Supplemental Digital Content is available in the text.
Background:
Exposure to higher levels of ambient air pollution is a known risk factor for cardiovascular disease but long-term effects of pollution exposure on the pulmonary vessels are unknown.
Methods:
Among 2428 Framingham Heart Study participants who underwent chest computed tomography (CT) between 2008 and 2011, pulmonary vascular volumes were calculated by image analysis, including the total vascular volume and small vessel volume (cross-sectional area <5 mm2; BV5 defined as small vessel volume). Using spatiotemporal models and participant home address, we assigned 1-year (2008) and 5-year (2004–2008) average concentrations of fine particulate matter (PM2.5), elemental carbon (EC), and ground-level ozone (O3), and distance to major roadway. We examined associations of 1- and 5-year exposures, and distance to road, with CT vascular volumes using multivariable linear regression models.
Results:
There was a consistent negative association of higher O3 with lower small vessel volumes, which persisted after adjustment for distance to road. Per interquartile range (IQR) of 2008 O3, BV5 was 0.34 mL lower (95% confidence intervals [CI], −0.61 to −0.06; P = 0.02), with similar results for 5-year exposure. One-year EC exposure and closer proximity to road were weakly associated with small vessel volumes; BV5 was 0.18 mL higher per IQR of 2008 EC (95% CI, −0.05 to 0.42; P = 0.13) and 0.40 mL higher per IQR closer proximity to road (95% CI: −0.10 to 0.89; P = 0.12). PM2.5 was not associated with small vascular volumes; BV5 was 0.26 mL lower per IQR of 2008 PM2.5 (95% CI: −0.68 to 0.16; P = 0.22).
Conclusions:
Among community-dwelling adults living in the northeastern United States, higher exposure to O3 was associated with lower small pulmonary vessel volumes on CT.
What this study adds
While ambient air pollution is associated with systemic vascular disease, it is not clear a similar relationship exists with the pulmonary vessels. Using data from more than 2400 participants in the Framingham Heart Study, our study investigated the association of markers of ambient air pollution—including fine particulate matter, elemental carbon, ground-level ozone, and distance to roadway—with pulmonary vascular volumes derived from quantitative computed tomographic image analysis. We found that ozone exposure was consistently inversely associated with small vessel volumes on computed tomography. This suggests that long-term ozone exposure may be associated with vasoconstriction and loss of the small pulmonary vessels.
Introduction
Exposure to higher levels of pollution is a known risk factor for cardiovascular morbidity and mortality, including greater risk of systemic hypertension, atherosclerosis, myocardial infarction, and heart failure.1–9 While the lungs are the site of entry of inhaled gaseous and particulate pollutants, and a site of inflammatory injury to the airways and lung parenchyma,8,10,11 the long-term effects of air pollution on pulmonary vascular structure is unknown. Even mild pulmonary vascular dysfunction is associated with poor outcomes including higher mortality,12–14 and therefore even subtle differences in pulmonary vascular structure and function attributable to pollution may be of relevance to public health.
Prior studies evaluating the associations of inhaled toxins, including tobacco and particulate air pollution, with pulmonary vascular structure and function have yielded inconsistent results. On one hand, smaller studies with controlled exposures have generally demonstrated vascular remodeling and higher pulmonary vascular tone in association with higher levels of pollution exposure. For example, in rodents, both exposure to particulate matter ranging from 24 hours to 4 months is associated with vascular remodeling in the pulmonary vessels,15–17 while a 2-hour air pollutant exposure in human volunteers has shown an increase in pulmonary vascular tone in 1 study,18 although not others.19 In addition, greater long-term exposure to PM2.5 has been linked to elevated pulmonary arterial pressures in children20 and reduced transplant-free survival in adults with pulmonary arterial hypertension.21 In contrast, two more recent, larger cohort studies have suggested that greater peripheral vascular volumes are linked to higher levels of exposure to tobacco and air pollution. Using image analysis of computed tomography (CT) scans, the Multi-Ethnic Study of Atherosclerosis (MESA) found that long-term exposure to black carbon was associated with greater vascular volumes of the small, peripheral pulmonary blood vessels.22 Using a related technique in the Framingham Heart Study, we reported a similar association of tobacco exposure with higher volumes of the pulmonary vessels on CT (including the total vascular volume and the volume of the small vessels alone).23
From this existing literature, it remains unclear how ambient exposures, particularly at the lower levels experienced by general populations in the United States, affect pulmonary vasculature structure. To address this gap, we investigated associations of long-term exposure to ambient pollutants, including fine particulate matter (PM2.5), elemental carbon (EC, a marker of traffic-related pollution), and ground-level ozone (O3) and CT-based pulmonary blood vessel volumes in participants of the Framingham Heart Study, a group not selected on the basis of pulmonary or cardiovascular disease and who are exposed to relatively low levels of ambient air pollution.
Methods
Study population
The study population consists of participants of the Framingham Heart Study Offspring and Third Generation cohorts who took part in the Multi-Detector CT 2 sub-study and underwent volumetric thoracic CT between 2008 and 2011 as previously described.23,24 A total of 2470 participants had CT vascular data; those without assessment of any air pollutant were excluded, leaving 2428 individuals in the analysis. This study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Boards of the Beth Israel Deaconess Medical Center and Boston University Medical Campus approved this study. All participants provided written informed consent.
