45d | 3.87 ± 0.13c | 2.52 ± 0.12c | 5.77 ± 0.31c | 228.80±1.77a |
| Group II | 4.10 ± 0.37d | 5.22 ± 0.11a | 2.41 ± 0.12c | 5.54 ± 0.22c | 229.40±2.25a |
| Group III | 10.13 ± 0.21a | 1.9 ± 0.09b | 1.20 ± 0.14a | 2.56 ± 0.29a | 211.60±4.15b |
| Group IV | 7.76 ± 0.08b | 3.07 ± 0.06d | 2.13 ± 0.21c | 4.75 ± 0.39c | 223.00±3.24a |
Values are mean ± standard errors.
Means in the same column with different letters are significantly different at p < 0.05.
Group I (control); Group II: Propolis 200 mg/kg B.W.; Group III: CCl4-treated rats 2 ml/kg B.W./i.p.; and Group IV: CCl4 + propolis.
The outcomes shown in Table 3 revealed that serum TC, TAG, and LDL-c levels were increased markedly (p < 0.05); however, serum HDL-c was significantly decreased (p < 0.05) in group III as compared to group I. Propolis induced a pronounced hypolipidemic activity as reflected in a significant decrease (p < 0.05) of serum TC, TAG, VLDL-c, and LDL-c levels and a significant increase (p < 0.05) of HDL-c compared to group III. Serum HDL-c increased significantly, while LDL-c level was decreased markedly (p < 0.05) in group II compared to group I.
Table 3. Effect of Propolis on Serum lipid profile in rats treated with CCl4.
| Groups | HDL-c (mg/dl) | LDL-c(mg/dl) | vLDL-c (mg/dl) | TAG (mg/dl) | TC(mg/dl) |
|---|---|---|---|---|---|
| Group I | 48.5 ± 1.59b | 34.27 ± 0.6b | 16.86 ± 0.66b | 84.3 ± 3.32b | 99.6 ± 3.15cb |
| Group II | 60.3 ± 1.96c | 16.18 ± 0.03c | 15.92 ± 1.42b | 79.6 ± 7.11b | 92.4 ± 3.21c |
| Group III | 22 ± 0.67a | 84.4 ± 1.18a | 29.2 ± 1.21a | 146.0 ± 6.07a | 136 ± 3.06a |
| Group IV | 35.2 ± 1.07d | 58.4 ± 1.61d | 24.4 ± 1.11d | 122 ± 5.54d | 118 ± 3.79d |
Values are mean ± standard errors.
Means in the same column with different letters are significantly different at p < 0.05.
Group I (control); Group II: Propolis 200 mg/kg. B.W.; Group III: CCl4-treated rats 2 ml/kg B.W./i.p.; and Group IV: CCl4 + propolis.
TC = Total cholesterol; TAG = Triacylglycerol; vLDL-c = Very low-density lipoprotein cholesterol; LDL-c = Low-density lipoprotein cholesterol; HDL-c = High-density lipoprotein cholesterol.
Data in Table 4 reveal that CCl4 significantly increased (p < 0.05) the testicular level of MDA and significantly decreased (p < 0.05) the GSH levels and activities of GPX, GSH, CAT, and SOD in contrast to group I. Propolis successfully decreased (p < 0.05) MDA concentration and showed significant antioxidant proprieties as reflected in the prominent increase (p < 0.05) in GSH level and antioxidant enzymes activities (GPX, CAT, and SOD) compared to group III. Besides, propolis significantly improved (p < 0.05) the antioxidant capacity and attenuated peroxidation in group II in comparison with group I.
Table 4. Effect of propolis on testes lipid peroxidation, GSH, and antioxidant enzymes in rats treated with CCl4.
| Groups | SOD (IU/gm wt tissue) | CAT (IU/gm wt tissue) | GSH (μmol/gm wt tissue) | GPX (IU/gm wt tissue) | MDA (nmol/gm wt tissue) |
|---|---|---|---|---|---|
| Group I | 131.23 ± 1.18e | 412.12 ± 3.16e | 37.89 ± 1.68c | 8.27 ± 0.23c | 24.40 ± 0.55de |
| Group II | 134.12 ± 1.23e | 409.5 ± 3.11e | 50.70 ± 2.33a | 12.20 ±0.22a | 14.42 ± 0.91a |
| Group III | 85.92 ± 1.1b | 135.72 ± 2.3b | 19.82 ± 1.61b | 3.08 ± 0.25b | 60.85 ± 5.88c |
| Group IV | 115.13 ± 1.2c | 310.37 ± 2.6c | 31.35 ± 2.15e | 6.04 ± 0.55e | 40.73 ± 4.73b |
Values are mean ± standard errors.
