Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection appears to increase the risk of adverse pregnancy outcomes, such as pre-eclampsia in pregnant women. The mechanism(s) by which this occurs remains unclear.
Methods
We investigated the pathophysiology of SARS-CoV-2 at maternal-fetal interface in pregnant women who tested positive for the virus using RNA in situ hybridization (viral RNA), immunohistochemistry, and hematoxylin and eosin staining. To investigate whether viral infection alters the renin angiotensin system (RAS) in placenta, which controls blood pressure, we treated human trophoblasts with recombinant spike protein or a live modified virus with a vesicular stomatitis viral backbone expressing spike protein (VSV-S).
Findings
Viral colonization was highest in maternal decidua, fetal trophoblasts, Hofbauer cells, and in placentas delivered prematurely. We localized SARS-CoV-2 to cells expressing angiotensin-converting enzyme 2 (ACE2) and demonstrate that infected placentas had significantly reduced ACE2. In response to both spike protein and VSV-S, cellular ACE2 decreased although angiotensin II receptor type 1 (AT1R) increased with concomitant increase in soluble fms-like tyrosine kinase-1 (sFlt1). Viral infection decreased pro-angiogenic factors, AT2R, and placental growth factor, which competitively binds to sFlt1. Sera from infected pregnant women had elevated levels of sFlt1 and angiotensin II type 1-receptor autoantibodies prior to delivery, both signatory markers of pre-eclampsia.
Conclusions
SARS-CoV-2 colonizes ACE2-expressing maternal and fetal cells in the placenta. Infection in pregnant women correlates with alteration of placental RAS. As RAS regulates blood pressure, SARS-CoV-2 infection may thus increase adverse hemodynamic outcomes, such as pre-eclampsia in pregnant women.
Funding
NIH/NICHD grants R01 HD091218 and 3R01HD091218-04S1 (RADx-UP Supplement).
Keywords: decidua, pregnancy, placenta, extravillous trophoblasts, ACE2, sFlt1, AT1R, PlGF, pre-eclampsia, AT1-AA
Graphical abstract
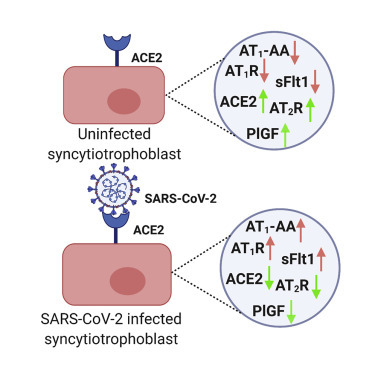
Context and significance
SARS-CoV-2 infection appears to increase the risk of pre-eclampsia, a dangerous condition in pregnancy characterized by high blood pressure. However, the mechanisms by which it may do so are currently unknown. Researchers at the Washington University School of Medicine in St. Louis, Missouri show that SARS-CoV-2 colonizes multiple cell types in the human placenta. In addition, infection is associated with alterations in the placental renin-angiotensin hormonal system, which regulates blood pressure leading to increased markers of pre-eclampsia. This study provides insights into the pathology of SARS-CoV-2 infection in the placenta and supports a link between SARS-CoV-2 infection and development of pre-eclampsia in pregnant women through changes in the renin-angiotensin system.
Verma et. al. show that the novel coronavirus, SARS-CoV-2, infects maternal and fetal cells in the human placenta, and infection is associated with decreased angiotensin-converting enzyme 2 (ACE2) expression and alterations in the blood-pressure-regulating renin angiotensin system (RAS). Alterations in this system are implicated in hypertensive disorders of pregnancy, such as pre-eclampsia. These findings shed light on how SARS-CoV-2 infection may increase risk of adverse pregnancy outcomes.
Introduction
The outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019 has led to a global health crisis, causing more than 115 million infections and 2.5 million deaths worldwide. In March 2020, the World Health Organization (WHO) declared a pandemic of coronavirus disease 2019 (COVID-19). The burden of disease has not been borne equally among the population, with more severe disease noted in the elderly, men, and those with cardiovascular comorbidities1, 2, 3, 4. Initially the majority of studies to date suggest that COVID-19 did not cause increased mortality in pregnant women compared to non-pregnant women5. There are studies which do not support evidence of SARS-CoV-2 infection in the placenta and also disprove the possibility of vertical transmission and pregnancy-related complications.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 However, a case report published early on in the pandemic showed the death of 7 pregnant women (out of 9), as well as one who was critically ill and one who recovered after prolonged hospitalization due to severe COVID-19 disease. Comparison of severity of infection to family members of all 9 women showed disease severity in COVID-19-infected pregnant women.20 Recent studies have reported that pregnant women are more likely to be positive for SARS-COV-2 and have both asymptomatic and severe disease outcomes as compared to non-pregnant women.21, 22, 23, 24, 25 SARS-CoV-2 infection has been reported to put pregnant women and their fetuses at risk by increasing the possibility of pregnancy-related complications, including pre-eclampsia and intrauterine growth restriction (IUGR).23 , 26, 27, 28, 29, 30, 31 A number of recent studies now indicate increased risk for perinatal complications in pregnant patients with severe COVID-19, including higher incidence of pre-eclampsia and preterm birth in SARS-CoV-2-infected women.18 , 32 That said, the risk for vertical transmission,33 , 34 although not nil, appears to be minimal.6 , 7 , 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 Thus, it remains imperative to understand and elucidate mechanisms of SARS-CoV-2 at the maternal-fetal interface dictating its pathogenesis during pregnancy.
Multiple studies have demonstrated the presence of SARS-CoV-2 in placental villi, mainly in syncytiotrophoblasts (STBs), placental submembranes (chorionic and amniotic membranes), maternal macrophages, and fetal macrophages (Hofbauer cells [HCs]) at different gestational stages of pregnancy.29 , 44 , 48, 49, 50, 51, 52, 53, 54, 55 A second-trimester miscarriage was reported in a case study of a woman with SARS-CoV-2 infection, and virus was found in placental submembranes with immune cell infiltration in placental cotyledons.56
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as a cellular entry receptor via viral spike (S) protein, which binds the N-terminal peptidase domain of ACE2.57, 58, 59 ACE2 is a key component of the renin-angiotensin system (RAS), a cascade of vasoactive peptides controlling angiogenesis, blood pressure, and fetal development by regulating inflammation.60 Viral endocytosis into host cells is also mediated by transmembrane protease, serine 2 (TMPRSS2), so maximum viral tropism for host cells depends on co-expression of ACE2 and TMPRSS2.57, 58, 59 Notably, placental trophoblasts are highly responsive to the changes in angiotensin receptor concentrations that occur during pregnancy.60 , 61 Expression of ACE2 and TMPRSS2 has been found during early pregnancy at the maternal-fetal interface in villous cytotrophoblasts (CTBs), STBs, and extravillous trophoblast cells (EVTs), but ACE2 expression decreases as the pregnancy progresses.44 , 62 , 63 Although some doubts have been raised regarding whether ACE2 is present in the placenta,64 a number of recent studies have confirmed the expression of ACE2 in placental cells.49 , 50 , 65, 66, 67, 68 The mechanism by which expression of ACE2 changes in the context of COVID-19 remains to be fully elucidated. The objective of our study was to determine whether SARS-CoV-2 affects expression of ACE2 in the placenta and, if so, whether alterations in ACE2 impacts the RAS system, which can contribute to pregnancy outcomes.
Here, we report SARS-CoV-2 colonization in term and preterm placentas from SARS-CoV-2-positive women. SARS-CoV-2 was found in all placental sections, colonizing the basal plate (BP) in EVTs, villous tissue (VT) in STBs and HCs, subchorionic (SC) region, amniotic epithelium of fetal membrane (FM), and tunica adventitia layer of umbilical cord (UC). Intracellular virus was found in placental trophoblasts and HCs expressing ACE2. Histopathological analyses showed that SARS-CoV-2-infected preterm placentas harbored inflammatory infiltrates, whereas inflammation was negligibly present in non-infected preterm or any term placentas. Transfection of human trophoblasts with SARS-CoV-2 S protein or infection with a live modified virus with a vesicular stomatitis viral (VSV) backbone expressing SARS-CoV-2 S protein triggers downregulation of ACE2 receptor expression while increasing expression of angiotensin II type 1 receptor 1 (AT1R) and soluble fms-like tyrosine kinase-1 (sFlt1), which are associated with pre-eclampsia pathogenesis. These findings were confirmed in SARS-CoV-2-infected pregnant patients. Thus, SARS-CoV-2, likely via viral S protein, disrupts the placental RAS pathway. In sum, we demonstrate that SARS-CoV-2 colonizes fetal trophoblasts, stromal cells, and macrophages in the placenta, which express the ACE2 receptor. S binding to ACE2 leads to reduction of the receptor expression and results in alterations of the RAS pathway—changes that are similar to those typically noted in pre-eclampsia.
