Abstract
Purpose
To evaluate metformin’s benefit on the incidence and survival of hepatocellular carcinoma (HCC) in cirrhosis with type 2 diabetes mellitus (T2DM) patients.
Patients and Methods
We conducted a retrospective study from 2006 to 2019. The patients were assigned to metformin exposure if they administered metformin at least 3 months after diagnosis of cirrhosis. The outcomes were incidence and survival of HCC in T2DM with cirrhosis treated with metformin compared with those who were not treated with metformin. For the incidence of HCC, the follow-up time was 5 years after cirrhosis was diagnosed. For the survival of HCC, we censored for vital status in June 2019.
Results
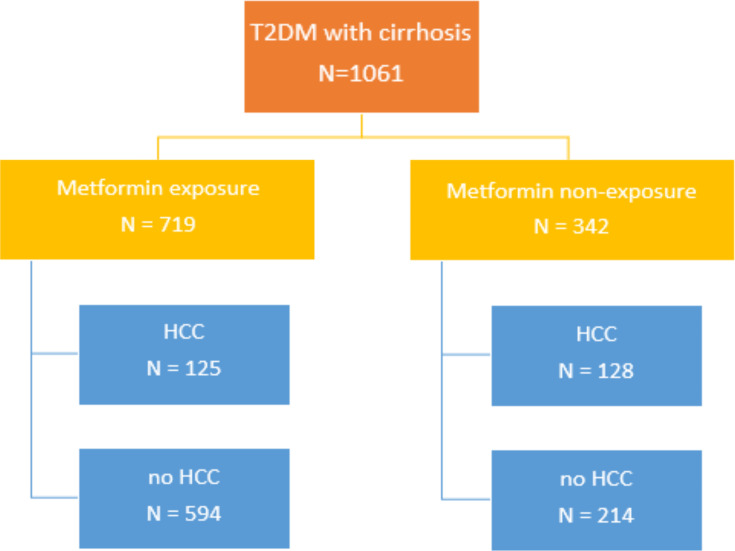
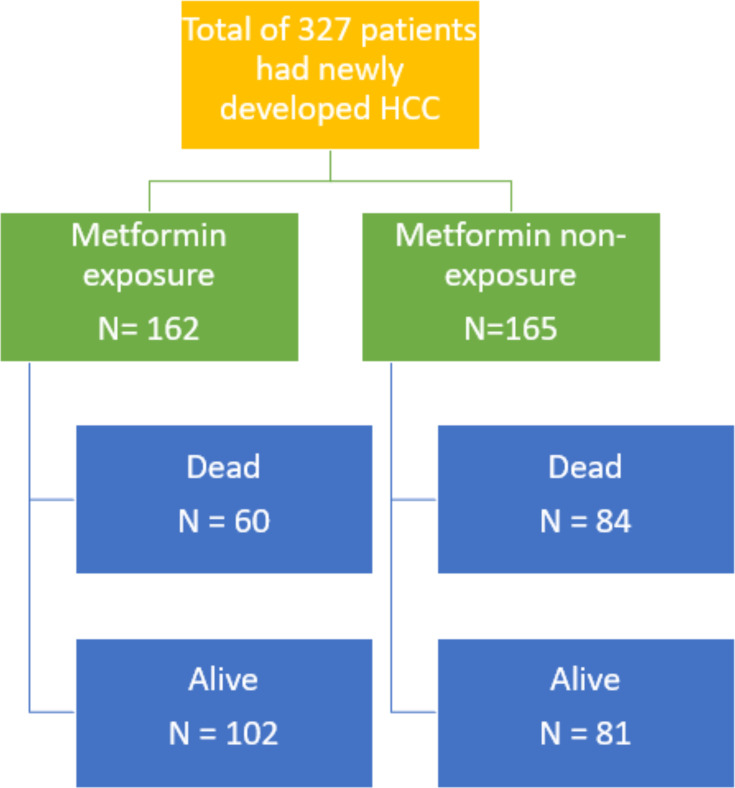
Of 1061 patients, the patients were divided into 719 patients with metformin exposure and 342 in metformin non-exposure. In metformin exposure, 125 patients (17.4%) developed HCC. In metformin non-exposure, 128 patients (37.4%) developed HCC. Metformin exposure had a significantly lower risk of developing HCC in multivariate analysis HR 0.48 (0.36–0.61); P<0.001. For the survival of HCC, 327 patients were recruited. One-hundred and sixty-two patients were in metformin exposure and 165 patients were in metformin non-exposure. Sixty patients (37%) in metformin exposure died, while 84 patients (50.9%) in metformin non-exposure died. The median survival of metformin exposure and metformin non-exposure were 6.9 years and 3.88 years, respectively; P=0.003. In univariate analysis, the metformin exposure was significantly associated with better survival than in the non-exposure group, HR 0.63 (0.45–0.88); P=0.006. No significant difference was observed in multivariate analysis between two groups, HR 1.07 (0.74–1.54); P=0.72.
Conclusion
Metformin exposure was associated with a lower incidence of HCC in cirrhosis with T2DM patients and seemed to extend survival. Continuing metformin in patients with cirrhosis with T2DM should be considered if there was no contraindication.
Keywords: type 2 diabetes mellitus, cirrhosis, hepatocellular carcinoma, metformin
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic disease that is a major problem for public health worldwide. The International Diabetes Federation predicting that by 2030, 522 million people will have diabetes.1 Diabetes can cause complications in important organs. Besides, it was found that T2DM and many cancers share some common risk factors.2–5 Indeed, diabetes is one of the risk factors for many cancer types, including HCC.6–10 Insulin resistance may affect the delivery and accumulation of free fatty acids to the liver and contribute to fatty liver. In addition, oxidative stress can lead to the inflammation of hepatocytes, with the risk the inflammation may lead to fibrosis and develop into cirrhosis and HCC.11–14 HCC is the fifth most common cancer in the world.15 Important risk factors for HCC are all causes of cirrhosis. One-third of patients with cirrhosis will develop HCC in the future.16 In addition, diabetes also increases mortality from HCC. Despite there being many methods for diagnosing and treating HCC, the mortality rate from HCC is still high and the treatment results are not as effective as they should be.
