Abbreviations
- CC
cholangiocarcinoma
- CSI
chemical shift imaging
- CT
computed tomography
- DWI
diffusion‐weighted imaging
- FAP
familial adenomatous polyposis
- FNH
focal nodular hyperplasia
- GFR
glomerular filtration rate
- GSD
glycogen storage disease
- HCA
hepatocellular adenoma
- HCC
hepatocellular carcinoma
- HNF‐1A
hepatocyte nuclear factor‐1 alpha
- LI‐RADS
Liver Imaging Reporting and Data System
- MODY3
maturity‐onset diabetes of the young
- MRCP
magnetic resonance cholangiopancreatography
- MRI
magnetic resonance imaging
- OCP
oral contraceptive pill
- SI
signal intensity
- T2WI
T2‐weighted imaging
Computed tomography (CT) and magnetic resonance imaging (MRI) are both extremely helpful in the diagnosis of liver lesions. Each has strengths and weaknesses for particular indications, and having an appropriate history is important in choosing the best modality for the patient.
In CT, there are several protocols to choose from when imaging the abdomen and, in particular, when imaging the liver. In addition to the unenhanced scan, a dedicated liver CT has three phases after intravenous contrast agent administration: the late arterial (25‐35 seconds), portal venous (60‐75 seconds), and delayed (3‐5 minutes) phases.
Liver parenchyma is mainly supplied by the portal vein, and thus predominantly enhances during the portal venous phase. Liver lesions, however, are supplied only by the hepatic artery. 1 The multiphasic CT and magnetic resonance protocols take advantage of the various enhancement patterns of each type of lesion to noninvasively characterize them. For instance, hypervascular lesions appear brighter than the background liver on the arterial phase, whereas hypovascular ones are darker on the portal venous phase when the background liver enhances maximally. Moreover, peculiar enhancement patterns are typically seen in some liver lesions, for example, early peripheral nodular enhancement and delayed fill‐in is characteristic of liver hemangioma.
For assessment of focal liver lesions, standard MRI protocol should include:
-
T2‐weighted sequences
Half‐Fourier‐acquired single‐shot turbo spin‐echo: provides an anatomic overview and highlights the bright signal of fluid‐containing spaces and structures, such as gallbladder and bile ducts
T2 fat‐saturated: assesses T2‐signal characteristics of liver lesions
-
T1‐weighted sequence
T1 in‐ and opposed‐phase: drop of signal intensity (SI) on opposed‐phase relative to in‐phase images indicates microscopic fat in a liver lesion, while a drop of signal on in‐phase images is detected in a siderotic liver nodule as a result of iron deposition
T1 fat‐saturated: T1 bright signal in a liver lesion can be seen in a hemorrhagic cyst, hemorrhagic metastasis, or melanoma metastasis
Diffusion‐weighted imaging (DWI): improves sensitivity for the detection of small, even subcentimeter, focal lesions 2
Multiphase imaging before and after intravenous injection of an extracellular contrast agent includes precontrast, late arterial, portal venous, and delayed phases
Newer hepatobiliary‐specific agents used in MRI are specifically taken up by hepatocytes and excreted in the bile. The enhancement of normal liver parenchyma and bile ducts peaks at the hepatobiliary phase (20‐60 minutes), helping to detect nonhepatocellular origin lesions, such as metastases, which have no functioning hepatocytes to take up the contrast and will be hypoenhancing during the hepatobiliary phase, while functioning hepatocytes of lesions of hepatocellular origin, such as focal nodular hyperplasia (FNH), take up the contrast and are enhancing during the hepatobiliary phase. 3
Liver MRI protocol varies according to the provided clinical history. In patients who have diffuse liver disease and a suspected liver lesion, a standard liver MRI is performed with multiphasic postcontrast imaging. If the patient presents with a cholestatic liver profile, magnetic resonance cholangiopancreatography (MRCP) should be added to assess the degree and level of biliary obstruction and to determine whether the obstructive lesion is periductal or endoluminal. In asymptomatic patients who have incidentally detected liver lesions, hepatobiliary‐specific agents may be used instead of extracellular fluid contrast agents to differentiate FNH from hepatocellular adenoma (HCA). Hepatobiliary contrast agent‐enhanced MRI is also recommended in oncology patients who need accurate detection and mapping of hepatic metastases.
