Abstract
COVID-19 has rarely been associated with immune-mediated phenomena such as autoimmune haemolytic anaemia (AIHA). Both cold hemolysis with cold agglutinin detection and warm haemolysis have been described with variable prognoses. Current treatment regimens are based on experience with other case series and case reports, which still represent a clinical challenge. Corticosteroids, red cell transfusions and rituximab have been successfully employed. We present 3 cases of AIHA in the context of COVID-19 disease, the first case successfully treated with plasma exchange and long-term follow-up of the 3 cases showing complete remission of anaemia.
Keywords: Autoimmune haemolytic anaemia, COVID-19, Plasma exchange, Corticosteroids
Introduction
COVID-19 is a new worldwide infectious disease caused by the SARS Cov2 coronavirus infection and is responsible for a great variety of systemic immune-mediated phenomena, such as Guillain-Barré syndrome or immune thrombocytopenia [1, 2]. In common with other viral infections, it has been related to the development of autoimmune haemolytic anaemia (AIHA). However, both mechanisms involving cold and warm hemolysis have been reported [3–8]. Treatment is based on case report experience: corticosteroids, red cell transfusions and rituximab have been used successfully [5]. Another issue is lack of data about prognosis of this phenomena. Herein, we present three cases of AHAI in the context of COVID-19, and 6 months following, showing complete remission of hemolysis. Also, one of them successfully treated with plasma exchange.
Case 1
A 54-year-old man with no previous relevant medical history was admitted to the emergency department for a cough, fever and dyspnoea. On physical examination, the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen ratio (PaFi) was 181 mmHg. In addition, the patient also had jaundice, without other stigmata of liver disease. A chest X-ray showed bilateral infiltrates, while a nasal swab PCR test was positive for SARS Cov2. The patient received hydroxychloroquine and tocilizumab as a treatment for COVID-19 pneumonia. Blood tests showed elevated inflammatory parameters, as well as progressive anaemia with evidence of haemolysis (Table 1). Results for acute infection by hepatotropic viruses, syphilis, HIV, cytomegalovirus, Epstein-Barr virus and Mycoplasma pneumoniae were negative. Direct antiglobulin test (DAT) was strongly positive for C3d and negative for IgG. Based on the DAT results, cold hemolysis-mediated phenomena was assumed, although cold agglutinins were not initially determined. Moreover, a battery of test was conducted without finding an underlying autoimmune, neoplastic or haematological disease. Treatment was initiated with methylprednisolone boluses (250 mg × 3 times) and methylprednisolone 1 mg/kg in a descending dose, with fluid warmer and plasma exchange for five sessions. Tocilizumab was initially administered due to the severity of COVID-19 pneumonia. Rituximab was not administered in order to avoid the simultaneous use of two biological monoclonal antibody treatments during the COVID-19 infection. The patient’s COVID-19 pneumonia improved favourably while their haemoglobin concentrations increased to 15 g/dl with normalization of haemolysis parameters. He had not presented relapses after corticosteroid withdrawal and after a 6-month follow-up.
Table 1.