Results
Clinical characteristics
In our study, we used five placentas each from SARS-CoV-2 uninfected and SARS-CoV-2-infected pregnant women. Of the five SARS-CoV-2-infected women, three were asymptomatic, one had only anosmia, and one had cough, dyspnea, chest pain, nausea/vomiting (n/v), and chills. SARS-CoV-2 infection was confirmed by qRT-PCR using nasopharyngeal swabs or saliva. There were no significant demographic differences between cases with positive maternal SARS-CoV-2 testing and our controls, and maternal age ranged from 19 to 33 years (uninfected) and 17 to 32 years (infected; Table 1 ). One patient (20%) in both the infected and uninfected groups delivered preterm and three (60%) in both groups delivered vaginally. With the exception of one patient, who tested positive and was symptomatic prior to delivery, the patients were positive at delivery. In addition, 3/5 (60%) pregnant patients were diagnosed with chronic hypertension, pre-eclampsia with severe features, or gestational hypertension.
Table 1.
Demographic and clinical features of COVID-19-negative and -positive women
| No. | Maternal age, years | Symptoms | Gestational age at delivery (weeks and days) | Pre-eclampsia features | Mode of delivery | Maternal race |
|---|---|---|---|---|---|---|
| COVID-19-negative women | ||||||
| P1 | 30 | NA | 37 and 3 | NA | vaginal | white |
| P2 | 33 | NA | 38 and 1 | NA | vaginal | white |
| P3 | 33 | NA | 39 and 2 | NA | C-section | white |
| P4 | 19 | NA | 24 and 4 (preterm) | NA | vaginal | African American |
| P5 | 24 | NA | 37 and 1 | NA | C-section | African American |
| COVID-19-positive women | ||||||
| S1 | 17 | anosmia (no cough or other symptoms) | 37 and 1 | NA | vaginal | white |
| S2 | 32 | asymptomatic | 37 and 5 | chronic hypertension, pre-eclampsia with severe features | vaginal | African American |
| S3 | 23 | asymptomatic | 39 and 3 | gestational hypertension | vaginal | African American |
| S4 | 28 | asymptomatic | 23 and 5 (preterm) | NA | C-section | African American |
| S5 | 32 | cough, dyspnea, chest pain, n/v, chills | 38 and 1 | gestational hypertension | C-section | African American |
SARS-CoV-2 colonizes multiple compartments in the maternal-fetal interface
Biopsies were taken from multiple sections: (1) BP (or decidua, which represents the maternal face of the placenta); placental VT (VT) (which represents the fetal compartment comprising STBs and CTBs); FMs; SC; and UC. The placental VTs also included fetal macrophages termed Hofbauer cells (HCs). The presence of SARS-CoV-2 was determined in term placental sections from SARS-CoV-2-positive women at RNA (Figures 1 A–1F) as well as protein level (Figures 1G–1L) using RNA probes for SARS-CoV-2-S RNA and an antibody against the S protein using RNAscope in situ hybridization (ISH) and immunohistochemistry (IHC), respectively. The corresponding controls for RNAscope ISH and IHC are presented in Figures 1A and 1G, respectively.
Figure 1.
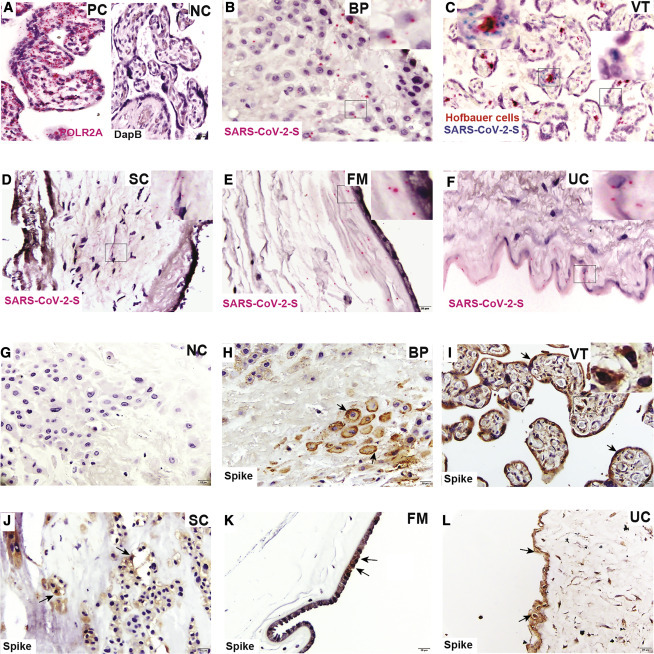
Term placenta from SARS-CoV-2-infected women shows SARS-CoV-2 localization
(A) RNAscope ISH positive control (PC) (RNA polymerase II subunit A [POLR2A]) and negative control (NC) (bacterial gene; DapB).
(B–F) RNA-ISH images of SARS-CoV-2-S RNA (B) in the basal plate (BP) in extravillous trophoblast cells (EVTs); (C) villous tissue (VT); syncytiotrophoblasts (STBs) shown in right side inset and Hofbauer cells (HCs) in left side inset; (D) subchorion (SC); (E) fetal membrane (FM); and (F) umbilical cord (UC).
(G) Immunohistochemical (IHC) NC.
(H–L) IHC staining for the presence of viral spike protein in (H) BP; (I) VT, including STBs (black arrow) and HCs (right side inset); (J) SC; (K) FM; and (L) UC. Black arrows show the location of spike protein in respective tissues.
Scale bar represents 20 μm.
SARS-CoV-2-S RNA and S protein localized to EVTs in the BP and STBs in the villous placenta (Figures 1B, 1C, 1H, and 1I), supporting recent published studies showing SARS-CoV-2 in the placenta.49 , 50 , 69 However, we determined that the virus was also found in fetal macrophages present in villous capillaries (Figure 1C, left inset, and 1I, right inset), stromal cells of the chorionic plate (Figures 1D and 1J), and amniotic epithelial cells of the FM (Figures 1E and 1K). Finally, we note evidence of the virus in the tunica adventitial layer of the UC (Figures 1F and 1L). Together, our findings indicate that the virus can colonize multiple compartments at the maternal-fetal interface, including trophoblasts, stromal cells, immune cells, and epithelial cells.
Preterm placenta harbors higher abundance of SARS-CoV-2 and histopathological abnormalities
The preterm placenta delivered at 23 weeks and 5 days harbored an abundance of S protein in EVTs (Figures 2A and 2G) and in STBs and HCs (Figures 2B, 2C, and 2H). SARS-CoV-2 S RNA and protein were present in higher abundance in stromal cells of the SC (Figures 2D and 2I), amniotic epithelial cells (Figures 2E and 2J), and the UC (Figures 2F, inset, and 2K). These findings indicate that SARS-CoV-2 can colonize all compartments at the maternal-fetal interface even earlier in gestation. Indeed, severity of SARS-CoV-2 infection appears to be greater in preterm than term tissue as observed by RNA-ISH staining of SARS-CoV-2-S (Figures 1 and 2). Consistent with this, pathological changes were evident in the preterm placenta relative to term placentas. The term placentas did not show any significant histopathological changes (Figures 3 A–3E, left panel). However, the preterm placenta had trophoblast giant cells in the BP (Figure 3A, right panel) but no changes in VT (Figure 3B, right panel). The subchorion harbored infiltration of neutrophils indicative of increased inflammation (Figure 3C, right panel). The FM showed amniotic edema and acute chorioamnionitis (inset, Figure 3D, right panel) and the presence of necrotic amniocytes in addition to pigmented macrophages (Figure 3D, right panel). The umbilical cord revealed acute funisitis or vasculitis (Figure 3E, right panel). There was no histological correlation between asymptomatic versus symptomatic placentas of SARS-CoV-2-positive women.
Figure 2.
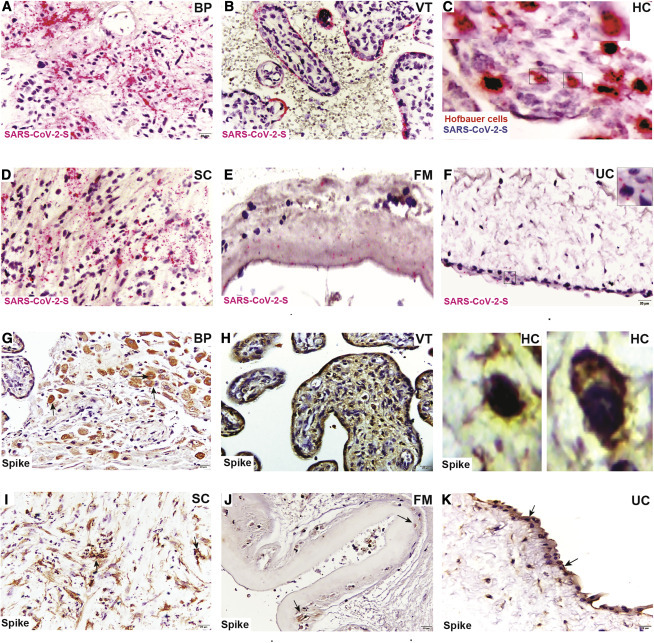
Preterm placenta from SARS-CoV-2-positive woman shows higher SARS-CoV-2 viral presence
(A–F) RNAscope ISH images depict presence of (A) SARS-CoV-2 spike RNA in BP (EVTs), (B) VT in STBs and (C) fetal HCs (insets), (D) SC, (E) FM, and (F) UC (inset).
