Abstract
Assisted reproductive technologies (ARTs) are widely used as a tool to improve reproductive performance in both humans and animals. In particular, in the veterinary field, ARTs are used to improve animal genetics, recover endangered animals, and produce offspring in the event of subfertility or infertility in males or females. However, the use of ARTs did not improve the fertilization rate in some animals due to various factors such as the difficulty in reproducing an anatomical and humoral substrate typical of the natural condition or due to the increase in catabolites and their difficult elimination. The in vitro environment allows the production and increase in the concentration of substances, including reactive oxygen species (ROS), which could be harmful to gametes. If produced in high concentration, the ROS becomes deleterious, both in vitro and in vivo systems. It has been seen that the use of antioxidants can help neutralize or counteract the production of ROS. The present study aims to report the latest findings regarding the use of antioxidants in ARTs of some domestic species, such as dogs, cats, and horses, compared to other animal species, such as cattle, in which ARTs have instead developed more widely.
Keywords: Antioxidants, Assisted reproductive technologies (ARTs), Animal reproduction, Oxidative stress (OS)
Introduction
The development of assisted reproductive technologies (ARTs) is crucial to improving domestic and wild animal reproduction. In domestic animals, ARTs are used for many reasons depending on the animal’s value, age, logistic, and practical health and economic reasons. ARTs are used in wild animals to protect and ensure the conservation of endangered animal species. Therefore, ARTs provide a new approach as the safeguard of biodiversity [1]. Nowadays, ARTs mainly include artificial insemination (AI), in vitro embryo production (IVEP), embryo transfer (ET), intracytoplasmic sperm injection (ICSI), gamete/embryo sexing, gamete/embryo cryopreservation, gamete/embryo micromanipulation, somatic cell nuclear transfer (SCNT), and genome resource banking (GRB) [2,3]. IVEP included oocyte in vitro maturation (IVM), in vitro fertilization (IVF), and in vitro culture (IVC) of embryos IVC. Most ARTs need to recreate an environment similar as much as possible to the natural one. However, objective impediments to this analogous reconstruction are the lack of an anatomical and humoral substrate typical of the natural condition. Moreover, there is the progressive increase of catabolites, which inevitably form the activation of metabolic functions, and the concomitant impossibility of their elimination. Therefore, in vitro environment allows the production and increase of the concentration of substances that could be detrimental to gametic cells or their products. If produced in high concentration, the reactive oxygen species (ROS) become deleterious both in in vitro and in vivo systems. Antioxidants could help in the neutralization of ROS or make them harmless, or counteract ROS production. Therefore, to counteract its harmful effects, many strategies have been tested in vivo, through food supplementation, and in vitro, by adding various antioxidants to the media (Fig. 1) [4–8]. The present study has highlighted the antioxidants used in ARTs, how they are used, and their effects on dogs, horses, and cats’ reproductive activities. These species require the implementation of ARTs much more than others, as for functional, health, and economic reasons, their rate of production and reproductive efficacy are still low.
Figure 1. Effects of the antioxidants on in vitro technologies. The addition of specific antioxidants to culture media containing cells for ARTs neutralizes and/or counteracts ROS’s harmful effects.
Assisted reproductive technologies (ARTs)
Artificial insemination (AI)
In 1784, Spallanzani was the first to perform an AI successfully in a dog. In 1922, Ivanoff examined AI in dogs, foxes, rabbits, domestic farm animals and poultry. Polge et al. [9] were the first to revolutionize the AI program through the worldwide transport of frozen semen. Today, AI is regularly being practiced in cattle, goats, sheep, pigs, turkeys, chickens, and rabbits [10]. Over 100 million cattle, 40 million pigs, 3.3 million sheep, and 0.5 million goats are subjected to AI annually worldwide [11].
In vitro embryo production
The IVF became an entirely in vitro method until the second half of the 1980s, called IVEP, including three procedures, namely oocyte IVM, IVF, and embryo IVC [12]. IVEP systems perform very well in bovine, but there is always space for improvement. Approximately 90% of the aspirated oocytes reach the metaphase II (MII) stage, and about 80% of these become fertilized in vitro. After oocyte IVC about 50% of the fertilized oocytes develop to blastocyst. Embryos are well-developed in sheep IVEP, but success rates are lower than bovines. Until now, the IVF technique has not been well established in the horse. The IVF’s failure in the horse is believed to be due to the inability of stallion sperm capacitation [13]. In pigs, IVM, IVF, and IVEP procedures often lead to low rates of embryonic development. During in vitro fertilization, polyspermia represents the major obstacle, in vitro, in the fertilization of porcine embryos [14]. In dogs, in vitro fertilization failure is due to anestrus, which is the most extended phase in this species, and their oocytes are ovulated in the metaphase I stage. It takes 2–3 days for those oocytes to mature in the oviduct to the MII stage. On the other hand, in cats, the oocytes are ovulated in MII, thus making in vitro fertilization, in this species, much more effective than in dogs [15].
Embryo transfer (ET)
The first successful generation of live young ET was achieved in rabbits in 1890 [10]. The first successful ET of sheep, pigs, and cattle was reported in the early 1950s. In 1972, researchers declared the birth of live mouse offspring produced from embryos that had been frozen, thawed, and then transferred. In the female, the efficacy of the association of multiple ovulation and embryo transfer (MOET) is similar to AI in the male. MOET allows the production of numerous progenies from genetically superior females [16]. Using ET or MOET techniques, faster livestock enhancement could be done more rapidly, elite animal extension, genetic gain, accelerated herd development, and conservation of rare genetic stocks could be achieved [17].
Intra-cytoplasmic sperm injection
The first successful ICSI was reported in hamsters [18]. This technique was applied to other species, including humans, cow, rabbit, mouse, sheep, horse, the domestic cat, and wild felids, pig, and goat. ICSI is a technique of micromanipulation. It includes the injection of a single spermatozoon into the cytoplasm of a mature oocyte. The success of this technique has been limited in farm animals [19].
Gamete and embryo cryopreservation
There are two methods for effective cryopreservation of mammalian gametes and embryos. Those are gradual freezing (programmed) and vitrification. Vitrification is an ultra-rapid cooling technique. In vitrification, cells and tissues are put directly into cryoprotective agents and then immersed into liquid nitrogen [20,21].
Gamete/embryo sexing
Molecular biology tools have gained significant importance in the early 1990s, but have lost significance in the past few years. Today, polymerase chain reaction technology is used for small-scale sexing of embryos [2]. This technology is likely being replaced by semen sexing [16]. In the past, various methods have been used to distinguish the X and Y sperm, which were based on the concept of difference in mass, size, swimming pattern, immunological structure, and surface charges. Flow cytometric sorting of sperm is the only verified commercial process with 90% accuracy based on the difference in deoxyribonucleic acid (DNA) content of X and Y. Still, it has certain limitations such as damage of sperm, straw costs, sorting rates, and so on [22].
Somatic cell nuclear transfer (SCNT)
Somatic cell cloning (cloning or nuclear transfer) is a technique characterized by the transfer of a somatic cell’s nucleus inside an enucleated MII oocyte to generate a new individual genetically identical to the somatic cell donor [23]. Fibroblasts used for SCNT come from an extensive range of donor cells from various sources and different tissues. These include fibroblasts from skin ovarian cumulus cells, granulosa cells, oviduct epithelial cells, uterine epithelial cells, mammary epithelial cells, cells from internal organs, Sertoli cells, macrophages, muscle cells, neural stem cells, blood leukocytes, lymphocytes, natural killer T cells, mature B and T cells, neural and embryonic stem cells, and olfactory cells [24].
Genome resource banking (GRB)
The GRB is the storage of frozen biological material, including embryos and semen; cryopreservation is the storage at ultra-low temperatures of viable cells, tissues, organs, and organisms, typically in liquid nitrogen at the temperature of −196°C [25]. Cryo-banking is often used with modern reproductive techniques, such as rederivation, oocyte IVC, and ET [26].
