Abstract
Many diseases, including cancers, AIDS, diabetes, asthma, Parkinson's, and lymphoma, are associated with the immune cell responses of patients suffering from them. Identifying the underlying immune response in such diseases is critical to correctly diagnose their root cause and determine the correct medications to target that root cause for personal therapy and immunotherapy. This work focuses on small molecular CF dyes to conjugate with antibodies, such as CD4 and CD19, for their application in flow cytometry. The CF dyes enable the expansion of flow cytometry reagent panels to support high dimensional flow cytometry analysis of the resulting emissions of 30–40 fluorescent colors, a record in flow cytometry. The CF dyes can be used along with existing flow cytometry dyes to provide a quick, accurate, and cost-effective method for the diagnosis and immunology treatment of diseases such as minimal residual disease (MRD) after cancer therapy. The CF dyes will also be an effective tool for the clinical studies of immune response to SARS-CoV-2 and the related vaccine development.
Keywords: CF fluorescence dyes, Flow cytometry, Cell analysis, Immunology
Graphical abstract
1. Introduction
Flow cytometers have been used to phenotype cells in genomics, immunology, immuno-oncology, infectious diseases and vaccine development. Many diseases, such as cancer, AIDS, diabetes, asthma, Parkinson's, lymphoma, and coronavirus induced cytokine storms are associated with the immune system and immune cell responses of the patients. Phenotyping the immune cells using flow cytometry can contribute to correctly diagnosing the root causes of the diseases and determining the right medications to target those root causes for personal therapy and immunotherapy.
Flow cytometry incorporates one or more lasers, typically at wavelengths of 355 nm, 405 nm, 488 nm, 561 nm, and 638 nm, to rapidly analyze immune cells in a fluid stream through laser excitation and fluorescence emission spectral analysis [1,2]. There are three main components in the flow cytometer: the fluidics system, the optical system, and the electronics. The fluidics system is used for lining up and transporting the bio-cell samples into a flow cell chamber. The optical system is used for illuminating the bio-cells that pass one by one in the flow cell chamber by the laser beams, detecting scattering light and fluorescence that are emitted [1]. Finally, the electronics system is used to control the laser beams and amplify the detected light signals, enabling an analysis of the bio-cells to an accuracy of a single unit. The instrument can analyze bio-cells at a rate of more than 30,000 cells per second.
Bio-cells phenotyped by the flow cytometers are labeled by reagents consisting of fluorescent biomarkers, which are dyes conjugated (tagged) with antibodies, such as CD3, CD4, and CD8, and used as test assays to attach to the bio-cells for flow cytometer analysis [[2], [3], [4]]. The lasers on the flow cytometer excite the bonded biomarkers, which absorb the laser light and emit the fluorescence corresponding to the emission spectra of the dyes. The flow cytometer captures the fluorescent light and determines the bio-cell immune profile based on the biomarker spectra. The number of bio-cell parameters that can be analyzed simultaneously depends on the number of biomarkers, which in turn depends on the number of conjugated dyes that can be excited and detected by the flow cytometer. Through the detection and analysis of the emitted fluorescence spectra, the bio-cells are phenotyped.
The human immune system is sophisticated, with multiple parameters involved in any immune response. Conventional flow cytometers may simultaneously analyze around 20 parameters of bio-cells from a tube of sample [5,6]. Multiple tubes of samples are needed to phenotype the immune cells and gain an in-depth understanding of all the parameters. For example, complete diagnosis of leukemia and lymphoma requires the immunophenotyping of more than 30 parameters of immune cells. EuroFlow, a consortium in Europe for standardizing the flow cytometry diagnosis of leukemia and lymphoma, has proposed a 7-tube test sequence, with each tube supporting 8 parameters, including a few backbone parameters repeated in each tube for correlation [7]. Such testing protocols and workflows are time-consuming, expensive, and insensitive for minimal residual disease (MRD), because the precious blood or bone marrow samples must be split among multiple testing tubes [[8], [9], [10], [11], [12], [13]]. For immuno-cell analysis in response to the COVID-19 pandemic, analysis of more than 30 parameters has been conducted by CYTOF [14], a mass cytometry technology. Conventional fluorescence flow cytometry is not able to efficiently support such analysis. Recently, spectral flow cytometers have been developed to overcome the deficiency of the conventional flow cytometers. A 5-laser spectral flow cytometer has been demonstrated to analyze up to 40 cell parameters from a single tube of sample. With spectral flow cytometry, the number of available photo fluorescence labels, or fluorochromes, that can be co-used in a single tube as biomarkers becomes the limit of the technical breadth and capabilities of flow cytometry and the number of cell parameters that flow cytometers can analyze.