(G–K) Immunohistochemical staining for SARS-CoV-2 spike protein demonstrating virus localization in (G) BP, (H) VT (including STBs and fetal HCs in insets), (I) SC, (J) FM, and (K) UC.
Scale bar represents 20 μm.
Figure 3.
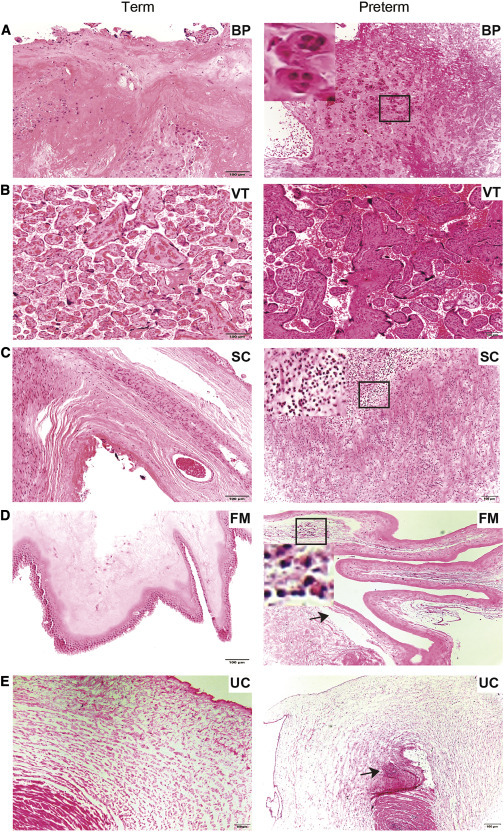
Histopathological findings in SARS-CoV-2-positive preterm placenta
(A–E) (Left) H&E staining of term placental compartments does not indicate obvious histopathological changes. (Right) H&E staining of preterm placental compartments is shown.
(A) Trophoblast giant cells (inset) BP.
(B) No histopathological defects found in VT.
(C) Acute chorioamnionitis, infiltration of neutrophils (inset) in SC.
(D) Necrotic amniocyte (inset), edema of amnion (arrow), and pigmented macrophages in FM.
(E) Acute funisitis or vasculitis in UC.
Black arrows indicate defects in respective tissue. Scale bar represents 100 μm.
SARS-CoV-2 entry receptor ACE2 is expressed in the placenta and is downregulated upon SARS-CoV-2 infection
SARS-CoV-2 uses ACE2 as entry receptor, which is abundantly expressed in different organs of the body.70, 71, 72, 73 A few studies predating the pandemic have suggested that the placenta is no exception.74 , 75 Here, using IHC with antibodies to ACE2, we analyzed SARS-CoV-2-positive and negative and preterm and term placentas for localization of ACE2 in multiple compartments. We found ACE2 expression in EVTs (Figures 4A and 4D), STBs (Figures 4B and 4E), HCs (Figures 4B and 4E), as well as in the FM (Figures 4C and 4F).
Figure 4.
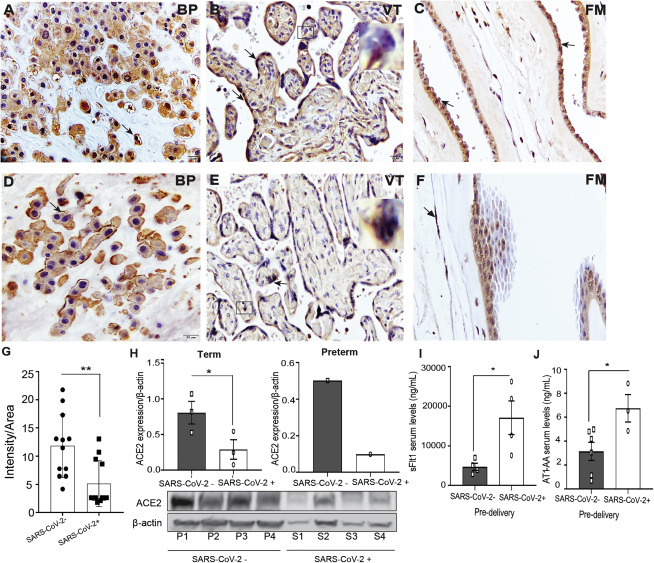
SARS-CoV-2 infection in pregnant women is associated with changes in RAS components
(A–F) Immunohistochemical (IHC) staining for (A) ACE2 in BP, (B) VT (STBs and HC; inset), and (C) FM in uninfected and (D) BP, (E) VT (STBs and HC; inset), and (F) FM in SARS-CoV-2-infected placenta. Arrows indicate expression of ACE2. Scale bar represents 20 μm.
(G) Semiquantitative analysis of ACE2 expression in uninfected and SARS-CoV-2-infected placenta.
(H) Densitometric analysis of ACE2 protein expression in term (left panel) and preterm (right panel) placenta of SARS-CoV-2-negative and positive pregnant women. Absolute quantification was performed after normalization with β-actin. The respective blots of ACE2 expression and loading control β-actin are shown.
(I and J) Pre-delivery soluble fms-like tyrosine kinase 1 (sFlt1) and angiotensin II type 1-receptor autoantibody (AT1-AA) levels in sera of uninfected and SARS-CoV-2-infected pregnant women.
P1–P3, SARS-CoV-2-negative placenta; P4, SARS-CoV-2-negative preterm placenta; S1–S3, SARS-CoV-2-positive placenta; S4, SARS-CoV-2-positive preterm placenta. Values are expressed as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Recent studies have shown that, upon SARS-CoV-2 infection, interaction between viral S protein and an intact and cell-surface-localized ACE2 leads to the internalization of ACE2, which facilitates SARS-CoV-2 entry into host cells, resulting in infection.76 Semiquantitative analysis of IHC images of infected (Figures 4D–4F) versus uninfected placental sections (Figures 4A–4C) showed a significant reduction in the level of ACE2 expression after SARS-CoV-2 infection (Figure 4G). A similar observation was found after western blotting quantification of ACE2 levels in SARS-CoV-2-positive and uninfected term placentas (Figure 4H, left panel). Furthermore, ACE2 levels were relatively lower in preterm placenta, and severe infection was associated with a greater decrease (Figure 4H, right panel). Thus, not only does the placenta express the viral entry receptor ACE2, it is actively responsive to infection, which results in its downregulation, which is itself indicative of infection.
SARS-CoV-2 infection is associated with dysregulation of the RAS pathway
The RAS is a complex cascade of vasoactive peptides controlling angiogenesis, blood pressure, and fetal development and is made up of two major axes: inflammatory angiotensin II (Ang II)/(AT1R; vasoconstrictive) and the anti-inflammatory protective axis ACE2/Ang-(1-7) (angiotensin II type 1 receptor 2 [AT2R]; vasodilatory).77 SARS-CoV-2 entry via ACE2 causes downregulation of membrane-bound ACE278 in other tissues, an occurrence we now show is recapitulated in the placenta. We reasoned that reduced ACE2 due to SARS-CoV-2 infection could impact the RAS system. Impaired RAS function and increase in the inflammatory AT1R could lead to increased production of pre-eclampsia markers, including soluble fms-like tyrosine kinase-1 (sFlt1).79, 80, 81, 82 Consistent with this, we observed increased levels of sFlt1 in the pre-delivery sera of SARS-CoV-2-infected versus uninfected pregnant women (Figure 4I). Pre-eclampsia is associated with increased serum secretion of an autoantibody (AT1-AA) that stimulates the AT1R receptor on trophoblasts, where it synergistically acts with Ang II to impair RAS function, leading to increased production of sFlt1.79, 80, 81, 82 Indeed, we note that the pre-delivery sera of SARS-CoV-2-infected women exhibited significantly increased levels of AT1 autoantibodiesAT1-AA (Figure 4J). Furthermore, the patients with increased AT1-AA and sFlt1 were diagnosed with chronic hypertension or gestational hypertension.
