Abstract
Background:
Patients with rheumatic aortic stenosis (AS) were excluded from transcatheter aortic valve replacement (TAVR) trials.
Objectives:
We sought to examine outcomes with TAVR versus surgical AVR (SAVR) in patients with rheumatic AS, and versus TAVR in non-rheumatic AS.
Methods:
We identified Medicare beneficiaries who underwent TAVR or SAVR from October 2015 to December 2017. We then identified patients with rheumatic AS utilizing prior validated ICD-10 codes. Overlap propensity score weighting analysis was utilized to adjust for measured confounders. The primary study outcome was all-cause mortality. Multiple secondary outcomes were also examined.
Results:
The final study cohort included 1159 patients with rheumatic AS who underwent AVR (SAVR n=554 and TAVR n=605), and 88,554 patients with non-rheumatic AS who underwent TAVR. Patients in the SAVR group were younger and with lower prevalence of most comorbidities and frailty scores. After median follow up of 19 months (IQR 13–26), there was no difference in all-cause mortality with TAVR versus SAVR (11.2 versus 7.0 per 100 person-year, aHR 1.53, 95% CI 0.84–2.79, P =0.2). Compared with TAVR in non-rheumatic AS, TAVR for rheumatic AS was associated with similar mortality (15.2 versus 17.7 deaths per 100 person-years (aHR 0.87, 95% CI 0.68–1.09, P=0.2) after median follow up of 17 months (IQR 11–24). None of the rheumatic TAVR patients, <11 SAVR patients, and 242 non-rheumatic TAVR patients underwent repeat AVR (124 redo-TAVR and 118 SAVR) at follow up.
Conclusion:
Compared with SAVR, TAVR could represent a viable and possibly durable option for patients with rheumatic AS. \
Keywords: Rheumatic aortic stenosis, Transcatheter aortic valve replacement, Surgical aortic valve replacement
Condensed abstract
We identified 1159 patients with rheumatic AS who underwent AVR (SAVR n=554 and TAVR n=605), and 88,554 patients with non-rheumatic AS who underwent TAVR from Medicare database. After adjustment using overlap propensity score weighting analysis, there was no difference in all-cause mortality with TAVR versus SAVR in rheumatic AS after median follow up of 19 months (IQR 13–26). Compared with TAVR in non-rheumatic AS, TAVR for rheumatic AS was associated with similar mortality after median follow up of 17 months (IQR 11–24). None of the rheumatic TAVR patients underwent repeat AVR at follow up.
Introduction
Transcatheter aortic valve replacement (TAVR) has become established as an excellent alternative to surgical aortic valve replacement (SAVR) for patients with severe calcific aortic stenosis (AS) and appropriate anatomy at all levels of the surgical risk spectrum (1–4). Patients with a rheumatic etiology for their AS were excluded from the pivotal randomized controlled trials (1,2). Further, due to the low prevalence of rheumatic AS in developed countries, our knowledge about the role of TAVR in those patients is limited to case reports or series.(5–7)
Aortic valves with rheumatic disease usually exhibit significant fibrosis, with calcification only occurring late in the degenerative process. The anatomical differences compared with degenerative AS may have a technical impact on the transcatheter heart valve deployment and anchoring. Although rheumatic heart disease (RHD) prevalence is low in the western world, it represents a significant burden in developing countries, with more than one million deaths per year.(8) TAVR offers a less invasive intervention in these patients, especially in light of the global unmet needs in cardiac surgery in the developing world (9).
To address the gap in knowledge in regard to the safety and efficacy of TAVR in patients with rheumatic AS, the current study sought to examine outcomes with TAVR versus SAVR in patients with rheumatic AS, and with TAVR in patients with rheumatic versus non-rheumatic AS, using a nationwide patient database.
Methods
Study cohort
We identified Medicare beneficiaries who underwent TAVR or SAVR from October 2015 through December 2017 using the 100% Medicare Provider and Analysis Review (MEDPAR) Part A files from the Center for Medicare and Medicaid Services (CMS), using ICD-10 procedure codes (02RF37Z, 02RF38Z, 02RF3JZ, 02RF3KZ, 02RF37H, 02RF38H, 02RF3JH, 02RF3KH, or X2RF332). We then identified patients who had a diagnosis of rheumatic AS using ICD-10 codes I06.0 and I06.2. These codes were validated in prior studies and showed positive predictive values >85%.(10) The study cohort was divided into three groups, patients with rheumatic AS who underwent SAVR, patients with rheumatic AS who underwent TAVR, and patients with non-rheumatic AS who underwent TAVR. We excluded patients who had concomitant mitral valve surgery in the same admission as SAVR. Important comorbidities were identified with algorithms defined by Elixhauser et al (11), using ICD-9 and ICD-10 diagnoses codes on inpatient claims during one year period prior to and including the index TAVR admission. We calculated a frailty score that was previously validated in TAVR patients using administrative claims data, using 105 clinical variables associated with frailty (such as falls, dementia, hemiplegia, etc.) (Supplementary Table 1).(12) This score classifies a patient into one of three categories; low (<5), intermediate (5–15), and high frailty (>15). The Institutional Review Board of the University of Iowa approved this study with waiver for individual informed consent.
Study outcomes
The primary study outcome was mortality at the longest follow-up available. Secondary outcomes included in-hospital mortality, acute kidney injury (AKI), blood transfusion, new-onset atrial fibrillation (AF), aortic annulus rupture, new permanent pacemaker (PPM) placement, conversion to open surgery and cardiogenic shock. All-cause mortality and ischemic stroke at 30-days, inpatient admission with heart failure, and need for repeat valve replacement at longest follow-up available were also examined. When assessing in-hospital outcomes, to ensure that an outcome was a new event and not a historic diagnosis, we utilized the “Present on Admission” indicator in the inpatient admission claims. Data on mortality were available through August 2018, while data on admissions for ischemic stroke was available through December 2017. Patients were censored at disenrollment from Medicare, if they experienced an event, or at end of the study follow-up period.