Means in the same column with different letters are significantly different at p < 0.05.
Group I (control); Group II: Propolis 200 mg/kg. B.W.; Group III: CCl4-treated rats 2 ml/kg B.W./i.p.; and Group IV: CCl4 + propolis.
MDA = Malondialdehyde; GPX = Glutathione peroxidase; GSH = Reduced glutathione; CAT = Catalase; SOD = Superoxide dismutase.
CCl4 caused marked inflammation as reflected on significant upregulation (p < 0.05) TNFα gene expression in contrast with group I (Table 5). The combined administration of propolis induced the downregulation (p < 0.05) of TNFα gene expression compared to group I (Table 5), attenuating the testicular inflammation caused by CCl4.
Table 5. Effect of propolis on testes gene expression of TNF-α in rats treated with CCl4.
| Groups | Expression fold change (2-∆∆ct) |
|---|---|
| Group I | 1 ± 0.02e |
| Group II | 0.35 ± 0.01a |
| Group III | 3.7 ± 0.12b |
| Group IV | 2.1 ± 0.11c |
Values are mean ± standard errors.
Mean in the same column with different letters are significantly different at p < 0.05.
Group I (control); Group II: Propolis 200 mg/kg B.W.; Group III: CCl4-treated rats 2 ml/kg B.W./i.p.; and Group IV: CCl4+ propolis. TNF-α: Tumor necrosis factor alpha.
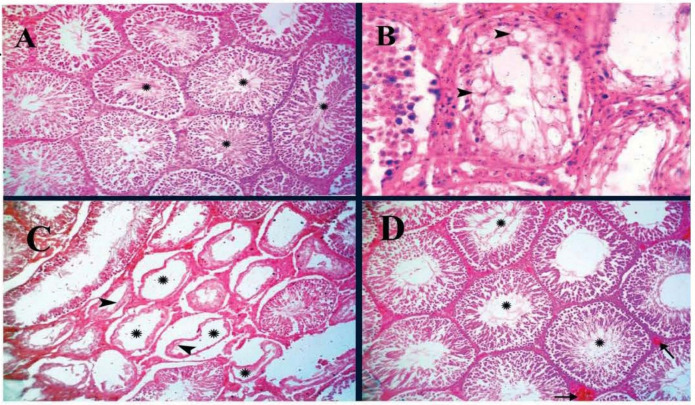
As indicated in Figure 1A, no histopathological alterations have been recorded in rat testes of group I and group II. Also, the seminiferous tubules and interstitial tissue showed standard histological criteria (Fig. 1A). On the contrary, the rats’ testes in group III revealed congestion in testicular blood vessels and interstitial capillaries. The interstitium showed edema as it was mildly extended by homogenous eosinophilic material. Multifocal, moderate numbers of seminiferous tubules exhibited marked degeneration of lining epithelial cells characterized by swollen pale discrete large vacuoles, usually replacing the cytoplasm and displaced the nucleus periphery of the cell (Fig. 1B). Occasionally, the degenerated tubules showed collapse, with thick buckled basement membrane and lined by single or two layers of degenerated germ cells. These changes were frequently accompanied by reduced spermatogenesis and spermatozoa’s absence in the degenerated tubules lumen (Fig. 1C). Testes of rats received combined doses of propolis and CCl4 (group IV) showed the normal histological appearance of seminiferous tubules, mild congestion of testicular blood vessels, and moderate active spermatogenesis (Fig. 1D)
Figure 1. The histopathological findings of rat testes. (A): Groups (I and II) showing a normal histological appearance of seminiferous tubules (asterisk); H&E stain ×200. (B): Testis of rat from CCl4-treated group (III) showing testicular degeneration characterized by swollen pale discrete large cytoplasmic vacuoles (arrowhead) in the germinal epithelium; H&E stain ×400. (C) Testis of rats from the group (III) showing degeneration of the lining epithelium (arrow) of few seminiferous tubules accompanied by reduced spermatogenesis and absence of spermatozoa in the lumen (asterisk); H&E stain ×100. (D): Group (IV) showed normal histological criteria of the seminiferous tubules with moderate active spermatogenesis (asterisk) and mild congestion of testicular blood vessels (arrow); H&E stain ×200.
