Abstract
Background:
Statins may be protective in viral infection and have been proposed as treatment in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection.
Objective:
We evaluated the effect of statins on mortality in four groups hospitalized with (SARS-CoV-2) infection (continued statin, newly initiated statin, discontinued statin, never on statin).
Design:
In a single center cohort study of 1179 patients hospitalized with SARS-CoV-2 infection, the outcome of death, Intensive Care Unit (ICU) admission or hospital discharge was evaluated. Patients’ statin use, laboratory data, and co-morbidities were determined via chart review and electronic health records. Using marginal structural models to account for timing of statin initiation and competing risks, we compared the likelihood of severe outcomes in the four statin exposure groups.
Setting:
Academic medical center in the United States
Participants:
Patients hospitalized with SARS-CoV-2 infection
Measurements:
28-day mortality, ICU admission, or discharge
Results:
Among 1179 patients, 360 were never on a statin, 311 were newly initiated on a statin, 466 were continued on a statin, and 42 had a statin discontinued. In this cohort, 154 (13.1%) patients died by 28-days. With marginal structural model analysis, statin use reduced the hazard of 28-day mortality (HR 0.566 [CI 0.372, 0.862], p = 0.008). Both new initiation of statins (HR 0.493 [CI 0.253, 0.963], p=0.038) and continuing statin therapy reduced the hazard of 28-day mortality (HR 0.270 [CI 0.114, 0.637], p=0.003). Sensitivity analysis found that statin use was associated with improved mortality for patients > 65 years, but not for patients 65 years or younger.
Limitation:
Observational design
Conclusion:
Statin therapy during hospitalization for SARS-CoV-2 infection, including new initiation and continuation of therapy, was associated with reduced short-term mortality.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the associated disease (COVID-19) has resulted in millions of deaths (1). During the initial rush for treatments, over 100 off-label and experimental drugs were used to treat patients with COVID-19 (2). Off-label statin use was considered early in the pandemic at Massachusetts General Hospital (MGH) for several reasons, including: reports of cardiac complications and death due to COVID-19 (3, 4) and the cardioprotective effect of statins (5, 6); statins are low cost, widely available and generally safe (7); and statins blunt the hyperinflammatory response from infection (8–10). It was also suggested that statins block SARS-CoV-2 infectivity via binding to the main protease mediating viral entry, inhibiting the virus’ ability to invade cells (11).
The safety and efficacy of statins for the treatment of patients hospitalized with COVID-19 has remained uncertain. Prior to the COVID-19 pandemic, the impact of statins in acute respiratory distress syndrome (ARDS) was unclear, with some trials showing no impact on mortality (12, 13), while others found a significant improvement (14, 15). Observational studies have similarly yielded mixed results regarding the effects of statins in COVID-19. These investigations have been limited by small sample size (16–19), insufficient adjustments for time varying confounders (16, 20, 21), and lack of a sub-cohort newly initiated on statins for COVID-19 (20, 22–24).
During the surge in Spring 2020, a multidisciplinary group of physicians at MGH created clinical guidelines that recommended starting statin therapy on patients hospitalized for COVID-19 (25, 26). Clinicians were advised to continue pre-hospital statins and to start atorvastatin 40 mg daily on patients who had an evidence-based indication (e.g. hyperlipidemia). At times, clinicians were also advised to start atorvastatin if the patient with COVID-19 had no contraindication, though this guidance changed over the study period. This placed MGH in a unique position to evaluate empirically the impact of starting patients on statin therapy during hospitalization due to COVID-19.
We aimed to evaluate the effect of statins on 28-day mortality in patients hospitalized with COVID-19, using robust causal inference methods (i.e., marginal structural Cox proportional hazards models) to account for variable timing of statin initiation and competing risks. Notably, our approach takes into account that the decision to initiate statins was influenced both by baseline confounding factors as well as patients’ changing health condition during hospitalization. We further aimed to evaluate the specific effect of new statin initiation on survival.
Methods
Patient Selection
This study is based on an MGH cohort described previously (27). Inclusion criteria included ≥ 18 years of age and confirmed SARS-CoV-2 infection with reverse transcriptase-polymerase chain reaction testing of nasopharyngeal or sputum specimens. For this study, we included patients hospitalized between March and June 2020. This study was approved by the local Institutional Review Board (IRB # 2020P000829); a waiver of informed consent was granted.
Statin Exposure
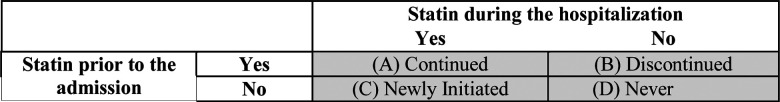
Patients were categorized into four statin groups: (A) continued home statin during hospitalization (“continued”), (B) discontinued home statin during hospitalization (“discontinued”), (C) newly initiated statin during hospitalization (“newly initiated”), and (D) did not use statins during or prior to hospitalization (“never”) [Figure 1]. Manual chart review, supplemented by extracted medication orders from electronic health records in the Mass General Brigham Enterprise Data Warehouse (EDW), was performed to assess statin use both prior to and during hospitalization.
Figure 1.
Definition of statin treatment groups
Covariates and Outcomes
Chart abstraction collected information on patient age, sex, smoking status, medical comorbidities, medications, hospitalization date, date of ICU admission, date of death or discharge, need for invasive mechanical ventilation, renal replacement therapy, and medications used at hospitalization. Laboratory values, self-reported race/ethnicity and body mass index (BMI) were extracted from the electronic health record.
Patients were followed from hospitalization until death, discharge, or 28 days from presentation to care. The primary outcome was time to death. Patients who were discharged to palliative care were classified as deceased on the day of their discharge. The secondary outcome was time to a composite outcome of ICU admission or death. For this secondary outcome, a patient must have received statins during hospitalization but prior to ICU admission to be categorized as newly initiated or continued.
Statistical Analysis
We examined the differences between baseline characteristics among the four statin treatment groups. The median and interquartile ranges are presented for continuous variables and the counts and percentage are presented for categorical variables. For statistical tests, we conducted Fisher’s exact test for categorical variables and Kruskal-Wallis test by ranks for continuous variables.