Each modality has advantages and specific limitations (Table 1). CT attenuation or MRI signal characteristics are exploited to characterize liver lesions. Awareness of the key imaging features would help to decide the modality of choice for each diagnostic category (Table 2). In this review, we discuss the spectrum of imaging features of the most common focal hepatic pathologies (Table 3).
TABLE 1.
Advantages and Limitations of CT and MRI in Liver Imaging
| CT | MRI | |
|---|---|---|
| Advantages |
|
|
| Limitations |
|
|
TABLE 2.
Pros and Cons of CT Versus MRI in Characterization of Liver Lesions
| CT | MRI | |
|---|---|---|
| Pros |
|
|
| Cons |
|
|
TABLE 3.
HCA Subtypes, Epidemiology, and Important Imaging Features at MRI
| Frequency | Risk Factors and Associations | Complications | Signal Dropout on CSI | T2 Signal | Enhancement | |
|---|---|---|---|---|---|---|
| Inflammatory HCA | 40%‐50% | More in women, OCPs, obesity, and systemic inflammatory syndromes | Highest risk for hemorrhage | Absent or only focal | Markedly hyperintense more toward the periphery “atoll sign” | Strong arterial enhancement, persists on the poral venous and delayed phases |
| HNF‐1A mutation HCA | 30%‐35% | Almost exclusively in women | Low risk for complications in tumors <5 cm | Diffuse signal dropout | Isointense to slightly hyperintense | Moderate arterial enhancement, does not persist on the portal venous phase |
| OCPs | ||||||
| MODY3 | ||||||
| β‐Catenin activated HCA | 10%‐15% | More in men | Highest risk for malignancy | No specific feature | ||
| Anabolic steroids, GSD, and FAP | May mimic HCC showing strong arterial enhancement and portal venous washout | |||||
| Unclassified HCA | <5% | No specific gene mutation | No specific imaging features |
Simple Hepatic Cyst
Cysts are incidentally discovered on cross‐sectional imaging of the liver and show CT fluid attenuation or MRI fluid SI without enhancement. The hyperintense T2 signal on MRI similar to cerebrospinal fluid is characteristic of benign cysts. CT is usually sufficient for detecting cysts, but in cases of lesions <1 cm, MRI may be more useful in differentiating cysts from subcentimeter metastases. 4 , 5
Hepatic Abscess
The imaging features of liver abscess vary according to its evolution. At an early stage, it can appear as a heterogeneous solid mass on CT, whereas a mature abscess has a necrotic hypoattenuating center and enhancing rim. 3 The clinical and laboratory findings, including fever, right upper quadrant abdominal pain, neutrophilic leukocytosis, and elevated alkaline phosphatase, can be very useful for the diagnosis of abscess. 6 In some cases, however, it may be quite challenging to differentiate an abscess from a necrotic tumor, and biopsy with tissue diagnosis may be required. 6 Some features on MRI may help to make this distinction. The “double target sign” refers to a layered‐wall enhancement surrounding the abscess cavity where the outer layer shows a delayed enhancement relative to the inner one. 6 In one study, this lesion was more frequently found in abscesses. However, delayed washout of the outer layer was more noted in malignant tumors. 7
Hemangioma
The typical features of hemangioma of early peripheral interrupted enhancement and delayed progressive enhancement (fill‐in) are sufficient to make the diagnosis on CT and MRI. 8 However, MRI may be required in some atypical hemangiomas, particularly in patients with a known malignancy or at risk for hepatocellular carcinoma (HCC). 9 Capillary hemangiomas appear as small hyperenhancing lesions and may be difficult to differentiate from small hypervascular tumors. On MRI, marked hyperintensity on heavily T2‐weighted imaging (T2WI) is helpful to diagnose a hemangioma. 8 Sclerosed hemangiomas have atypical imaging appearance on CT and MRI. A comparison with the previous examination is advisable to determine whether typical features of hemangioma were present in this location previously. A “bright dot sign” is a helpful feature to diagnose this type of hemangioma and refers to a peripheral enhancing dot without complete delayed enhancement. However, a biopsy may still be needed to confirm the diagnosis because of overlapping appearances between sclerosed hemangiomas, cholangiocarcinoma (CC), and metastases. 8 , 9
