ABSTRACT
Introduction: Patients admitted with COVID-19 often have severe hypoxemic respiratory insufficiency and it can be difficult to maintain adequate oxygenation with oxygen supplementation alone. There is a physiological rationale for the use of Continuous Positive Airway Pressure (CPAP), and CPAP could keep some patients off mechanical ventilation. We aimed to examine the physiological response to CPAP and the outcome of this treatment.
Methods: Data from all patients admitted with COVID-19 and treated with CPAP, from March to July 2020 were collected retrospectively. CPAP was initiated on a medical ward when oxygen supplementation exceeded 10 liters/min to maintain oxygen saturation (SpO2) ≥92%. CPAP was administered with full face masks on a continuous basis until stable improvement in oxygenation or until intubation or death.
Results: CPAP was initiated in 53 patients (35 men, 18 women) with a median (IQR) age of 68 (57–78) years. Nine patients were not able to tolerate the CPAP treatment. Median duration for the 44 patients receiving CPAP was 3 (2–6) days. The PaO2/FiO2 ratio was severely reduced to an average of 101 mmHg at initiation of treatment. A positive response of CPAP was seen on respiratory rate (p = 0.002) and on oxygenation (p < 0.001). Of the 44 patients receiving CPAP, 12 (27%) avoided intubation,13 (29%) were intubated, and 19 (43%) died. Of the patients with a ceiling of treatment in the ward (26 of 53) only 2 survived. Older age and high initial oxygen demand predicted treatment failure.
Discussion: CPAP seems to have positive effect on oxygenation and respiratory rate in most patients with severe respiratory failure caused by COVID-19. Treatment with CPAP to severely hypoxemic patients in a medical ward is possible, but the prognosis for especially elderly patients with high oxygen requirement and with a ceiling of treatment in the ward is poor.
KEYWORDS: Coronavirus disease, Continuous positive airway pressure, Physiotherapy, noninvasive ventilation, hypoxemia
Introduction
On 11 March 2020, Denmark closed the society as a response to the pandemic of coronavirus disease 2019 (COVID-19). The healthcare system prepared for an anticipated load of patients requiring medical attention and hospital admission. Hvidovre Hospital in the Capital region of Denmark is the largest hospital receiving acute COVID-19 patients from a catchment area comprising around 500.000 people. The early reports from the World Health Organization (WHO) estimated that 5% of the admitted patients would develop critical illness and require intensive care with the need for respiratory support[1]. There were concerns about the lack of beds in the Intensive Care Units (ICU) and with the international reports of the poor outcome from mechanical ventilation, noninvasive ventilation became appealing. The physiological rationale for a positive end-expiratory pressure(PEEP), delivered by a noninvasive device intended for sleep apnea treatment, had been discussed from the beginning of the pandemic. With the first recommendations, based on experiences from Italy, suggesting Continuous Positive Airway Pressure (CPAP) as a beneficial treatment for patients with COVID-19 [2] it was decided to offer CPAP as a standard treatment at Hvidovre Hospital. To prepare for this, the specialized ward for infectious diseases worked as a semi-intensive care unit. Soon after, more recommendations followed supporting the use of CPAP [3]. The Italian authors recommended applying CPAP 24 hours a day and with a PEEP of initially 10 cmH2O to patients with COVID-19 and hypoxemia [2,3]. Even before the pandemic, physiotherapists were experienced in administering CPAP treatment given intermittently for shorter duration e.g. to patients with atelectasis. Therefore, the physiotherapists played a key role in the management of this noninvasive respiratory support when implemented in a medical ward without prior knowledge of the 24 hours CPAP treatment.
The objective of CPAP treatment is to increase functional residual capacity (FRC), improve oxygenation, increase lung compliance, and possibly delay or avoid intubation [3,4]. It was given from the start that not all patients could survive intubation and thus had a ceiling of the treatment offered in the ward. Our aim was to keep the patients off mechanical ventilation and instead treat with less invasive respiratory support. CPAP is a simple way to apply a positive end- expiratory- pressure with noninvasive devices and therefore has the potential of contributing significantly to the management of respiratory failure. However, evidence is lacking as well as knowledge on which factors should be considered when treating patients.