Analyses that stratify patients by in-hospital statin use are subject to both immortal time bias and time-varying confounding, as a patient’s changing health condition affected when and whether they initiated statins (28). To address these challenges, we used marginal structural Cox proportional hazards models to evaluate the effect of statin initiation on the primary and secondary outcomes (29). Patients who were discharged alive or to a non-palliative care facility were categorized together as discharged. We fit a pooled multinomial regression to account for discharge as a competing risk alongside the outcome to fit the weighted Cox model (30). If patients did not experience the outcome and were not discharged by 28 days, they were treated as administratively censored.
Using the marginal structural Cox model, we estimated the hazard ratio of each outcome for initiating statins vs. not initiating statins by the previous day. We excluded patients who experienced the outcome on the day of admission, as there was no opportunity for in-hospital statin use to affect the risk of the outcome. The model we fit assumes that once initiated, the duration of in-hospital statin use does not affect the hazard of the outcome.
To fit the marginal structural Cox model, we used inverse probability weighting with stabilized weights trimmed at the 5th and 95th percentiles. Because all patients were followed until 28 days unless they experienced the outcome or were discharged, censoring weights were not used. The following baseline variables were included in the weighting models: demographic variables (sex, age>65 years, race, active smoker, BMI≥30), comorbidities on admission (coronary artery disease, congestive heart failure, hypertension, diabetes, dyslipidemia, chronic liver disease, active cancer, pulmonary disease), ACE inhibitor medication status at presentation to care, number of days from March 1st, 2020 to the date of hospitalization to account for era effect, and prior statin usage. The following time-varying daily lab measurements were also included and log-transformed: absolute lymphocyte count, white blood cell count (WBC), aspartate aminotransferase (AST), C-reactive protein (CRP), creatine kinase (CK) and alanine aminotransferase (ALT). To account for missingness in both baseline and time-varying data, we used multiple imputation with 25 imputations. Peak lab values were calculated for each statin group, defined as the highest lab measurement observed between hospital admission and death, discharge, or end of follow-up, whichever came first. These lab values were calculated irrespective of the exact timing of statin initiation.
For the primary outcome of mortality, we adjusted for ICU admission status each day as a time-varying covariate. 95% confidence intervals and p-values were calculated, accounting for the uncertainty due to estimation of the weighting models (30), and results across imputations were combined using Rubin’s rules (31).
The analysis was repeated for the primary and secondary outcomes on the subset of patients who were not prior users (“Newly initiated” vs. “Never”) and patients who were prior users (“Continued” vs. “Discontinued”). In total, six models were fit. An additional analysis was performed using E-values to assess robustness of the observed results to unmeasured confounding. The E-value is a measure that assesses how strong an unmeasured confounder would have to be to fully explain away the observed results (32, 33).Stratified analysis was also performed to assess whether the effect of statins in patients above and below 65 years of age. All analyses were conducted in R Version 4.0.2 (34). The nnet package (35) was used to fit the multinomial regression model and the Jomo package (36) was used to perform multiple imputation of multilevel data, which included demographic characteristics as well as time-varying laboratory measures.
Role of the Funding Source
Support for this study was provided by the MGH Division of Clinical Research. Funding was used primarily to support the MGH COVID-19 registry development and statistical analysis.
Results
Baseline Characteristics
Overall, 1179 adult patients with confirmed SARS-CoV-2 infection were included, after the exclusion of 7 patients who were deceased, discharged, or censored on the day of their hospital admission (Appendix Table 1). 676 (57.3%) were male, 443 (37.6%) were at least 65 years old, and 493 (45.9%) had a BMI ≥30. Patient characteristics differed by statin group (Table 1). Patients on statins prior to hospitalization (groups A and B) were older (median age 69 vs. 52 years, p < 0.001), and had higher rates of coronary artery disease (28.7% vs 3.1%, p < 0.001), congestive heart failure (18.9% vs. 5.1%, p < 0.001), hypertension (74.4% vs. 34.3%, p < 0.001), type 2 diabetes (55.9% vs. 17.1%, p < 0.001), and dyslipidemia (66.7% vs. 15.6%, p < 0.001) than those not on statins prior to hospitalization (groups C and D). White/non-Hispanics were more likely to be on statins prior to hospitalization than Hispanics (56.4% of White/non-Hispanics on statins vs. 32.9% of Hispanics on statins, p < 0.001).
Table 1: Patient Characteristics at Hospitalization for COVID-19.
Stratified by (A) continued statin during hospitalization (Continued), (B) discontinued statin during hospitalization (Discontinued), (C) newly initiated statin during hospitalization (Newly Initiated), and (D) no statin prior to admission nor during hospitalization (Never).