Blood test at time of diagnosis of AIHA
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Hemoglobin (g/dl) | 6.5 | 7.4 | 8 |
| Lymphocytes per mm3 | 630 | 2320 | 3550 |
| Platelets per mm3 | 169,000 | 770,000 | 93,000 |
| LDH (UI/l) | 724 | 1456 | 423 |
| Haptoglobin (mg/dl) | < 7.75 | < 7.75 | < 7.75 |
| Bilirrubin (mg/dl) | 1 | 3.5 | 0,35 |
| C3 (mg/dl) | 72 | 81,4 | - |
| C4 (mg/dl) | 15 | 13.4 | - |
| IL-6 (μg/ml) | 138 | 89 | 82.9 |
| DAT specificity | IgG | C3d | IgG |
DAT direct antiglobulin test
Case 2
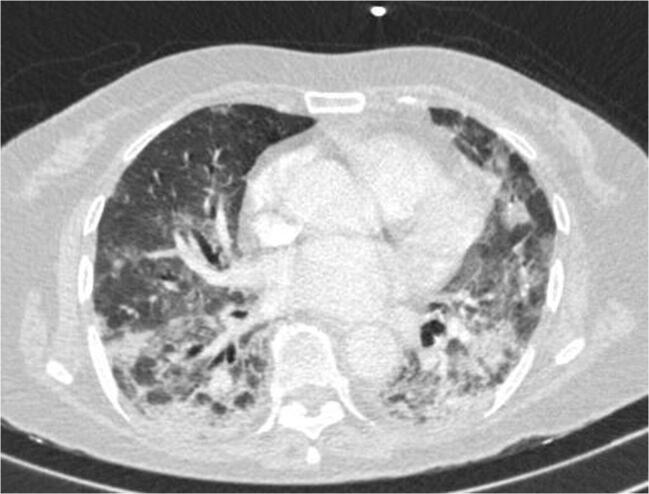
A 72-year-old woman with no previous relevant medical history was brought to the Emergency department due to dyspnea, peripheral oxygen saturation of 79% and breathing 100% FiO2 when recorded at the patient’s home. Thorax computed tomography showed bilateral infiltrates (Fig. 1) while a nasal swab PCR test was positive for SARS Cov2. The patient was diagnosed with acute respiratory syndrome related to COVID-19. Owing to the severity of the acute respiratory syndrome, the patient required orotracheal intubation and treatment with hydroxychloroquine, tocilizumab 600 mg (two doses), and dexamethasone 20 mg with descending dose was started. During admission to the intensive care unit (ICU), the patient presented progressive anaemia with no signs of macroscopic haemorrhagia while blood test values were consistent with hemolysis (Table 1). DAT was positive for IgG and negative for C3d, and the patient was diagnosed with AIHA. Results for acute infection by hepatotropic viruses, syphilis, HIV, cytomegalovirus, Epstein-Barr virus and Mycoplasma pneumoniae were negative. The patient was still receiving dexamethasone treatment at time of AIHA diagnosis and red blood cell transfusion was carried out.
Fig. 1.
Computed tomography of case 2, showing bilateral infiltrates by COVID-19 pneumonia
Both the respiratory condition and the haemolytic anaemia of the patient improved favourably and were subsequently discharged from the ICU without requiring additional therapy. The patient’s blood returned to normal hemoglobin levels (15 mg/dl) at the 6-month follow-up period.
Case 3
A 76-year-old woman with a previous history of arterial hypertension, hypothyroidism and chronic lymphocytic leukaemia (CLL) Rai-0, Binet B (no previous treatment) was admitted to the emergency department with a fever, cough and dyspnea. Peripheral oxygen saturation of 89% was recorded at arrival, requiring immediate oxygen therapy via a nasal cannula resulting in a PaFI of 360 mmHg. A positive PCR test for SARS Cov2 was recorded, while chest X-rays showed bilateral infiltrates of the lungs. A diagnosis of COVID-19 pneumonia was determined. Hydroxychloroquine and corticosteroid treatment with methylprednisolone 20 mg daily was administered for the treatment of the COVID-19 pneumonia. During hospitalization, the patient presented progressive anaemia and hemolysis as determined by blood tests, which had not been previously during CLL diagnosis and treatment (Table 1). DAT was positive for IgG and negative for both C3d and IgM, resulting in an AIHA diagnosis. Results for acute infection by hepatotropic viruses, syphilis, HIV, cytomegalovirus, Epstein-Barr virus and Mycoplasma pneumoniae were negative. Corticosteroid treatment was maintained and red blood cell transfusion was administered. Both the respiratory condition and the haemolytic anaemia of the patient improved favourably resulting in a discharge from the ICU without further therapeutic interventions. The patient’s blood had returned to normal haemoglobin concentrations (12 mg/dl) at the 6-month follow-up period.
Discussion
In the present study, three patients with concurrent AIHA and COVID19 infection are reported. One of them presumably by cold hemolysis and the other two by warm hemolysis. The patient with cold hemolysis was treated successfully with plasma exchange and, to our knowledge, is the first case of COVID-19-related AIHA treated with plasma exchange reported. We also describe the 6-month follow-up of these patients with all showing complete recovery of hemoglobin levels.
AHAI onset associated with a viral infection is a well-known phenomenon. Although warm haemolysis is more frequently presented, cold haemolysis has also been previously reported by others [9]. A molecular mimicry mechanism has been recently proposed [10].
