Abstract
Background
In a recent study, autoantibodies neutralizing type I interferons (IFNs) were present in at least 10% of cases of critical COVID-19 pneumonia. These autoantibodies neutralized most type I IFNs but rarely IFN-beta.
Objectives
We aimed to define the prevalence of autoantibodies neutralizing type I IFN in a cohort of patients with severe COVID-19 pneumonia treated with IFN-beta-1b during hospitalization and to analyze their impact on various clinical variables and outcomes.
Methods
We analyzed stored serum/plasma samples and clinical data of COVID-19 patients treated subcutaneously with IFN-beta-1b from March to May 2020, at the Infanta Leonor University Hospital in Madrid, Spain.
Results
The cohort comprised 47 COVID-19 patients with severe pneumonia, 16 of whom (34%) had a critical progression requiring ICU admission. The median age was 71 years, with 28 men (58.6%). Type I IFN-alpha- and omega-neutralizing autoantibodies were found in 5 of 47 patients with severe pneumonia or critical disease (10.6%), while they were not found in any of the 118 asymptomatic controls (p = 0.0016). The autoantibodies did not neutralize IFN-beta. No demographic, comorbidity, or clinical differences were seen between individuals with or without autoantibodies. We found a significant correlation between the presence of neutralizing autoantibodies and higher C-reactive protein levels (p = 5.10e−03) and lower lymphocyte counts (p = 1.80e−02). No significant association with response to IFN-beta-1b therapy (p = 0.34) was found. Survival analysis suggested that neutralizing autoantibodies may increase the risk of death (4/5, 80% vs 12/42, 28.5%).
Conclusion
Autoantibodies neutralizing type I IFN underlie severe/critical COVID-19 stages in at least 10% of cases, correlate with increased C-RP and lower lymphocyte counts, and confer a trend towards increased risk of death. Subcutaneous IFN-beta treatment of hospitalized patients did not seem to improve clinical outcome. Studies of earlier, ambulatory IFN-beta treatment are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-021-01036-0.
Keywords: COVID-19, subcutaneous interferon-beta 1b, type I IFN neutralizing autoantibodies, severity biomarkers
Introduction
In December 2019, infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in the city of Wuhan, China. One year later, as of December 22, 2020, it has caused over 79 million reported cases and at least 1.7 million deaths globally [1]. A striking feature is the vast interindividual clinical variability in the course of infection. The vast majority of infected individuals remain asymptomatic or develop mild, self-healing, ambulatory disease of the upper respiratory tract. In approximately 3% of subjects, SARS-CoV-2 infection results in pneumonia, which in approximately 0.3% of subjects evolves into acute respiratory distress syndrome (ARDS) with systemic inflammation [2]. The identification of the determinants of COVID-19 severe pneumonia could change the current treatment and prognosis of the disease.
The contribution of inborn errors of immunity to the determinism of severe infectious diseases is well established, particularly for viral infections, such as severe influenza pneumonia [3, 4]. Autoimmune phenocopies of inborn errors of cytokines, with neutralizing autoantibodies to cytokines, are also well known [5]. In this context, two studies of the COVID Human Genetic Effort consortium have recently reported that in a cohort of nearly 1000 patients with life-threatening COVID-19 pneumonia, at least 10% showed neutralizing autoantibodies against type I interferons (IFNs) [6], while another 3.5% carried rare deleterious variants in 8 genes governing TLR3- and IRF7-dependent type I IFN immunity to influenza virus [7]. These data provided evidence that defective type I IFN immunity could underlie life-threatening COVID-19 pneumonia in a significant proportion of cases [8].
The 17 individual type I IFNs are part of both intrinsic and innate immunity. They are known to increase cell defenses in response to various viruses, blocking viral spread [9]. Insufficient type I IFN immunity during the first days of infection with SARS-CoV-2 may result in viral spread to the lungs and via the bloodstream. This spread may in turn unleash excessive inflammation, including a cytokine storm, when leukocytes are recruited to infected tissues. Defects in type I IFN immunity may therefore explain why some infected subjects develop a severe pneumonia, and progress to an acute respiratory distress syndrome (ARDS), systemic inflammation, and critical disease, requiring admission to the intensive care unit (ICU). Importantly, autoantibodies to type I IFN neutralize most but not all individual IFNs. They typically neutralize the 13 IFN-alpha proteins and a single IFN-omega but rarely neutralize IFN-beta, IFN-kappa, and IFN-epsilon [6].
The detailed epidemiological and clinical characteristics of patients with autoantibodies to type I IFNs, as well as their response to type I IFN therapy, have not been reported. We set out to study patients from a single hospital in Madrid, Spain, one of the global epicenters of COVID-19 during the first wave. We analyzed the presence of autoantibodies against type I IFNs and compared patients with severe pneumonia with and without these autoantibodies, in terms of clinical features and outcome, as well as responses to subcutaneous IFN-beta therapy during hospitalization. Indeed, in the context of emerging SARS-CoV2 infection, IFN-beta was administered to severe hospitalized patients in the first months of the pandemic, alone or combined with other drugs, due to the unavailability of a specific treatment, and their unspecific antiviral effects [9]. Interferons, mainly IFN-beta, have shown to have in vitro activity against SARS-CoV and MERS-CoV [10, 11] and to reduce the mortality rate and need for intensive respiratory support in hospitalized patients [12].
