Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ACR
American College of Radiology
- APHE
arterial phase hyperenhancement
- CEUS
contrast‐enhanced ultrasound
- CT
computed tomography
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- LI‐RADS
Liver Imaging Reporting and Data System
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- OPTN
Organ Procurement and Transplantation Network
- TIV
tumor in vein
Contrast‐enhanced ultrasound (CEUS) uses intravenously administered microbubbles to evaluate the enhancement characteristics of focal lesions visible on ultrasound. In 2016, the US Food and Drug Administration approved the use of sulfur hexafluoride lipid type A microspheres (Lumason, Bracco Diagnostics Inc., Monroe Township, NJ, USA) for the evaluation of a focal liver lesion in a pediatric or adult patient. Subsequently, the American College of Radiology (ACR) Liver Imaging Reporting and Data System (LI‐RADS) published CEUS LI‐RADS and updated it in 2017. 1 , 2 CEUS LI‐RADS, like computed tomography/magnetic resonance (MR) imaging (CT/MRI) LI‐RADS, is a framework for classifying untreated liver observations with different likelihood levels in patients at high risk for hepatocellular carcinoma (HCC). The components of CEUS LI‐RADS, accuracy of CEUS for diagnosing HCC, and indications for CEUS LI‐RADS are highlighted in this review.
Primary Liver Cancer and Components of CEUS LI‐RADS
Primary liver cancer is the sixth leading cause of cancer worldwide and the fourth leading cause of cancer‐related mortality. 3 Accurate characterization of early‐stage HCC allows appropriate treatment and improves patient survival. Per‐observation CEUS LI‐RADS categories range from LR‐1 through LR‐5 and also include LR‐M (probably malignant but not HCC specific), LR‐TIV (tumor in vein), and LR‐NC (not characterizable because of image omission or degradation). LR‐1 and LR‐2 represent definitely and probably benign, respectively. LR‐3 indicates an intermediate probability for HCC, and LR‐4 and LR‐5 represent probably and definitely HCC, respectively. Major criteria for CEUS LI‐RADS include: (1) size of lesion; (2) presence or absence of arterial phase hyperenhancement (APHE) (nonrim; nondiscontinuous peripheral nodular enhancement); and (3) presence or absence of, as well as degree of, washout (mild or marked/punched out) and timing of washout, if present (early or late). APHE and mild, late washout (>60 seconds) are typical for HCC, whereas early (<60 seconds) or marked washout (<2 minutes) typically occurs in intrahepatic cholangiocarcinoma (ICC) and other nonhepatocellular malignancies. Please refer to the ACR CEUS LI‐RADS website for further details. 1
Accuracy of CEUS LI‐RADS
CEUS LI‐RADS has a high positive predictive value for diagnosing HCC in patients with cirrhosis and other high‐risk patients without cirrhosis, comparable with CT/MRI LI‐RADS. The overarching goal of CEUS LR‐5 diagnosis is high specificity for HCC and exclusion of non‐HCC malignancy, including ICC and combined HCC/ICC. Because LR‐5 designation is intended to guide management without biopsy, the sensitivity for diagnosing HCC is sacrificed to maintain a high specificity, resulting in some HCCs characterized as LR‐M, LR‐4, or rarely, LR‐3.
A review of 21 pathologically proved ICCs, published in 2010 (prior to CEUS LI‐RADS), claimed CEUS was unable to differentiate ICC from HCC, 4 resulting in removal of CEUS from a 2011 American Association for the Study of Liver Diseases (AASLD) practice guideline for HCC management. 5 However, subsequent research has shown that ICC can be effectively differentiated from HCC by assessing the timing and degree of onset of washout, and thus the importance of these features in CEUS LI‐RADS. 6 , 7 Additional recent retrospective reviews have shown sensitivity of CEUS LR‐5 for diagnosing HCC ranging between 62% and 75%, positive predictive values of 97% and 98.5%, and specificity of 96%, 8 , 9 , 10 equivalent to CT/MRI LI‐RADS. In these studies, HCC represented between 48% and 75% of all LR‐M observations. The latest AASLD guidance document now includes CEUS as a recommended diagnostic tool to characterize observations suspicious for HCC. 11 However, at this time, Organ Procurement and Transplantation Network (OPTN) does not recognize CEUS for transplant considerations. Multiphase contrast‐enhanced CT or MRI may be necessary to verify a CEUS LR‐5 observation in liver transplant candidates. Incongruent CT/MRI and CEUS cases may require discussion and/or appeal to the regional review board.