It has been shown that the viral S protein can induce internalization of ACE2, which initiates the fusion of viral particles and host cells.76 , 77 , 83, 84, 85 To model the complex interaction between host receptor and viral S protein and investigate the mechanistic link with RAS pathway activity, we treated placental trophoblast cells (JEG-3 cells) with the recombinant SARS-CoV-2 S protein in culture. We noted a significant reduction in the expression of ACE2 at 3 and 6 h post-S protein treatment compared to untreated control cells (Figure 5 A). Immunofluorescence staining and western blotting for ACE2 showed that S treatment leads to reduction of ACE2 levels in placental cells, corroborating our placental data (Figures 5C and 5D).
Figure 5.
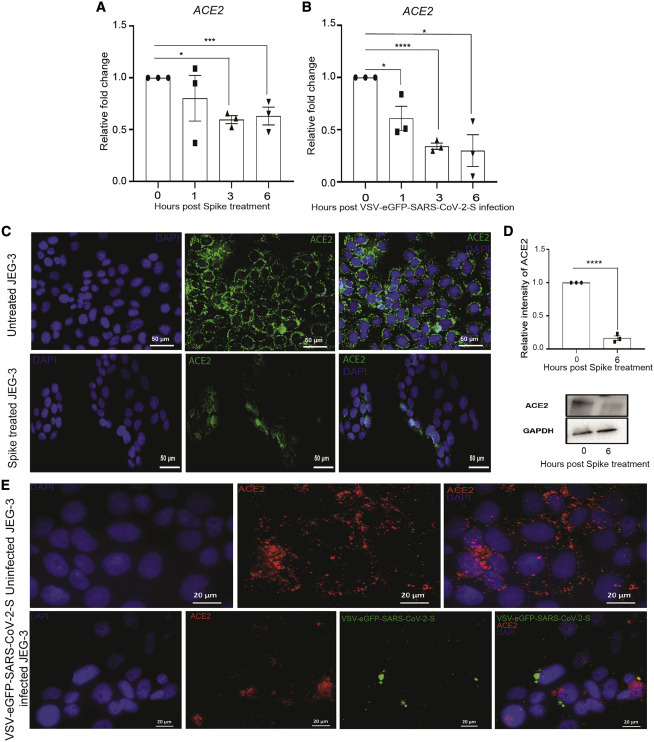
ACE2 expression decreases when treated or infected with spike or VSV-EGFP-SARS-CoV-2-S
(A and B) Relative expression of ACE2 normalized with GAPDH in JEG-3 cells treated or infected with spike or VSV-EGFP-SARS-CoV-2-S at 0, 1, 3, and 6 h treatment or post-infection.
(C and D) Expression of ACE2 in untreated and spike-protein-treated JEG-3 cells after 6 h of treatment by (C) immunofluorescence and (D) western blot, respectively (magnification scale 20×; scale bar represents 50 μm).
(E) Immunofluorescence images of uninfected and VSV-EGFP-SARS-CoV-2-S-infected JEG-3 cells after 6 h post-infection, showing expression of ACE2 (magnification scale 63×; scale bar represents 20 μm).
Values are expressed as mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
In addition, to model whole-virus interaction with ACE2 in placental cells, we also employed a replication-competent virus to study entry and neutralization of SARS-CoV-2.86 This pseudovirus (VSV-EGFP-SARS-CoV-2-S) permits folding and presentation of SARS-CoV-2 S protein in its native form. Infection of placental cells with VSV-EGFP-SARS-CoV-2-S at 1 multiplicity of infection (MOI) shows a significant reduction in the expression of ACE2 at all the time points (1, 3, and 6 h) studied compared to 0 h control cells (Figure 5B). Immunofluorescence analysis confirmed that the expression of ACE2 is reduced after VSV-EGFP-SARS-CoV-2-S infection (Figure 5E). Together, these cellular findings confirmed that viral S protein interaction with ACE2 on placental cells leads to ACE2 downregulation. Next, we determined the consequences of ACE2 decrease on RAS activity in placental trophoblasts. We found a significant increase in expression of the inflammatory AT1R (1 h) after the addition of recombinant SARS-CoV-2 S protein; expression of AT1R was significantly high at all time points compared to 0 h JEG-3 cells (Figure S1A). We confirmed the same findings in the presence of VSV-EGFP-SARS-CoV-2-S infection at 1 MOI as compared to 0 h control (Figure S1B). In contrast, expression of the protective angiotensin II type 1 receptor 2 (AT2R) and placental growth factor (PlGF) was significantly downregulated after S treatment (Figures S1C and S1E) and VSV-EGFP-SARS-CoV-2-S infection (Figures S1D and S1F). S protein treatment was sufficient to induce a significant increase in sFlt1 (Figure S1G). Furthermore, levels of sFlt1 were upregulated after VSV-EGFP-SARS-CoV-2-S at 1 and 3 h of infection compared to uninfected controls (Figure S1H). The data here strongly suggest that, even in the absence of viral nucleic acid, the presence of SARS-CoV-2 S antigen is enough to induce pre-eclamptic signatory markers in trophoblasts.
Together, our in vitro and tissue findings demonstrate that SARS-CoV-2 infection leads to reduced expression of ACE2, resulting in downregulation of the protective AT2R and PlGF and upregulation of the proinflammatory AT1R, AT1-AA, and sFlt1 arms of the RAS cascade.
Discussion
In this study, we detected both viral S RNA and protein in all five placentas (four term and one preterm) from SARS-CoV-2-infected women. We described the presence of SARS-CoV-2 S RNA and protein at the maternal-fetal interface, showing virus colonization in EVTs, STBs, HCs, stromal cells, amniotic epithelium, and tunica adventitia layer of the UC. Earlier studies have shown evidence of SARS-CoV-2 S or nucleocapsid protein in STBs, EVTs, FM, and HCs, which support our findings.44 , 49, 50, 51 The abundance of viral S RNA and protein was high in preterm placenta compared to term infected placenta of SARS-CoV-2-infected women. The colonization of virus was greater in the maternal decidua (BP) and fetal VT compared to other compartments of the placenta. Although viral infection at term did not appear to be associated with significant histopathological changes consistent with a number of recent studies,7 , 8 , 44 , 45 , 49 the preterm placenta exhibited significant inflammation, including neutrophil infiltration. A second-trimester case study has also reported abnormalities in the placenta, including perivillous fibrin deposition and inflammatory infiltrates.43 , 56 Together, these studies suggest the possibility that SARS CoV-2 infection is more severe in preterm placenta. Whether the virus would also show similarly increased abundance in the term placentas examined earlier in gestation or whether there is a more causal relationship between the virus and the time of delivery remains unclear. Detection of virus in HCs supports the possibility of fetal infection as HCs, which serve as vectors that transmit many viruses (Zika virus [ZIKV], human immunodeficiency virus [HIV], and human cytomegalovirus [HCMV]), although HCs can also induce immunomodulatory cytokines that limit viral replication.87, 88, 89, 90, 91
The high affinity of SARS-CoV-2 toward ACE2 aids in viral entry that subsequently triggers downregulation of ACE2 expression, disrupting the equilibrium of the RAS pathway.76 , 77 , 92 The importance of ACE2 and RAS in pregnancy is supported by several pieces of evidence. First, expression of renin and angiotensin increases as pregnancy progresses.93 Second, mice lacking Ace2 exhibit placental dysfunction, as evidenced by abnormal uterine remodeling, placental hypoxia, and uterine artery reactivity to vasoconstrictors.94 Third, the circulating levels of renin and angiotensin 1 are lower in women with pre-eclampsia than in normotensive women.95 Fourth, ACE2 is expressed in multiple cell types throughout the body, and its expression can be altered due to pregnancy. A recent study showed the downregulation of ACE2 in the nasal epithelium of pregnant rats compared with non-pregnant rats.96 Finally, reduction in ACE2 can pivot the RAS to become more inflammatory with increased activity of AT1 97 and a concomitant reduction in AT2 receptor. Indeed, a 2005 study on SARS-CoV infection showed that reduced ACE2 levels and increased expression of AT1R promotes disease pathogenesis in the lung.98
In our studies, treatment or infection of trophoblast cells with SARS-CoV-2 S recombinant protein and modified virus (VSV-EGFP-SARS-CoV-2-S) led to reduced ACE2 expression levels. Furthermore, SARS-CoV-2-infected placentas harbored reduced ACE2 levels. Reduced ACE2 correlated with an imbalance in the RAS pathway reflected in increased expression of the proinflammatory AT1R arm of the RAS pathway and concomitant downregulation of the AT2R protective arm. Thus, the increased expression of sFlt1 and downregulation of PlGF found in SARS-CoV-2-infected patients further suggests a potential mechanism for the endothelial dysfunction associated with multi-organ failure.76 , 99 , 100 Given the critical role of balanced angiogenic (e.g., PlGF) and antiangiogenic (e.g., sFlt1) factors required for normal placental angiogenesis, the dysregulation of PlGF and sFlt1 in SARS-CoV-2-infected placentas specifically suggests a mechanism for pre-eclampsia in COVID-19 patients supported by increased secretion of AT1 autoantibodies. Therefore, targeting RAS and the various specific components dysregulated by viral infection could improve pregnancy outcomes in pregnant patients with COVID-19. However, a number of recent clinical studies are uncovering a strong link between SARS-CoV-2 infection and the probability of pre-eclampsia in SARS-CoV-2-infected severe and non-severe pregnant groups.31 , 47 , 80 , 101, 102, 103, 104, 105 Our study provides mechanistic support for this link. In addition, large and diverse cohort studies, including from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network, suggest that patients with severe COVID-19 are at increased risk of perinatal complications.22 , 31 In sum, our study provides the foundation to understand the mechanism by which SARS-CoV-2 affects pregnancy by downregulating ACE2 expression in placental trophoblast cells and subsequently dysregulating the RAS pathway.
