Abstract
Objective:
Anticoccidial drugs may lead to the development of drug resistance and drug residues. Herbal extracts could be an attractive alternative. This research was undertaken to evaluate the anticoccidial outcome of Carica papaya compared with the anticoccidial drug (Toltazuril) in Sonali chickens.
Materials and Methods:
A total of 80 Sonali chickens were evenly and equally allocated into four groups, namely T1 (non-infected control), T2 (infected control), T3 (treated with C. papaya), and T4 (treated with Toltrazuril). All groups were experimentally infected with oocysts of mixed Eimeria spp. orally except T1, and the mixed Eimeria spp. oocyst load (OL), body weight (BW) gain, and hematological parameters were calculated.
Results:
In the findings, the highest OL reduction rate in T4 was 100%, while the T3 was 83.44%. Nevertheless, BW differed significantly (p < 0.01) among the different groups, while the daily BW gain was higher in T3 amounting to 8.10 gm. In the case of hematological parameter, total erythrocyte count (TEC), hemoglobin (Hb), packed cell volume (PCV), and erythrocyte sedimentation rate in different groups were almost the same and were also statistically insignificant (p > 0.05) barring total leukocyte count resulting as significant (p < 0.05) at day 30. Additionally, the results of Pearson’s correlation in T3 at day 30 indicated a strong significant (p < 0.01) negative correlation between OL and BW (r = −0.780) with the following regression equation: y = −0.16*x + 433.665. Moreover, the correlation of TEC, PCV, and Hb with OL was significantly (p < 0.01) negative, r = −0.786, r = −0.752 and r = −0.633, where the regression equations were y = −0.03*x + 4.51, y = −0.03*x + 27.42, and y = −0.04*x + 11.40, respectively.
Conclusion:
Long-term use of C. papaya leaves’ extracts effectively controls coccidiosis in Sonali chickens and can act as an effective growth promoter.
Keywords: Carica papaya, Toltrazuril, hematological, Sonali, Eimeria spp.
Introduction
The poultry business in Bangladesh is a prospective and effective considerable income-generating sector, especially for the rural people [1]. Besides this, poultry meats supplement about 37% of this large population’s total meat production and protein demand in Bangladesh [2]. Among the poultry in Bangladesh, the Sonali chicken population gradually enhances to fulfill consumers’ demand [3]. Sonali chickens are more likely to local chickens of Bangladesh, from the cross of Rhode Island Red cocks and Fayoumi hens [4]. However, poultry production has been hampered by many enteric diseases like coccidiosis [5]. A protozoan parasite belonging to the genus Eimeria of the Eimeridae family under the Apicomplexa phylum is mainly responsible for coccidiosis in poultry [6]. Most commonly, poultry is susceptible to some species of Eimeria, including Eimeria tenella, Eimeria acervulina, Eimeria maxima, and Eimeria brunette [7]. Although the chickens of 3–8 weeks of age are most susceptible to coccidiosis, all ages are also vulnerable [8]. Accordingly, coccidiosis reduced feed conversion efficiency, weakness, severe physiological damages, diarrhea, weight loss, and anemia, followed by death [9,7]. Moreover, coccidiosis occurrences are high in poultry of commercial farm, starting from 5% up to 70%, leading to increased morbidity and mortality [10,11], which increased production cost per chicken by £0.16 [12].
Therefore, the chemical coccidiostats in poultry feed are the primary approach for controlling coccidiosis in chicken [13]. However, these drugs’ routine practice and abuse are driven toward Eimeria strains’ emersion having drug-resistant properties [14] with many other negative implications of these drugs [15]. Along with the drug resistance, anticoccidial drug residues are also present in poultry products, which have potentially harmful effects on public health and food safety concerns [16]. Alternatively, during the last decades, the plant extracts were widely investigated to substitute the preventive methods for limiting avian coccidiosis and progressing poultry’s performance [17]. Among the botanical elements as sustainable alternatives, C. papaya belongs to the family Caricaceae, which has been acquainted with be feasible against coccidiosis in some states due to less bitterness and the presence of carotene [18]. It can also enhance the palatability of feed and subsequently boost up the growing performance of chick. Besides this, it has anti-inflammatory properties, protects the caecal epithelial cells, and prevents the coccidial reproduction [19]. However, only some limited findings have been reported on C. papaya leaf extract as a potential agent against coccidiosis, especially in Sonali chickens in Bangladesh. Additionally, updated information on plant extract’s comparative efficacy is essential because Eimeria spp. can change their resistant properties to the conventional anticoccidial agents day-by-day and raise a continuous necessity to track down new anticoccidials [20]. Hence, the current study was conducted to assess C. papaya leaf extract’s anticoccidial effect compared to the anticoccidial drug (Toltazuril) on body weight (BW), hematological parameters, and oocyst (mixed Eimeria spp.) per gm feces in Sonali chickens.
Materials and Methods
Ethical approval
This research was according to the ethics and guidelines, including animal care followed by the Department of Physiology and Pharmacology of Hajee Mohammad Danesh Science and Technology University (HSTU), Dinajpur-5200, Bangladesh. The approval number is HSTU/VAS/PPH-1068, Date: 07-01-2019 (Resolution No: 08).
Study site and duration
This trial was carried out from 3rd January to 2nd February 2019 at the Department of Physiology and Pharmacology, HSTU, Dinajpur-5200, Bangladesh, and the laboratory of the Department of Pathology and Parasitology of this university where protozoal egg count was carried out.
Experimental design
In this research, the experimental design was entirely randomized. All the empirical birds (n = 80) were equally and randomly allocated into the four groups, namely T1, T2, T3, and T4, with 20 chickens each. Although the group T1 was non-infected and considered Eimeria negative control, the other treatment groups were challenged by the sporulated oocysts inoculating 1 ml suspension containing (3,200 oocysts per ml) [18] of mixed Eimeria spp. directly in the pharynx. Besides this, group T3 was treated with papaya leaves suspension, and group T4 was treated with the anti-coccidial drug, while group T2 remained infected as Eimeria positive control. All the experimental chickens under each group were reared for 35 days in separate experimental sheds under strict biosecurity measures without any vaccination during the research period.
