Abstract
Background and Objectives:
This study was aimed to isolate Rhizobium spp., from the plant rhizosphere and to investigate their effects on the growth of peanut (Arachis hypogaea L.) as plant growth-promoting rhizobacteria (PGPR).
Materials and Methods:
The isolates were characterized using YEMA, YEMA + Congo Red, and YEMA + Bromothymol blue (BTB) media. The Rhizobium was tested qualitatively for their ability to produce indole acetic acid (IAA), siderophores, proteases, nitrogenases as well as phosphate solubilizing activity. A greenhouse experiment was carried out to elucidate the effect of Rhizobium inoculation on Arachis hypogaea L. growth.
Results:
Eleven isolates were obtained in YEMA media and they were red-pink in the YEMA + Congo Red media. The YEMA + BTB test showed that 2 isolates were slow-growing and the rest were fast-growing isolates. Seven isolates produced siderophores, 5 were capable of phosphate solubilizing, 9 isolates produced protease enzyme, 4 isolates could produce IAA, and 7 isolates could fix nitrogen. The B1 and the combination of some high trait-isolate treatments in Y gave the best results on Arachis hypogaea L. growth.
Conclusion:
These isolates can be developed as biological fertilizer agents for the peanut plant.
Keywords: Arachis, Fertilizers, Greenhouse, Rhizobium, Yeast extract mannitol agar
INTRODUCTION
Dieng is a hilly highland region in Central Java, Indonesia that produces various vegetables, such as peanuts (Arachis hypogaea L.). Continuous peanut planting up to the top of the hills brings yield losses compared to planting other types of crops. Besides the substantial production costs, pesticides and fertilizer usage are also continuous and excessive, which has caused chemical contamination. Thus, efforts need to be taken to improve the level of soil fertility. Among other ways, planting crops and using microorganisms to improve soil fertility without damaging the environment while still increasing the plants’ productivity is the preferred method (1).
There are a lot of soil microbes that could fertilize the soil and increase plant’s yields, one of them is Rhizobium. These bacteria are capable to live in the soil or inside the legume root nodules and fix nitrogen (N) from the atmosphere to supply N to plants (2). Biological nitrogen fixation is estimated to contribute more than 170 million tons of nitrogen to the biosphere annually, 80% of which is the result of Rhizobium bacteria and leguminous plants symbiosis (3).
Each Rhizobium species or strain has different abilities in symbiosis. A Rhizobium strain capable to form root nodules and nitrogen fixation is called a useful strain. Since not all Rhizobium species are capable to form root nodules with every legume, there is a need to isolate indigenous species and characterize them into cross-inoculation groups with the potential of developing a symbiotic relationship with specific legumes (4).
Rhizobium inoculation can meet the nitrogen needs in plants and reduce the need for inorganic nitrogen fertilizer (5). Effective inoculation with Rhizobium can provide up to 50–70% of the total nitrogen needs to increase yields (6). Besides fixing nitrogen, Rhizobium produces vitamins, amino acids, siderophores, and auxins (7). Besides Rhizobium is capable to produce indole acetic acid (IAA) and gibberellic acid (GA), which are growth hormones for germination and plant growth (8) and enhancing the growth of root hair and root branches that extend the root’s range. Rhizobium can increase the absorption of phosphate (9). Phosphate is a primary nutrient in root development and the formation of soybean pods (10). The application of rhizobium inoculum in peanut plants can increase the root nodules, which function to fix nitrogen for plants. Nitrogen has an important role in the growth of leaves, stems, roots, flowers, and fruit. Besides, inoculation is expected to improve soil structure, balance soil pores, and increase soil resistance (11).
The effectiveness of Rhizobium in plants is influenced by several factors, such as the competition with native microbial populations and biotic agents like diseases and insects (12); the lack of limiting factors in the soil, such as phosphorus and various microelements (13); and low soil pH (below 5.0). Another problem is the diverse efficiency of Rhizobium inoculants for certain types of leguminous crops (14).
Thus, this research was aimed to understand the activity of the plant growth-promoting rhizobacteria (PGPR) Rhizobium spp. from the plant rhizosphere and soil, obtain pure cultures, and study their effects on the growth of peanut (Arachis hypogaea L.). In the end, this study is aimed to obtain potential isolates that can be developed as biological organic fertilizer agents. This study would help in the exploration of native rhizobium strains in promoting peanut yield and improving soil fertility.