Hemoglobin A1c (HbA1c) levels and the different treatments of diabetes carry different risks of developing HCC.17,18 Metformin is classified as the first-line drug for the treatment of T2DM by the practice guidelines of many agencies around the world, such as the American Diabetes Association (ADA),19 the International Diabetes Federation (IDF),20 and the European Association for the Study of Diabetes (EASD).21 It works by reducing insulin resistance, which is the pathogenesis of T2DM, due to there being much evidence supporting its long-term safety and therapeutic efficacy. However, metformin use is limited in some patients with impaired renal function, as it may cause lactic acidosis. The older biguanides were discontinued because they were reportedly found to be associated with lactic acidosis. However, lactic acidosis is rarely reported with the use of metformin and it is more likely to occur in patients with some conditions that can cause poor tissue perfusion.22
Metformin has a potential effect on anti-tumor activity by two main mechanisms 1) indirect effect may be related with insulin/IGF-1 pathway. Metformin increasing muscle uptake of glucose and reducing plasma insulin levels by decreasing hepatic gluconeogenesis, which can reduce and slow cancer cells proliferation; and 2) direct effect of metformin in decreased cell proliferation by against respiratory Complex I of the electron transport chain in mitochondria of cancer cells, which interfere cancer-promoting signaling pathway. However from animal cell study and epidemiological studies showed evidence of the mechanism of action of metformin could be mixed both direct and indirect effects involve the activation of AMP-activated protein kinase (AMPK), which inhibits the mammalian target of the rapamycin (mTOR) pathway, reducing cell proliferation, and inducing apoptosis and cell-cycle arrest.23 Many researchers have studied the relationship between metformin and cancer prevention and treatment. For instance, in 2005, Evans et al found a 23% decrease in cancer incidence among T2DM patients who received metformin than those who received sulfonylurea.24
Although there have been many studies covering the use of metformin to reduce the incidence of various types of cancer, including HCC, no current studies have been conducted on T2DM with cirrhosis patients. T2DM and liver cirrhosis are considered important risk factors for HCC. Additionally, the survival rate of patients with HCC who use metformin is currently controversial.25,26 Consequently, we performed this study to investigate two clinical questions: The incidence rate of HCC in patients with liver cirrhosis with T2DM who were exposed compared to those unexposed to metformin therapy and the survival rate of HCC patients who were exposed compared to those unexposed to metformin therapy. This study results may be useful for further treatment in the future.
Patients and Methods
Study Participants
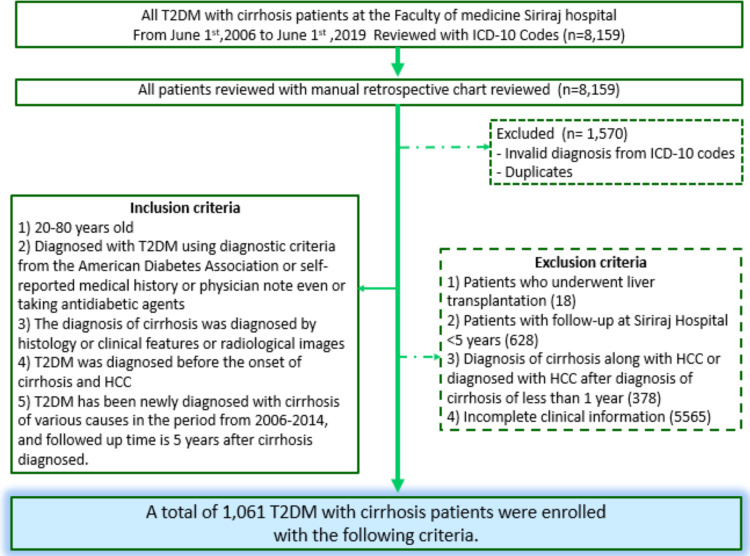
Participants required to meet the ICD-10 research criteria for cirrhosis, T2DM, and presenting with all of the following criteria: 1) aged 20–80 years old; 2) diagnosed with T2DM using diagnostic criteria from ADA;19 3) cirrhosis diagnosed by histology or clinical features or radiological images; 4) T2DM diagnosed before the occurrence of cirrhosis and 5) newly diagnosed with cirrhosis from various causes in the period from 2006–2014.
Exclusion criteria were as follows: 1) patients who had undergone liver transplantation; 2) patients who were followed up less than five years; 3) diagnosis of cirrhosis along with HCC or diagnosed with HCC after a diagnosis of cirrhosis in less than one year, or 4) incomplete clinical information. The diagnosis of HCC was made according to the American Association for the Study of Liver Diseases (AASLD)27 or The European Association for the Study of the Liver (EASL) guidelines.16 A continuous exposure to metformin for at least three months since the cirrhosis was diagnosed was defined as significant metformin exposure (Figure 1).
Figure 1.
Flow chart of the subjects selection process showing the exclusion criteria and the number of subjects considered for inclusion.
Outcomes
The first outcome was the 5-year overall incidence of HCC in T2DM with cirrhosis patients. The second outcome was the survival outcome in HCC patients who were exposed to metformin.
Study Procedures
All participants were recruited between January 2006 and June 2014. We divided participants into two cohorts according to the objectives. The first cohort was for the incidence of HCC. We recruited the participants between January 2006 and June 2014. Their follow-up time was 5 years after cirrhosis was diagnosed. The second cohort was for HCC survival. The participants were T2DM with cirrhosis who developed HCC within 5 years (from the first cohort) and developed HCC after 5 years. We censored for vital status in June 2019.
