Abstract
OBJECTIVES
Our goal was to study long-term observed and relative survival after first-time aortic valve replacement surgery with or without concomitant coronary artery bypass surgery with reference to valve morphology (i.e. bicuspid vs tricuspid).
METHODS
Consecutive patients (n = 5086) from 3 Swedish hospitals, operated on between 1 January 2005 and 31 December 2016, were included. The 30-day mortality (n = 116, 2.3%) was excluded from the analysis of long-term observed and relative survival (n = 4970). Observed survival was analysed using Cox regression. Relative survival was calculated as the ratio between observed and expected survival based on data from the general Swedish population, matched for age, sex and calendar year. Risk factors for death were explored using multivariable analysis.
RESULTS
During the follow-up (median 4.7 years) period, 1157 (23%) patients died. Observed survival excluding 30-day mortality was 96.6%, 82.7% and 57.6% after 1, 5 and 10 years. Compared with the general Swedish population, the relative 1-, 5- and 10-year survival rates were 99.0%, 97.5% and 89.0%. Bicuspid morphology was independently associated with higher observed and relative long-term survival. Renal dysfunction, diabetes, chronic obstructive pulmonary disease, heart failure, smoking and atrial fibrillation were associated with higher long-term mortality. Combined surgery was not associated with higher observed or relative mortality.
CONCLUSIONS
Patients with a bicuspid morphology had better prognosis, matching that of the general population. With increased age, long-term relative survival compared favourably with survival in the general Swedish population. Adding coronary artery bypass surgery to an aortic valve replacement procedure did not affect long-term outcome.
Keywords: Aortic stenosis, Aortic valve replacement, Bicuspid valve, Observed survival, Relative survival
INTRODUCTION
Bicuspid aortic valve is the most common congenital heart condition and leads to premature valve failure in a significant number of patients [1]. In recent years, several publications focused on comparing results of transcatheter aortic valve implantation (TAVI) in patients with bicuspid versus tricuspid aortic valves in an effort to expand indications for TAVI [2]. In contrast, there is a lack of data on an equivalent comparison of long-term results for bicuspid versus tricuspid morphology in surgical aortic valve replacement (SAVR). Such a comparison is challenging because the underlying aetiology, congenital versus pure degeneration, is different. This difference results in a marked variation in baseline data for populations of surgically treated patients with bicuspid versus tricuspid morphology with regards to age at surgery and concomitant coronary artery disease (CAD).
Long-term survival in cardiac surgery patients must be seen in context with that expected in the general population. Comparing survival data with SAVR to that in the general population (relative survival) may help to explore differences between SAVR in bicuspid versus tricuspid morphology in a meaningful manner.
The goal of this study was to assess observed and relative survival in a large group of Swedish patients with bicuspid and tricuspid morphology who underwent SAVR between 2005 and 2016. The aim of this study was to provide a benchmark for contemporary SAVR in patients with severe aortic valve disease, keeping future catheter-based aortic intervention in mind. Patients requiring aortic root or ascending aortic intervention are currently not eligible for catheter-based treatment. Thus, patients requiring concomitant aortic surgery were excluded. However, patients with or without concomitant coronary artery bypass grafting (CABG) were included. Potential risk factors for outcome were explored with a focus on valve morphology (i.e. bicuspid/tricuspid) and the effects of patient age and sex and the impact of concomitant CABG.