| Total 1,179 subjects | (A) Continued (n=466) | (B) Discontinued (n=42) | (C) Newly Initiated (n=311) | (D) Never (n=360) | p-value* |
|---|---|---|---|---|---|
| Demographics | |||||
| Male sex, n/N (%) | 298/466 (63.9%) | 26/42 (61.9%) | 173/311 (55.6%) | 179/360 (49.7%) | 0.001 |
| Age, median (IQR) | 68 (59–78) [n=466] | 70 (58–78) [n=42] | 55 (43–66) [n=311] | 48 (35–62) [n=360] | <0.001 |
| Age > 65, n/N (%) | 265/466 (56.9%) | 24/42 (57.1%) | 83/311 (26.7%) | 71/360 (19.7%) | <0.001 |
| Race/Ethnicity, n/N (%) | <0.001 | ||||
| 1. White | 233/457 (51%) | 23/42 (54.8%) | 84/301 (27.9%) | 114/355 (32.1%) | |
| 2. Black or African American | 40/457 (8.8%) | 7/42 (16.7%) | 43/301 (14.3%) | 37/355 (10.4%) | |
| 3. Hispanic | 131/457 (28.7%) | 10/42 (23.8%) | 129/301 (42.9%) | 159/355 (44.8%) | |
| 4. Other (American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander, others) | 53/457 (11.6%) | 2/42 (4.8%) | 45/301 (15%) | 45/355 (12.7%) | |
| Active smoker, n/N (%) | 24/442 (5.4%) | 7/37 (18.9%) | 15/275 (5.5%) | 39/330 (11.8%) | <0.001 |
| BMI, median (IQR) | 29 (26–34) [n=430] | 29 (25–32) [n=38] | 30 (26–34) [n=290] | 29 (25–34) [n=316] | 0.735 |
| BMI >= 30, n/N (%) | 192/430 (44.7%) | 14/38 (36.8%) | 141/290 (48.6%) | 146/316 (46.2%) | 0.500 |
| Comorbidities on Admission, n/N (%) | |||||
| Coronary Artery Disease | 140/466 (30%) | 6/42 (14.3%) | 9/311 (2.9%) | 12/360 (3.3%) | <0.001 |
| Congestive Heart Failure | 90/466 (19.3%) | 6/42 (14.3%) | 15/311 (4.8%) | 19/360 (5.3%) | <0.001 |
| Hypertension | 349/466 (74.9%) | 29/42 (69%) | 130/311 (41.8%) | 100/360 (27.8%) | <0.001 |
| Diabetes | 261/466 (56%) | 24/42 (57.1%) | 76/311 (24.4%) | 42/360 (11.7%) | <0.001 |
| Dyslipidemia | 320/466 (68.7%) | 19/42 (45.2%) | 59/311 (19%) | 46/360 (12.8%) | <0.001 |
| Chronic kidney disease | 121/458 (26.4%) | 9/41 (22%) | 31/299 (10.4%) | 32/356 (9%) | <0.001 |
| Dialysis | 18/458 (3.9%) | 1/41 (2.4%) | 7/299 (2.3%) | 6/356 (1.7%) | 0.238 |
| Chronic liver Disease** | 37/458 (8.1%) | 5/41 (12.2%) | 30/299 (10%) | 36/353 (10.2%) | 0.567 |
| Alcohol-related Cirrhosis | 4/458 (0.9%) | 2/41 (4.9%) | 5/299 (1.7%) | 4/353 (1.1%) | 0.161 |
| NAFLD | 22/458 (4.8%) | 0/41 (0%) | 16/299 (5.4%) | 14/353 (4%) | 0.499 |
| Current Viral Hepatitis | 18/466 (3.9%) | 1/42 (2.4%) | 11/311 (3.5%) | 25/360 (6.9%) | 0.125 |
| HIV | 6/413 (1.5%) | 0/40 (0%) | 4/283 (1.4%) | 6/329 (1.8%) | 0.947 |
| History of Cancer | 88/459 (19.2%) | 10/42 (23.8%) | 43/298 (14.4%) | 32/354 (9%) | <0.001 |
| Pulmonary Disease | 167/461 (36.2%) | 14/41 (34.1%) | 76/301 (25.2%) | 91/357 (25.5%) | 0.001 |
| COPD | 71/461 (15.4%) | 6/41 (14.6%) | 20/301 (6.6%) | 23/357 (6.4%) | <0.001 |
| Asthma | 60/461 (13%) | 7/41 (17.1%) | 37/301 (12.3%) | 55/357 (15.4%) | 0.548 |
| ILD | 3/461 (0.7%) | 0/41 (0%) | 3/301 (1%) | 3/357 (0.8%) | 0.938 |
| Home oxygen supplementation | 14/461 (3%) | 1/41 (2.4%) | 3/301 (1%) | 3/357 (0.8%) | 0.065 |
| Obstructive Sleep Apnea (OSA) | 46/466 (9.9%) | 4/42 (9.5%) | 16/311 (5.1%) | 12/360 (3.3%) | 0.001 |
| History of organ transplant | 13/462 (2.8%) | 2/41 (4.9%) | 6/301 (2%) | 7/356 (2%) | 0.480 |
| Treatment with immunosuppressing agent in the past 6 months | 36/448 (8%) | 4/38 (10.5%) | 16/295 (5.4%) | 22/353 (6.2%) | 0.366 |
| Medications at Presentation to Care, n/N (%) | |||||
| Type and dose of statin (at PTC) | 0.358 | ||||
| Atorvastatin | 309/458 (67.5%) | 28/41 (68.3%) | |||
| Atorvastatin 10mg-20mg | 95/309 (30.7%) | 7/28 (25%) | |||
| Atorvastatin 40mg-60mg | 116/309 (37.5%) | 12/28 (42.9%) | |||
| Atorvastatin 80mg | 85/309 (27.5%) | 7/28 (25%) | |||
| Unknown Dosage | 13/309 (4.2%) | 2/28 (7.1%) | |||
| Rosuvastatin | 39/458 (8.5%) | 1/41 (2.4%) | |||
| Other Statins | 110/458 (24%) | 12/41 (29.3%) | |||
| Type and dose of statin (during hospitalization) | |||||
| Atorvastatin | 331/434 (76.3%) | 274/285 (96.1%) | <0.001 | ||
| Atorvastatin 10mg-20mg | 76/331 (23%) | 53/274 (19.3%) | |||
| Atorvastatin 40mg-60mg | 138/331 (41.7%) | 147/274 (53.6%) | |||
| Atorvastatin 80mg | 117/331 (35.3%) | 74/274 (27%) | |||
| Unknown Dosage | 0/331 (0%) | 0/274 (0%) | |||
| Rosuvastatin | 33/434 (7.6%) | 6/285 (2.1%) | |||
| Other Statins | 70/434 (16.1%) | 5/285 (1.8%) | |||
| Duration of pre-admission statin use at PTC | |||||
| <= 1 year | 54/466 (11.6%) | 4/42 (9.5%) | |||
| >1 year | 244/466 (52.4%) | 23/42 (54.8%) | 0.973 | ||
| Unknown | 168/466 (36.1%) | 15/42 (35.7%) | |||
| ACE Inhibitor | 116/466 (24.9%) | 4/42 (9.5%) | 30/311 (9.6%) | 33/360 (9.2%) | <0.001 |
| Azithromycin | 17/457 (3.7%) | 2/42 (4.8%) | 18/302 (6%) | 22/353 (6.2%) | 0.326 |
| Immunosuppressants | 42/466 (9%) | 3/42 (7.1%) | 20/311 (6.4%) | 22/360 (6.1%) | 0.396 |
| Oral Steroid | 29/466 (6.2%) | 3/42 (7.1%) | 13/311 (4.2%) | 17/360 (4.7%) | 0.512 |
| Immunomodulators | 13/466 (2.8%) | 2/42 (4.8%) | 10/311 (3.2%) | 8/360 (2.2%) | 0.598 |
| Hydroxychloroquine | 4/466 (0.9%) | 0/42 (0%) | 3/311 (1%) | 3/360 (0.8%) | 1.000 |
| Presentation to Care, n/N (%) | |||||
| Symptomatic (yes/no) | 439/462 (95%) | 36/40 (90%) | 305/309 (98.7%) | 333/357 (93.3%) | 0.001 |
| First Available Labs, median (IQR)*** | |||||
| White blood cell count, /μL | 6.5 (4.9–8.3) [n=466] | 7.1 (5.2–10.5) [n=40] | 6.8 (5.2–9.2) [n=311] | 6.7 (4.9–9.2) [n=349] | 0.152 |