Our sample consisted of 47 hospitalized clinically severe COVID-19 patients with pneumonia and oxygen support who were treated subcutaneously with IFN-beta-1b from March to May 2020. We tested for the presence of autoantibodies against IFN, and analyzed the possible impact of these autoantibodies in different clinical variables and the response to IFN-beta, in terms of bad prognosis or mortality.
Materials and Methods
We studied data from hospitalized adult patients with COVID-19 pneumonia treated subcutaneously with recombinant IFN-beta-1b (Betaferon) during admission at the Infanta Leonor University Hospital in Madrid, Spain, from the first wave of the pandemic (March to May 2020), in a retrospective manner. Based on the local treatment protocol at that time, when no drug had yet been proven efficacious, IFN-beta-1b was administered only to patients with severe respiratory failure and lack of initial response to the standard treatment (lopinavir/ritonavir, hydroxychloroquine, azithromycin).
We initially selected, from a cohort of 1549 COVID-19 patients admitted to the hospital [13], 47 clinically severe patients who were treated with IFN-beta-1b (250 μg every 48 h for up to 14 days) and who had an available plasma sample collected prospectively during hospitalization and stored in the hospital sample collection; this criterion was mandatory to guarantee the possibility to perform a test to determine the presence of anti-IFN autoantibodies.
All 47 patients were considered clinically severe at admission following the US National Institutes of Health (NIH) classification [14]. The patients presented SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, respiratory frequency > 30 breaths/min, or lung infiltrates >50%. Patients who progressed to an ARDS were defined as critical following the NIH guidelines [14], with 200 mmHg <PaO2/FiO2 ≤ 300 mmHg to PaO2/FiO2 ≤ 100 mmHg, and required support with invasive or noninvasive mechanical ventilation at the ICU.
The primary outcome of this retrospective analysis of the IFN-beta-treated cohort was to evaluate the presence of anti-IFN autoantibodies. The secondary outcome was the effect of the presence of these antibodies on different clinical and laboratory variables and disease course.
Clinical data included demographics (age, sex, and ancestry), comorbidities (arterial hypertension, diabetes, dyslipidemia, overweight-obesity, and cardiac, renal, immunological, or tumor diseases), symptoms (fever and cough), analytics (d-dimer, C-reactive-protein, ferritin, and lymphocyte counts), treatment needs (corticosteroids, tocilizumab, and response to IFN-beta), oxygen needs (nasal cannula and mask or mechanical ventilation), and clinical outcomes (days of hospitalization, critical stage, and total deaths). The response to IFN-beta-1b was evaluated as a clinical improvement in no more than the median 9 days of hospitalization and no need for escalation in treatment after IFN-beta treatment [13].
The clinical data were collected retrospectively from the electronic medical records and entered into an anonymous electronic database (REDCap, Research Electronic Data Capture) [15]. Different classes of variables were used for this study: (a) response variables (presence of antibodies: dichotomous variable (presence/absence) and antibody titer: qualitative variable, factor); (b) explanatory variables: (survival: measured as time to death or discharge from hospital (censored data), requirements of O2, the severity of the disease, positive response to IFN (qualitative variables), and lack of response to IFN) (assumed with no clinical improvement and the need for escalation in treatment with a bolus of corticosteroids or tocilizumab according to the local protocol or more than the mean 10 days of hospitalization after interferon administration); and (c) covariates: different demographic variables: comorbidities, laboratory values, symptoms, and treatments.
The biological blood samples were processed for the determination of anti-IFN autoantibodies (IFN-alpha, IFN-beta, and IFN-omega) as described by P. Bastard et al. (2020) [6]. In brief, 96-well ELISA plates (MaxiSorp; Thermo Fisher Scientific) were coated by incubation overnight at 4 °C with 2 μg/ml rhIFN-α2 (Invitrogen), rhIFN-ω (Merck), or rh-IFN-β (Invitrogen). Plates were then washed (PBS/0.005% Tween), blocked by incubation with 5% nonfat milk powder in the same buffer, washed again, and incubated with 1:50 dilutions of plasma from the patients or controls for 2 h at room temperature (or with specific mAbs as positive controls). Each sample was tested once. Plates were thoroughly washed. HRP-conjugated Fc-specific (Fc, fragment crystallizable region) IgG fractions from polyclonal goat antiserum against human IgG (Nordic Immunological Laboratories) were added to a final concentration of 2 μg/ml. Plates were incubated for 1 h at room temperature and washed. Substrate was added, and the OD was measured.