MATERIALS AND METHODS
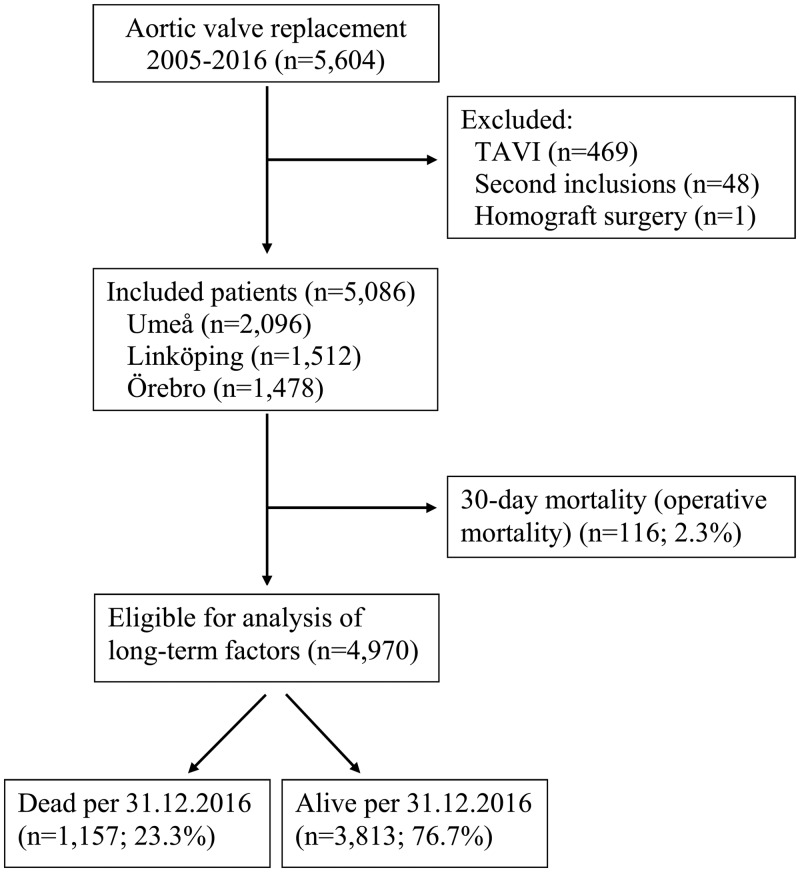
This study is based on a prospective database, originally designed for hospital quality control and epidemiological research. The database is regularly validated and monitored by a quality control officer. The study was approved by the ethics committee (Regionala etikprövningsnämnden i Umeå 2016/514-31). Patients were informed about compulsory registration of data in the institutional database and the national registry. Formal written consent for the study was waived by the ethics committee. After excluding patients who had TAVI, patients included twice by mistake and patients undergoing homograft surgery, we included 5086 consecutive patients who had their first SAVR, with or without coronary surgery, in 3 Swedish heart surgery centres (Linköping, Umeå and Örebro) between 1 January 2005 and 31 December 2016. The aim of this study was to analysis long-term survival and mortality. Previous studies have shown differences in associated risk factors for short- and long-term mortality [3]. To address our aim of assessing factors associated with long-term mortality, we therefore excluded deaths within 30 days from surgery (operative mortality, n = 116/2.3%). Thus, the final analyses included 4970 patients (Fig. 1).
Figure 1:
Flow diagram of patient inclusion process. TAVI: transcatheter aortic valve implantation.
All clinical data were recorded prospectively, and all 3 centres used the same cardiac surgery database named ‘Carath’. This database was established in January 2005 and is used by 4 out of 8 cardiac surgery centres in Sweden. It comprises procedural- and patient-related characteristics, intraoperative and postoperative events and laboratory results. An overview of relevant variable definitions and missing data is given in Supplementary Material, Table S1.
Date of death through 31 December 2016 was obtained through linkage to the Swedish population registry. Data on cause of death was not obtained in this study. Expected survival and mortality rates were calculated from life tables compiled from the Swedish population stratified on age, sex and calendar year, obtained from the Human Mortality Database [4].
The primary end point of this study was long-term all-cause mortality, referred to as observed long-term mortality. Follow-up for the primary end point was 100%. The secondary end point was long-term relative mortality, defined as the ratio between the observed and expected mortality in the Swedish population, as a measure of mortality of SAVR.
Surgical procedure and postoperative management
The heart was accessed through a median or partial sternotomy. Cardiopulmonary bypass with light to moderate hypothermia was established, and a standard aortic valve replacement (AVR) was performed during cardioplegic arrest. Cold crystalloid or cold blood cardioplegia was delivered antegrade and/or retrograde. In cases with combined procedures, CABG was most often performed first. Patients ∼70 years of age or older usually received a biological valve prosthesis whereas younger patients received a mechanical prosthesis, with exceptions due to the patient’s and/or the surgeon’s preferences. Reheparinization and the initiation of anticoagulation therapy were started within 24 h after surgery in patients with a mechanical valve prosthesis, whereas the anticoagulation strategy after bioprosthesis implantation differed between surgeons and between centres. If anticoagulants were initiated, they were usually continued for 3 months, whereas other patients received aspirin only. Patients also received perioperative antibiotic prophylaxis for 1–2 days. Valve morphology was determined during surgical exploration and prospectively registered in the database.