Discussion
Normal cellular function is maintained through keeping equilibrium between the reactive oxygen species (ROS) and the antioxidants [38]. ROS formed either typically during cellular metabolism or particular chemicals exposure [39]. Spermatozoa are permanently exposed to the “oxygen paradox”, resulting in excessive generation of ROS involved in male infertility [40].
Cell damages induced by ROS could be slowed or prevented by antioxidants [41]. Propolis has many biological activities as anti-inflammatory, anticancer, antioxidant, antibiotic, and antifungal activities [20,42]. Recently, propolis was stated as a potent scavenger of ROS [43]. The observed significant reduction of testes weight, testes/B.W. ratio, and testosterone concentration in rats were administered with CCl4 compared to control ones caused by the CCl4 toxic effect. CCl4 can generate free radicals leading to OS affecting testicular germline, testicular weight, or even testosterone level. Male infertility could be caused directly by free radicals or indirectly through disrupting male hormonal balance leading to reproductive dysfunction [44]. A pronounced drop in testosterone confirmed alterations in rats, reproductive physiology. The reduction in testosterone production may activate P450 aromatase, which favors estrogen production from androgen, thereby decreasing androgen levels [19]. The recorded significant increase of estradiol level may indicate dysregulation of the pituitary function as an insult of CCl4 exposure [45–47]. The current finding regarding testicular weight, testosterone, and estrogen concentrations confirmed by histopathological findings of the testis indicated a degeneration of testicular tissue and the lining epithelium of a few seminiferous tubules accompanied by reduced spermatogenesis and absence of spermatozoa in group III. A previous report [48] stated that administration of CCl4 to normal rats induced a marked reduction in body weight, testes, seminal vesicles, and prostate glands; moreover, there was marked testicular degeneration, lowered semen quality and quantity, a marked reduction of testosterone, luteinizing hormone and follicle-stimulating hormone concentrations with a prominent increase of rat serum concentration of prolactin and estradiol.
The propolis defensive role against the detrimental impact of CCl4 in testes weight, testes/B.W. ratio, and sex hormones (testosterone and estradiol) may be explained by the antioxidant capacities of phenols and flavonoids or other described constituents [49–53] protecting the reproductive system against toxicity. Other reports [20,54] revealed that propolis provides elevation in testosterone level, body, and testes weights with reductions of oxidants levels. The observed antioxidant effects came in harmony with histopathological findings that indicated the typical architecture of testes of propolis-treated rats.
The significant increase of TC, TAG, LDL-c, and VLDL-c and pronounced drop of HDL-c in rats treated with CCl4 following the previous reports [55,56] demonstrated that CCl4 increased peroxidation of polyunsaturated fatty acid and oxidants concentrations. The increased level of cholesterol is owed to reduced androgen concentration, as mentioned in Table 2, which agrees with ElMazoudy et al. [19]. The observed ameliorative impact of Propolis on the detrimental effect of CCl4 on lipid profile has been documented earlier in rats [57,58], particularly TAG level. Propolis hypolipidemic role might be exhibited through lipase stimulation and modulation of metabolism of lipid [59]. Moreover, Alves et al. [60] cited that propolis showed hypocholesterolemic effect attributed either directly to the hepatic role or indirectly to the thyroid hormones, as these hormones affect the fat metabolism.