There have been recently published studies reporting COVID-19 cases with positive DAT results for IgG, C3d or both, even with determination of cold agglutinin in some cases. In many of the incidences of AIHA associated with COVID-19, the patients presented comorbidities such as idiopathic thrombotic purpura, chronic lymphatic leukaemia or lymphomas, especially in those associated with C3d-positive DAT [3–5, 8]. However, herein, we present two patients who did not have an underlying disease despite an exhaustive screening for autoimmune disease and malignancies, even during follow-up visits. This phenomenon suggests that an underlying immunological disorder is not necessary to present a COVID-19-related AIHA.
With respect to the SARS-CoV2 pneumonia treatment, corticosteroids and tocilizumab were employed due to the worsening of respiratory symptoms, as it was the best treatment available at the time of the disease occurrence. The use of steroids, rituximab and red blood cell transfusions with good responses has been previously described in the context of COVID-19-related AHAI [3, 5]. Patients two and three were treated with corticosteroids and red blood cell transfusions with both showing a good response in agreement with previously published cases. However, in patient 1, rituximab, which is usually the first-line treatment when cold agglutinins are suspected [11], was not administered this in order to avoid the simultaneous combination of two biological treatments. This decision was made after taking into account the risks of the hyperinflammatory syndrome associated with COVID-19. A total of five sessions of plasma exchange were performed, in combination with high-dose corticosteroids resulting in a significant improvement in the patient.
As much of the information about COVID-19-related AIHA has been obtained from case reports and case studies, there is a lack of information about its prognosis. In the present study, we have followed these patients for 6 months and have verified the complete resolution of the anaemia, as shown by a return to normal hemoglobin concentrations (Fig. 2). The results from this case study build on the information about the prognosis of these phenomena which is likely to be related to the acute phase of COVID-19.
Fig. 2.
Hemoglobin levels during follow-up
In summary, despite its rarity, coexistence of AIHA and COVID-19 is a recently described phenomenon and it must be suspected in the context of an unexplained or persistent anaemia in patients with COVID-19 disease. In our experience, plasma exchange might be an option when cold hemolysis is suspected and AIHA might resolve in time once COVID-19 acute phase is over.
Author Contribution
LRR and CBA collaborated in the redaction of the manuscript. All authors have revised and corrected the manuscript.
Data availability
Not applicable, as is a case study report.
Code Availability
Not applicable.
Declarations
Ethical approval
The Ethics Committee of the Hospital Universitario La Paz waived the need for ethics approval for this non-interventional study.
Consent to Participate
Not applicable, as there was no intervention. Patient informed consent was obtained for the publication of the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A, Micieli G. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zulfiqar A-A, Lorenzo-Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med. 2020;382(18):e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez C, Kim J, Pandey A, Huang T, DeLoughery TG. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190(1):31–32. doi: 10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zagorski E, Pawar T, Rahimian S, Forman D. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19) Br J Haematol. 2020;190:e183–e184. doi: 10.1111/bjh.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, Merabet F, Mekinian A, Braun T, Damaj G, Delmer A, Cymbalista F. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190(1):29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capes A, Bailly S, Hantson P, Gerard L, Laterre PF. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol. 2020;99(7):1679–1680. doi: 10.1007/s00277-020-04137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Nguyen CB, Yeung Z, Sanchez K, Rosen D, Bushan S. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190:0–3. doi: 10.3324/haematol.2020.258574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahlster L, Weichert-Leahey N, Trissal M, Grace RF, Sankaran VG. COVID-19 presenting with autoimmune hemolytic anemia in the setting of underlying immune dysregulation. Pediatr Blood Cancer. 2020 Sep;67(9):e28382. 10.1002/pbc.28382. [DOI] [PMC free article] [PubMed]
- 9.Brodsky RA. Warm autoimmune hemolytic anemia. N Engl J Med. 2019;381(7):647–654. doi: 10.1056/NEJMcp1900554. [DOI] [PubMed] [Google Scholar]
- 10.Angileri F, Légaré S, Marino Gammazza A, Conway de Macario E, Macario AJL, Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol. 2020 Jul;190(2):e92–e93. 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed]
- 11.Berentsen S. New insights in the pathogenesis and therapy of cold agglutinin-mediated autoimmune hemolytic anemia. Front Immunol. 2020;11. Available from:. 10.3389/fimmu.2020.00590/full. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable, as is a case study report.
Not applicable.