Statistical analyses
The Kaplan–Meier method was used to calculate the observed survival curves. Group differences were tested using the log-rank test. Expected survival was calculated from life tables compiled from the Swedish population stratified on age, sex and calendar year [4]. Relative survival was calculated as the ratio between the observed and expected survival rates, using the strs command in Stata (StataCorp LP, College Station, TX, USA) [5].
Factors associated with long-term observed mortality were investigated using multivariable Cox proportional hazards modelling. The selection of candidate risk factors was guided by clinical knowledge and literature, a method recommended to avoid overfitting and confounders as found with selection based on univariable analysis [6]. Violations to the linearity assumption were assessed graphically by categorizing into quantiles, as well as with Stata’s linktest. Serum creatinine levels were not linearly associated with the outcome. Therefore, the estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation [7]. The eGFR showed a linear trend with observed mortality and was therefore considered a more appropriate measure of renal function in our study. Deviations from the proportionality assumption were assessed graphically and by the inclusion of interaction terms between adjustment factors and time. Both age and eGFR violated the proportionality assumption. Thus, we performed time-split analyses (separate analysis of years 0–1, 1–5 and >5) to evaluate time-dependent effects.
Model fit and complexity were compared using log likelihood, the Bayesian and Akaike information criteria. Goodness of fit was evaluated with Harrell’s concordance statistic and Somers’ D correlation coefficient, which are measures of the concordance of ranked predicted and observed outcomes [8].
The Cox proportional model was used to identify possible confounding factors. We then used these factors to analyse long-term relative mortality. We applied multiplicative modelling of relative mortality as described by Pohar and Stare [9, 10], using the relsurv package in R [11]. Differences in relative mortality between patients with different covariate levels are expressed as relative mortality ratios (RMR).
Categorical variables are described as n (%); continuous variables are described as median (lower quartile–upper quartile). Group and centre differences were tested using the χ2 test for categorical data, and the Mann–Whitney U-test or Kruskal–Wallis test was used for continuous data. P-values <0.05 were considered significant. All statistical analyses were performed with Stata (version 16.0, StataCorp LP) and R (version 3.5.2, The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The 30-day mortality for all patients undergoing SAVR (n = 5086) was 2.3% (2.6% and 1.0% for tricuspid and bicuspid valve operations, respectively; P = 0.001), and these were excluded from further analyses of long-term survival and mortality (remaining n = 4970). The median total follow-up was 4.7 (2.3–7.6, max. 12.0) years, with a total of 25 129 patient-years. A total of 1157 patients (23.0%) died during the follow-up period. Valve morphology was not registered for 57 individuals (1.1%; Supplementary Material, Table S1). A total of 3782 patients (77.0%) had a tricuspid morphology and 1131 (23.0%) had a bicuspid morphology confirmed at the time of the operation. There were no significant differences between groups with regards to endocarditis and predominant aortic valve incompetence as an indication for the index operation (Table 1). The number of patients who were previously operated on for non-valvular heart disease was evenly distributed between the groups. However, patients with tricuspid valves were older, had more comorbidity and had significantly higher 30-day mortality. The tricuspid valve group had a higher incidence of concomitant coronary surgery. Comparison of patient characteristics between the 3 centres is provided in Supplementary Material, Table S2.
Table 1:
Comparison of patient characteristics in terms of tricuspid and bicuspid valve morphology
| Tricuspid valve (n = 3882) | Bicuspid valve (n = 1142) | P-value | |
|---|---|---|---|
| Age (years) | 75 (68–79) | 63 (56–71) | <0.001 |
| Female gender | 1550 (39.9) | 331 (29.0) | <0.001 |
| Smoking (current/past) | 1651 (46.0) | 528 (49.4) | 0.046 |
| COPD | 302 (8.0) | 59 (5.3) | 0.002 |
| NYHA functional class III/IV | 2600 (67.4) | 570 (50.3) | <0.001 |
| Heart failure | 795 (21.4) | 190 (17.3) | 0.003 |
| Diabetes mellitus | 858 (22.6) | 153 (13.5) | <0.001 |
| Atrial fibrillation | 627 (16.5) | 107 (9.5) | <0.001 |
| Endocarditis | 83 (2.1) | 23 (2.0) | 0.80 |
| Preoperative serum creatinine (mg/dl) | 84 (71–101) | 84 (71–96) | 0.075 |
| Previous cardiac surgery | 138 (3.6) | 43 (3.80) | 0.79 |
| Combined CABG/AVR | 1684 (43.4) | 251 (22.0) | <0.001 |
| Mechanical valve | 461 (11.9) | 417 (36.5) | <0.001 |
| Primary aortic insufficiencya | 400 (10.4) | 113 (10.1) | 0.72 |
| 30-Day mortality | 100 (2.6) | 11 (0.96) | 0.001 |
| Follow-up time (years) | 4.5 (2.1–7.3) | 4.9 (2.3–8.2) | <0.001 |
Categorical variables are given as n (%), continuous variables as median (p25–p75). Gender differences were tested with the χ2 test and the Mann–Whitney U-test for categorical and continuous data, respectively.