| Aspartate aminotransferase, U/L | 40 (28–56) [n=463] | 58 (30–121) [n=38] | 44 (31–65) [n=311] | 48 (30–72) [n=338] | <0.001 |
| Alanine aminotransferase, U/L | 27 (18–42) [n=463] | 34 (23–62) [n=38] | 34 (21–58) [n=311] | 34 (21–66) [n=338] | <0.001 |
| Total bilirubin, mg/dL | 0.5 (0.4–0.7) [n=463] | 0.5 (0.4–0.8) [n=38] | 0.5 (0.4–0.7) [n=311] | 0.5 (0.3–0.6) [n=339] | 0.125 |
| Alkaline phosphatase, U/L | 86 (67–108) [n=463] | 91 (69–127) [n=38] | 75 (59–95) [n=311] | 80 (62–108) [n=338] | <0.001 |
| Troponin, ng/L | 17 (8–39) [n=451] | 39 (14–90) [n=38] | 8 (6–17) [n=305] | 7 (6–18) [n=322] | <0.001 |
| C-reactive protein, mg/L | 74 (33–142) [n=459] | 85 (48–152) [n=36] | 89 (49–152) [n=311] | 68 (31–136) [n=330] | 0.001 |
| Erythrocyte sedimentation rate, mm/h | 41 (27–63) [n=426] | 49 (35–84) [n=32] | 42 (28–65) [n=297] | 35 (21–54) [n=295] | <0.001 |
| Creatine kinase, U/L | 114 (63–217) [n=461] | 298 (82–702) [n=36] | 114 (67–223) [n=310] | 132 (65–349) [n=331] | 0.003 |
| D-dimer, ng/mL | 1063 (695–1812) [n=455] | 1554 (826–6580) [n=37] | 1074 (693–1770) [n=305] | 949 (608–1661) [n=329] | 0.002 |
| Absolute lymphocyte count, K/μL | 0.9 (0.6–1.3) [n=466] | 1 (0.6–1.5) [n=40] | 1 (0.7–1.3) [n=311] | 1 (0.7–1.4) [n=341] | 0.132 |
| Calendar Days Since 03/01/2020, median (IQR) | |||||
| Number of days from 03/01/2020 to hospital admission date | 43 (33–52) [n=466] | 50 (41–56) [n=42] | 37 (30–44) [n=311] | 46 (40–54) [n=360] | <0.001 |
Non-alcoholic Fatty Liver Disease (NAFLD), HIV (Human Immunodeficiency Virus), ILD (interstitial lung disease), COPD (chronic obstructive pulmonary disease)
p-values were calculated comparing all 4 groups using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables.
Unknown and other chronic liver disease not presented in the table
Note that this is first available lab result following admission
In total, 777 patients (65.9%) received a statin during their hospitalization for COVID-19. Atorvastatin was the most common statin prescribed before and during hospitalization. 274/285 (96.1%) patients newly initiated on statins and 331/434 (76.3%) patients continued on statins were prescribed atorvastatin. The most common dosage was moderate intensity (atorvastatin 40mg daily). Among those continued on statins, the majority were prescribed statins for more than a year prior to hospitalization (244/466, 52.4%). Appendix Table 2 contains information on when statins were initiated during hospitalization relative to admission date.
According to first laboratory measurements obtained during hospitalization, the group of patients who discontinued statins at hospitalization had higher erythrocyte sedimentation rate (p = 0.046), CK (p = 0.001), troponin (p < 0.001), and D-dimer (p = 0.002) compared to the other cohorts (Table 1).
Unadjusted Analysis
In the complete cohort, 154 (13.1%) patients died and 841 (71.3%) were discharged within 28 days of follow up. In unadjusted analyses, patients on statins during hospitalization (groups A and C) had similar rates of death (108 (13.9%) vs. 46 (11.4%), p = 0.273), but higher rates of ongoing hospitalization at 28 days (144 (18.5%) vs. 40 (10.0%), p < 0.001) and ICU admission (276 (35.5%) vs. 85 (21.1%), p < 0.001) than those not on statins during hospitalization (groups B and D) (Table 2).
Table 2:
Unadjusted Patient Outcomes at 28 Days
| Total 1,179 subjects | (A) Continued (n=466) | (B) Discontinued (n=42) | (C) Newly Initiated (n=311) | (D) Never (n=360) | p-value** |
|---|---|---|---|---|---|
| 28-day Outcomes from Presentation to Care | |||||
| Patient status at 28 days, n (%) | |||||
| Deceased* | 78/466 (16.7%) | 14/42 (33.3%) | 30/311 (9.6%) | 32/360 (8.9%) | <0.001 |
| Discharged alive | 252/466 (54.1%) | 20/42 (47.6%) | 176/311 (56.6%) | 257/360 (71.4%) | |
| Transfer to other facility (non-palliative care) | 61/466 (13.1%) | 4/42 (9.5%) | 36/311 (11.6%) | 35/360 (9.7%) | |
| Still hospitalized at 28 days after presentation to care | 75/466 (16.1%) | 4/42 (9.5%) | 69/311 (22.2%) | 36/360 (10.0%) | |
| Days from hospital admission to death, median (IQR) | 10 (6–15) [n=78] | 3 (1–4) [n=14] | 12 (7–16) [n=30] | 6 (3–13) [n=32] | <0.001 |
| Days from hospital admission to discharge or transfer to non-palliative facility, median (IQR) | 7 (4–10) [n=313] | 4 (1–6) [n=24] | 7 (4–10) [n=212] | 5 (3–8) [n=292] | <0.001 |
| Patient events during 28 days of follow-up, n (%) | |||||
| ICU admission | 145/466 (31.1%) | 8/42 (19.0%) | 131/311 (42.1%) | 77/360 (21.4%) | <0.001 |
| Invasive intubation | 122/466 (26.2%) | 6/42 (14.3%) | 110/311 (35.4%) | 65/360 (18.1%) | <0.001 |
| Bacterial Pneumonia | 132/466 (28.3%) | 9/42 (21.4%) | 106/311 (34.1%) | 71/360 (19.7%) | <0.001 |
| Acute Respiratory Distress Syndrome | 112/466 (24.0%) | 5/42 (11.9%) | 105/311 (33.8%) | 61/360 (16.9%) | <0.001 |
| Stroke / Cerebrovascular accident | 3/466 (0.6%) | 1/42 (2.4%) | 3/311 (1.0%) | 2/360 (0.6%) | 0.390 |
| Cardiac arrest | 2/466 (0.4%) | 1/42 (2.4%) | 2/311 (0.6%) | 2/360 (0.6%) | 0.390 |
| Rhabdomyolysis/myositis | 18/466 (3.9%) | 2/42 (4.8%) | 18/311 (5.8%) | 21/360 (5.8%) | 0.499 |
| Liver dysfunction | 80/466 (17.2%) | 11/42 (26.2%) | 71/311 (22.8%) | 64/360 (17.8%) | 0.123 |
IQR (Interquartile range)
Deceased includes 3 patients who were discharged to hospice care
p-values were calculated comparing all 4 groups using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables.