Experimental birds and management
For this study, 80 commercial, 7-day-old Sonali chickens were sourced from a local farm in Bahadur Bazar, Dinajpur district of Bangladesh. After purchase, the birds were transferred to an experimental shed, which was previously well designed and having 16 h of continuous light facilities, both natural and artificial, in an open-sided house system. Additionally, glucose and vitamin C were supplied with drinking water for the first 3 days to overcome the transportation stress and acclimatize to their new environment before the experiment’s commencement. The experimental birds were fed with coccidiostat-free Sonali Mash commercial feeds, collected from Griholokkhi Poultry Feed, Kalitola, Dinajpur, and feed ingredients are described by Roy et al. [21]. In the entire experimental period, all the birds of each group were maintained with ad libitum of mash feed and safe drinking water in similar care and management practices. Besides this, adequate hygiene and sanitation were appropriately maintained.
Collection and isolation of protozoal oocysts
The oocysts of mixed Eimeria spp. were isolated from few coccidiosis suspected birds collected from a different Dinajpur district regions to source protozoal oocysts. Two different types of qualitative tests examined feces and intestinal contents with lesions, namely direct smear and flotation techniques using sodium chloride solution were applied to identify the morphological features of eggs, cyst, and oocysts [22]. After that, a shallow Petri dish containing 2.5% potassium dichromate (K2Cr2O7) solution was used to transfuse the identified Eimeria spp. oocysts and then incubated for sporulation, according to Conway and McKenzie [23]. After collecting the sporulated oocysts, the oocysts were preserved in 2.5% K2Cr2O7 and stored at 4°C to prevent harmful bacteria growth. The sporulated oocysts were then allowed to count by using the McMaster chamber according to the method described by Holdsworth et al. [24] which determined the mixed Eimeria spp. oocysts load (OL) in per gm of feces.
Papaya leaves’ extract and anti-coccidiosis drug
The fresh young green papaya (Carica papaya) leaves were picked and washed with running fresh water. Then, they were soaked with cotton and kept in a well-ventilated room for air drying. The air-dried leaves were chopped into small pieces and mashed by a pestle and mortar. Finally, the leaves’ extracts were obtained by squeezing and pressing mashed leaves. Then, 0.5% of suspension was produced by dissolving the ground papaya leaves in distilled water. On the other hand, the commercially available anti-coccidiosis drug named Coxitril®-Vet liquid (Toltrazuril INN 2.5%), a product Square Pharmaceuticals Ltd. Bangladesh, was considered as the source of Toltrazuril. The drug was preserved in a dry place at room temperature and administered at a dose rate of 1 ml per liter of drinking water for 2 consecutive days.
Hematological study
The samples (blood) were collected aseptically from each chicken wing vein of all groups on days 1, 15, and 30 of post-treatment. Approximately 2 ml of blood samples were collected in heparinized vials containing anticoagulant ethylenediaminetetraacetate for hematological analysis. The hematological study was carried out on the indices such as total erythrocyte count (TEC), erythrocyte sedimentation rate (ESR), packed cell volume (PCV), hemoglobin estimation (Hb), and total leukocyte count (TLC). All the hematological parameters were observed as per methods described by Feldman et al. [25] and Benjamin [26].
BW measurement
Each bird of all the groups was subjected to recording the BW on days 0, 7, 14, 21, and 30 of the experiment with an electrically operated weighing machine. The BW was taken three times in the morning, noon, and afternoon before feeding. Then, the averages of the three weights were made and kept in a record. Finally, each bird’s BW gain was calculated by subtracting the initial BW (at day 0) from the BW at day 30.
Statistical analysis
The results obtained from the experiment were inserted into a spreadsheet (Microsoft Excel-2010), and subsequently, all statistical analysis was conducted using Statistical Package for Social Sciences version 25.0. At first, the assumption of normality was checked using the Shapiro–Wilk test, where the result (p > 0.05) indicated that the normality assumptions of data distribution were not violated. Thus, data were compared between the groups by performing the one-way analysis of variance (ANOVA). The difference between each group’s means to other groups was evaluated by multiple comparison tests Student–Newman–Keuls test. On the other hand, the effect of treatment within the groups in every 15-day interval up to 30 days was compared by the repeated measure ANOVA. The Bonferroni test assessed the mean impact among the different days in each group. Additionally, all the variables under group T3 at 30 days were subjected to determine the relationships with the protozoal load simultaneously using Pearson’s correlation (r) test. Before calculating r, the assumptions of normality, linearity, and homoscedasticity were checked and found to be permitted. Notably, a visual inspection of the scatterplot of data obtained from group T3 at day 30 suggested that the relationship between the variables was linear and heteroscedastic. However, a p-value of ≤ 0.5 was considered significant for the entire test. The reduction rate of oocytes per gm of feces at day 30 of treatment in percentages and average daily weight gain (AWG) was measured as per methods narrated by Nghonjuyi et al. [18].
Result and Discussion
The present study demonstrated that the C. papaya leaves affect coccidial infection treatment in Sonali chickens. The result of the protozoal load of mixed Eimeria spp. in feces of different treatment groups is presented in Table 1, showing that protozoal load per gm of feces was significantly different from those of treated groups. The protozoal load was significantly (p < 0.05) elevated in the T2 (infected but control) group, indicated by a reduction rate of −5.49%. On the other hand, the protozoal load was significantly (p < 0.05) reduced in both T3 and T4 groups provided with the suspension of 0.5% papaya leaves and Toltrazuril, respectively. In these findings, the utmost oocyst decline rate was perceived in the T4 group by 100%.
Table 1. Mixed Eimeria species OL in per gm of feces of experimentally induced coccidiosis in different treatment groups.
| Group | Mean ± SEM | F-value | Level of Significance | Reduction Rate (%) | ||
|---|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 30 | ||||
| T1 | 0.000c ± 0.000 | 0.000c ± 0.000 | 0.000c ± 0.000 | – | – | – |
| T2 | 331.600abx ± 8.678 | 341.8ax ± 10.374 | 349.800ax ± 16.347 | 0.474 | NS | −5.49 |
| T3 | 315.2bx ± 7.953 | 152.4by ± 7.081 | 52.200bz ± 5.702 | 411.147 | ** | 83.44 |
| T4 | 347.0a ± 6.703 | 0.000c ± 0.000 | 0.000c ± 0.000 | - | - | 100.00 |
| F-value | 601.735 | 667.622 | 376.722 | |||
| Level of Significance | ** | ** | ** | |||
NS = Insignificant; SEM = standard error of the mean; T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, T4 = Infected and treated with Toltrazuril group.
x,y,zMeans within the same row having different superscript letters are significantly different.
a,b,c,dMeans within the same column having different superscript letters are significantly different.