Air pollution assessment
We used a spatiotemporal model to estimate ambient PM2.5 concentrations for the residential address of each participant. This model provides estimates of daily PM2.5 at 200 × 200 m resolution by utilizing satellite-based aerosol optical depth data (retrieved using the Multi-Angle Implementation of Atmospheric Correction algorithm at 1 × 1 km resolution), as well as ground-level daily PM2.5 mass measurements, meteorological covariates, and land use terms.25,26 The mean out-of-sample R2 for predictions from this model was 0.88.26 We estimated ambient elemental carbon (EC) using a hybrid neural network model, which predicts ground-level EC exposure at a 1 × 1 km resolution. This model uses GEOS-Chem (Goddard Earth Observing System) three-dimensional chemical transport simulations and a neural network incorporating daily meteorological variables and land use terms, with calibration at monitoring sites.27,28 The total annual R2 for EC estimates was 0.71–0.75 for 2004–2008.29 Twenty-four hour estimates of ground-level ozone (O3) exposure (at a 1 × 1 km resolution) was assigned using a prediction model, which also used a hybrid neural network using GEOS-Chem transport simulations, land use terms, and satellite-based estimates of total column and vertical profile O3 (to estimate the fraction of ground-level O3 in the total column O3).30 Finally, to account for tropospheric reactions of precursors that produce and deplete O3, the neural network model included emissions inventory daily United States Environmental Protection Agency (USEPA) Air Quality System measures of sulfur dioxide, nitrogen dioxide, nitrogen oxides, and volatile organic compounds.29 The cross-validated annual R2 for 8-hour maximum O3 for this model was 0.76–0.78 for 2004–2008.30
Exposures were assigned to each participant based on their residential address recorded at the time of their Framingham Heart Study exam. As our primary exposure, we used the annual mean concentrations for 2008 (the year the CT sub-study began) as a measure of recent, intermediate-term particulate matter exposure. In additional analyses, we also examined associations with the annualized average exposure over a five-year period from 2004 to 2008 as a measure of longer-term exposure. We used the same one- and five-year exposure periods for all participants to reduce bias related to downward temporal trends in air pollutant concentrations and to avoid confusing differences in exposure due to residential location versus choice of year, which is consistent with our prior work.25,29
Proximity to nearest major roadway
Using data from the Framingham study visit nearest to the CT, we geocoded each participants’ home address using ArcGIS (Esri, Redlands, CA). As a measure of traffic-related emissions, we calculated the distance from the home to the nearest major roadway (A1, A2, or A3), as defined by the US Census feature classification system as previously described.25 To reflect the known exponential decay of traffic emissions as a function of distance to roadway, we examined the negative natural log of distance to roadway as a continuous exposure (with higher values therefore indicating potentially greater traffic-related pollution exposure) as in our prior work.25,31
Radiographic pulmonary vascular assessment
Inspiratory CT examinations covering the entire thorax were performed in the supine position without contrast using a 64-detector-row CT scanner (Discovery, GE Healthcare, Waukesha, WI) as previously described.23,24,32 Using software based on the Chest Imaging Platform (www.chestimagingplatform.org), vascular image analysis was performed in the Applied Chest Imaging Laboratory at Brigham and Women’s Hospital.32 Using automated algorithms, three-dimensional reconstructions of the pulmonary vasculature were generated (Figure 1). We calculated the total volume of all intraparenchymal vessels (TBV) and the volume of smaller pulmonary vessels, including those with cross-sectional area <5 mm2 (BV5) and those with area between 5 and 10 mm2 (BV10). The small vessel ratios (BV5/TBV and BV10/TBV, respectively) were used to represent the relative distribution of blood vessel volume in the smallest, most peripheral blood vessels detectable by CT. A lower small vessel ratio indicates more severe radiographic pulmonary vascular pruning.23,24,32,33
Figure 1.
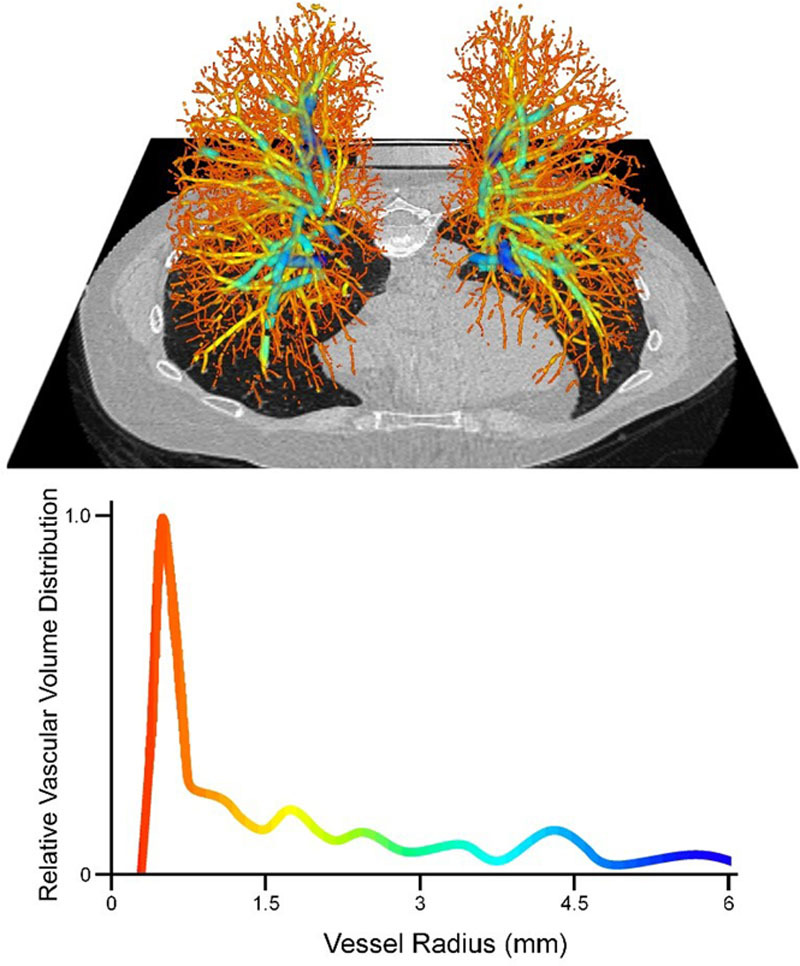
(Top) Three-dimensional volumetric reconstruction of the pulmonary vascular tree from a Framingham Heart Study participant, overlaid onto axial CT section. (Bottom) Representative quantitative histogram demonstrating distribution of vascular volume as a function of vessel size. The drop in the volume distribution for vessels with radius 0–0.8 mm reflects the limit of resolution of CT. CT indicates computed tomography.