Statistical analysis
Continuous variables are reported as mean and standard deviation and compared using ANOVA, or median and interquartile range and compared using Mann-Whitney U test if not normally distributed. Categorical variables are reported as frequencies and were compared using Chi-square or Fisher-Exact test as appropriate. We utilized overlap propensity score (PS) weighting analysis to adjust for measured confounders between different groups in each comparison (TAVR versus SAVR and TAVR in rheumatic versus non-rheumatic AS).(13,14) First, a non-parsimonious multivariable logistic regression model of 38 independent variables, including frailty score, was performed, with the dependent variable being receipt of TAVR in the first comparison (Table 1), or having a rheumatic AS in the second comparison (Table 1), to calculate the propensity for each patient to be in the dependent variable group. Then, patients’ weights were derived from the overlap PS weighting methods, in which each patient’s weight is the probability of that patient being assigned to the opposite treatment group. The balance between groups after overlap weighting adjustment was demonstrated by reporting the weighted covariate means (or proportions) for the two groups being compared. Subsequently, a Cox proportional hazards regression model was constructed to assess the risk of the primary outcome, using patients’ weights to adjust for age, sex, and comorbidities. We performed a sensitivity analysis by performing propensity score matching analysis between patients with rheumatic AS who underwent TAVR versus SAVR and between patients with and without rheumatic AS who underwent TAVR. The validity of the proportional hazards assumption was evaluated by inspecting the plot of Schoenfeld residuals against time with Locally Weighted Scatterplot Smoothing (LOESS) curve and by log (-log (survival) plot against log (time). For the secondary outcomes, we measured the explained variation and predictive accuracy of the Cox model to assess the competing risk of death.(15) We performed a competing risk regression analysis using the Fine Gray proportional subhazards model and sub distribution hazard ratios (sHR) were calculated with 95% CI.(16) Weighted KM curves for events were generated and compared with log-rank or generalized Wilcoxon statistic. A P value of 0.05 was the cutoff for statistical significance. The analysis was done with SAS version 9.4 (SAS Institute, North Carolina) and R 3.4.3 (R Foundation, Austria).
Table 1:
Baseline characteristics of rheumatic AS patients who underwent SAVR versus TAVR, before and after PS weighting adjustment
| Variable | Before adjustment | After adjustment | Standardized differences | |||
|---|---|---|---|---|---|---|
| SAVR (N=554) | TAVR (N=605) | P value | SAVR (N=554) | TAVR (N=605) | ||
| Age | 73.4±7.2 | 79.4±8.1 | <0.001 | 76.3 (7.8) | 76.3 (6.7) | 0.0 |
| Race | 0.0 | |||||
| White | 90.6% | 90.4% | 0.4 | 0.91 | 0.91 | |
| Black | 4.5% | 4.3% | 0.43 | 0.43 | ||
| Male sex | 56.0% | 40.0% | <0.001 | 0.47 (0.50) | 0.47 (0.50) | 0.0 |
| Hypertension | 88.1% | 93.7% | <0.001 | 0.92 (0.28) | 0.92 (0.28) | 0.0 |
| Diabetes mellitus | 35.4% | 44.8% | 0.001 | 0.40 (0.49) | 0.40 (0.49) | 0.0 |
| Heart failure | 40.3% | 82.6% | <0.001 | 0.65 (0.48) | 0.65 (0.48) | 0.0 |
| Lung disease | 26.7% | 44.0% | <0.001 | 0.35 (0.48) | 0.35 (0.48) | 0.0 |
| Liver disease | 3.4% | 4.1% | 0.5 | 0.04 (0.20) | 0.04 (0.20) | 0.0 |
| Chronic kidney disease | 13.4% | 29.6% | <0.001 | 0.20 (0.40) | 0.20 (0.40) | 0.0 |
| End stage renal disease | -- | 4.6% | <0.001 | 0.03 (0.16) | 0.03 (0.16) | 0.0 |
| Peripheral arterial disease | 21.8% | 32.9% | <0.001 | 0.28 (0.45) | 0.28 (0.45) | 0.0 |
| Prior ischemic stroke | 6.0% | 11.8% | <0.001 | 0.07 (0.26) | 0.07 (0.26) | 0.0 |
| Coronary artery disease | 22.6% | 56.4% | <0.001 | 0.36 (0.48) | 0.36 (0.48) | 0.0 |
| Prior revascularization | 4.7% | 21.0% | <0.001 | 0.11 (0.31) | 0.11 (0.31) | 0.0 |
| Preexisting atrial fibrillation | 26.0% | 53.0% | <0.001 | 0.41 (0.49) | 0.41 (0.49) | 0.0 |
| Depression | 10.5% | 19.5% | <0.001 | 0.12 (0.32) | 0.12 (0.32) | 0.0 |
| Hypothyroidism | 21.3% | 28.9% | 0.003 | 0.26 (0.44) | 0.26 (0.44) | 0.0 |
| Drug abuse | -- | -- | 0.7 | 0.01 (0.11) | 0.01 (0.11) | 0.0 |
| Anemia | 18.6% | 50.9% | <0.001 | 0.31 (0.46) | 0.31 (0.46) | 0.0 |
| Connective tissue disease | 6.5% | 8.8% | 0.2 | 0.08 (0.27) | 0.08 (0.27) | 0.0 |
| Alcohol abuse | 2.7% | 3.5% | 0.5 | 0.03 (0.18) | 0.03 (0.18) | 0.0 |
| Lymphoma | -- | 2.3% | 0.02 | 0.01 (0.09) | 0.01 (0.09) | 0.0 |
| Electrolytes abnormality | 45.1% | 57.9% | <0.001 | 0.48 (0.50) | 0.48 (0.50) | 0.0 |
| Obesity | 30.1% | 27.1% | 0.2 | 0.28 (0.45) | 0.28 (0.45) | 0.0 |
| Psychosis | 2.9% | 3.8% | 0.4 | 0.03 (0.17) | 0.03 (0.17) | 0.0 |
| Pulmonary hypertension | 2.5% | 11.2% | <0.001 | 0.06 (0.23) | 0.06 (0.23) | 0.0 |
| Tumor without metastasis | 2.2% | 5.1% | 0.008 | 0.03 (0.18) | 0.03 (0.18) | 0.0 |
| Metastatic disease | -- | 2.0% | 0.01 | 0.01 (0.09) | 0.01 (0.09) | 0.0 |
| Peptic ulcer disease | -- | 2.6% | 0.2 | 0.02 (0.13) | 0.02 (0.13) | 0.0 |
| Weight loss | 4.7% | 12.6% | <0.001 | 0.08 (0.27) | 0.08 (0.27) | 0.0 |
| Prior bleeding | 11.2% | 26.5% | <0.001 | 0.17 (0.38) | 0.17 (0.38) | 0.0 |
| Prior cerebral bleeding | -- | -- | 0.01 (0.08) | 0.01 (0.08) | 0.0 | |
| Prior gastrointestinal bleed | 4.7% | 16.2% | <0.001 | 0.10 (0.30) | 0.10 (0.30) | 0.0 |
| Prior defibrillator | -- | 3.5% | 0.007 | 0.02 (0.13) | 0.02 (0.13) | 0.0 |
| Prior pacemaker | 2.9% | 10.9% | <0.001 | 0.06 (0.25) | 0.06 (0.25) | 0.0 |
| Prior sleep apnea | 7.4% | 14.7% | <0.001 | 0.10 (0.31) | 0.10 (0.31) | 0.0 |
| Smoking | 10.3% | 20.0% | <0.001 | 0.15 (0.36) | 0.15 (0.36) | 0.0 |
| Frailty score, median (IQR) | 5.3 (2.3–9.3) | 11.3 (4.9–19.6) | <0.001 | 9.17 (8.53) | 9.17 (8.27) | 0.0 |
| High frailty | 9.4% | 39.0% | <0.001 | 0.19 (0.39) | 0.19 (0.39) | 0.0 |
SAVR= surgical aortic valve replacement; TAVR= transcatheter aortic valve replacement
Values are presented as percentages, mean (SD) or median (IQR)
Cells with N<11 were suppressed with (--)per CMS policy.