Conventional biomarkers used for flow cytometry are labeled by dyes like PE, PerCP, FITC, APC, Alexa Fluor, Pacific Blue/Green/Orange, Brilliant Violet (BV), and Brilliant Ultraviolet (BUV), which span organic, to protein, polymer, and tandem. The required dye properties include brightness, photostability, water-solubility, and compatibility to each other. Many of these dyes cannot be co-used in conventional flow cytometers due to overlapping spectral profiles, which makes it difficult to distinguish one cell parameter from the other. Spectral flow cytometers have made it possible to co-use many of the existing dyes. However, due to the limiting varieties of the existing flow cytometry dyes, it is challenging to put together a panel of reagents to maximize the analysis capability provided by the spectral flow cytometer. For example, cancer studies and diagnoses like the Euroflow panels require the phenotyping of immune cells on more than 30 parameters. Conventional approaches have been to divide those parameters into 4 to 12 panels, with each panel containing 4 to 8 parameters. This process requires multiple tubes of samples to complete the analysis. To analyze all those parameters using a single tube as a faster, more accurate and cost-effective solution, high dimensional reagent panels beyond 30 colors should be developed using additional new dyes to improve flow cytometry analysis.
This work focuses on the development of new flow cytometry reagents using small molecular CF dyes to conjugate with antibodies, such as CD4, CD19, and CD45. The CF dyes enable high dimensional reagent panels in fluorescence flow cytometry. With the development of new flow cytometry reagents, spectral flow cytometry can be utilized to its full extent and achieve the unprecedented rapid cell analysis of more than 40 parameters using only one tube of sample. The CF dyes can be used along with the existing flow cytometry dyes to provide a quick, accurate, and cost-effective method for the diagnosis and treatment of immunology-related diseases such as cancers, including the diagnosis of MRD. It will also provide a more viable and effective single-panel solution to detect human immune response to COVID-19 [15], thereby helping develop new medications and vaccines to cope with the COVID-19 pandemic.
CF dyes are a new class of small-molecule organic dyes developed for improving water solubility, brightness, and stability, as well as providing excellent specificity when the dyes are conjugated to proteins and oligonucleotides. The CF dyes have been used previously for imaging cell analysis [[16], [17], [18], [19]]. The molecular structure of CF dyes can be divided into three components:
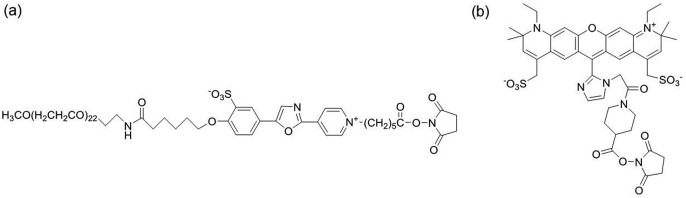
a) a dye core structure (i.e., the aromatic ring skeleton that defines the dye colors or absorption/emission wavelengths), b) core structure-modifying elements, and c) a reactive functional group for bio-conjugation. The core structures of CF dyes resemble those of classic organic dyes, such as coumarin, pyrene, rhodamine, and cyanine dyes. Blue fluorescent CF dyes are based on a coumarin or pyrene dye core structure, and green to near-IR CF dyes are based on the core structures of either cyanine or rhodamine dye. Various chemical attachments to the core structure are the key aspect of the CF dyes and make the performances of the CF dyes superior to those of conventional dyes. The reactive group is typically an amine-reactive succinimidyl ester (SE) that can form an amide bond with a lysine side chain of a protein. There are portfolios of CF dyes with various absorption and emission spectrums to cover wavelength gaps for flow cytometry applications. Our experiments start with developing reagent technology using two CF dyes, CF405L and CF633, and can be extended across the remaining CF dyes for spectral flow cytometry applications. The molecular structures of the two CF dyes are shown in Fig. 1 , and their absorption and emission spectrums are shown in Supplemental Fig. 1(a) and (b).