This study was approved by the Siriraj Institutional Review Board, Faculty of Medicine Siriraj Hospital, Mahidol University. The patient consent to review their medical records was not required by the Siriraj Institutional Review Board because this research does not affect the rights and welfare of the subjects. The trial was conducted according to the Declaration of Helsinki and patient data was kept confidential.
Data were collected retrospectively as recorded in electronic medical records, including age, gender, body mass index (BMI), etiology of cirrhosis, average %HbA1c level in the first year after being diagnosed with cirrhosis, metformin exposure or non-exposure, Child-Pugh classification, smoking and alcohol consumption, age when diagnosed with T2DM, cirrhosis, HCC, Barcelona clinic liver cancer (BCLC) staging for HCC patients. Death and causes of death reports in this cohort were derived from the Bureau of Policy and Strategy database, Thai Ministry of Public Health.
Statistical Analysis
The clinical data of all cirrhosis with T2DM patients were retrospectively collected in case record form and followed for five years from their cirrhosis diagnosis. The baseline clinical characteristics of cirrhosis with T2DM patients who were exposed vs non-exposed to metformin were compared using the Students t-test or Mann–Whitney test for comparing continuous variables and the chi-square test for categorical variables. The associations among gender, age at cirrhosis diagnosis, BMI, Child-Pugh classification, etiology of cirrhosis, average %HbA1c level in the first year after being diagnosed with cirrhosis, smoking history, alcohol consumption, BCLC staging, metformin exposure and risk of developing HCC, and risk of death were determined by hazard ratio (HR) and 95% confidence interval (95% CI) calculated using Cox proportional hazards analysis. For each variable with a p-value less than 0.05 in the univariate analysis, the predicted outcomes were entered into stepwise Cox regression multivariable models. In all the tests, the survival of patients in both groups was estimated using the Kaplan–Meier method and compared with the use of the Log rank test. Follow-up data were censored in June 2019 for patients who had developed HCC and so the survival time of those HCC patients was prospectively calculated from the date of HCC diagnosis to the date of their death or last follow-up. A p-value<0.05 was considered statistically significant. All the statistical analyses were conducted using SPSS version 19.
Results
Study Participants
A Total of 1061 cirrhosis with T2DM patients was recruited. There were 719 patients in the metformin exposure group and 342 patients in the metformin non-exposure group. The metformin exposure group has a significantly higher rate of Child-Pugh classification A and a lower rate of smokers. Sex, BMI, %HbA1c, etiology of cirrhosis, and alcohol consumption history were not significantly different between metformin exposure and non-exposure groups. The baseline clinical characteristics were shown in Table 1.
Table 1.
Baseline Clinical Characteristics
| Variables | Metformin Exposure (n = 719) | Metformin Non-Exposure (n = 342) | p-value |
|---|---|---|---|
| Male | 419 (66.6%) | 220 (64.3%) | 0.057 |
| Age | 59.0±9.9 | 60.6±9.9 | 0.834 |
| BMI | 707 | 328 | 0.502 |
| <18.5 | 23 (3.3%) | 18 (5.5%) | |
| 18.5–22.9 | 151 (21.4%) | 93 (28.4%) | |
| 23–24.9 | 144 (20.4%) | 78 (23.8%) | |
| ≥ 25 | 389 (55.0%) | 139 (42.4%) | |
| Child–Pugh class | <0.001 | ||
| A | 629 (87.5%) | 257 (75.2%) | |
| B | 72 (10.0%) | 71 (20.8%) | |
| C | 18 (2.5%) | 14 (4.1%) | |
| HbA1c | 699 | 325 | 0.645 |
| <7.5% | 453 (64.8%) | 213 (65.5%) | |
| ≥7.5% | 246 (35.2%) | 112 (32.8%) | |
| Etiology of cirrhosis | 0.568 | ||
| Alcoholism | 143 (19.9%) | 84 (24.6%) | |
| HBV | 227 (31.6%) | 115 (45.3%) | |
| HCV | 133 (18.5%) | 60 (17.5%) | |
| NASH | 136 (18.9%) | 34 (9.9%) | |
| Others | 80 (11.1%) | 49 (14.3%) | |
| Alcohol | 675 | 334 | 0.230 |
| No | 374 (55.4%) | 162 (48.5%) | |
| Yes | 199 (29.5%) | 107 (32.0%) | |
| Quit ≥5 years | 102 (15.1%) | 65 (19.5%) | |
| Smoking | 605 | 301 | 0.004 |
| No | 428 (70.7%) | 189 (62.8%) | |
| Yes | 93 (15.4%) | 48 (16.0%) | |
| Quit ≥5 years | 84 (13.9%) | 64 (21.3%) |
Incidence of HCC
We followed the 1061 cirrhosis with T2DM patients after they had been diagnosed with cirrhosis for a period of five years. The 5-year overall incidence of HCC was 253 patients (23.9%). In the metformin exposure group, 125 patients (17.4%) developed HCC. In the metformin non-exposure group, 128 patients (37.4%) developed HCC within 5 years. In the univariate analysis, the 5-year incidence of HCC was lower in the metformin exposure group compare with the metformin non-exposure group (HR: 0.42; 95% CI: 0.33–0.54; p<0.001) (Table 2). For other variables, male gender (HR: 1.64; 95% CI: 1.25–2.14; p<0.001), average %HbA1c in the first year after diagnosis with cirrhosis ≥ 7.5% (HR: 1.30; 95% CI: 1.00–1.68; p=0.049), smoking cessation ≥ 5 years (HR: 1.78; 95% CI: 1.31–2.42; p=0.001), and alcohol cessation ≥ 5 years (HR: 1.74; 95% CI: 1.27–2.38; p=0.002) were risk factors of developing HCC. The etiology of cirrhosis was also a risk factor of HCC development, p<0.001. HBV cirrhosis had a HR of 1.80 (95% CI: 1.13–2.85) and HCV cirrhosis had a HR of 2.31 (95% CI: 1.43–3.74). In addition, BMI, Child-Pugh classification and age at diagnosis with cirrhosis were not risked of HCC development (Table 2).