Oxidative stress (OS)
OS is determined by an imbalance between pro-and antioxidants, which may be due to high levels of ROS and/or reactive nitrogen species, or a reduction in antioxidant defenses [27]. Typically the ROS are produced physiologically, but their excessive production can be detrimental. For example, ROS physiological levels in reproduction have an essential role in capacitation, hyperactivation, acrosome reaction, and sperm–oocyte fusion [27]. Instead, high levels of ROS can determine Adenosine Triphosphate consumption and decrease in motility and vitality of the spermatozoa; ROS can break down the cell’s natural antioxidant defense system, promoting an environment unable for normal reproductive physiological reactions [28]. There is often confusion in the definition of OS and neuro-endocrine stress. The first case is the stress of “chemical nature,” which, if it occurs, needs to be neutralized to go back to the physiological range. It is necessary to carry out periodic checks to avoid that, over time and with a prolonged state of OS, deleterious chronic alterations can occur which do not have the possibility of being recovered. Neuro-endocrine stress, which mainly depends on the hypothalamic–pituitary–adrenal axis function, also involves a behavioral sphere. When it occurs, the body quickly implements all actions to resolve discomfort induced by stress factors and restore homeostasis [29,30]. The beginning and/or evolution of pathological processes involving the female and male reproductive sphere [31] resulting in embryonic reabsorption, repeated pregnancy interruption, preeclampsia, intrauterine growth limitation, fetal death, and male infertility are often caused by OS [32,33]. The most potent ROS, generated during the consumption of oxygen (O2), are superoxide anion (O2−•), hydroxyl radical (•OH), peroxyl (ROO•), alkoxyl (RO•), and hydroperoxyl radical (HO2•).
ROS, unstable and highly reactive molecules, can stabilize by stripping electrons from macromolecules such as carbohydrates, proteins, lipids, and nucleic acids, triggering chain reactions that lead to cell damage disease [34].
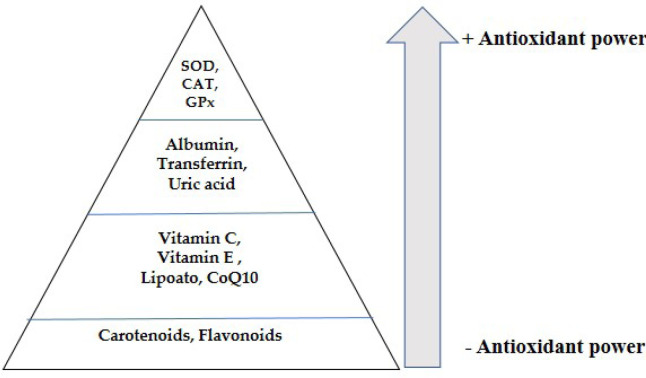
OS is responsible for DNA damage, denaturation of proteins, and lipid peroxidation. Antioxidants can counteract the harmful effects of reactive species. Two types of antioxidants are present in the body: enzymatic and non-enzymatic antioxidants. Among the enzymatic antioxidants there is superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Non-enzymatic antioxidants include β-carotene, vitamin C, vitamin E, zinc, selenium (Se), taurine, and glutathione (GSH) [35]. Figure 2 shows the power hierarchy of antioxidants: the antioxidant power grows progressively, proceeding from the base toward the pyramid’s apex where the power reaches its maximum.
Figure 2. The antioxidant pyramid. Endogenous enzymes are positioned at the top of the pyramid and are the elements with the greatest antioxidant power; at the pyramid’s base, there are elements with less antioxidant capacity.
Enzymatic antioxidants
SOD, CAT, and GPx neutralize ROS in surplus and avoid cell damage. They have a metal center, which allows them to have different valences while transferring electrons to balance molecules for the detoxification process.
SOD catalyzes the dismutation reaction of the superoxide anion to H2O2. There are three isoenzymes: SOD 1, SOD 2, and SOD 3. SOD 1 (cytoplasmic) and SOD 3 (extracellular) contain copper (Cu) and zinc (Zn) as cofactors, SOD 2 (mitochondrial) contains Mn [36]. CAT eliminates H2O2 from the cell whenever its concentration increases, and performs its action, especially in peroxisomes, endoplasmic reticulum, mitochondria, and cytosol in different cell types [37].
GPx catalyzes the reduction of H2O2 and organic-free hydroperoxides that require GSH as a co-substrate and expresses five isoforms [38]. GPx1, the cytosolic isoform, is mainly present in tissues. GPx2 encodes a gastrointestinal form without definite function. GPx3 is located in epididymal fluid and plasma. GPx4 detoxifies mainly phospholipid hydroperoxide inside the biological membranes; in the epididymis is GPx5 [6,8].
Non-enzymatic antioxidants
Among non-enzymatic antioxidants, there are vitamin C (ascorbic acid), vitamin E (α-tocopherol), GSH, Zn, taurine, selenium, and carotenoids [39]. Vitamin C is an antioxidant that catalyzes redox reactions, reduces and neutralizes ROS. Its principal function is to scavenge the superoxide radical anion, singlet oxygen, and hydrogen peroxide [40]. Vitamin E has eight isoforms that counteract the lipid radicals derived from the lipid peroxidation process. The reaction, catalyzed by vitamin E, leads to the formation of α-tocopheroxyl oxidized radicals that can again be reduced by reacting with antioxidants such as vitamin C, ubiquinol, or retinol [41]. GSH, a tripeptide, is the main non-enzymatic antioxidant present in oocytes and embryos. The presence of a thiol group of cysteine, which represents a reducing agent capable of being reversibly oxidized and reduced to its stable form, makes it an efficient antioxidant [42]. Taurine and hypotaurine neutralize the products deriving from lipid peroxidation and hydroxyl radicals, allowing to keep the redox homeostasis in the gametes [43]. Transferrin and ferritin, which bind iron, are considered antioxidants since they prevent free radicals by chelation reaction.
Furthermore, micronutrients such as Se, Cu, and Zn are essential for some antioxidant enzyme activity. The hormone melatonin is also a very effective antioxidant that, unlike other antioxidants, cannot undergo redox reactions. Once oxidized, it cannot return to the reduced state since, after the response, it forms stable final products [44]. Melatonin enhances the transport chain of electrons and reduces mitochondrial DNA damage, acting mainly in the mitochondria.
Assisted reproductive technologies (ARTs) in dog
ARTs are primarily used in large animals and laboratory species, while they are partially used in most pets. Generally, the success rates are due to the pet species and the differences in the optimal conservation of fertility after the freeze–thawing processes. The success also depends on the choice of the cryoprotectant in the freezing solution, the medium composition, and the difficulty in having standardized, repetitive procedures [45]. The dog (Canis lupus familiaris) is one of the leading domestic animals which plays a fundamental role in human society [46], with a high socio-economic value that has led to advances in dog ARTs [47]. IVF has become one of the routine techniques used in dogs. Although there is a lot of data reporting ARTs in carnivores, the dog represents a big problem for the researcher working in reproduction. One of the reasons is that the canine follicular oocytes have a low meiotic capacity with an estrous cycle characterized by estrus, followed by a mandatory period of anestrous, with an inter-estrus period of about 7 months [48,49]. Therefore, determining the induction of estrus and females’ synchronization is difficult, as they do not react to the standard gonadotropin protocols employed in other animals [49,50]. Besides the long interval of interestrus and difficulty in manipulating, the canine cycle involves a very high number of females to obtain at least one female in a phase suitable for receiving embryos. Other than IVF, ET is arduous to apply in the dogs for the limited possibility of obtaining synchronized recipients.
In a fascinating study, Tsutsui et al. [51] reported that divided dog embryos were posed in the uterine horn, which led to the birth of two cubs from one of the eight females involved in this study. This result was seen as a huge success, while in human ARTs, the transfer of cut embryos into the uterus is a common practice. The first live births of dogs through IVF, embryo cryopreservation, and transfer techniques were reported by Mukai et al. [52]. These techniques can be of great applicability, especially for the conservation of endangered canids and the development of gene modification/repair technologies, improving animal welfare by restoring normal gene function, thus counteracting the predisposition to diseases. In the ARTs of canine species, the technique of cryopreservation of sperm is used to ensure genetic conservation. However, during this technique, DNA damage is still high, to the point of limiting the fertilization rate [53].