Luciferase Reporter Assays
The blocking activity of anti-IFN-α and anti-IFN-ω autoantibodies was determined by assessing a reporter luciferase activity. Briefly, HEK293T cells were transfected with the firefly luciferase plasmids under the control human ISRE promoters in the pGL4.45 backbone, and a constitutively expressing Renilla luciferase plasmid for normalization (pRL-SV40). Cells were transfected in the presence of the X-tremeGene 9 transfection reagent (Sigma-Aldrich) for 36 h. The, Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific) medium supplemented with 10% healthy control or patient serum/plasma and were either left unstimulated or were stimulated with IFN-α, IFN-ω, or IFN-β (10 ng/mL) for 16 h at 37 °C. Each sample was tested once. Finally, Luciferase levels were measured with the Dual-Glo reagent, according to the manufacturer’s protocol (Promega). Firefly luciferase values were normalized against Renilla luciferase values, and fold induction is shown relative to controls transfected with empty plasmids.
Statistical analysis was carried out using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, New York, USA). The variables that did not follow a normal distribution were expressed using the median and interquartile range (IQR). Nominal variables were expressed as numbers and percentages. Different models were used to study the association among the two response variables and the explanatory variables, as well as the covariates. To analyze the effect of dichotomous variables, the Chi2 test was used with a Markov chain correction for small sample sizes. The nonparametric Kruskal-Wallis test was used to explore the effect of continuous variables on the presence of antibodies. Likewise, the nonparametric Kaplan-Meier estimator was used to univariably analyze the survival function. Survival models with covariates were tested using the Cox proportional hazards model and the additive model of Aalen.
Informed consent was obtained orally when clinically possible. In the remaining cases, the informed consent waiver was authorized by the ethics committee. The study was approved by the Committee for Ethical Research of the Infanta Leonor University Hospital, code 008-20, and the Bellvitge University Hospital code PR127/20.
Results
A total of 47 adult patients with severe COVID-19 and with a positive nasopharyngeal swab PCR test for SARS-CoV-2 who were treated with IFN-beta-1b (Betaferon) were included in the study and assayed for the presence of neutralizing autoantibodies against type I IFN in plasma, as described by Bastard P et al. [6], (Fig. 1). Descriptive demographic and clinical variables of the included patients are shown in Table 1. In our cohort, the mean age was 66 years old, the median age was 71 years old (IQR: 57–75), male sex represented 59.6% (28) of the study population, the ancestry of 91.4% (43) of the patients was European (41/43 Spaniards), and only 6.3% (3) were Latin Americans. Preexisting comorbidities, as described before, were registered in 93.6% of patients (44/47). At admission to the hospital, all patients (47/47) were clinically classified as severe COVID-19 after the NIH classification [14], and 34% (16/47) of them had an unfavorable course, progressing to critical illness.
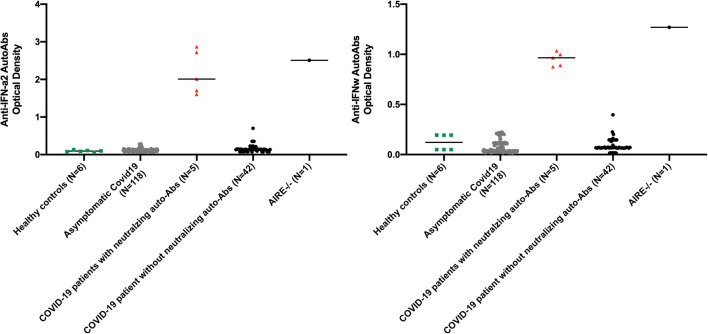
Fig. 1.
Neutralizing autoantibodies against IFN-α2 and/or IFN-ω in patients with life-threatening COVID-19. Multiplex particle-based assay for auto-Abs against IFN-α2 and IFN-ω in patients with life-threatening COVID-19 treated with IFN-beta (N = 47), or asymptomatic or mild SARS-CoV-2 infection (N = 18), and in healthy controls not infected with SARS-CoV2 (N = 6)
Table 1.