The objective of this study was to investigate the physiological response to CPAP therapy in patients with COVID-19 and hypoxemic respiratory failure. Additionally, the aim was to evaluate prognostic factors regarding positive response and CPAP success/CPAP failure.
Methods
This study is a retrospective observational study including data from the CPAP treatment consecutively offered to patients with COVID-19 admitted at the DepartmentofInfectious Diseases during the period March 31st to July 1st, 2020.
Patients who were diagnosed with COVID-19 were offered face-mask CPAP if they had hypoxemia with the need of oxygen supply >10 liters per minute (l/min) to maintain SpO²≥92%. Contraindications for starting CPAP were pneumothorax, systolic blood pressure (BP) below 90 or diastolic BP below 50, nausea/vomiting, or coma. The treatment was stopped if the patients did not tolerate the CPAP-mask.
CPAP was implemented as standard of care for patients with COVID-19 and hypoxemia, thus the regional ethics committee did not require informed consent from the patients, but only approval from the hospital management. Retrospective access to the medical records of patients was granted by the hospital data protection manager (WZ no. 20,017,637–2020–53).
The CPAP treatment
The patients were closely monitored with noninvasive BP measurement and pulse oximetry during treatment. The staff could monitor vital sign on screens both in the patient room and on the central monitoring screen in the staffroom. The duration of CPAP was basically aimed to be 24 hours a day but with breaks to eat, drink and for oral hygiene. The length of the break and how often it was repeated depended on patient compliance, oxygen demand, and stability of oxygen saturation when the mask was removed.
The CPAP treatment was handled by trained respiratory physiotherapists in close collaboration with the nurses, doctors on the ward and intensivists. The physiotherapists were present in the ward 24/7 (3 shifts a day) and were experienced in treating patients with atelectasis or secretions with intermittent CPAP. To reduce the risk of staff being infected when treating patients with CPAP the staff used Filtering Facepiece (FFP2) masks and visor. An exhalation valve and a virus filter were added to the patient’s non-vented facemask (Figure 1).
Figure 1.
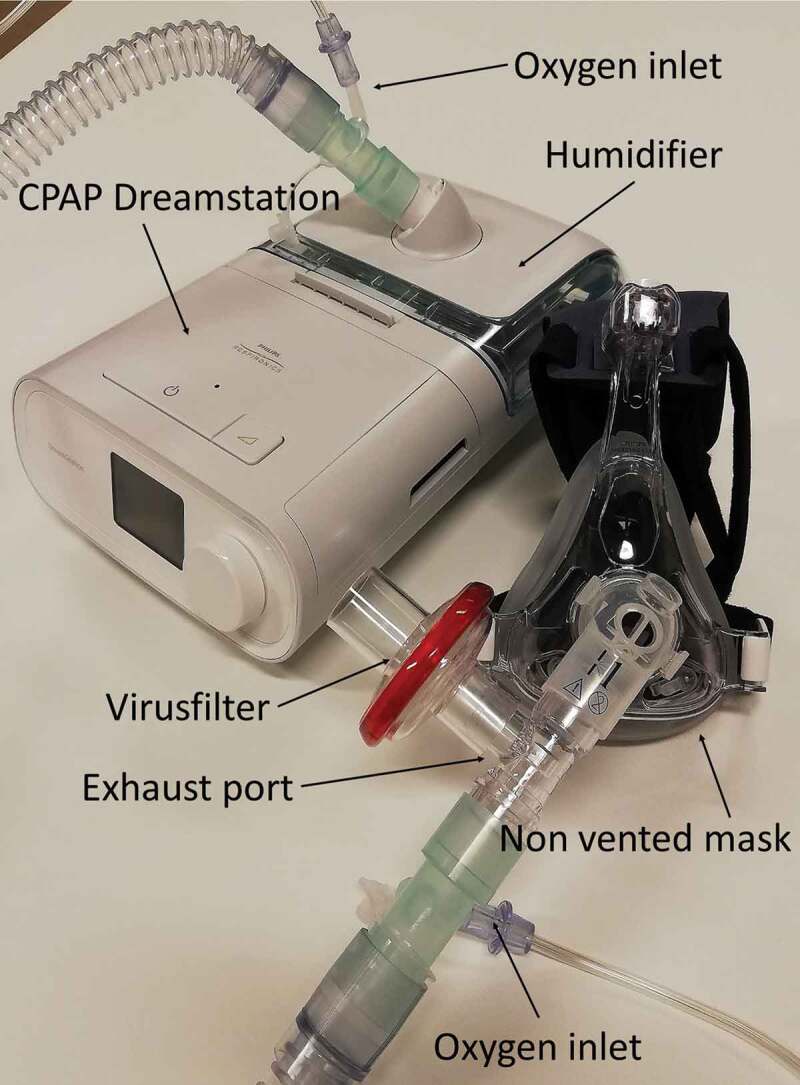
The mask used for CPAP was non vented (no exhalation port on the mask), so that all exhaled air passed through the expiratory port on the tube. A virus filter was fitted to the expiration port to eliminate the spread of virus-aerosol in the room. The exhalation port was placed close to the mask to reduce dead space. With oxygen supplements above 20–25 l/min two oxygen ports were attached. Humidifier was supplied on the dreamstation