Aortic insufficiency was coded if the primary indication for surgery was aortic insufficiency. If patients also had aortic stenosis, aortic stenosis was the main diagnosis.
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease, NYHA: New York Heart Association.
Survival following aortic valve surgery
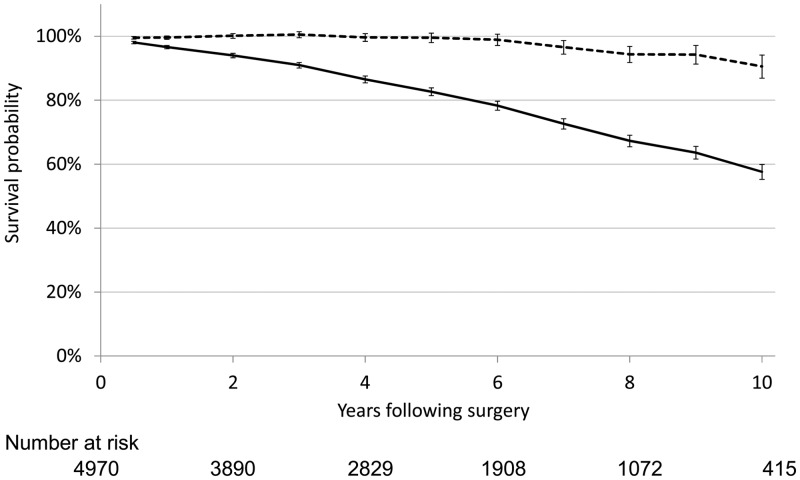
Observed long-term survival with 30-day mortality excluded was 96.6%, 82.7% and 57.6% after 1, 5 and 10 years, respectively. Adjusting for the background mortality in the general Swedish population, relative 1-, 5- and 10-year survival rates were 99.0%, 97.5% and 89.0%, respectively (Fig. 2).
Figure 2:
Long-term observed and relative survival following aortic valve surgery. Comparison of long-term observed (continuous line) and relative survival (dotted line) for patients undergoing aortic valve replacement (n = 4970). The 95% confidence intervals for estimated survival are provided as well as the number at risk (n) at the start of even follow-up years.
Patients with bicuspid morphology had a marked long-term survival advantage compared to those with tricuspid morphology, shown both in analyses of observed and relative survival (Fig. 3). Relative survival for patients with bicuspid morphology was close to the survival in the general population during the whole observation period.
Figure 3:
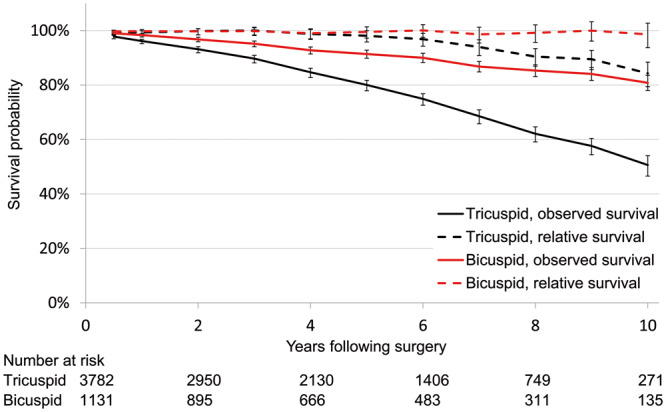
Long-term observed and relative survival following aortic valve surgery stratified on valvular morphology. Long-term observed (continuous line) and relative (dotted line) survival for patients undergoing aortic valve replacement (n = 4970), shown for tricuspid valves (black line) and bicuspid valves (red), separately. Number at risk (n) at the start of even follow-up years.