Limitations of study
Our study is limited by the small number of placentas analyzed, which are not powered to determine an association of infection and changes in ACE2 expression with expression of pre-eclampsia markers. Further studies are warranted to validate the impact of SARS-CoV-2 on pregnancy and on adverse hemodynamic outcomes, such as pre-eclampsia in pregnant women.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-ACE2 | Proteintech | Cat#21115-1-AP; RRID: AB_10732845 |
| anti- SARS-CoV/SARS-CoV-2 Spike Protein S2 | Thermo Scientific | Cat#MA5-35946; RRID: AB_2866558 |
| anti-Rabbit IgG HRP | Cell Signaling | Cat# 7076S, RRID: AB_330924 |
| anti-Mouse IgG HRP | Cell Signaling | Cat# 7074S, RRID: AB_2099233 |
| anti-rabbit Alexa 594 | Thermo Scientific | Cat# A11012, RRID: AB_2534079 |
| anti-rabbit Alexa 488 | Thermo Scientific | Cat# A11008, RRID: AB_143165 |
| Bacterial and virus strains | ||
| VSV-eGFP-SARS-CoV-2-S | Case et al.86 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| 4% buffered formalin | Fisher Scientific | Cat# 316-156 |
| Tissue Tek O.T.C. Compound | Fisher Bioreagents | Cat# BP1426 |
| Hydrogen peroxide | Sigma Aldrich | Cat# 216763 |
| Hematoxylin | Thermo Scientific | Cat# 7221 |
| Eosin | Thermo Scientific | Cat# 7111 |
| CytosealTM-60 | Thermo Scientific | Cat# 8310-4 |
| 1X Phosphate Buffer Saline | GIBCO | Cat# 14190-136 |
| Bovine Serum Albumin | Sigma Aldrich | Cat# 126593 |
| 3,3′-diaminobenzidine (DAB) | Dako | Cat# K346711-2 |
| DMEM/F12 media | Sigma Aldrich | Cat# D6421 |
| DAPI (4’, 6’-diamidino-2-phenylindole) | Sigma Aldrich | Cat#32670 |
| ProLong Diamond Antifade | Thermo Scientific | Cat# P36930 |
| Paraformaldehyde | Sigma Aldrich | Cat#P6148 |
| Xylene | Sigma Aldrich | Cat# 247642 |
| SsoAdvanced Universal SYBR Green Supermix | Bio-Rad | Cat# 1725275 |
| SARS-CoV-2 Spike protein | Sino Biological | Cat #40589-V08B1 |
| Experimental models: cell lines | ||
| JEG3 | ATCC | ATCC®HTB-36™ |
| Oligonucleotides | ||
| ACE2 Forward: 5′CAAGAG CAAACGGTTGAACAC 3′ |
Integrated DNA Technologies | N/A |
| ACE2 Reverse: 5′ CCAGAGCCTCTCATTGTAGTCT 3′ | Integrated DNA Technologies | N/A |
| AT1R Forward: 5′ CAGAAAGT CGGCACCAGATGAAG 3′ |
Integrated DNA Technologies | N/A |
| AT1R Reverse: 5′ CTGAGCTG TGGAGCCTTTGGA 3′ |
Integrated DNA Technologies | N/A |
| AT2R Forward: 5′ CTGGCAACA ATGAGTCTACCTT 3′ |
Integrated DNA Technologies | N/A |
| AT2R Reverse: 5′ GCCACAGC GAGGTTGAAGAT 3′ |
Integrated DNA Technologies | N/A |
| sFlt1 Forward: 5′ GAAAACGCA TAATCTGGGACAGT 3′ |
Integrated DNA Technologies | N/A |
| sFlt1 Reverse: 5′ GCGTGGTGTG CTTATTTGGA 3′ |
Integrated DNA Technologies | N/A |
| PlGF Forward: 5′ CAGAGGTGGA AGTGGTACCCTTCC 3′ |
Integrated DNA Technologies | N/A |
| PlGF Reverse: 5′CGGATCTTTAGG AGCTGCATGGTGAC 3′ |
Integrated DNA Technologies | N/A |
| SPIKE Forward: 5′ GCTGGTGCTGC AGCTTATTA 3′ |
Integrated DNA Technologies | N/A |
| SPIKE Reverse: 5′ AGGGTCAAGT GCACAGTCTA 3′ |
Integrated DNA Technologies | N/A |
| VSV Forward: 5′ GATAGTACCGGA GGATTGACGACTA 3′ |
Integrated DNA Technologies | N/A |
| VSV Reverse: 5′ TCAAACCA TCCGAGCCATTC 3′ |
Integrated DNA Technologies | N/A |
| Critical commercial assays | ||
| RNAscope® 2.5 HD Detection Reagent | ACD | Cat# 322360 |
| RNAscope® 2.5 HD Duplex Detection Reagents | ACD | Cat# 322500 |
| Human VEGFR1/Flt-1 DuoSet ELISA kit | R&D systems | Cat# DY321B |
| AT1-AA ELISA kit | Mybiosource.com | Cat# MBS722452 |
| QIAamp® Viral RNA kit | QIAGEN | Cat# 52904 |
| RNeasy® plus Mini Kit | QIAGEN | Cat# 74134 |
| Superscript II Reverse Transcriptase | Invitrogen | Cat# 18064-014 |
| DNase Amplification Grade | Thermo Fisher Scientific | Cat# 18068-015 |
| Bicinchoninic acid assay (BCA) | Thermo Fisher Scientific | Cat# 23225 |
| Software and algorithms | ||
| Prism 8.2.1 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| Image Lab software (6.0.1) | Life science research Bio-Rad | https://www.bio-rad.com/en-us/product/image-lab-software |
| Fiji-ImageJ | ImageJ | https://imagej.net/Fiji |
| Others | ||
| RNA-ISH Probe: RNA Polymerase II Subunit A, polr2a | ACD | Cat# 310451 |
| Bacterial gene, dapB | ACD | Cat# 310043 |
| SARS-CoV2-Spike | ACD | Cat# 848561 |
| CD163 | ACD | Cat# 417061 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Indira U. Mysorekar (imysorekar@wustl.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any unique datasets or code.
Experimental model and subject details
Study approval
This study was approved by Washington University School of Medicine Institutional Review Board (IRB #202003130). Informed consent has been signed by all the subjects involved in the study. Selected patient demographic data and institutional pathology reports were collected for all cases and controls. Universal testing for SARS- CoV-2 by PCR of nasopharyngeal swabs and later saliva was performed for all women admitted to our labor and delivery service at the Barnes Jewish Hospital. Placental samples and blood were collected from women who tested positive at the time of delivery, and contemporary controls who tested negative.
Placental tissue processing
Placentas were collected upon vaginal or C-section delivery. Multiple biopsies were isolated from basal plate (maternal decidua); villous placenta, fetal membranes, sub chorion, umbilical cord and processed as follows: 1) placed in 4% buffered formalin and embedded in paraffin for histological analysis and 2) placed in Tissue-Tek O·C.T. compound (Sakura, Finetek, USA) and frozen at −80°C. The placenta was delivered to Pathology for formal grossing, staging and histological evaluation by a board-certified placental pathologist (Dr. Mai He).
Virus propagation and titration
VSV-eGFP-SARS-CoV-2-S was kindly provided by Dr. Sean P.J. Whelan, Washington University in Saint Louis. The VSV-eGFP-SARS-CoV-2-S was synthesized by incorporating SARS-CoV-2 S gene (Wuhan-Hu-1 isolate) in an infectious molecular clone of VSV86. VSV-eGFP-SARS-CoV-2-S was propagated in Vero cells and titration of virus was performed following viral RNA isolation (QIAamp® Viral RNA kit, #52904), cDNA synthesis and qPCR using VSV and Spike specific primers 106. Experiments were performed under biosafety level 2 (BSL2) conditions.