Before starting the treatment, respiratory rate (RR), heart rate (HR), oxygen supplement, oxygen saturation (SpO2) and BP were recorded. ADreamstation CPAP Machine®(Philips Respironics, Murrysville, USA) was used to deliver flow and pressure. Oxygen was delivered via an oxygen-port attached to the mask and the oxygen fraction (FiO2) in the mask was estimated through single FiO2- measurement in a lab setting using TSI Certifier FA Plus High Flow Module (TSI Incorporated, Shoreview, US).An initial pressure of 10 cmH2O was chosen and the mask was fitted to the head of the patient. The patient was observed for approximately 20 minutes with focus on RR, SpO2, mask leakage, and compliance. The pressure was adjusted if necessary and vital signs were registered again. The pressure was increased if there was insufficient effect on SpO2. The pressure was decreased(e.g. from 10 to 8 cmH2O) if the RR increased or the patient found the treatment too demanding. However, a slightly increased RR was acceptable if the effect on oxygen demand and SpO2was present. In the beginning of the COVID-19 pandemic the CPAP was not humidified due to the risk of aerosol dispersal, but especially at higher oxygen concentrations, humidification was necessary to reduce dryness in the airways and patient discomfort. The need for and the influence of the CPAP-treatment changed throughout the course of the disease and it was required to evaluate and adjust the treatment continuously. Positioning of the patient was carefully considered and position changes from e.g. Fowler’s position (upright position at 60 degrees in bed with the knees flexed) to a flat lateral position or even prone position was seen to have major immediate positive impact on oxygenation.
Difficulties accepting the mask were handled with breaks, agreements, care or in some cases sedatives like promethazine, morphine or midazolam in small doses. The CPAP treatment was stopped if the medical condition improved with oxygen supplement below 10 l/min and stable SpO2, if the patient was unable to tolerate the mask or in case of CPAP failure.
Data collection
Data from the electronic medical records were collected and registered in Research Electronic Data Capture (REDCap) (REDCap Consortium, Vanderbilt University Medical Centre, Nashville, US). The following data were registered:
Demographics: gender, age, body mass index (BMI), length of hospital stay, comorbidity and mobility level before admission
Ceiling of treatment when received in the ward after agreement between doctors, the patient, and relatives. Ceiling of treatment was registered as either ‘full treatment’, including intensive care, intubation and resuscitation or ‘ceiling treatment in the ward’ meaning do not intubate (DNI) and do not resuscitate (DNR)
Vital signs before initiating CPAP: RR, oxygen supplement, SpO2, HR and BP
Vital signs 10–20 minutes after the initiation ofCPAP
Length of CPAP treatment and CPAP settings
The reason for CPAP termination: treatment failure (death or intubation); Successful treatment (<10 l/min and stable SpO2); lack of compliance
The duration of treatment with CPAP was registered as ‘full treatment’ meaning that the patients had the mask on for 18–24 hours.‘Lack of treatment’ meaning that the patient was not able to tolerate the mask and treatment and therefore received less than 1 hour of treatment. The remaining patients received CPAP between 10 and 18 hours a day.