Table 2.
Cox Proportional Hazards Analysis of Variables Associated with 5-Year Incidence of Hepatocellular Carcinoma in Cirrhosis with Type 2 Diabetes Patients
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Gender | ||||||
| Male | 1.636 | 1.249–2.142 | <0.001 | 1.373 | 0.977–1.929 | 0.068 |
| Female | Ref | Ref | Ref | Ref | Ref | Ref |
| BMI | 0.980 | |||||
| <18.5 | 1.038 | 0.545–1.974 | 0.911 | |||
| 18.5–22.9 | 0.943 | 0.689–1.290 | 0.713 | |||
| 23.0–24.9 | 0.962 | 0.697–1.328 | 0.815 | |||
| ≥25 | Ref | Ref | Ref | |||
| Child–Pugh score | 0.152 | |||||
| A | Ref | Ref | Ref | |||
| B | 1.364 | 0.981–1.895 | 0.065 | |||
| C | 0.825 | 0.367–1.859 | 0.643 | |||
| HbA1c | ||||||
| <7.5% | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥7.5% | 1.296 | 1.002–1.677 | 0.049 | 1.188 | 0.910–1.552 | 0.205 |
| Age at diagnosis of cirrhosis | 0.245 | |||||
| <55 | Ref | Ref | Ref | |||
| 55–64 | 1.246 | 0.926–1.675 | 0.146 | |||
| ≥65 | 1.012 | 0.733–1.397 | 0.942 | |||
| Etiology of cirrhosis | <0.001 | <0.001 | ||||
| Alcoholism | 0.913 | 0.537–1.551 | 0.735 | 0.736 | 0.395–1.373 | 0.335 |
| CHB | 1.797 | 1.133–2.851 | 0.013 | 1.706 | 1.045–2.786 | 0.033 |
| CHC | 2.308 | 1.431–3.742 | 0.001 | 2.197 | 1.323–3.647 | 0.002 |
| NASH | 0.805 | 0.451–1.435 | 0.462 | 0.856 | 0.469–1.562 | 0.612 |
| Other | Ref | Ref | Ref | Ref | Ref | Ref |
| Smoke | 0.001 | 0.350 | ||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.128 | 0.786–1.619 | 0.513 | 0.821 | 0.519–1.298 | 0.399 |
| Quit ≥5 years | 1.777 | 1.305–2.419 | <0.001 | 1.172 | 0.770–1.783 | 0.459 |
| Alcohol | 0.002 | 0.419 | ||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.127 | 0.843–1.508 | 0.420 | 1.308 | 0.845–2.025 | 0.229 |
| Quit ≥5 years | 1.736 | 1.267–2.378 | 0.001 | 1.278 | 0.822–1.988 | 0.276 |
| Metformin | ||||||
| Non-exposure | Ref | Ref | Ref | Ref | Ref | Ref |
| Exposure | 0.423 | 0.331–0.542 | <0.001 | 0.475 | 0.364–0.619 | <0.001 |
Multivariate Cox regression analysis was used to assess the independent risk with the different variables of the cirrhosis with T2DM patients. We focused on metformin exposure, gender, etiology of cirrhosis, smoking cessation, and alcohol cessation. Metformin exposure was still associated with a decreased incidence of HCC, with a HR of 0.48 (95% CI: 0.36–0.62, p<0.001). The cirrhosis etiology was a risk factor of HCC with p<0.001. HBV cirrhosis had a HR of 1.71 (95% CI: 1.05–2.79) and HCV cirrhosis had a HR of 2.20 (95% CI: 1.32–3.65) (Table 2).
HCC Survival
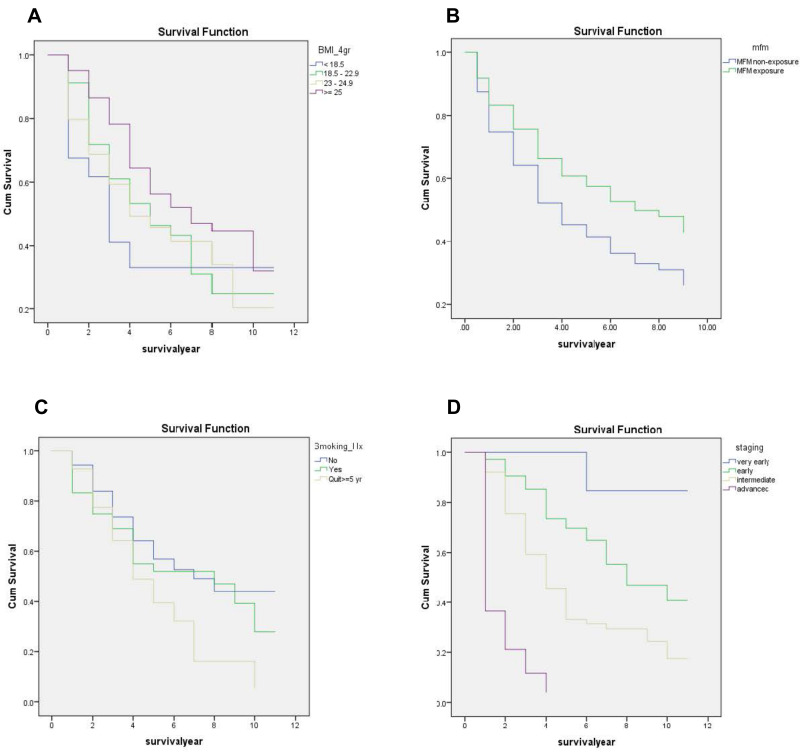
Among the 1061 cirrhosis with T2DM patients, we prospectively followed these patients until June 2019 and found that a total of 327 patients had newly developed HCC (253 patients developed HCC within 5 years and 74 patients developed HCC after 5 years). The number of HCC patients with metformin exposure vs metformin non-exposure was 162 vs 165, respectively (Figure 2). By the time of data censure, 144 of the 327 patients (44.0%) died from HCC or its complication (Figure 3). The median survival of the total cohort was five years (3.65–6.35 years). The median survival of the HCC patients in the metformin exposure group significantly greater than that of the HCC patients in the metformin non-exposure group (6.90 vs 3.88 years, p=0.003). In univariate analysis, the metformin exposure group was significantly associated with better survival than the metformin non-exposure group (HR: 0.63; 95% CI: 0.45–0.88), p=0.006. Other than metformin exposure, BMI lower than 25 kg/m2, smoking and advance stage of HCC were associated with poor survival (Figure 4). Only the stage of HCC remained statistically significant in the survival of HCC in multivariate analysis, p<0.001. No significant difference was observed in HCC patients’ survival with cirrhosis with T2DM between the metformin exposure and non-exposure groups (Table 3).