Using LI‐RADS in CEUS—Daily Practice
The basic CEUS LI‐RADS imaging protocol includes: (1) bolus intravenous injection of contrast agent; (2) timer started at onset of saline flush; (3) continuous imaging of the arterial phase, up to 1 minute; and (4) intermittent imaging at 60 seconds and then every 30 to 60 seconds thereafter, until liver or observation washout. Benefits of CEUS are listed in Table 1. In brief, the ability of CEUS to continuously image during the entire arterial phase represents a key advantage of CEUS over multiphase contrast‐enhanced CT/MRI. Because CT/MRI arterial phase images are obtained at set points in time, the chance of missing peak APHE of an observation is increased. Furthermore, microbubble ultrasound contrast is nonnephrotoxic and cleared by the lung, with a short half‐life, allowing for multiple injections during a single examination. These advantages make CEUS invaluable for imaging liver patients. The following are common indications for performing CEUS in a patient at high risk for HCC:
Thrombus: to differentiate TIV from bland thrombus (Fig. 1)
LR‐1: to confirm classic enhancement pattern of a flash‐filling hemangioma (peripheral, discontinuous nodular enhancement with centripetal fill‐in)
Improve characterization of indeterminate CT/MRI LI‐RADS categories: LR‐3, LR‐4, and LR‐M (Figs. 2 and 3)
Immediately evaluate observation or nodule ≥10 mm detected on surveillance ultrasound
Inability to obtain multiphase CT/MRI (e.g., due to renal insufficiency) (Fig. 4)
Evaluate CT/MRI observation for APHE when arterial phase mistiming is suspected (Fig. 5), or if observation/nodule is occult on CT/MRI
Monitor LR‐3 and/or LR‐4 observations for longitudinal changes
Monitor known observations for longitudinal changes in patients currently listed for liver transplant
Improve localization of known liver observation for percutaneous intervention, particularly if difficult to visualize on grayscale sonography, and help select viable/appropriate component for biopsy
TABLE 1.
Benefits and Drawbacks of Using CEUS for Liver Imaging
| Benefits | Drawbacks |
|---|---|
| Continuous imaging of arterial phase | Operator dependent, requires CEUS imaging expertise |
| High safety profile: nonnephrotoxic contrast; no ionizing radiation | Not recognized by OPTN for evaluating transplant patients |
| Can be performed immediately after surveillance ultrasound | Limitations in setting of severe steatosis, deep lesions, or large body habitus |
| Can guide interventional radiology procedures | Not suitable for HCC screening at this time |
| Rapid contrast clearance, allowing for multiple injections in one examination | Targets small handful of observations per examination; not suitable for complete disease staging |
FIG 1.
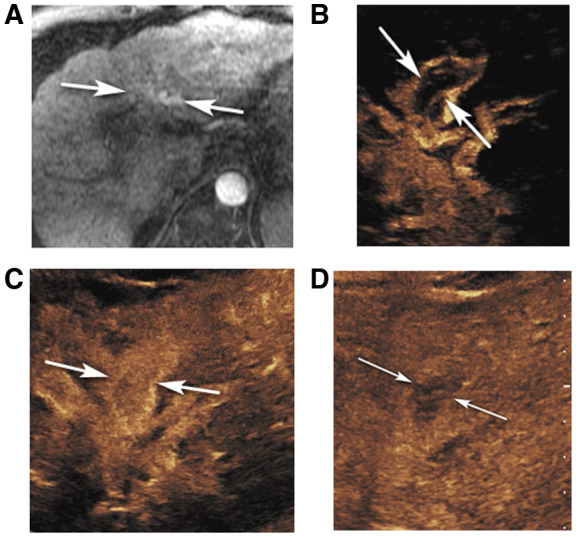
CEUS LR‐TIV in a 61‐year‐old man with alcoholic cirrhosis. (A) An axial arterial phase contrast‐enhanced T1‐weighted fat‐suppressed MR image shows arterial hyperenhancement in the left portal vein area (arrows) suspicious for TIV. Delayed MR images were nondiagnostic due to motion artifact. CEUS images of the left portal vein show curvilinear arterial enhancement (arrows in B, 8 seconds) with complete enhancement later in the arterial phase (arrows in C, 18 seconds) and washout in the delayed phase (arrows in D, 3 minutes 45 seconds), diagnostic of TIV.
FIG 2.