Statistics
Statistical analyses were conducted using SPSS, version 25 (IBM, New York, US). Data distributions were tested using histograms and Q-Q-plots. Demographic variables were analyzed using non-parametric statistics and reported as median with interquartile range (IQR) or counted numbers with percentage. Paired t-test was used to examine normal distributed data, such as the immediate effect (initial effect) of CPAP and is presented as mean ± SD. P < 0.05 was considered significant.
In order to evaluate the physiological response of the treatment we looked at the initial response on the vital parameters from before starting CPAP and until 20 minutes of CPAP treatment.
Simple and multivariable logistic regression analyses were used to examine prediction of ‘positive initial response’ and ‘CPAP success’.
Positive initial responders of CPAP were defined as: Oxygen reduction AND SpO2 unchanged or better, or oxygen supplement unchanged AND SpO2increased (>2%)
For positive initial response prediction, the following variables were entered in the multivariable logistic regression analysis: age, BMI, initial RR, initial oxygen supplement, gender, pre-COVID mobility level.
CPAP success was defined as: CPAP treatment alone was enough to maintain the patients SpO2 level and lower the oxygen supplement. CPAP failure: When CPAP was inadequate, and patients required intubation or if they switched to palliative care. The following data were entered in a multivariable logistic regression analysis (enter method): age, BMI, initial RR, initial oxygen supplement, gender, and initial response. The patients unable to cooperate with the mask were excluded from this analysis.
Results
Fifty-three patients admitted with COVID-19 developed hypoxemia with oxygen need >10 l/min and were offered CPAP. Characteristics are presented in Table 1. In nine patients, CPAP was discontinued after a couple of unsuccessful trials, due to lack of cooperation with the mask due to discomfort, anxiety or claustrophobia. Patients that survived (n = 22) stayed a median (IQR) of 20 (14–26) days in hospital, whereas in-hospital days for patients who died was 8 [5–12]. CPAP was ceiling of treatment in the ward for 26 (49%) patients of whom 24 died. Figure 2 shows the reason for discontinuing the CPAP treatment in all the included patients and the length of stay for each group.
Table 1.
Characteristics of the patients receiving CPAP, n = 53
| Age, years | 68 (57–78) |
| Gender, male/female | 35/18 |
| Height, cm | 172.5(166.0–180.0) |
| Weight, kg | 83.5 (70.0–102.5) |
| Body Mass Index, kg per m2 | 28.2 (24.6 − 32.6) |
| Independent mobility level before COVID | 39 (74) |
| Comorbidities, no (%) | |
| Heart disease (IHD, HF, AF) | 19 (36) |
| Hypertension | 21 (40) |
| Diabetes | 21 (40) |
| Cancer | 3 (6) |
| COPD | 7 (13) |
| Asthma | 1 (2) |
| Other (Dementia, stroke, alcohol or drug abuse, renal failure, hip fracture, multiple sclerosis) | 15 (27) |
| No comorbidity | 11 (21) |
| Caucasian/other ethnicity | 37 (70)/16 (30) |
| Obese (BMI > 30) | 15 (28) |
Data presented as median with interquartile rate (IQR) or counted number with percentage (%). Abbreviations: IHD: ischemic heart disease; HF: heart failure; AF: atrial fibrillation; COPD: chronic obstructive pulmonary disease
Figure 2.

The figure shows the reason for discontinuing the CPAP in the 53 patients who started the treatment and the median (IQR) time in hospital in days until discharge (LOS) or death
The treatment
The patients able to complete the CPAP treatment (n = 44), spent a median of 3 (2–6) days in CPAP. The median initial pressure was 10.5 (10–12) cmH2O. Eleven patients had difficulties accepting the mask and needed extra support (see description under methods). Fifteen patients received sedatives. Thirty patients received full treatment with CPAP between 18 and 24 hours a day and in 16 cases the patients did not tolerate breaks in the CPAP treatment without heavy desaturation and dyspnea.
Initial response
A positive and significant (p ≤ 0.002) immediate response of CPAP was seen on RR, oxygen flow, and SpO2, while no change was observed in BPor HR (Table 2 and Table 3).
Table 2.