Summary of the variables used in this analysis and their statistical association. The different variables were defined according to their nature. Continuous variables are shown with the median and interquartile range [median (IQR)]. Dichotomous variables are presented with the number of events, the number of total samples and % [no. events/N (%)]. Additionally, we show the p values of the association tests (Chi2 test for dichotomous variables, used with a Markov chain correction for small sample sizes and nonparametric Kruskal-Wallis test for continuous variables) of the different variables with the presence/absence of neutralizing autoantibodies
| IFN-beta-1b treated cohort | No antibodies | Neutralizing antibodies | p values | |
|---|---|---|---|---|
| Demographics | ||||
| N | 47 | 42 | 5 | |
| Age (years) | 71 (18) | 71 (18.5) | 64 (17) | 0.113 |
| Sex (male) | 28/47 (59.6) | 25/42 (59.5) | 3/5 (60.0) | 0.854 |
| Comorbidities | ||||
| Arterial hypertension | 29/47 (61.7) | 25/42 (59.5) | 4/5 (80.0) | 0.669 |
| Diabetes | 9/47 (19.1) | 9/42 (21.4) | 0/5 (0.0) | 0.885 |
| Dyslipidemia | 18/47 (38.3) | 15/42 (35.7) | 3/5 (60.0) | 0.636 |
| Overweight (BMI >25) | 36/39 (92.3) | 32/35 (91.4) | 4/4 (100) | 0.744 |
| Obesity (BMI >30) | 21/39 (83) | 18/35 (51.4) | 3/4 (75.0) | 0.601 |
| Renal disease | 3/29 (6.4) | 2/25 (8.0) | 1/4 (25.0) | 0.368 |
| Heart disease | 8/29 (17.0) | 6/25 (24.0) | 2/4 (50.0) | 0.542 |
| Autoimmune disease | 11/47 (23.4) | 10/42 (23.8) | 1/5 (20.0) | 0.874 |
| Tumor disease | 14/47 (29.8) | 11/35 (31.4) | 2/5 (40.0) | 0.634 |
| Symptoms at admission | ||||
| Days with symptoms | 5 (4.7) | 7 (6) | 5 (2) | 0.117 |
| Fever | 46/47 (97.9) | 41/42 (97.6) | 5/5 (100) | 0.887 |
| Cough | 46/47 (97.9) | 41/42 (97.6) | 5/5 (100) | 0.887 |
| Analytics | ||||
| C-RP maximum (mg/L) | 226.3 (172.7) | 212.5 (168.1) | 360 (63.6) | 5.10e−03 |
| Ferritin maximum (ng/ml) | 1024 (1.118) | 791 (1192.5) | 1324 (577) | 0.315 |
| d-Dimer maximum (μg/L) | 3610 (13,292) | 2565 (8850) | 191,910 (31,070) | 0.144 |
| Lymphocyte count minimum (10E3/μL) | 0.5 (0.4) | 0.6 (0.3) | 0.3 (0) | 1.80e−02 |
| Treatments needs | ||||
| Corticosteroids* | 28/47 (59.6) | 23/42 (54.7) | 5/5 (100) | 0.144 |
| Bolus of corticosteroids** | 12/47 (25.5) | 10/42 (23.8) | 4/5 (80) | 0.613 |
| Tocilizumab | 17/47 (36.2) | 14/42 (33.3) | 3/5 (60) | 0.327 |
| Response to IFN-beta | 11/47 (23.4) | 11/42 (26.1) | 0/5 (0) | 0.340 |
| O2 requirements | ||||
| Nasal cannula | 17/47 (36.2) | 17/42 (40.4) | 0/5 (0) | 1.10e−03 |
| High flow reservoir mask | 24/47 (29.8) | 12/42 (28.5) | 2/5 (40) | 0.142 |
| Invasive or non-invasive mechanical ventilation | 16/47 (34.0) | 13/42 (30.9) | 3/5 (60) | 0.414 |
| Clinical outcomes | ||||
| Days of hospitalization | 14(11) | 14 (10.7) | 14 (5) | 0.258 |
| Critical stage | 16/47 (34) | 13/42 (30.9) | 3/5 (60) | 0.339 |
| Total deaths | 16/47 (34) | 12/42 (28.5) | 4/5 (80) | 3.40e−02 |
BMI body mass index, IFN interferon, O2 oxygen. *Dexamethasone 6 mg or methylprednisolone 40 mg daily, oral or intravenous; **intravenous 250 mg of methylprednisolone
We tested all patients for the presence of high titer autoantibodies against IFN-alpha2 and/or IFN-omega. We then tested their neutralizing capacity against 10 ng/ml of the corresponding type I IFNs. The prevalence of neutralizing anti-type I IFN autoantibodies against IFN-alpha proteins and the single IFN-omega was 10.6% (5 out of 47 in both types) in our cohort of IFN-beta-treated patients (Fig. 1), while they were not found in any of the 118 asymptomatic controls (p = 0,0016, Fisher exact test). This finding includes 2 out of 31 severe patients hospitalized (6.4%), and 3 out of 16 patients with critical progression to an ARDS (18.7%). No neutralizing autoantibodies against IFN-beta proteins were detected (Fig. S1). The median age of the patients with autoantibodies was 64 years (IQR: 58–75 years), 60% (3/5) were males, and 100% were of Caucasian European descent. The patients that were autoantibody negative (42/47) included 25 men (59.5%), the median age was 71 years (IQR: 56.7–76 years), 92.8% (39/42) were European, and 7.9% (3/42) were Latin Americans.
Our data indicate that most of the patients’ baseline characteristics or status at hospital admission did not show a statistically significant effect on the presence/absence of neutralizing autoantibodies according to the Kruskal-Wallis test. No demographic differences were seen between the individuals who generated such anti-IFN autoantibodies and those who did not.
We also did not find significant differences regarding the presence of a high proportion of comorbidities, including arterial hypertension, obesity, and heart, immunological, or tumor pathology, among the patients who presented autoantibodies in general, those who presented neutralizing autoantibodies and those who did not (Table 1).
