ABSTRACT
Spontaneous Coronary Artery Dissection (SCAD) is one of the nonatherosclerotic causes of Acute Coronary Syndrome. It’s extremely rare for SCAD to present in an asymptomatic male, with incidental finding of Left Ventricular (LV) thrombus on echocardiogram. This report presents the case of a 36-year-old male with such an atypical presentation of Spontaneous Coronary Artery Dissection with Left Ventricular apical thrombus as a complication. The patient received successful medical management, with excellent clinical outcomes. This case highlights the importance of an early recognition and treatment strategy for both conditions using medical therapy.
KEYWORDS: Spontaneous coronary artery dissection, left ventricular thrombus, myocardial infarction
1. Introduction
Spontaneous coronary artery dissection (SCAD) is a nonatherosclerotic cause of acute coronary syndrome. It is extremely rare for SCAD to present in asymptomatic male with incidental finding of left ventricle (LV) thrombus on echocardiogram. We present a case of spontaneous coronary artery dissection complicated with apical thrombus. The patient received medical management with excellent clinical outcomes.
2. History of presentation
This is a 36-year-old male with a past medical history of schizophrenia who was brought by the police into the emergency room after an acute episode of psychosis. The patient was very agitated and presented auditory hallucinations. He denied chest pain, shortness of breath, palpitations, additional symptoms, or drug use. His vital signs were within normal limits except for a heart rate of 105/minutes. His physical exam was remarkable only for mild distress and agitation. The rest of the exam was normal. During his stay in the emergency room, the patient underwent blood work which was remarkable for elevated creatine kinase levels > 4500 u/l (Normal 38–174 U/L) and an EKG which showed an inverted T-wave in inferior and anterolateral leads (Image 1). Patient had a peak troponin of 0.38 ng/ml (normal< 0.5 ng/ml). The echocardiogram showed a left ventricular ejection fraction of 50 to 55%, akinesis of the apex, and well-circumscribed 2.2 × 2.5 cm echogenic mass attached to the endocardial border of the apex that could represent thrombus versus myxoma (Images 2, 3). He was started on a therapeutic dose of enoxaparin and in order to rule out any ischemic etiology causing wall motion abnormality, the patient underwent cardiac catheterization which showed distal left anterior descending(LAD) artery dissection with thrombus versus intramural hematoma (Image 4). The patient was continued on therapeutic enoxaparin and bridged to warfarin, aspirin, high-intensity statin, and beta-blocker as well as antipsychotic medications. The patient continued asymptomatic and mentally stable. He was discharged on warfarin, aspirin, beta-blocker, and high-intensity statin.
3. Discussion
Spontaneous coronary artery dissection (SCAD) is defined as a tear in the coronary arterial wall that is not related to trauma or medical instrumentation. The dissection causes blood entry in the vascular wall with the consequent formation of the false lumen and intramural hematoma (IMH) blocking blood flow to the heart. In the general population, although the true incidence of SCAD is still uncertain, in modern series it has been reported to represent 1.1%–4% of all acute coronary syndrome (ACS) cases [1] and has been identified as the cause of 15%–35% of ACS in women under 50 years [2].
The spectrum of clinical presentation can range from chest pain symptoms alone to ST-segment–elevation myocardial infarction (STEMI), ventricular fibrillation, and sudden death [3,4]. The most common association with SCAD are fibromuscular dysplasia (FMD), pregnancy, emotional stressors, and physical activities but the etiology and pathogenesis of SCAD are still uncertain. The coronary distribution of SCAD has been well described. Although any artery can be affected, the left anterior descending artery is the most commonly affected (32%–46% of cases) [5]. LV thrombus is a complication usually seen six to ten days after myocardial infarction (MI), especially after LAD MI associated with anterior wall and apical motion abnormalities, this can occur even in the absence of aneurysm [6].