Antioxidants relieve stress conditions produced from increased oxidants over antioxidants through either enzymatic or non-enzymatic pathways. Excessive ROS could produce lipid peroxidation, damage DNA, RNA, and denatured protein in the spermatozoa [44,45,61]. Testicular lipid peroxidation was increased after CCl4 exposure as the testicular MDA was increased parallel to depletion of GSH content and the reduction activities of GPX, CAT, and SOD. This could be referred to as the increase in polyunsaturated fatty acids peroxidation that presents in the testicle cell membrane. The phase activates the CCl4 I cytochrome P450 system to form reactive metabolic trichloromethyl radicals (CCL3), which is the principal initiator of lipid peroxidation. CCl4 consumed GSH as an outcome of enhancing lipid peroxidation, as mentioned by Park et al. [62]. In the current research, the antioxidant system (SOD, CAT, GPX, and GSH) in CCl4-treated groups was prominently reduced compared to group I. CCl4 generated cellular ROS subsequently depleted of the antioxidant cellular system as mentioned by El-Boshy et al. [63]. OS indicated by elevated testicular MDA may also be caused by elevated estradiol in group III due to high estradiol testicular fatty acid degeneration [47]. Chronic inflammation is linked with OS as ROS is considered as second messengers that propagate pro-inflammatory signals that’s why rats treated with CCl4 showed upregulation of TNFα expression compared to control. This was confirmed by current histopathological findings that cleared inflammatory testicular degeneration characterized by swollen pale discrete large cytoplasmic vacuoles and edema as indicated by expanded interstitium due to homogenous eosinophilic material.
Infertility has been associated with testicular OS recommending the need to develop powerful antioxidant therapies facing this condition [64]. Preceding results cleared that propolis treatment ameliorated the CCl4-induced imbalance in the oxidant-antioxidant system of testes due to its antioxidant properties and successfully increased the intracellular concentration of glutathione. Glutathione has crucial participation in maintaining antioxidant and down-regulating cytokine transcription and biosynthesis. Therefore, pre-treatment of propolis downregulated expression of TNF-α and successfully increased SOD, CAT, and GPX activities in CCl4-treated rats. These findings came in parallel with other works [7,65,66], which stated that propolis increased concentration of reduced glutathione and SOD activity and reduced lipid peroxidation processes in plasma, liver, lungs, and brain of mice in a dose and tissue-dependent manner. Propolis showed potent anti-inflammatory properties due to CAPE, a chief element of propolis, and may act through suppressing pro-inflammatory cytokines or mediators like TNFα and interleukins [67,68]. Validation of approach of the present research as clinical therapies has to be performed in the future. Besides expression of antioxidants and cytokines genes during propolis supplementation in CCl4 intoxicated rats has to be conducted.
Conclusion
The current research concluded that the used dose of propolis (200 mg/kg/B.W.) is safe and capable of ameliorating CCl4 testicular damage in rats. Propolis showed a protective effect. It successfully improved antioxidants and alleviated OS conditions, reduced TNFα, corrected hormone levels, and replaced damaged tissues with healthy ones; however, constructive studies must be accomplished to approve these trials as clinical treatments.
List of abbreviations
CCl4: Carbon tetrachloride, TNFα: Tumor necrosis factor alpha, CCL3: trichloromethyl radical, IL-1: Interleukine-1, CAPE: Caffeic acid phenyl ester, TC: Total cholesterol, TAG: TriAcylGlycerol, HDL-c: High-density lipoprotein cholesterol, MDA: Malondialdehyde, GSH: Reduced glutathione, GPX: Glutathione peroxidase, SOD: Superoxide dismutase, VLDL-c: Very low-density lipoprotein cholesterol, LDL-c: Low-density lipoprotein cholesterol, CAT: Catalase, qRT-PCR: Quantitative real-time polymerase chain reaction, H&E: Hematoxyline and eosin, OS: Oxidative stress, ROS: Reactive oxygen species.
Acknowledgments
The author is grateful to the Department of Biochemistry, Faculty of Veterinary Medicine, Alexandria University.
Conflict of interest
The author declares that there is no conflict of interest related to this research.
Author’s contribution
ASH is responsible for designing, experimentation, analysis, and manuscript preparation and finalization.