Statistical methods
Multivariable linear regression models were used to examine associations of air pollutants with pulmonary vascular volumes on CT. All models included covariates that were selected a priori based on known or suspected associations with abnormalities of pulmonary vessels. These included age at time of CT, sex, height, weight, smoking status (current, former, or never), total cumulative pack-years of cigarette exposure, second-hand tobacco exposure during adulthood, personal educational attainment, occupation category, median value of owner-occupied housing, study cohort (Offspring vs. Third Generation), and any history of cardiovascular disease (including congestive heart failure, myocardial infarction, angina pectoris, and cerebrovascular accident). We also adjusted for the date of the CT and sine and cosine day of the year to account for seasonality and time. As nearly all participants are of European ancestry, we were unable to adjust for race/ethnicity. Alongside our primary analysis, we created models that additionally adjusted for the forced expiratory volume in 1 second (FEV1)—lung function is a potential confounder as it is known to be associated with air pollution exposure and with pulmonary vascular pathology but could also lie along the pathway between pollution exposure and pulmonary vascular structure. Using generalized additive models with a Gaussian distribution, we plotted penalized splines with three degrees of freedom to evaluate departures from linearity for the association of air pollutants and CT vascular volumes.
In secondary analyses, given prior work demonstrating that associations of tobacco exposure with higher pulmonary vascular volumes and associations of air pollutants and respiratory outcomes may differ by sex,23,34,35 we tested for differential associations of air pollution exposure with blood vessel volumes by sex and by smoking status. We also performed sensitivity analyses excluding those participants who moved between Framingham study visits (and thus did not reside at the same address for the full period from 2004 to 2008) and those who reported being current smokers (to exclude the possibility that the acute inflammatory effects of smoking may mask the association of air pollution and the appearance of the pulmonary vasculature on CT). Given that ozone levels exhibit seasonal variation and are highest during warmer months, we investigated associations of warm-season ozone exposure (April–September) with CT vascular volumes.36,37 Finally, nitrogen oxides, a product of fresh vehicle exhaust, may potentially “scavenge” ground-level ozone38 (and ozone may thus be an inverse marker of vehicle exhaust). Therefore, we constructed multipollutant models, adjusted for both ozone concentration and distance to major roadway, to investigate whether any associations of ozone were explained by traffic-related emissions. For PM2.5, EC, O3, and distance to roadway, regression coefficients were scaled by the width of the interquartile range (IQR) of exposure and reported with 95% confidence intervals. For interaction terms, a threshold P value of <0.10 for the Wald test was used as statistically significant evidence of effect modification. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC), and spline plots were produced using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study participant characteristics
Details of the study cohort are provided in Table 1. Roughly half of the participants were never smokers (48.5%), while only 7.5% were current smokers. Two hundred seventy participants (11.1%) had a history of any cardiovascular disease, and average lung function in the cohort was normal, including a mean percent-predicted FEV1 of 97.9% ± 15.8%. The exposure distributions and correlation matrices for this study are shown in Tables 2 and 3. The median PM2.5, EC, and O3 concentrations for 2008 were 9.6 μg/m3, 0.4 μg/m3, and 38.1 ppb, respectively, and the five-year annualized averages were similar. The median distance to the closest major roadway was 211.4 m.
Table 1.
Characteristics of study participants (n = 2,428)
| Age (y, mean ± SD) | 59.2 ± 11.7 |
|---|---|
| Female (n, %) | 1,242 (51.2) |
| Body-mass index (kg/m2, mean ± SD) | 28.4 ± 5.4 |
| Occupation category (n, %) | |
| Laborer | 193 (8.0) |
| Sales/clerical | 616 (25.4) |
| Professional/executive/supervisory/technical | 1000 (41.2) |
| Other | 619 (25.5) |
| Educational attainment (n, %) | |
| High school or less | 521 (21.5) |
| Some college | 769 (31.7) |
| College/grad school | 1,135 (46.8) |
| Median value of owner-occupied housing unit ($, median, IQR) | 198,600 (90,400) |
| Offspring cohort (n, %) | 1,133 (46.7) |
| Smoking status (n, %) | |
| Never | 1,170 (48.5) |
| Former | 1,062 (44.0) |
| Current | 181 (7.5) |
| Pack-years of smoking (median, IQR) | |
| Entire sample | 0 ± 15.0 |
| Former smokers | 12.0 ± 21.0 |
| Current smokers | 32.9 ± 15.6 |
| Second-hand smoke exposure (n, %) | 624 (25.9) |
| CT pulmonary vascular measures | |
| TBV (mL, mean ± SD) | 143.1 ± 30.9 |
| BV5 (mL, mean ± SD) | 55.9 ± 11.6 |
| BV5/TBV (%, mean ± SD) | 39.3 ± 4.1 |
| BV10 (mL, mean ± SD) | 81.8 ± 15.9 |
| BV10/TBV (%, mean ± SD) | 57.6 ± 3.8 |
CT indicates computed tomography; IQR, interquartile range.
Table 2.
Distributions of air pollutants and distance to roadway
| Annual average of 2008 | Median | IQR width | Range |
| PM2.5 (μg/m3) | 9.6 | 1.6 | 1.0–15.6 |
| Elemental carbon (μg/m3) | 0.4 | 0.1 | 0.07–3.0 |
| Ozone (ppb) | 38.1 | 1.7 | 27.2–50.2 |
| Annualized average from 2004 to 2008 | Median | IQR width | Range |
| PM2.5 (μg/m3) | 9.9 | 1.3 | 2.0–15.9 |
| Elemental carbon (μg/m3) | 0.4 | 0.1 | 0.1–1.4 |
| Ozone (ppb) | 38.4 | 1.9 | 24.4–50.7 |
| Median | IQR width | Range | |
| Distance to major roadwaya (m) | 211.4 | 363.0 | 0.02–1000 |
aThe primary analysis excluded 292 participants (12.0%) who lived ≥1,000 m from a major roadway.
IQR indicates interquartile range.
Table 3.
Correlation matrices of air pollution exposures
| PM2.5 | Elemental carbon | Ozone | Distance to roadwaya | |
|---|---|---|---|---|
| 2008 annual average (spearman correlation coefficient, P value) | ||||
| PM2.5 | 1 | |||
| Elemental carbon | 0.27 (<0.0001) | 1 | ||
| Ozone | −0.18 (<0.0001) | −0.23 (<0.0001) | 1 | |
| Distance to roadwaya | 0.31 (<0.0001) | 0.15 (<0.0001) | −0.20 (<0.0001) | 1 |
| PM2.5 | Elemental carbon | Ozone | Distance to roadwaya | |
| 2008 annual average (spearman correlation coefficient, P value) | ||||
| PM2.5 | 1 | |||
| Elemental carbon | 0.46 (<0.0001) | 1 | ||
| Ozone | −0.16 (<0.0001) | −0.29 (<0.0001) | 1 | |
| Distance to roadwaya | 0.30 (<0.0001) | 0.27 (<0.0001) | −0.24 (<0.0001) | 1 |
aDistance to roadway is defined as the negative natural log of distance to roadway (with higher values therefore indicating greater traffic-related pollution exposure).
