ABSTRACT
Hand, Foot and Mouth Disease (HFMD) is usually a self-limiting, mild childhood disease that is caused mainly by Coxsackie virus A16 (CVA16) and Enterovirus A71 (EV-A71), both members of the Picornaviridae family. However, recurring HFMD outbreaks and epidemics due to EV-A71 infection in the Western Pacific region, and the propensity of EV-A71 strains to cause severe neurological complications have made this neurotropic virus a serious public health concern in afflicted countries. High mutation rate leading to viral quasispecies combined with frequent intra- and inter-typic recombination events amongst co-circulating EV-A71 strains have contributed to the great diversity and fast evolution of EV-A71 genomes, making impossible any accurate prediction of the next epidemic strain. Comparative genome sequence analyses and mutagenesis approaches have led to the identification of a number of viral determinants involved in EV-A71 fitness and virulence. These viral determinants include amino acid residues located in the structural proteins of the virus, affecting attachment to the host cell surface, receptor binding, and uncoating events. Critical residues in non-structural proteins have also been identified, including 2C, 3A, 3C proteases and the RNA-dependent RNA polymerase. Finally, mutations altering key secondary structures in the 5’ untranslated region were also found to influence EV-A71 fitness and virulence. While our current understanding of EV-A71 pathogenesis remains fragmented, these studies may help in the rational design of effective treatments and broadly protective vaccine candidates.
KEYWORDS: Hand, Foot and Mouth Disease (HFMD), Coxsackie virus A16 (CVA16), neurovirulence, intertypic recombination, viral quasispecies, RNA-dependent RNA polymerase (RdRp), VP1 capsid, 5’UTR
1. Introduction to HFMD and Enterovirus-A71
Enterovirus-A71 (EV-A71) is the second most common causative agent after Coxsackievirus A16 (CVA16) of Hand, Foot and Mouth Disease (HFMD), a childhood disease that occurs worldwide but prominently affects countries within the Asia-Pacific region including China, Taiwan, Vietnam, Thailand, Malaysia, Singapore, Japan, Korea and Australia [1]. Numerous outbreaks and cyclical epidemics have been reported in these countries, with the latest EV-A71 outbreak in Singapore in 2018 during which 1,249 cases were reported within a single week [2]. Infections are usually followed by fever and sore throat before occurrence of characteristic blisters and lesions on the palms, soles and oral mucosa [3]. HFMD is highly transmissible via bodily fluids or contaminated objects and even asymptomatic adults are able to transmit the virus [3].
While HFMD is usually a self-limiting disease, serious complications have been reported with involvement of the central nervous system (CNS), including aseptic meningitis, brainstem encephalitis, acute flaccid paralysis and cardiopulmonary dysfunction of neurogenic origin [4]. When not fatal, HFMD-associated neurological complications may lead to long-term cognitive and motor disorders [5]. Up to 90% of HFMD cases with neurological complications have been attributed to EV-A71 infection [4]. This makes EV-A71 an important neurotropic virus after the eradication of its close cousin poliovirus (PV) from most of the surface of our planet. Three vaccines have made it to the Chinese market so far [1]. They are inactivated whole virus vaccines that cover a single genogroup (C4). These monovalent vaccines however, may not confer long-term pan-genogroup protection, and may influence epidemiological dynamics of EV-A71 strains [6]. Hence, efforts to develop multivalent vaccines including various EV-A71 genogroups and CVA16 have been pursued [7].
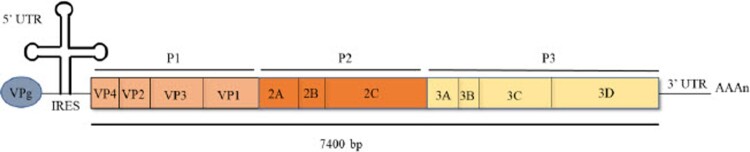
A member of the Picornaviridae family, EV-A71 is a non-enveloped, positive sense, single-stranded RNA virus. Its genome is approximately 7.4kb-long and the coding region, which encompasses sub-regions P1, P2 and P3 is preceded by a short, structured 5’ untranslated region (UTR), while the 3’UTR ends with a ploy-A tail (Figure 1). The P1 region codes for four structural proteins VP1 to VP4, which make up the protein capsid where the exposed VP1-3 are antigenic. The P2 and P3 regions code for seven non-structural proteins that include viral proteases and the RNA-dependent RNA polymerase (RdRp). The infection cycle of EV-A71 in its host cell starts with receptor-mediated entry involving the capsid protein VP1, followed by uncoating that allows release of the viral genome into the cytoplasm. After a first round of translation to produce structural and non-structural proteins, the viral genome is replicated followed by virus assembly and maturation, before the newly formed virions exit the cells upon apoptosis-induced cell lysis or via non-lytic exit pathway [8]. The role of each viral proteins during EV-A71 infection cycle has been recently reviewed elsewhere [9].
Figure 1.
Schematic illustration of EV-A71 genome organization.
Infection outcome typically results from the interplay between the intrinsic virulence of the pathogen, and the genetic make-up and immune status of the host. This review focuses on the viral determinants that play a critical role in EV-A71 fitness and virulence. An earlier study on the circulation of EV-A71 lineages in different countries and geographical areas since 1960 indicated that no association could be drawn between a particular genogroup or subgenogroup and the increased risk of neurological complications [10]. More recently however, complete genome analyses of EV-A71 strains have been conducted to identify genetic determinants responsible for different clinical patterns. Nucleotide differences in the 5’-UTR of the viral genome, and amino acid changes in viral proteins have been proposed to play a role in virulence. Additionally, mutagenesis studies have allowed explore the role of a number of nucleotides or amino acids in EV-A71 fitness and virulence, both in vitro and in animal models. We provide here an overview of these latest findings and the gaps in our knowledge that remain to be addressed.