The initial response of CPAP, n = 53
| Respiratory rate | 28.6 ± 7.6 | 26.9 ± 6.2 | −1.7 ± 3.6 | 0.002 |
| Oxygen supplement, l/min | 27.4 ± 13.3 | 23.3 ± 10.7 | −4.1 ± 7.4 | <0.001 |
| SpO2, % | 90.7 ± 3.5 | 92.7 ± 3.2 | 2.0 ± 3.8 | <0.001 |
| Blood pressure, systolic mm Hg | 133.6 ± 19.1 | 129.3 ± 15.2 | −4.3 ± 18.4 | NS |
| Heart rate | 89.5 ± 24.4 | 94.9 ± 18.9 | 5.4 ± 23.0 | NS |
Data are mean ± SD. Vital values collected before starting CPAP and immediately after. The nine patients unable to receive full treatment are included. Abbreviations: SpO2: oxygen saturation measured with pulsoximeter.
Table 3.
The initial response of CPAP excluding the patients unable to cooperate, n = 44
| Respiratory rate | 29.7 ± 7.1 | 27.7 ± 5.7 | −2.0 ± 3.7 | 0.001 |
| Oxygen supplement, l/min | 27.9 ± 13.2 | 23.3 ± 10.5 | −4.5 ± 7.5 | <0.001 |
| SpO2, % | 90.4 ± 3.5 | 93.1 ± 2.5 | 2.8 ± 3.4 | <0.001 |
| Blood pressure, systolic mm Hg | 135.2 ± 21.5 | 129.4 ± 15.2 | −5.8 ± 20.3 | NS |
| Heart rate | 91.2 ± 26.8 | 98.5 ± 19.4 | 7.3 ± 25.9 | NS |
Data are mean ± SD. Vital values collected before starting CPAP and immediately after. The nine patients unable to receive full treatment are excluded. Abbreviations: SpO2: oxygen saturation measured with pulsoximeter.
Prediction of initial response
Thirty-five (66%) patients had an immediate positive response in terms of oxygenation during the CPAP treatment. Gender, age, BMI, initial oxygen supplement, and mobility level were similar in the initial positive response group and in the no initial positive response group. Only a higher RR was significantly associated with a reduced risk of no initial positive response in the multivariable analysis, Odds Ratio = 0.87 (95%CI; 0.78–0.97) (Table 4)
Table 4.
Simple and multivariable logistic regression analysis of factors with potential influence on initial positive response of CPAP
| Crude | Adjuste | |||||
|---|---|---|---|---|---|---|
| Variables | Exp (B) | 95% CI | P-value | Exp (B) | 95% CI | P-value |
| Male | 2.0 | (0.61, 6.54) | 0.3 | 0.95 | (0.2, 4.44) | 0.95 |
| Age | 1.022 | (0.98, 1.07) | 0.3 | 1.05 | (0.98, 1.12) | 0.19 |
| BMI | 1.022 | (0.94, 1.12) | 0.6 | 1.09 | (0.96, 1.24) | 0.16 |
| Initial RR | 0.9 | (0.79, 0.96) | 0.005 | 0.87 | (0.78, 0.97) | 0.01 |
| Initial O2 suppl. | 0.97 | (0.93, 1.02) | 0.194 | 0.98 | (0.92, 1.04) | 0.5 |
| Not independent mobility level | 0.9 | (0.25, 3.24) | 0.87 | 0.9 | (0.16, 5.06) | 0.9 |
No-initial-positive-response as dependent variable. CI: Confidence Interval. RR: Respiratory Rate
Prediction of CPAP success
Forty-four (83%) patients were able to receive treatment with CPAP. Among these, 12 (27%) patients were treated with CPAP until the oxygen supplement was below 10 l/min. Thirty-two (73%) patients were in the CPAPfailure group (13 required intubation, 19 died, Figure 2). Gender, BMI, initial RR and amount of positive initial responders were similar in the CPAP failure group and the CPAP success group. However, the risk ofCPAPfailureincreased significantly with the age of patients, OR = 1.19 (95%CI; 1.03–1.37)) and for those with a higher initial oxygen requirement, OR = 1.26 (95%CI; 1.04–1.52) before starting CPAP treatment (Table 5).
Table 5.