References
- [1].Ganie SA, Haq E, Hamid A, Qurishi Y, Mahmood Z, Zargar BA. Carbon tetrachloride induced kidney and lung tissue damages and antioxidant activities of the aqueous rhizome extract of Podophyllum hexandrum. BMC Complement Altern Med. 2011;11:1–10. doi: 10.1186/1472-6882-11-17. https://doi.org/10.1186/1472-6882-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Khaki A, Fathiazad F, Nouri M, Khaki AA, Ghanbari Z, Ghanbari Anti-oxidative effects of citro-flavonoids on spermatogenesis in rat. Afr J Pharm Pharmacol. 2011;5:721–5. https://doi.org/10.5897/AJPP11.277. [Google Scholar]
- [3].Al-Olayan E, El-Khadragy M, Othman M, Aref A, Kassab R, Abdel Moneim AE. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxid Med Cell Longev. 2014;2(1):12–7. doi: 10.1155/2014/381413. https://doi.org/10.1155/2014/381413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abouzied MM, Eltahir HM, Taye A, Abdelrahman MS. Experimental evidence for the therapeutic potential of tempol in the treatment of acute liver injury. Mol Cell Biochem. 2016;6,411(1–2):107–15. doi: 10.1007/s11010-015-2572-2. https://doi.org/10.1007/s11010-015-2572-2. [DOI] [PubMed] [Google Scholar]
- [5].Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. https://doi.org/10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- [6].Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28(10):1456–62. doi: 10.1016/s0891-5849(00)00252-5. https://doi.org/10.1016/S0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- [7].Ma JQ, Ding J, Zhang L, Liu CM. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem Toxicol. 2014;64:41. doi: 10.1016/j.fct.2013.11.017. https://doi.org/10.1016/j.fct.2013.11.017. [DOI] [PubMed] [Google Scholar]
- [8].Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13(2):135–41. doi: 10.1016/s1359-6101(01)00020-x. https://doi.org/10.1016/S1359-6101(01)00020-X. [DOI] [PubMed] [Google Scholar]
- [9].Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106(9):1127–37. doi: 10.1172/JCI9914. https://doi.org/10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. J Comp Physiol. 2013;3(2):977–1010. doi: 10.1002/cphy.c120020. https://doi.org/10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Devi R, Boruah DC, Sharma DK, Kotoky J. Leaf extract of Clerodendron colebrookianum inhibits intrinsic hypercholesterolemia and extrinsic lipid peroxidation. Int J Pharm Technol Res. 2011;3(2):960–7. [Google Scholar]
- [12].Shang H, Bhagavathula AS, Aldhaleei WA, Rahmani J, Karam G, Clark C. Effect of propolis supplementation on c reactive protein level and other inflammatory factors: a systematic review and meta-analysis of randomized controlled trials. J King Saud Univ Sci. 2020;32(2):1694–1701. https://doi.org/10.1016/j.jksus.2020.01.003. [Google Scholar]
- [13].Wang T, Chen L, Wu W, Long Y, Wang R. cytoprotection: antioxidant defense by caffeic acid phenethyl ester against free radical-induced damage of lipids, DNA, and proteins. Can J Physiol Pharmacol. 2008;86:279–87. doi: 10.1139/y08-029. https://doi.org/10.1139/Y08-029. [DOI] [PubMed] [Google Scholar]
- [14].Naama HJ, Nima ZA, Suleiman MG. Effects of active materials in alcoholic extract of Iraqi propolis on growth of some cancer lines in the laboratory and cancer of mammary gland in mice. Rep Opin. 2010;2(5):12–20. [Google Scholar]
- [15].Kurek-Gorecka A, Rzepecka-Stojko A, Gorecki M, Stpiko J, Sosada M, Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules. 2013;19:78–101. doi: 10.3390/molecules19010078. https://doi.org/10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marinotti S, Ranzato E. Propolis: a new frontier for wound healing? Int J Burns Trauma. 2015;3:9. doi: 10.1186/s41038-015-0010-z. https://doi.org/10.1186/s41038-015-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barlak U, Deger O, Ucar M, Cakiroglu TN. Effects of Turkish propolis extract on secretion of polymorphonuclear elastase following respiratory burst. Res Art Turk J Biol. 2015;39(4):1–8. https://doi.org/10.3906/biy-1402-48. [Google Scholar]