Figure 2.
The 5-year overall incidence of hepatocellular carcinoma in T2DM with cirrhosis patients.
Figure 3.
Survival of hepatocellular carcinoma in type 2 diabetes patients with cirrhosis who were treated with and without metformin. Follow-up was censored in June 2019.
Figure 4.
Kaplan–Meier survival curves of patients with hepatocellular carcinoma according to grouping using (A) metformin exposure and metformin non-exposure. (B) The BMI: (1) <18.5, (2) 18.5–22.9, (3) 23–24.9, (4) ≥25 kg/m2. (C) Smoking history: (1) non-smoking, (2) current smoker, (3) quit ≥5 years. (D) BCLC staging of HCC: (1) very early, (2) early, (3) intermediate, (4) advanced/terminal.
Table 3.
Univariate and Multivariate Cox Regression Analysis of Prognostic Factors of Survival in HCC Patients
| Prognostic Factor | Number of Deaths/Total | Median Survival (Years) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| Gender | 0.347 | |||||||
| Male | 106/233 | 4.87 | 1.195 | 0.825–1.731 | ||||
| Female | 38/94 | 7.09 | Ref | Ref | ||||
| BMI | 0.031 | 0.734 | ||||||
| <18.5 | 11/19 | 2.57 | 1.981 | 1.041–3.769 | 1.276 | 0.619–2.629 | ||
| 18.5–22.9 | 36/70 | 4.47 | 1.521 | 1.009–2.291 | 1.234 | 0.796–1.914 | ||
| 23.0–24.9 | 34/70 | 3.93 | 1.630 | 1.073–2.476 | 1.199 | 0.752–1.913 | ||
| ≥25 | 63/168 | 6.41 | Ref | Ref | Ref | Ref | ||
| Child–Pugh score | 0.152 | |||||||
| A | 107/266 | 6.13 | Ref | Ref | ||||
| B | 30/52 | 3.15 | 1.364 | 0.981–1.895 | ||||
| C | 7/9 | 0.90 | 0.825 | 0.367–1.859 | ||||
| %HbA1c | 0.216 | |||||||
| <7.5 | 95/206 | 5.13 | Ref | Ref | ||||
| ≥7.5 | 49/121 | 5.00 | 0.908 | 0.643–1.282 | ||||
| Age at diagnosis | 0.796 | |||||||
| HCC | 23/63 | 9.37 | Ref | Ref | ||||
| <55 | 63/129 | 4.73 | 1.386 | 0.859–2.236 | ||||
| 55–64 | 58/135 | 4.86 | 1.296 | 0.799–2.104 | ||||
| ≥65 | ||||||||
| Etiology of cirrhosis | 0.972 | |||||||
| Alcoholism | 23/54 | 3.79 | 0.906 | 0.459–1.790 | ||||
| CHB | 56/127 | 5.73 | 0.816 | 0.446–1.493 | ||||
| CHC | 38/85 | 4.72 | 0.852 | 0.453–1.602 | ||||
| NASH | 14/37 | 8.00 | 0.727 | 0.341–1.547 | ||||
| Other | 13/24 | 3.99 | Ref | Ref | ||||
| Smoke | 0.024 | 0.059 | ||||||
| No | 67/181 | 6.77 | Ref | Ref | Ref | Ref | ||
| Yes | 29/70 | 7.42 | 1.243 | 0.803–1.923 | 0.981 | 0.622–1.547 | ||
| Quit ≥5 years | 34/57 | 3.93 | 1.776 | 1.175–2.685 | 1.620 | 1.055–2.486 | ||
| Alcohol | 0.286 | |||||||
| No | 65/146 | 4.86 | 0.780 | 0.501–1.215 | ||||
| Yes | 47/128 | 7.55 | 0.686 | 0.429–1.095 | ||||
| Quit ≥5 years | 28/49 | 4.28 | Ref | Ref | ||||
| Metformin | 0.006 | 0.720 | ||||||
| Non-exposure | 84/165 | 3.88 | Ref | Ref | Ref | Ref | ||
| Exposure | 60/162 | 6.90 | 0.629 | 0.451–0.877 | 1.069 | 0.741–1.544 | ||
| Stage | <0.001 | <0.001 | ||||||
| Very early | 1/17 | 11.00 | Ref | Ref | Ref | Ref | ||
| Early | 46/146 | 7.62 | 5.718 | 0.788–41.468 | 5.931 | 0.812–43.298 | ||
| Intermediate | 70/131 | 3.68 | 12.909 | 1.791–93.027 | 13.206 | 1.817–95.994 | ||
| Advanced/Terminal | 27/33 | 0.79 | 58.361 | 7.483–434.277 | 44.694 | 5.844–341.825 | ||
Discussion
T2DM and cancer remarkably influence public health worldwide. T2DM is associated with various types of cancers, one of which is HCC.9,10,12,15,17,28 T2DM has been shown to incur a 2–3-fold increase in the risk of HCC.29 The risk may be increased more in people who have both cirrhosis and T2DM by ten-fold.30 Previous observation studies about diabetes treatment have shown that treatment with metformin is associated with a reduced risk of many cancers, including HCC. However, the risks and benefits of metformin treatment in cirrhosis with T2DM patients are still unclear. Metformin-associated lactic acidosis (MALA) is rarely reported (6.3/100,000 patient-years).31 The mortality rate of MALA is reported to be 50% and the mechanism of lactic acidosis is still uncertain.32 Physicians often discontinue metformin once patients are diagnosed with cirrhosis due to the side effects and potential risk for lactic acidosis. We aimed to determine whether metformin use had a better outcome in cirrhosis with T2DM and survival in HCC patients.