**Level of significance at 1% (p < 0.01).
*Level of significance at 5% (p < 0.05).
In comparison, the T3 group was 83.44%, indicating the commercial anticoccidial drug (Toltrazuril) was highly effective. The C. papaya leaves’ extract has anticoccidial properties to some extent close to Toltrazuril. Nghonjuyi et al. [18] used the ethanolic extract of C. papaya leaves to control coccidiosis in Kabir chickens. Still, our findings are close to them. They reported that the commercial anticoccidial drug available was very active, and the plant’s efficient (C. papaya) extracts also improved with increasing the dosages. Furthermore, C. papaya is familiar with an anti-inflammatory property with riches in Vitamin A, which can accelerate wound healing [27]. These properties protect the caecal epithelium cells that hinder the coccidial reproductive activities and improve the C. papaya-treated birds compared with infected untreated control [6].
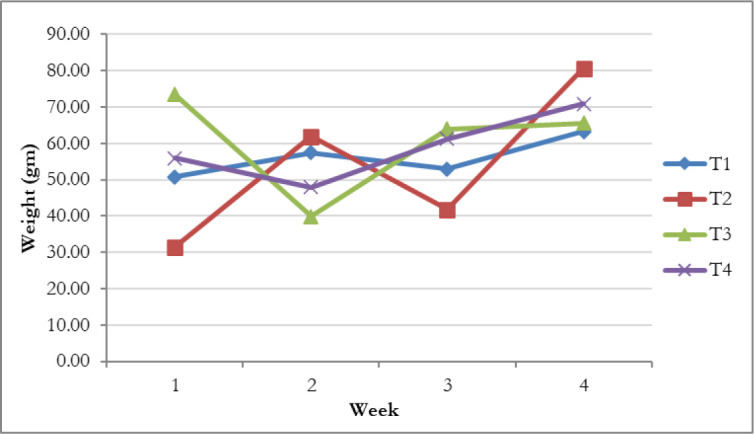
The consequence of experimental treatments on BW is summarized in Table 2, indicating that the significant (p < 0.01) growth in BW was in each group. Besides, the BWs of Sonali chickens treated with C. papaya (T3) were significantly higher than other groups at day 30 of post-treatment. As shown in Figure 1, the BW gain was higher, amounting to 243.06 gm in birds fed diets supplemented with C. papaya compared to the other experimental groups. Likewise, the highest daily average BW gain was observed in group T3 by 8.10 gm, whereas next was in birds fed with Toltrazuril (T4), amounting to 7.87 gm (Fig. 2). The weight gain could also be due to the C. papaya leaf extract comprising papain enzyme. This protease-like enzyme hydrolyzes proteins to tiny peptides, thus assisting protein digestion augmenting free amino acids essential to growth factors [28,29]. However, the weekly BW gain observed in Figure 3 shows the infected control had the highest fluctuation. Accordingly, in group 3, after a sudden drop in the second week, the weekly BW gain was increased gradually and peaked in the fourth weeks, later amounting to 65.57 gm. Additionally, the C. papaya leaves have the properties of limiting the intestinal coliform count [30] and increasing the feed palatability due to organoleptic characteristics [31], which directly affects feed utilization and BW gain.
Table 2. Effects of C. papaya and anticoccidial drug on BW (gm) in experimental groups at different days of post-infection.
| Group | Mean ± SEM | F-value | LS | ||||
|---|---|---|---|---|---|---|---|
| Day-0 | Day-7 | Day-14 | Day-21 | Day-30 | |||
| T1 | 182.78az ± 0.66 | 233.56cy ± 0.53 | 290.94bx ± 0.61 | 343.87cw ± 0.93 | 407.12cv ± 0.84 | 19813.31 | ** |
| T2 | 182.77az ± 0.73 | 214.10dy ± 0.82 | 276.16cx ± 0.97 | 317.98dw ± 0.85 | 398.33dv ± 0.89 | 10144.30 | ** |
| T3 | 182.42az ± 1.64 | 255.22ay ± 1.08 | 295.90ax ± 1.14 | 359.91aw ± 0.87 | 425.49av ± 1.15 | 11380.23 | ** |
| T4 | 185.95az ± 0.96 | 241.52by ± 0.68 | 289.83bx ± 0.86 | 351.17bw ± 0.90 | 421.96bv ± 0.79 | 13663.33 | ** |
| F-value | 2.391 | 456.090 | 85.075 | 412.747 | 189.216 | ||
| Level of significance | NS | ** | ** | ** | ** | ||
NS = Insignificant; SEM = Standard error of the mean; T1 = Non-infected control group; T2 = Infected control group; T3 = Infected and treated with C. papaya group; T4 = Infected and treated with Toltrazuril group.
v,w,x,y,zMeans within the same row having different superscript letters are significantly different.
a,b,c,dMeans within same column having different superscript letters are significantly different.
**Level of significance at 1% (p < 0.01).
*Level of significance at 5% (p < 0.05).
Figure 1. Final BW gain (gm) in different experimental groups. T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, and T4 = Infected and treated with Toltrazuril group.
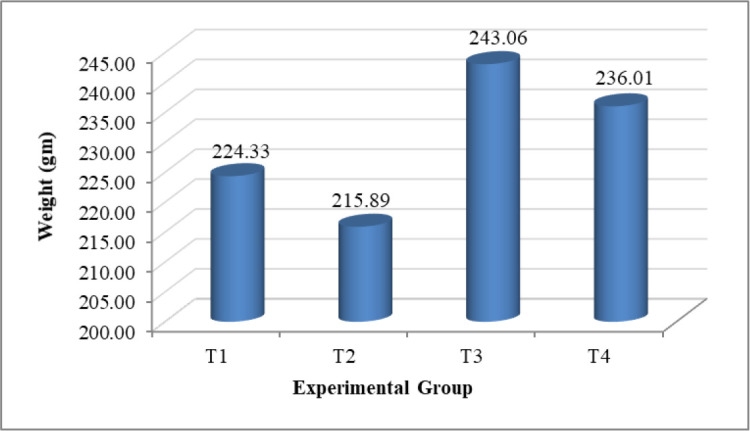
Figure 2. AWG (gm) in different experimental groups. T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, and T4 = Infected and treated with Toltrazuril group.