Observed mortality
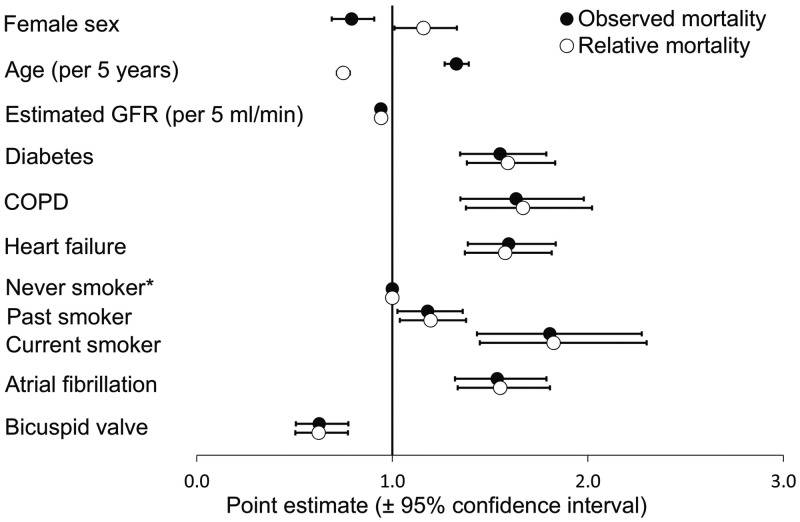
The multivariable analysis of observed mortality confirmed that bicuspid morphology was independently associated with reduced mortality (P < 0.001) (Fig. 4). Moreover, increasing age, male sex, diabetes, preoperative atrial fibrillation, chronic obstructive pulmonary disease (COPD), heart failure, current smoking and reduced eGFR were associated with increased mortality. Peripheral vascular disease, valve prosthesis type (biological vs mechanical), combined surgery (AVR + CABG) and previous cardiac surgery were not significantly associated with increased mortality (P = 0.47, P = 0.27, P = 0.84 and P = 0.84, respectively; Supplementary Material, Table S3).
Figure 4:
Predictors of long-term mortality in patients undergoing aortic valve replacement surgery. Estimated hazard ratios (HR, black dots) and relative mortality ratios (hollow dots) for predictor variables of long-term observed and relative mortality, respectively. Corresponding 95% confidence intervals are provided. COPD; chronic obstructive pulmonary disease; GFR; glomerular filtration rate. *Never smoker was used as the reference category (HR = 1.0).
The time-dependent analysis showed that the survival advantage among patients with a bicuspid morphology was only significant after the first postoperative year (P = 0.09 in year 0–1 vs P < 0.001 > 1 year; Supplementary Material, Fig. S1). The effect of ageing increased with observation time. The female survival advantage decreased with increasing observational time. Reduced eGFR was no longer associated with increased mortality after 5 years, indicating that the influence of eGFR on mortality due to valvular surgery was mainly related to early mortality.
Relative mortality
When adjusting for the expected mortality in the general Swedish population based on age, sex and year, bicuspid valve morphology was also associated with lower RMR [RMR 0.62 (0.50–0.77); P < 0.001; Fig. 4]. Increasing age was associated with lower relative mortality [RMR 0.75 per 5 years (0.72–0.78); P < 0.001]. Female sex was associated with increased mortality [RMR 1.16 (1.01–1.33); P = 0.04]. Combined surgery (AVR + CABG) was not associated with relative mortality [RMR 1.07 (0.94–1.22); P = 0.33]. The other risk factors for observed mortality remained unchanged when adjusting for the expected mortality.
In the time-dependent analysis of relative mortality, the survival advantage with bicuspid valve morphology was consistent through all time intervals (Supplementary Material, Fig. S2). Female sex was no longer associated with increased RMR during the later years.
DISCUSSION
In this study of a substantial number of consecutive patients (n = 4970) who underwent AVR with or without concomitant CABG, survival rates returned towards the average expected survival of their matched cohort in the general population during the first 6 years. Thereafter, relative survival rates declined. Bicuspid morphology was independently associated with higher long-term survival. Renal dysfunction, diabetes, COPD, heart failure, smoking and atrial fibrillation were associated with higher long-term mortality. Combined surgery was not associated with higher observed or relative mortality.