Results
SAVR versus TAVR in Rheumatic AS
The final study cohort included 1159 patients with rheumatic AS who underwent aortic valve replacement (SAVR n=554 and TAVR n=605). Patients in the SAVR group were younger (mean age 73.4±7.2 versus 79.4±8.1 years, P<0.001), and had lower prevalence of most comorbidities including hypertension, diabetes, heart failure, lung disease, kidney disease, peripheral arterial disease, stroke, coronary artery disease, atrial fibrillation, anemia, and pulmonary hypertension, compared to TAVR group. SAVR patients were less frail than patients in the TAVR group (median frailty score 5.3 versus 11.3, and high frailty 9.4% versus 39.0%, P<0.001 both). After propensity score overlap weighing adjustment, both groups were balanced on all baseline characteristics (Table 1).
After a median follow-up of 19 months (IQR 13–26), there was no difference in all-cause mortality with TAVR versus SAVR for rheumatic AS (11.2 versus 7.0 per 100 person-year, aHR 1.53, 95% CI 0.84–2.79, P =0.2). Similarly, no difference was observed in in-hospital and 30-day mortality (2.4% versus 3.5%, P=0.6; and 3.6% versus 3.2%, P=0.9, respectively), as well as 30-day stroke (2.4% versus 2.8%, P=0.8). After median follow-up of 10.5 months (IQR 4–17.3 months), there was no difference in risk of HF admission between TAVR and SAVR (14.8 versus 19.4 events per 100 person-year, sHR 0.71, 95% CI 0.41–1.23, P=0.2). Less than 11 patients who underwent SAVR, and none of the patients who underwent TAVR, required repeat valve replacement in follow up.
Patients who underwent SAVR had higher weighted risk of in-hospital acute kidney injury (AKI) (22.3% versus 11.9%, P=0.02), blood transfusion (19.8% versus 7.6%, P=0.002), cardiogenic shock (5.7% versus 1.5%, P=0.047) and new onset AF (21.1% versus 2.2%, P<0.001) and had longer hospital stay (median 8 (IQR 6–12) versus 3 (2–6) days, P<0.001) compared with those who underwent TAVR (Table 2).
Table 2:
In-hospital and short-term outcomes in rheumatic AS patients who underwent SAVR versus TAVR, before and after PS weighting adjustment
| Before adjustment | After adjustment | |||||
|---|---|---|---|---|---|---|
| Outcome | SAVR (N=554) | TAVR (N=605) | P value | SAVR (N=554) | TAVR (N=605) | P value |
| AKI, % | 22.1 | 14.4 | 0.02 | 22.3 | 11.9 | 0.02 |
| Blood transfusion, % | 20.1 | 9.1 | 0.002 | 19.8 | 7.6 | 0.002 |
| Cardiogenic shock, % | 5.6 | <1.7 | 0.04 | 5.7 | 1.5 | 0.047 |
| New onset AF, % | 28.3 | 2.2 | <0.001 | 21.1 | 2.2 | <0.001 |
| New PPM, % | 6.0 | 12.2 | 0.001 | 7.2 | 12.5 | 0.1 |
| Length of hospital stay | 7 (5–10) | 3 (2–7) | <0.001 | 8 (6–12) | 3 (2–6) | <0.001 |
| In-hospital mortality, % | 2.2 | 2.2 | 0.8 | 3.5 | 2.4 | 0.6 |
| 30-day stroke, % | 2.2 | 2.0 | 0.8 | 2.8 | 2.4 | 0.8 |
| 30-day mortality, % | 2.4 | 3.6 | 0.3 | 3.2 | 3.6 | 0.9 |
| One-year mortality | 6.1 | 16.0 | <0.001 | 8.9 | 13.1 | 0.2 |
AF= atrial fibrillation; AKI= acute kidney injury; PPM= permanent pacemaker; SAVR= surgical aortic valve replacement; TAVR= transcatheter aortic valve replacement
Cells with N<11 were suppressed with (--)per CMS policy.
TAVR in Rheumatic versus Non-rheumatic AS
Overall, 88,554 patients with non-rheumatic AS, including 1098 (1.2%) with bicuspid AV, underwent TAVR compared to 605 patients with rheumatic AS. Patients with rheumatic AS were younger (79.4±8.1 versus 81.2±8.1, P<0.001), less likely to be male (40% versus 53.3%, P<0.001), and had higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease. There was no difference in prevalence of diabetes, kidney, or liver disease between the two groups. TAVR patients with rheumatic AS were more frail than patients with non-rheumatic AS (median frailty score 11.3 versus 6.9, P<0.001, and high frailty 39% versus 19.7%, P<0.001). After propensity score overlap weighing adjustment, both groups were balanced on all baseline characteristics (Table 3).