Associations with radiographic vascular outcomes
Associations of 2008 annual average air pollutant exposure and distance to roadway with pulmonary vascular volumes on CT, including adjustment for FEV1, are presented in Table 4, Figure 2, and eFigure 1; http://links.lww.com/EE/A125. Results of models examining 2004–2008 annual average exposures, and in models without adjustment for FEV1, are presented in the Supplemental Digital Content (eTables 1 and 2; http://links.lww.com/EE/A125). We found no associations of PM2.5 with radiographic pulmonary vascular volumes, including one- and five-year averages and in models with and without adjustment for FEV1. There was a weak association of 2008 EC exposure with higher small vessel volumes (BV5 and BV10) that did not reach statistical significance. We also found consistent negative associations of O3 with small vessel volumes. For example, per IQR of 2008 O3, BV5 was 0.34 mL lower (95% confidence intervals [CI]: −0.61 to −0.06; P = 0.02), and BV10 was 0.44 mL lower (95% CI: −0.80 to −0.09; P = 0.01). These associations were similar when examining the 2004–2008 five-year averages and in models without adjustment for FEV1. We found a weak association of closer proximity to road with higher BV5 and TBV (Table 4). Penalized spline plots demonstrated roughly linear relationships for these associations (Figure 2).
Table 4.
Associations of air pollutants (2008 Annual average) and distance to roadway with pulmonary vascular volumes on CTa
| Exposure | Model N | Difference in TBV, mL (95% CI) | P | Difference in BV5, mL (95% CI) | P | Difference in BV10, mL (95% CI) | P |
|---|---|---|---|---|---|---|---|
| PM2.5 | 2017 | −0.48 (−1.42 to 0.47) | 0.32 | −0.26 (−0.68 to 0.16) | 0.22 | −0.30 (−0.83 to 0.24) | 0.28 |
| Elemental carbon | 2018 | 0.24 (−0.29 to 0.78) | 0.37 | 0.18 (−0.05 to 0.42) | 0.13 | 0.19 (−0.11 to 0.50) | 0.22 |
| Ozone | 2240 | −0.55 (−1.17 to 0.06) | 0.08 | −0.34 (−0.61 to −0.06) | 0.02 | −0.44 (−0.80 to −0.09) | 0.01 |
| Distance to roadwayb | 1985 | 0.90 (−0.21 to 2.01) | 0.11 | 0.40 (−0.10 to 0.89) | 0.12 | 0.45 (−0.18 to 1.08) | 0.16 |
| Exposure | Model N | Difference in TBV, mL (95% CI) | P | Difference in BV5, mL (95% CI) | P | ||
| PM2.5 | 2017 | −0.08 (−0.21 to 0.06) | 0.27 | −0.03 (−0.16 to 0.10) | 0.62 | ||
| Elemental carbon | 2018 | 0.06 (−0.01 to 0.14) | 0.11 | 0.05 (−0.02 to 0.12) | 0.18 | ||
| Ozone | 2240 | −0.05 (−0.14 to 0.04) | 0.27 | −0.06 (−0.15 to 0.03) | 0.17 | ||
| Distance to roadwayb | 1985 | 0.03 (−0.14 to 0.19) | 0.76 | −0.03 (−0.19 to 0.12) | 0.66 |
aAll models adjusted for age at time of CT, sex, height, weight, smoking status, total pack-years of cigarette exposure, second-hand tobacco exposure during adulthood, personal educational attainment, occupation category, median value of owner-occupied housing, study cohort, date of CT and sine and cosine day of the year terms (to account for seasonality and time), any history of cardiovascular disease, and FEV1. Distance to roadway analysis excludes 292 participants (12.0%) who lived ≥1,000 m from a major roadway. Results expressed per IQR of exposure.
bDistance to roadway is defined as the negative natural log of distance to roadway (with higher values therefore indicating greater traffic-related pollution exposure).
CI indicates confidence interval; CT, computed tomography; IQR, interquartile range.
Figure 2.
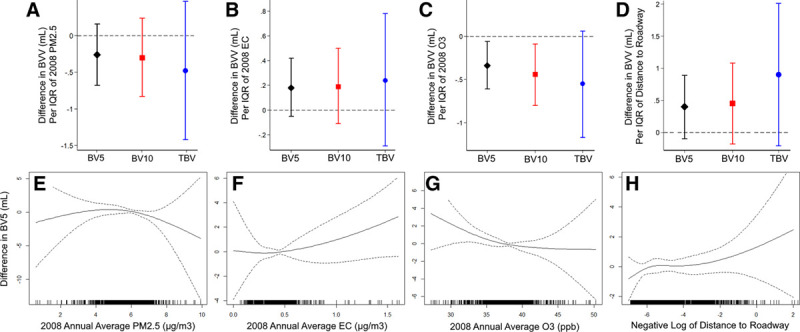
Associations of PM2.5, elemental carbon, ozone (2008 Annual Averages), and distance to roadway with pulmonary vessel volumes on CTa. Upper panes demonstrate difference in BVV (BV5, BV10, and TBV) by IQR of PM2.5 (A), elemental carbon (B), ozone (C), and distance to roadway (D). Lower panes demonstrate difference in BV5b by PM2.5 (E), elemental carbon (F), ozone (G), and distance to roadway (H). aAll results from models adjusted for age at time of CT, sex, height, weight, smoking status, total pack-years of cigarette exposure, second-hand tobacco exposure during adulthood, personal educational attainment, occupation category, median value of owner-occupied housing, study cohort, date of CT and sine and cosine day of the year terms (to account for seasonality and time), any history of cardiovascular disease, and FEV1. Distance to Roadway is defined as the negative natural log of distance to roadway (with higher values therefore indicating greater traffic-related pollution exposure), and those living ≥1 km from a major road were excluded. Results expressed per IQR of exposure. bPenalized splines demonstrating difference in BV5 as a function of air pollutant exposure. Data were fitted using a penalized spline with 3 degrees of freedom. The solid line represents adjusted difference in BV5 and the dashed lines indicate the 95% confidence interval bands. The distribution of the exposure is displayed by the rug plot along the x axis. BVV indicates blood vessel volumes; CT, computed tomography; IQR, interquartile range.
