Abbreviations
- APHE
arterial phase hyperenhancement
- CT
computed tomography
- HCC
hepatocellular carcinoma
- HU
Hounsfield unit
- iCCA
intrahepatic cholangiocarcinoma
- MRI
magnetic resonance imaging
- T2W
T2‐weighted
Cirrhosis represents the end stage of chronic liver disease with a projected 1‐year mortality rate of 57% in patients with uncompensated cirrhosis. 1 Cirrhosis results in replacement of normal liver parenchyma by fibrous and regenerative tissue, which in turn complicates imaging interpretation. This article reviews the common imaging pitfalls related to technique or misinterpretation of focal lesions in the cirrhotic liver and provides clues to the correct diagnosis.
Technical Pitfalls
Arterial Phase Timing
Arterial phase timing presents a challenge in patients with cirrhosis because of variability of hemodynamics and third‐spacing of fluids. Hepatocellular carcinoma (HCC) typically demonstrates late arterial phase hyperenhancement (APHE) and may appear isoenhancing to surrounding hepatic parenchyma outside the late arterial phase. Failure to demonstrate the presence of APHE precludes imaging‐based diagnosis of HCC. 2 One technique to avoid this pitfall is bolus‐triggered tracking to determine the optimal late arterial phase. Bolus‐triggered tracking allows individualized timing of scan acquisition based on detecting threshold enhancement of a specific artery. With computed tomography (CT) liver imaging, the late arterial phase is commonly achieved by scanning 15 to 18 seconds after the aorta reaches an enhancement of 100 to 150 HU. Bolus‐triggered tracking may also be used with magnetic resonance liver imaging. 3
Delayed Phase Enhancement
In the setting of cirrhosis, fibrosis surrounding regenerative or dysplastic nodules can mimic the appearance of an “enhancing capsule.” This may lead to a false interpretation of benign lesions as HCC.
Another pitfall is that 10% to 15% of HCCs do not demonstrate APHE and, therefore, are visible only on venous or delayed phase images as areas of washout. 4
Malignant Lesions Pitfalls
Malignant Lesions Mimicking Benign Entities
HCC typically develops in a background of liver cirrhosis from a regenerative nodule in a multistep process. The benign regenerative nodule evolves into a dysplastic nodule, which may then turn into early HCC and, ultimately, progressed HCC. 5 When detected early, HCC may appear as an intermediately enhancing internal nodule within a less enhancing larger dysplastic nodule. 6 The inner HCC nodule may be interpreted as a benign or indeterminate lesion. If biopsy is warranted, targeting the inner nodule should be attempted to avoid false‐negative results.
HCCs are typically hypervascular with APHE in a nonrim pattern. Approximately 10% to 15% of HCCs are hypovascular and appear slightly hypoenhancing or isoenhancing on late arterial phase images, mimicking regenerative nodules. 7 The clue to the correct diagnosis involves careful review of the delayed phase, which may show nonperipheral washout and possibly an enhancing capsule (Fig. 1).
FIG 1.
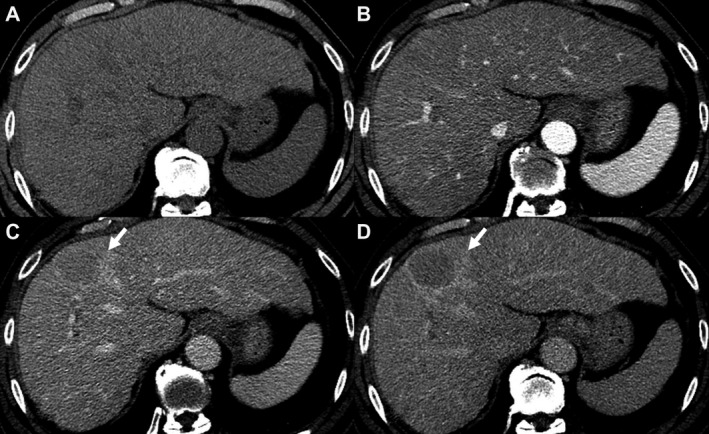
A 62‐year‐old patient with hepatitis C cirrhosis. Multiphasic CT of the liver demonstrates a 42‐mm observation (arrows) with no APHE relative to precontrast images (A and B). This observation demonstrates nonperipheral washout on portovenous phase (C) and an enhancing capsule on delayed phase (D). This lesion is proved pathologically to be HCC.