In conventional freezing, it is essential to choose an optimal temperature that prevents damage to the semen and the use of cryoprotectants that maintain the effectiveness of the semen once thawed. Liquid nitrogen is primarily used in conventional freezing, one of the techniques allowing the conservation of canine sperm for long periods and maintaining the vital sperm. In this technique, it is essential to establish the semen freezing time since higher exposure can damage the spermatozoa’s fertilization capacity. González et al. [54] showed that using freezers at −80°C to store frozen dog sperm allows for preserving sperm characteristics for at least 15 days, while motility is maintained only 1 day. The cryopreservation advantage is the possibility of storing sperm for a long time and the possible shipment to avoid the inconvenience associated with the donor’s journey. All this leads to a reduction in costs, while limiting the sanitary restrictions on live animals’ transport [55].
Currently, one of the goals of freezing sperm for short- and long-term availability is to prevent damage to intact sperms from the freezing process. In this view, vitrification gives better results due to less manipulation of the sperm and shorter freezing times than conventional freezing techniques. Indeed, this new technique can guarantee the cryo-survival of the spermatozoa with a large number of benefits such as low cost and rapid alternative to the expensive methods of cryopreservation of the dog spermatozoa [56,57]. Therefore, the cryopreservative medium’s choice is essential as it considers the physiology of semen capacity (membrane quality, motility, OS, DNA conservation, and fertilization). In particular, canine sperm has a low resistance to sperm cooling [58]. The freezing–thawing process can lead to morphological changes, such as ice crystals, and functional changes, such as reduced sperm motility.
Aparnak and Saberivand [59] have reported the effects of curcumin on sperm motility and total count, among other factors of frozen dog semen. They concluded that curcumin in 2.50 mM concentration might help protect dog sperm from possible damages caused by cryopreservation procedures. Recently, Grandhaye et al. [53] reported that the use of metformin, a molecule that affects metabolic activities, ameliorates the quality of the frozen–thawed dog semen during the cryopreservation procedure. In particular, metformin was added to the extender before and during the freezing process. After thawing, the sperm maintained the same viability without altering the membrane’s integrity or acrosomal reaction. The use of different cryoprotectants during vitrification has also taken researchers’ attention in improving this freezing procedure. The cryoprotectants supplementation to semen extenders ameliorates cell survival afterward the freezing. Gharajelar et al. [60] compare the effect of three cryoprotectants such as milk, glycerol, and egg yolk on the quality of the canine semen after vitrification and thawing processes. The milk and egg yolk are impermeable cryoprotectants, preventing ice crystals from forming outside the cells and supplying the spermatozoa with enough energy.
On the contrary, the glycerol acts as a permeable cryoprotectant. It is utilized to freeze semen of various species because it prevents ice crystals inside the cells [61]. This cryoprotectant can be toxic to spermatozoa because it damages the plasma membrane with consequently reduced fertility. The results showed that milk has better impacts on the cryopreservation process of dog sperm compared to the egg yolk and glycerol [60]. Later Caturla-Sánchez et al. [56] have studied the effects of various impermeable cryoprotectants on the vitrification of dog spermatozoa using different concentrations of trehalose and sucrose. The disaccharide of glucose and trehalose keeps cells from being damaged during the cryopreservation process [62]. The samples were also frozen in liquid nitrogen. The results obtained showed that the vitrification process greatly influences the motility of the dog’s spermatozoa.
On the contrary, the data relating to the plasma membrane’s integrity were comparable to those achieved with conventional freezing [56]. Instead, the cause of low motility seems to be suggested because the spermatozoa in the diluents with disaccharides leak the motility faster during the post-thawing incubation concerning the diluents with either glucose or fructose [56]. This last condition perhaps would be due to that monosaccharides are more easily metabolized by spermatozoa. Recently Zakošek Pipan et al. [63] reported the effects of different sucrose and soy lecithin concentrations utilized to confront different vitrification techniques for canine semen cryopreservation. The results obtained showed that the vitrification media with soy lecithin and sucrose cause minimal wane in the quality of the dog seed after devitrification, as they keep unchanged physiological parameters of the sperm, such as morphology, vitality, total and progressive motility, acrosome, the integrity of the sperm tail membrane, and DNA.
Besides, soy lecithin is an attractive protein to replace albumin and egg yolk in the means of vitrification to obtain a good sperm quality after devitrification. Del Prete et al. [5] correlated OS status and dog sperm parameters and the effect of supplementation of SOD, CAT, and GPx in egg yolk glucose tris-citrate extender on sperm stored at 4°C for 10 days. The dogs were separated into two groups: fertile and subfertile. Adding SOD, CAT, and GPx to the extender maintains semen quality for 10 days of storage at 4°C in both fertile and subfertile dogs.
Qamar et al. [64] showed the effect of myoinositol involved in the process of sperm maturation, capacity, motility, and acrosomal reaction [65] because it neutralizes free radicals developed in the cryopreservation of canine semen. The result of this study showed that the integration of myoinositol in the freezing medium improved sperm motility, concluding that this procedure can protect against OS and improve the quality of the dog’s post-thaw sperm [64]. In Table 1, it is reported a comparison of the treatment, and the results during the ARTs obtained in the dog.
Table 1. A comparison of the treatment and the results during the ARTs obtained in the dog.
| Species | Treatment | Results | References |
|---|---|---|---|
| Dog | Metformin | The frozen sperm preserved viability without altering the integrity of the membrane or the acrosome reaction. | Grandhaye et al. [53] |
| Dog | Sucrose and/or trehalose | Non-permeable cryoprotectants | Yamashiro et al. [62]; Caturla-Sánchez et al. [56] |
| Dog | Curcumin | Preserves sperm from reactive oxygen stress and increases NOX5 gene expression. | Aparnak and Saberivand [59] |
| Dog | Glycerol, milk, and egg yolk | Cryoprotectants | Gharajelar et al. [60] |
| Dog | Soy lecithin and sucrose | Ameliorate sperm motility, sperm tail membrane integrity, and DNA integrity | Zakošek Pipan et al. [63] |
| Dog | Myoinositol | Enhances sperm motility and sperm tail, membrane integrity | Bevilacqua et al. [65]; Qamar et al. [64] |
| Dog | SOD, CAT e GPx | Preservation of semen quality | Del Prete et al. [86] |
Assisted reproductive technologies (ARTs) in cat
The model for the development of ARTs in the Felidae species is the cat. ARTs are used in threatened species conservation projects for endangered species, but the efficacy of ARTs for semi-domestic, non-domestic, and significantly compromised felid species is poor [2,4,66]. In males, the two primary endogenous potential sources of ROS are immature spermatozoa and leukocytes. In females, oocyte, cumulus mass cells, and follicular fluid are ROS sources [67]. Male and female reproductive systems of cats produce significant ROS sources; therefore, the OS in classical clinical ARTs in felid depends on culture medium composition, the pH, temperature, oxygen concentration, technique type, and cryopreservation processes [68]. In females, enzymatic and non-enzymatic antioxidants are within the ovary, in the fluids of the follicle, uterine tubes, and peritoneum, and also in the endometrial epithelium [69]; while they are within in the testis, epididymis, secretion of the male accessory glands, and seminal plasma [70]. SOD, CAT, and GPx, endogenous antioxidants, effectively prevent ROS harmful impacts in vivo conditions. Thus, several possibilities were investigated to use antioxidants to maximize outcomes of reproduction technologies in vitro. The balance between antioxidants and ROS is critical for the cumulus/oocyte complex’s functional integrity [71]. GSH, an antioxidant tripeptide, increases as oocyte maturation progresses; thus, mature oocytes have a greater quantity of GSH than immature ones [72].