Cell Culture and infection
JEG-3 cells were purchased from ATCC (HB-36) and grown in 10% DMEM/F12 media at 37°C, 5% CO2 and 70% relative humidity as described in107. JEG3 cells (0.15 X 106) were infected with VSV-eGFP-SARS-CoV-2-S at a multiplicity of infection (MOI) 1 for 1, 3 and 6 h. After each incubation supernatant was collected and cells were washed with PBS and harvested for RNA, protein, and immunofluorescence experiments.
Method details
RNA- In situ hybridization (RNA-ISH)
RNA-ISH was performed using RNAscope® 2.5 HD Detection Reagent – RED (#322360) and RNAscope® 2.5 HD Duplex Detection Reagents (#322500) as per manufacturer’s instructions. Briefly, Formalin-Fixed Paraffin-Embedded (FFPE) tissues were deparaffinized followed by dehydration and rehydration using ethanol–water gradient followed by H2O2 incubation for 10 min for quenching peroxidase activity at room temperature (RT). Slides were boiled in target retrieval solution for 20 min followed by protease treatment for 30 min at 40°C. After protease treatment slides were incubated in SARS-CoV2-Spike probe (#848561), CD163 probe (#417061) Positive probe (RNA Polymerase II Subunit A, polr2a, #310451) and negative probe (bacterial gene, dapB, #310043) synthesized by ACD. Amplification of signal was performed using Amp1-6 probes for single staining and Amp1-10 for dual staining. Slides were counterstained using Hematoxylin and mounted using Eco mount solution. Slides were scanned using standard bright field Nikon Eclipse E800 microscope.
H & E staining and Immunohistochemical (IHC) analysis
Tissue section slides of various parts of the placenta were sequentially rehydrated using ethanol-water gradients. For H & E staining, slides were stained using hematoxylin and Eosin, mounted using Cytoseal™-60 and scanned using a Nikon Eclipse E800 microscope.
For IHC, indigenous peroxidase activity was quenched using 1% H2O2 for 30 min. Sections were washed twice with 1X Phosphate Buffer Saline (PBS). Tissue sections were blocked with 1% Bovine Serum Albumin (BSA) at RT for 1 h. Next, sections were incubated with primary antibodies overnight at 4°C. The next day, sections were washed three times with 1X PBS and the respective secondary antibodies were incubated for 1 h at RT. Sections were developed using liquid 3,3′-diaminobenzidine (DAB) and counterstained with Hematoxylin. They were sequentially dehydrated and cleared in Xylene and mounted using Cytoseal™-60. Finally, images were captured using a Nikon Eclipse E800 microscope.
For image analysis histology images were imported into ImageJ (1.53c), color deconvolution was used to process images, H-DAB module was used to isolate DAB and hematoxylin layer. DAB Intensity was analyzed and normalized to the area of the selected region of interest (ROI). One representative image of BP, VT and FM from four COVID-19 negative and COVID-19 positive placentas was selected. Parametric unpaired t test was applied to calculate statistical significance. p < 0.05 was considered significant.
sFlt1 and AT1 –AA Elisa
Pre-delivery serum samples from uninfected and SARS-CoV-2 infected pregnant women were used for Elisa analysis using Human VEGFR1/Flt-1 DuoSet (R&D systems, Minneapolis, MN, USA) and AT1-AA (Mybiosource.com, Southern California, San Diego, USA) Elisa kits as per manufacturer’s instruction. Optical density was measured using microplate reader at 450 nm and 540 nm. The concentration of sFlt1 and AT1-AA was calculated using 4-parametric logistic curve-fit. The experiment was performed in duplicate.
Immunofluorescence (IF)
Indirect immunofluorescence was performed to check infection of VSV-eGFP-SARS-CoV-2-S and the expression of ACE2. Briefly, JEG-3 cells were seeded in 2-chamber slides and kept overnight at 37°C, 5% CO2 and 70% relative humidity. The next day, cells were infected/treated with VSV-eGFP-SARS-CoV-2-S (1 MOI)/Spike protein (2 μg) and incubated for 6 h in the CO2 incubator. After 6 h, cells were fixed with 4% PFA (15 min) at 4°C followed by blocking with 1% BSA in PBS for 1h at RT. After 1h, cells were incubated with ACE2 antibody (1:200) overnight at 4°C. The following day, cells were washed with PBS and incubated with anti-rabbit Alexa 594 or 488 (1:400) secondary antibody for 1h at RT. Further, cells were washed and counterstained with DAPI (4’, 6’-diamidino-2-phenylindole) and fixed in ProLong Diamond Antifade mounting medium. The images were captured using an epifluorescence microscope using 20X or 63X objectives.
qPCR analysis
Total mRNA was isolated after VSV-eGFP-SARS-CoV-2-S infection/Spike protein treatment at 0, 1, 3, and 6 h using RNeasy® plus Mini Kit (QIAGEN kit #74134) as per manufacturer’s instructions. The cDNA was made using 1000 ng of RNA and Superscript II Reverse Transcriptase (Invitrogen, #18064014). The qPCR reaction was performed in triplicates using primers shown in Table S1 using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, #1725275) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The fold change expression (-ΔΔCt) was calculated after normalization with GAPDH expression.
Western Blotting
Placental samples (BP, VT, FM, SC) from uninfected and SARS-CoV-2 infected women were collected and homogenized in RIPA buffer with protease and phosphatase inhibitor cocktails. Proteins isolated from individual section of placenta (BP, VT, FM, SC) were combined equally to study ACE2 expression.
JEG-3 cells were harvested after each time point in RIPA buffer with protease and phosphatase inhibitor cocktails. The total protein estimation was performed using Bicinchoninic acid assay (BCA) (Thermo Fisher Scientific, #23225) and separated on 4%–20% Mini-PROTEAN precast TGX gels (Bio-Rad Laboratories, #4561093). The proteins were transferred on Polyvinylidene fluoride (PVDF) membrane for 2 h at 90 Volts. After transfer, the membrane was blocked in 5% skimmed milk followed by incubation in primary antibody for ACE2 (1:1000) overnight at 4°C. The next day the membrane was washed with 0.1% TBST 3 times followed by incubation in HRP conjugated anti-rabbit secondary antibody (1:3000) for 1 h at RT. After incubation, the membrane was washed and developed using immobilon® Western Chemiluminescent HRP substrate (Millipore corporations, #WBKLS0100). The blots were captured using Chemidoc (Bio-Rad Laboratories) and bands were quantified using ImageJ software and plotted after normalization with loading control (β-actin/GAPDH).
Chemicals
Rabbit polyclonal anti- ACE2 was purchased from Proteintech (#21115-1-AP, 1:100 dilution). anti- SARS-CoV/SARS-CoV-2 Spike Protein S2 (1:100 dilution) were purchased from Thermo Scientific (#MA5-35946) respectively. HRP labeled anti-mouse (#7076S) and anti-rabbit (#7074S) antibodies were procured from Cell Signaling. Hydrogen peroxide was purchased from Sigma Aldrich (#216763, 1:200 dilution). Liquid DAB was procured from Dako (#K346711-2, 1:200 dilution). Mounting medium Cytoseal™-60 was obtained from Thermo Scientific (#8310-4).
Quantification and statistical analysis
All experiments were performed at least three times, and results were presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using unpaired t-Test for qPCR experiments. One way Anova was performed for western blotting and IHC experiments. Graphpad Prism 8.0 was used for all analyses. p < 0.05 was considered significant.
Acknowledgments
This work was funded in part by NIH/NICHD grants R01 HD091218 and 3R01HD091218-04S1 (RADx-UP Supplement; to I.U.M.), McDonnell International Academy Award (to I.U.M.), and Washington University CTSA (BJF/ICTS) award (to E.B.C.). We thank Dr. Jason Mills for comments and Dr. Sean Whelan for the kind gift of the VSV-chimeric virus stock.
Author contributions
S.V., E.B.C., and I.U.M. conceived the study; S.V. performed the principal experiments supported by C.S.J. and R.B.S.; M.H. provided expertise on placental pathology analysis; E.B.C. provided patient samples and clinical data; and S.V. and I.U.M. wrote the full draft of the manuscript. All authors edited and agreed with the final version.
Declaration of interests
I.U.M. serves on the Scientific Advisory Board of Luca Biologics. The authors declare no competing interests.
Published: April 13, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.medj.2021.04.009.