Valve morphology
The survival of individuals with diagnosed bicuspid aortic valve disease has been investigated previously. A recent meta-analysis by Masri et al. [12] showed that survival in populations with bicuspid valve matched that of the general population. This result was ascribed to successful surveillance and surgical intervention in patients with significant valve dysfunction. However, in contrast to our data, this publication did not focus specifically on outcomes following surgery. Moreover, the study by Masri et al. did not include patients with tricuspid morphology. Mentias et al. [13] focused on surgery for aortic incompetence only. They found excellent survival in patients operated on for aortic incompetence with preserved left ventricular ejection fraction.
Little is known about the results of SAVR in patients with bicuspid compared to tricuspid morphology. In the present cohort, bicuspid morphology conveyed an independent survival advantage in the univariable and multivariable analyses. Survival following surgery for bicuspid valve pathology was close to that of the matched general population during the whole observation period. This result would suggest that surgery for this condition offers excellent treatment with low 30-day mortality and good long-term results with respect to survival. Possible complications of valve replacement, such as bleeding and thromboembolic complications in mechanical valves and structural valve degeneration in biological valves, apparently only had a small impact on long-term survival in this patient cohort. It is noteworthy that 36.5% of the patients in the bicuspid valve group received a mechanical prosthesis. Patients with tricuspid morphology were older and had a higher incidence of comorbidity. However, results were inferior to those of patients with bicuspid morphology, even when adjusting for known risk factors in the multivariable analysis. These findings seem particularly relevant in the current era, where the role of TAVI in bicuspid valves and in low-risk populations is being explored.
There was a clear negative impact of known risk factors [14], such as diabetes, COPD, heart failure, reduced renal function and smoking at the time of surgery, both for observed and relative survival. Observed and relative survival were reduced in patients with atrial fibrillation. These results have been found previously in publications on surgical AVR [15–17]. Tricuspid morphology was nevertheless an independent risk factor.
Identifying individuals with bicuspid morphology is challenging in the context of SAVR, because valve morphology has little impact on how surgery is performed. In contrast, a correct preoperative evaluation of valve morphology is important when TAVI is considered. In this study, information on valve morphology was collected from intraoperative assessment and prospective inclusion in the database. In addition, the number of missing entries was low, further strengthening the results.
Combined surgery
Populations who have AVR with and without concomitant CABG differ significantly in most studies. In an extensive analysis of Society of Thoracic Surgeons data, Brennan et al. [18] observed higher mean age, more comorbidity and a lower percentage of women in the AVR with concomitant CABG cohort. In our population, concomitant CABG was not a significant risk factor for mortality in the multivariable analysis. Surgical treatment of CAD in conjunction with AVR effectively seems to eliminate the risk of CAD in this patient population. Studies in coronary surgery without AVR have repeatedly shown that surgery has a substantial effect on the mortality risk of CAD. In a Norwegian population, Enger et al. [14] showed that CABG conveyed persistently better relative survival than that in the general population.
Relative survival in surgical aortic valve replacement
Only a few published series compare data on relative and observed survival in patients undergoing AVR. Some studies were published many years ago, based on surgical patients who do not reflect today’s population [15, 16]. Others describe series that are not large enough to allow subgroup analysis [19]. A recent study by Glaser et al. [20], based on data from the SWEDEHEART registry, analysed a larger but overlapping data set, compared to this study. The authors provided a summative assessment of observed and expected survival in 13 727 patients who underwent primary AVR without concomitant coronary surgery. They concluded that after a mean follow-up period of 6.8 years, AVR is associated with a significant loss of life expectancy, particularly in younger patients. However, valve morphology was not investigated in the publication by Glaser et al. The study of Kvidal et al. and the study of Lindblom et al. revealed a large difference in results depending on whether patients in a Swedish population were operated on for predominant aortic stenosis versus aortic incompetence [15, 16]. In our more recent analysis, results in patients with aortic incompetence were considerably improved. Predominant aortic incompetence had no significant effect on survival.