2. Mechanisms driving EV-A71 strains diversity
2.1. EV-A71 strains classification and circulation
EV-A71 strains have been isolated in various countries all over the world, and have been classified into genogroups (A-G) and subgenogroups, based on their VP1 gene sequence similarity. A Bayesian tree was generated that traced the time of origin and evolutionary history of these strains [11]. Furthermore, reports of EV-A71 outbreaks worldwide have allowed monitor the circulation and evolution of EV-A71 strains over time [12]. Extensive and rapid shifts in genogroup and subgenogroup dominance have been noted. Intra-genogroup dominance shifts have also been reported over time in a single country. Furthermore, it is important to note that within a single outbreak, co-circulation of several genogroups and subgenogroups is common [12]. China, however, has seen persistent predominance of subgenogroup C4 over the past 15 years, and represents more the exception than the rule. The introduction of three monovalent C4 vaccines in China may however drive strain replacement and lead to a shift in EV-A71 genogroup or subgenogroup dominance, as seen with other infectious diseases [13].
Observation of rapid shifts in EVA-71 genogroup or subgenogroup dominance in the same country or geographical location is likely facilitated by the fact that several lineages co-circulate at any one point of time and take turn to become predominant. Herd immunity may represent an important factor that drives the shift in genogroup prevalence. Additionally, evidence of sequence recombination between EV-A71 strains, as well as spontaneous mutations in the viral genome contribute to the genetic diversity and drive evolution of EV-A71 strains.
2.2. Recombination
Studies employing recombination analysis techniques found evidence that strongly supported horizontal acquirement of sequences for almost every tested EV-A71 strains [14,15]. Sequence analysis of a single subgenogroup revealed that a group of isolates with similar sequences sampled from a single location were interspersed with sequences from strains found in geographically distant areas [15].
Recombination events in EV-A71 can be intra- or intertypic and occur non-randomly in both structural and non-structural protein gene sequences, or at the 5’ UTR, and with the highest frequency found in the 3D region that encodes for the RdRp [14,16]. Recombination events appear to drive the fitness and virulence of EV-A71 strains, and lead to the emergence of new EVA-71 strains responsible for major outbreaks. An EV-A71 C4 strain that had undergone recombination with CVA16 in the 3D region was responsible for fatal outbreaks in China where neurological complications were reported [17]. A similar observation was made upon the analysis of HFMD cases in China between 2011 and 2012, where viruses resulting from recombination between EV-A71 and CVA16 were isolated in severe cases [18]. In addition, a double recombination event between EV-A71 genogroups B and C, and a CVA16 strain was suspected to be the cause of an epidemic in China [19]. Intertypic recombination events in EV-A71 are not limited to its close relative CVA16, as recombination with other Human Enterovirus-A (HEA) strains has also been reported [20]. Copy-choice recombination, also known as template switching, can also occur when RdRp switches templates during synthesis, resulting in a mosaic-like genome [21]. This has been reported in PV, and could potentially occur in EV-A71 as well [22].
2.3. Spontaneous mutations and quasispecies
The P2 and P3 regions coding for non-structural proteins in enteroviruses are expected to be sites where recombination occurs most frequently due to their higher degree of sequence homology. In contrast, the more variable P1 region, coding for the viral capsid proteins is believed to undergo mutations more frequently, particularly at the VP1 region as interactions with antibodies and host cell receptors require the virus to evolve quickly in order to evade the host immune system while maintaining its ability to bind to its host receptors [19]. Mutations occur at high rates in RNA viruses due to the lack of proofreading activity in the RdRp, which leads to approximately 1 × 10−4 substitutions per nucleotide copied [23]. Such high mutation rate allows EV-A71 to adapt rapidly to selection pressures, which select for beneficial mutations [24]. A study on the potential antiviral activity of two capsid-binding compounds showed that EV-A71 acquired resistance mutations in VP1 upon successive passages in the presence of the compounds [25]. The resistance mutations acquired however, had a fitness cost and they were quickly lost upon removal of the selection pressure, highlighting the high plasticity of EV-A71 genome. The ability of EV-A71 to rapidly acquire mutations in response to specific culture conditions has also been documented in another study where EV-A71 acquired four mutations in VP1 sequence at position 104, 145, 146, and 241 that facilitated its replication after only three sub-passages in Rhabdomyosarcoma (RD) cells [26]. Mutations in the P1 region can also improve viral fitness by promoting interaction with attachment receptors such as heparan sulfate (HS) or conferring resistance to capsid-targeting drugs [27–29]. Mutations driven by selection pressure are not strictly limited to the P1 region. A point mutation in the RdRp from EV-A71 was reported to confer resistance to Ribavirin, a broad range antiviral compound effective against a variety of RNA viruses [30]. Ribavirin is a nucleoside analogue that is mistakenly incorporated into the nascent RNA strand and stops RNA synthesis [35]. The point mutation in RdRp was found to increase fidelity of the enzyme, hence avoiding incorporation of the nucleoside analogue [31].
In addition, the error-prone RdRp of enteroviruses resulting in high mutation rates enables the formation of quasispecies, where the viral progeny consists of a spectrum of closely related genome variants [32]. The existence of quasispecies in enteroviruses has been well documented and is evidenced by the formation of plaques of different sizes from a single viral culture suspension [33]. Importantly, the genetic diversity arising from quasispecies dynamics was found to play a critical role in the virus fitness and virulence [24,31]. Consistently, higher-fidelity RdRp EV-A71 and PV mutants, which limited the generation of quasispecies, were found to be attenuated in vivo [31,34]. The genetic diversity resulting from quasispecies dynamics was recently proposed to have driven the development of neurovirulent EV-A71 isolates in humans [35]. Similarly, genetic diversity in PV was demonstrated to be critical for neuroinvasion in order to overcome the host antiviral activities triggered upon entry of the first few viral particles into the CNS [34]. This was further supported by findings that high fidelity PV mutants were less neurovirulent and pathogenic, or elicited higher titres of neutralizing antibodies [36].