Fig. 1.
Molecular structure of (a) CF405L dye and (b) CF633 dye.
CF405L has an absorption spectrum suitable for being excited by the flow cytometer violet laser (405 nm) with emission centered around 550 nm. CF633 has an absorption spectrum suitable for being excited by the flow cytometer red laser (638 nm) with emission centered around 660 nm. The CF405L spectral peak matches that of the Pacific Orange dye, and the CF633 spectral peak matches that of the APC dye, as shown in Supplemental Fig. 1 (c) and (d).
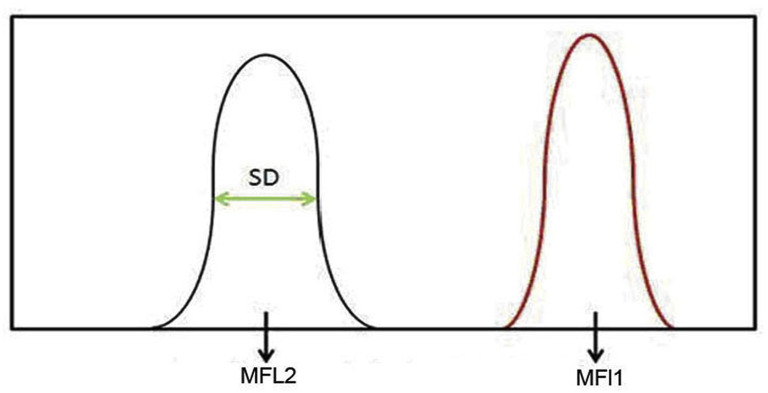
A key parameter for the evaluation of a flow cytometry reagent is the stain index (SI) after the fluorochrome dye is conjugated with a specific antibody. SI is defined as the difference between the positive and negative cell populations (MFl1-MFl2) divided by 2 times the standard
deviation of the negative population (2× SD) (Fig. 2 ) when used for cell analysis by a flow
Fig. 2.
Definition of Stain Index (SI): SI = (MFL1 - MFL2)/2xSD, where MFL1 is the positive population value, MFL2 is the negative population value, and SD denotes the standard deviation.
cytometer. The larger the SI, the better the dye-antibody combination in the construction of reagent panels of flow cytometry.
Here we report a technology developed to conjugate CD19 antibodies to CF633 and CD45 antibodies to CF405L. The materials and the conjugation processes are reported in detail in the Experimental Materials section of the Supplemental Materials and summarized in Supplemental Fig. 2. These two reagents are incorporated into a four-color panel together with CD3/FITC and CD4/PE-CY7 to study the properties of CD19/CF633 and CD45/CF405L. Three-color panel using CD3, CD4 and CD45 has been used to study T-cell immunodeficiency such as HIV AIDS [20]. This four-color panel adds CD19 to the study for additional B-cell analysis. The SIs of CD19/CF633 and CD45/CF405L are obtained from the experiment. To validate the general effectiveness of the CF dyes and the conjugation process for their applications in flow cytometry, the CD4 antibody is used as a standard protein to conjugate to a wide series of CF dyes and Alexa FluorTM 532, a common flow cytometry fluorochrome dye. The SIs of those conjugated CF dye reagents are obtained and compared to that of CD4/Alexa 532.