The exact pathological mechanism of the association between T2DM and HCC remains not completely understood. One of the reasons for the association between both diseases is that they share common risk factors, such as obesity, alcohol consumption, smoking, and fatty liver. The following process may be involved in cancer formation. T2DM increases the formation of free fatty acids and hyperinsulinemia. T2DM leads to the accumulation of ROS. An excessive generation of ROS will activate NF-kB and STATs signaling pathways and hepatic stellate cells, which leads to hepatocarcinogenesis. Insulin resistance; endogenous and exogenous hyperinsulinemia in T2DM lead to an increased level of IGF-1, which stimulates the proliferation of hepatocytes and inhibits cellular apoptosis within the liver. The increased release of pro-inflammatory cytokines, JNK-1 activation, altered gut microbiota, and immune mechanisms also play roles in the development and progression of HCC.3,33
Metformin is most commonly used for initial therapy in T2DM. The mechanism of metformin’s action is to reduce the levels of both glucose and insulin plasma in T2DM patients and, consequently, decrease insulin resistance, which is the clinical feature of T2DM. On the contrary, insulin therapy or some oral hypoglycemic agents are used to increase insulin plasma levels. In vitro and in vivo studies have shown chemopreventive and chemotherapeutic effects of metformin in many cancers, such as colorectum, breast, and liver. In laboratory studies, it has been shown that metformin has an anti-oxidant effect, inhibits tumor proliferation, apoptosis, activates AMP-activated protein kinase (AMPK), and inhibits the mTORC1 signaling pathway and downstream effectors, which may describe the anti-tumor effects of metformin.24,34,35
Our study revealed the novel observation of an association between metformin exposure and the incidence of HCC in patients with cirrhosis with T2DM. Moreover, we observed an association between metformin exposure and survival in HCC patients, particularly in a large cohort at the Faculty of Medicine Siriraj Hospital.
For the baseline characteristics, significant differences were seen in the Child-Pugh classification and smoking history between the two groups. The metformin exposure group had a significantly higher number of patients with Child-Pugh classification A. The baseline clinical characteristic of this cohort showed that metformin was significantly used in Child-Pugh classification A, but not in B or C. These results might affect the incidence of HCC because a higher Child-Pugh score is associated with a higher rate of HCC development.36
The metformin non-exposure group was more likely to smoke than the metformin exposure group. A previous study showed that smoking is an independent risk factor of HCC.37 Thus the incidence of HCC might be increased in the metformin non-exposure group from this condition. Our study showed that metformin exposure was significantly associated with a 58% reduced risk of HCC within 5 years in cirrhosis with T2DM patients. Metformin exposure remained significant after adjusting by multivariate analysis. This result suggests that metformin exposure may decrease the incidence of HCC within 5 years after a diagnosis of cirrhosis. This hypothesis is consistent with the previous case-control study by Donadon et al, which shows that metformin treatment was associated with a strong and statistically significant reduction of the risk of HCC compared with the use of sulfonylureas or insulin.24 Chen et al reported that metformin use could reduce the risk of HCC development in patients with diabetes by 7%.38 A meta-analysis study by Zhang et al showed that people who had been treated with metformin had a reduced risk of developing HCC by 76%.39 For these reasons, metformin may have a protective effect on HCC development. So, continuing metformin should be considered in cirrhosis with T2DM patients if there is no contraindication, such as liver failure or renal impairment, due to the better benefit from reducing HCC occurrence. Although lactic acidosis is a concern in patients with liver disease when using metformin, lactic acidosis did not occur in our cohort.
For other variables, the univariate analysis showed that the male gender, % HbA1c average, etiology of cirrhosis, smoking history, and alcohol consumption were all factors associated with the incidence of HCC in cirrhosis with T2DM patients. In the univariate analysis, we found that the risk of HCC developing in patients with cirrhosis with T2DM was higher in males than in females. The reason for this gender disparity is controversial. HCC shows a predominance in males worldwide, with male-to-female ratios of 2:1 to 4:1.40 The higher risk of HCC in males could be explained by environmental risk factors, eg, alcohol use, smoking, and sex hormones. However, gender was not statistically significant in our multivariate analysis. Surprisingly, an average HbA1C in the first year after being diagnosed with cirrhosis lower than 7.5% indicated a higher risk of developing HCC than in the other groups. This result was inconsistent with previous studies, which showed that hyperglycemia increased the risk of HCC development.41 Applying HbA1c may not properly represent the glycemic control status in cirrhosis patients due to the short lifespan of red blood cells caused by hypersplenism.42–44 Also, cirrhotic patients can easily develop low blood glucose due to insufficient hepatic glycogen storage.