Table 3:
Baseline characteristics of the non-rheumatic and rheumatic AS patients who underwent TAVR, before and after PS weighting adjustment
| Variable | Before adjustment | After adjustment | Standardized differences | |||
|---|---|---|---|---|---|---|
| Non-rheumatic AS (N=88,554) | Rheumatic AS (N=605) | P value | Non-rheumatic AS (N=88,554) | Rheumatic AS (N=605) | ||
| Age | 81.2±8.1 | 79.4±8.1 | <0.001 | 79.41 (8.05) | 79.41 (8.85) | 0.0 |
| Race | ||||||
| White | 92.2% | 90.4% | 0.2 | 0.91 | 0.91 | 0.0 |
| Black | 4.0% | 4.3% | 0.04 | 0.04 | ||
| Male sex | 53.3% | 40.0% | <0.001 | 0.40 (0.49) | 0.40 (0.49) | 0.0 |
| Hypertension | 94.3% | 93.7% | 0.5 | 0.94 (0.24) | 0.94 (0.24) | 0.0 |
| Diabetes mellitus | 41.6% | 44.8% | 0.1 | 0.45 (0.50) | 0.45 (0.50) | 0.0 |
| Heart failure | 78.5% | 82.6% | 0.01 | 0.83 (0.38) | 0.83 (0.38) | 0.0 |
| Lung disease | 36.4% | 44.0% | <0.001 | 0.44 (0.50) | 0.44 (0.50) | 0.0 |
| Liver disease | 4.1% | 4.1% | 0.9 | 0.04 (0.20) | 0.04 (0.20) | 0.0 |
| Chronic kidney disease | 28.8% | 29.6% | 0.7 | 0.30 (0.46) | 0.30 (0.46) | 0.0 |
| End stage renal disease | 3.7% | 4.6% | 0.2 | 0.05 (0.21) | 0.05 (0.21) | 0.0 |
| Peripheral arterial disease | 33.3% | 32.9% | 0.9 | 0.33 (0.47) | 0.33 (0.47) | 0.0 |
| Prior ischemic stroke | 9.2% | 11.8% | 0.03 | 0.12 (0.32) | 0.12 (0.32) | 0.0 |
| Coronary artery disease | 47.2% | 56.4% | <0.001 | 0.56 (0.50) | 0.56 (0.50) | 0.0 |
| Prior revascularization | 19.9% | 21.0% | 0.5 | 0.21 (0.41) | 0.21 (0.41) | 0.0 |
| Atrial fibrillation | 42.0% | 53.0% | <0.001 | 0.53 (0.50) | 0.53 (0.50) | 0.0 |
| Depression | 15.1% | 19.5% | 0.003 | 0.19 (0.40) | 0.19 (0.40) | 0.0 |
| Hypothyroidism | 25.2% | 28.9% | 0.04 | 0.29 (0.45) | 0.29 (0.45) | 0.0 |
| Drug abuse | 0.9% | -- | 0.3 | 0.01 (0.11) | 0.01 (0.11) | 0.0 |
| Anemia | 38.7% | 50.9% | <0.001 | 0.51 (0.50) | 0.51 (0.50) | 0.0 |
| Connective tissue disease | 6.7% | 8.8% | 0.04 | 0.09 (0.28) | 0.09 (0.28) | 0.0 |
| Alcohol abuse | 2.4% | 3.5% | 0.09 | 0.03 (0.18) | 0.03 (0.18) | 0.0 |
| Lymphoma | 1.6% | 2.3% | 0.2 | 0.02 (0.15) | 0.02 (0.15) | 0.0 |
| Electrolytes abnormality | 40.6% | 57.9% | <0.001 | 0.58 (0.49) | 0.58 (0.49) | 0.0 |
| Obesity | 25.6% | 27.1% | 0.4 | 0.27 (0.44) | 0.27 (0.44) | 0.0 |
| Psychosis | 2.1% | 3.8% | 0.004 | 0.04 (0.19) | 0.04 (0.19) | 0.0 |
| Pulmonary hypertension | 7.4% | 11.2% | <0.001 | 0.11 (0.31) | 0.11 (0.31) | 0.0 |
| Tumor without metastasis | 4.9% | 5.1% | 0.8 | 0.05 (0.22) | 0.05 (0.22) | 0.0 |
| Metastatic disease | 1.2% | 2.0% | 0.08 | 0.02 (0.14) | 0.02 (0.14) | 0.0 |
| Peptic ulcer disease | 2.4% | 2.6% | 0.7 | 0.03 (0.16) | 0.03 (0.16) | 0.0 |
| Weight loss | 7.9% | 12.6% | <0.001 | 0.12 (0.33) | 0.12 (0.33) | 0.0 |
| Prior bleeding | 18.5% | 26.5% | <0.001 | 0.26 (0.44) | 0.26 (0.44) | 0.0 |
| Prior cerebral bleeding | 0.5% | -- | 0.002 | 0.01 (0.12) | 0.01 (0.12) | 0.0 |
| Prior gastrointestinal bleed | 10.3% | 16.2% | <0.001 | 0.16 (0.37) | 0.16 (0.37) | 0.0 |
| Prior defibrillator | 2.6% | 3.5% | 0.2 | 0.03 (0.18) | 0.03 (0.18) | 0.0 |
| Prior pacemaker | 8.0% | 10.9% | 0.008 | 0.11 (0.31) | 0.11 (0.31) | 0.0 |
| Prior sleep apnea | 11.9% | 14.7% | 0.04 | 0.15 (0.35) | 0.15 (0.35) | 0.0 |
| Smoking | 15.1% | 20.0% | <0.001 | 0.20 (0.40) | 0.20 (0.40) | 0.0 |
| Frailty score median (IQR) | 6.9 (3.0–13.1) | 11.3 (4.9–19.6) | <0.001 | 13.4 (10.7) | 13.43(10.7) | 0.0 |
| High frailty | 19.7% | 39.0% | <0.001 | 0.39 (0.49) | 0.39 (0.49) | 0.0 |
Values are presented as percentages, mean (SD) or median (IQR)
Cells with N<11 were suppressed with (--)per CMS policy.
After median follow-up of 17 months (IQR 11–24), there was no difference between TAVR in patients with rheumatic versus non-rheumatic AS in mortality (15.2 versus 17.7 deaths per 100 person-years (aHR 0.87, 95% CI 0.68–1.09, P=0.2). There was also no difference in weighted risk of 30-day stroke (2.0% versus 3.3%, P=0.1), in-hospital mortality (2.2% versus 2.6%, P=0.7), or mortality at 30 days (3.6% versus 3.7%, P=0.9). After median follow-up of 9 months (IQR 4–16 months), there was no difference in risk of HF admissions between rheumatic and non-rheumatic AS with TAVR (14.1 versus 17.3 events per 100 person-year, sHR 0.82, 95% CI 0.60–1.12, P=0.2). Two hundred and forty-two patients (0.3%) with non-rheumatic AS who underwent TAVR required repeat valve replacement (124 redo-TAVR and 118 SAVR), while none of the patients with rheumatic AS required repeat valve replacement.
There was no difference between rheumatic and non-rheumatic patients in weighted risk outcomes assessed during the hospitalization, including conversion to open surgery (2.1% versus 4.0%, P=0.07), AKI (14.4% versus 13.7%, P=0.7), blood transfusion (9.0% versus 9.5%, P=0.8), cardiogenic shock (2.0% versus 2.4%, P=0.6), new onset AF (2.2% versus 2.4%, P=0.8), aortic annular rupture (1.7% vs 0.9%, P=0.3), and risk of new PPM (12.2% versus 11.4%, P=0.7) (Table 4).