2. Discussions
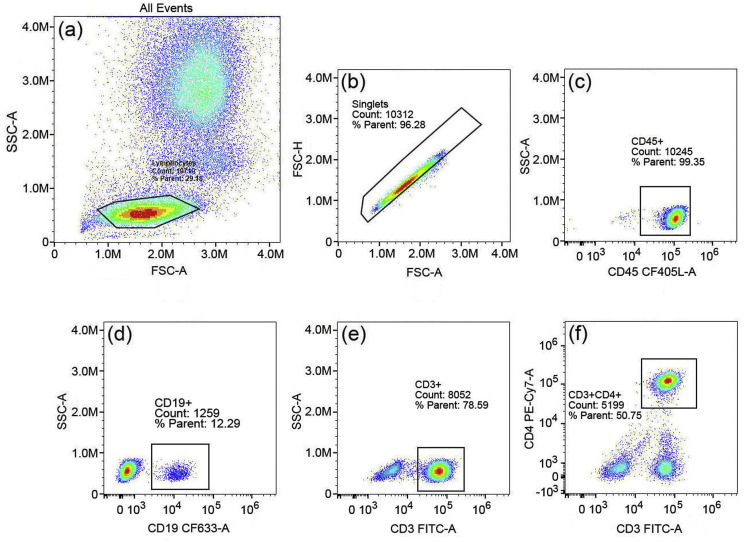
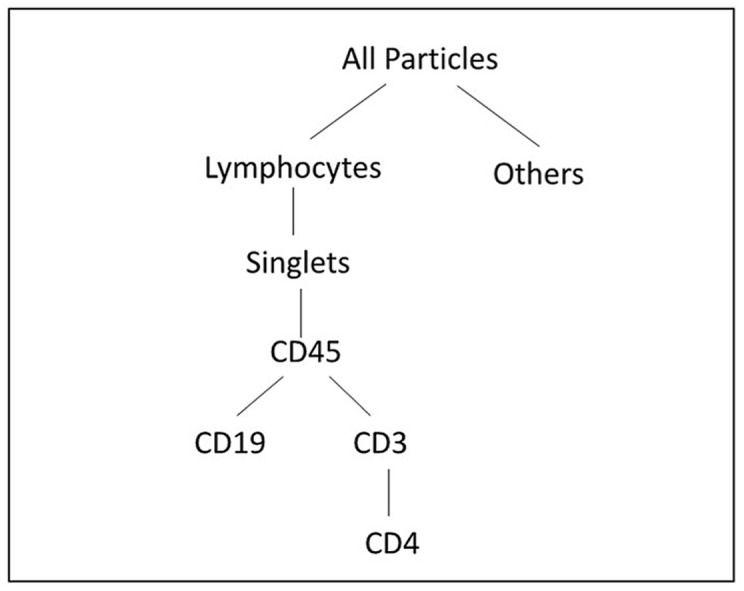
The newly developed single-color reagent markers, CD19-CF633 and CD45-CF405L, are individually calibrated by NL3000 spectral flow cytometer along with unstained whole blood, CD3-FITC and CD4-PE-Cy7, as single-color references as shown in Supplemental Figs. 3–7. The reference spectral profiles are stored in a library of the NL3000, which are used for unmixing the four-color panel data by SpectroFlo software equipped with the NL3000 flow cytometer, as shown in Fig. 3 . The SIs of CD19-CF633 and CD45-CF405L are then obtained according to the definition in Fig. 2. The average SI value of CD19-CF633 is 18.4, and the average SI value of CD45-CF405L is 1.4. This four-color panel has worked well in correctly phenotyping the four types of immune proteins on the cells in this experiment. Fig. 3(a) shows the dot plot of all events between side scatter light (SSC-A) vs. forward scattering light (FSC-A) with three distinct groups of populations. The gated lymphocyte population count is 29% of the total events. Fig. 3(b) shows the dot plot of the gated lymphocytes in (a) between the forward scattering signal height (FSC–H) vs. the forward scattering signal area (FSC-A). The singlet lymphocyte count is at 96% of the total lymphocytes. Fig. 3(c) shows the dot plot of the SSC-A vs. the fluorescence light signal from CF405L, which is conjugated with CD45. The CD45+ count is at 99% of the singlet lymphocytes, setting a baseline for the leukocyte count. Fig. 3(d) shows the dot plot of SSC-A vs. the fluorescence light signal from CF-633, which is conjugated with CD19. The CD19+ count shows that the B-cell singlet count is at 12% of the total leukocyte singlets. Fig. 3(e) shows the dot plot of SSC-A vs. the fluorescence light signal from FITC, which is conjugated with CD3. The CD3+ count shows that the T-cell co-receptor count is at 79% of the total leukocyte singlets. Fig. 3(f) shows the dot plot of the fluorescence light signal from PE-Cy7 vs. the fluorescence light signal from FITC. The PE-Cy7 is conjugated with CD4. The double positive CD3+ and CD4+ indicates that the T-cell helper singlet count at 51% of the total leukocyte singles.
Fig. 3.