3. Molecular determinants that influence EVA-71 fitness and virulence
The dynamic changes occurring in the genome of EVA-71 resulting from recombination events and mutations have led to the emergence of strains with distinct fitness and virulence, and a distinct ability to cause neurological disease in their host. Earlier studies have reported that EV-A71 isolates that caused neurological complications displayed greater resistance to high temperature and enhanced replication capacity compared to milder strains [37–39]. These features were proposed to help the virus survive in its host during the febrile stage and ensure successful neuroinvasion and replication in the CNS. Temperature-adapted strains were obtained in in vitro culture systems, supporting that the temperature-resistant phenotype could be acquired through mutation [40].
More recently, comparative genome sequence analyses and mutagenesis approaches have allowed the identification of mutations in both structural and non-structural proteins, as well as in the UTRs that significantly influenced EV-A71 fitness and virulence (Table 1). In our literature survey, we have employed the following keywords: Enterovirus 71 virulence; molecular determinant; EV-A71 mutation affecting virulence; comparative studies on EV-A71.
Table 1.
Mutations in EV-A71 genome that influence fitness and virulence.
| Phenotype/Mechanism | |||||
|---|---|---|---|---|---|
| Region | Mutation | In vitro | In vivo | References | |
| VP1 | 31G |
DLD-1, RD and SK-N-SH cells ➢Reduced virion stability at 35°C, 36°C, 37°C, and 39°C ➢Reduced viral titer in DLD-1 cells ➢Increased growth in SK-N-SH cells |
– | [35] | |
| L97R | SH-SY5Y cells ➢Replicative advantage ➢Improved binding to host cells ➢Associated with E167G/A (Entry, or assembly) |
– | [66] | ||
| E98K |
L929 and Neuro-2a cells ➢Improved growth kinetic |
Two-week-old (BALB/c mice) ➢Non-fatal ➢No visible damage to liver and lungs and were comparable to control mice infected with PBS |
[67] | ||
| 107A |
Vero cells ➢Reduced viral growth and titre ➢Regulates the cleavage of precursor VP0 and potentially affects virus uncoating and maturation |
– | [50] | ||
| 145G |
RD cells overexpressing hSCARB2 ➢Improved replication ➢Enhanced HS binding compared to 145E |
Neonatal mice (ICR mice) ➢Lower viral loads in organs ➢Non-fatal hSCARB2 transgenic mice ➢High adsorption to non-susceptible cells and rapid decrease in plasma of hSCARB2 transgenic mice ➢Lowered viral load in organs ➢Non-fatal |
[44] | ||
| 145E |
RD cells overexpressing hSCARB2 ➢Lower virus titer produced in mutants compared to 145G |
Neonatal mice (ICR) ➢Higher viral load in organs ➢Lethal in mice hSCARB2 transgenic mice ➢More efficient replication and greater neuroinvasion compared to 145G |
|||
|
RD cells ➢Mouse-adapted strain ➢Lower titers compared to parental strain ➢Decreased binding to cell |
1-week old mice (BALB/c) ➢Caused 100% mortality |
[45,46] | |||
|
Jurkat T cells ➢Molecular switch that changes virus from PSGL-binding (145G/Q) to non-PSGL-binding |
– | [68] | |||
| – |
5-day-old BALB/c mice Caused 100% mortality |
[69] | |||
| – |
Cynomolgous monkeys ➢CNS inflammation and damage ➢Neurological manifestations (tremors) ➢145E mutants recovered from monkeys inoculated with 145G mutant, while those inoculated with 145E maintained the same mutation, suggesting a selection pressure for 145E. ➢Innate immune system response with rise in IFNα, TNFα and IL-6 ➢More resistant to neutralizing antibodies |
[47] | |||
| Double mutant VP1 145E & VP2 149M |
Neuro-2a cells ➢Higher viral titers ➢Higher viral protein expression |
1-day old mice (ICR) ➢149M increased effect of 145E for mouse lethality |
[48] | ||
| Double mutant 145E & 98E |
Murine NIH/3T3 and Neuro-2a cells ➢Productive infection ➢Addition of 169F mutation further enhanced infectivity |
1-week old mice (BALB/c) ➢No significant signs of disease and non-lethal in mice |
[51] | ||
| K162A |
Computer simulated Heparin ➢Reduced strength of interaction with heparin |
– | [28] | ||
| 192M |
Vero cells ➢Resistance to EV-A71 inhibitor pyridyl imidazolidinone |
– | [27] | ||
| K215A |
Vero cells Increased thermostability where infectivity of mutant is higher than wild type after treatment at 420C |
– | [70] | ||
| K242A |
Computer simulated Heparin ➢Reduced strength of interaction with heparin RD cells ➢Reduced binding on cell surface |
– | [28] | ||
| K244A | |||||
| K244E | – |
1-day-old (BALB/c mice) ➢Increased virulence 5-day-old (BALB/c mice) ➢Caused 100% mortality at virus doses greater than 8.7 × 101 TCID50 6-week-old (AG129 mice) ➢Increased virulence |
[71] [72] |
||
| A289T |
Human Brain Microvascular Endothelial Cells (HBMECs) ➢Decreased viral attachment, replication, protein synthesis, and virus particle secretion. |
1-week-old (BALB/c&Sv129) ➢Decreased morbidity; decreased CNS infectivity |
[73] | ||
| Q172A |
RD cells ➢Loss in both binding and infection |
– | [49] | ||
| Q152A R166A W171A T173A T175A N176A S178A F180A R236A |
RD cells ➢Loss in binding and infection ➢No impairment of viral assembly except for N176A |
– | |||
| VP2 | K149I |