Table 4:
In-hospital and short-term outcomes with TAVR in non-rheumatic versus rheumatic AS, before and after PS weighting adjustment
| Before adjustment | After adjustment | |||||
|---|---|---|---|---|---|---|
| Outcome | Non-rheumatic AS (N=88,554) | Rheumatic AS (N=605) | P value | Non-rheumatic AS (N=88,554) | Rheumatic As (N=605) | P value |
| AKI, % | 11.3 | 14.4 | 0.02 | 13.7 | 14.4 | 0.7 |
| Cardiac arrest, % | 2.0 | <1.7 | 0.3 | 2.3 | 1.3 | 0.2 |
| Conversion to surgery, % | 3.5 | 2.2 | 0.07 | 4.0 | 2.1 | 0.07 |
| Blood transfusion, % | 7.4 | 9.1 | 0.1 | 9.5 | 9.0 | 0.8 |
| Cardiogenic shock, % | 1.9 | 2.0 | 0.9 | 2.4 | 2.0 | 0.6 |
| Aortic annulus rupture, % | 1.1 | 1.7 | 0.2 | 0.9 | 1.7 | 0.3 |
| New onset AF, % | 2.9 | 2.2 | 0.3 | 2.4 | 2.2 | 0.8 |
| New PPM, % | 11.6 | 12.2 | 0.6 | 11.4 | 12.2 | 0.7 |
| Length of hospital stay | 3 (2–5) | 3 (2–7) | <0.01 | 3 (2–6) | 3 (2–7) | <0.01 |
| In-hospital mortality, % | 1.9 | 2.2 | 0.7 | 2.6 | 2.2 | 0.6 |
| 30-day stroke, % | 2.5 | 2.0 | 0.4 | 3.3 | 2.0 | 0.1 |
| 30-day mortality, % | 2.9 | 3.6 | 0.3 | 3.7 | 3.6 | 0.95 |
| 1-year mortality, % | 13.6 | 16.0 | 0.09 | 17.1 | 16.0 | 0.6 |
AF= atrial fibrillation; AKI= acute kidney injury; PPM= permanent pacemaker; SAVR= surgical aortic valve replacement; TAVR= transcatheter aortic valve replacement
Cells with N<11 were suppressed with (--)per CMS policy.
Sensitivity analysis
The results of the propensity score matching analysis are presented in the online supplement. After adjustment, baseline characteristics were well balanced between patients with rheumatic AS who underwent TAVR and SAVR (supplemental Table 2). There was no difference in in-hospital, 30-day and 1-year mortality between TAVR and SAVR (supplemental Table 3). There was also no difference in mortality in the longest follow up [HR 1.41, 95% CI 0.84–2.35, P=0.2] (Supplemental Figure 1). Similarly, baseline characteristics were well balanced between patients with and without rheumatic AS who underwent TAVR (supplemental Table 4). There was no difference in in-hospital, 30-day and 1-year mortality between the two groups (supplemental Table 5). There was also no difference in mortality in the longest follow up [HR 0.90, 95% CI 0.71–1.13, P=0.4] (Supplemental Figure 2).
Discussion
In this study, we demonstrated the following important findings. First, compared with SAVR for rheumatic AS, TAVR was associated with similar risk of mortality (in-hospital, 30-day, and midterm), but with lower rates of in-hospital complications, with the exception of permanent pacemaker implantation. Second, there was no difference in short and mid-term mortality between TAVR in rheumatic versus non-rheumatic AS, as well as no difference in risk of procedural complications (Central Illustration). Finally, TAVR in rheumatic AS appears to be feasible and durable with lack of need for a redo-AVR at mid-term follow-up.
Central illustration:
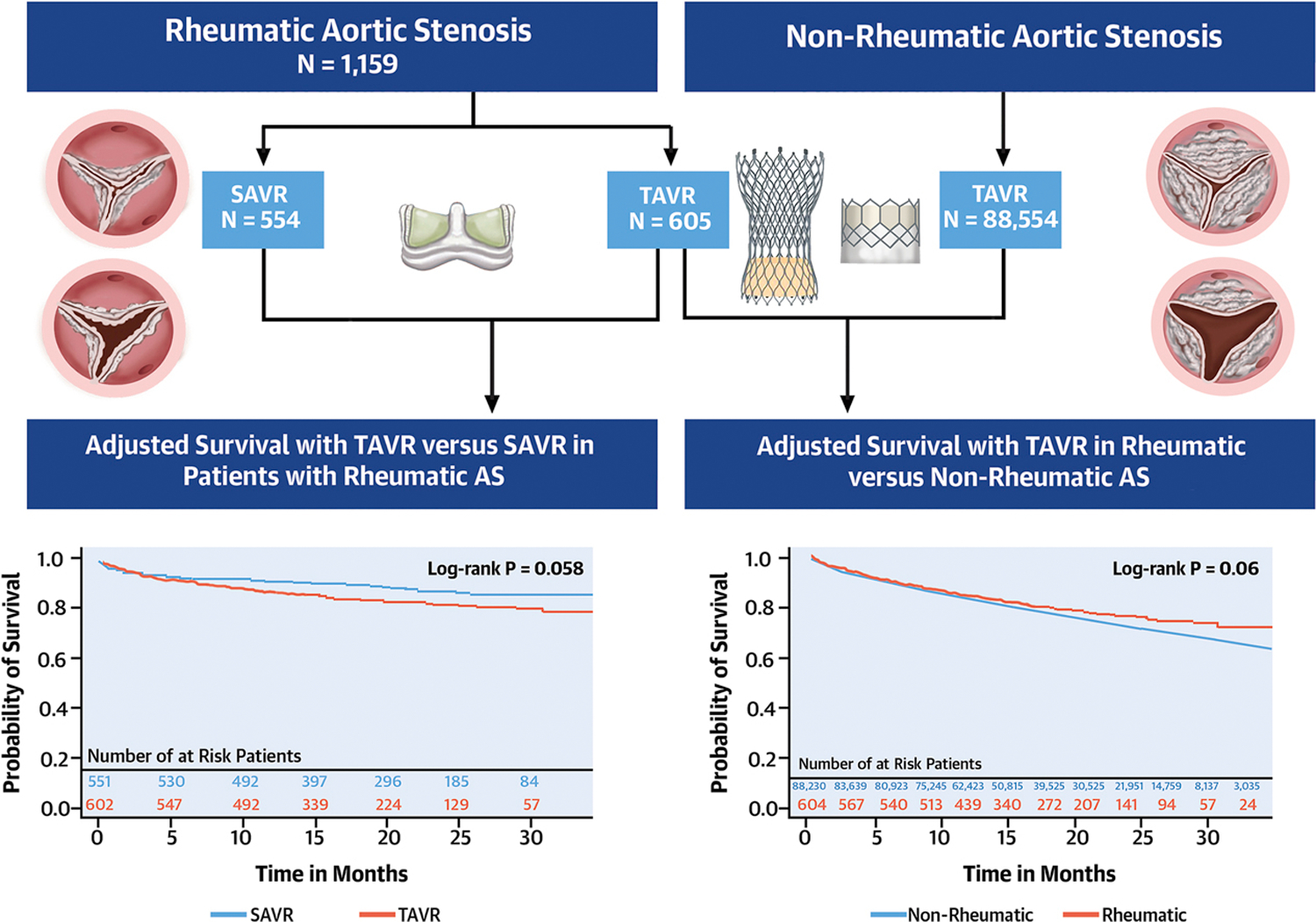
Main study findings. A) Flowchart of study cohort, B) Adjusted survival with transcatheter aortic valve replacement (TAVR) versus surgical aortic valve replacement in patients with rheumatic aortic stenosis. C), Adjusted survival with TAVR in patients with rheumatic AS versus non-rheumatic AS.