- [18].Yousef MI, Salama AF. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem Toxicol. 2009;47:1168–75. doi: 10.1016/j.fct.2009.02.006. https://doi.org/10.1016/j.fct.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [19].ElMazoudy RH, Attia AA, El-Shenawy NS. Protective role of propolis against reproductive toxicity of chlorpyrifos in male rats. Pestic Biochem Physiol. 2011;101(3):175–81. https://doi.org/10.1016/j.pestbp.2011.09.003. [Google Scholar]
- [20].Yousef MI, Kamel KI, Hassan MS, El-Morsy A. Protective role of propolis against reproductive toxicity of triphenyltin in male rabbits. Food Chem Toxicol. 2010;48:1846–52. doi: 10.1016/j.fct.2010.04.018. https://doi.org/10.1016/j.fct.2010.04.018. [DOI] [PubMed] [Google Scholar]
- [21].Fitzpatrick LR, Wang J, Le T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-kappaB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. J Pharmacol Exp Ther. 2001;299:915–20. [PubMed] [Google Scholar]
- [22].Murtaza G, Sajjad A, Mehmood Z, Shah SH, Siddiqi AR. Possible molecular targets for therapeutic applications of caffeic acid phenethyl ester in inflammation and cancer. J Food Drug Anal. 2015;23(1):11–8. doi: 10.1016/j.jfda.2014.06.001. https://doi.org/10.1016/j.jfda.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bhadauria M. Propolis prevents hepatorenal injury induced by chronic chronic exposure to carbon tetrachloride. Res Art Evid Based Complement Altern Med. 2012;12(4):1–12. doi: 10.1155/2012/235358. https://doi.org/10.1155/2012/235358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huda MA, Rahmat AK, Muhammad RK, Sumaira S. CCl4 induced genotoxicity and DNA oxidative damages in rats: hepatoprotective effect of Sonchus arvensis. BMC Complement Altern Med. 2014;21(14):45. doi: 10.1186/1472-6882-14-452. https://doi.org/10.1186/1472-6882-14-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yoshihiko Inaoka Y, Yazawa T, Uesaka M, Mizutani T, Yamada K, Miyamoto K. Regulation of NGFI-B/Nur77 gene expression in the rat ovary and in Leydig tumor cells MA-10. Mol Reprod Dev. 2008;75:931–9. doi: 10.1002/mrd.20788. https://doi.org/10.1002/mrd.20788. [DOI] [PubMed] [Google Scholar]
- [26].Searcy RL. New York, NY: McGraw-Hill; 1969. Diagnostic biochemistry. [Google Scholar]
- [27].Stein EA. 3rd. Philadelphia, PA: WB Saunders; 1987. Lipids, lipoproteins and apolipoproteinsz; Tiet NW, Fundement clin chem; pp. 448–81. [Google Scholar]
- [28].Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem Res. 1972;18:499–502. https://doi.org/10.1093/clinchem/18.6.499. [PubMed] [Google Scholar]
- [29].Bauer JD. 9th. Maryland Heights, MO: The C.V. Company; 1982. Clinical laboratory methods; p. 555. [Google Scholar]
- [30].Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Int J Clin Chem. 1978;90:37–42. doi: 10.1016/0009-8981(78)90081-5. https://doi.org/10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- [31].Beutler E, Duron O, Kellin BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- [32].Palgia DE, Valentine WN. Studies on quantitative and qualitative characteriztion of erythrocyte peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- [33].Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. https://doi.org/10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- [34].Nishikimi M, Roa NA, Yogi K. The occurrence of supeoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophes Res. 1972;46:849–54. doi: 10.1016/s0006-291x(72)80218-3. doi:10.1016/S0006-291X(72)80218-3 . [DOI] [PubMed] [Google Scholar]
- [35].Yuan JS, Reed A, Chen F, Stewart CN. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. https://doi.org/10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Culling CF. 3rd. London, UK: Butterworth; 1983. Handbook of histopathological and histochemical techniques. [Google Scholar]
- [37].SAS. Cary, NC: SAS Institute, INC.; 2002. Statistical analysis system, version 9, users guide. [Google Scholar]