MATERIALS AND METHODS
Bacterial isolation and purification.
The composite soil samples were taken randomly at the depth of 0–15 cm from Dieng, Wonosobo, Central Java (Table 1). The samples were taken in March 2015. The bacterial isolation was prepared by diluting a 1 ml solution of soil and root sample into 9 ml of 0.85% NaCl until a 10−1 to 10−5 dilution series. About 0.1 ml of the final diluted solution was poured into a petri dish containing YEMA media and incubated at 27–28°C for 2–5 days. The colonies formed were observed and counted daily by the plate count method. The purification was carried out by putting the sample colony in 5 ml of sterile diluted dH O and 0.1 ml of dilution was poured in a petri dish containing three kinds of yeast extract mannitol agar (YEMA) media, namely YEMA, YEMA + Congo Red, and YEMA + Bromthymol Blue (BTB) media. The YEMA media was used as selection media for Rhizobium. YEMA + Congo Red was used to check the colony color that represents Rhizobium; the pink Rhizobium colony is a colony that did not absorb the congo red dye. YEMA and BTB were used to check the growth speed of the Rhizobium; the yellow-colored colony indicated a quick-growing colony, while the blue one did not. Isolated single colonies were grown in a slanted medium in a test tube as a pure culture.
Table 1.
Rhizobium isolates and the origins of samples
| Rhizobium isolate | Origin of sample | Rhizobium population (× 107 CFU/g of soil) |
|---|---|---|
| 1A and B | Peanut Dieng, Dieng (Wonosobo) | 65 |
| 2A and B | Solanum sp., Dieng (Wonosobo) | 103 |
| 3A and B | Soil crater of Sikidang 1, Dieng (Wonosobo) | 40 |
| 4A and B | Soil crater of Sikidang 2, Dieng (Wonosobo) | 35 |
| 5A and B | Carica papaya, Dieng Kulon (Banjarnegara) | 77 |
| 6A and B | Solanum sp., Dieng kulon (Banjarnegara) | 55 |
| 7A and B | Solanum sp. + mulch, no microsol (Banjarnegara) | 81 |
| 8A and B | Zea mays, Wanaraja (Banjarnegara) | 53 |
| 9A and B | Solanum sp + mulch + microsol 1 (Banjarnegara) | 66 |
| 10A and B | Solanum sp + mulch + microsol 2 (Banjarnegara) | 46 |
| DW A and B | Daucus Carota, Wanaraja, Banjarnega | 37 |
Rhizobium activity test: Siderophores production.
Siderophores production was tested qualitatively using the chrome sulfate azurol (CAS) selective media and incubated at 27–28°C for 7 days. A positive result was indicated by the formation of clear zones around the bacterial colony (15).
Phosphate solubilizing activity test.
Phosphate solubilizing activity was tested qualitatively using Pikosvkaya media as described in Gupta et al. (16) and incubated at 27–28°C for 2–7 days. A positive result was indicated by a halo zone production by a colony, indicating that the bacteria can solubilize phosphate.
Protease production.
Protease production activity was tested qualitatively using a skim milk agar (SMA) as in Bhowmik et al. (17). The bacteria were inoculated and incubated at 28–30°C for 2–5 days. A positive result was a clear zone around the colony, indicating that these isolates were able to produce a protease enzyme.
Indole acetic acid (IAA) production.
The IAA production activity was tested using TSB (tryptone soy broth) agar and incubated at 28–30°C for 2–5 days. Approximately 1 ml of Salkowsky solution was dropped on top of the growing colonies and then incubated in a dark condition for 1 hour. A positive result was noted by a color change to pink indicating that these isolates were able to produce IAA (18).
The effectiveness of Rhizobium on peanut growth.
This study was conducted by a completely randomized design with three replications. The identified Rhizobium was labelled based on the origin area as 1B, 2B, 3B, 4B, 5B, 6B, 7B, 8A, 9A, 10A, DW 2, X (a mix of isolates 1B + 2B + 3B + 4B + 5B + 6B), and Y (a mix of isolates 7B + 8A + 9A + 10A + DW2) (Table 1 and 2) and then used as treatments. Each of these Rhizobium was inoculated in a peanut (Arachis hypogaea L.) growing medium by submerging the peanut in Rhizobium solution with 106/mL CFU concentration. The controls were not inoculated and fertilized with N (K1) or fertilized with urea fertilizer equivalent to 100 kg/ha (K2). The plants were harvested after 40 days and the following parameters were observed: plant height (cm), leaf number, the dry weight of shoot (g), root (g), root nodules (g), and total plant (g),. The measurement of chlorophyll index was done using SPAD-502 Plus (19). An average of 24% of soil humidity was maintained by watering every day and using a nutrient solution without N (20).