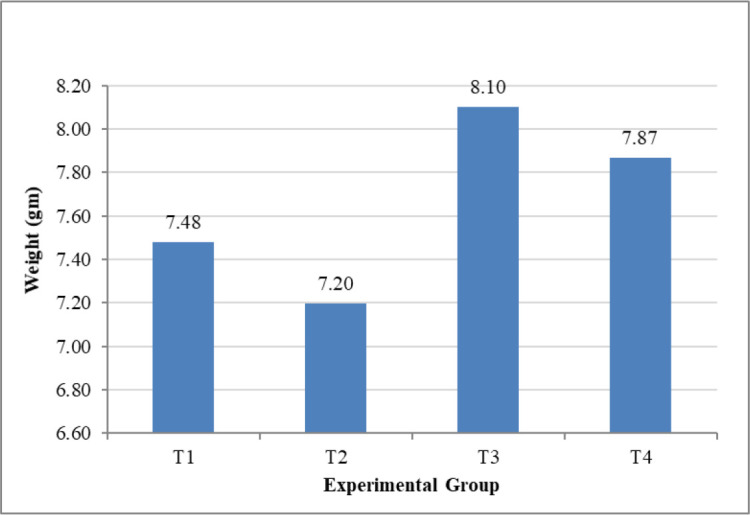
Figure 3. Weekly BW gain (gm) in different experimental groups. T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, and T4 = Infected and treated with Toltrazuril group.
In this study, the results obtained on TEC are presented in Table 3, which show that the values of the experimented groups (T3 and T4) and the infected control group (T1) are in the normal range [32]. Although group T4 had the highest mean and group T2 had the lowest in (infected and no treatment), there was no significant (p > 0.05) variance in these concerning groups at days 1, 15, and 30 of post-treatment. The lower mean in the positive coccidiosis group without any treatment (T2) might be due to the internal bleeding that ensued at the acute phase of infection from the inoculation of mixed Eimeria spp. [33]. Moreover, within the groups, TEC values changed at days 1, 15, and 30 after post-inoculation but were not statistically significant (p > 0.05). On the contrary, Melkamu et al. [34] reported that TEC significantly decreased in all infected groups compared to the control group (non-infected), particularly on day 7 of post-infection. This variation might be due to the differences in sample size and days considered after post-infection. However, our findings have similarities with Adamu et al. [35], who showed lower counts of TEC in coccidiosis-infected chickens than in the uninfected controls. However, Nghonjuyi et al. [18] observed that the RBC counts were not significantly influenced by the ethanolic extract of C. papaya leaf. At the same time, our findings showed that the TEC in the papaya-treated group was higher than the T1 (non-infected with no treatment) group and close to the value of the T4 group. Our study is in line with Kumar et al. [36], who reported that C. papaya eliminated the blood-sucking gastrointestinal parasites and increased iron absorption from the gastrointestinal tract, increasing TEC. Moreover, as shown in Table 4, the Hb level had not significantly (p > 0.05) differed among the groups and within the groups at different days of post-infection. Nevertheless, the Hb level values indicated that the highest was in group T4 and both groups T3 and T4 had values near to the non-infected group (T1), which were higher than the infected group (T2). These findings give hints that C. papaya leaves reduce the coccidiosis infection as anticoccidial drugs did, whose outcome was an approximate value of Hb level in group T3 and T4. Similarly, Hirani et al. [37] reported that the acute phase of infection had decreased the level of Hb in the infected groups and the values also returned to usual after recovery. Coccidiosis results dysfunction of spleen, leading to acute massive hemorrhage manifested by bloody diarrhea [38], which might be one of the reasons for a decreased Hb level.
Table 3. Effects of C. papaya and anticoccidial drug on TEC (million/mm3) level in blood of experimental groups at different days of post-infection.
| Group | Mean ± SEM | F-value | Level of Significance | ||
|---|---|---|---|---|---|
| Day-0 | Day-15 | Day-30 | |||
| T1 | 2.307ax ± 0.229 | 2.456ax ± 0.206 | 2.715ax ± 0.242 | 1.381 | NS |
| T2 | 2.237ax ± 0.170 | 2.178ax ± 0.358 | 2.129ax ± 0.182 | 0.106 | NS |
| T3 | 2.417ax ± 0.274 | 2.528ax ± 0.265 | 2.740ax ± 0.246 | 1.016 | NS |
| T4 | 2.516ax ± 0.237 | 2.634ax ± 0.261 | 2.862ax ± 0.244 | 0.710 | NS |
| F-value | 0.284 | 0.493 | 2.031 | ||
| Level of Significance | NS | NS | NS | ||
NS = Insignificant; SEM = Standard error of the mean; T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, T4 = Infected and treated with Toltrazuril group.
x,y,z Means within the same row having different superscript letters are significantly different.
a,b,c,d Means within the same column having different superscript letters are significantly different.
**Level of significance at 1% (p < 0.01).
*Level of significance at 5% (p < 0.05).
Table 4. Effects of C. papaya and anticoccidial drug on Hb level (gm/dl) in the blood of experimental groups at different days of post-infection.
| Group | Mean ± SEM | F-value | Level of Significance | ||
|---|---|---|---|---|---|
| Day-0 | Day-15 | Day-30 | |||
| T1 | 9.331ax ± 0.357 | 9.378ax ± 0.327 | 9.504ax ± 0.347 | 0.120 | NS |
| T2 | 9.013ax ± 0.317 | 8.998ax ± 0.358 | 8.974ax ± 0.348 | 0.007 | NS |
| T3 | 9.298ax ± 0.368 | 9.374ax ± 0.265 | 9.476ax ± 0.332 | 0.129 | NS |
| T4 | 9.810axy ± 0.347 | 9.013ax ± 0.261 | 9.612ay ± 0.311 | 3.010 | NS |
| F-value | 0.902 | 0.491 | 0.720 | ||
| Level of Significance | NS | NS | NS | ||
NS = Insignificant; SEM = Standard error of the mean; T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, T4 = Infected and treated with Toltrazuril group.
x,y,z Means within the same row having different superscript letters are significantly different.
a,b,c,dMeans within the same column having different superscript letters are significantly different.