FNH
On CT/MRI, FNH shows marked arterial phase hyperenhancement and becomes isoenhancing to the liver parenchyma during the portal venous and delayed phases. 3 Hepatobiliary contrast agent‐enhanced MRI has the advantage over CT by showing contrast taken up by the functioning hepatocytes within the FNH, which enhances during the hepatobiliary phase. This allows confident diagnosis of FNH and differentiating it from HCAs, HCC, and metastasis. 10 In a systematic review, gadoxetic acid (hepatobiliary contrast agent)‐enhanced MRI was able to discriminate between HCA and FNH on the hepatobiliary phase with 91% to 100% sensitivity and 87% to 100% specificity. 11
HCA
HCAs are difficult to accurately diagnose on CT due to a similar appearance to benign lesions (e.g., FNH) and malignant tumors (e.g., HCC). 9 However, MRI can be useful in diagnosing HCA and in recognizing HCA subtypes. 12 Inflammatory HCAs usually demonstrate high SI on T2WI and marked hyperenhancement on the arterial phase, which persists on the portal and delayed phases. These MRI features have 85% to 88% sensitivity and 88% to 100% specificity for the diagnosis of inflammatory HCA. 13 , 14 In HCA with hepatocyte nuclear factor‐1 alpha (HNF‐1A) mutation, diffuse intratumoral microscopic fat is shown as diffuse and homogeneous signal dropout on T1 opposed‐phase relative to in‐phase images (Fig. 1), with a sensitivity of 87% to 91% and specificity of 89% to 100%. 13 , 14 β‐Catenin‐activated HCA and unclassified HCA have no specific imaging features on MRI. Thus, if a suspected HCA cannot be confidently classified as either an inflammatory subtype or an HNF‐1A mutated subtype, a biopsy may be considered for definitive diagnosis. 10
FIG 1.
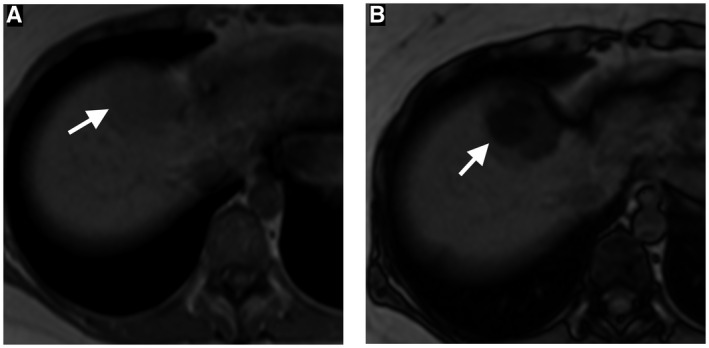
A 35‐year‐old woman with HNF‐1A mutation hepatic adenoma. Axial in‐phase (A) and out‐of‐phase (B) chemical shift MRIs show significant drop of signal on out‐of‐phase image because of intravoxel fat, a characteristic imaging feature of HNF‐1A mutation hepatic adenoma.
Cholangiocarcinoma
On CT/MRI, CC may appear as a biliary stricture in periductal infiltrative (perihilar) CC, hypovascular tumor with peripheral enhancement in mass‐forming CC, or intraductal nodular mass. 15 CT provides an accurate assessment of vascular invasion but tends to underestimate the biliary extent of perihilar CC. 16 MRCP is an accurate, noninvasive method for evaluation of the biliary extension of the tumor and the entire biliary tree, with an accuracy rate of 95%. However, MRCP may suffer motion artifact. 17 Although vascular invasion also can be identified on MRI and magnetic resonance angiography, it may be overestimated because of misinterpretation of the peritumoral inflammation and fibrosis as tumor infiltration, especially in patients after biliary stenting. Therefore, unless immediately required, stenting should be delayed after staging MRI. 18 In mass‐forming CC, hepatobiliary‐specific agent MRI, including DWI, is more accurate in detecting intrahepatic metastasis. On the contrary, it may mask perihilar CC because of the simultaneous enhancement of liver parenchyma during the hepatobiliary phase. 17
Metastasis
CT is the preferred imaging modality for the initial assessment and posttreatment surveillance because it allows an excellent overview of the primary tumor and other potential sites for metastases. 19 Calcified metastases, such as colorectal metastases, are better assessed with CT 20 (Fig. 2). MRI has better sensitivity in the detection of small liver metastasis that can be missed on CT 21 (Fig. 3). MRI is also useful in presurgical planning for resection of metastasis or when hepatic metastasis precludes resection of the primary tumor (e.g., pancreatic cancer). 19 , 22 In one study, gadoxetic acid (hepatobiliary contrast agent)‐enhanced MRI had a sensitivity of 86% for the detection of colorectal hepatic metastases ≤1 cm compared with 50% with contrast‐enhanced CT. Gadoxetic acid‐enhanced MRI combined with DWI had the highest sensitivity (95%). 23 Moreover, liver steatosis is a known limitation for the assessment of small hypoattenuating metastases on CT; therefore, MRI is a problem‐solving tool in this condition. 19 , 22
FIG 2.