Chinese Hamster Ovary cells ➢More efficient replication with higher virus titre |
– | [45] | |
| 5’ UTR | C158U | – |
Three-day-old ICR mice ➢Prolonged survival of infected mice ➢Reduced viral replication and virulence |
[53] |
|
| C115T | – |
1-day old BALB/c mice ➢Reduced virulence |
[60] | ||
| A158T C475T A486G G487A |
RD cells ➢Reduced CPE ➢Lower viral titers |
– | [65] | ||
| Helicase 2C | K135A D176N C270A C281A C286A I141R S282R E325A L327K F328A F328Y |
Vero cells ➢Reduced viral production ➢Reduced ATPase activity of protein 2C |
– | [74] | |
| Protease 3A | A5262G |
RD cells ➢Absence of CPE ➢Reduced viral titer |
– | [65] | |
| Protease 3C | N69D |
RD cells ➢Decreased enzymatic/catalytic activity ➢Decreased viral production |
– | [75] | |
| T79V |
RD cells ➢Increased viral replication Immunoprecipitation ➢Increased interaction with TRIM21 |
– | [58] | ||
| R84Q |
Vero cells ➢Loss of RNA-binding activity ➢No plaque formation at 8 d.p.i. |
– | [76] | ||
| I86A |
Vero cells ➢Loss of proteolytic activity ➢No plaque formation at 8 d.p.i. |
– | [76] | ||
| 3D polymerase | I251T |
SK-N-SH cells ➢Viral replication rate reduced by up to 100-fold at 39.5°C |
ICR (newborn) ➢Reduced virulence |
[77] | |
| L123F |
RD cells ➢Increased replication fidelity ➢In vitro growth not altered |
Ten-day-old (AG129 mice) ➢Reduced virulence ➢Reduced viral titers |
[31] | ||
| Double mutation G64R & L123F |
RD cells ➢Increased replication fidelity ➢Attenuated in vitro growth |
Ten-day-old (AG129 mice) ➢Reduced virulence ➢Reduced viral titer compared to L123F single mutation. |
[31] | ||
3.1. Mutagenesis approaches
3.1.1. Structural proteins VP1-4
Mutations that influence EV-A71 binding and entry through interaction with cellular receptors have been found to play an important role in EV-A71 infectivity and virulence. EV-A71 entry into human cells is primarily mediated by SCARB2 and PSGL-1 while other surface molecules such as Annexin2 and HS function as attachment receptors [41]. Specifically, HS has garnered a lot of interest with recent studies highlighting the important role of this surface attachment receptor in EV-A71 virulence and pathogenesis [29], and the potential antiviral activity of HS mimetic compounds [42].
The receptor binding domain lies within the VP1 capsid protein, and numerous mutations in this region have been found to affect receptor binding [41], with consequences on cell/tissue tropism and replication efficacy both in vitro and/or in vivo (Table 1). Mutations in VP1 alter capsid-receptor interactions either directly by changing the charge of the critical residues, or indirectly by changing the orientation of the critical residues when the neighbouring residues are mutated [28]. Other VP1 mutations were found to destabilize the capsid, impair VP1-VP2 interactions and affect virus uncoating [43].
One notable residue is VP1 145, which resides within the DE loop and is surrounded by positively charged residues. A glycine (G) residue at that position increased significantly EV-A71 binding affinity to HS due to the highly positive charge of the five-fold pentamer, which facilitates electrostatic interactions with HS [43,44]. In contrast, presence of the negatively charged glutamate (E) at position 145 in VP1 led to decreased binding affinity to HS, resulting in lower viral titres in the culture supernatant [28, 44–46]. However, VP1 145E mutant surprisingly performed better in vivo compared to VP1 145G virus as evidenced by higher viral loads and increased neuroinvasion in neonatal mice and cynomolgous monkeys [44,47]. The seemingly contradictory in vitro and in vivo observations led the authors to propose that the increased adherence property of VP1 145G virus to HS-expressing cells limited its ability to disseminate and invade the CNS [43,44]. In addition, Huang et al reported increased virulence with a VP1 145E / VP2 149M double mutant, supporting cooperative interaction between VP2 and VP1 during the entry step [48].
Other VP1 mutations have also been reported to alter binding efficacy to surface receptors. In silico simulation models predicted that replacement of lysine (K) residues with alanine (A) at VP1 162, 242 or 244 would lead to reduced strength of the electrostatic interactions with HS, and this was experimentally confirmed in RD cells [28]. Furthermore, although a number of key residues in VP1 have been found to play a critical role in the viral entry step including attachment and uncoating [49–51], the molecular and structural insights are lacking to explain their role.
It is important to note that EV-A71 displays weak binding affinity to murine SCARB2 (mSCARB2) and PSGL-1 (mPSGL-1), and it is likely that the virus uses different surface entry receptors in mice [48,51]. Therefore, whether the findings on the role of various VP1/VP2 mutations in EV-A71 virulence in mouse models hold true in human settings remains to be demonstrated. Some of these findings however were generated in hSCARB2 transgenic mice [44] or in NHP models [47], the latter share high homology with hSCARB2 and are thus expected to be more predictive.