**Level of significance at 1% (p < 0.01).
*Level of significance at 5% (p < 0.05).
According to the obtained results on PCV and ESR of the treatments group (Tables 5 and 6), albeit the values of PCV were not differed significantly (p > 0.05) between the groups, within the groups on certain days after post-treatment there was a significant (p < 0.05) difference indicating the PCV percentages are increasing according to the gradual development of ages. The PCV% in group T3 was approximating to the non-infected control group (T1), and the percentages in each of the groups were below the average reference level. A decrement percentage of PCV, less than 35% in birds, was generally envisaged as an anemic condition [35]. Although, in the initial stage, the ESR count had a significant (p < 0.05) difference among the group at day 0, even so, there was no significant difference after the post-infection. On the other hand, by the progress of days after post-treatment, the ESR changes within the group of T1 and T4 were significant (p < 0.05). As observed in Table 7, all the experimental groups had no (p > 0.05) alteration in TLC count except at day 30, where group T3 was higher than other groups. However, Zulpo et al. [39] noted a non-significant variation in TLC when the infected groups were assessed with the control group. Simultaneously, TLC within the groups increased but not significantly, while it decreased in the anticoccidial drug-treated group (T4). Another study by Mohammed [40] reported that TLC significantly increased in coccidial-infected chickens compared to control. Similarly, Ahmad et al. [41] found higher TLC in the Eimeria spp.-positive group.
Table 5. Effects of C. papaya and anticoccidial drug on PCV percentages in experimental groups’ blood at different days of post-infection.
| Group | Mean ± SEM | F-value | Level of significance | ||
|---|---|---|---|---|---|
| Day-0 | Day-15 | Day-30 | |||
| T1 | 20.394az ± 0.317 | 21.990ay ± 0.357 | 25.610ax ± 0.327 | 169.803 | ** |
| T2 | 20.788az ± 0.358 | 21.416ay ± 0.368 | 25.038ax ± 0.265 | 76.843 | ** |
| T3 | 20.698ay ± 0.347 | 21.862ay ± 0.347 | 25.625ax ± 0.261 | 127.777 | ** |
| T4 | 19.802ay ± 0.332 | 21.842ax ± 0.311 | 25.580ax ± 0.311 | 166.818 | ** |
| F-value | 1.724 | 0.519 | 0.943 | ||
| Level of Significance | NS | NS | NS | ||
NS = Insignificant; SEM = Standard error of the mean; T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, T4 = Infected and treated with Toltrazuril group.
x,y,zMeans within the same row having different superscript letters are significantly different.
a,b,c,dMeans within the same column having different superscript letters are significantly different.
**Level of significance at 1% (p < 0.01).
*Level of significance at 5% (p < 0.05).
Table 6. Effects of C. papaya and anticoccidial drug on ESR (mm/1st h) in the blood of experimental groups at different days of post-infection.
| Group | Mean ± SEM | F-value | Level of significance | ||
|---|---|---|---|---|---|
| Day-0 | Day-15 | Day-30 | |||
| T1 | 10.520by ± 0.304 | 10.656ay ± 0.275 | 11.734ax ± 0.283 | 9.358 | ** |
| T2 | 10.492bx ± 0.288 | 10.590ax ± 0.272 | 10.914ax ± 0.278 | 1.446 | NS |
| T3 | 10.994abx ± 0.308 | 10.896ax ± 0.260 | 10.776ax ± 0.228 | 0.246 | NS |
| T4 | 11.73ax ± 0.318 | 10.590ay ± 0.272 | 11.472ay ± 0.428 | 9.310 | ** |
| F-value | 3.636 | 0.289 | 2.101 | ||
| Level of Significance | * | NS | NS | ||
NS = Insignificant; SEM = Standard error of the mean; T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, T4 = Infected and treated with Toltrazuril group.
x,y,zMeans within the same row having different superscript letters are significantly different.
a,b,c,dMeans within the same column having different superscript letters are significantly different.
**Level of significance at 1% (p < 0.01).
*Level of significance at 5% (p < 0.05).
Table 7. Effects of C. papaya and anticoccidial drug on TLC (Thousand/mm3) in the blood of experimental groups at different days of post-infection.
| Group | Mean ± SEM | F-value | Level of significance | ||
|---|---|---|---|---|---|
| Day-0 | Day-15 | Day-30 | |||
| T1 | 6.454ax ± 0.357 | 6.476ax ± 0.327 | 6.722abx ± 0.347 | 0.339 | NS |
| T2 | 7.132ax ± 0.317 | 7.452ax ± 0.358 | 7.588ax ± 0.348 | 0.933 | NS |
| T3 | 6.778ax ± 0.261 | 6.544ax ± 0.311 | 7.254abx ± 0.347 | 1.223 | NS |
| T4 | 6.668ax ± 0.265 | 6.486ax ± 0.332 | 6.176bx ± 0.368 | 0.915 | NS |
| F-value | 0.877 | 2.046 | 3.077 | ||
| Level of significance | NS | NS | * | ||
NS = Insignificant; SEM = Standard error of the mean; T1 = Non-infected control group, T2 = Infected control group, T3 = Infected and treated with C. papaya group, T4 = Infected and treated with Toltrazuril group.
x,y,zMeans within the same row having different superscript letters are significantly different.
a,b,c,dMeans within the same column having different superscript letters are significantly different.
**Level of significance at 1% (p < 0.01).
*Level of significance at 5% (p < 0.05).