Furthermore, GSH promotes, during IVF, sperm decondensation, oocyte activation, and male pronuclear formation. During IVC, the oocytes expose themselves to ROS’s harmful effects, whereby Luvoni et al. [73] added as a supplement cysteine, known to be one of the precursors of GSH, to improve the endogenous system of cell defenses. In a set of experiments, the cysteine was associated with other compounds containing thiols, such as β-mercaptoethanol and cysteamine, to avoid the GSH depletion during oocyte culture and to maintain cysteine, necessary for GSH synthesis. Ochota et al. [74] showed that the in vitro supplementation of IVM and IVC media with SOD and taurine improved blastocysts’ production from poor-quality cat oocytes. In domestic cats, Cocchia et al. [4] detected that adding SOD to the ovary transport medium reduced cellular apoptosis with increased survival of the cumulus/oocyte complex and IVEP. They noted the lower ovarian expression of apoptotic Bax and a higher anti-apoptotic, Bcl-2 in the SOD-treated group compared to the control group. Bcl-2 is an essential anti-apoptotic protein that inhibits cell death by reducing the generation of ROS. The same authors verified the positive impact of the SOD and CAT addition to the cat oocyte IVM medium. They showed an increase of cleaved embryo and blastocyst formation of 70% and 77%, respectively.
Furthermore, the addition of GPx to the IVF medium increased the rate of embryo and blastocyst formation; cleaved embryos doubled, with a 96% increase in embryos [6]. Thiangtum et al. [75] assessed in the semen extender the effect of the antioxidants, SOD and CAT, on the motility, vitality, and acrosomal integrity of frozen and thawed cats spermatozoa. The semen extender treated with glycerol and CAT or SOD or without antioxidants did not show any effect.
There have also been attempts to utilize exogenous antioxidants. Indeed, Tris egg yolk extender treatment with cysteine or vitamin E was used to improve the motility and integrity of the sperm membrane and DNA of the frozen and thawed epididymal cat spermatozoa [76]. Macente et al. [77] found that the addition of cysteine or Trolox, a water-soluble vitamin E analog, to Tris egg yolk extender improved the motility, membrane, and DNA integrity but had no effect on acrosome integrity in cats [77]. Recently, resveratrol, a phytoalexin with antioxidant activity, has been used as an IVM supplement and has been able to prevent OS’s adverse effects on domestic cat oocytes and improve embryo development after storage of the ovary at 4°C for 48 h [78]. In Table 2, it is reported a comparison of the treatment, and the results during the ARTs obtained in the cat.
Table 2. A comparison of the treatment and the results during the ARTs obtained in the cat.
| Species | Treatment | Results | References |
|---|---|---|---|
| Cat | SOD and Taurine | Improve the production of blastocysts of low-quality oocytes | Ochota et al. [74] |
| Cat | SOD | Reduces cell apoptosis and improves the survival of the cumulus/oocyte complexes | Cocchia et al. [4] |
| Cat | SOD and CAT | Increase formation of cleaved embryos and blastocysts by 70% and 77%, respectively | Cocchia et al. [4] |
| Cat | Glycerol and CAT or SOD | No consequence on sperm motility, viability and acrosome integrity. | Thiangtum et al. [75] |
| Cat | Cysteine or vitamin E | Improves motility and integrity of the sperm membrane of frozen and thawed epididymal cat spermatozoa | Thuwanut et al. [76] |
| Cat | Cysteine or Trolox | Improves membrane and DNA integrity and sperm motility, with no effect on the integrity of acrosomes | Macente et al. [77] |
| Cat | Resveratrol | Prevents the adverse effects of OS and improves embryo development after storage of the ovary at 4°C for 48 h | Piras et al. [78] |
Assisted reproductive technologies (ARTs) in horse
Among the ARTs, AI is a widely used alternative and an effective tool for horses’ natural mounts. It allows fertilizing mares avoiding all the inconveniences and costs linked to the transport of animals. AI also enables an improvement of the offspring for several reasons. It avoids infections, enhances the characteristics of a breed, and manages and chooses fertile and adequate doses of male semen for protecting the well-being of the mare. Besides, AI increases the chances of pregnancy for mares with a low fertility index due to uterine deficiencies or simple aging; it uses a reduced amount of gametic material from the male for each insemination and allows to plan pregnancies the needs of the farm. Antioxidants are an essential tool to ease the quality and quantity of raw and cooled horse semen; they are employed as a supplement in the diet or added to the media for in vitro studies. The equine breeding season is influenced by the length of the hours of light. The beginning of the breeding season is related to the increase in hours of light, and on the contrary, the end is determined by the progressive increase of the hours of darkness. The estrus cycle of the mares, of about 22 days, is evident only during the breeding season [79].
Stallions, unlike other species, do not exhibit testicular degeneration during the non-breeding season, so sperm is often collected for freezing storage when fresh sperm is not required for AI. During the breeding season, the sperm collection rate can be daily or every 2–3 days. At the end of the breeding season, sporadic decreases in infertility are seen in stallions. For mares, a false impression of subfertility can sometimes be obtained at the end of the breeding season. This is because if they do not conceive early in the season, they will be over-represented toward the end of the breeding season, and it can be more difficult for mares to conceive during this time. There are few studies concerning the quality of refrigerated sperm throughout the breeding season. However, various studies have evaluated the quality of sperm for cryopreservation outside the breeding season. During the breeding season, it is not easy to obtain biological material due to the high economic value of sperm, resulting in a reduction in earnings for breeders. Janett et al. [80] collected semen from warm-blooded stallions across the year and evaluated semen volume and concentration, motility parameters, morphological aspects, frozen–thawed semen characteristics. In spring, the sperm resulted in good quality, but surprisingly, the best results were obtained in autumn, which seems to be the best season to cryopreserve stallion semen. A similar study found that the period in which the sperm had the best morpho-functional and motility characteristics is the spring that coincides with the regular season for the breeding horse [81].
The biotechnology of in vitro reproduction applied to sperm inevitably leads to an increase in ROS levels and a decrease in natural antioxidant defenses, since the in vitro system is a closed system in which molecular turnover does not occur naturally but is altered, and this leads to the accumulation of substances which, if they reach high concentrations, cause deleterious effects. De Andrade et al. [82] evaluated that nitric oxide ensured the spermatozoa motility in cryopreserved equine sperm. According to the authors, nitric oxide is also involved in modulating several variables related to equine sperm capacitation.
Morte et al. [83] carried out a study of infertile and subfertile stallions during breeding and non-breeding season. They evaluated in stallions sperm DNA damage by TUNEL assay and sperm lipid and protein oxidation. Interestingly, during the breeding season, stallion semen showed increased protein oxidation correlated with sperm motility and viability. During the non-breeding season, in subfertile stallions, a correlation between protein and lipid oxidation levels in both sperm and seminal plasma was detected. This suggests that protein and lipid oxidation could be markers during the non-breeding season to identify subfertile stallions. The stallion semen is damaged by OS during cooling and transport, compromising fertilizing semen capacity [84].
Contri et al. [85] evaluated the effects of dietary supplementation with exogenous antioxidants on the sperm quality of stallions; they suggested that such antioxidant supplementation influences the ability of spermatozoa to resist the impact of OS. Del Prete et al. [86] have shown that it is possible to prevent oxidative injuries by administering a natural antioxidant as dietary supplementation. For the first time, the authors highlighted dietary supplementation effects with Lepidium meyenii (Maca) on fresh and refrigerated stallion sperm features. Finally, this study suggests that dietary supplementation with Maca increased the production of sperm during chilled storage, stabilizing semen quality. Recently, Tafuri et al. [87] reported that Maca contains a high percentage of glucosinolates and macamides, which may be responsible for the plant’s antioxidant activity. Maca is believed to have antioxidant activity and fertility-enhancing properties due to its metabolites, some of which are present only in this tuber [88].