A 4-color panel test with (a) the 4 color panel for all cells between side scattering signal area (SSC-A) vs. forward scattering signal area (FSC-A), showing three groups of cells and gated for lymphocytes with count at 29% of the total cells; (b) forward scattering signal height (FSC–H) vs. forward scattering signal area (FSC-A) for lymphocytes and gated for singlet count at 96% of the total lymphocytes; (c) side scattering light vs. CD45-CF405L fluorescent light, showing CD45+ leukocyte count at 99% of the total singlet lymphocytes; (d) side scattering light vs. CD19-CF633 fluorescence light, showing CD19+ B-cell count at 12% of the singlet leukocytes; (e) side scattering light vs. CD3-FITC fluorescence light, showing CD3+ T-cell co-receptor count at 79% of the singlet leukocytes; and (f) CD4 PE-Cy7 fluorescence light vs. CD3-FITC fluorescence light, showing CD4+ T-helper cell count at 51% of the singlet leukocytes. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
This 4-color panel study has been repeated twice with two additional tubes of samples, as shown by Supplement Figs. 8 and 9. The cell count vs. parent for lymphocytes, lymphocyte singlets, leukocytes, B-cells, T-cell co-receptors and T helper cells are summarized in Table 1 . The count percentage variances for leukocytes, B-cells, T-cells and T helper cells vs. the parent cells are 1–2%, validating the repeatability and stability of the reagent panels. The data in Table 1 match the typical distributions of lymphocyte populations for CD3 (61–85%), CD19 (7–23%), and CD4 (28–58%), as reported by Reichert et al. [21].
Table 1.
Cell Count vs. Parent.
| Tube 1 | Tube 2 | Tube 3 | |
|---|---|---|---|
| Lymphocyte | 29% | 43% | 30% |
| Singlet Lymphocyte | 96% | 97% | 97% |
| Leukocyte (CD45) | 99% | 98% | 99% |
| B-Cell (CD19) | 12% | 14% | 12% |
| Total T-cell (CD3) | 79% | 78% | 79% |
| T-cell helper (CD4) | 51% | 51% | 50% |
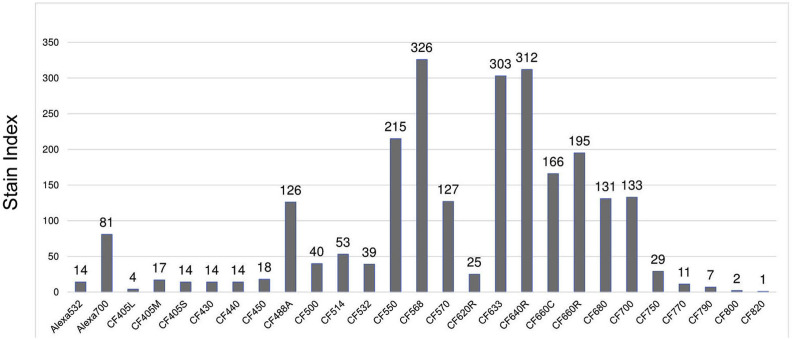
Using the same conjugation process developed for the 4-color panel study, a series of CF dyes are conjugated to CD4 as a common antibody for comparison against the dim Alexa Fluor dyes. Specifically, the control variables of the study are Alexa 532 and Alexa 700. Under the same condition, the conjugated dyes are prepared with whole blood samples and tested using the NL3000 spectral flow cytometer. Stain indexes of these dyes are calculated according to the formula in Fig. 2 and are shown in Fig. 4 . In this same condition comparison study, Alexa 532 and Alexa 700 have SIs of 14 and 81, respectively. Most of the CF dyes have achieved a SI higher than that of Alexa 532, and half of them have a SI higher than that of Alexa 700. The dyes with SIs higher than Alexa 700 include: CF488A, CF550, CF568, CF570, CF633, CF640R, CF660C, CF660R, CF680, CF680R, and CF700, among which CF568, CF633, and CF640R have SIs over 300, while a few of other dyes have SIs between 100 and 200, and all the dyes have higher SIs than those of the Alexa Fluor dyes. The CF633/CD4 SI of 303 is significantly larger than that of the CF633/CD19 in the 4-color panel study due to improved formulation process for producing the CF dyes, making the CF633 dye brighter.
Fig. 4.
Stain indexes (SIs) of various CF dyes in conjugation with CD4 are shown in comparison with Alexa 532 and Alexa 700 conjugated with CD4.