Peak Laboratory Values
Unadjusted peak liver biochemistries and inflammatory markers differed across statin groups (Table 3), with the highest peaks of AST and ALT in the newly initiated statin group. Patients newly initiated on statins had a lower peak CK than those who discontinued statins (median and interquartile range: 222 [90–636] U/L vs. 374 [89–838] U/L), but higher peak CK than those who continued (178 [85–507] U/L) or were never on statins (173 [78–578] U/L).
Table 3:
Unadjusted Peak Laboratory Values by Statin Group.
| Total 1,179 subjects | (A) Continued (n=466) | (B) Discontinued (n=42) | (C) Newly Initiated (n=311) | (D) Never (n=360) | p-value* |
|---|---|---|---|---|---|
| Peak Labs, median (IQR) | |||||
| White blood cell count, /μL | 8.7 (6.6–12.5) [n=466] | 7.9 (6.3–16) [n=40] | 9.9 (7.1–15.2) [n=311] | 8.3 (6.2–12.1) [n=349] | 0.001 |
| Aspartate aminotransferase, U/L | 65 (40–119) [n=463] | 60 (32–208) [n=38] | 84 (45–150) [n=311] | 66 (35–127) [n=338] | 0.008 |
| Alanine aminotransferase, U/L | 46 (26–85) [n=463] | 47 (23–121) [n=38] | 68 (31–120) [n=311] | 57 (26–118) [n=338] | <0.001 |
| Total bilirubin, mg/dL | 0.7 (0.5–1.1) [n=463] | 0.5 (0.4–0.8) [n=38] | 0.6 (0.5–1) [n=311] | 0.6 (0.4–0.9) [n=339] | 0.011 |
| Alkaline phosphatase, U/L | 105 (79–166) [n=463] | 101 (77–155) [n=38] | 97 (72–156) [n=311] | 96 (72–140) [n=338] | 0.017 |
| Troponin, ng/L | 23 (10–52) [n=451] | 40 (14–90) [n=38] | 12 (6–29) [n=305] | 8 (6–23) [n=322] | <0.001 |
| C-reactive protein, mg/L | 145 (69–252) [n=459] | 141 (50–231) [n=36] | 151 (82–283) [n=311] | 117 (52–175) [n=330] | <0.001 |
| Erythrocyte sedimentation rate, mm/h | 57 (36–104) [n=426] | 68 (36–90) [n=32] | 72 (41–114) [n=297] | 44 (26–74) [n=295] | <0.001 |
| Creatine kinase, U/L | 178 (85–507) [n=461] | 374 (89–838) [n=36] | 222 (90–636) [n=310] | 173 (78–578) [n=331] | 0.028 |
| D-dimer, ng/mL | 1863 (986–3596) [n=455] | 1944 (1075–7380) [n=37] | 2090 (1038–5098) [n=305] | 1241 (713–3270) [n=329] | <0.001 |
| Absolute lymphocyte count, K/μL | 1.6 (1.1–2.2) [n=466] | 1.5 (1–2.5) [n=40] | 1.9 (1.4–2.4) [n=311] | 1.7 (1.3–2.3) [n=341] | <0.001 |
p-values were calculated comparing all 4 groups using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables.
Primary Outcome Analysis
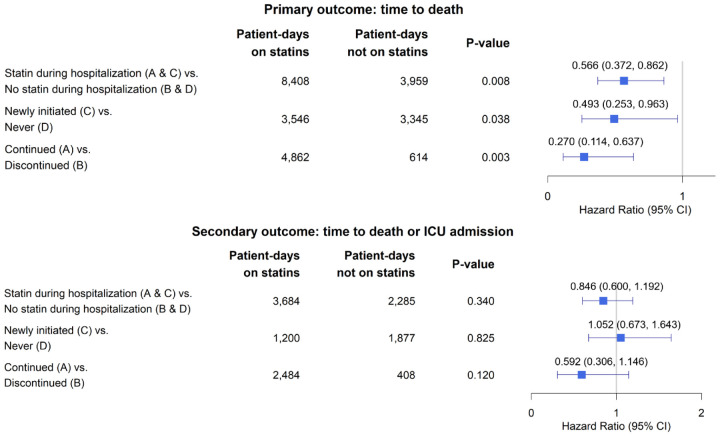
The median time to death in each statin group can be found in Table 1. Overall, statin usage during hospitalization (groups A and C) decreased the hazard of death (HR 0.566 [95% CI 0.372, 0.862], p=0.008). In the sub-group of patients not on statin therapy prior to hospitalization (groups C and D), new statin initiation at hospitalization (group C) decreased the hazard of death (HR 0.493 [95% CI 0.253, 0.963], p=0.038). The sub-group of patients on statin therapy prior to hospitalization (groups A and B), continued statin usage (group A) also decreased the hazard of death (HR 0.270 [95% CI 0.114,0.637], p=0.003) (Figure 2). A summary of the distribution of missing laboratory measurements can be found in Appendix Table 3.
Figure 2:
Marginal Structural Model Outputs for Primary and Secondary Outcomes.