Regarding alcohol and smoking histories, patients with cessation ≥ 5 years had the highest risk of HCC development. These results could be explained by the cumulative effect of using alcohol and smoking. In the multivariate analysis, only the etiology of cirrhosis was an independent risk factor for HCC occurrence, especially HBV and HCV cirrhosis. The etiology of cirrhosis was also significant in HCC occurrence: in our patients, the 5-year cumulative incidence of HCC for chronic HCV was 36.8%, chronic HBV was 29.2%, alcoholism was 15.9%, and other causes were 17.1%, followed by NASH cirrhosis at 14.1%. In both our univariate and multivariate analysis, patients with cirrhosis related to chronic viral hepatitis (B and C) had a statistically significantly higher incidence of HCC than those with non-viral diseases. These results were consistent with the epidemiology study, which showed that one-third of cirrhotic patients would develop HCC in the long-term follow-up. The incidence of HCC among patients with cirrhosis was about 1–8% per year; 2–3% in HBV cirrhosis, 3–8% in HCV cirrhosis, 2% in alcoholic cirrhosis, and 1–2% in NASH cirrhosis.16,45
We also determined the overall survival of the 327 patients who had developed HCC after being diagnosed with cirrhosis between the metformin exposure and non-exposure groups. In the univariate analysis, HCC patients in the metformin exposure group seemed to have a better survival rate than those in the non-exposure group. After adjusting in the multivariate analysis, metformin was no longer a significant predictor of overall survival in HCC. BMI lower than 25 kg/m2 and smoking seemed to be associated with the overall survival of HCC, but the multivariate analysis showed that these were not independent risk factors for HCC survival. Unsurprisingly, BCLC staging of HCC was a strong predictor for the overall survival of HCC in cirrhosis with T2DM patients. This is consistent with the fact for HCC that the median survival time progressively decreased from a very early stage to an advanced stage.46,47 So when we adjusted metformin exposure with BCLC staging, the metformin exposure was not statistically significant in the multivariate analysis. In addition, the small number in each BCLC stage of HCC may not be adequate to show up as statistically significant in the subgroup analysis. This evidence was supported by a prior meta-analysis study that reported that metformin significantly prolonged the survival of HCC patients with T2DM in curative HCC; albeit, the efficacy of metformin in non-curative therapies for HCC patients with T2DM was not significant.48 A recent multi-center study reported that HCC patients with T2DM on metformin had a significantly longer median overall survival than patients who were not treated with metformin (22 vs 15 months, p=0.019). However, this effect was the most outstanding among patients at the potentially curative stages of HCC.49
The overall survival progressively decreased with patients with a lower BMI. This could be explained by the symptoms of the patient. Patients with an advanced stage of HCC may also have some degree of malnutrition and cancer cachexia, with these risks providing lower BMI. Usually, the direct carcinogenic effects of hyperglycemia promote carcinogenesis, resulting in tumor progression. One cohort study revealed a linear relationship between HCC occurrence and HbA1c. HCC itself (4–27%) can develop hypoglycemia due to paraneoplastic effects, with hypoglycemia typically found in the advanced or terminal stage, and hence it’s associated with a poor prognosis and short survival.17 This is consistent with our result in the univariate analysis that showed that an HbA1c average lower than 7.5% was associated with a lower survival outcome.
In conclusion, this study aimed to contribute knowledge to fill some gaps in inconclusive information about whether metformin can lead to a reduced incidence of HCC in patients with cirrhosis with T2DM. Also, we aimed to investigate for patients who had developed HCC in our cohort whether metformin could prolong survival or not. Our study provides the novel observation that using metformin as an anti-diabetic agent in cirrhosis with T2DM patients shows the significant trend of its anti-tumoral properties and strong evidence of diminishing the risk of HCC development. From the univariate analysis, metformin use seemed to increase the survival rate in HCC patients; however, it showed no significance in HCC survival in our multivariate analysis. Our study had some limitations. We could not study the dose or duration of metformin treatment due to the nature of the study being a retrospective study design. We could not observe a longer study period as some information was not always available in the electronic database. For higher quality data and validation of these results, a randomized control trial with a large cohort is recommended in the future to confirm this finding.
Acknowledgments
We thank Ms. Khemjira Karaketklang of the Division of Clinical Research and Academic, Department of Medicine, Faculty of Medicine Siriraj Hospital, for assisting with the statistical analysis. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
T2DM, Type 2 diabetes mellitus; HCC, Hepatocellular carcinoma; HbA1c, Hemoglobin A1c; BMI, Body mass index; SD, Standard deviation; HBV, Hepatitis B virus; HCV, Hepatitis C virus; NASH, Nonalcoholic steatohepatitis; HR, Hazard ratio; 95% CI, 95% confidence interval; MALA, Metformin-associated lactic acidosis; BCLC, Barcelona clinic liver cancer.
Data Sharing Statement
Data is available from the corresponding author upon reasonable request.