Table 2.
Rhizobium isolates characteristics
| Rhizobium isolate | YEMA | YEMA + CR | YEMA + | BTB | Activity test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth | Color | Siderophores (cm) | PK (cm) | Protease (cm) | IAA | N-fixation | |||||
| 1B | + | P | F | Y | 2.2 | - | - | No | Yes | ||
| 2B | ++ | P | F | Y | 2.2 | 0.1 | - | No | Yes | ||
| 3B | + | P | S | B | 3.4 | - | 1.7 | No | Yes | ||
| 4B | + | P | S | B | - | - | 1.8 | No | No | ||
| 5B | + | P | F | Y | 2.1 | - | 2.2 | No | Yes | ||
| 6B | +++ | P | F | Y | 3.4 | 0.2 | 1.8 | No | No | ||
| 7B | +++ | P | F | Y | - | 0.2 | 0.4 | Yes | No | ||
| 8A | +++ | P | F | Y | - | - | 2.3 | Yes | Yes | ||
| 9A | ++ | P | F | Y | 1.1 | 0.1 | 2.8 | Yes | No | ||
| 10A | ++ | P | F | Y | 1.0 | 0.1 | 2.5 | Yes | Yes | ||
| DW2 | ++ | P | F | Y | - | - | 2.6 | No | Yes | ||
Note. YEMA: Yeast extract mannitol agar; CR: Congo red; BTB: Bromothymol blue; +: less fertile; ++: fertile; +++: high fertile; P: pink; F: fast; S: slow; Y: yellow; B: blue; PK: phosphate solubility; IAA: indole acetic acid production.
Data analysis.
The observed data were tested using a one-way analysis of variance (ANOVA) in 5% probability level to determine the significance of the treatment’s effect on the observed parameters.
RESULTS
Characterization of Rhizobium isolate.
As shown in Table 1, the population of bacteria in the sampled soils and plants was 35 to 103 × 107 CFU/g of soil. The samples taken from soil showed a smaller amount compared to the rooting areas. As shown in Table 2, four Rhizobium isolates grew slowly in YEMA media; 4 isolates had a moderate growth rate, and 3 isolates were fast-growing. Two slow-growing isolates were characterized by blue colonies in YEMA + Bromthymol Blue while 9 rapid growth isolates were a yellow-colored colony. The eleven isolates grown in YEMA + Congo Red media were all pink, indicating that the isolates were Rhizobium.
Rhizobium activity.
As shown in Table 2, 11 isolates of Rhizobium had high activity in promoting peanut growth, although the results varied considerably. Regarding plant nutrient availability, it was showed that 7 isolates could produce siderophores. Regarding the nutrient tests, nine isolates grown in protease media showed that they could produce protease enzymes. There were 7 isolates capable to form colonies in NFB (Nitrogen Free Medium) for N-fixation assay, indicated by the white ring or a pellicle formation below the media’s surface and the change in the media color from a cream color to blue. The activities of the isolate were shown in Fig. 1.
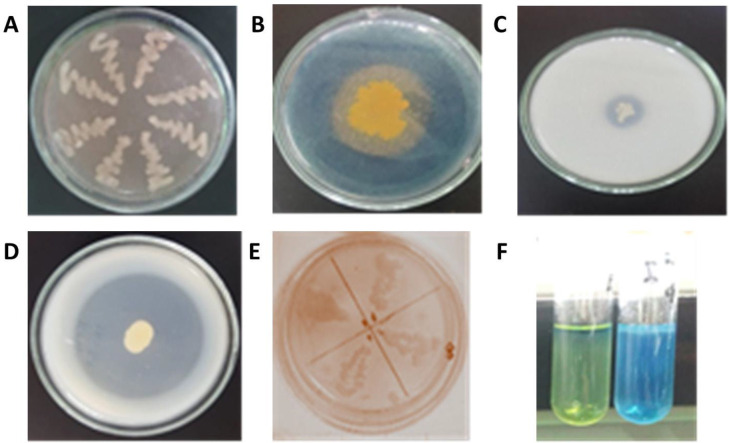
Fig. 1.