Estimates were obtained from fitting marginal structural Cox models adjusted for the following baseline covariates: sex, age>65 years, race, active smoker, BMI≥30, comorbidities on admission (coronary artery disease, congestive heart failure, hypertension, diabetes, dyslipidemia, chronic liver disease, active cancer, pulmonary disease), ACE inhibitor use, number of days since March 1st, 2020, and prior statin usage. The following time-varying covariates were adjusted for as well: ALC, WBC, AST, CRP, CK, ALT, and ICU admission status. Models were fit accounting for immortal time bias, time-varying confounding, and discharge as a competing risk.
Secondary Outcome Analysis
To fit the marginal structural model for the secondary outcome of time to death or ICU admission, 198 patients were excluded because they were admitted to the ICU on their day of hospitalization and therefore did not contribute time at risk for the secondary outcome (Appendix Table 1). Of the 981 patients remaining, 588 (59.9%) had recorded statin use during their hospitalization. Statin use during hospitalization (groups A and C) did not change the hazard of the composite outcome of death or ICU admission (HR 0.846 [95% CI 0.600,1.192], p=0.340) (Figure 2 and Appendix Table 4).
Analysis of Unmeasured Confounding
For the primary outcome assessing mortality, the point estimate and the upper confidence limit of the E-value associated with statin use during hospitalization is 2.326 and 1.455, respectively. Given that the E-value of 2.326 is much greater than any observed known risk factors examined in the current study (with the exception of age), it is unlikely that an unmeasured confounder exists that would explain away the observed effect in the present analysis. The point estimate and the upper confidence limit of the E-value associated with new initiation of statins is 2.639 and 1.191, respectively. The point estimate and the upper confidence limit of the E-value associated with continuation of statins is 4.305 and 2.073, respectively.
Sensitivity Analyses for Age
We fit a marginal structural model for the primary outcome of time to death in both a subgroup of patients 65 years of age or younger (746 patients) and patients older than 65 years (443 patients). Statin use during hospitalization did not change the hazard of death in patients ≤ 65 years (HR 1.175 [95% CI 0.520, 2.655], p=0.699) (Appendix Table 5); however, patients > 65 years were found to have a decreased hazard of death with any statin use during hospitalization (HR 0.477 [95% CI 0.292, 0.78], p=0.003) and within the subgroup of patients newly initiated on statins during hospitalization (HR 0.321 [ 95% CI 0.137, 0.752], p= 0.009) (Appendix Table 6).
Discussion
In this large cohort from a single tertiary medical center, we found that statin use during hospitalization for COVID-19 was associated with improved short-term mortality. The survival benefit was seen in those who continued statin therapy, as well as those who newly initiated therapy while hospitalized. A sensitivity analysis found that statin use was associated with improved mortality for patients older than 65 years, but not for patients 65 years old or younger.
Our study confirms and expands on prior work. A recent propensity score-matched analysis found that statin use prior to hospitalization reduces the risk of short-term in-hospital mortality from COVID-19 (23). Our study probed further, into whether in-hospital statin use had a similar effect on mortality. To investigate the impact of statins administered during hospitalization, we used marginal structural models, which account for both survivorship bias (i.e., patients need to survive long enough to begin statins) and time-varying confounding bias (i.e., patient health status during hospitalization changes over time, affecting the propensity of initiating treatment). Our study accounted for a wide variety of time-varying confounders, which we believe accurately captures shifts in the propensity of initiating treatment. We further expanded on prior work by specifically evaluating the effect of statin initiation during hospitalization without prior use, because 26% of our cohort was newly initiated on statins during COVID-19 hospitalization. Overall, the novel findings presented here are that statin therapy during hospitalization, whether it be a new or continued prescription, was associated with improved mortality.
The composition of our cohort was similar to other published patient databases of patients with COVID-19 (17, 22, 24). The median age of patients was 60 years old (IQR 47–73 years). Obese patients with a BMI ≥30 represented almost half the population, consistent with evidence that obesity is a risk factor for hospitalization with COVID-19 (37). Racial and ethnic demographics vary immensely across the literature. In this study, White/Non-Hispanic (38.5%) and Hispanic patients (36.4%) were most common, followed by Black/Non-Hispanic patients (10.8%). This cohort’s mortality rate (13.1%) was similar to the mean published United States hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic (11.8%) among 955 hospitals (38).
Our findings contradict some prior reports. One meta-analysis of 3,449 patients in 9 observational studies found that statin use did not improve severe outcomes or mortality in COVID-19 (39). The majority of these studies were small in size (as few as 50 patients) and most failed to control for potential confounders. Comorbidities such as diabetes, cardiovascular disease and obesity are established risk factors for more severe COVID-19 disease, and patients prescribed statins are more likely to have these comorbidities, highlighting the critical need to control for confounding in this analysis (18). Additionally, most prior work either examined the relationship with antecedent statin usage prior to hospitalization (19, 21–24) or exclusively during hospitalization (16, 18, 20). Our study had several advantages over prior reports: our study investigated the influence of statin use both prior to and during hospitalization, minimized immortal time bias, adjusted for time-varying confounders, and minimized era effect (or the potential changes in COVID-19 care over time). These advantages provide clarity not offered by prior work.
The duration of statin therapy required to provide mortality benefit in COVID-19 remains unclear. This study suggests that statins are associated with a survival benefit even after brief exposure during hospitalization. Interestingly, in a large trial measuring mortality in US Veterans over the age of 75, statins did not affect mortality from cardiovascular events until at least 2 years of daily usage (40). However, a cardiology trial found that administration of atorvastatin within 24 to 96 hours of hospitalization for myocardial ischemia, compared with placebo, reduced the incidence of cardiovascular events including death, further myocardial infarction, and cardiac arrest (41). The main therapeutic mechanism of action of statins may be endothelial stabilization as well as its effects on inflammation (41). Given the association between COVID-19 infection and a hyperinflammatory response that provokes cardiovascular disorders (6), it is possible that new initiation of statins even for a short duration could have provided a positive impact on both mortality and secondary complications.
Another proposed explanation for statins’ mechanism of action in COVID-19 is the drug’s ability to inhibit hydroxymethylglutaryl-coenzyme A reductase, which may interfere with the virus’ invasion into cells by compromising the lipid-rich membrane required for SARS-CoV-2 to interact with the cellular receptor angiotensin-converting enzyme 2 (42, 43). If statins improve COVID-19 outcomes by inhibiting viral cell invasion, the benefit may be enhanced when statins are prescribed before infection.