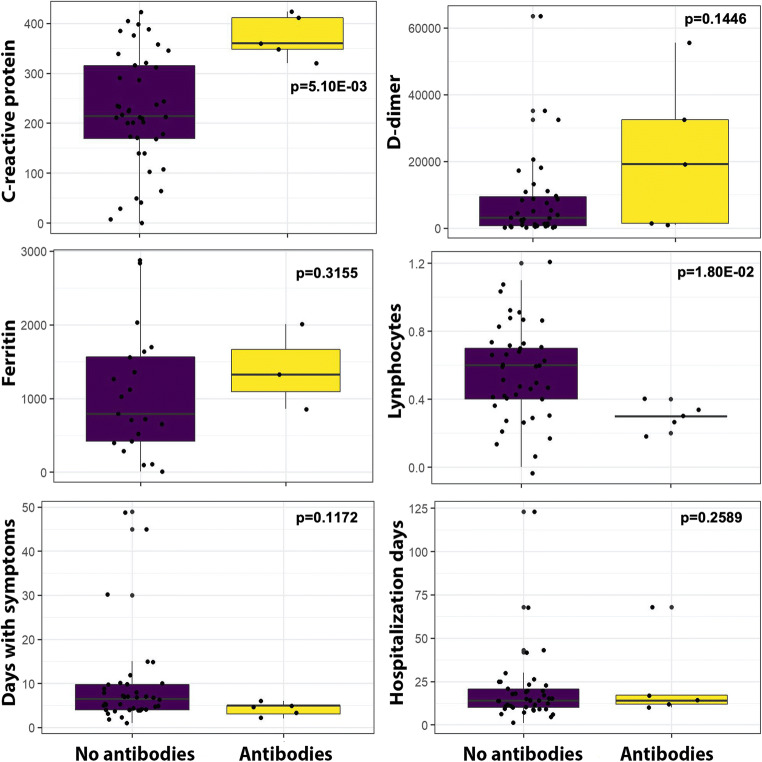
In contrast, we found a highly significant correlation between the presence of autoantibodies neutralizing type I IFNs and raised levels of C-reactive protein (C-RP), over 300 (mg/L) (p = 5.10e-03), as well as lower lymphocyte counts, well below 0.5 10E3/μL (p = 1.80e−02). Figure 2 shows the distribution of different acute phase reactants, lymphocyte counts, days of symptoms, and days of hospitalization in a graphic display.
Fig. 2.
Box chart. The distribution of continuous variables from Table 1 is presented here, with significantly different levels of C-reactive protein and lymphocyte counts
Moreover, a significant correlation was found between the O2 treatment with nasal cannula (p = 1.10e-03) instead of more aggressive measures, and absence of autoantibodies neutralizing type I IFNs. Indeed, patients with neutralizing autoantibodies needed higher oxygen requirements during hospitalization (40% vs 28.5%). Furthermore, when analyzing the treatments received by the patients, we did not find a significant association between the presence of neutralizing autoantibodies and the administration of corticosteroids (p = 0.14), bolus of corticosteroids (p = 0.61), tocilizumab against the IL-6 receptor (p = 0.32), or even response to IFN-beta-1b therapy (p = 0.34). These factors have been repeatedly related to the severity and high rates of mortality [16–18].
During hospitalization, the larger proportion of patients 31/47 (65.9%) remained at severe stage, whereas critical progression occurred in 34% of patients (16/47), of whom 18.7% (3/16) presented neutralizing autoantibodies. The median age of critical patients was 65 years old (IQR: 14.5 years), 63.5 years old (IQR: 20 years) in the patients with neutralizing autoantibodies, and 68 years old (IQR: 14.7 years) in patients without them. Male sex represented 75% (12/16) of critical patients, and in the autoantibody and non-autoantibody subgroups, males accounted for 3/4 and 9/12, respectively.
The global mortality rate in our cohort was 34% (16/47). It corresponded to 28.6% (12/42) in the non-autoantibody group and 80% (4/5) in the patients with neutralizing autoantibodies. The mortality in critical patients was 75% (12/16) globally, and 3/3 (100%) and 9/12 (75%) in the autoantibody and non-autoantibody subgroups, respectively.
Of note, we found a significant association between global mortality and the presence of neutralizing autoantibodies by Kruskal Wallis (p = 3.4e−02), Table 1. Furthermore, using the Cox regression, we found that the presence of neutralizing autoantibodies increased the hazard/ odds ratio (3.73) (p = 0.04), and we found also significance in age related to mortality (p = 0.0291), with hazard ratio 1.05, although Aalen regression was not significant (Table 2). Because of the limitations of the sample size, we applied additional statistics to analyze survival. In Fig. 3, Kaplan-Meier statistics shows a trend pointing to an effect of neutralizing autoantibodies as a factor increasing the risk of death from COVID-19, although failing to show statistical significance (p = 0.11).
Table 2.
Survival analysis: Cox and Aalen regressions. Cox (risk ratio model) and Aalen (additive model) regressions were adjusted
| Cox model | Aalen model | |||
|---|---|---|---|---|
| OR | P-val | Coef. | P-val | |
| Neutralizing antibodies | 3.73 | 0.0401 | 0.04 | 0.4837 |
| Age | 1.05 | 0.0291 | 0.001 | 0.095 |
| Sex | 0.64 | 0.4384 | −0.01 | 0.512 |
Fig. 3.