The prevalence of RHD has declined in developed countries in the past two decades. However, it remains a major public heart burden in low-income countries. It is estimated that 15 million people have RHD in Africa, with approximately 250,000 mortality per year and 100,000 patients who need valve replacement per year.(17) Patients in developing countries have a higher burden of poorly-controlled or undiagnosed chronic diseases and thus may be at higher risk for SAVR(18). Furthermore, patients in developing countries have less access to cardiac surgical services, and appropriate post-operative care capabilities.(9) It is estimated that >20% of patients with RHD in low-income countries require valve intervention within 30 months after the initial diagnosis.(19) The feasibility of TAVR in RHD would offer a potential treatment for patients with rheumatic AS in low and middle-income countries.(20)
The challenges with TAVR in rheumatic AS are several. First, RHD results in fibrosis and retraction of AV leaflets, but causes less calcification compared to degenerative calcific AS. Successful deployment of TAVR valve depends on annular and leaflet calcifications to act as an anchor. Lack of calcification in the native valve could result in transcatheter heart valve migration or paravalvular regurgitation. However, newer generations of TAVR prosthesis have advanced designs to improve this outcome.(21) Our study included a contemporary cohort of TAVR patients and hence reflecting outcomes with newer generations of TAVR valves. Second, patients with rheumatic AS are likely to have concomitant aortic regurgitation. Although these patients were excluded from some TAVR trials, recent studies suggest that TAVR is feasible and safe in patients with mixed aortic valve disease.(22) The lack of extensive annular and leaflet calcification in rheumatic AS may offer a benefit toward a safer deployment with appropriate oversizing to reduce the risk of residual paravalvular leakage without increased risk of annular injury/rupture as compared with calcific AS especially in patients with concomitant/pure aortic regurgitation and dilated aortic root. Third, patients with RHD also tend to have concomitant disease of multiple valves, and hence making the clinical correlation of patients’ symptoms to a specific valve malfunction is challenging.
In the current study, SAVR for rheumatic AS was associated with higher risk of cardiogenic shock compared to TAVR. RHD is commonly associated with right ventricular (RV) dysfunction.(23) Post-surgical RV failure can lead to cardiogenic shock and was shown to be associated with significant morbidity and mortality after open-heart surgery.(24) In the PARTNER IIA trial, the odds of RV dysfunction were four times higher with SAVR compared to TAVR, and had important prognostic implications in mortality.(25) It is likely that the higher rates cardiogenic shock with SAVR versus TAVR in our study is related to the higher rates of biventricular dysfunction in this unique population, however, this was not possible to confirm due to lack of information about echocardiographic parameters.
Patients with RHD are at high risk for atrial fibrillation and ischemic strokes.(26) This is due to structural changes in the left ventricle and atrium, either directly from rheumatic myocarditis, or indirectly through valvular disease. This is evident in our study population, as TAVR patients with rheumatic AS had up to 50% prevalence of pre-existing AF and 12% prevalence of prior ischemic stroke. However, it is important to note that TAVR was associated with lower incidence of postoperative new-onset AF compared with SAVR. Prior research has shown higher risk of new-onset AF after SAVR compared with TAVR(27). In TAVR, new-onset AF is associated with poor outcomes.(28) Whether new-onset AF after SAVR impacts long-term outcomes remains debatable, especially if transient and efforts are implemented early to restore sinus rhythm.(29,30) Interestingly, despite the difference in new-onset AF after TAVR and SAVR for rheumatic AS in our study, the rates of ischemic stroke and mortality were similar, which may suggest more benign effect of transient post-surgical new-onset AF.
Our study is the largest to date to highlight the feasibility of TAVR patients with rheumatic AS. While one could expect inferior technical success with TAVR in those patients due to the above-mentioned anatomical differences, the lack of need for repeat AVR after TAVR in our population is encouraging. Furthermore, the similar outcomes with TAVR for rheumatic versus non-rheumatic AS confirms the safety and feasibility of TAVR in this group. An interesting observation is the numerically, however without statistically, lower incidence of conversion to open surgery in rheumatic versus non-rheumatic patients. The theory of less annular and leaflet calcification in rheumatic AS could be behind this observation since annular injury and rupture are among the major reasons for emergent conversion to open-heart surgery.
The current study raises important questions that need to be explored in future studies conducted in countries where rheumatic AS is predominant. In developing countries, despite lack of such information, one must assume that a large proportion of those with AS is due to rheumatic heart disease. The differential criteria on echocardiogram as well as lack of severe calcification can confirm such theory in large cross-sectional cohorts. Introducing TAVR for rheumatic AS in these countries can offer a good alternative to surgical valves especially in areas with low surgical capacity or for patients with concerns over lack of adequate anticoagulation monitoring with mechanical valves. On the other hand, the durability of TAVR valves needs to be adequately studied before wide-spread use in this relatively younger population.
Our study has several limitations. Information on type of valve implanted (balloon or self-expanding in case of TAVR, mechanical versus bioprosthetic in case of SAVR) was not available. Similarly, we lacked information on pre-procedure echocardiography to quantify grade of aortic regurgitation or concomitant valve disease. It is important to note that, in our study, the majority of patients who underwent TAVR for rheumatic AS exhibited moderate to high frailty and were probably deemed high risk for SAVR. Although we used propensity score overlap weighting analysis to adjust for measured confounders, the risk of unmeasured confounding cannot be entirely excluded. Thus, caution should be exercised when extrapolating such results to younger patients with RHD, as outcomes might be different. Finally, longer duration of follow-up would be of interest to understand the durability of TAVR versus SAVR in such population.
Conclusion
Compared to surgical replacement, TAVR could represent a viable and durable option for patients with rheumatic AS. Future studies aiming to specifically examine the outcomes of TAVR versus SAVR in this unique cohort of patients are highly encouraged.