On a separate project, one of the CF dyes developed in this study, CF568, which is renamed as cFluor YG584, has been conjugated with CD4 for the demonstration of the first fluorescence-based 40-color panel using a full spectrum flow cytometer [22]. The results there, along with the 4-color panel study in this report, have demonstrated the feasibility of CF dyes for applications in flow cytometry, enabling high dimensional panels along with existing reagents to support cell phenotyping with more than 30 parameters, paving the way to improve the diagnosis accuracy and efficiency of leukemia MRD, as well as to allow for high dimensional flow cytometry panels to study the immune response to the SARS-CoV-2 virus.
3. Conclusion
We have demonstrated small molecular CF dyes conjugated to antibodies as biomarkers using a 4-color panel, which has prepared for flow cytometry applications on 30+ color panels for multi-parameter bio-cell analysis and bio-cell phenotyping. Reagents of 11 CF dyes have achieved higher SIs than those of the conventional Alexa Fluor dyes. The CF dyes significantly expand the capability of the spectral flow cytometry technology; facilitate efficient, quick, and low-cost flow cytometry diagnosis of immune system-related diseases such as leukemia; and provide a quick and efficient way to study the immune cell response to SARS-CoV-2.
Statement of Ethical Use of Human Samples: All human PBMCs used in this study were obtained from AllCells Alameda, 1301 Harbor Bay Parkway, Suite 200, Alameda, CA 94502, Phone: 510-726-2700. Ethical review and regulatory compliance were conducted by Alpha Independent Review Board, 1001 Avenida Pico, Suite C #497, San Clemente, CA 92673, Phone: 888-265-5766, under Protocol number: 7000-SOP-045 (effective through April 26, 2021).
Author contributions
Janine Jiang - Primary contributor; conducting the experiment, analyzing data, writing the paper. Xue Li - Collaborator; support on the dye conjugation process. Fei Mao - Contributed on resources. Xingyong Wu - Supported on flow cytometer operation. Yong Chen - Advisor.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ab.2020.114063.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gefvert B. Cytometry/Spectroscopy: optics and photonics advance spectral flow cytometry, StackPath. 1 Feb. 2019. https://www.bioopticsworld.com/biophotonics-techniques/spectroscopy/article/16429892/cytometryspectroscopy-optics-and-photonics-advance-spectral-flow-cytometry
- 2.Maecker H.T., Frey T., Nomura L.E., Trotter J. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry. 2004;62A(2):169–173. doi: 10.1002/cyto.a.20092. [DOI] [PubMed] [Google Scholar]
- 3.Flor A.C., Williams J.H., Blaine K.M., Duggan R.C., Sperling A.I., Schwartz D.A., Kron S.J. DNA-directed assembly of antibody-fluorophore conjugates for quantitative multiparametric flow cytometry. Chembiochem. 2013;15(2):267–275. doi: 10.1002/cbic.201300464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mckinnon K.M. Flow cytometry: an overview. Curr. Protoc. Im. 2018;120(1) doi: 10.1002/cpim.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robillard N., Bene M.C., Morean P., Wuilleme S. A single-tube multiparameter seven-colour flow cytometry strategy for the detection of malignant plasma cells in multiple myeloma. Blood Canc. J. 2013;3:e134. doi: 10.1038/bcj.2013.33. www.ncbi.nlm.nih.gov/pmc/articles/PMC3763387/ Nature Publishing Group, 16 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staser K.W., Eades W., Choi J., Karpova D., F Dipersio J. OMIP-042: 21-color flow cytometry to comprehensively immunophenotype major lymphocyte and myeloid subsets in human peripheral blood. Cytometry. 2017;93(2):186–189. doi: 10.1002/cyto.a.23303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dongen J.J.M., Lhermitte L., Bottcher S., Almeida J., Vhj van der Velden, Flores-Montero J., Rawstron A., Asnafi V., Lecrevisse Q., Lucio P., Mejstrikova E., Szczepanski T., Kalina T., de Tute R., Bruggemann M., Sedek L., Cullen M., Langerak A.W., Mendonca A., Macintyre E., Martin-Ayuso M., Hrusak O., Vidriales M.B., Orfao A. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal reactiveand malignantleukocytes. Leukemia. 2012;26:1908–1975. doi: 10.1038/leu.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee T., Mallhi R.S., Venkatesan S. Minimal residual disease detection using flow cytometry: applications in acute leukemia, Medical Journal, Armed Forces India, Elsevier. 2016. www.ncbi.nlm.nih.gov/pmc/articles/PMC4878947/ [DOI] [PMC free article] [PubMed]
- 9.Immunophenotypic analysis by flow cytometry, Ped-Onc Resource Center, www.ped-onc.org/diseases/MRD/flowcyto.html.