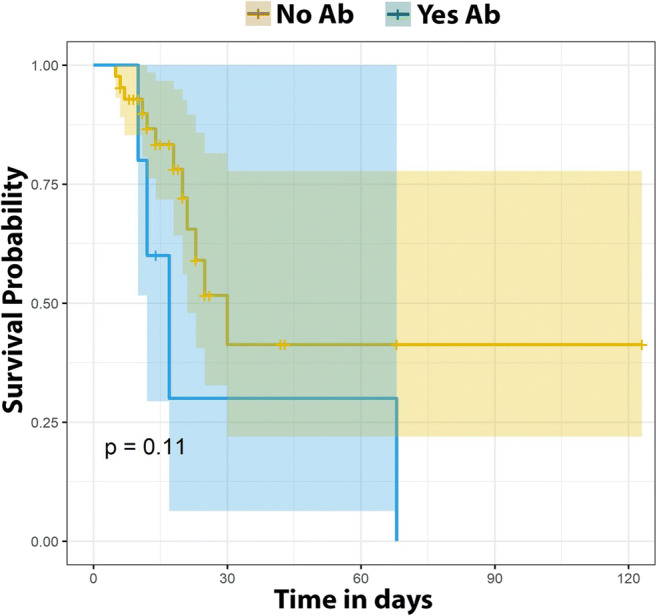
Survival analysis by Kaplan-Meier. Survival analysis of the presence/absence of anti-interferon autoantibodies (Ab). The p value of the Kaplan-Meier estimators is presented. In this figure, although not significant, a trend of a higher risk of death can be found with the presence of neutralizing autoantibodies
Discussion
The prevalence of neutralizing anti-IFN-alpha and anti-IFN-omega autoantibodies in our cohort of severe IFN-beta-treated patients was 10.6%, confirming the findings in a larger sample of a recent publication [6] from the COVID Human Genetic Effort consortium. Our data show that no demographic differences were present between the individuals who generated neutralizing anti-IFN autoantibodies (IFN-alpha, IFN-omega) and those who did not, in terms of age, sex, and ancestry, but globally, the median age was over 70 years old, and the predominant sex was male. We also did not detect differences in terms of comorbidities. The high presence of comorbidities in our cohort (93.6% of patients) may likely be explained by the advanced age of the sample, with a median age over 70 years in both groups. In published cohorts, male patients and elderly patients or patients ≥50 years are at higher risk of developing severe disease, whereas comorbidities and clinical manifestations could significantly affect the prognosis and severity of COVID-19 [19, 20].
Importantly, we found a statistically significant association between the presence of neutralizing autoantibodies against IFN-alpha and IFN-omega, with higher C-reactive protein values and lower lymphocyte counts. High C-reactive protein and ferritin concentrations and low lymphocyte counts have been repeatedly identified as risk predictors of severity and mortality [21–23].
Additional findings of this study relate to the lack of differences in the response to the various treatments used for COVID-19, including subcutaneous IFN-beta. One possible explanation may be that patients arrived to the hospital well advanced in the course of disease, after 2 weeks of symptoms, and thus, IFN-beta treatment was given late in the course of infection, restricted to patients with severe pneumonia. This aspect raises the possibility of using IFN-beta as an earlier, ambulatory therapeutic option in patients with anti-IFN autoantibodies [8]. Indeed, IFN-beta could improve symptoms, shorten the duration of viral shedding and thus improve prognosis, as recently reported [24, 25]. In this sense, IFN treatment may be a safe and affordable option to improve treatment, especially in the early ambulatory stages of infection. Furthermore, the absence of neutralizing autoantibodies against IFN-beta excludes that a putative interference of such autoantibodies would have counteracted the treatment with IFN-beta-1b.
The mortality of patients without autoantibodies was 28.5%, similar to published data on severe or critical patients during the first months of the pandemic [26]. However, in patients with neutralizing autoantibodies, mortality significantly increased to 80%, much higher than the 33.6% previously described in a larger cohort (37 deaths out of the 101 autoantibody-positive patients in the Bastard study [6]). Despite the significantly higher mortality in patients with neutralizing autoantibodies when using the Kruskal-Wallis test or Cox regression, we could not find a statistically significant correlation between the presence of autoantibodies and higher mortality when using Kaplan-Meier, or Aalen regression. Nevertheless, we detect a trend correlating the presence of neutralizing anti-IFN autoantibodies with the worst clinical prognosis, although a higher statistical power would be needed to unequivocally demonstrate this point. This study did not detect effects on survival related to sex, in contrast to the published data with male patients at higher risk of poor prognosis [19, 27].
The limitation of this pilot study is the small sample size, compounded by dealing with unbalanced groups with very dissimilar patient numbers in the groups with or without autoantibodies. However, the similarities we found should be interpreted as strong evidence that the mechanisms at play in patients with and without autoantibodies are similar. This finding could suggest that the patients without neutralizing autoantibodies who died, could have inborn errors of IFN or autoantibodies not detected by the method used. Moreover, our results suggest a trend pointing to the presence of antibodies as a risk marker for a worse clinical prognosis.
In sum, larger cohorts will be necessary to confirm the relationship of anti-IFN autoantibodies and their real impact on the prognosis and mortality of COVID-19 patients [28, 29]. Our estimates (R software, utility for estimating population samples) indicate that a sample of at least 192 patients, 96 patients with and 96 patients without autoantibodies, would be necessary to ascertain a putative correlation with increased mortality.