Turunen et al. [89] reported that the coenzyme Q10 associated with the a-tocopherol effectively maintains plasma membrane integrity and functionality. Franco et al. [90] used a-tocopherol added into the extender and observed beneficial effects to stallion semen parameters. Nogueira et al. [91] evaluated the impact of the addition of the a-tocopherol and coenzyme Q10 to the refrigerating extender on the stallion semen features. The data showed that coenzyme Q10 and a-tocopherol were influential during the cooling processes of equine semen, increasing total motility levels and reducing lipid peroxidation. Research groups [92] have evaluated both sperm motility and viability and semen and extender oxidation state with and without additives in the extender to detect antioxidant properties. To this end, the protective and antioxidant effects of lactoferrin and caseinate milk proteins adjunct to the equine semen cooling extender were investigated on sperm parameters and nitrite and H2O2 concentrations. The results demonstrated that the supplementation with lactoferrin as an antioxidant to a modified caseinate extender did not benefit both in membrane integrity and on sperm motility and of the refrigerated semen [92]. Nonetheless, they suggest that the caseinate can protect the stallion sperm during refrigeration in a similar way as milk. Table 3 describes a comparison of the treatment and the results during the ARTs obtained in the horse.
Table 3. A comparison of the treatment and the results during the ARTs obtained in the horse.
| Species | Treatment | Results | References |
|---|---|---|---|
| Horse | Nitric oxide | Ensures the spermatozoa motility in cryopreserved equine sperm | de Andrade et al. [82] |
| Horse | Lipid and protein peroxidation in semen and seminal plasma | Lipid and protein peroxidation on breeding season markers for subfertile stallions | Morte et al. [83] |
| Horse | Exogenous antioxidants | Ensures spermatozoa ability to withstand the effects of OS | Contri et al. [85] |
| Horse | Lepidium meyenii (Maca) | Prevents oxidative damages and stabilizes semen quality | Del Prete et al. [86] |
| Horse | Glucosinolates and macamides present in Maca | Fertility enhancing properties | Tafuri et al. [88] |
| Horse | Coenzyme Q10 with the a-tocopherol | Maintenance of plasma membrane integrity and functionality | Turunen et al. [89] |
| Horse | a-tocopherol into the semen extender | Beneficial effects to equine semen parameters | Franco et al. [90] |
| Horse | Q10 and the a-tocopherol to the semen cooling extender | Increase the levels of total motility and reducing lipid peroxidation of cooled semen | Nogueira et al. [91] |
| Horse | Caseinate and lactoferrin to equine semen cooling extenders | Beneficial effect on sperm motility and membrane integrity of cooled semen | Martins et al. [92] |
Conclusion
ARTs are based on manipulating the oocytes, sperm, and/or embryos to overcome the problems due to female and male infertility. Inevitably, the biotechnology of in vitro reproduction leads to increased ROS levels with a consequent decrease in natural antioxidant defenses. The in vitro reproduction is a closed system in which molecular turnover does not occur naturally but is altered with the resultant accumulation of substances that can cause harmful effects at high concentrations. In the present study, the use and results of various antioxidants for the improvement of reproductive performance in some animals were highlighted. Although there have been numerous studies on animals, different types of antioxidants are continuously tested to prevent and contrast the effects of ROS formed during in vitro reproduction techniques.
List of abbreviations
AI: Artificial Insemination, ARTs: Assisted Reproductive Technologies, CAT: Catalase, Cu: Copper, DNA: Deoxyribonucleic Acid, ET: Embryo Transfer, GPx: Glutathione Peroxidase, GRB: Genome Resource Banking, GSH: Glutathione, ICSI: Intracytoplasmic Sperm Injection, IVC: In vitro Culture, IVEP: In vitro Embryo Production, IVF: In vitro fertilization, IVM: In vitro Maturation, MII: Metaphase II, MOET: Multiple Ovulation and Embryo Transfer, OS: Oxidative stress, ROS: Reactive Oxygen Species, SCNT: Somatic Cell Nuclear Transfer, Se: Selenium, SOD: Superoxide Dismutase, Zn: Zinc.
Acknowledgments
This research was funded by a grant from the Department of Veterinary Medicine and Animal Production (N. 37-15/6/2015), University of Naples Federico II, Italy.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
Conceptualization: FC, LM, NC, DdA, DC, LA, AAN, and ST; Design: FC, LM, NC, and ST; Data curation: FC, LM, NC, DdA, AAN, and ST; Writing the review: FC, LM, DdA, AAN, and ST. Critically revised: FC, LM, NC, DdA, DC, LA, AAN and ST. All authors read and agreed to the final version of the manuscript.
References
- [1].Cocchia N, Ciani F, El-Rass R, Russo M, Borzacchiello G, Esposito V, et al. Cryopreservation of feline epididymal spermatozoa from dead and alive animals and its use in assisted reproduction. Zygote. 2010;18(1):1–8. doi: 10.1017/S0967199409990256. https://doi.org/10.1017/S0967199409990256. [DOI] [PubMed] [Google Scholar]
- [2].Ciani F, Cocchia N, Rizzo M, Ponzio P, Tortora G, Avallone L, et al. Sex determining of cat embryo and some feline species. Zygote. 2008;16(2):169–77. doi: 10.1017/S0967199408004681. https://doi.org/10.1017/S0967199408004681. [DOI] [PubMed] [Google Scholar]
- [3].Ciani F, Cocchia N, Esposito L, Avallone L. Fertility cryopreservation. In: Wu B, editor. Advances in embryo transfer book. London, UK: IntechOpen; 2012. pp. 225–48. Intech. [Google Scholar]
- [4].Cocchia N, Corteggio A, Altamura G, Tafuri S, Rea S, Rosapane I, et al. The effects of superoxide dismutase addition to the transport medium on cumulus-oocyte complex apoptosis and IVF outcome in cats (Felis catus) Reprod Bio, 2015;15:56–64. doi: 10.1016/j.repbio.2014.10.002. https://doi.org/10.1016/j.repbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- [5].Del Prete C, Ciani F, Tafuri S, Pasolini MP, Della Valle G, Palumbo V, et al. Effect of superoxide dismutase, catalase and glutathione peroxidase supplementation in the extender on chilled semen of fertile and hypofertile dogs. J Vet Sci. 2018;19(5):667–75. doi: 10.4142/jvs.2018.19.5.667. https://doi.org/10.4142/jvs.2018.19.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cocchia N, Tafuri S, Del Prete C, Palumbo V, Esposito L, Avallone L, et al. Antioxidant supplementation to medium for in vitro embryo production in Felis catus. Pol J Vet Sci. 2019;22(3):573–9. doi: 10.24425/pjvs.2019.129966. [DOI] [PubMed] [Google Scholar]
- [7].Costantino M, Giuberti G, Caraglia A, Caraglia M, Lombardi A, Misso G, et al. Possible antioxidant role of SPA therapy with chlorine–sulphur–bicarbonate mineral water. Amino Acids. 2009;36:161–5. doi: 10.1007/s00726-008-0032-y. https://doi.org/10.1007/s00726-008-0032-y. [DOI] [PubMed] [Google Scholar]
- [8].Del Prete C, Stout T, Montagnaro S, Pagnini U, Uccello M, Florio P, et al. Combined addition of superoxide dismutase, catalase and glutathione peroxidase improves quality of cooled stored stallion semen. Anim Reprod Sci. 2019;210:106195. doi: 10.1016/j.anireprosci.2019.106195. https://doi.org/10.1016/j.anireprosci.2019.106195. [DOI] [PubMed] [Google Scholar]