In this study, we found an association with mortality benefit with statin use in adults over 65 years old but not in patients 65 years or younger. Given these findings were generated from a sensitivity analysis, we hesitate to provide age cut-offs for statin initiation for the treatment of COVID-19, but do not believe that age should be a contraindication to statin use during COVID-19 hospitalization.
Liver biochemistries are frequently elevated in severe COVID-19 disease and are associated with worse clinical outcomes (44). Separately, statins are known to increase liver biochemistries in certain patients (42). In our study, newly initiated statin use was associated with higher levels of peak AST and ALT throughout hospitalization; however, only 16% of patients on statins developed an ALT level greater than 5 times the upper limit of normal, which is a similar rate compared other cohorts with severe COVID (45). These findings are limited in that peak levels could have occurred before or after statin initiation and were unadjusted for confounding. Despite these limitations, there is no clear evidence that statin exposure during infection is associated with clinically important hepatotoxicity.
Myotoxicity is a known, albeit rare, complication of statins. In the context of COVID-19, where creatinine kinase elevations are prevalent, there is concern that statins could increase myotoxicity and subsequent creatinine kinase-induced nephrotoxicity. This was not seen among individuals newly initiated on statins or continued on statin therapy, with creatinine kinase peak levels never reaching three times the upper limit of normal, clinically considered the threshold for acute kidney injury due to pigment associated nephropathy (46).
Our findings must be interpreted in the context of study design. Although we utilized two data sources to confirm demographics, medications, laboratory data and clinical outcomes, misclassification errors are possible. We limited this error rate by employing physician review of discrepant data. Additionally, we were unable to account for patients who had dose escalations in their statin usage or who had their statin temporarily held during hospitalization. It is important to note that peak liver biochemistry levels (AST and ALT) as well as peak CK level were compared with unadjusted analyses and timing of statin initiation was not taken into account. Finally, patients who were on statin therapy prior to hospitalization but had the medication discontinued during hospitalization, presumably due to organ dysfunction, are a unique sub-cohort of patients that warrant further exploration. We utilized matched weighting within our marginal structural model to adjust for potential bias introduced by the extreme illness of this group.
After adjusting for cofounding factors, our study found that inpatient statin therapy was associated with improved 28-day mortality in COVID-19. This benefit was found in those who continued and newly initiated statins. Given the safety and availability of statins worldwide, a randomized controlled trial of statins in COVID-19 should be considered.
Supplementary Material
Disclosures:
ZM has no disclosures to report. JL receives funding through the Training Program in Environmental Health Statistics at the Harvard School of Public Health funded through the National Institute of Environmental Health Sciences (T32 ES007142). AF receives funding through the National Institute of Health/National Institute of General Medical Sciences (R01 GM127862).RC received research grants from Abbvie, Gilead, Merck, Boehringer, BMS, Janssen, Roche, GSK, Synlogic, Kaleido and receives funding through the MGH Research Scholars program. TT has no disclosures to report. PB consults for Synlogic Inc and is supported by the AASLD Transplant Hepatology Award and the ACG Junior Faculty Development Award (though neither supported this work).
Abbreviations:
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- COVID-19
Coronavirus disease 2019
- MGH
Massachusetts General Hospital
- ACE-2
Angiotensin-converting enzyme-2
- AST
Aspartate Aminotransferase test
- ALT
Alanine Aminotransferase test
- ICU
Intensive Care Unit
- HR
Hazard Ratio
- CI
Confidence Interval
- ARDS
acute respiratory distress syndrome
- EDW
Enterprise Data Warehouse
- BMI
Body Mass Index
- WBC
white blood cell count
- CRP
C-reactive protein
- HIV
Human Immunodeficiency Virus
- COPD
Chronic Obstructive Pulmonary Disease
- ILD
Interstitial Lung Disease
- IQR
Interquartile range
- NAFLD
Non-alcoholic Fatty Liver Disease
- ALC
Absolute lymphocyte count
- CK
Creatine Kinase
References:
- 1.United States COVID-19 Cases and Deaths by State [Internet]. U.S. Department of Health & Human Services. 2020. Available from: https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days.
- 2.Fajgenbaum DC, Khor JS, Gorzewski A, Tamakloe MA, Powers V, Kakkis JJ, et al. Treatments Administered to the First 9152 Reported Cases of COVID-19: A Systematic Review. Infect Dis Ther. 2020;9(3):435–49. 10.1007/s40121-020-00303-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and Cardiovascular Disease. Circulation. 2020;141(20):1648–55. 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 4.Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation. 2020;141(23):1930–6. 10.1161/CIRCULATIONAHA.120.047164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–10. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized with COVID-19 Infection. J Am Coll Cardiol. 2020. 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salami JA, Warraich H, Valero-Elizondo J, Spatz ES, Desai NR, Rana JS, et al. National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013: Insights From the Medical Expenditure Panel Survey. JAMA Cardiol. 2017;2(1):56–65. doi: 10.1001/jamacardio.2016.4700 [DOI] [PubMed] [Google Scholar]
- 8.Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25(6):1231–6. Epub 2005/03/24. doi: 10.1161/01.ATV.0000163840.63685.0c [DOI] [PubMed] [Google Scholar]
- 9.Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and antiinflammatory properties of statins. Trends Cardiovasc Med. 2005;15(6):202–6. doi: 10.1016/j.tcm.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Sapey E, Patel JM, Greenwood H, Walton GM, Grudzinska F, Parekh D, et al. Simvastatin Improves Neutrophil Function and Clinical Outcomes in Pneumonia. A Pilot Randomized Controlled Clinical Trial. Am J Respir Crit Care Med. 2019;200(10):1282–93. doi: 10.1164/rccm.201812-2328OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiner Ž, Hatamipour M, Banach M, Pirro M, Al-Rasadi K, Jamialahmadi T, et al. Statins and the COVID-19 main protease:. Arch Med Sci. 2020;16(3):490–6. 10.5114/aoms.2020.94655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–200. 10.1056/NEJMoa1401520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371(18):1695–703. 10.1056/NEJMoa1403285 [DOI] [PubMed] [Google Scholar]
- 14.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131(4):1006–12. doi: 10.1378/chest.06-1997 [DOI] [PubMed] [Google Scholar]
- 15.Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205(1):13–9. 10.1093/infdis/jir695 [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, Chung CW, Trelles-Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24(1):429. 10.1186/s13054-020-03154-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song SL, Hays SB, Panton CE, Mylona EK, Kalligeros M, Shehadeh F, et al. Statin Use Is Associated with Decreased Risk of Invasive Mechanical Ventilation in COVID-19 Patients: A Preliminary Study. Pathogens. 2020;9(9). 10.3390/pathogens9090759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan WYT, Young BE, Lye DC, Chew DEK, Dalan R. Statin use is associated with lower disease severity in COVID-19 infection. Sci Rep. 2020;10(1):17458. 10.1038/s41598-020-74492-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, et al. The Effects of ARBs, ACEis, and Statins on Clinical Outcomes of COVID-19 Infection Among Nursing Home Residents. J Am Med Dir Assoc. 2020;21(7):909–14.e2. 10.1016/j.jamda.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, et al. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020. 10.1016/j.cmet.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan H, Valdes AM, Vijay A, Wang S, Liang L, Yang S, et al. Role of Drugs Used for Chronic Disease Management on Susceptibility and Severity of COVID-19: A Large Case-Control Study. Clin Pharmacol Ther. 2020;108(6):1185–94. 10.1002/cpt.2047 [DOI] [PubMed] [Google Scholar]
- 22.Daniels LB, Sitapati AM, Zhang J, Zou J, Bui QM, Ren J, et al. Relation of Statin Use Prior to Admission to Severity and Recovery Among COVID-19 Inpatients. Am J Cardiol. 2020. 10.1016/j.amjcard.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta A, Madhavan MV, Poterucha TJ, DeFilippis EM, Hennessey JA, Redfors B, et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):1325. 10.1038/s41467-021-21553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butt JH, Gerds TA, Schou M, Kragholm K, Phelps M, Havers-Borgersen E, et al. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open. 2020;10(12):e044421. 10.1136/bmjopen-2020-044421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hospital MG. Rationale for Consideration of Statins for COVID-19 Patients: The General Hospital Corporation; 2020. Available from: https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/rationale-for-consideration-of-statins-for-COVID-19-patient.pdf.