To our knowledge, this is the first study that describes a significant association between the presence of anti-IFN autoantibodies and analytical markers of clinical severity and poor prognoses, such as higher C-reactive protein and lower lymphocyte counts. Moreover, the survival analysis suggests a trend pointing to the presence of high titers of antibodies as a risk marker for adverse outcome. Late treatment with subcutaneous IFN-beta in hospitalized patients with severe or critical respiratory condition did not improve the clinical response or outcomes, as described previously in clinical trials [25]. These results suggest that a rapid determination of anti-IFN autoantibodies at admission would be a very useful tool in the stratification of patients, to adopt early IFN-beta and/or early intensification of their treatment to minimize the risks of adverse outcomes, as described recently in a patient with incontinentia pigmenti and autoantibodies to type I IFNs [30]. This may be even more pertinent in patients with lower lymphocyte counts well below 0.5 10E3/μL and C-RP higher than 300 (mg/L), analytes which show a strong correlation with the presence of neutralizing autoantibodies. Alternatively, ambulatory treatment of all infected subjects >65 years of age with subcutaneous IFN-beta might be considered, as this short-term treatment appears to be safe, with side-effects mostly resolving within 3 days after drug initiation, and no severe adverse events or related deaths reported [25].
Supplementary Information
Neutralizing effect on luciferase induction, after stimulation with IFN-β, in the presence of plasma from patients with life-threatening COVID-19 and autoantibodies against IFN-α2 and IFN-ω (N = 5), and one patient previously identified with autoantibodies against IFN-β. (JPG 377 kb)
Acknowledgements
We thank all the study participants, who donated blood, and all the clinicians who attended COVID-19 patients at Infanta Leonor University Hospital. We thank the members of both branches of the Laboratory of Human Genetics of Infectious Diseases: Adrian Gervais, Jérémie Rosain, Quentin Phillipot, Tom Le Voyer, Mélanie Migaud, Lazaro Lorenzo-Diaz, Yelena Nemirovskaya, Qian Zhang, Anne Puel, Emmanuelle Jouanguy, Shen-Ying Zhang, and Aurélie Cobat for their help and fruitful discussions.
Author Contribution
Authors have participated in (a) conception and design, (b) analysis and interpretation of the data; (c) drafting the article; (d) revising it critically for important intellectual content; and (e) approval of the final version. Jesús Troya: conception and design, analysis and interpretation of the data, drafting the article, revising it critically for important intellectual content, and approval of the final version. Paul Bastard: analysis and interpretation of the data and revising it critically for important intellectual content. Laura Planas-Serra: analysis and interpretation of the data. Montse Ruiz: analysis and interpretation of the data. Pablo Ryan, María de Carranza, Juan Torres, Amalia Martínez, Laurent Abel, and Jean-Laurent Casanova: revising it critically for important intellectual content. Aurora Pujol: conception and design, analysis and interpretation of the data, drafting the article, revising it critically for important intellectual content, and approval of the final version.
Funding
The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (R01AI088364), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award (CTSA) program (UL1 TR001866), a Fast Grant from Emergent Ventures, Mercatus Center at George Mason University, the Yale Center for Mendelian Genomics and the GSP Coordinating Center funded by the National Human Genome Research Institute (NHGRI) (UM1HG006504 and U24HG008956), the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the FRM and ANR GENCOVID project, ANRS-COV05, the Square Foundation, Grandir - Fonds de solidarité pour l’enfance, the SCOR Corporate Foundation for Science, Institut National de la Santé et de la Recherche Médicale (INSERM) and the University of Paris. PB was supported by the MD-PhD program of the Imagine Institute (with the support of the Fondation Bettencourt-Schueller). Work at the Neurometabolic Diseases Lab received funding from the Horizon 2020 program under grant no. 824110 (EasiGenomics grant no. COVID-19/PID12342) to A.P. We also thank the CERCA Program/Generalitat de Catalunya for institutional support to A.P.
Data Availability
Clinical data files are stored at Infanta Leonor University hospital. It may be shared if needed.
Declarations
Ethics Approval
The study was approved by the Committee for Ethical Research of the Infanta Leonor University Hospital, code 008-20, and the Bellvitge University Hospital code PR127/20.
Consent to Participate
Informed consent was obtained orally when clinically possible. In the remaining cases, the informed consent waiver was authorized by the ethics committee.
Consent for Publication
This consent was obtained with consent to participate.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jesús Troya and Aurora Pujol contributed equally to this work.
References
- 1.WHO. Coronavirus disease 2019 (COVID-19). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 22 Feb 2021.