- [9].Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. doi: 10.1038/164666a0. https://doi.org/10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- [10].Foote RH. The history of artificial insemination: aelected notes and notables. J Anim Sci. 2002;80:1–10. https://doi.org/10.2527/animalsci2002.80E-Suppl_21a. [Google Scholar]
- [11].Thibier M, Wagner HG. World statistics for artificial insemination in cattle. Livest Prod Sci. 2002;74(2):203–21. https://doi.org/10.1016/S0301-6226(01)00291-3. [Google Scholar]
- [12].Zhu J, Moawad AR, Wang CY, Li HF, Ren JY, Dai YF. Advances in in vitro production of sheep embryos. Int J Vet Sci Med. 2018;6(Suppl):S15–26. doi: 10.1016/j.ijvsm.2018.02.003. https://doi.org/10.1016/j.ijvsm.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sjunnesson Y. In vitro fertilisation in domestic mammals-a brief overview. Ups J Med Sci. 2020;125(2):68–76. doi: 10.1080/03009734.2019.1697911. https://doi.org/10.1080/03009734.2019.1697911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oberlender G, López SR, De Ondiz Sánchez AD, Vieira LA, Pereira MB, de Fátima Silva L, et al. In vitro fertilization of porcine oocytes is affected by spermatic coincubation time. Pesqui Vet Bras. 2016;36:58–64. https://doi.org/10.1590/S0100-736X2016001300009. [Google Scholar]
- [15].Van Soom A, Rijsselaere T, Filliers M. Cats and dogs: two neglected species in this era of embryo production in vitro? Reprod Domest Anim. 2014;49:87–91. doi: 10.1111/rda.12303. https://doi.org/10.1111/rda.12303. [DOI] [PubMed] [Google Scholar]
- [16].Bertolini M, Bertolini LR. Advances in reproductive technologies in cattle: from artificial insemination to cloning. Rev Fac Med Vet Zootecnia. 2009;56(3):184–94. [Google Scholar]
- [17].Nicholas FW, Smith C. Increased rates of genetic change in dairy cattle by embryo transfer and splitting. Anim Prod. 1983;36:341–53. https://doi.org/10.1017/S0003356100010382. [Google Scholar]
- [18].Uehara T, Yanagamachi R. Microsurgical injection of transformation. Biol Reprod. 1976;470:467–70. doi: 10.1095/biolreprod15.4.467. https://doi.org/10.1095/biolreprod15.4.467. [DOI] [PubMed] [Google Scholar]
- [19].Salamone DF, Canel NG, Rodriguez B. Intracytoplasmic sperm injection in domestic and wild mammals. Reproduction. 2017;154:111–24. doi: 10.1530/REP-17-0357. https://doi.org/10.1530/REP-17-0357. [DOI] [PubMed] [Google Scholar]
- [20].Cocchia N, Ciani F, Russo M, El Rass R, Rosapane I, Avallone L, et al. Immature cat oocyte vitrification in open pulled straws (OPSs) using a cryoprotectant mixture. Cryobiology. 2010;60(2):229–34. doi: 10.1016/j.cryobiol.2010.01.003. https://doi.org/10.1016/j.cryobiol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- [21].Huang Z, Gao L, Hou Y, Zhu S, Fu X. Cryopreservation of farm animal gametes and embryos: recent updates and progress. Front Agric Sci Eng. 2019;6(1):42–53. https://doi.org/10.15302/J-FASE-2018231. [Google Scholar]
- [22].Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist. 2016;21(9):1050–62. doi: 10.1634/theoncologist.2016-0067. https://doi.org/10.1634/theoncologist.2016-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tian XC, Kubota C, Enright B, Yang X. Cloning animals by somatic cell nuclear transfer - biological factors. Reprod Biol Endocrinol. 2003;1:1–7. doi: 10.1186/1477-7827-1-98. https://doi.org/10.1186/1477-7827-1-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Campbell KHS, Fisher P, Chen WC, Choi I, Kelly RDW, Lee JH, et al. Somatic cell nuclear transfer: past, present and future perspectives. Theriogenology. 2007;68:S214–31. doi: 10.1016/j.theriogenology.2007.05.059. https://doi.org/10.1016/j.theriogenology.2007.05.059. [DOI] [PubMed] [Google Scholar]
- [25].Benson EE. Cryopreservation of phytodiversity: a critical appraisal of theory & practice. Crit Rev Plant Sci. 2008;27(3):141–219. https://doi.org/10.1080/07352680802202034. [Google Scholar]
- [26].Amstislavsky SY, Brusentsev EYu, Abramova TO, Ragaeva DS, Rozhkova IN, Igonina TN, et al. Applying reproductive technologies and genome resource banking to laboratory animals. Russ J Genet Appl Res. 2016;6(4):373–7. https://doi.org/10.1134/S2079059716040031. [Google Scholar]
- [27].Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224C:164–75. doi: 10.1016/j.cbi.2014.10.016. https://doi.org/10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- [28].Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17(2):87–97. doi: 10.1080/2090598X.2019.1599624. https://doi.org/10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Esposito L, Auletta L, Ciani F, Pelagalli A, Pasolini MP, Lamagna B, et al. Hair cortisol levels in captive brown hare (Lepus europaeus): potential effect of sex, age, and breeding technology. Eur J Wildl Res. 2017;63:62. https://doi.org/10.1007/s10344-017-1121-6. [Google Scholar]
- [30].Esposito L, Tafuri S, Cocchia N, Fasanelli R, Piscopo N, Lamagna B, et al. Assessment of living conditions in wild boars by analysis of oxidative stress markers. J Appl Anim Welf Sci. 2020;10:1–8. doi: 10.1080/10888705.2020.1790365. https://doi.org/10.1080/10888705.2020.1790365. [DOI] [PubMed] [Google Scholar]
- [31].Wang S, He G, Chen M, Zuo T, Xu W, Liu X. The role of antioxidant enzymes in the ovaries. Oxid Med Cell Longev. 2017;2017:4371714. doi: 10.1155/2017/4371714. https://doi.org/10.1155/2017/4371714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16:80. doi: 10.1186/s12958-018-0391-5. https://doi.org/10.1186/s12958-018-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tafuri S, Ciani F, Iorio EL, Esposito L, Cocchia N. Reactive oxygen species (ROS) and male fertility. New discoveries in embryology. InTech. 2015;2:19–40. https://doi.org/10.5772/60632. [Google Scholar]
- [34].Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–42. doi: 10.1021/bi9020378. https://doi.org/10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev. 2012;70(5):257–65. doi: 10.1111/j.1753-4887.2012.00476.x. https://doi.org/10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- [36].Van Raamsdonk JM, Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci U S A. 2012;109:5785–90. doi: 10.1073/pnas.1116158109. https://doi.org/10.1073/pnas.1116158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Scibior D, Czeczot H. Catalase: structure, properties, functions. Postepy hig Med Dośw. 2006;60:170–80. [PubMed] [Google Scholar]
- [38].Galecka E, Jacewicz R, Mrowicka M, Florkowski A, Galecki P. Antioxidative enzymes - structure, properties, functions. Pol Merkur Lekarski. 2008;25:266–8. [PubMed] [Google Scholar]
- [39].He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44(2):532–53. doi: 10.1159/000485089. https://doi.org/10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- [40].Barros AI, Nunes FM, Gonçalves B, Bennett RN, Silva AP. Effect of cooking on total vitamin C contents and antioxidant activity of sweet chestnuts (Castanea sativa Mill.) Food Chem. 2011;128(1):165–72. doi: 10.1016/j.foodchem.2011.03.013. https://doi.org/10.1016/j.foodchem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- [41].Mustacich DJ, Bruno RS, Traber MG. Vitamin E. Vitam Horm. 2007;76:1–21. doi: 10.1016/S0083-6729(07)76001-6. https://doi.org/10.1016/S0083-6729(07)76001-6. [DOI] [PubMed] [Google Scholar]