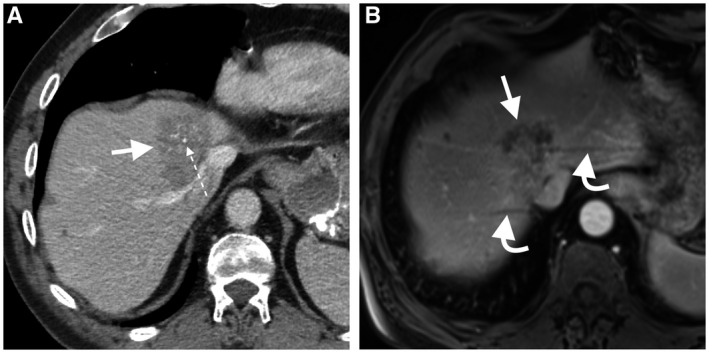
A 65‐year‐old woman with hepatic metastasis from mucinous colorectal cancer. Central calcification (dotted arrow) within a hypoattenuating liver mass is seen only on portal venous phase CT image (A). The mass is heterogeneous hypoenhancing on the axial dynamic postcontrast portal venous‐phase MRI (B), but calcification is not seen. Also, note the respiratory motion artifacts on MRI (curved arrows), a recognized limitation of MRI in the assessment of liver lesion close to the hepatic dome.
FIG 3.
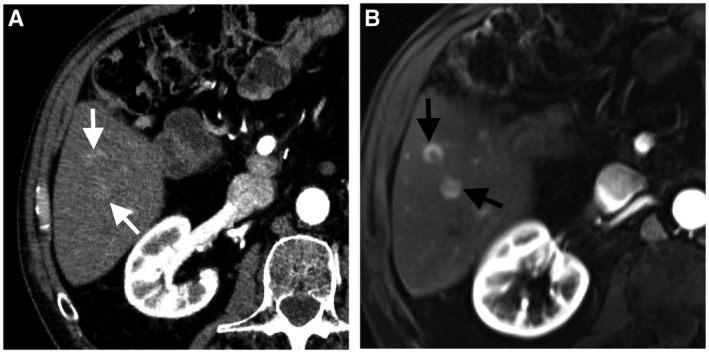
A 43‐year‐old man with hepatic metastasis from pancreatic neuroendocrine tumor. Axial arterial‐phase CT image (A) shows two subtle arterial‐phase hyperenhancing metastatic nodules in hepatic segment 6 (white arrows). Axial arterial‐phase dynamic postcontrast MRI (B) shows improved visibility of subtle metastases (black arrows).
Conclusions
CT and MRI have been established cross‐sectional imaging modalities useful for the evaluation and characterization of liver lesions. CT is usually more easily available, and scan time is short. MRI has a better contrast resolution and is an excellent problem‐solving tool. Choosing which modality to start with is dependent on many factors and requires good communication between requesting physicians and the radiologist to optimize the imaging modality and technique.
Potential conflict of interest: Nothing to report.