The first-line management of SCAD consists of conservative medical therapy. This is based on evidence that angiographic healing occurs in the majority of cases and reaching up to 100% if the control angiography is performed 26 days after SCAD onset [7,8]. Medical management of SCAD includes antiplatelets and beta-blockers, The role of anticoagulation for SCAD is controversial with the risk of dissection extension balanced by the potential benefit of resolution of overlying thrombus and improvement in the true lumen patency [7]. Once SCAD is proven on angiography the anticoagulation is usually avoided to prevent the extension of IMH, however, in this case with the presence of LV thrombus which presents a high risk for embolization and stroke, the use of anticoagulation is warranted. Joo and colleagues reported a case of SCAD with LV thrombus managed medically with antiplatelet and anticoagulation, the course was successful with coronary artery wall healing and a complete resolution with repeat angiography at 4 months consistent with current evidence [9].
American College of Cardiology (ACC) recommends conservative management in clinically stable patients without high-risk anatomy. Extended inpatient monitoring for 3–5 days is also recommended. In patients with ongoing ischemia, left main artery dissection, or hemodynamic instability, urgent intervention with a percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) can be considered [10].
This case highlights the successful clinical course of SCAD using medical therapy with antiplatelet and anticoagulation correlating with current recommendations. Early treatment of LV thrombus with anticoagulation is reasonable to decrease the risk of embolization including stroke.
In the setting of SCAD, historically PCI is associated with low success and CABG retains high success, but CABG is usually performed as a salvaged procedure in case of PCI failure. A previous retrospective analysis in patients presenting with SCAD had a 53% technical failure of PCI [8]; however, most patients in these studies presented with non-STEMI. A recent analysis between 2003 and 2017 identified patients with STEMI due to atherosclerosis versus SCAD. This study demonstrates that a traditional primary PCI strategy for STEMI-SCAD is effective in most patients, with technical success only modestly lower than STEMI due to atherosclerosis. Successful revascularization and final TIMI flow grade 3 was achieved in 91% of PCI treated STEMI-SCAD versus 98% of STEMI-atherosclerosis [11].
The decision for revascularization is considered by angiographic and clinical aspects: in case of persistent ST elevation or ischemia, and hemodynamic instability, also an invasive approach is recommended particularly when left main or the proximal tracts of the major coronary arteries are involved [8].
There is limited data regarding follow up after SCAD however Gad and colleagues reported the incidence and cause of 30 day readmission following hospitalization of acute myocardial infarction (AMI) secondary to SCAD versus non-SCAD. The incidence of 30-day readmission following AMI and SCAD is real and occurs early post-discharge. Most readmissions are due to cardiac causes, especially recurrence of AMI. Among the SCAD readmissions, 50.6% of patients were readmitted in the first-week post-discharge, with 54.5% of AMI readmissions occurring in the first 2 days post-discharge. Unfortunately, there is no specific data reported about the incidence of LV thrombus after SCAD-MI [12].
In summary, SCAD remains a medical condition with limited evidence available to further guide medical decision; however, significant efforts are being made to improve awareness and comprehension of the prevalence, causes, prognosis, recurrence rate, and optimal management of SCAD; in part, due to many works published by Mayo Clinic SCAD registry with accessible and reliable sources of information available to patients and families. In the long term, much is needed to be learned. image 1image 2image 3image 4
Image 1.
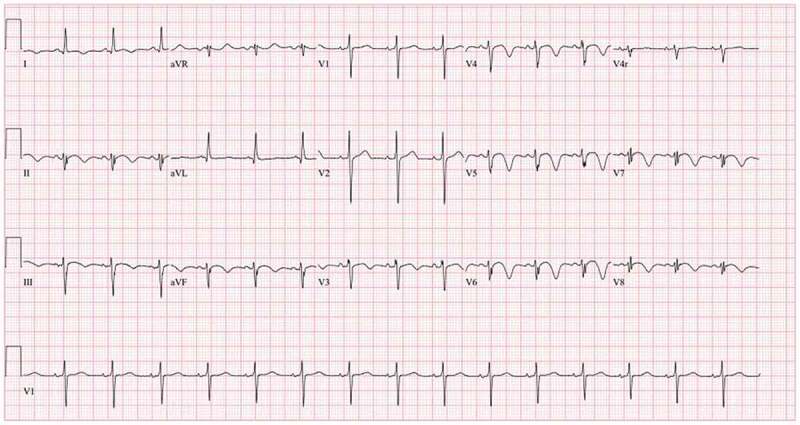
EKG: T wave inversion in anterior-lateral and inferior leads
Image 2.