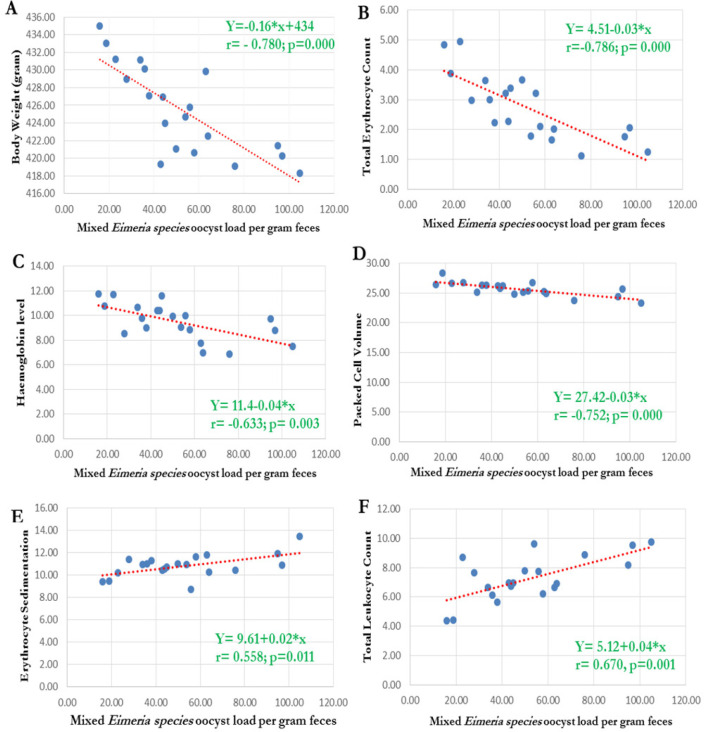
The results of Pearson’s correlation on hematological parameter and BW with mixed Eimeria spp. oocyst load (OL) at day 30 of post-treatment in C. papaya-treated group (T3) are shown in Figure 4. The results show a strong significant (p < 0.01) negative correlation between coccidial OL and BW (r = −0.780) with regression equation, y = −0.16*x + 433.665, indicating that the BW of birds will be decreased significantly when the protozoal load will increase. Our findings are in line with the Nurzaty et al. [42], who narrated a highly significant correlation (r = −0.49, p < 0.01) between coccidian count and the BW of Dorper sheep. Moreover, TEC and PCV’s correlation with coccidial OL was significantly (p < 0.01) strong negative, r = −0.786 and r = −0.752, where regression equation was y = −0.03*x + 4.51 and y = −0.03*x + 27.42, respectively. Also a moderately negative association was between Hb concentration and protozoal load (r = −0.633, p < 0.01) with regression equation, y = −0.04*x + 11.40. However, Nurzaty et al. [42] reported the correlation (r = −0.081, p < 0.01) between the PCV and OL are in line with the findings of this study. Still, Alexey et al. [43] reported Eimeria oocysts per gm of caecal contents had no significant correlation with Hb and TEC of the immunized broiler, which is contrary to our findings. These controversial findings might be due to broilers vaccinated by using attenuated strains or sometimes sporozoite proteins. On the other hand, ESR and TLC had a significantly (p < 0.01) positive correlation with mixed Eimeria spp. OL, r = 0.558 and r = 0.670, respectively. Simultaneously, the regression equation for these two parameters was y = 9.61 + 0.02*x and y = 5.12 + 0.04*x, respectively. These results indicate that ESR and TLC will increase by increasing mixed Eimeria spp. OL in birds.
Figure 4. Scatter diagrams showing the correlations of mixed Eimeria species OL per gm feces with (A) BW, (B) TEC, (C) Hb level, (D) PCV percentages, (E) ESR, and (F) TLC at 30 days of post-treatment with C. papaya leaves extract.
Conclusion
The present study revealed that the C. papaya leaves’ extract had effectiveness in reducing the mixed Eimeria spp. oocyst count per gm of feces. Still, the oocyst reduction rate was higher in Toltrazuril. Additionally, these had a significant outcome on increasing the BW and hematological parameters in Sonali chickens. The coccidiosis-infected birds treated with C. papaya leaves’ extract for 1 month had a strong significant correlation with BW and hematological values. Remarkably, the papaya leaves suspension at a dose rate of 0.5% had no adverse effect on herbal use during this experimental period. To sum up, afore-narrated findings signify that papaya leaves’ (C. papaya) extract effectively controls coccidiosis in Sonali chickens. It can also be considered an effective growth promoter. These studies justify further research on this herb in the mechanism of action, contra-indication, histopathological changes, and effects on intestinal microbiota in different species, especially in diverse dosages.
List of abbreviations
Oocyst Load = OL, Body Weight = BW, Rhode Island Red = RIR, Total Erythrocyte Count = TEC, Erythrocyte Sedimentation Rate = ESR, Packed Cell Volume = PCV, Hemoglobin = Hb, Total Leukocyte Count = TLC, Average Daily Weight Gain = AWG.
Acknowledgment
The authors are happy to acknowledge the Department of Physiology and Pharmacology and the Department of Pathology and Parasitology, HSTU, Dinajpur-5200, for the research facility.
Conflict of interests
All the authors declare that they have no competing interests.
Authors’ Contribution
MJA carried out the experiment and animal trial and contributed to the sample and data collection. FBA edited and approved the experimental design, methodology and supervised the overall research work. MMH participated in proposing and designing the experiment. RI proposed and approved the methodology. SS, MJA, and MMMP prepared the herb extract C. papaya, carried out the hematological analysis, and counted the protozoal egg. MMM arranged the data as well as performed all the statistical analysis, wrote and equipped the manuscript. Finally, all authors read and agreed to the manuscript submission.