3.1.2. Non-structural proteins and UTRs
While capsid proteins primarily drive virulence by influencing interactions with the host receptors, mutations within non-structural proteins are expected to affect virulence by altering intracellular steps of the infection cycle such as genome replication, protein translation, and virus assembly and maturation. Mutations in proteins 2C, 3A, 3C, and in 3D polymerase have been reported to influence the in vitro fitness of EV-A71 strains (Table 1). Mutations within UTRs of the viral genome could also affect EV-A71 fitness and virulence (Table 1). For example, nucleotide changes that affect the stem loop structure in the 5’UTR were associated with reduced IRES-dependent translational activity [52]. A single point mutation at position 158 (C158U) in domain V of the stem loop structure led to reduced virulence of an EV-A71 strain from genogroup B1 in three-day-old ICR mice [53]. Interestingly, the same C158U substitution was associated with a fatal case, highlighting the lack of direct correlation between virulence in a mouse model and clinical outcome in patients [54]. In PV, C158U substitution was predicted to cause a change in the secondary structure of the upper stem loop II, which altered binding affinity to host factors, and subsequently attenuated viral genome replication [55,56]. Similar results were obtained with B4 genogroup strains where A158T mutants caused minimal CPE and led to reduced viral RNA copy number, plaque forming units (pfu) and VP1 production in RD cells [57].
In addition, polymorphism at the 79th amino acid in EV-A71 3C protease was associated with varying levels of clinical severity [58]. Mechanistically, the study found that T79V substitution in 3C led to higher viral replication while reducing interaction with TRIM21, a component of antibody-dependent intracellular neutralization.
3.2. Comparative genome sequences analyses
In addition to experimental mutagenesis approaches, numerous genome comparative studies have been conducted over the years. While these studies failed to correlate a particular genogroup with disease severity [59], they have allowed identify viral determinants of EV-A71 virulence in its human host. Upon comparing clinical isolates that led to mild versus more severe disease outcome in patients, differences were observed in virtually all parts of EV-A71 genome, including coding and non-coding regions. In some cases, follow up experiments have been conducted to verify the potential role of the viral determinants identified (Table 2). For example, a single nucleotide difference located in the 5’ UTR stem loop structure at position 115 between two EV-A71 strains of different clinical severity was associated with a differential virulence phenotype in BALB/c mouse neonates [60]. In another study, Li et al [38] replaced the protease 2A region from a severe EV-A71 strain with that of a milder strain. The recombinant virus displayed slower replication rate compared to the severe strain at two different temperatures (37 and 39.5 °C) and in three different cell lines. One-day-old ICR mice infected with this mutant also showed less severe pathological features and lower viral loads compared to mice infected with the severe strain [38]. Three amino acid differences at positions 64, 68 and 85 were mapped between both protease 2A sequences. However, the study did not investigate further the mechanisms involved in the differential virulence phenotype observed, and the relative contribution of each of these residues.
Table 2.
Comparative studies of EV-A71 genome sequences and their phenotypic differences.
| Phenotypic Differences | |||
|---|---|---|---|
| Mutations | In vitro | In vivo | References |
| Protease 2A from a severe strain → protease 2A from a mild strain Protease 2A Position 64 (Y64H) Position 68 (M68R) Position 85 (D85E) |
RD cells, Vero cells and SH-SY5Y cells ➢Slower replication compared to the severe strain at 37 °C and 39.5 °C ➢Less viral RNA compared to the severe strain at 37 °C and 39.5 °C |
1-day-old ICR mice ➢Less severe pathological changes compared to severe strain ➢Lower viral load compared to severe strain |
[78] |
| 3CD region from severe strain → 3CD region from mild strain Protease 3C Position 138 (V138I) 3D polymerase Position 37 (S37N) Position 143 (R143K) Position 176 (I176V) Position 261 (E261G) Position 360 (G360A) |
RD cells and Vero cells ➢Milder CPE as compared to severe strain at 37 °C and 39.5 °C ➢Decreased replication rate compared to severe strain at 39.5 °C |
1-day-old ICR mice ➢Mice showed no paralysis, no death, less body weight loss, and milder symptoms as compared to the mice injected with the severe strain ➢Lower viral load as compared to mice infected with the severe strain |
[38] |
| Weakly pathogenic strain → Highly pathogenic strain 5’ UTR Position 115 (115C>T) Position 132 (132T>C) Position 811 (811T>C) VP1 Position 639 (G639S) VP3 Position 485 (Q485R) Position 504 (A504V) Position 537 (V537I) Protease 2A Position 937 (G937S) Protease 2B Position 1014 (A1014V) Position 1045 (I1045V) Position 1062 (T1062I) Protease 2C Position 1135 (K1135R) Position 1145 (E1145D) Position 1155 (V1155A) Position 1361 (V1361M) Protease 3C Position 1597 (I1597V) Position 1617 (D1617N) 3D Polymerase Position 1746 (L1746F) Position 2146 (A2146V) 3’ UTR Position 7347 (7347A>G) |
Nucleotide differences when a weakly pathogenic strain (JN200803) is compared to a highly pathogenic strain (JN200804) MA104 cells ➢Both strains (weakly and highly pathogenic strain) exhibited similar CPE ➢No difference in virulence as determined by TCID50 titration |
1-day-old BALB/c mice ➢Mice infected with the highly pathogenic strain exhibited hind limb paralysis by day 4 post-infection. ➢Mice infected with the weakly pathogenic strain had no evident neurological symptoms and remained healthy. |
[79] |
4. Concluding remarks and future perspectives
This review provided an update on the main viral determinants that have been found to influence EV-A71 fitness and virulence. Unsurprisingly, majority of the residues identified mapped in the main capsid protein VP1, affecting attachment or receptor binding. A number of mutations in non-structural proteins have also been described to influence in vitro replication of the virus but their in vivo phenotype has yet to be reported. Nevertheless, this incomplete picture still provides useful information and helps improve our understanding of EV-A71 virulence mechanisms.