References
- 1. Asayama Y, Yoshimitsu K, Nishihara Y, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic‐pathologic correlation. Am J Roentgenol 2008;190:W28‐W34. [DOI] [PubMed] [Google Scholar]
- 2. Galea N, Cantisani V, Taouli B. Liver lesion detection and characterization: role of diffusion‐weighted imaging. J Magn Reson Imaging 2013;37:1260‐1276. [DOI] [PubMed] [Google Scholar]
- 3. Venkatesh SK, Chandan V, Roberts LR. Liver masses: a clinical, radiologic, and pathologic perspective. Clin Gastroenterol Hepatol 2014;12:1414‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegelman ES, Chauhan A. MR characterization of focal liver lesions: pearls and pitfalls. Magn Reson Imaging Clin N Am 2014;22:295‐313. [DOI] [PubMed] [Google Scholar]
- 5. Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics 2001;21:895‐910. [DOI] [PubMed] [Google Scholar]
- 6. Bächler P, Baladron MJ, Menias C, et al. Multimodality imaging of liver infections: differential diagnosis and potential pitfalls. Radiographics 2016;36:1001‐1023. [DOI] [PubMed] [Google Scholar]
- 7. Park HJ, Kim SH, Jang KM, et al. Differentiating hepatic abscess from malignant mimickers: value of diffusion‐weighted imaging with an emphasis on the periphery of the lesion. J Magn Reson Imaging 2013;38:1333‐1341. [DOI] [PubMed] [Google Scholar]
- 8. Mamone G, Cortis K, Sarah A, et al. Hepatic morphology abnormalities: beyond cirrhosis. Abdom Radiol (NY) 2018;43:1612‐1626. [DOI] [PubMed] [Google Scholar]
- 9. Jang H‐J, Yu H, Kim TK. Imaging of focal liver lesions. Semin Roentgenol 2009;44:266‐282. [DOI] [PubMed] [Google Scholar]
- 10. Belghiti J, Cauchy F, Paradis V, et al. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol 2014;11:737‐749. [DOI] [PubMed] [Google Scholar]
- 11. McInnes MD, Hibbert RM, Inacio JR, et al. Focal nodular hyperplasia and hepatocellular adenoma: accuracy of gadoxetic acid–enhanced MR imaging—a systematic review. Radiology 2015;277:413‐423. [DOI] [PubMed] [Google Scholar]
- 12. Dharmana H, Saravana‐Bawan S, Girgis S, et al. Hepatocellular adenoma: imaging review of the various molecular subtypes. Clin Radiol 2017;72:276‐285. [DOI] [PubMed] [Google Scholar]
- 13. Laumonier H, Bioulac‐Sage P, Laurent C, et al. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology 2008;48:808‐818. [DOI] [PubMed] [Google Scholar]
- 14. Ronot M, Bahrami S, Calderaro J, et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 2011;53:1182‐1191. [DOI] [PubMed] [Google Scholar]
- 15. Valls C, Ruiz S, Martinez L, et al. Radiological diagnosis and staging of hilar cholangiocarcinoma. World J Gastrointestinal Oncol 2013;5:115‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruys AT, Van Beem BE, Engelbrecht M, et al. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta‐analysis. Br J Radiol 2012;85:1255‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges. Radiology 2018;288:7‐13. [DOI] [PubMed] [Google Scholar]
- 18. Chryssou E, Guthrie JA, Ward J, et al. Hilar cholangiocarcinoma: MR correlation with surgical and histological findings. Clin Radiol 2010;65:781‐788. [DOI] [PubMed] [Google Scholar]
- 19. Kaur H, Hindman NM, Al‐Refaie WB, et al. ACR Appropriateness criteria® suspected liver metastases. J Am College Radiol 2017;14:S314‐S325. [DOI] [PubMed] [Google Scholar]
- 20. Martinez L, Puig I, Valls C. Colorectal liver metastases: radiological diagnosis and staging. Eur J Surg Oncol 2007;33:S5‐S16. [DOI] [PubMed] [Google Scholar]
- 21. Dromain C, de Baere T, Lumbroso J, et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol 2004;23:70‐78. [DOI] [PubMed] [Google Scholar]
- 22. Albiin N. MRI of focal liver lesions. Curr Med Imaging 2012;8:107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HJ, Lee SS, Byun JH, et al. Incremental value of liver MR imaging in patients with potentially curable colorectal hepatic metastasis detected at CT: a prospective comparison of diffusion‐weighted imaging, gadoxetic acid–enhanced MR imaging, and a combination of both MR techniques. Radiology 2015;274:712‐722. [DOI] [PubMed] [Google Scholar]