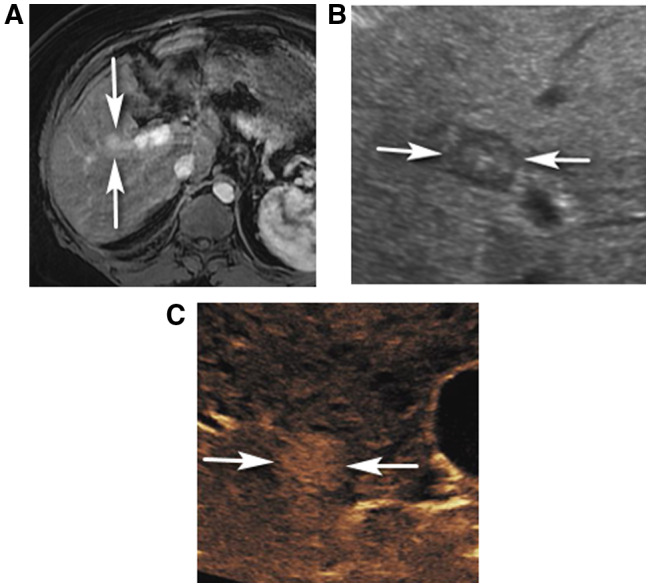
CEUS LR‐4 in a 68‐year‐old man with nonalcoholic steatohepatitis (NASH) cirrhosis. (A) An axial arterial phase contrast‐enhanced T1‐weighted fat‐suppressed MR image shows a vague arterial hyperenhancing observation (arrows), characterized as CT/MRI LR‐3. Delayed MR images were nondiagnostic due to motion artifact. (B) Grayscale ultrasound shows a circumscribed mixed echogenicity though predominantly hypoechoic nodule (arrows), corresponding to MRI finding. CEUS shows APHE of the nodule (arrows in C, 20 seconds), which was characterized as CEUS LR‐4. No washout was identified up to 5 minutes (image not shown).
FIG 3.
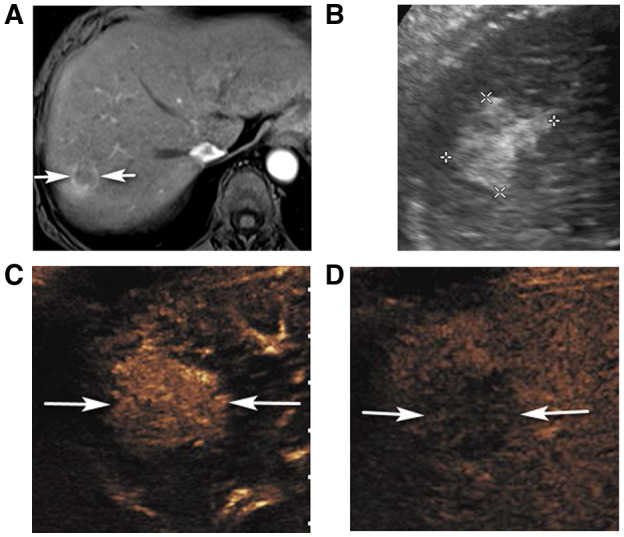
CEUS LR‐5 in a 67‐year‐old woman with hepatitis C cirrhosis and CT/MRI LR‐M observation. (A) An axial arterial phase contrast‐enhanced T1‐weighted fat‐suppressed MR image shows a rim enhancing observation (arrows). (B) Corresponding ultrasound image shows an irregularly marginated solid, echogenic nodule (between calipers). CEUS shows diffuse, heterogeneous APHE of the nodule (arrows in C, 23 seconds) with definite washout on late phase (arrows in D, 4 minutes 7 seconds) enabling characterization as CEUS LR‐5. Biopsy showed HCC.
FIG 4.
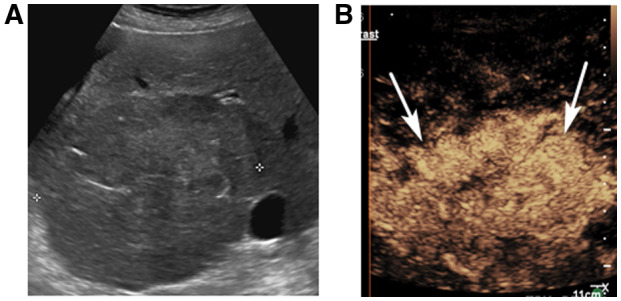
CEUS LR‐5 in a 70‐year‐old woman with cryptogenic cirrhosis and a liver observation on screening ultrasound. (A) A transverse ultrasound image shows a large area of architectural distortion (between calipers) in the right lobe of the liver. Patient was unable to lay flat due to hepatohydrothorax and could not undergo CT/MRI. CEUS shows APHE of the nodule (arrows in B, 13 seconds), with mild, late washout (not shown), characterized as CEUS LR‐5.
FIG 5.