Colony activity test. (a) Rhizobium isolates in YEMA+CR. (b) qualitative test of Siderophore. (c) Phosphate solubilizing. (d) Protease. (e) IAA hormone. (f) N-Fixation of Rhizobium bacteria.
Peanut growth.
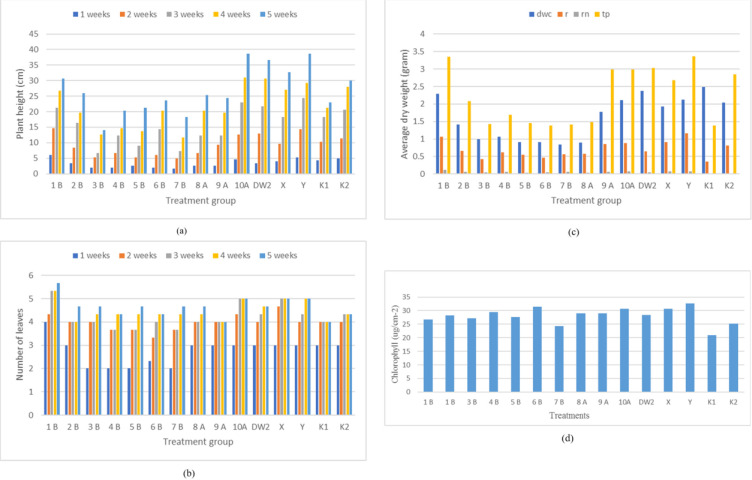
The result showed that the difference in Rhizobium isolates significantly affected peanut growth and development (p < 0.05). The highest plant yields were on plants inoculated with isolates 10A and Y. Isolate 1B gave the highest value in the number of leaves and the dry weight of canopy, root nodule, and total dry weight. The highest dry weights and chlorophyll index were found in plants inoculated with isolate Y. The Y treatments gave the best results in plant growth based on all observed parameters (Fig. 2).
Fig. 2.
Peanut growth and development based on the given treatment. (a) Plant height and (b) the number of leaves observed from: ( ): 1 week; (
): 1 week; ( ): 2 weeks; (
): 2 weeks; ( ): 3 weeks; (
): 3 weeks; ( ): 4 weeks; (
): 4 weeks; ( ): 5 weeks. (c) Dry weight of plant from: (
): 5 weeks. (c) Dry weight of plant from: ( ): dry weight of canopy (dwc); (
): dry weight of canopy (dwc); ( ): root (r); (
): root (r); ( ): root nodule (rn); (
): root nodule (rn); ( ): total biomass dry weight (tp). (d) Chlorophyll content
): total biomass dry weight (tp). (d) Chlorophyll content
DISCUSSION
Characterization of Rhizobium isolate.
The various Rhizobium population in Dieng indicated that there are different nutrient content in soil and on plant roots (11), which causes the different compositions and bacteria concentrations (21). The higher population density of Rhizobium bacteria in soil samples is likely due to the higher nutrients availability or the available nutrients are more suitable for Rhizobium growth. Dakora and Donald stated that root exudates consist of anions, sugar compounds, amino acids, organic acids, glycosides, nucleotide compounds and their bases, enzymes, vitamins, indol compounds, and others that can affect the population of bacteria in the soil (22). In other words, the metabolic activity and metabolite compounds released by plant roots into the soil determine the bacterial population. Also, the soil fertility factor, O2 content, pH, nutrients as well as physical and biological factors of soil affect the population of bacteria in the soil (23).
Rhizobium spp. bacteria can be identified from some selective media supplemented with specific dyes indicator. Our study observed that the Rhizobium sp. was dyed pink in YEMA + Congo Red media (Fig. 1). The result was following Somasegaran and Hoben’s (24) statement, one of the characteristics of Rhizobium bacteria is that they are pink-colored on YEMA + Congo Red media because they do not absorb the red dye. BTB supplemented YEMA medium could be used to identify the bacterial ability to produce acidic or basic metabolite. The medium color will turn from red to blue if they produce basic substances or red to yellow for acidic substances (25).