- [38].Fidan AF, Dundar Y. The effects of Yucca schidigera and Quillaja saponaria on DNA damage, protein oxidation, lipid peroxidation and some biochemical parameters in streptozotocin-induced diabetic rats. J Diabetes Complications. 2008;22(5): 348–56. doi: 10.1016/j.jdiacomp.2007.11.010. https://doi.org/10.1016/j.jdiacomp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- [39].Qian ZJ, Jung WK, Kim SK. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Ran catesbeiana shaw. Bioresour Technol. 2008;99:1690–8. doi: 10.1016/j.biortech.2007.04.005. https://doi.org/10.1016/j.biortech.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [40].Maneesh M, Jayalekshmi H. Role of reactive oxygen species and antioxidants on pathophysiology of male reproduction. Indian J Clin Biochem. 2006;21:80–89. doi: 10.1007/BF02912918. https://doi.org/10.1007/BF02912918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Grigorov B. Reactive oxygen species and their relation to carcinogenesis. Trakia J Sci. 2012;10:83–92. [Google Scholar]
- [42].Marquele FD, Di Mambro VM, Georgetti SR, Casagrande R, Valim YM, Fonseca MJV. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J Pharm Biomed Anal. 2005;39:455–62. doi: 10.1016/j.jpba.2005.04.004. https://doi.org/10.1016/j.jpba.2005.04.004. [DOI] [PubMed] [Google Scholar]
- [43].Ozguner F, Armagan A, Koyu A, Calıskan S, Koylu H. A novel antioxidant agent caffeic acid phenethyl ester (CAPE) prevents shock wave-induced renal tubular oxidative stress. Urol Res. 2005;33:239–43. doi: 10.1007/s00240-005-0470-x. https://doi.org/10.1007/s00240-005-0470-x. [DOI] [PubMed] [Google Scholar]
- [44].Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, et al. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol. 2018;16(1):87. doi: 10.1186/s12958-018-0406-2. https://doi.org/10.1186/s12958-018-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khan MR, Ahmed DH. Protective effects of Allium ampe-loprasum Mart on testis against oxidative stress of carbon tetra-chloride in rat. Food Chem Toxicol. 2011;47:1393–9. doi: 10.1016/j.fct.2009.03.020. https://doi.org/10.1016/j.fct.2009.03.020. [DOI] [PubMed] [Google Scholar]
- [46].Usman J, Dauda F, Nwenfulu K. Study effects of silymarin on reproductive variables in male wistar rats with carbon tetra chloride (CCl4) - induced liver fibrosis. Am J Innov Res Appl Sci. 2016;1(6):214–21. [Google Scholar]
- [47].Leavy M, Trottmann M, Liedl B, Rees S, Steif C, Fertige Baugh J, et al. Effects of elevated β-estradiol levels on the functional morphology of the testis - new insights. Sci Rep. 2017;7:39931. doi: 10.1038/srep39931. https://doi.org/10.1038/srep39931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jaffat H, Salih A, Sabah N, Adhraa BH. Protective effect of allium ampeloprasum against toxicity induced by CCl4 in male white rats. Int J Sci Eng Res. 2014;5(10):825–28. [Google Scholar]
- [49].Capucho C, Sette R, de Souza Predes F, de Castro Monteiro J, Pigoso AA, Barbieri R. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem Toxicol. 2012;50:3956–62. doi: 10.1016/j.fct.2012.08.027. https://doi.org/10.1016/j.fct.2012.08.027. [DOI] [PubMed] [Google Scholar]
- [50].Cristina CF, Souza P, Renata B, Mary A, Heidi D, Grasiela DC. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem Toxicol. 2012;50:3956–62. doi: 10.1016/j.fct.2012.08.027. https://doi.org/10.1016/j.fct.2012.08.027. [DOI] [PubMed] [Google Scholar]
- [51].Tatli SP, Yilmaz S, Seven I, Tuna KG. Effects of propolis in animals exposed oxidative stress. In: Lushchak VI, editor. Oxidative stress-environment induct dietary antioxidants. InTech. Rijeka, Croatia: 2012. pp. 267–88. doi:10.5772/34850. [Google Scholar]
- [52].Sherine M, Rizk A, Hala F, Zaki B, Mary AM. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem Toxicol. 2014;67:176–86. doi: 10.1016/j.fct.2014.02.031. https://doi.org/10.1016/j.fct.2014.02.031. [DOI] [PubMed] [Google Scholar]
- [53].Baykalir GB, Tatli S, Gur I. The effects of propolis on sperm quality, reproductive organs and testicular antioxidant status of male rats treated with cyclosporine-A. Anim Reprod Res. 2016;13(2):105–11. https://doi.org/10.21451/1984-3143-AR736. [Google Scholar]
- [54].Meurer F, Costa MMD, Barros DAD, Oliveira STL, Paixao PS. Brown propolis extract in feed as a growth promoter of Nile tilapia (Oreochromis niloticus, Linnaeus 1758) fingerlings. Aquac Res. 2009;40:603–8. https://doi.org/10.1111/j.1365-2109.2008.02139.x. [Google Scholar]