Despite its public health significance and economic impact on afflicted societies, HFMD has remained under-studied. Great majority of the research efforts have been led by academic institutions supported by governmental funding, as HFMD is not part of pharmaceutical companies’ R&D portfolio. As a result, patchy knowledge and limited understanding of the virulence mechanisms have hampered the development of effective treatments and the rational design of vaccine candidates. The limited availability of relevant animal models [61] has also contributed to the difficulty in translating pre-clinical findings.
Furthermore, while the ability to invade the CNS represents the cornerstone of EV-A71 neurovirulence, an increasing body of evidence has underscored the importance of the host immune response in EV-A71 pathogenesis. Indeed, severe HFMD cases have been associated with heightened production of pro-inflammatory mediators in EV-A71 patients with CNS complications [62]. EV-A71 immunopathogenesis has not been fully elucidated yet, although a number of viral proteins have been identified to interact with, and manipulate the host immune system [63]. Thus, it appears that the ability of EV-A71 to manipulate its host immune system represents an integral part of its virulence strategy, and influences the clinical outcome. However, while some recent evidence support that EV-A71 strains associated with severe clinical outcome manipulate differently their host immune signalling pathways [64], the viral determinants involved have yet to be reported.
Beyond whole inactivated virus formulations, the identification of key viral determinants involved in EV-A71 neurovirulence and immunopathogenesis represents an opportunity to develop live attenuated vaccine candidates [65] that may offer complete and strong protection upon a single immunization, which would improve the vaccine take up rate and control more effectively HFMD epidemics.
Funding Statement
This work was supported by National Research Foundation: [grant number NRF-CRP21-2018-0004].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Yi EJ, Shin YJ, Kim JH, et al. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res. 2017;6(1):4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh F. Weekly HFMD cases hit record high this year. AUG 16, 2018; Available from: https://www.straitstimes.com/singapore/weekly-hfmd-cases-hit-record-high-this-year.
- 3.Xu J, Qian Y, Wang S, et al. EV71: an emerging infectious disease vaccine target in the Far east? Vaccine. 2010;28(20):3516–3521. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Ju Y, Chen M, et al. Epidemiological and genetic characteristics of EV71 in hand, foot, and mouth disease in guangxi, southern China, from 2010 to 2015. PLoS One. 2017;12(12). e0188640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang LY, Huang LM, Gau SS, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007;356(12):1226–1234. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Chen Z, Zheng K, et al. Epidemiology of hand, foot, and Mouth Disease before and after the introduction of enterovirus 71 vaccines in chengdu, China, 2009-2018. Pediatr Infect Dis J. 2020;39(10):969–978. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Tong X, Huang Z.. Towards broadly protective polyvalent vaccines against hand, foot and mouth disease. Microbes Infect. 2015;17(2):155–162. [DOI] [PubMed] [Google Scholar]
- 8.Too IH, Yeo H, Sessions OM, et al. Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci Rep. 2016;6:36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Li Y.. Recent progress on functional genomics Research of enterovirus 71. Virol Sin. 2019;34(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMinn PC. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr Opin Virol. 2012;2(2):199–205. [DOI] [PubMed] [Google Scholar]
- 11.Bessaud M, Razafindratsimandresy R, Nougairède A, et al. Molecular comparison and evolutionary analyses of VP1 nucleotide sequences of new african human enterovirus 71 isolates reveal a wide genetic diversity. PLoS One. 2014;9(3):e90624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SW, Cheng D, Wang JR.. Enterovirus A71: virulence, antigenicity, and genetic evolution over the years. J Biomed Sci. 2019;26(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martcheva M, Bolker BM, Holt RD.. Vaccine-induced pathogen strain replacement: what are the mechanisms? J R Soc Interface. 2008;5(18):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Zhang Q, Li J, et al. Analysis of recombination and natural selection in human enterovirus 71. Virology. 2010;398(2):251–261. [DOI] [PubMed] [Google Scholar]
- 15.Tee KK, Lam TT, Chan YF, et al. Evolutionary genetics of human enterovirus 71: origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J Virol. 2010;84(7):3339–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Huo X, Dai Y, et al. Evidences for intertypic and intratypic recombinant events in EV71 of hand, foot and mouth disease during an epidemic in hubei province, China, 2011. Virus Res. 2012;169(1):195–202. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Tan X, Cui A, et al. Complete genome analysis of the C4 subgenotype strains of enterovirus 71: predominant recombination C4 viruses persistently circulating in China for 14 years. PLoS One. 2013;8(2):e56341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Wu S, Xiong Y, et al. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot, and mouth disease in Central China. PLoS One. 2014;9(4):e96051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yip CC, Lau SK, Zhou B, et al. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: first evidence for combination of intratypic and intertypic recombination events in EV71. Arch Virol. 2010;155(9):1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoke-Fun C, AbuBakar S.. Phylogenetic evidence for inter-typic recombination in the emergence of human enterovirus 71 subgenotypes. BMC Microbiol. 2006;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon-Loriere E, Holmes EC.. Why do RNA viruses recombine? Nat Rev Microbiol. 2011;9(8):617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis TC, Kirkegaard K.. Poliovirus RNA recombination: mechanistic studies in the absence of selection. EMBO J. 1992;11(8):3135–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domingo E, Wain-Hobson S.. The 30th anniversary of quasispecies. meeting on ‘quasispecies: past, present and future’. EMBO Rep. 2009;10(5):444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domingo E, Martin V, Perales C, et al. Coxsackieviruses and quasispecies theory: evolution of enteroviruses. Curr Top Microbiol Immunol. 2008;323:3–32. [DOI] [PubMed] [Google Scholar]