In secondary analyses, we found no consistent evidence of effect modification by sex in the associations of PM2.5, O3, or distance to nearest major roadway with radiographic pulmonary vascular volumes (Pinteraction > 0.10). However, we found that the association of 2008 EC and higher small vessel volumes (BV5 and BV10) differed based on sex. For example, in men, per IQR higher 2008 EC, BV5 was 0.48 mL higher (95% CI: 0.12 to 0.84), whereas no association was observed among women (95% CI: −0.42 to 0.23) (Pinteraction = 0.046). A similar pattern was seen for BV10 (Pinteraction = 0.049) and TBV (Pinteraction = 0.04). We also found that the association of EC (both one- and five-year averages) with radiographic vascular volumes differed based on smoking status. For example, among current smokers, per IQR higher 2008 EC, BV5 was 1.45 mL higher (95% CI: 0.56 to 2.33), while a weaker association was seen among former smokers (0.28 mL; 95% CI: −0.13 to 0.69) and no association was seen among never-smokers (Pinteraction = 0.003). Similar patterns were seen for associations involving BV10 and TBV, and for 2004–2008 EC exposure. We found no evidence of effect modification by smoking status in the associations involving PM2.5, O3, or distances to nearest major roadway (Pinteraction > 0.10).
We found that ozone concentrations were higher during the warm season (April–September). The warm-season ozone concentrations for 2008 and 2004–2008 were 44.5 and 44.7 ppb, respectively, compared with 38.1 and 38.4 ppb for the full-year averages. In models evaluating associations with warm-season ozone exposure and CT vascular volumes, we found that ozone remained associated with small vessel volumes. For example, per IQR of 2008 warm-season O3, BV5 was 0.40 mL lower (95% CI: −0.79 to −0.12; P = 0.006). Similarly, in multipollutant models, we found that one- and five-year ozone exposure remained consistently associated with small vessel volumes after adjustment for distance to roadway.
In sensitivity analyses excluding those who moved between Framingham exam visits, we found that the negative associations of one- and five-year O3 exposure with BV5 and BV10 were strengthened, with greater differences in volumes per IQR of O3 compared with the full analysis. When excluding current smokers, the associations of EC with BV5 and BV10 are attenuated and lose significance, while the inverse associations of O3 with BV5 and BV10 remained similar.
Discussion
In this large cohort of community-dwelling adults living in the northeastern United States, higher ozone exposure was consistently associated with smaller vascular volumes of the small pulmonary vessels on CT imaging. The association of ozone and small vessel volumes was also more robust when additionally adjusting for FEV1 and when examining only those participants who did not move from their residence during the study period, did not differ based on smoking status, and persisted after adjustment for distance to roadway and when examining warm-season ozone exposure.
In both animal models and in human studies, ozone exposure has been associated with vasoconstriction, impaired vasorelaxation, and remodeling of the systemic vasculature.39–46 In rodents, acute exposure to ozone resulted in vasoconstriction (due to increased vascular tone and augmented response to vasoconstrictors)47 and impaired vasorelaxation,48 while prolonged exposure was linked to increases in arterial tone, diastolic blood pressure, and decreased tissue NO.43 In humans, similar findings have been observed, including associations of both short- and long-term exposure to ozone with higher endothelin-1 levels (a potent vasoconstrictor), reduced brachial artery diameter, decreased arterial elasticity, and higher blood pressure.42,45,49–51 Additionally, a recent study by Wang et al demonstrated associations of ozone with structural remodeling of the carotid artery.52
However, very little is known about the effect of long-term ozone exposure on the pulmonary vasculature in adults. In a small group of both healthy and hypertensive volunteers who underwent catheterization of the pulmonary and radial artery, short-term ozone exposure (at a concentration of 0.3 ppm for three hours) did not acutely result in differences in pulmonary artery pressure, pulmonary vascular resistance, or cardiac output.53 Recently, Aaron et al also found a similar, albeit weaker, relationship between higher annual average ozone exposure and lower peripheral pulmonary vascular volumes on CT in participants of the Multi-Ethnic Study of Atherosclerosis (MESA).22 In the MESA analysis, per IQR higher one-year average ozone exposure, peripheral vessel volume was 0.27 mL lower (95% CI: −0.60 to 0.05; P = 0.10) in the limited model; these results were slightly attenuated in the full model (95% CI: −0.59 to 0.08; P = 0.14).
Our findings, taken together with the analogous MESA results, suggest that long-term ozone exposure may be associated with changes in the systemic and pulmonary vessels, including vasoconstriction, remodeling, and vascular loss. Plausible mechanisms include upregulation of vasoactive/inflammatory mediators, thrombosis, nitric oxide (NO) scavenging, and oxidative damage associated with ozone.39–46,54,55 For the pulmonary vasculature specifically, hypoxic vasoconstriction may also play a role, as suggested by results from Gong et al, who found that ozone exposure was associated with an increase in the alveolar-arterial oxygen gradient.53 Each of these mechanisms, singly or in combination, may result in lower vascular volumes of the smallest pulmonary vessels.
However, an alternative explanation may also be possible. While ozone is known to have direct effects, ozone is also known to be “scavenged” by products of vehicle exhaust, primarily nitrogen oxides.38,56–59 Ozone may thus be an inverse marker of vehicle exhaust, and our study results could be interpreted to mean that exposure to vehicle exhaust is associated with higher pulmonary vascular volumes. This may be consistent with the primary result of the MESA study by Aaron et al, which found that long-term exposure to black carbon (attributed to traffic emissions) was consistently associated with higher peripheral pulmonary vascular volumes.22 However, in our secondary models simultaneously adjusting for ozone and distance to roadway, we found that the associations of higher ozone and lower small vessel volumes persisted, which suggests that this is a primary effect of ozone on the pulmonary vasculature. This conclusion is further supported by the fact that the associations with ozone in our analysis are stronger than the associations with PM2.5 and EC, both of which are also known components of vehicle exhaust.