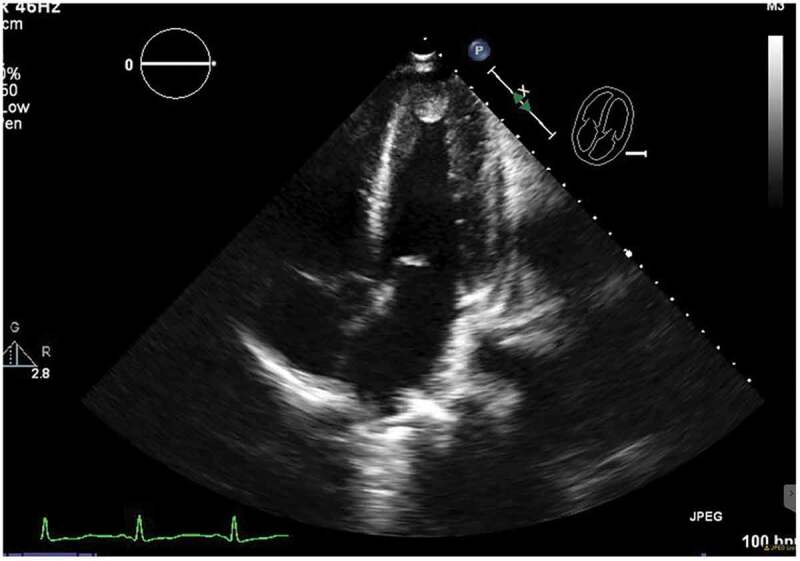
ECHO:Non contrast apical four chamber view showing echo density in the left ventricular apex which represents thrombus vs mass
Image 3.
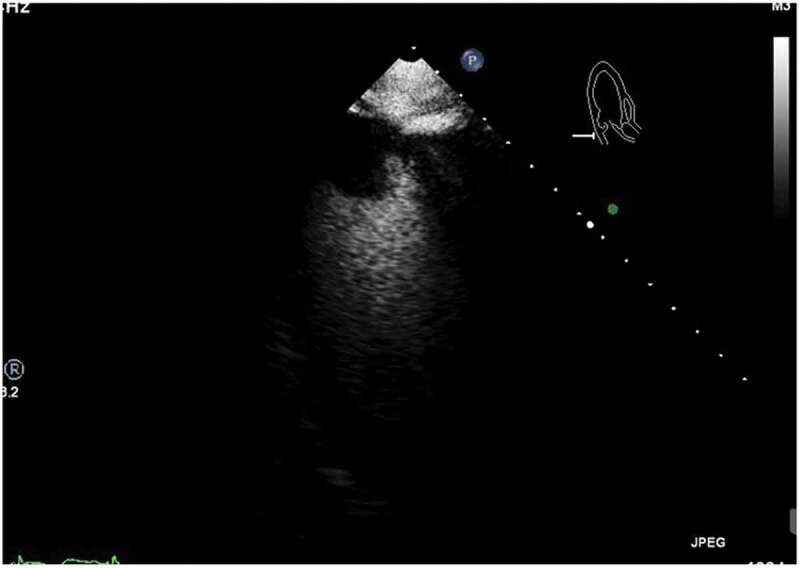
ECHO: Image obtained after administration of contrast agent, showing spherical filling defect noted in apex, consistent with apical thrombus
Image 4.