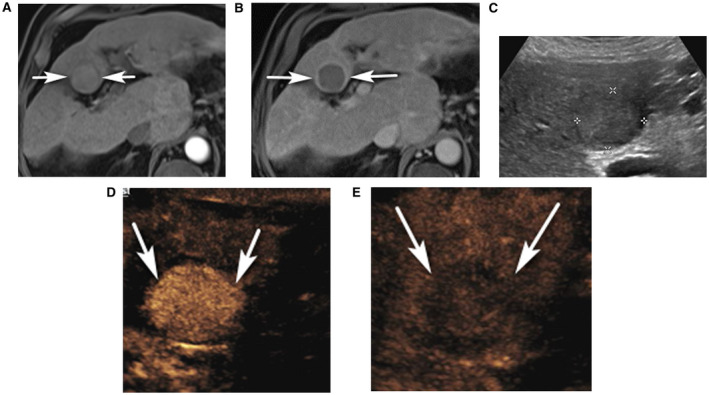
CEUS LR‐5 in a 68‐year‐old man with ethanol cirrhosis and CT/MR LR‐4 observation with suspected arterial mistiming. (A) An axial arterial phase contrast‐enhanced T1‐weighted fat‐suppressed MR image shows an observation (arrows) with no definite APHE. (B) Axial 5‐minute delayed phase contrast‐enhanced T1‐weighted fat‐suppressed MR image shows washout with capsule (arrows), and the observation was characterized as CT/MRI LR‐4. (C) Ultrasound image shows a solid isoechoic nodule (between calipers). CEUS shows APHE of the nodule (arrows in D, 23 seconds), with definite washout on late phase (arrows in E, 4 minutes 7 seconds), enabling characterization as CEUS LR‐5.
Challenges for CEUS IN LI‐RADS
Some drawbacks of CEUS are listed in Table 1. In brief, the small field of view renders CEUS best for focused examinations and not for staging. Other limitations may include: (1) CEUS of an observation or nodule occult on grayscale ultrasound; in this scenario, the sonographer can use anatomic landmarks to evaluate the area of interest and perform an initial injection to help localize the observation; if APHE or washout is observed, a repeat injection can be performed to further assess enhancement characteristics; (2) CEUS of an observation or nodule deep in the liver, or in a background of severe hepatic steatosis; and a (3) large body habitus. If the observation or nodule is difficult to visualize on grayscale ultrasound, it will likely be difficult to evaluate with CEUS.
Future Directions
CEUS LI‐RADS currently evaluates nodules visible on grayscale ultrasound. Future versions of CEUS may include: (1) how to evaluate nodules occult on grayscale imaging, (2) assessment of treatment response, (3) use in liver HCC screening, and (4) use of Kupffer cell–specific contrast agents.
Conclusion
LI‐RADS now includes a system for performance and interpretation of CEUS in patients at risk for HCC. The CEUS LI‐RADS algorithm has high positive predictive value and specificity for diagnosing HCC and differentiating from non‐HCC malignancy. An invaluable imaging tool and problem solver in the patient at risk for HCC, CEUS particularly excels in characterizing indeterminate CT/MRI liver observations and improves patient care.
Potential conflict of interest: D.T.F. has research agreements with Philips Healthcare and Siemens Healthineers. Y.K. received research support from GE Healthcare, Canon Medical Systems, Lantheus Medical Imaging, and Bracco Imaging Inc.
References
- 1. LI‐RADS ® v2017 CEUS Core CEUS Diagnostic Table . Available at: https://www.acr.org/‐/media/ACR/Files/RADS/LI‐RADS/CEUS‐LI‐RADS‐2017‐Core.pdf. Accessed August 19, 2020.
- 2. Wilson SR, Lyshchik A, Piscaglia F, et al. CEUS LI‐RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol 2018;43:127‐142. [DOI] [PubMed] [Google Scholar]
- 3. International Agency for Research on Cancer . Globacan 2018 11‐Liver‐fact‐sheet. Available at: https://gco.iarc.fr/today/data/factsheets/cancers/11‐Liver‐fact‐sheet.pdf. Accessed August 21, 2020.
- 4. Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast‐enhanced ultrasound. Hepatology 2010;51:2020‐2029. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu G‐J, Wang W, Lu M‐D, et al. Contrast‐enhanced ultrasound for the characterization of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Liver Cancer 2015;4:241‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wildner D, Bernatik T, Greis C, et al. CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients – early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med 2015;36:132‐139. [DOI] [PubMed] [Google Scholar]
- 8. Terzi E, Iavarone M, Pompili M, et al. Contrast ultrasound LI‐RADS LR‐5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 2018;68:485‐492. [DOI] [PubMed] [Google Scholar]
- 9. Zheng W, Li Q, Zou X‐B, et al. Evaluation of contrast‐enhanced US LI‐RADS version 2017: application on 2020 liver nodules in patients with hepatitis b infection. Radiology 2020;294:299‐307. [DOI] [PubMed] [Google Scholar]
- 10. Huang J‐Y, Li J‐W, Lu Q, et al. Diagnostic accuracy of CEUS LI‐RADS for the characterization of liver nodules 20 mm or smaller in patients at risk for hepatocellular carcinoma. Radiology 2020;294:329‐339. [DOI] [PubMed] [Google Scholar]
- 11. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723‐750. [DOI] [PubMed] [Google Scholar]