References
- [1].Kabir SML. The role of probiotics in the poultry industry. Int J Mol Sci. 2009;10(8):3531–46. doi: 10.3390/ijms10083531. https://doi.org/10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Begum IA, Alam MJ, Buysse J, Frija A, Van Huylenbroeck G. Contract farmer and poultry farm efficiency in Bangladesh: a data envelopment analysis. Appl Econ. 2012;44(28):3737–47. https://doi.org/10.1080/00036846.2011.581216. [Google Scholar]
- [3].Huque Q. Commercial poultry production in Bangladesh. 2011 Souvenir of the 7th International Poultry Show Seminar, Dhaka, Bangladesh, pp 25–7. [Google Scholar]
- [4].Uddin MT, Rahman MH, Saleque MA, Thieme O. Comparative performance of Sonali chickens, commercial broiler, layers and local non-descript (deshi) chickens in selected areas of Bangladesh. 2015 FAO Animal Production and Health Working, Rome, Italy, p 14. [Google Scholar]
- [5].Saima MZ, Jabbar MA, Mehmud A, Abbas MM, Mahmood A. Effect of lysine supplementation in low protein diets on the performance of growing broilers. Pak Vet J. 2010;30:17–20. [Google Scholar]
- [6].Dakpogan HB, Mensah S, Attindehou S, Chysostome C, Aboh A, Naciri M, et al. Anticoccidial activity of Carica papaya and Vernonia amygdalina extract. Int J Biol Chem Sci. 2018;12(5):2101–8. https://doi.org/10.4314/ijbcs.v12i5.12. [Google Scholar]
- [7].Remmal A, Achahbar S, Bouddine L, Chami N, Chami F. In vitro destruction of Eimeria oocysts by essential oils. Vet Parasitol. 2011;182(2–4):121–6. doi: 10.1016/j.vetpar.2011.06.002. https://doi.org/10.1016/j.vetpar.2011.06.002. [DOI] [PubMed] [Google Scholar]
- [8].Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol. 2000;24(2–3):303–24. doi: 10.1016/s0145-305x(99)00080-4. https://doi.org/10.1016/S0145-305X(99)00080-4. [DOI] [PubMed] [Google Scholar]
- [9].Patra G, Rajkhowa TK, Ayub Ali M, Tiwary JG, Sailo L. Studies on clinical, gross, histopathological and biochemical parameters in broiler birds suffered from Eimeria necatrix infection in Aizawl district of Mizoram, India. Int J Poult Sci. 2009;8:1104–11. https://doi.org/10.3923/ijps.2009.1104.1106. [Google Scholar]
- [10].Michels MG, Bertolini LC, Esteves AF, Moreira P, Franca SC. Anticoccidial effects of coumestans from Eclipta alba for sustainable control of Eimeria tenella parasitosis in poultry production. Vet Parasitol. 2011;177(1–2):55–60. doi: 10.1016/j.vetpar.2010.11.022. https://doi.org/10.1016/j.vetpar.2010.11.022. [DOI] [PubMed] [Google Scholar]
- [11].Jadhav BN, Nikam SV, Bhamre SN, Jaid EL. Study of Eimeria necatrix in broiler chicken from Aurangabad district of Maharashtra State India. Int Multidiscip Res J. 2011;1(11):11–2. [Google Scholar]
- [12].Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, et al. Re-calculating the cost of coccidiosis in chickens. Vet Res. 2020;51(1):1–14. doi: 10.1186/s13567-020-00837-2. https://doi.org/10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15(1):58–65. doi: 10.1128/CMR.15.1.58-65.2002. https://doi.org/10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shirley MW, Smith AL, Blake DP. Challenges in the successful control of the avian coccidia. Vaccine. 2007;25(30):5540–7. doi: 10.1016/j.vaccine.2006.12.030. https://doi.org/10.1016/j.vaccine.2006.12.030. [DOI] [PubMed] [Google Scholar]
- [15].Hafeez A, Ullah Z, Khan RU, Ullah Q, Naz S. Effect of diet supplemented with coconut essential oil on performance and villus histomorphology in broiler exposed to avian coccidiosis. Trop Anim Health Prod. 2020;52:2499–504. doi: 10.1007/s11250-020-02279-6. https://doi.org/10.1007/s11250-020-02279-6. [DOI] [PubMed] [Google Scholar]
- [16].Mortier L, Huet AC, Charlier C, Daeseleire E, Delahaut P, Van Peteghem C. Incidence of residues of nine anticoccidials in eggs. Food Addit Contam. 2005;22(11):1120–5. doi: 10.1080/02652030500199355. https://doi.org/10.1080/02652030500199355. [DOI] [PubMed] [Google Scholar]
- [17].Giannenas I, Papadopoulos E, Tsalie E, Triantafillou E, Henikl S, Teichmann K, et al. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet Parasitol. 2012;188(1–2):31–40. doi: 10.1016/j.vetpar.2012.02.017. https://doi.org/10.1016/j.vetpar.2012.02.017. [DOI] [PubMed] [Google Scholar]
- [18].Nghonjuyi NW, Tiambo CK, Kimbi HK, Manka’a CN, Juliano RS, Lisita F. Efficacy of ethanolic extract of Carica papaya leaves as a substitute of sulphanomide for the control of coccidiosis in KABIR chickens in Cameroon. J Anim Health Prod. 2015;3(1):21–7. https://doi.org/10.14737/journal.jahp/2015/3.1.21.27. [Google Scholar]
- [19].AL-Fifi ZIA. Effect of leaves extract of Carica papaya, Vernonia amigdalina and Azadiratcha indica on the coccidiosis in free-range chickens. Asian J Anim Sci. 2007;1(1):26–32. https://doi.org/10.3923/ajas.2007.26.32. [Google Scholar]
- [20].El-Shazly KA, El-Latif AA, Abdo W, El-Morsey A, El-Aziz MIA, El-Mogazy H. The anticoccidial activity of the fluoroquinolone lomefloxacin against experimental Eimeria tenella infection in broiler chickens. Parasitol Res. 2020;119(6):1955–68. doi: 10.1007/s00436-020-06692-6. https://doi.org/10.1007/s00436-020-06692-6. [DOI] [PubMed] [Google Scholar]
- [21].Roy R, Hasan MM, Aziz FB, Islam R, Sarkar S. Comparative study of neem leaf (Azadirachta indica) suspension and toltrazuril against coccidiosis in Sonali chicken. Bangladesh J Vet Med. 2019;17(2):97–105. https://doi.org/10.33109/bjvmjd19am1. [Google Scholar]
- [22].Hendrin CM, Robinson N. 3rd. Edinburgh, Scotland: Elsevier; 2006. Diagnostic parasitology for veterinary technicians; pp. 255–60. [Google Scholar]