Supplemental information
References
- 1.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napoli C., Tritto I., Benincasa G., Mansueto G., Ambrosio G. Cardiovascular involvement during COVID-19 and clinical implications in elderly patients. A review. Ann. Med. Surg. (Lond.) 2020;57:236–243. doi: 10.1016/j.amsu.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4.Bonafè M., Prattichizzo F., Giuliani A., Storci G., Sabbatinelli J., Olivieri F. Inflamm-aging: Why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33–37. doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elshafeey F., Magdi R., Hindi N., Elshebiny M., Farrag N., Mahdy S., Sabbour M., Gebril S., Nasser M., Kamel M., et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int. J. Gynaecol. Obstet. 2020;150:47–52. doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y., Nie X., Huang B.X. [Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases] Zhonghua Bing Li Xue Za Zhi. 2020;49:418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 8.Baergen R.N., Heller D.S. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., Bao Y., Sun Y., Huang J., Guo Y., et al. Perinatal transmission of 2019 coronavirus disease-associated severe acute respiratory syndrome coronavirus 2: should we worry? Clin. Infect. Dis. 2021;72:862–864. doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galang R.R., Chang K., Strid P., Snead M.C., Woodworth K.R., House L.D., Perez M., Barfield W.D., Meaney-Delman D., Jamieson D.J., et al. Severe coronavirus infections in pregnancy: a systematic review. Obstet. Gynecol. 2020;136:262–272. doi: 10.1097/AOG.0000000000004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L., Ren J., Xu L., Ke X., Xiong L., Tian X., Fan C., Yan H., Yuan J. Placental pathology of the third trimester pregnant women from COVID-19. Diagn. Pathol. 2021;16:8. doi: 10.1186/s13000-021-01067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A., Ferdosian F., Bahrami R. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr. Pathol. 2020;39:246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020;144:799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 14.Smithgall M.C., Liu-Jarin X., Hamele-Bena D., Cimic A., Mourad M., Debelenko L., Chen X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020;77:994–999. doi: 10.1111/his.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong X., Wei H., Zhang Z., Chang J., Ma X., Gao X., Chen Q., Pang Q. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID--19. J. Med. Virol. 2020;92:1657–1659. doi: 10.1002/jmv.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., Feng L., Li C., Chen H., Qiao Y., et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020;223:111.e1–111.e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Jiang Y., Wei M., Cheng B.H., Zhou X.C., Li J., Tian J.H., Dong L., Hu R.H. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province] Zhonghua Fu Chan Ke Za Zhi. 2020;55:166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 18.Jering K.S., Claggett B.L., Cunningham J.W., Rosenthal N., Vardeny O., Greene M.F., Solomon S.D. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern. Med. 2021 doi: 10.1001/jamainternmed.2020.9241. Published online January 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adhikari E.H., Moreno W., Zofkie A.C., MacDonald L., McIntire D.D., Collins R.R.J., Spong C.Y. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw. Open. 2020;3:e2029256. doi: 10.1001/jamanetworkopen.2020.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E., Pooransari P., Ghotbizadeh F., Aalipour S., Soleimani Z., et al. Maternal death due to COVID-19. Am. J. Obstet. Gynecol. 2020;223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lokken E.M., Taylor G.G., Huebner E.M., Vanderhoeven J., Hendrickson S., Coler B., Sheng J.S., Walker C.L., McCartney S.A., Kretzer N.M., et al. Washington COVID-19 in Pregnancy Collaborative Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.02.011. Published online February 16, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lokken E.M., Huebner E.M., Taylor G.G., Hendrickson S., Vanderhoeven J., Kachikis A., Coler B., Walker C.L., Sheng J.S., Al-Haddad B.J.S., et al. Washington State COVID-19 in Pregnancy Collaborative Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2020.12.1221. Published January 27, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodworth K.R., Olsen E.O., Neelam V., Lewis E.L., Galang R.R., Oduyebo T., Aveni K., Yazdy M.M., Harvey E., Longcore N.D., et al. CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team. COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT) Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., Woodworth K.R., Nahabedian J.F., 3rd, Azziz-Baumgartner E., Gilboa S.M., Meaney-Delman D., CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraiolo A., Barra F., Kratochwila C., Paudice M., Vellone V.G., Godano E., Varesano S., Noberasco G., Ferrero S., Arioni C. Report of positive placental swabs for SARS-CoV-2 in an asymptomatic pregnant woman with COVID-19. Medicina (Kaunas) 2020;56:E306. doi: 10.3390/medicina56060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma S., Carter E.B., Mysorekar I.U. SARS-CoV2 and pregnancy: an invisible enemy? Am. J. Reprod. Immunol. 2020;84:e13308. doi: 10.1111/aji.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan N.N., Ansari M., Gaikwad C., Jadhav P., Tirkey D., Pophalkar M.P., Bhurke A.V., Modi D.N., Mahale S.D., Gajbhiye R.K. Impact of SARS-CoV-2 on multiple gestation pregnancy. Int. J. Gynaecol. Obstet. 2021;152:220–225. doi: 10.1002/ijgo.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capobianco G., Saderi L., Aliberti S., Mondoni M., Piana A., Dessole F., Dessole M., Cherchi P.L., Dessole S., Sotgiu G. COVID-19 in pregnant women: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:543–558. doi: 10.1016/j.ejogrb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulinx B., Kieffer D., Michiels I., Petermans S., Strybol D., Delvaux S., Baldewijns M., Raymaekers M., Cartuyvels R., Maurissen W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:2441–2445. doi: 10.1007/s10096-020-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamaniyan M., Ebadi A., Aghajanpoor S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat. Diagn. 2020;40:1759–1761. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbloom J.I., Raghuraman N., Carter E.B., Kelly J.C. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.001. Published online March 3, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metz T.D., Clifton R.G., Hughes B.L., Sandoval G., Saade G.R., Grobman W.A., Manuck T.A., Miodovnik M., Sowles A., Clark K., et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet. Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirtsman M., Diambomba Y., Poutanen S.M., Malinowski A.K., Vlachodimitropoulou E., Parks W.T., Erdman L., Morris S.K., Shah P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:E647–E650. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM. 2020;2:100145. doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., Liu Y., Xiao J., Liu H., Deng D., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J., Feng L. A case report of neonatal 2019 coronavirus disease in China. Clin. Infect. Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K., Yue L., Li Q., Sun G., Chen L., Yang L. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin. Infect. Dis. 2020;71:2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H., Liu Y. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H., Sun G., Tang F., Peng M., Gao Y., Peng J., Xie H., Zhao Y., Jin Z. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J. Infect. 2020;81:e40–e44. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narang K., Enninga E.A.L., Gunaratne M.D.S.K., Ibirogba E.R., Trad A.T.A., Elrefaei A., Theiler R.N., Ruano R., Szymanski L.M., Chakraborty R., Garovic V.D. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin. Proc. 2020;95:1750–1765. doi: 10.1016/j.mayocp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., Dygulska B., Heyman T., Salafia C., Shen D., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He M., Skaria P., Kreutz K., Chen L., Hagemann I.S., Carter E.B., Mysorekar I.U., Nelson D.M., Pfeifer J., Dehner L.P. Histopathology of third trimester placenta from SARS-CoV-2-positive women. Fetal Pediatr. Pathol. 2020 doi: 10.1080/15513815.2020.1828517. Published online October 12, 2020. [DOI] [PubMed] [Google Scholar]
- 46.Yang R., Mei H., Zheng T., Fu Q., Zhang Y., Buka S., Yao X., Tang Z., Zhang X., Qiu L., et al. Pregnant women with COVID-19 and risk of adverse birth outcomes and maternal-fetal vertical transmission: a population-based cohort study in Wuhan, China. BMC Med. 2020;18:330. doi: 10.1186/s12916-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jing H., Ackerman W.E., 4th, Zhao G., El Helou Y., Buhimschi C.S., Buhimschi I.A. Connecting the dots on vertical transmission of SARS-CoV-2 using protein-protein interaction network analysis - Potential roles of placental ACE2 and ENDOU. Placenta. 2021;104:16–19. doi: 10.1016/j.placenta.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buonsenso D., Costa S., Sanguinetti M., Cattani P., Posteraro B., Marchetti S., Carducci B., Lanzone A., Tamburrini E., Vento G., Valentini P. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am. J. Perinatol. 2020;37:869–872. doi: 10.1055/s-0040-1710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., Yasumoto Y., Vogels C.B., Casanovas-Massana A., Vijayakumar P., et al. SARS-CoV-2 infection of the placenta. J. Clin. Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taglauer E., Benarroch Y., Rop K., Barnett E., Sabharwal V., Yarrington C., Wachman E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. doi: 10.1016/j.placenta.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Facchetti F., Bugatti M., Drera E., Tripodo C., Sartori E., Cancila V., Papaccio M., Castellani R., Casola S., Boniotti M.B., et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59:102951. doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linehan L., O’Donoghue K., Dineen S., White J., Higgins J.R., Fitzgerald B. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–266. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu A.L., Guan M., Johannesen E., Stephens A.J., Khaleel N., Kagan N., Tuhlei B.C., Wan X.F. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J. Med. Virol. 2021;93:1038–1044. doi: 10.1002/jmv.26386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michel A.S., De Logiviere V., Schnuriger A., Lefebvre M., Maisonneuve E., Kayem G. Description of a late miscarriage case at 16 weeks of gestation associated with a SARS-CoV-2 infection. J. Gynecol. Obstet. Hum. Reprod. 2021;50:102064. doi: 10.1016/j.jogoh.2021.102064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., Meyer J.A., Roman A.S. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020;2:100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 58.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Irani R.A., Xia Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta. 2008;29:763–771. doi: 10.1016/j.placenta.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lumbers E.R., Delforce S.J., Arthurs A.L., Pringle K.G. Causes and consequences of the dysregulated maternal renin-angiotensin system in preeclampsia. Front. Endocrinol. (Lausanne) 2019;10:563. doi: 10.3389/fendo.2019.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bloise E., Zhang J., Nakpu J., Hamada H., Dunk C.E., Li S., Imperio G.E., Nadeem L., Kibschull M., Lye P., et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am. J. Obstet. Gynecol. 2021;224:298.e1–298.e8. doi: 10.1016/j.ajog.2020.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weatherbee B.A.T., Glover D.M., Zernicka-Goetz M. Expression of SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 suggests susceptibility of the human embryo in the first trimester. Open Biol. 2020;10:200162. doi: 10.1098/rsob.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pique-Regi R., Romero R., Tarca A.L., Luca F., Xu Y., Alazizi A., Leng Y., Hsu C.D., Gomez-Lopez N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife. 2020;9:e58716. doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ashary N., Bhide A., Chakraborty P., Colaco S., Mishra A., Chhabria K., Jolly M.K., Modi D. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 2020;8:783. doi: 10.3389/fcell.2020.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W., Yuan P., Yang M., Yan Z., Kong S., Yan J., Liu X., Chen Y., Qiao J., Yan L. SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal-fetal interface. Engineering (Beijing) 2020;6:1162–1169. doi: 10.1016/j.eng.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edlow A.G., Li J.Z., Collier A.Y., Atyeo C., James K.E., Boatin A.A., Gray K.J., Bordt E.A., Shook L.L., Yonker L.M., et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open. 2020;3:e2030455. doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komine-Aizawa S., Takada K., Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45–49. doi: 10.1016/j.placenta.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menter T., Mertz K.D., Jiang S., Chen H., Monod C., Tzankov A., Waldvogel S., Schulzke S.M., Hösli I., Bruder E. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiology. 2021;88:69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albini A., Di Guardo G., Noonan D.M., Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020;15:759–766. doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu H., Wang L.L., Zhao S.J., Kwak-Kim J., Mor G., Liao A.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020;139:103122. doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kreis N.N., Ritter A., Louwen F., Yuan J. A message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells. 2020;9:E1777. doi: 10.3390/cells9081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lanza K., Perez L.G., Costa L.B., Cordeiro T.M., Palmeira V.A., Ribeiro V.T., Simões E Silva A.C. Covid-19: the renin-angiotensin system imbalance hypothesis. Clin. Sci. (Lond.) 2020;134:1259–1264. doi: 10.1042/CS20200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banu N., Panikar S.S., Leal L.R., Leal A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to macrophage activation syndrome: therapeutic implications. Life Sci. 2020;256:117905. doi: 10.1016/j.lfs.2020.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dragun D., Philippe A. From mother to child--transplacental effect of AT1R-AA in preeclampsia. Nephrol. Dial. Transplant. 2010;25:1774–1776. doi: 10.1093/ndt/gfq167. [DOI] [PubMed] [Google Scholar]
- 80.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F., Schisterman E.F., Thadhani R., Sachs B.P., Epstein F.H., et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 82.Zeisler H., Llurba E., Chantraine F., Vatish M., Staff A.C., Sennström M., Olovsson M., Brennecke S.P., Stepan H., Allegranza D., et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 83.Haga S., Nagata N., Okamura T., Yamamoto N., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 2010;85:551–555. doi: 10.1016/j.antiviral.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 85.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Case J.B., Rothlauf P.W., Chen R.E., Liu Z., Zhao H., Kim A.S., Bloyet L.M., Zeng Q., Tahan S., Droit L., et al. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe. 2020;28:475–485.e5. doi: 10.1016/j.chom.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson E.L., Chakraborty R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology. 2012;9:101. doi: 10.1186/1742-4690-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwartz D.A. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch. Gynecol. Obstet. 2017;295:1361–1368. doi: 10.1007/s00404-017-4361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simoni M.K., Jurado K.A., Abrahams V.M., Fikrig E., Guller S. Zika virus infection of Hofbauer cells. Am. J. Reprod. Immunol. 2017;77 doi: 10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zulu M.Z., Martinez F.O., Gordon S., Gray C.M. The elusive role of placental macrophages: the Hofbauer cell. J. Innate Immun. 2019;11:447–456. doi: 10.1159/000497416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parker E.L., Silverstein R.B., Verma S., Mysorekar I.U. Viral-immune cell interactions at the maternal-fetal interface in human pregnancy. Front. Immunol. 2020;11:522047. doi: 10.3389/fimmu.2020.522047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franco R., Rivas-Santisteban R., Serrano-Marín J., Rodríguez-Pérez A.I., Labandeira-García J.L., Navarro G. SARS-CoV-2 as a factor to disbalance the renin-angiotensin system: a suspect in the case of exacerbated IL-6 production. J. Immunol. 2020;205:1198–1206. doi: 10.4049/jimmunol.2000642. [DOI] [PubMed] [Google Scholar]
- 93.Pavličev M., Wagner G.P., Chavan A.R., Owens K., Maziarz J., Dunn-Fletcher C., Kallapur S.G., Muglia L., Jones H. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res. 2017;27:349–361. doi: 10.1101/gr.207597.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamaleyeva L.M., Pulgar V.M., Lindsey S.H., Yamane L., Varagic J., McGee C., daSilva M., Lopes Bonfa P., Gurley S.B., Brosnihan K.B. Uterine artery dysfunction in pregnant ACE2 knockout mice is associated with placental hypoxia and reduced umbilical blood flow velocity. Am. J. Physiol. Endocrinol. Metab. 2015;309:E84–E94. doi: 10.1152/ajpendo.00596.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valdés G., Neves L.A., Anton L., Corthorn J., Chacón C., Germain A.M., Merrill D.C., Ferrario C.M., Sarao R., Penninger J., Brosnihan K.B. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 96.Palanisamy A., Giri T. Reduced severe acute respiratory syndrome coronavirus 2 entry factors and enhanced innate immune gene expression in the nasal epithelium of pregnant rats. Am. J. Obstet. Gynecol. 2021;224:118–120. doi: 10.1016/j.ajog.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallukat G., Homuth V., Fischer T., Lindschau C., Horstkamp B., Jüpner A., Baur E., Nissen E., Vetter K., Neichel D., et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samavati L., Uhal B.D. ACE2, much more than just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020;10:317. doi: 10.3389/fcimb.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giardini V., Carrer A., Casati M., Contro E., Vergani P., Gambacorti-Passerini C. Increased sFLT-1/PlGF ratio in COVID-19: a novel link to angiotensin II-mediated endothelial dysfunction. Am. J. Hematol. 2020;95:E188–E191. doi: 10.1002/ajh.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amaral W.N.D., Moraes C.L., Rodrigues A.P.D.S., Noll M., Arruda J.T., Mendonça C.R. Maternal coronavirus infections and neonates born to mothers with SARS-CoV-2: a systematic review. Healthcare (Basel) 2020;8:E511. doi: 10.3390/healthcare8040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beys-da-Silva W.O., da Rosa R.L., Santi L., Tureta E.F., Terraciano P.B., Guimarães J.A., Passos E.P., Berger M. The risk of COVID-19 for pregnant women: evidences of molecular alterations associated with preeclampsia in SARS-CoV-2 infection. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:165999. doi: 10.1016/j.bbadis.2020.165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herraiz I., Llurba E., Verlohren S., Galindo A., Spanish Group for the Study of Angiogenic Markers in Preeclampsia Update on the diagnosis and prognosis of preeclampsia with the aid of the sFlt-1/ PlGF ratio in singleton pregnancies. Fetal Diagn. Ther. 2018;43:81–89. doi: 10.1159/000477903. [DOI] [PubMed] [Google Scholar]
- 104.Ahlberg M., Neovius M., Saltvedt S., Söderling J., Pettersson K., Brandkvist C., Stephansson O. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324:1782–1785. doi: 10.1001/jama.2020.19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C., Garcia-Manau P., Serrano B., Lopez-Martinez R.M., Balcells J., Fernandez-Hidalgo N., Carreras E., Suy A. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Puglia A.L., Rezende A.G., Jorge S.A., Wagner R., Pereira C.A., Astray R.M. Quantitative RT-PCR for titration of replication-defective recombinant Semliki Forest virus. J. Virol. Methods. 2013;193:647–652. doi: 10.1016/j.jviromet.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 107.Cao B., Parnell L.A., Diamond M.S., Mysorekar I.U. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med. 2017;214:2303–2313. doi: 10.1084/jem.20170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any unique datasets or code.