Rhizobium activity.
In terms of helping plant nutrient availability, isolates that could produce siderophores may have an essential role in the host plant’s survival. Siderophores are specific iron-defensive compounds, produced by microbes to sequester iron in the rhizosphere environment so that this element is not available to plants (26). The Rhizobium sp. isolates grown in PK medium were characterized by the formation of halo zones around the colony, indicating that the bacteria could solubilize bound phosphate to provide phosphate for the plant (27).
Few isolates showed the capacity of producing protease enzymes and hydrolyzing proteins into simpler molecules, such as oligopeptides or amino acids, which are needed for plant growth (28). The isolates that were grown in TSB media for the IAA test showed nine positive, characterized by pink colony color. Such characteristics can help to influence many physiological processes, including cell enlargement and division, and produce more lateral roots and roots hairs, which aiding the plant to uptake the nutrients (29).
Peanut growth.
Based on the peanut growth bioassay, the Y treatment that combined five isolates (7B, 8A, 9A, 10A, and DW2) induced the best growth promotion of peanut plants. The mixture of these strains could support the nutrient uptake since they were fertile, able to produce siderophores and hydrolyze protein as well as showing P-solubilizing effect. The inoculation had a significant effect on growth and yield, yet the level of effectiveness varies greatly depending on the symbiosis between the plant and the given inoculant (30).
Rhizobium inoculation plays an important role in providing nutrient N by forming root nodules, which bind atmosphere N into a readily-absorbed N. Besides, inoculation will have a significant effect on growth if there is a match between the inoculated Rhizobium and the host plant (31). Effective and efficient Rhizobium inoculation will able to compete with the indigenous Rhizobium, and able to synergize with its host plants (32).
The inoculated Rhizobium is expected to support plant growth, either directly or indirectly. The direct growth support by nitrogen fixation from the atmosphere, dissolving minerals like phosphate, production of siderophore, and the synthesis of growth hormones, such as IAA, gibberellin. On the other hand, the indirect ways were biocontrol of root pathogens, microbial destruction through the production of antibiotics, catalase, and siderophores (33).
CONCLUSION
This study concluded that the best inoculants for Arachis hypogaea L. growth were obtained from strain B1 of Rhizobium sp. The combination of several strains showed different beneficial characteristics. These isolates can be utilized to develop a formulation of biological fertilizer agents for peanuts.
ACKNOWLEDGEMENTS
The authors thank the Microbiology Division, Research Center for Biology, Indonesian Institute of Research for facilitating this research.
REFERENCES
- 1.Sobti S, Belhadj HA, Djaghoubi A. Isolation and characterization of the native rhizobia under hyper-salt edaphic conditions in Ouargla (Southest Algeria). Energy Procedia 2015; 74: 1434–1439. [Google Scholar]
- 2.Ulzen J, Abaidoo RC, Mensah NE, Masso C, Gadir AHA. Bradyrhizobium inoculants enhance grain yields of soybean and cowpea in Northern Ghana. Front Plant Sci 2016;7:1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 1999; 63: 968–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng C. Perspectives in biological nitrogen fixing research. J Integr Plant Biol 2008; 50: 786–798. [DOI] [PubMed] [Google Scholar]
- 5.Abbasi MK, Tahir MM. Economizing nitrogen fertilizer in wheat through combinations with organic manures in Kashmir-Pakistan. Agron J 2012; 104: 169–177. [Google Scholar]
- 6.Asei R, Nana EM, Clement AR. Response of soybean (Glycine max L.) to Rhizobia inoculation and molybdenum application in the Northern Savannah zones of Ghana. J Plant Sci 2015; 3: 64–70. [Google Scholar]
- 7.Boiero L, Perrig D, Masciarelli O, Penna C, Cassan F, Luna V. Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl Microbiol Biotechnol 2007; 74: 874–880. [DOI] [PubMed] [Google Scholar]
- 8.Egambedieva D, Wirth SJ, Alqarawi AA, Abd-Allah EF, Hashem A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front Microbiol 2017;8:2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiharn M, Lumyong S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from Rhizobacteria aimed at improving plant growth. Curr Microbiol 2011; 62: 173–181. [DOI] [PubMed] [Google Scholar]