- [42].Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig. 2001;8:S40–2. doi: 10.1016/s1071-5576(00)00106-4. https://doi.org/10.1177/1071557601008001S13. [DOI] [PubMed] [Google Scholar]
- [43].Orsi NM, Gopichandran N, Leese HJ, Picton HM, Harris SE. Fluctuations in bovine ovarian follicular fluid composition throughout the oestrous cycle. Reproduction. 2005;129:219–28. doi: 10.1530/rep.1.00460. https://doi.org/10.1530/rep.1.00460. [DOI] [PubMed] [Google Scholar]
- [44].Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. https://doi.org/10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Seidel GE. Lessons from reproductive technology research. Annu Rev Anim Biosci. 2015;3:467–87. doi: 10.1146/annurev-animal-031412-103709. https://doi.org/10.1146/annurev-animal-031412-103709. [DOI] [PubMed] [Google Scholar]
- [46].Jang G, Kim MK, Lee BC. Current status and applications of somatic cell nuclear transfer in dogs. Theriogenology. 2010;74:1311–20. doi: 10.1016/j.theriogenology.2010.05.036. https://doi.org/10.1016/j.theriogenology.2010.05.036. [DOI] [PubMed] [Google Scholar]
- [47].Thomassen R, Farstad W. Artificial insemination in canids: a useful tool in breeding and conservation. Theriogenology. 2009;71:190–9. doi: 10.1016/j.theriogenology.2008.09.007. [DOI] [PubMed] [Google Scholar]
- [48].England GCW. England GCW, Von Heimendahl A, editors. Physiology and endocrinology of the female. BSAVA manual of canine and feline reproduction and neonatology. 2nd edition, British Small Animal Veterinary Association, Gloucester, UK, pp 1–12, 2010; https://doi.org/10.22233/9781905319541.1. [Google Scholar]
- [49].Chastant-Maillard S, Viaris de Lesegno C, Chebrout M, Thoumire S, Meylheuc T, Fontbonne A, et al. The canine oocyte: uncommon features of in vivo and in vitro maturation. Reprod Fertil Dev. 2011;23:391–402. doi: 10.1071/RD10064. https://doi.org/10.1071/RD10064. [DOI] [PubMed] [Google Scholar]
- [50].Kutzler MA. Estrus induction and synchronization in canids and felids. Theriogenology. 2007;68:354–74. doi: 10.1016/j.theriogenology.2007.04.014. https://doi.org/10.1016/j.theriogenology.2007.04.014. [DOI] [PubMed] [Google Scholar]
- [51].Tsutsui T, Hori T, Endo S, Hayama A, Kawakami E. Intrauterine transfer of early canine embryos. Theriogenology. 2006;66:1703–5. doi: 10.1016/j.theriogenology.2006.01.009. https://doi.org/10.1016/j.theriogenology.2006.01.009. [DOI] [PubMed] [Google Scholar]
- [52].Mukai C, Nelson JL, Cheong SH, Diel de Amorim M, Travis AJ. Impacts of oocyte/zygote timing for in vitro fertilization and gene editing in the dog. Theriogenology. 2020;150:347–52. doi: 10.1016/j.theriogenology.2020.02.003. https://doi.org/10.1016/j.theriogenology.2020.02.003. [DOI] [PubMed] [Google Scholar]
- [53].Grandhaye J, Partyka A, Ligocka Z, Dudek A, Nizanski W, Jeanpierre E, et al. Metformin improves quality of post-thaw canine semen. Animals. 2020;10:287. doi: 10.3390/ani10020287. https://doi.org/10.3390/ani10020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].González A, Arando A, Acosta A, Alcalá CJ, Arrebola FA, Pérez-Marína CC. Frozen dog spermatozoa are negatively affected during storage at −80, −21 and −8°C. Anim Reprod Sci. 2019;210:106197. doi: 10.1016/j.anireprosci.2019.106197. https://doi.org/10.1016/j.anireprosci.2019.106197. [DOI] [PubMed] [Google Scholar]
- [55].Pena FJ, Nunez-Martinez I, Moran JM. Semen technologies in dog breeding: an update. Reprod Domest Anim. 2006;41(2):21–9. doi: 10.1111/j.1439-0531.2006.00766.x. https://doi.org/10.1111/j.1439-0531.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- [56].Caturla-Sánchez E, Sánchez-Calabuiga MJ, Pérez-Gutiérreza JF, Cerdeira J, Castaño C, Santiago-Moreno J. Vitrification of dog spermatozoa: effects of two cryoprotectants (sucrose or trehalose) and two warming procedures. Cryobiology. 2018;80:126–9. doi: 10.1016/j.cryobiol.2017.11.001. https://doi.org/10.1016/j.cryobiol.2017.11.001. [DOI] [PubMed] [Google Scholar]
- [57].Cerdeira J, Sanchez-Calabuig MJ, Perez-Gutierrez JF, Hijon M, Castano C, Santiago-Moreno J. Cryopreservation effects on canine sperm morphometric variables and ultrastructure: comparison between vitrification and conventional freezing. Cryobiology. 2020;95:164–70. doi: 10.1016/j.cryobiol.2020.03.007. https://doi.org/10.1016/j.cryobiol.2020.03.007. [DOI] [PubMed] [Google Scholar]
- [58].Hori T, Yoshikuni R, Kobayashi M, Kawakami E. Effects of storage temperature and semen extender on stored canine semen. J Vet Med Sci. 2014;76:259–631. doi: 10.1292/jvms.13-0303. https://doi.org/10.1292/jvms.13-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aparnak P, Saberivand A. Effects of curcumin on canine semen parameters and expression of NOX5 gene in cryopreserved spermatozoa. Vet Res Forum. 2019;10(3):221–6. doi: 10.30466/vrf.2019.76137.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gharajelar SN, Rajab Ali Sadrkhanloo RA, Masoud Onsori M, Adel Saberivand A. A comparative study on the effects of different cryoprotectants on the quality of canine sperm during vitrification process. Vet Res Forum. 2016;7(3):235–9. [PMC free article] [PubMed] [Google Scholar]
- [61].England GCW. Semen evaluation, artificial insemination. In: Etinger JS, Feldman EC, editors. Veterinary internal medicine. Philadelphia, PA: WB Saunders Company; 2000. pp. 1571–85. [Google Scholar]
- [62].Yamashiro H, Narita K, Sugimura S, Han YJ, Sugawara A, Morohaku K, et al. Trehalose enhanced the freezability of poodle dog sperm collected by an artificial vagina (AV) Anim Reprod Sci. 2007;102:165–71. doi: 10.1016/j.anireprosci.2007.02.026. https://doi.org/10.1016/j.anireprosci.2007.02.026. [DOI] [PubMed] [Google Scholar]
- [63].Zakošek Pipan M, Casal ML, Šterbenc N, Virant Klun I, Mrkun J. Vitrification using oy lecithin and sucrose: a new way to store the sperm for the preservation of canine reproductive function. Animals. 2020;10:653. doi: 10.3390/ani10040653. https://doi.org/10.3390/ani10040653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Qamar AY, Fang X, Kim MJ, Cho J. Myoinositol supplementation of freezing medium improves the quality-related parameters of dog sperm. Animals (Basel). 2019;9(12):1038. doi: 10.3390/ani9121038. https://doi.org/10.3390/ani9121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bevilacqua A, Carlomagno G, Gerli S, Montanino Oliva M, Devroey P, Lanzone A, et al. Results from the international consensus conference on myo-inositol and D-chiro-inositol in obstetrics and gynecology-assisted reproduction technology. Gynecol Endocrinol. 2015;31:441–6. doi: 10.3109/09513590.2015.1006616. https://doi.org/10.3109/09513590.2015.1006616. [DOI] [PubMed] [Google Scholar]
- [66].Pope CE. Aspects of in vivo oocyte production, blastocyst development, and embryo transfer in the cat. Theriogenology. 2014;81(1):126–37. doi: 10.1016/j.theriogenology.2013.09.006. https://doi.org/10.1016/j.theriogenology.2013.09.006. [DOI] [PubMed] [Google Scholar]
- [67].Kothari S, Thompson A, Agarwal A, du Plessis SS. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48:425–35. [PubMed] [Google Scholar]