Rarely, HCC can be associated with peliosis hepatis, which is characterized by multiple blood‐filled cavities within the hepatic parenchyma. 8 This may lead to erroneous misinterpretation of HCC as a hemangioma because of enhancement that follows the blood pool throughout the imaging phases, a characteristic typical of hemangiomas. 9 The clue to the correct diagnosis is that hemangiomas demonstrate markedly high signal intensity on T2‐weighted images, as opposed to peliotic changes, which typically exhibit mild‐to‐moderate increased T2 signal intensity.
Malignant Lesions Mimicking HCC
Intrahepatic cholangiocarcinoma (iCCA) often demonstrates ringlike APHE with a targetoid appearance on diffusion‐weighted imaging and hepatobiliary phase. Small iCCAs can be indistinguishable from HCC with nonrim APHE and nonperipheral delayed washout. 10 Clues that favor HCC over iCCA are intrinsic T1 hyperintensity and the presence of intralesional fat.
Although metastases to the cirrhotic liver are rare, they may display mild hyperintensity on T2‐weighted (T2W) images and ringlike APHE. In these instances, when a malignant‐appearing lesion does not demonstrate classic HCC features, biopsy is usually warranted. 11
Benign Lesions Pitfalls
Siderotic Nodules
Siderotic nodules are dysplastic nodules that contain iron. Siderotic nodules appear hyperattenuating on CT and can mimic hyperenhancement on postcontrast images. Careful review of precontrast images provides the clue to the correct diagnosis by identifying lesional hyperattenuation prior to contrast administration. On MRI, siderotic nodules appear hypointense on T2W images because of their iron content. 12 There is also signal loss on gradient‐echo sequences that should not be mistaken for areas of washout.
Macroregenerative Nodules
Regenerative nodules develop in response to toxic liver parenchymal injury and progress to HCC in a stepwise pattern, as discussed previously. They range in size from micronodules (<3 mm) to macronodules (≥3 mm). On MRI, regenerative nodules can be hyperintense on T1‐weighted images, making true postcontrast enhancement difficult to discern. Subtraction images, created by subtracting the precontrast images from the postcontrast images such that only true enhancement is depicted, are helpful in differentiating true APHE of HCC from inherent T1 hyperintensity of regenerative nodules (Fig. 2). In addition, regenerative nodules typically appear hypointense on T2W images, in contrast with HCC, which typically appear moderately hyperintense on T2W images. 13
FIG 2.
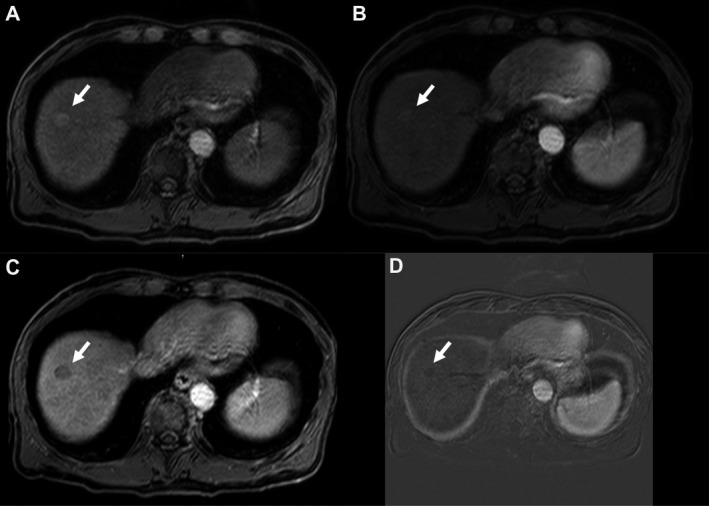
A 64‐year‐old patient with hepatitis C and alcoholic cirrhosis. Multiphasic MRI of the liver shows an 18‐mm observation (arrows) with slight T1 precontrast hyperintensity (A) and questionable postcontrast APHE (B). The venous phase (C) demonstrates nonperipheral washout with no enhancing capsule. Subtraction images (D) where precontrast images are subtracted from arterial phase images confirm the absence of true APHE, thus favoring a more benign entity, such as a regenerative nodule, over HCC.
Hemangioma With Pseudo‐washout
Hemangiomas are the most common benign tumor of the liver. Their incidence is rare within cirrhotic livers, and they can appear as rapidly enhancing lesions on the arterial phase, thus mimicking HCC. On MRI with hepatobiliary contrast agents, hemangiomas may display pseudo‐washout in the transitional phase because of rapid uptake of contrast medium by the background liver parenchyma relative to the hemangioma. 14 This pseudo‐washout phenomenon is more gradual compared with true washout in HCC. An additional clue to favor hemangioma over HCC is that the enhancement pattern follows the blood pool on all postcontrast phases.