- 10.Masciarelli O, Lianes A, Luna V. A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol Res 2014; 169: 609–615. [DOI] [PubMed] [Google Scholar]
- 11.Biswas B, Gresshoff PM. The role of symbiotic nitrogen fixation in sustainable production of biofuels. Int J Mol Sci 2014; 15: 7380–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afzal I, Shinwari ZK, Sikandar S, Shahzad S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol Res 2019; 221: 36–49. [DOI] [PubMed] [Google Scholar]
- 13.Kleiber T, Komosa A. Differentiated microelements content in Anthurium (Anthurium cultorum Birdsey) leaves. J Elem 2010; 15: 301–311. [Google Scholar]
- 14.Lindstrom K, Mousavi SA. Effectiveness of nitrogen fixation in rhizobia. Microb Biotechnol 2020;13: 1314–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem 1987; 160: 47–56. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RS, Rekha S, Aparna A, Kuhad RC. A modified plate assay for screening phosphate solubilizing microorganisms. J Gen Appl Microbiol 1994; 40: 255–260. [Google Scholar]
- 17.Bhowmik S, Islam S, Ahmed MM, Hossain M, Hossain MA. Protease producing bacteria and activity in gut of tiger shrimp (Penaeus monodon). JFAS 2015; 10: 489–500. [Google Scholar]
- 18.Gravel V, Aunton H, Tweddell RJ. Effect of indole-acetic acid (IAA) on the development of symptoms caused by Pythium ultimum on tomato plants. Eur J Plant Pathol 2007; 119: 457–462. [Google Scholar]
- 19.Cerovic ZG, Guillaume M, Ghozlen NB, Latouche G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol Plant 2012; 146: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin BB, Egerer MH, Liere H, Jha S, Philpott SM. Soil management is key to maintaining soil moisture in urban gardens facing changing climatic conditions. Sci Rep 2018; 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju W, Lei L, Linchuan F. Impact of co-inoculant with plant growth promoting Rhizobacteria and Rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol Environ Saf 2019; 167: 218–226. [DOI] [PubMed] [Google Scholar]
- 22.Dakora FD, Donald AP. Root exudates as mediators of mineral acquisition in low nutrient environment. Plant Soil 2002; 245: 35–47. [Google Scholar]
- 23.Singh B, Kaur R, Singh K. Characterization of Rhizobium strain isolated from the roots of Trigonella foenumgraecum (fenugreek). Afr J Biotechnol 2008; 7: 3671–3676. [Google Scholar]
- 24.Somasegaran P, Hoben HJ. (1984). Methods in legum rhizobium technology. University of Hawaii, Hawai. [Google Scholar]
- 25.Kawaka F, Dida MM, Opala PA, Ombori O, Maingi J, Osoro N, et al. Symbiotic efficiency of native Rhizobia nodulating common bean (Phaseolus vulgaris L.) in soils of Western Kenya. Int Sch Res Notices 2014;2014:258497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed E, Holmstrom SJM. Siderophores in environmental research: Roles and applications. Microb Biotechnol 2014; 7: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baig KS, Arshad M, Zahir ZA, Cheema MAM. Comperative efficacy of qualitative and quantitative methods for rock phosphate solubilization with phosphate solubilizing rhizobacteria. Soil Environ 2010; 29: 82–86. [Google Scholar]
- 28.Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 1998; 62: 597–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maor R, Haskin S, Levi-Kedmi H, Sharon A. In planta production of indole-3 acetic acid by Colletotrichum gloeosporioides f. sp. Aeschynomene. Appl Environ Microbiol 2004; 70: 1852–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Argaw A. Effectiveness of Rhizobium inoculation on common bean productivity as determined by inherent soil fertility status. J Crop Sci Biotechnol 2016; 19: 311–322. [Google Scholar]
- 31.Santos MS, Nogueira MA, Hungria M. Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019;9:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco RA, Sicardi M, Frioni M. Competition for nodule occupancy between introduced and native strains for Rhizobium leguminosarum biovar trifolii. Biol Fertil Soils 2010; 46: 419–425. [Google Scholar]
- 33.Mahdhaiyan M, Reddy BVS, Anandham R, Sentthilkumar M, Poonguzhali S, Sundaram SP, et al. Plant growth-promoting Methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot pathogens. Curr Microbiol 2006; 53: 270–276. [DOI] [PubMed] [Google Scholar]