- [68].Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol. 2014;12:1–19. doi: 10.1186/1477-7827-12-112. https://doi.org/10.1186/1477-7827-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rakhit M, Gokul SR, Agarwal A, du Plessis SS. Antioxidant strategies to overcome OS in IVF-Embryo transfer. In: Agarwal A, Aziz N, Rizk B, editors. Studies on women’s health. Totowa, NJ: Humana Press; 2013. pp. 237–62. https://doi.org/10.1007/978-1-62703-041-0_13. [Google Scholar]
- [70].Tremellen K. Oxidative stress and male infertility - a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. https://doi.org/10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- [71].Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51(1):57–64. doi: 10.1080/15216540119253. https://doi.org/10.1080/15216540152035073. [DOI] [PubMed] [Google Scholar]
- [72].Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev. 2003;64(1):106–12. doi: 10.1002/mrd.10214. https://doi.org/10.1002/mrd.10214. [DOI] [PubMed] [Google Scholar]
- [73].Luvoni GC, Chigioni S. Culture strategies for maturation of carnivore oocytes. Theriogenology. 2006;66:1471–5. doi: 10.1016/j.theriogenology.2006.02.004. https://doi.org/10.1016/j.theriogenology.2006.02.004. [DOI] [PubMed] [Google Scholar]
- [74].Ochota M, Pasieka A, Nizanski W. Superoxide dismutase and taurine supplementation improves in vitro blastocyst yield from poor-quality feline oocytes. Theriogenology. 2016;85:922–7. doi: 10.1016/j.theriogenology.2015.10.042. https://doi.org/10.1016/j.theriogenology.2015.10.042. [DOI] [PubMed] [Google Scholar]
- [75].Thiangtum K, Pinyopummin A, Hori T, Kawakami E, Tsutsui T. Effect of catalase and superoxide dismutase on motility, viability and acrosomal integrity of frozen-thawed cat spermatozoa. Reprod Domest Anim. 2009;44:369–72. doi: 10.1111/j.1439-0531.2009.01420.x. https://doi.org/10.1111/j.1439-0531.2009.01420.x. [DOI] [PubMed] [Google Scholar]
- [76].Thuwanut P, Chatdarong K, Techakumphu M, Axnér E. The effect of antioxidants on motility, viability, acrosome integrity and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology. 2008;70(2):233–40. doi: 10.1016/j.theriogenology.2008.04.005. https://doi.org/10.1016/j.theriogenology.2008.04.005. [DOI] [PubMed] [Google Scholar]
- [77].Macente BI, Ribeiro Gutierrez R, Apparício M, de Carvalho Balieiro C, Menegasso Mansano CF, Pereira MM, et al. Cat epididymal semen cryopreserved with and without vitamin E: effect on sperm parameters and lipid peroxidation. Anim Reprod. 2018;15(4):1193–8. doi: 10.21451/1984-3143-AR2018-0001. https://doi.org/10.21451/1984-3143-AR2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Piras AR, Ariu F, Falchi L, Zedda MT, Pau S, Schianchi E, et al. Resveratrol treatment during maturation enhances developmental competence of oocytes after prolonged ovary storage at 4°C in the domestic cat model. Theriogenology. 2020;44:152–7. doi: 10.1016/j.theriogenology.2020.01.009. https://doi.org/10.1016/j.theriogenology.2020.01.009. [DOI] [PubMed] [Google Scholar]
- [79].Aurich C. Recent advances in cooled semen technology. Anim Reprod Sci. 2008;107:268–75. doi: 10.1016/j.anireprosci.2008.04.015. https://doi.org/10.1016/j.anireprosci.2008.04.015. [DOI] [PubMed] [Google Scholar]
- [80].Janett F, Thun R, Niederer K, Burger D, Hassig M. Seasonal changes in semen quality and freezability in the Warmblood stallion. Theriogenology. 2003;60:453–61. doi: 10.1016/s0093-691x(03)00046-3. https://doi.org/10.1016/S0093-691X(03)00046-3. [DOI] [PubMed] [Google Scholar]
- [81].Gamboa S, Rodrigues AS, Henriques L, Batista C, Santos JR. Seasonal functional relevance of sperm characteristics in equine spermatozoa. Theriogenology. 2010;73:950–8. doi: 10.1016/j.theriogenology.2009.11.023. https://doi.org/10.1016/j.theriogenology.2009.11.023. [DOI] [PubMed] [Google Scholar]
- [82].de Andrade AFC, Arruda RP, Torres MA, Pieri NCG, Leite TG, Celeghini ECC, et al. Nitric oxide in frozen-thawed equine sperm: effects on motility, membrane integrity and sperm capacitation. Anim Reprod Sci. 2018;195:176–84. doi: 10.1016/j.anireprosci.2018.05.022. https://doi.org/10.1016/j.anireprosci.2018.05.022. [DOI] [PubMed] [Google Scholar]
- [83].Morte MI, Rodrigues AM, Soares D, Rodrigues AS, Gamboa S, Ramalho-Santosa J. The quantification of lipid and protein oxidation in stallion spermatozoa and seminal plasma: seasonal distinctions and correlations with DNA strand breaks, classical seminal parameters and stallion fertility. Anim Reprod Sci. 2008;106:36–47. doi: 10.1016/j.anireprosci.2007.03.020. https://doi.org/10.1016/j.anireprosci.2007.03.020. [DOI] [PubMed] [Google Scholar]
- [84].Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function in sickness and in health. J Androl. 2012;33:1096–106. doi: 10.2164/jandrol.112.016535. https://doi.org/10.2164/jandrol.112.016535. [DOI] [PubMed] [Google Scholar]
- [85].Contri A, De Amicis I, Molinari A, Faustini M, Gramenzi A, Robbe D, et al. Effect of dietary antioxidant supplementation on fresh semen quality in stallion. Theriogenology. 2011;75(7):1319–26. doi: 10.1016/j.theriogenology.2010.12.003. https://doi.org/10.1016/j.theriogenology.2010.12.003. [DOI] [PubMed] [Google Scholar]
- [86].Del Prete C, Tafuri S, Ciani F, Pasolini MP, Ciotola F, Albarella S, et al. Influences of dietary supplementation with Lepidium meyenii (Maca) on stallion sperm production and on preservation of sperm quality during storage at 5°C. Andrology. 2018;6(2):351–61. doi: 10.1111/andr.12463. https://doi.org/10.1111/andr.12463. [DOI] [PubMed] [Google Scholar]
- [87].Tafuri S, Cocchia N, Carotenuto D, Vassetti A, Staropoli A, Mastellone V, et al. Chemical analysis of Lepidium meyenii (Maca) and its effects on redox status and on reproductive biology in stallions. Molecules. 2019a;1981;24 doi: 10.3390/molecules24101981. https://doi.org/10.3390/molecules24101981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tafuri S, Cocchia N, Vassetti A, Carotenuto D, Esposito L, Maruccio L, et al. Lepidium meyenii (Maca) in male reproduction. Nat Prod Res. 2019b;5:1–10. doi: 10.1080/14786419.2019.1698572. https://doi.org/10.1080/14786419.2019.1698572. [DOI] [PubMed] [Google Scholar]
- [89].Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660(1–2):171–99. doi: 10.1016/j.bbamem.2003.11.012. https://doi.org/10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- [90].Franco JSV, Chaveiro A, Gois A, Silva FM. Effects of a-tocopherol and ascorbic acid on equine semen quality after cryopreservation. J Equine Vet Sci. 2013;33:787–93. https://doi.org/10.1016/j.jevs.2012.12.012. [Google Scholar]
- [91].Nogueira BG, Sampaio BFB, Souza MIL, Costa E, Silva EV, Zuccari CESN. Coenzyme Q10 and a-tocopherol prevent the lipid peroxidation of cooled equine semen. Reprod Domest Anim. 2015;50:1003–10. doi: 10.1111/rda.12627. https://doi.org/10.1111/rda.12627. [DOI] [PubMed] [Google Scholar]
- [92].Martins HS, Souza MR, Penna CFAM, da Silva GC, Cortes SF, Stahlberg R, et al. Milk, caseinate and lactoferrin addition to equine semen cooling extenders. Andrologia. 2016;48:950–6. doi: 10.1111/and.12523. https://doi.org/10.1111/and.12523. [DOI] [PubMed] [Google Scholar]