- [23].Conway DP, McKenzie ME. 3rd. Ames, IA: Blackwell Publishing; 2007. Poultry coccidiosis: diagnostic and testing procedures [Internet] pp. 37–40. https://doi.org/10.1002/9780470344620. [Google Scholar]
- [24].Holdsworth PA, Conway D, McKenzie ME, Dayton AD, Chapman HD, Mathis GF, et al. World Association for the Advancement of Veterinary Parasitology (WAAVP): guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol. 2004;121(3–4):189–212. doi: 10.1016/j.vetpar.2004.03.006. https://doi.org/10.1016/j.vetpar.2004.03.006. [DOI] [PubMed] [Google Scholar]
- [25].Feldman BF, Zinkl JK, Jain NC. Schalm’s veterinary haematology. 5th edition. Walters Kluwer Company, Alphen aan den Rijn, Netherlands, pp 1344–8, 2000. [Google Scholar]
- [26].Benjamin MM. Outlines of veterinary clinical pathology. 3rd edition, The Iowa State University Press, Ames, IA, p 7–8, 1985. [Google Scholar]
- [27].Beuth J, Ost B, Pakdaman A, Rethfeldt E, Bock PR, Hanisch J, et al. Impact of complementary oral enzyme application on the postoperative treatment results of breast cancer patients - results of an epidemiological multicentre retrolective cohort study. Cancer Chemother Pharmacol. 2001;47(7):S45–54. doi: 10.1007/s002800170009. https://doi.org/10.1007/s002800170009. [DOI] [PubMed] [Google Scholar]
- [28].Elneney BA, El-Neney, Awadien NB, Ebeid TA. The Productive performance and immunological traits of local chicken strain by using natural enzymes (plant papain) and remnants of plant papaya. 1-effect of papaya latex on laying period. Egypt Poult Sci J. 2015;35(1):1–24. https://doi.org/10.21608/epsj.2015.5371. [Google Scholar]
- [29].Onyimonyi AE, Ernest O. An assessment of pawpaw leaf meal as protein ingredient for finishing broiler. Int J Poult Sci. 2009;8(10):995–8. https://doi.org/10.3923/ijps.2009.995.998. [Google Scholar]
- [30].Omidiwura BRO, Agboola AF, Omotosho OY, Mustapha-Olosho JA. Influence of diets supplemented with Carica papaya and Chromolaena odorata leaf meals on performance, blood profile and gut integrity of broiler chickens. Medcina Int. 2020;4(2):211–7. [Google Scholar]
- [31].Muazu U, Aliyu-Paiko M. Evaluating the potentials of Carica papaya seed as phytobiotic to improve feed efficiency, growth performance and serum biochemical prameters in broiler chickens. IOSR J Biotechnol Biochem. 2020;6(1):8–18. [Google Scholar]
- [32].Wakenell PS. Weiss DJ, Wardrop KJ, editors. Hematology of chickens and turkeys. Veterinary hematology. 6th edition, John Wiley & Sons, pp 957–67, Ames, IA. [Google Scholar]
- [33].Ogbe AO, Atawodi SE, Abdu PA, Oguntayo BO, Dus N. Oral treatment of Eimeria tenella -infected broilers using aqueous extract of wild mushroom (Ganoderma sp.): Effect on haematological parameters and histopathology lesions. Afr J Biotechnol. 2010;9(52):8923–7. [Google Scholar]
- [34].Melkamu S, Chanie M, Asrat M. Haematological changes caused by coccidiosis in experimentally infected broiler chickens. J Anim Res. 2018;8(3):345–51. https://doi.org/10.30954/2277-940X.06.2018.2. [Google Scholar]
- [35].Adamu M, Boonkaewwan C, Gongruttananun N, Vongpakorn M. Hematological, biochemical and histopathological changes caused by coccidiosis in chickens. Kasetsart J Nat Sci. 2013;47(2):238–46. [Google Scholar]
- [36].Kumar B, Sahni YP, Kumar N. Effect of Indigenous anthelmintic Carica papaya against gastrointestinal nematodes in cattle. World J Pharm Res. 2014;3(10):998–1007. [Google Scholar]
- [37].Hirani ND, Hasnani JJ, Pandya SS, Patel PV. Haematological changes in broiler birds with induced caecal coccidiosis following prophylaxis with different coccidiostats. Int J Curr Microbiol Appl Sci. 2018;7(4):1094–100. https://doi.org/10.20546/ijcmas.2018.704.119. [Google Scholar]
- [38].Wang L, Guo Z, Gong Z, Cai J, Yang F, Wei X, et al. Efficacy of an oral solution prepared from the ultrasonic extract of radix dichroae roots against Eimeria tenella in broiler chickens. Evid Based Complement Altern Med. 2020;2020:3870902. doi: 10.1155/2020/3870902. https://doi.org/10.1155/2020/3870902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zulpo DL, Peretti J, Ono LM, Longhi E, Oliveira MR, Guimarães IG, et al. Pathogenicity and histopathological observations of commercial broiler chicks experimentally infected with isolates of Eimeria tenella, E. acervulina and E. maxima. Semina: Ciênc Agrárias. 2007;28(1):97–104. https://https://doi.org/10.5433/1679-0359.2007v28n1p97. [Google Scholar]
- [40].Mohammed AK. Study of hematological and some biochemical values changing with administration of salinomycin and poultrystar probiotics in broiler chickens challenged with cocciodsis (Eimeria tenella) Al-Qadisiyah J Vet Med Sci. 2012;11(1):42–6. [Google Scholar]
- [41].Ahmad Z, Hafeez A, Ullah Q, Naz S, Khan RU. Protective effect of Aloe vera on growth performance, leucocyte count and intestinal injury in broiler chicken infected with coccidiosis. J Appl Anim Res. 2020;48(1):252–6. https://doi.org/10.1080/09712119.2020.1773473. [Google Scholar]
- [42].Nurzaty EAH, Ariff OM, Sani RA, Rasedee A. Relationship between coccidiosis infection and hematological profile, body weight and famacha scores in dorper sheep. Malays J Anim Sci. 2014;17(1):103–10. [Google Scholar]
- [43].Alexey LGB, João LG, Patrícia FN, da S, Mara RSB, José da SGJ. Post-challenge hematological evaluation with virulent strain of Eimeria tenella in broilers immunized with attenuated strain or sporozoite proteins from homologous strain. Rev Bras Parasitol Vet. 2010;19(1):1–6. doi: 10.1590/s1984-29612010000100002. https://doi.org/10.1590/S1984-29612010000100002. [DOI] [PubMed] [Google Scholar]