Supplementary Material
Figure 1:
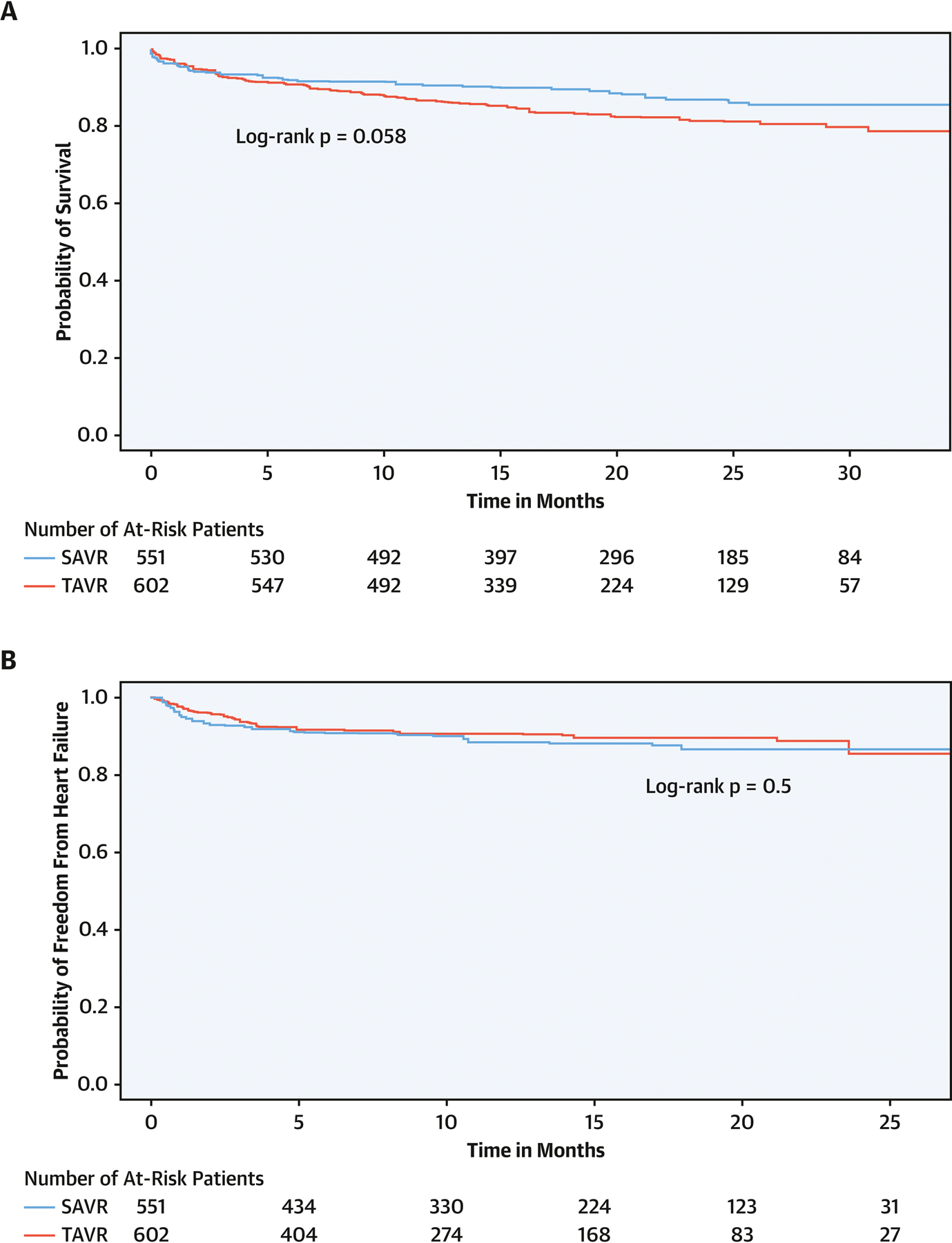
Mid-term outcomes of transcatheter versus surgical aortic valve replacement in patients with rheumatic aortic stenosis. A) all-cause mortality, B) heart failure admissions.
Figure 2:
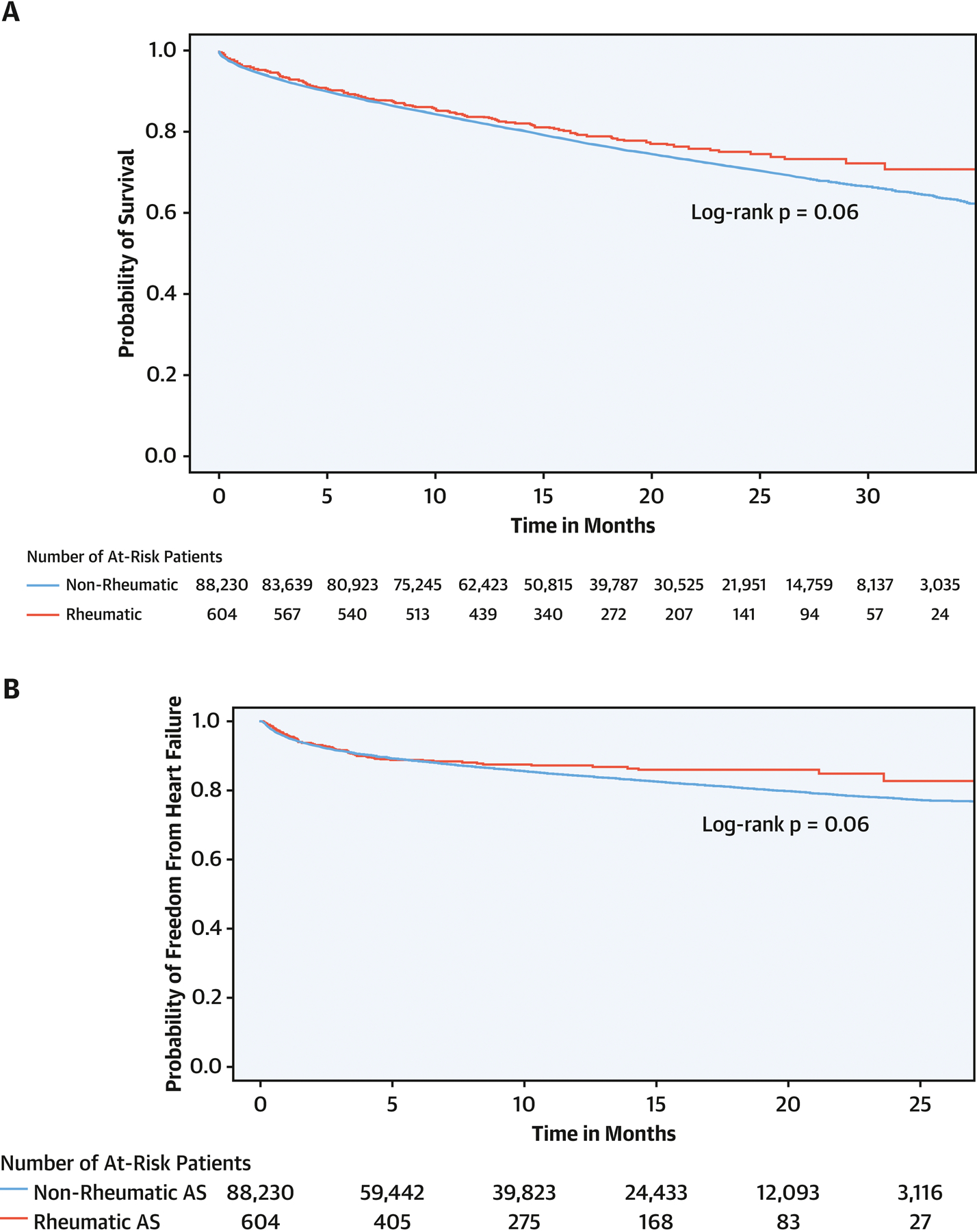
Mid-term outcomes of transcatheter aortic valve replacement in patients with rheumatic aortic stenosis versus patients with non-rheumatic aortic stenosis. A) all-cause mortality, B) heart failure admissions.