Simple and multivariable logistic regression analysis of factors with potential influence on CPAP failure
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variables | Exp (B) | 95% CI | P-value | Exp (B) | 95% CI | P-value |
| Male | 1.36 | (0.30, 6.14) | 0.7 | 1.31 | (0.04, 39.65) | 0.9 |
| Age | 1.08 | (1.02, 1.14) | 0.01 | 1.19 | (1.03, 1.37) | 0.01 |
| BMI | 0.99 | (0.89, 1.09) | 0.8 | 1.21 | (0.97, 1.5) | 0.08 |
| Initial RR | 1.03 | (0.94, 1.13) | 0.5 | 1.06 | (0.9, 1.24) | 0.5 |
| Initial O2 suppl. | 1.17 | (1.05, 1.30) | 0.003 | 1.26 | (1.04, 1.52) | 0.01 |
CPAP failure as dependent variable. CI: Confidence Interval. RR: Respiratory Rate
The average FiO2 in the CPAP mask, of the 44 patients at the beginning of the treatment was 75 ± 18% and the PaO2/FiO2 ratio was 101 ± 36 mmHg. The initial PaO2/FiO2 ratio was 105 ± 33.4 mmHg in the group without limitations in treatment (n = 24) and 97 ± 39.9 mmHg in the group with ceiling of treatment in the ward (n = 20). Over the course of the disease the FiO2fluctuated. Overall, the PaO2/FiO2increased significantly (p = 0.02) for the success group and decreased for the CPAPfailure group (p < 0.001) (Figure 3).
Figure 3.

The average PaO2/FiO2 in mmHg at the start of the CPAP treatment, at the middle and at the end of the treatment for the patients in the CPAP success (n = 12) group and the CPAP failure group (n = 32). PaO2 is estimated from SpO2. PaO2/FiO2 < 200 is considered low
Other observations
CPAP was given without using negative pressure rooms, but no increased COVID-19 infection rate was observed among staff in the COVID-19 ward, compared to the staff in the rest of the hospital.
Discussion
We aimed to examine the physiological response to CPAP treatment in a cohort of patients admitted with COVID-19 and severe hypoxemic respiratory failure and to evaluate prognostic factors regarding initial response and outcome. CPAP seems to have a positive effect on oxygenation and respiratory rate in most patients. We were not able to predict a positive response to CPAP treatment, but a high respiratory rate when commencing CPAP seems to be a factor leading to a positive response. Equally, predicting CPAP success without the need for intubation seems very difficult, but factors like age and high oxygen supplementation before starting CPAP is in the present study associated with a poorer outcome, including death.
Correspondingly, age and the severity of the disease in general are associated with high mortality in patients with COVID-19 [5]. The low PaO2/FiO2 ratio of 101.4 ± 36.3 indicates that patients receiving CPAP in the present study were severely affected by the COVID-19 disease. The low ratio combined with many patients with a ceiling of treatment in the ward (considered unable to profit of intubation or with pre-defined decision of no resuscitation) suggests poor odds for survival and, in fact, 31 (58%) of the patients in the present study died.
Prediction of initial response
By initiating CPAP, the respiratory rate decreased, especially in the group of patients able to cooperate. A high respiratory rate was also the only predictor of a positive response to CPAP. Possibly, CPAP decreases the work of breathing by recruiting closed alveoli, increasing FRC, and increasing the ventilation of the lungs [4], which could be the explanation for its immediate effect on patients with a high respiratory rate. In the beginning of the epidemic, high CPAP pressure from 12 to 15 cm H2O was chosen in accordance with the recommendation from Italian Thoracic Society [2]and the aim was to achieve an effect on SpO2. Based on our experiences from clinical practice and the results of the present and recent studies [6–8]our treatment is now more customized with focus on the lowest possible pressure (6.5–12 cmH2O). After recruiting alveoli at a pressure of 10–12 cmH2O, the pressure can often be reduced after 20–30 minutes to 6.5 or 8 cmH2O.A low pressure will prevent risk of adding load to the respiratory system but can still have effect on oxygenation (SpO2 or oxygen supplement) and possibly also on the respiratory rate.