- 25.Kelly JT, De Colibus L, Elliott L, et al. Potent antiviral agents fail to elicit genetically-stable resistance mutations in either enterovirus 71 or Coxsackievirus A16. Antiviral Res. 2015;124:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CK, Wu SR, Chen YC, et al. Mutations in VP1 and 5'-UTR affect enterovirus 71 virulence. Sci Rep. 2018;8(1):6688, doi: 10.1038/s41598-018-25091-7. Erratum in: Sci Rep. 2018;8(1):8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih SR, Tsai MC, Tseng SN, et al. Mutation in enterovirus 71 capsid protein VP1 confers resistance to the inhibitory effects of pyridyl imidazolidinone. Antimicrob Agents Chemother. 2004;48(9):3523–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan CW, Sam IC, Lee VS, et al. VP1 residues around the five-fold axis of enterovirus A71 mediate heparan sulfate interaction. Virology. 2017;501:79–87. [DOI] [PubMed] [Google Scholar]
- 29.Tseligka ED, Sobo K, Stoppini L, et al. A VP1 mutation acquired during an enterovirus 71 disseminated infection confers heparan sulfate binding ability and modulates ex vivo tropism. PLoS Pathog. 2018;14(8):e1007190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crotty S, Cameron CE, Andino R.. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A. 2001;98(12):6895–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng T, Kwang J.. Attenuation of human enterovirus 71 high-replication-fidelity variants in AG129 mice. J Virol. 2014;88(10):5803–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domingo E, Sheldon J, Perales C.. Viral quasispecies evolution. Microbiol Mol Biol Rev. 2012;76(2):159–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsingh AI, Caggana M, Ronstrom S.. Genetic mapping of the determinants of plaque morphology of coxsackievirus B4. Arch Virol. 1995;140(12):2215–2226. [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer JK, Kirkegaard K.. Bottleneck-mediated quasispecies restriction during spread of an RNA virus from inoculation site to brain. Proc Natl Acad Sci U S A. 2006;103(14):5520–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang SW, Huang YH, Tsai HP, et al. A selective Bottleneck shapes the evolutionary mutant spectra of enterovirus A71 during viral dissemination in humans. J Virol. 2017;91(23):e01062–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vignuzzi M, Stone JK, Arnold JJ, et al. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439(7074):344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kung CM, King CC, Lee CN, et al. Differences in replication capacity between enterovirus 71 isolates obtained from patients with encephalitis and those obtained from patients with herpangina in Taiwan. J Med Virol. 2007;79(1):60–68. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Qiao Q, Hao SB, et al. Nonstructural protein 2A modulates replication and virulence of enterovirus 71. Virus Res. 2018;244:262–269. [DOI] [PubMed] [Google Scholar]
- 39.Arita M, Shimizu H, Nagata N, et al. Temperature-sensitive mutants of enterovirus 71 show attenuation in cynomolgus monkeys. J Gen Virol. 2005;86(Pt 5):1391–1401. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Cheng Y, Liang X, et al. Molecular characterization of enterovirus 71 sibling strains for thermal adaption in Vero cells with adaptive laboratory evolution. Infect Genet Evol. 2019;67:44–50. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi K, Koike S.. Cellular receptors for enterovirus A71. J Biomed Sci. 2020;27(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pourianfar HR, Poh CL, Fecondo J, et al. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against enterovirus 71. Virus Res. 2012;169(1):22–29. [DOI] [PubMed] [Google Scholar]
- 43.Tee HK, Tan CW, Yogarajah T, et al. Electrostatic interactions at the five-fold axis alter heparin-binding phenotype and drive enterovirus A71 virulence in mice. PLoS Pathog. 2019;15(11):e1007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi K, Sudaka Y, Takashino A, et al. Amino acid Variation at VP1-145 of enterovirus 71 determines attachment receptor usage and neurovirulence in human scavenger receptor B2 transgenic mice. J Virol. 2018;92(15):e00681–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chua BH, Phuektes P, Sanders SA, et al. The molecular basis of mouse adaptation by human enterovirus 71. J Gen Virol. 2008;89(Pt 7):1622–1632. [DOI] [PubMed] [Google Scholar]
- 46.Arita M, Ami Y, Wakita T, et al. Cooperative effect of the attenuation determinants derived from poliovirus sabin 1 strain is essential for attenuation of enterovirus 71 in the NOD/SCID mouse infection model. J Virol. 2008;82(4):1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii K, Sudaka Y, Takashino A, et al. VP1 amino acid residue 145 of enterovirus 71 Is a Key residue for Its receptor attachment and resistance to neutralizing antibody during cynomolgus monkey infection. J Virol. 2018;92(15):e00682–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang SW, Wang YF, Yu CK, et al. Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement. Virology. 2012;422(1):132–143. [DOI] [PubMed] [Google Scholar]