Perspectives.
Competency in Patient Care:
Transcatheter aortic valve replacement can be accomplished with midterm outcomes comparable to surgical replacement in patients with rheumatic aortic stenosis.
Translational Outlook:
Randomized trials are needed to compare the safety and long-term efficacy of TAVR vs SAVR in patients with rheumatic aortic stenosis.
Funding:
Dr. Mentias received support from National Institute of Health NRSA institutional grant (T32 HL007121) to the Abboud Cardiovascular Research Center. Dr. Sarrazin is supported by funding from the National Institute on Aging (NIA R01AG055663-01), and by the Health Services Research and Development Service (HSR&D) of the Department of Veterans Affairs.
List of Abbreviations:
- AF
Atrial fibrillation
- AKI
Acute kidney injury
- AS
Aortic stenosis
- CMS
Center for Medicare and Medicaid Services
- PPM
Permanent pacemaker
- RHD
Rheumatic aortic stenosis
- SAVR
Surgical aortic valve replacement
- TAVR
Transcatheter aortic valve replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Horwitz receives grant support from Edwards Lifesciences and Boston Scientific. The remaining authors do not have any conflicts of interest or financial disclosures.
Ethics approval: The Institutional Review Board at the University of Iowa approved the study with waiver of informed consent
References:
- 1.Leon MB, Smith CR, Mack MJ et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 2.Reardon MJ, Van Mieghem NM, Popma JJ et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 3.Mack MJ, Leon MB, Thourani VH et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 4.Popma JJ, Deeb GM, Yakubov SJ et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 5.Saji M, Highchi R, Iguchi N et al. Transcatheter aortic valve replacement in patients with degenerative calcified rheumatic aortic stenosis: A 10-patient case series. Int J Cardiol 2019;280:38–42. [DOI] [PubMed] [Google Scholar]
- 6.Asami M, Windecker S, Praz F et al. Transcatheter aortic valve replacement in patients with concomitant mitral stenosis. Eur Heart J 2019;40:1342–1351. [DOI] [PubMed] [Google Scholar]
- 7.Daly MJ, Blair PH, Modine T et al. Carotid-access transcatheter aortic valve replacement in a patient with a previous mitral valve replacement. J Card Surg 2015;30:256–9. [DOI] [PubMed] [Google Scholar]
- 8.Ntsekhe M, Scherman J. TAVI for rheumatic aortic stenosis - The next frontier? Int J Cardiol 2019;280:51–52. [DOI] [PubMed] [Google Scholar]
- 9.Zilla P, Yacoub M, Zuhlke L et al. Global Unmet Needs in Cardiac Surgery. Glob Heart 2018;13:293–303. [DOI] [PubMed] [Google Scholar]
- 10.Andell P, Li X, Martinsson A et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017;103:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12.Kundi H, Popma JJ, Reynolds MR et al. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J 2019;40:2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas LE, Li F, Pencina MJ. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Thomas LE, Li F. Addressing Extreme Propensity Scores via the Overlap Weights. Am J Epidemiol 2019;188:250–257. [DOI] [PubMed] [Google Scholar]
- 15.Schemper M, Henderson R. Predictive accuracy and explained variation in Cox regression. Biometrics 2000;56:249–55. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association 1999;94:496–509. [Google Scholar]
- 17.Sliwa K, Zilla P. Rheumatic heart disease: the tip of the iceberg. Circulation 2012;125:3060–2. [DOI] [PubMed] [Google Scholar]
- 18.Hajat C, Stein E. The global burden of multiple chronic conditions: A narrative review. Prev Med Rep 2018;12:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sliwa K, Carrington M, Mayosi BM, Zigiriadis E, Mvungi R, Stewart S. Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: insights from the heart of Soweto study. Eur Heart J 2010;31:719–27. [DOI] [PubMed] [Google Scholar]
- 20.Jacques Scherman DB, Ofoegbu Chima, Williams David F, Zilla Peter. TAVI for low to middle income countries. Eur Heart J 2017;38:1182–1184. [Google Scholar]
- 21.Gomes B, Geis NA, Chorianopoulos E et al. Improvements of Procedural Results With a New-Generation Self-Expanding Transfemoral Aortic Valve Prosthesis in Comparison to the Old-Generation Device. J Interv Cardiol 2017;30:72–78. [DOI] [PubMed] [Google Scholar]
- 22.Chahine J, Kadri AN, Gajulapalli RD et al. Outcomes of Transcatheter Aortic Valve Replacement in Mixed Aortic Valve Disease. JACC Cardiovasc Interv 2019;12:2299–2306. [DOI] [PubMed] [Google Scholar]
- 23.Konstam MA, Kiernan MS, Bernstein D et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018;137:e578–e622. [DOI] [PubMed] [Google Scholar]
- 24.Mandoli GE, Cameli M, Novo G et al. Right ventricular function after cardiac surgery: the diagnostic and prognostic role of echocardiography. Heart Fail Rev 2019;24:625–635. [DOI] [PubMed] [Google Scholar]
- 25.Cremer PC, Zhang Y, Alu M et al. The incidence and prognostic implications of worsening right ventricular function after surgical or transcatheter aortic valve replacement: insights from PARTNER IIA. Eur Heart J 2018;39:2659–2667. [DOI] [PubMed] [Google Scholar]
- 26.Gupta A, Bhatia R, Sharma G, Prasad K, Singh MB, Vibha D. Predictors of Ischemic Stroke in Rheumatic Heart Disease. J Stroke Cerebrovasc Dis 2015;24:2810–5. [DOI] [PubMed] [Google Scholar]
- 27.Motloch LJ, Reda S, Rottlaender D et al. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg 2012;93:124–31. [DOI] [PubMed] [Google Scholar]
- 28.Mentias A, Saad M, Girotra S et al. Impact of Pre-Existing and New-Onset Atrial Fibrillation on Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019;12:2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filardo G, Hamilton C, Hamman B, Hebeler RF Jr., Adams J, Grayburn P. New-onset postoperative atrial fibrillation and long-term survival after aortic valve replacement surgery. Ann Thorac Surg 2010;90:474–9. [DOI] [PubMed] [Google Scholar]
- 30.Swinkels BM, de Mol BA, Kelder JC, Vermeulen FE, Ten Berg JM. New-onset postoperative atrial fibrillation after aortic valve replacement: Effect on long-term survival. J Thorac Cardiovasc Surg 2017;154:492–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