Several countries have treated patients with CPAP during the COVID-19 pandemic and research is already being shared [6–15].None of the studies were prospective studies with a control group. In general, the studies have shown that the treatment with CPAP was well tolerated with few patients dropping out. Still, the intubation rate was high between 42% and 57% [7,8,10,12] among those without a ceiling of treatment in the ward and comparable to our study. Only one study had 83% recovering from CPAP treatment alone [11]. In our study, there was a marked difference in success rate with CPAP according to treatment limitations, as 46% of the patients without limitation in treatment avoided intubation and death, and thus were considered successful, whereas only 5% of CPAP treatments were successful for patients with a ceiling of treatment in the ward. When comparing success rates, the severity of the disease, and especially the severity of the hypoxemic failure, must be considered. Our study, with an average PaO2/FiO2 ratio of 101 mmHg, had the lowest ratio compared to other studies reporting this outcome, where average PaO2/FiO2 was in the range 119 to 248 mmHg [6,11,12].
The rationale for offering CPAP lies in a physiological response which could lead into long term improvements and the first step for an evidence in the CPAP treatment is to certify this physiological response. Four studies, including the present, report a significant improvement in the oxygenation by application of CPAP regardless of CPAP success or failure [6,10,13].
Our experience is that careful titration of the pressure reduces the patient’s respiratory work and increases oxygenation. Our study design does not allow us to conclude whether CPAP prevented death or intubation. However, it seems that, without CPAP, more patients would have required intubation sooner. Whether this delay in intubation bought time and therefore reduced time on mechanical ventilators is uncertain. It is clearly difficult to find the optimal time for intubation and no unique markers can be used. Considerations include e.g. elevation of PaCO2, but it is rarely seen in patients with COVID-19. Elevated lactate levels may indicate insufficient tissue oxygenation. Increasing respiratory rate and superficial respiration may indicate fatigue. Lack of effect of CPAP on SpO2 and oxygen supplement may indicate that the functional residual capacity is sufficiently high and cannot be further optimized. When CPAP is used in a medical ward in patients with severe hypoxemic respiratory failure, it is therefore necessary to work very closely together with the intensive care unit so that the intensive care physicians monitor the patients closely and assess the optimal time for intubation. The argument for early intubation is, among other things that spontaneous respiration with extensive respiratory work leads to increased transpulmonary pressure with a risk of patient self-induced lung injury [16,17]. The argument for late intubation is that long-term mechanical ventilation carries the risk of infection and ventilator-induced lung injury and is generally associated with high morbidity and mortality due to immobilization and sedation [18,19].
The treatment with CPAP in a medical ward in a group of patients so severely affected by respiratory insufficiency must be seen in the light of the rare situation where ICU beds are scarce, and an unusual high number of critically ill patients exists. A requirement for this to succeed is close monitoring of the patients and a multidisciplinary teamwork, and positively, CPAP is possible outside an ICU setting, with acceptable outcome, as this study and others have shown [20].
Our study has limitations. First, lack of a control group in a retrospective design makes it difficult to make a firm conclusion on the role of CPAP. Second, data on the exact number of hours per day in CPAP and the pressure levels could have contributed to a greater knowledge of the treatment. Third, in some patients, treatment in general, were a desperate attempt to rescue an elderly patient with comorbidities already in respiratory distress and therefore not fit for inclusion in a study – yet again a part of this real-life study. During the ‘second wave’ of COVID-19, our hospital was better prepared, armed with knowledge and equipment regarding CPAP, therefore the treatment could be started much earlier. CPAP has been offered at the medical ward as an essential treatment for patients with hypoxemia and there are clear effects on respiratory rate and oxygenation and, compared to the ‘first wave’, with fewer patients requiring intubation.
Positively, no increased COVID-19 infection rate was observed among staff in the COVID-19 ward, compared to the staff in the rest of the hospital, despite that CPAP was given without using negative pressure rooms.
In conclusion, the present study demonstrates that CPAP to patients with COVID-19 and severe respiratory failure reduces respiratory work and optimizes oxygenation, the latter to an extent where only mechanical ventilation is an alternative for acceptable oxygenation to be maintained. Treatment with CPAP can in some cases delay or avoid intubation, however, the prognosis for especially elderly patients with high oxygen requirement and with a ceiling of treatment in the ward is poor.
Biographies
Linette Marie Kofod is a respiratory physiotherapist at Copenhagen University Hospital - Hvidovre, Denmark and a PhD- student at Örebro University, Sweden.
Klaus Nielsen Jeschke is a pulmonologist and Senior Consultant at the Department of Pulmonology, Copenhagen University Hospital – Hvidovre, Denmark. He specialises in NIV and is also the first author of the Danish COVID guideline.