- 49.Chen P, Song Z, Qi Y, et al. Molecular determinants of enterovirus 71 viral entry: cleft around GLN-172 on VP1 protein interacts with variable region on scavenge receptor B 2. J Biol Chem. 2012;287(9):6406–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang YX, Huang YM, Li QJ, et al. A highly conserved amino acid in VP1 regulates maturation of enterovirus 71. PLoS Pathog. 2017;13(9):e1006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Victorio CB, Xu Y, Ng Q, et al. Cooperative effect of the VP1 amino acids 98E, 145A and 169F in the productive infection of mouse cell lines by enterovirus 71 (BS strain). Emerg Microbes Infect. 2016;5(6):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levengood JD, Tolbert M, Li ML, et al. High-affinity interaction of hnRNP A1 with conserved RNA structural elements is required for translation and replication of enterovirus 71. RNA Biol. 2013;10(7):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh MT, Wang SW, Yu CK, et al. A single nucleotide in stem loop II of 5'-untranslated region contributes to virulence of enterovirus 71 in mice. PLoS One. 2011;6(11):e27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong CY, Bible JM, Platt C.. A fatal case of enterovirus 71 infection with a single nucleotide variation in domain V of the 5’ untranslated region. Pediatr Infect Dis J. 2011;30(11):1013–1014. [DOI] [PubMed] [Google Scholar]
- 55.Gutiérrez AL, Denova-Ocampo M, Racaniello VR, et al. Attenuating mutations in the poliovirus 5’ untranslated region alter its interaction with polypyrimidine tract-binding protein. J Virol. 1997;71(5):3826–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skinner MA, Racaniello VR, Dunn G, et al. New model for the secondary structure of the 5’ non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989;207(2):379–392. [DOI] [PubMed] [Google Scholar]
- 57.Yee PT, Tan KO, Othman I, et al. Identification of molecular determinants of cell culture growth characteristics of enterovirus 71. Virol J. 2016;13(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma HY, Lu CY, Tsao KC, et al. Association of EV71 3C polymorphisms with clinical severity. J Microbiol Immunol Infect. 2018;51(5):608–613. [DOI] [PubMed] [Google Scholar]
- 59.Yip CC, Lau SK, Woo PC, et al. Human enterovirus 71 epidemics: what's next? Emerg Health Threats J. 2013;6:19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li P, Yue Y, Song N, et al. Genome analysis of enterovirus 71 strains differing in mouse pathogenicity. Virus Genes. 2016;52(2):161–171. [DOI] [PubMed] [Google Scholar]
- 61.Wang YF, Yu CK.. Animal models of enterovirus 71 infection: applications and limitations. J Biomed Sci. 2014;21(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W, Huang Z, Huang M, et al. Predicting severe enterovirus 71-infected hand, foot, and Mouth Disease: cytokines and chemokines. Mediators Inflamm. 2020;2020:9273241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox JA, Hiscox JA, Solomon T, et al. Immunopathogenesis and virus-host interactions of enterovirus 71 in patients with hand, Foot and Mouth disease. Front Microbiol. 2017;8:2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amrun SN, Tan JJL, Rickett NY, et al. TREM-1 activation is a potential key regulator in driving severe pathogenesis of enterovirus A71 infection. Sci Rep. 2020;10(1):3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yee PT, Tan SH, Ong KC, et al. Development of live attenuated enterovirus 71 vaccine strains that confer protection against lethal challenge in mice. Sci Rep. 2019;9(1):4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cordey S, Petty TJ, Schibler M, et al. Identification of site-specific adaptations conferring increased neural cell tropism during human enterovirus 71 infection. PLoS Pathog. 2012;8(7). e1002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ke X, Zhang Y, Liu Y, et al. A single mutation in the VP1 gene of enterovirus 71 enhances viral binding to heparan sulfate and impairs viral Pathogenicity in mice. Viruses. 2020;12(8):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishimura Y, Lee H, Hafenstein S, et al. Enterovirus 71 binding to PSGL-1 on leukocytes: VP1-145 acts as a molecular switch to control receptor interaction. PLoS Pathog. 2013;9(7). e1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaini Z, McMinn P.. A single mutation in capsid protein VP1 (Q145E) of a genogroup C4 strain of human enterovirus 71 generates a mouse-virulent phenotype. J Gen Virol. 2012;93(Pt 9):1935–1940. [DOI] [PubMed] [Google Scholar]
- 70.Yuan S, Li G, Wang Y, et al. Identification of positively charged residues in enterovirus 71 capsid protein VP1 essential for production of Infectious particles. J Virol. 2015;90(2):741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaini Z, Phuektes P, McMinn P.. Mouse adaptation of a sub-genogroup B5 strain of human enterovirus 71 is associated with a novel lysine to glutamic acid substitution at position 244 in protein VP1. Virus Res. 2012;167(1):86–96. [DOI] [PubMed] [Google Scholar]
- 72.Caine EA, Moncla LH, Ronderos MD, et al. A single mutation in the VP1 of enterovirus 71 Is responsible for increased virulence and neurotropism in adult interferon-deficient mice. J Virol. 2016;90(19):8592–8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu H, Cao Y, Su W, et al. Enterovirus A71 VP1 Variation A289 T decreases the Central nervous system infectivity via attenuation of interactions between VP1 and vimentin In vitro and In vivo. Viruses. 2019;11(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guan H, Tian J, Qin B, et al. Crystal structure of 2C helicase from enterovirus 71. Sci Adv. 2017;3(4). e1602573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li B, Yue Y, Zhang Y, et al. A novel enterovirus 71 (EV71) virulence determinant: The 69th residue of 3C Protease modulates pathogenicity. Front Cell Infect Microbiol. 2017;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shih SR, Chiang C, Chen TC, et al. Mutations at KFRDI and VGK domains of enterovirus 71 3C protease affect its RNA binding and proteolytic activities. J Biomed Sci. 2004;11(2):239–248. [DOI] [PubMed] [Google Scholar]
- 77.Kung YH, Huang SW, Kuo PH, et al. Introduction of a strong temperature-sensitive phenotype into enterovirus 71 by altering an amino acid of virus 3D polymerase. Virology. 2010;396(1):1–9. [DOI] [PubMed] [Google Scholar]
- 78.Li J, Gao F, Hao SB, et al. Contribution of 3CD region to the virulence of enterovirus 71. Biomed Environ Sci. 2017;30(10):767–771. [DOI] [PubMed] [Google Scholar]
- 79.Yue Y, Li P, Song N, et al. Genomic and immunologic factors associated with viral pathogenesis in a lethal EV71 infected neonatal mouse model. Mol Med Rep. 2016;13(5):4183–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]