- 26.McCarthy CP, Murphy S, Jones-O’Connor M, Olshan DS, Khambhati JR, Rehman S, et al. Early clinical and sociodemographic experience with patients hospitalized with COVID-19 at a large American healthcare system. EClinicalMedicine. 2020;26:100504. 10.1016/j.eclinm.2020.100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassett IV, Triant VA, Bunda BA, Selvaggi CA, Shinnick DJ, He W, et al. Massachusetts general hospital Covid-19 registry reveals two distinct populations of hospitalized patients by race and ethnicity. PLoS One. 2020;15(12):e0244270. 10.1371/journal.pone.0244270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolkewitz M, Lambert J, von Cube M, Bugiera L, Grodd M, Hazard D, et al. Statistical Analysis of Clinical COVID-19 Data: A Concise Overview of Lessons Learned, Common Errors and How to Avoid Them. Clin Epidemiol. 2020;12:925–8. 10.2147/CLEP.S256735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–70. doi: 10.1097/00001648-200009000-00012 [DOI] [PubMed] [Google Scholar]
- 30.Moodie EE, Stephens DA, Klein MB. A marginal structural model for multiple-outcome survival data:assessing the impact of injection drug use on several causes of death in the Canadian Co-infection Cohort. Stat Med. 2014;33(8):1409–25. 10.1002/sim.6043 [DOI] [PubMed] [Google Scholar]
- 31.Little RJ, Tchetgen EJT, Troxel AB. University of Pennsylvania 11th annual conference on statistical issues in clinical trials: Estimands, missing data and sensitivity analysis (afternoon panel session). Clin Trials. 2019;16(4):381–90. 10.1177/1740774519853565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 33.Ding P, VanderWeele TJ. Sensitivity Analysis Without Assumptions. Epidemiology. 2016;27(3):368–77. doi: 10.1097/EDE.0000000000000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Team RC. R: A language and environment for statistical computing. Vienna, Austria: 2020. p. R Foundation for Statistical Computing. [Google Scholar]
- 35.Venables W, BD R. Modern Applied Statistics with S. Fourth edition ed. New York: Springer; 2002. [Google Scholar]
- 36.Quartagno M, Grund S, Carpenter J. Jomo: a flexible package for two-level joint modelling multiple imputation. R Journal 9.1; 2019.
- 37.Fresán U, Guevara M, Elía F, Albéniz E, Burgui C, Castilla J, et al. Independent Role of Severe Obesity as a Risk Factor for COVID-19 Hospitalization: A Spanish Population-Based Cohort Study. Obesity (Silver Spring). 2021;29(1):29–37. 10.1002/oby.23029 [DOI] [PubMed] [Google Scholar]
- 38.Asch DA, Sheils NE, Islam MN, Chen Y, Werner RM, Buresh J, et al. Variation in US Hospital Mortality Rates for Patients Admitted With COVID-19 During the First 6 Months of the Pandemic. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.8193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hariyanto TI, Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(6):1613–5. 10.1016/j.dsx.2020.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orkaby AR, Driver JA, Ho YL, Lu B, Costa L, Honerlaw J, et al. Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA. 2020;324(1):68–78. doi: 10.1001/jama.2020.7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711–8. doi: 10.1001/jama.285.13.1711 [DOI] [PubMed] [Google Scholar]
- 42.Guo H, Huang M, Yuan Q, Wei Y, Gao Y, Mao L, et al. The Important Role of Lipid Raft-Mediated Attachment in the Infection of Cultured Cells by Coronavirus Infectious Bronchitis Virus Beaudette Strain. PLoS One. 2017;12(1):e0170123. 10.1371/journal.pone.0170123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Y, Liu DX, Tam JP. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem Biophys Res Commun. 2008;369(2):344–9. 10.1016/j.bbrc.2008.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra K, Naffouj S, Gorgis S, Ibrahim H, Gill S, Fadel R, et al. Liver Injury as a Surrogate for Inflammation and Predictor of Outcomes in COVID-19. Hepatol Commun. 2021;5(1):24–32. 10.1002/hep4.1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Z F, L C, J L, C T, Y Z, S H. Clinical Features of COVID-19 Related Liver Damage. 2020. [Google Scholar]
- 46.Sakthirajan R, Dhanapriya J, Varghese A, Saravanakumar K, Dineshkumar T, Balasubramaniyan T, et al. Clinical profile and outcome of pigment-induced nephropathy. Clin Kidney J. 2018;11(3):348–52. 10.1093/ckj/sfx121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.