Disclosure
The authors declared that there are no conflicts of interest.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029 [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5(13):270. doi: 10.21037/atm.2017.04.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15(2):223–243, vii–x. doi: 10.1016/j.cld.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 6.Fabiani S, Fallahi P, Ferrari SM, Miccoli M, Antonelli A. Hepatitis C virus infection and development of type 2 diabetes mellitus: systematic review and meta-analysis of the literature. Rev Endocr Metab Disord. 2018;19(4):405–420. doi: 10.1007/s11154-017-9440-1 [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nkontchou G, Cosson E, Aout M, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96(8):2601–2608. doi: 10.1210/jc.2010-2415 [DOI] [PubMed] [Google Scholar]
- 9.Tseng CH. Type 2 diabetes, smoking, insulin use, and mortality from hepatocellular carcinoma: a 12-year follow-up of a national cohort in Taiwan. Hepatol Int. 2013;7(2):693–702. doi: 10.1007/s12072-012-9405-0 [DOI] [PubMed] [Google Scholar]
- 10.Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018;38(11):2018–2027. doi: 10.1111/liv.13872 [DOI] [PubMed] [Google Scholar]
- 11.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S97–103. doi: 10.1053/j.gastro.2004.09.021 [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–1167. doi: 10.1093/aje/kwh161 [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Giovannucci E. RESPONSE: re: prospective study of adult onset diabetes mellitus (Type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91(15):1334a–1334. doi: 10.1093/jnci/91.15.1334A [DOI] [PubMed] [Google Scholar]
- 14.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. 2003;157(12):1092–1100. doi: 10.1093/aje/kwg100 [DOI] [PubMed] [Google Scholar]
- 15.Montella M, Crispo A, Giudice A. HCC, diet and metabolic factors: diet and HCC. Hepat Mon. 2011;11(3):159–162. [PMC free article] [PubMed] [Google Scholar]
- 16.European Association For The Study Of The Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 17.Li CI, Chen HJ, Lai HC, et al. Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes–National cohort of Taiwan Diabetes Study. Int J Cancer. 2015;136(11):2668–2679. doi: 10.1002/ijc.29321 [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108(6):881–891; quiz 892. doi: 10.1038/ajg.2013.5 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association.Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement1):S73–S85. doi: 10.2337/dc18-S008 [DOI] [PubMed] [Google Scholar]
- 20.Force ICGT. Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23(6):579–593. doi: 10.1111/j.1464-5491.2006.01918.x [DOI] [PubMed] [Google Scholar]
- 21.Herman WH. Response to comment on Inzucchi et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149. Diabetes Care. 2015;38(9):e143. doi: 10.2337/dc15-1234 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy MSN, Masharani U. Pancreatic Hormones & Antidiabetic Drugs. In: Katzung BG, Trevor AJ, editors. Basic & Clinical Pharmacology, 13e. New York, NY: McGraw-Hill Medical; 2015.: [Google Scholar]
- 23.Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60(9):1639–1647. doi: 10.1007/s00125-017-4372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR,Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhat M, Chaiteerakij R, Harmsen WS, et al. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol. 2014;20(42):15750–15755. doi: 10.3748/wjg.v20.i42.15750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60(6):2008–2016. doi: 10.1002/hep.27199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 28.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 29.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–539. doi: 10.1136/gut.2004.052167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15(3):280–288. doi: 10.3748/wjg.15.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asif S, Bennett J, Marakkath B. Metformin-associated lactic acidosis: an unexpected scenario. Cureus. 2019;11(4):e4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016;65(2):20–29. doi: 10.1016/j.metabol.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 33.Li X, Wang X, Gao P. Diabetes mellitus and risk of hepatocellular carcinoma. Biomed Res Int. 2017;2017:5202684. doi: 10.1155/2017/5202684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat A, Sebastiani G, Bhat M. Systematic review: preventive and therapeutic applications of metformin in liver disease. World J Hepatol. 2015;7(12):1652–1659. doi: 10.4254/wjh.v7.i12.1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30(5):750–758. doi: 10.1111/j.1478-3231.2010.02223.x [DOI] [PubMed] [Google Scholar]
- 36.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer. 2014;120(22):3485–3493. doi: 10.1002/cncr.28832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Rahman O, Helbling D, Schöb O, et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10(4):245–254. doi: 10.1111/jebm.12270 [DOI] [PubMed] [Google Scholar]
- 38.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62(4):606–615. doi: 10.1136/gutjnl-2011-301708 [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48(1):78–87. doi: 10.3109/00365521.2012.719926 [DOI] [PubMed] [Google Scholar]
- 40.Liu P, Xie S-H, Hu S, et al. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget. 2017;8(40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosokawa T, Kurosaki M, Tsuchiya K, et al. Hyperglycemia is a significant prognostic factor of hepatocellular carcinoma after curative therapy. World J Gastroenterol. 2013;19(2):249–257. doi: 10.3748/wjg.v19.i2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57(9):751–762. doi: 10.1507/endocrj.K10E-138 [DOI] [PubMed] [Google Scholar]
- 43.Koga M, Kasayama S, Kanehara H, Bando Y. CLD (chronic liver diseases)-HbA1C as a suitable indicator for estimation of mean plasma glucose in patients with chronic liver diseases. Diabetes Res Clin Pract. 2008;81(2):258–262. doi: 10.1016/j.diabres.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 44.Nomura Y, Nanjo K, Miyano M, et al. Hemoglobin A1 in cirrhosis of the liver. Diabetes Res. 1989;11(4):177–180. [PubMed] [Google Scholar]
- 45.Tarao K, Nozaki A, Ikeda T, et al. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019;8(3):1054–1065. doi: 10.1002/cam4.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalaf N, Ying J, Mittal S, et al. Natural history of untreated hepatocellular carcinoma in a US cohort and the role of cancer surveillance. Clin Gastroenterol Hepatol. 2017;15(2):273–281 e271. doi: 10.1016/j.cgh.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 47.Wang CY, Li S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: a single center 14 years experience from China. Medicine (Baltimore). 2019;98(4):e14070. doi: 10.1097/MD.0000000000014070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J, Ke Y, Lei X, et al. Meta-analysis: the efficacy of metformin and other anti-hyperglycemic agents in prolonging the survival of hepatocellular carcinoma patients with type 2 diabetes. Ann Hepatol. 2019;19(3):320–328. doi: 10.1016/j.aohep.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 49.Schulte L, Scheiner B, Voigtländer T, et al. Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma. Liver Int. 2019;39(4):714–726. doi: 10.1111/liv.14048 [DOI] [PubMed] [Google Scholar]