Confluent Hepatic Fibrosis
Confluent hepatic fibrosis is associated with longstanding cirrhosis caused by chronic alcohol consumption or biliary obstruction. Early on, it starts as peripheral focal wedge‐shaped areas of inflammation that later progress to fibrosis with capsular retraction. These areas of inflammation can result in heterogeneous APHE mimicking malignancy. The clue to the correct diagnosis on CT is isoattenuation to surrounding parenchyma on venous phase and persistent delayed enhancement typical of fibrotic lesions. 15 In addition, typical features can help identify this entity, including location within the left medial segment or right anterior segment, wedge‐shaped morphology, progressive capsular retraction over time, and geographical extension from porta hepatis to the liver capsule.
Conclusion
Cirrhosis can complicate liver imaging because of parenchymal distortion and vascular supply alterations that can alter the typical imaging characteristics of benign and malignant lesions. Being mindful of potential pitfalls and their solutions related to liver imaging in cirrhosis allows for more accurate and timely diagnosis and avoids extraneous biopsy.
Potential conflict of interest: Nothing to report.
References
- 1. D’Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217‐231. [DOI] [PubMed] [Google Scholar]
- 2. Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI‐RADS) version 2018: imaging of hepatocellular carcinoma in at‐risk patients. Radiology 2018;289:816‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan R, Kumar G, Abdullah B, et al. Optimising the scan delay for arterial phase imaging of the liver using the bolus tracking technique. Biomed Imaging Interv J 2011;7:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim JH, Choi D, Kim SH, et al. Detection of hepatocellular carcinoma: value of adding delayed phase imaging to dual‐phase helical CT. AJR Am J Roentgenol 2002;179:67‐73. [DOI] [PubMed] [Google Scholar]
- 5. Willatt JM, Hussain HK, Adusumilli S, et al. MR imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 2008;247:311‐330. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell DG, Rubin R, Siegelman ES, et al. Hepatocellular carcinoma within siderotic regenerative nodules: appearance as a nodule within a nodule on MR images. Radiology 1991;178:101‐103. [DOI] [PubMed] [Google Scholar]
- 7. Hanna RF, Aguirre DA, Kased N, et al. Cirrhosis‐associated hepatocellular nodules: correlation of histopathologic and MR imaging features. RadioGraphics 2008;28:747‐769. [DOI] [PubMed] [Google Scholar]
- 8. Fujimoto M, Nakashima O, Komuta M, et al. Clinicopathological study of hepatocellular carcinoma with peliotic change. Oncol Lett 2010;1:17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brancatelli G, Baron RL, Peterson MS, et al. Helical CT screening for hepatocellular carcinoma in patients with cirrhosis: frequency and causes of false‐positive interpretation. AJR Am J Roentgenol 2003;180:1007‐1014. [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Pan Y, Shen K‐R, et al. Contrast‐enhanced multiple‐phase imaging features of intrahepatic mass‐forming cholangiocarcinoma and hepatocellular carcinoma with cirrhosis: a comparative study. Oncol Lett 2017;14:4213‐4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galia M, Taibbi A, Marin D, et al. Focal lesions in cirrhotic liver: What else beyond hepatocellular carcinoma? Diagn Interv Radiol 2014;20:222‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegelman ES, Chauhan A. MR characterization of focal liver lesions: pearls and pitfalls. Magn Reson Imaging Clin N Am 2014;22:295‐313. [DOI] [PubMed] [Google Scholar]
- 13. Hussain SM, Semelka RC, Mitchell DG. MR imaging of hepatocellular carcinoma. Magn Reson Imaging Clin N Am 2002;10:31‐52. [DOI] [PubMed] [Google Scholar]
- 14. Doo KW, Lee CH, Choi JW, et al. “Pseudo washout” sign in high‐flow hepatic hemangioma on gadoxetic acid contrast‐enhanced MRI mimicking hypervascular tumor. AJR Am J Roentgenol 2009;193:W490‐W496. [DOI] [PubMed] [Google Scholar]
- 15. Brancatelli G, Federle MP, Ambrosini R, et al. Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 2007;61:57‐69. [DOI] [PubMed] [Google Scholar]