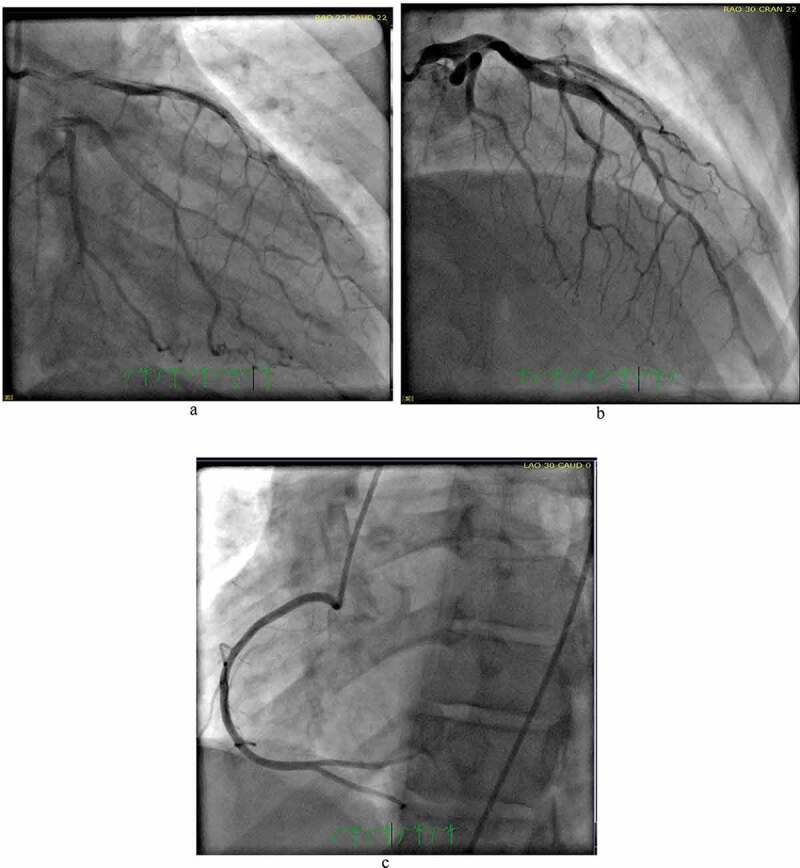
Cath: Coronary angiogram showing arterial wall contrast staining with multiple radiolucent lumen in mid to distal left anterior descending artery consistent with type 1 spontaneous coronary artery dissection (SCAD) . Normal right coronary artery
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Rashid HNZ, Wong DTL, Wijesekera H, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome — a single-centre Australian experience. Int J Cardiol. 2016;202:336–338. [DOI] [PubMed] [Google Scholar]
- [2].Motreff P, Malcles G, Combaret N, et al. How and when to suspect spontaneous coronary artery dissection: novel insights from a single-centre series on prevalence and angiographic appearance. EuroIntervention. 2017;12:e2236–e2243. [DOI] [PubMed] [Google Scholar]
- [3].Tweet MS, Hayes SN, Pitta SR.. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- [4].Guragai N 1, Patel H 2, Patel K 2, et al. Spontaneous coronary dissection of left anterior descending artery complicated by retrograde extension of dissection and involvement of left main artery: successful management with percutaneous coronary intervention. Minerva Cardioangiol. 2017. August;65(4):444–446. [DOI] [PubMed] [Google Scholar]
- [5].Hayes S, Kim ESH, Saw J, et al. Spontaneous coronary artery dissection: current state of the science A scientific statement from the American heart association. Circulation. 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carpenter K, Adams D.. Adams DApical mural thrombus: technical pitfalls. Heart. 1998;80:S6–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saw J. Coronary angiogram classification of spontaneous coronary artery dissection.Catheter. Cardiovasc Interv. 2014;84:1115–1122. [DOI] [PubMed] [Google Scholar]
- [8].Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–786. [DOI] [PubMed] [Google Scholar]
- [9].Joo JH, Caldera AE, Divakaran VG. Spontaneous coronary artery dissection with left ventricular thrombus. Proc (Bayl Univ Med Cent). 2018;32(1):99–100. Published 2018 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American heart association. Circulation. 2018. February 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lobo A, Cantu SM, Sharkey SW, et al. Revascularization in patients with spontaneous coronary artery dissection and ST-segment elevation myocardial infarction. September 10, 2019:. Journal of the American College of Cardiology. 2019;74(10):1290–1300. [DOI] [PubMed] [Google Scholar]
- [12].Gad MM, Mahmoud AN, Saad AM, et al. Incidence, clinical presentation, and causes of 30-day readmission following hospitalization with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2020. April 27;13(8):921–932.PMID: 32327089. [DOI] [PubMed] [Google Scholar]