Morten Tange Kristensen is physiotherapist, PhD at Copenhagen University Hospital - Hvidovre and a Professor at Department of Clinical Medicine, University of Copenhagen, Denmark.
Rikke Krogh-Madsen is a MD, PhD at the Department for Infectious diseases, Copenhagen University Hospital – Hvidovre, Denmark.
Carsten Monefeldt Albek is anesthesiologist and Senior Consultant at Copenhagen University Hospital - Hvidovre and an Associate Professor at University of Copenhagen, Denmark.
Ejvind Frausing Hansen is pulmonologist and Senior Consultant at the Department of Pulmonology, Copenhagen University Hospital – Hvidovre, Denmark. He specialises in treatment of Respiratory Failure, which is also his field of research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- ([1]).World Health Organization. (2020) . Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331446.
- [2].Harari S, Vitacca M, Blasi F, et al. Managing the respiratory care of patients with COVID-19: Italian recommendations. Italian Thoracic Society and Italian Respiratory Society. Version - 2020. March 08. Available at http://www.aiponet.it and http://www.siprirs.it. [Google Scholar]
- [3].NHS England . Guidance for the role and use of non-invasive respiratory support in adult patients with coronavirus (confirmed or suspected). Version. 2020. April;3:6. [Google Scholar]
- [4].Kofod LM, Jeschke KN, Krogh-Madsen R, et al. CPAP for patients with COVID-19. Ugeskr Laeger. 2020. August 10;182(33): V05200358. [PubMed] [Google Scholar]
- [5].Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. 2020. August 13;56(2):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020. April 28;323(16):1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Aliberti S, Radovanovic D, Billi F, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. 2020. October 15;56(4):2001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alviset S, Riller Q, Aboab J, et al. Continuous Positive Airway Pressure (CPAP) face-mask ventilation is an easy and cheap option to manage a massive influx of patients presenting acute respiratory failure during the SARS-CoV-2 outbreak: a retrospective cohort study. PLoS One. 2020. 14; 15(10): Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nightingale R, Nwosu N, Kutubudin F, et al. Is continuous positive airway pressure (CPAP) a new standard of care for type 1 respiratory failure in COVID-19 patients? A retrospective observational study of a dedicated COVID-19 CPAP service. BMJ Open Respir Res. 2020. July;7(1):e000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ashish A, Unsworth A, Martindale J, et al. CPAP management of COVID-19 respiratory failure: a first quantitative analysis from an inpatient service evaluation. BMJ Open Respir Res. 2020. November;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arina P, Baso B, Moro V, et al. UCL Critical Care COVID-19 Research Group. Discriminating between CPAP success and failure in COVID-19 patients with severe respiratory failure. Intensive Care Med 2021 Feb;47(2):237-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brusasco C, Corradi F, Di Domenico A, et al. Continuous positive airway pressure in COVID-19 patients with moderate-to-severe respiratory failure. Eur Respir J 2021; 57: 2002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Di Domenico SL, Coen D, Bergamaschi M, et al. Clinical characteristics and respiratory support of 310 COVID-19 patients, diagnosed at the emergency room: a single-center retrospective study. Intern Emerg Med. 2020. November 11;1–9. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Noeman-Ahmed Y, Gokaraju S, Powrie DJ, et al. Predictors of CPAP outcome in hospitalized COVID-19 patients. Respirology. 2020. December;25(12):1316–1319. [DOI] [PubMed] [Google Scholar]
- [15].Duca A, Memaj I, Zanardi F, et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the emergency department during Italian novel coronavirus SARS-CoV2 outbreak: preliminary data on the role of helmet CPAP and non-invasive positive pressure ventilation. EClinicalMedicine. 2020;18:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020. May;46(5):854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marini JJ, Gattinoni L.. Management of COVID-19 respiratory distress. JAMA. 2020. April;323(22):24. [DOI] [PubMed] [Google Scholar]
- [18].Gattinoni L, Quintel M, Marini JJ. Volutrauma and atelectrauma: which is worse? Crit Care. 2018. October 25;22(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020. April 14;46(6):1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020. November;56(5):5. [DOI] [PMC free article] [PubMed] [Google Scholar]