Our findings suggest a possible link between ozone exposure and structural changes in the small pulmonary vessels, and corroborate prior results from Aaron and colleagues in a similar cohort of generally healthy adults.22 The potential relationship between ambient pollution exposure and pulmonary vascular change is of increasing relevance given recent work that showed some measures of traffic-related air pollution are associated with poorer clinical outcomes in individuals with pulmonary arterial hypertension (although that study did not assess ozone exposure).21 In addition, we found larger associations with ozone exposure when excluding those participants who had moved (who would be expected to have a less accurate exposure assessment), which further supports the plausibility of our results. Finally, our findings were robust to adjustment for tobacco exposure (including smoking status, total pack-years of exposure, and second-hand exposure), and we found no evidence that the association of ozone with small vessel volume differed based on smoking status. Of note, while the association of ozone and lower small vessel volume was consistent and robust, there was also a roughly proportional (albeit much weaker) association with lower total pulmonary vascular volume (TBV) which did not reach statistical significance, leading to null associations with the small vessel ratios. While prior work has indicated that a lower small vessel ratio (i.e., BV5/TBV) was indicative of vascular pruning that was linked to more severe pulmonary disease,24,32,60,61 other studies have used the absolute small vessel volume (i.e., BV5) as the primary measure of pruning.62–64 Our findings may suggest that differences in absolute BV5 may be more sensitive than vascular ratios, although further investigation is necessary to determine which radiographic measures best capture clinically relevant vascular disease.
While long-term ozone exposure was consistently associated with lower small vessel volumes on CT in all our primary and secondary models, PM2.5 and EC were not associated with small vessel volume. The reason for this may be due to the relatively low overall level and narrow range of exposure present in the Framingham study region of the northeastern United States, which reduces the ability to discriminate small differences in vessel volume attributable to long-term ambient pollutant exposures. Our findings are consistent with some of the results in MESA by Aaron and colleagues, which found no associations with PM2.5 and CT vascular volumes in their fully adjusted models.22 Another factor may be related to limitations in our method of estimating PM2.5 and EC exposure. In particular, for EC our models predict ground-level EC exposure at a 1 × 1 km resolution, which may not be fine enough to capture variations in exposures related to traffic and other sources. Our models do not account for indoor sources of PM2.5 and EC exposure, including wood-burning stoves and fireplaces, which may be especially relevant in colder months given the northerly distribution of our study sample.65 In contrast, since ground-level ozone is the result of stratospheric reactions,57 variations in ozone exposure are more smoothly distributed across the study area even at a 1 × 1 km resolution.
Although we did not find consistent associations with EC and CT vascular volumes in our primary models, our secondary analysis suggested that this relationship may differ based on smoking status, with an association of EC (both one- and five-year annual averages) with higher vascular volumes in smokers compared to nonsmokers. These findings suggest that there may be a synergistic effect of tobacco exposure and ambient EC concentrations, although the potential basis for this interaction is not well-understood. There is some evidence from animal models which suggests that diesel particulate exposure (which includes EC) is synergistic with tobacco exposure,66 and more consistent evidence that EC specifically is associated with additive risk of lung cancer when combined with cigarette smoke exposure.67 Further research is necessary to elucidate the important question of whether there is a synergistic effect of EC and tobacco smoke in pulmonary vascular pathology.
To our knowledge, our study is one of very few studies of ozone exposure and pulmonary vascular volumes on CT. Strengths of our study include the large sample size, control for multiple demographic, socioeconomic, and behavioral potential confounders in all our models, examination of both absolute and relative pulmonary vascular volumes, and the use of a single scanner model (and uniform acquisition protocols) for all participants. This latter factor may reduce imprecision or bias introduced by differences in image acquisition and reconstruction algorithms, which is a potential limitation of CT biomarkers.68
There are several limitations. Our cross-sectional study design limits conclusions regarding the causality of the associations. Although our models were adjusted for relevant potential confounders, residual or unmeasured confounding cannot be excluded in this observational study. In addition, our study does not adjust for the use of certain cardiovascular and respiratory medications, including bronchodilators, diuretics, and calcium channel blockers, which may be related to the appearance of the pulmonary vessels on imaging. However, these medications are used almost exclusively to treat cardiopulmonary disease, and since our models adjust for cardiovascular disease and lung function, the effect of these medication classes is accounted for to some degree. Due to the original recruitment strategy of the Framingham Heart Study, our sample is essentially entirely of European ancestry and our findings may not be generalizable to the population at large. Finally, while radiographic BV5 has been associated with histologic indices of structural vascular remodeling in the pulmonary microvessels,64 BV5 itself is not a direct indicator of pulmonary hypertension or pulmonary hemodynamics and the clinical relevance of our findings, particularly given the relatively modest difference in BV5 per IQR of ozone exposure, remains to be determined. However, prior work has demonstrated that BV5 (both absolute small vessel volume and relative to total volume) is linearly associated with clinical outcomes, including right ventricular size, all-cause mortality, and presence of interstitial lung abnormalities (which are also known to be associated with air pollution exposure).29,60–62 This suggests that even the small differences in BV5 found in our study may have clinical relevance. If the linear association we found between ozone and pulmonary vascular volume is present across a wider range of ozone exposure, then the absolute difference in pulmonary vascular volume attributable to ozone may be greater in populations exposed to higher levels of ozone than our study population in the northeastern United States. Clearly, more studies are warranted to confirm our findings in other regions, identify susceptible subgroups (e.g., those with cardiopulmonary disease), and to assess for consistency of the effect size to determine clinical and public health significance. Future work may address the clinical implications of differences in BV5, and further investigation into the direct role of ozone in the development of pulmonary vascular pathology.
Supplementary Material
Footnotes
Published online 18 March 2021
Process to replicate results: Research applications can be submitted to the Framingham Heart Study Research Review Committees online at https://www.framinghamheartstudy.org.
The authors declare that they have no conflicts of interest with regard to the content of this report.