- 2.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciancanelli MJ, Abel L, Zhang SY, Casanova JL. Host genetics of severe influenza: from mouse Mx1 to human IRF7. Curr Opin Immunol. 2016;38:109–120. doi: 10.1016/j.coi.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jouanguy E, Béziat V, Mogensen TH, Casanova JL, Tangye SG, Zhang SY. Human inborn errors of immunity to herpes viruses. Curr Opin Immunol. 2020;62:106–122. doi: 10.1016/j.coi.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku CL, Chi CY, von Bernuth H, Doffinger R. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum Genet. 2020;139(6–7):783–794. doi: 10.1007/s00439-020-02180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Bastard P, Bolze A, Jouanguy E, Zhang SY, COVID Human Genetic Effort. Cobat A, Notarangelo LD, Su HC, Abel L, Casanova JL. Life-threatening COVID-19: defective interferons unleash excessive inflammation. Med (NY) 2020;1(1):14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesev EV, LeDesma RA, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4(6):914–924. doi: 10.1038/s41564-019-0421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JF, Chan KH, Kao RY, To KK. Zheng BJ, Li CP, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Inf Secur. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY, HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez E, Fontán-Vela M, Valencia J, Fernandez-Jimenez I, Álvaro-Alonso EA, Izquierdo-García E, Lazaro Cebas A, Gallego Ruiz-Elvira E, Troya J, Tebar-Martinez AJ, Garcia-Marina B, Peña-Lillo G, Abad-Motos A, Macaya L, Ryan P, Pérez-Butragueño M, COVID@HUIL Working Group. COVID@HUIL Working Group Characteristics, complications and outcomes among 1549 patients hospitalised with COVID-19 in a secondary hospital in Madrid, Spain: a retrospective case series study. BMJ Open. 2020;10(11):e042398. doi: 10.1136/bmjopen-2020-042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical Spectrum of SARS-CoV-2 Infection. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 22 Feb 2021.
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Antorán B, Sancho-López A, Torres F, Moreno-Torres V, de Pablo-López I, García-López P, et al. Combination of tocilizumab and steroids to improve mortality in patients with severe COVID-19 infection: a Spanish, multicenter, cohort study. Infect Dis Ther. 2020;10:1–16. doi: 10.1007/s40121-020-00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smoke SM, Raja K, Hilden P, Daniel NM. Early clinical outcomes with tocilizumab for severe Covid-19: a two-center retrospective study. Int J Antimicrob Agents. 2020;57(2):106265. doi: 10.1016/j.ijantimicag.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen Jeschke K, Bonnesen B, Hansen EF, Jensen JS, Lapperre TS, Weinreich UM, et al. Guideline for the management of COVID-19 patients during hospital admission in a non-intensive care setting. Eur Clin Respir J. 2020;7(1):1761677. doi: 10.1080/20018525.2020.1761677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6(12):e05684. doi: 10.1016/j.heliyon.2020.e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu R, Hu L, Ling Y, Fang H, Zhang H, Liang S, et al. C-reactive protein concentration as a risk predictor of mortality in intensive care unit: a multicenter, prospective, observational study. BMC Anesthesiol. 2020;20(1):292. doi: 10.1186/s12871-020-01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y, Li Y, Sun J, Pan H, Yao F, Jiao X. Selection of an optimal combination panel to better triage COVID-19 hospitalized patients. J Inflamm Res. 2020;13:773–787. doi: 10.2147/JIR.S273193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao P, Wu Y, Wu S, Wu T, Zhang Q, Zhang R, Wang Z, Zhang Y. Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia. Biomarkers. 2020;26:1–18. doi: 10.1080/1354750X.2020.1861098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1604. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, Cattaneo S, Cereda D, Colombo S, Coluccello A, Crescini G, Forastieri Molinari A, Foti G, Fumagalli R, Iotti GA, Langer T, Latronico N, Lorini FL, Mojoli F, Natalini G, Pessina CM, Ranieri VM, Rech R, Scudeller L, Rosano A, Storti E, Thompson BT, Tirani M, Villani PG, Pesenti A, Cecconi M, COVID-19 Lombardy ICU Network Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mussini C, Cozzi-Lepri A, Menozzi M, Meschiari M, Franceschini E, Rogati C, et al. Better prognosis in females with severe COVID-19 pneumonia: possible role of inflammation as potential mediator. Clin Microbiol Infect. 2020:S1198-743X(20)30765–5. 10.1016/j.cmi.2020.12.010. [DOI] [PMC free article] [PubMed]
- 28.Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filardo TD, Khan MR, Krawczyk N, Galitzer H, Karmen-Tuohy S, Coffee M, Schaye VE, Eckhardt BJ, Cohen GM. Comorbidity and clinical factors associated with COVID-19 critical illness and mortality at a large public hospital in New York City in the early phase of the pandemic (March-April 2020) PLoS One. 2020;15(11):e0242760. doi: 10.1371/journal.pone.0242760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastard P, Lévy R, Henriquez S, Bodemer C, Szwebel T-A, Casanova JL, et al. Interferon-β therapy in a patient with Incontinentia Pigmenti and autoantibodies against type I IFNs infected with SARS-CoV-2. JoCI. 2021; 25:1–3. 10.1007/s10875-021-01023-5. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neutralizing effect on luciferase induction, after stimulation with IFN-β, in the presence of plasma from patients with life-threatening COVID-19 and autoantibodies against IFN-α2 and IFN-ω (N = 5), and one patient previously identified with autoantibodies against IFN-β. (JPG 377 kb)
Data Availability Statement
Clinical data files are stored at Infanta Leonor University hospital. It may be shared if needed.