- [55].Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride-induced kidney injury in wistar rats. Afri J Biomed Res. 2007;10:153–64. https://doi.org/10.4314/ajbr.v10i2.50619. [Google Scholar]
- [56].Hosseinzadeh H, Parvardeh S, Asl M. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomed. 2007;14(9):621–7. doi: 10.1016/j.phymed.2006.12.005. https://doi.org/10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- [57].Azab AE, Algridi MA, Lashkham NM. Hypolipidemic and antiatherogenic effects of aqueous extract of Libyan propolis in lead acetate intoxicated male albino mice. Int J Scie Res. 2015;4(3):1060–8. [Google Scholar]
- [58].Albokhadaim I. Influence of dietary supplementation of propolis on hematology, biochemistry and lipid profile of rats fed high cholesterol diet. J Adv Vet Anim Res. 2015;2(1):56–63. https://doi.org/10.5455/javar.2015.b49. [Google Scholar]
- [59].Cetin E, Kanbur M, Silici S, Eraslan G. Propetamphos induced changes in haematological and biochemical parameters of female rats: protective role of propolis. Food Chem Toxicol. 2010;48:1806–10. doi: 10.1016/j.fct.2010.04.010. https://doi.org/10.1016/j.fct.2010.04.010. [DOI] [PubMed] [Google Scholar]
- [60].Alves M, Mesquita F, Sakaguti M, Tardivo A. Hypocholesterolemic effect of propolis caffeic acids. Braz J Med Plants. 2008;10:100–5. [Google Scholar]
- [61].Khan RA. Protective effects of Launaea procumbens on rat testis damage by CCl4. Lipids Health Dis. 2012;11:103. doi: 10.1186/1476-511X-11-103. doi:10.1186/1476-511X-11-103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Park WH, Lee SK, Kim CH. Akorean herbal medicine, panax notoginseng, prevent liver fibrosis and hepatic microvascular dysfunction in rats. Life Sci. 2005;76:1675–90. doi: 10.1016/j.lfs.2004.07.030. doi:10.1016/j.lfs.2004.07.030 . [DOI] [PubMed] [Google Scholar]
- [63].El-Boshy ME, Abdelhamid F, Richab E, Ashshia A, Gaitha M, Qustya N. Attenuation of CCl4 induced oxidative stress, immunosuppressive, hepatorenal damage by Fucoidan in Rats. J Clin Toxicol. 2017;7:3. https://doi.org/10.4172/2161-0495.1000348. [Google Scholar]
- [64].Turner TT, Lysiak JL. Oxidative stress: a common factor in testicular dysfunctions. J Androl. 2008;29:488–98. doi: 10.2164/jandrol.108.005132. https://doi.org/10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- [65].Shinohara R, Ohta Y, Hayashi T, Ikeno T. Evaluation of antilipid peroxidative action of propolis ethanol extract. Phytother Res. 2002;16(4):340–7. doi: 10.1002/ptr.894. https://doi.org/10.1002/ptr.894. [DOI] [PubMed] [Google Scholar]
- [66].Abeer ME, Amal AS, Faiza AM. The potential protective effect of propolis on experimentally induced hepatitis in adult male albino rats. histological and immunohistochemical study. J Histol Histopathol. 2015;2(4):1–9. https://doi.org/10.7243/2055-091X-2-14. [Google Scholar]
- [67].El-Shobaki FA, Refaat OG, Saleh ZA, Abd-Elfatahand AS, El-Hagar EF. The effect of consuming a cake containing propolis on gut micro flora and toxicity. J Amer Sci. 2011;7(7):421–29. [Google Scholar]
- [68].Pahlavani N, Malekahmadi M, Firouzi S, Rostami D, Sedaghat A, Moghaddam AB, et al. Molecular and cellular mechanisms of the effects of propolis in inflammation, oxidative stress and glycemic control in chronic diseases. Nutr Metab (Lond), 2020;17:65. doi: 10.1186/s12986-020-00485-5. https://doi.org/10.1186/s12986-020-00485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wentzel P, Gäreskog M, Eriksson UJ. Decreased cardiac glutathione peroxidase levels and enhanced mandibular apoptosis in malformed embryos of diabetic rats. Diabetes. 2008;57(120):112–8. doi: 10.2337/db08-0830. https://doi.org/10.2337/db08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Khan HA, Abdelhalim MAK, Alhomida AS, Al Ayed MS. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet Mol Res. 2013;12(4):5851–7. doi: 10.4238/2013.November.22.12. https://doi.org/10.4238/2013.November.22.12. [DOI] [PubMed] [Google Scholar]