The results reported herein correspond to specific aims of grant K23 ES026204 to investigator M.B.R. from NIEHS. A.J.S. is supported by grants from the American Lung Association (CA-626548) and the NIH (grant F32 HL143819). D.R.G. reports grants from the NIH. R.S.J.E. is supported by the NIH (grants 1R01 HL116931 and R01 HL116473) and reports personal fees from Toshiba, Boehringer Ingelheim, Eolo Medical, and Leuko Labs, outside the submitted work. He is also a founder and co-owner of Quantitative Imaging Solutions, which is a company that provides image based consulting and develops software to enable data sharing. G.R.W. is supported by the NIH (grants R01 HL122464 and R01 HL116473) and reports grants and other support from Boehringer Ingelheim, Genentech, Quantitative Imaging Solutions, PulmonX, Regeneron, ModoSpira, BTG Interventional Medicine, Janssen Pharmaceuticals, Toshiba, GlaxoSmithKline, outside the submitted work. G.R.W.’s spouse works for Biogen which is focused on developing therapies for fibrotic lung disease. G.T.O’C. reports personal fees from AstraZeneca and grants from Janssen Pharmaceuticals, outside the submitted work. M.A.M. reports grants from the NIH, US EPA, during the conduct of the study; grants from PCORI, Kellogg Foundation, and US National Institute of Justice, outside the submitted work. This work is supported in part by the National Heart, Lung and Blood Institute (Framingham Heart Study contract numbers N01-HC-25195 and HHSN268201500001I).
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
REFERENCES
- 1.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007; 356:447–458 [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Kim J, Kim S, et al. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J Am Heart Assoc. 2017; 6:e007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adar SD, Sheppard L, Vedal S, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013; 10:e1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund AK, Lucero J, Lucas S, et al. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol. 2009; 29:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Q, Wang A, Jin X, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005; 294:3003–3010 [DOI] [PubMed] [Google Scholar]
- 6.Pope CA, 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004; 109:71–77 [DOI] [PubMed] [Google Scholar]
- 7.Newby DE, Mannucci PM, Tell GS, et al. ; ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation; ESC Heart Failure Association. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015; 36:83–93b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills NL, Donaldson K, Hadoke PW, et al. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009; 6:36–44 [DOI] [PubMed] [Google Scholar]
- 9.Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013; 187:1226–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YC, Ghio AJ. Vascular effects of ambient pollutant particles and metals. Curr Vasc Pharmacol. 2006; 4:199–203 [DOI] [PubMed] [Google Scholar]
- 11.Grigg J. Where do inhaled fossil fuel-derived particles go? Am J Respir Crit Care Med. 2017; 196:804–806 [DOI] [PubMed] [Google Scholar]
- 12.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016; 133:1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018; 197:509–516 [DOI] [PubMed] [Google Scholar]
- 14.Huston JH, Maron BA, French J, et al. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol. 2019; 4:1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemos M, Mohallen SV, Macchione M, et al. Chronic exposure to urban air pollution induces structural alterations in murine pulmonary and coronary arteries. Inhal Toxicol. 2006; 18:247–253 [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez Ferreira Rivero DH, Castro Soares SR, Lorenzi-Filho G, et al. Acute cardiopulmonary alterations induced by fine particulate matter of São Paulo, Brazil. Toxicol Sci. 2005; 85:898–905 [DOI] [PubMed] [Google Scholar]
- 17.Batalha JR, Saldiva PH, Clarke RW, et al. Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ Health Perspect. 2002; 110:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wauters A, Vicenzi M, De Becker B, et al. At high cardiac output, diesel exhaust exposure increases pulmonary vascular resistance and decreases distensibility of pulmonary resistive vessels. Am J Physiol Heart Circ Physiol. 2015; 309:H2137–H2144 [DOI] [PubMed] [Google Scholar]
- 19.Barath S, Langrish JP, Lundbäck M, et al. Short-term exposure to ozone does not impair vascular function or affect heart rate variability in healthy young men. Toxicol Sci. 2013; 135:292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect. 2007; 115:1248–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sofianopoulou E, Kaptoge S, Gräf S, et al. Traffic exposures, air pollution and outcomes in pulmonary arterial hypertension: a UK cohort study analysis. Eur Respir J. 2019; 53:1801429. [DOI] [PubMed] [Google Scholar]
- 22.Aaron CP, Hoffman EA, Kawut SM, et al. Ambient air pollution and pulmonary vascular volume on computed tomography: the MESA Air Pollution and Lung cohort studies. Eur Respir J. 2019; 53:1802116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Synn AJ, Zhang C, Washko GR, et al. Cigarette smoke exposure and radiographic pulmonary vascular morphology in the Framingham Heart Study. Ann Am Thorac Soc. 2019; 16:698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Synn AJ, Li W, San José Estépar R, et al. Radiographic pulmonary vessel volume, lung function, and airways disease in the Framingham Heart Study. Eur Respir J. 2019; 54:1900408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice MB, Li W, Dorans KS, et al. Exposure to traffic emissions and fine particulate matter and computed tomography measures of the lung and airways. Epidemiology. 2018; 29:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloog I, Chudnovsky AA, Just AC, et al. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across Northeastern USA using high resolution aerosol optical depth data. Atmos Environ (1994). 2014; 95:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Q, Koutrakis P, Schwartz J. A hybrid prediction model for PM2.5 mass and components using a chemical transport model and land use regression. Atmos Environ. 2016; 131:390–399 [Google Scholar]
- 28.Bey I, Jacob DJ, Yantosca RM, et al. Global modeling of tropospheric chemistry with assimilated meteorology: model description and evaluation. J Geophys Res Atmos. 2001; 106D1923073–23095 [Google Scholar]
- 29.Rice MB, Li W, Schwartz J, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham Heart Study. Thorax. 2019; 74:1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Q, Rowland S, Koutrakis P, Schwartz J. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc. 2017; 67:39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice MB, Ljungman PL, Wilker EH, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015; 191:656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estépar RS, Kinney GL, Black-Shinn JL, et al. ; COPDGene Study. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013; 188:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ash SY, Rahaghi FN, Come CE, et al. ; SARP Investigators. Pruning of the pulmonary vasculature in asthma. The Severe Asthma Research Program (SARP) Cohort. Am J Respir Crit Care Med. 2018; 198:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin HH, Parajuli RP, Gogna P, Maquiling A, Dehghani P. Pollutant-sex specific differences in respiratory hospitalization and mortality risk attributable to short-term exposure to ambient air pollution. Sci Total Environ. 2021; 755pt 2143135. [DOI] [PubMed] [Google Scholar]