- 10.Jaso J.M., Wang S.A. Multi-color flow cytometry for minimal residual disease detection in acute myeloid leukemia, MD Anderson Cancer Center Experience. ICCS e-Newsletter. 2014;5(3) www.cytometry.org/public/newsletters/eICCS-5-3/article4.php [Google Scholar]
- 11.Minimal residual disease, Leukemia and lymphoma society. www.lls.org/sites/default/files/National/USA/Pdf/Publications/FS35_MRD_Final_2019.pdf
- 12.Ommen H.B. Monitoring minimal residual disease in acute myeloid leukaemia: a review of the current evolving strategies. Therapeutic Advances in Hematology. 2015;7(1):3–16. doi: 10.1177/2040620715614529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theunissen T., Mejstrikova E., Sedek L., van der Sluijs-Gelling A.J., Gaipa G., Bartels M., da Costa E.S., Kotrova M., Novakova M., Sonneveld E., Buracchi C., Bonaccorso P., Oliveira E., te Marvelde J.G., Szczepanski T., Lhermitte L., Hrusak O., Lecrevisse Q., Grigore G.E., Fronkova E., Trka J., Bruggemann M., Orfao A., van Dongen J.J.M., van der Velden V.H.J. American Society of Hematology; 19 Jan. 2017. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia, Blood.www.ncbi.nlm.nih.gov/pmc/articles/PMC5291958/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., Su B., Pang L., Qiao L., Feng Y., Ouyang Y., Guo X., Shi H., Wei F., Su X., Yin J., Jin R., Chen D. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell. Mol. Immunol. 2020;17:650–652. doi: 10.1038/s41423-020-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvin A. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182(6):1401–1408. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takenaka N., Araki N., Satoh T. Involvement of the protein kinase Akt 2 in insulin-stimulated Rac 1 activation leading to glucose uptake in mouse skeletal muscle. PloS One. 2019;14(2):e0212219. doi: 10.1371/journal.pone.0212219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellmann J., Goswami R.Y., Girardo S., Rein N., Hosseinzadeh Z., Hicks M.R., Busskamp V., Pyle A.D., Werner C., Sterneckert J. A customizable microfluidic platform for medium-throughput modeling of neuromuscular circuits. Biomaterials. 2019;225 doi: 10.1016/j.biomaterials.2019.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoess P., Mund M., Reitberger M., Ries J. In: Dual-color and 3D Super-resolution Microscopy of Multi-Protein Assemblies, Chapter 14, Protein Complex Assembly: Methods and Protocols. Marsh J.A., editor. vol. 1764. Springer Science Business Media; 2018. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 19.Zanetti-Domingues L.C., Tynan C.J., Rolfe D.J., Clarke D.T., Martin-Fernandez M. Hydrophobic fluorescent probes introduce artifacts into single molecule tracking experiments due to non-specific binding. PloS One. 2013;8(9) doi: 10.1371/journal.pone.0074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson J.K.A., Jones B.M., Hubbard M. CD4 T-lymphocyte determinations on whole bolld specimens using a single-tube three-color assay. Journal of Quantitative Cell Science: Cytometry. 1993;14:685–689. doi: 10.1002/cyto.990140614. [DOI] [PubMed] [Google Scholar]
- 21.Reichert T., DeBruyere M., Deneys V., Totterman T., Lydyard P., Yuksel F., Chapel H., Jewell D., Van Hove L., Linden J. Lymphocyte subset reference ranges in adult Caucasians. Clin. Immunol. Immunopathol. 1991;60:190–208. doi: 10.1016/0090-1229(91)90063-g. [DOI] [PubMed] [Google Scholar]
- 22.Park L.M., Lanigan J., Jaimes M. Forty-color full spectrum flow cytometry panel for deep immunophenotyping of major cell subsets in human peripheral blood. Journal of Quantitative Cell Science: Cytometry. 2020 doi: 10.1002/cyto.a.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.