Age
As expected, age had a negative influence on observed survival, confirming previous research results. Nevertheless, several studies have focused on the excellent results achieved following AVR, with survival approaching that of the general population, particularly in the elderly [18, 21]. This result was also confirmed in our investigation where increasing age was associated with a survival advantage. The most likely explanation for this phenomenon is a selection bias for surgery in older patients, which implies that patients who were accepted for surgery on average appear to be healthier than their peers. However, our data do not provide direct means to substantiate this assumption. Although most surgeons in Scandinavian cardiothoracic practices would state that age per se is not a contraindication for AVR, other surveys have shown that 30–60% of patients with severe symptomatic aortic stenosis are not offered surgery [22–24]. The most common reason for withholding surgery is advanced age, reduced left ventricular function and neurological dysfunction [22]. Certainly, frailty is also a reason for patients being denied surgery. The disease burden in the general population has a strong correlation to age [25]. This is particularly true for stroke and dementia [25]. We therefore find that the possibility of selection bias must be considered when discussing the results of AVR in older patients. Most of our data are from the pretranscatheter era, and we speculate that more transcatheter aortic valve implants will lead to an even more pronounced selection bias in surgical populations.
Strengths and limitations
Some strengths of the study should be emphasized. Compared to several previous studies, it was large, included several variables and had few missing data (Supplementary Material, Table S1). Furthermore, the study population was unselected and consisted of consecutive patients.
Unfortunately, no information on the patient’s quality of life was available in the database.
No information on the cause of death was obtained because all-cause mortality rather than cardiac-related death was analysed. However, the study by Enger et al. [14] found that neither survival trends nor predictors of long-term mortality changed when all-cause mortality was used as an alternate end point to cardiovascular death. Furthermore, the main limitation of cause-specific survival analysis is its dependence on reliable information of the cause of death, which is often not available [26, 27]. Finally, for analysis of relative survival, information about the individual’s cause of death is not required.
Calculation of relative survival assumes that survival in the general population is unaffected by deaths related to the disease being studied. Due to the relative rarity of aortic valve disease, the influence on survival in the general population should be only marginal, at least in the younger population.
Furthermore, selection bias could be suspected because many surgeons preferably accept patients with few comorbidities for aortic valve surgery, especially among older patients. Diagnosed heart failure was analysed in this study, but left ventricular function, a significant predictor of long-term survival after AVR, was not considered. Although known risk factors for premature death in surgical populations were analysed, residual confounding factors cannot be excluded. Nevertheless, our results are still relevant because they underline the fact that these patient groups are different and that different results are achieved when operating on them.
CONCLUSION
Survival in patients undergoing AVR compared favourably with survival in the general Swedish population with the same composition of age, gender and year of operation. The presence of bicuspid morphology was associated with lower observed mortality compared with a tricuspid valve and a relative survival matching that in the general population. There was a clear negative impact of known risk factors, i.e. diabetes, COPD, atrial fibrillation, heart failure, reduced renal function and smoking at the time of surgery, on both observed and relative survival. Finally, we found no significant difference in mortality between patients undergoing AVR and combined AVR and CABG.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors appreciate the assistance provided by RTA Jan Hentschel, Heart Centre, Umeå.
Conflict of interest: none declared.
Author contributions
Anders Holmgren: Conceptualization; Project administration; Resources; Writing—original draft; Writing—review & editing. Tone Bull Enger: Formal analysis; Methodology; Software; Visualization; Writing—original draft; Writing—review & editing. Ulf Näslund: Conceptualization; Funding acquisition; Supervision; Writing—review & editing. Vibeke Videm: Formal analysis; Methodology; Writing—review & editing. Solveig Valle: Data curation; Validation. Karen Julie Dybvad Evjemo: Data curation; Validation. Örjan Friberg: Resources. Alexander Wahba: Conceptualization; Project administration; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Rafael Garcia-Fuster, Tadashi Isomura and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- AVR
Aortic valve replacement
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- COPD
Chronic obstructive pulmonary disease
- eGFR
Estimated glomerular filtration rate
- RMR
Relative mortality ratios
- SAVR
Surgical aortic valve replacement
- TAVI
Transcatheter aortic valve implantation
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
REFERENCES
- 1. Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM. et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoon SH, Bleiziffer S, De Backer O, Delgado V, Arai T, Ziegelmueller J. et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017;69:2579–89. [DOI] [PubMed] [Google Scholar]
- 3. Gao D, Grunwald GK, Rumsfeld JS, Schooley L, MacKenzie T, Shroyer AL.. Time-varying risk factors for long-term mortality after coronary artery bypass graft surgery. Ann Thorac Surg 2006;81:793–9. [DOI] [PubMed] [Google Scholar]
- 4.Statistics Sweden: Life Table by Sex and Age. Year 1960–2018 (Data Obtained through the Human Mortality Database). www.mortality.org (16 February 2018, date last accessed).