- 35.Rice MB, Ljungman PL, Wilker EH, et al. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med. 2013; 188:1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloomer BJ, Vinnikov KY, Dickerson RR. Changes in seasonal and diurnal cycles of ozone and temperature in the eastern U.S. Atmos Environ. 2010; 44:2543–2551 [Google Scholar]
- 37.Li W, Dorans KS, Wilker EH, et al. Short-term exposure to ambient air pollution and biomarkers of systemic inflammation: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2017; 37:1793–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConnell R, Berhane K, Yao L, Lurmann FW, Avol E, Peters JM. Predicting residential ozone deficits from nearby traffic. Sci Total Environ. 2006; 363:166–174 [DOI] [PubMed] [Google Scholar]
- 39.Arjomandi M, Wong H, Donde A, et al. Exposure to medium and high ambient levels of ozone causes adverse systemic inflammatory and cardiac autonomic effects. Am J Physiol Heart Circ Physiol. 2015; 308:H1499–H1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devlin RB, Duncan KE, Jardim M, Schmitt MT, Rappold AG, Diaz-Sanchez D. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation. 2012; 126:104–111 [DOI] [PubMed] [Google Scholar]
- 41.Bourdrel T, Bind MA, Béjot Y, Morel O, Argacha JF. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. 2017; 110:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia Y, Niu Y, Cai J, et al. Effects of personal short-term exposure to ambient ozone on blood pressure and vascular endothelial function: a mechanistic study based on DNA methylation and metabolomics. Environ Sci Technol. 2018; 52:12774–12782 [DOI] [PubMed] [Google Scholar]
- 43.Chuang GC, Yang Z, Westbrook DG, et al. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L209–L216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kodavanti UP, Thomas R, Ledbetter AD, et al. Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles. Environ Health Perspect. 2011; 119:312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balmes JR, Arjomandi M, Bromberg PA, et al. Ozone effects on blood biomarkers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: the Multicenter Ozone Study in oldEr Subjects (MOSES). PLoS One. 2019; 14:e0222601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srebot V, Gianicolo EA, Rainaldi G, Trivella MG, Sicari R. Ozone and cardiovascular injury. Cardiovasc Ultrasound. 2009; 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paffett ML, Zychowski KE, Sheppard L, et al. Ozone inhalation impairs coronary artery dilation via intracellular oxidative stress: evidence for serum-borne factors as drivers of systemic toxicity. Toxicol Sci. 2015; 146:244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robertson S, Colombo ES, Lucas SN, et al. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci. 2013; 134:304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002; 105:1534–1536 [DOI] [PubMed] [Google Scholar]
- 50.Day DB, Xiang J, Mo J, et al. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med. 2017; 177:1344–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirowsky JE, Carraway MS, Dhingra R, et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ Health. 2017; 16:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M, Sampson PD, Sheppard LE, Stein JH, Vedal S, Kaufman JD. Long-term exposure to ambient ozone and progression of subclinical arterial disease: the Multi-Ethnic Study of atherosclerosis and air pollution. Environ Health Perspect. 2019; 127:57001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong H, Jr, Wong R, Sarma RJ, et al. Cardiovascular effects of ozone exposure in human volunteers. Am J Respir Crit Care Med. 1998; 158:538–546 [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal S, Gross CM, Sharma S, Fineman JR, Black SM. Reactive oxygen species in pulmonary vascular remodeling. Compr Physiol. 2013; 3:1011–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiegman CH, Li F, Clarke CJ, et al. A comprehensive analysis of oxidative stress in: the ozone-induced lung inflammation mouse: model. Clin Sci. 2014; 126:425–440 [DOI] [PubMed] [Google Scholar]
- 56.Han S, Bian H, Feng Y, et al. Analysis of the relationship between O3, NO and NO2 in Tianjin, China. Aerosol Air Qual Res. 2011; 11:128–139 [Google Scholar]
- 57.Zhang JJ, Wei Y, Fang Z. Ozone pollution: a major health hazard worldwide. Front Immunol. 2019; 10:2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson E. Cars and ground-level ozone: how do fuels compare? Eur Transp Res Rev. 2017; 9:47 [Google Scholar]
- 59.Munir S, Chen H, Ropkins K. Modelling the impact of road traffic on ground level ozone concentration using a quantile regression approach. Atmos Environ. 2012; 60:283–291 [Google Scholar]
- 60.Synn AJ, Li W, Hunninghake GM, et al. Vascular pruning on CT and interstitial lung abnormalities in the Framingham Heart Study. Chest. 2021; 159:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Synn AJ, Li W, San José Estépar R, et al. Pulmonary vascular pruning on computed tomography and risk of death in the Framingham Heart Study. Am J Respir Crit Care Med. 2021; 203:251–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease. A Longitudinal Observational Study. Am J Respir Crit Care Med. 2019; 200:454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahaghi FN, Winkler T, Kohli P, et al. Quantification of the pulmonary vascular response to inhaled nitric oxide using noncontrast computed tomography imaging. Circ Cardiovasc Imaging. 2019; 12:8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahaghi FN, Argemí G, Nardelli P, et al. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur Respir J. 2019; 54:1900370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rau JA. Composition and size distribution of residential wood smoke particles. Aerosol Sci Technol. 1989; 10:181–192 [Google Scholar]
- 66.Gondim FL, Moura MF, Ferreira RM, et al. Exposure to total particulate matter obtained from combustion of diesel vehicles (EURO 3 and EURO 5): effects on the respiratory systems of emphysematous mice. Environ Toxicol Pharmacol. 2021; 83:103583. [DOI] [PubMed] [Google Scholar]
- 67.Ge C, Peters S, Olsson A, et al. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. A pooled exposure-response analysis of 14 case-control studies. Am J Respir Crit Care Med. 2020; 202:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bodduluri S, Reinhardt JM, Hoffman EA, Newell JD, Jr, Bhatt SP. Recent advances in computed tomography imaging in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018; 15:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