- 5. Dickman PW, Coviello E.. Estimating and modeling relative survival. Stata J 2015;15:186–215. [Google Scholar]
- 6. Harrell FE Jr, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Stevens LA, Schmid CH, Zhang Y(L), Castro AF, Feldman HI. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers' D. Stata J 2010;10:339–58. [Google Scholar]
- 9. Pohar M, Stare J.. Relative survival analysis in R. Comput Methods Programs Biomed 2006;81:272–8. [DOI] [PubMed] [Google Scholar]
- 10. Pohar M, Stare J.. Making relative survival analysis relatively easy. Comput Biol Med 2007;37:1741–9. [DOI] [PubMed] [Google Scholar]
- 11.Perme. relsurv: Relative Survival: R Package Version 2.0-6; 2015. htttp://CRANR-projectorg/package=relsurv (6 June 2016, date last accessed).
- 12. Masri A, Svensson LG, Griffin BP, Desai MY.. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart 2017;103:1323–30. [DOI] [PubMed] [Google Scholar]
- 13. Mentias A, Feng K, Alashi A, Rodriguez LL, Gillinov AM, Johnston DR. et al. Long-term outcomes in patients with aortic regurgitation and preserved left ventricular ejection fraction. J Am Coll Cardiol 2016;68:2144–53. [DOI] [PubMed] [Google Scholar]
- 14. Enger TB, Pleym H, Stenseth R, Greiff G, Wahba A, Videm V.. Reduced long-term relative survival in females and younger adults undergoing cardiac surgery: a prospective cohort study. PLoS One 2016;11:e0163754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kvidal P, Bergström R, Horte LG, Ståhle E.. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000;35:747–56. [DOI] [PubMed] [Google Scholar]
- 16. Lindblom D, Lindblom U, Qvist J, Lundström H.. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990;15:566–73. [DOI] [PubMed] [Google Scholar]
- 17. Ahlsson A, Bodin L, Fengsrud E, Englund A.. Patients with postoperative atrial fibrillation have a doubled cardiovascular mortality. Scand Cardiovasc J 2009;43:330–6. [DOI] [PubMed] [Google Scholar]
- 18. Brennan JM, Edwards FH, Zhao Y, O'Brien SM, Douglas PS, Peterson ED.. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 1991 to 2007. Circulation 2012;126:1621–9. [DOI] [PubMed] [Google Scholar]
- 19. Lassnigg A, Hiesmayr M, Frantal S, Brannath W, Mouhieddine M, Presterl E. et al. Long-term absolute and relative survival after aortic valve replacement: a prospective cohort study. Eur J Anaesthesiol 2013;30:695–703. [DOI] [PubMed] [Google Scholar]
- 20. Glaser N, Persson M, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U.. Loss in life expectancy after surgical aortic valve replacement: SWEDEHEART study. J Am Coll Cardiol 2019;74:26–33. [DOI] [PubMed] [Google Scholar]
- 21. Cappabianca G, Ferrarese S, Musazzi A, Terrieri F, Corazzari C, Matteucci M. et al. Predictive factors of long-term survival in the octogenarian undergoing surgical aortic valve replacement: 12-year single-centre follow-up. Heart Vessels 2016;31:1798–805. [DOI] [PubMed] [Google Scholar]
- 22. Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P. et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714–20. [DOI] [PubMed] [Google Scholar]
- 23. Charlson E, Legedza AT, Hamel MB.. Decision-making and outcomes in severe symptomatic aortic stenosis. J Heart Valve Dis 2006;15:312–21. [PubMed] [Google Scholar]
- 24. Bach DS, Cimino N, Deeb GM.. Unoperated patients with severe aortic stenosis. J Am Coll Cardiol 2007;50:2018–19. [DOI] [PubMed] [Google Scholar]
- 25. Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R. et al. The burden of disease in older people and implications for health policy and practice. Lancet (London, England). 2015;385:549–62. [DOI] [PubMed] [Google Scholar]
- 26. Lloyd-Jones DM, Martin DO, Larson MG, Levy D.. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med 1998;129:1020–6. [DOI] [PubMed] [Google Scholar]
- 27. Mant J, Wilson S, Parry J, Bridge P, Wilson R, Murdoch W. et al. Clinicians didn't reliably distinguish between different causes of cardiac death using case histories. J Clin Epidemiol 2006;59:862–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.