Abstract
Background and Objectives:
Dental caries is one of the most common chronic diseases around the world. Inhibitory effects of Magnolia Grandiflora bark extract has been proved on tooth decay both in vitro and by using free sugar chewing gum. This research aimed to examine the effect of Magnolia Grandiflora bark mouth-wash on the prevalence of Streptococcus mutans in dental plaque.
Materials and Methods:
This crossover, placebo-controlled, clinical trial study, was performed on a total of twenty participants (aged 18 to 35 years) in both control and intervention groups and four phases. The prevalence of S. mutans was measured in a certain volume of volunteer’s dental plaque at the beginning of the project (phase 1), after the first prescription (phase 2), following the washout period (phase 3) and finally after the second prescription (phase 4) by culture on bacteriology medium. Plaque index and saliva sampling were carried out in follow-up visits by a dentist. The data were analyzed using T-Test (paired and independent) quantitatively.
Results:
There was a significant difference in S. mutans frequency in dental plaque between when the participants used Magnolia mouthwash and when they washed out or used a placebo (p<0.005). Results also showed a significant difference between Magnolia and Placebo groups in the mean count of saliva bacterial colony counts after oral administration in the first and second time (P<0.001 and P<0.004, respectively).
Conclusion:
The current trial showed that Magnolia Grandiflora %0.3 mouthwash tends to decrease the number of S. mutans in dental plaque significantly. Therefore, its mass production and release to the oral health community are suggested. However, further studies with larger sample sizes and varying treatment are required to substantiate the findings of this study.
Keywords: Magnolia, Mouthwashes, Streptococcus mutans, Dental plaque, Clinical trial
INTRODUCTION
Dental caries and periodontitis are the most common chronic diseases. Dental plaque is a biofilm that plays an important role in causing mentioned diseases by adhering to the teeth surfaces (1). The most common diseases of tooth-supporting structures are the plaque-induced inflammatory changes in gingival and periodontal tissues (2). Completion of the condition completely without plaque in the oral environment is inaccessible and even non-physiological. However, if the plaque buildup and pathogenic organisms become lower and safe immune response is provided, the health of the gingival and periodontal can be maintained (3).
As a resident microflora in dental plaque, Streptococcus mutans creates an acidic environment through the metabolizing of different carbohydrates. The ability of this species to synthesize extracellular glucans is a major factor in the formation and development of dental plaque (4). Studies have shown that adhesion of S. mutans to tooth surfaces and its role in dental plaque formation consequently leads to dental caries and periodontal diseases (5, 6). One of the most effective ways to reduce the number of germs in the mouth is mouth washing along with brushing and flossing regularly. This decreases oral microbial populations and dental plaque accumulations, which in turn reduces chances for tooth decay and periodontal diseases (7). In addition to antimicrobial effects, a good mouthwash should also affect much less on normal oral microflora and reveal the lowest level of drug resistance to it (8).
In recent years the use of herbal medicines has become more acceptable in oral health due to the antibacterial, antifungal, anticancer, and fewer side effects (9). Magnolia plant is used widely in traditional and herbal medicine with many applications listed for it. This plant is scientifically named Magnolia grandiflora, grows as a tree, and is classified in Magnoliaceae family. In addition to antibacterial properties, the magnolia bark extract is also characterized as an antioxidant, anti-cancer, and Antidepressant. Magnolia is located in the group of polyphenolic plants. Magnolol and Honokiol are two major polyphenols found in this herb and are well-known for their beneficial characteristics. In various studies, some properties, such as antioxidant, anti-cancer, anti-bacterial, anti-caries, and anti-periodontal diseases have been reported for these components (10–12). In general, antibacterial effects of Magnolia have been shown in vitro studies but no clinical study is available in relation to the minimum inhibitory concentration (MIC) of the plant for S. mutans bacterium. This study aimed to determine the minimum inhibitory concentration (MIC) of the large-leaved magnolia against S. mutans and to evaluate the plaque index and the prevalence of S. mutans in dental plaque and saliva after exposure with Magnolia mouthwash.
MATERIALS AND METHODS
Extract preparation and authentication.
Initially, the Magnolia bark was collected from Rasht, Gilan Province, Iran. The collected species was confirmed by botanists of the Botanical Garden of Mashhad University after comparison with samples of large-leaved Magnolia (MAG704). In the next stage, using a Soxhlet extractor (Electrothermal England, Model ME1000), the hydro-ethanol extract was obtained from the bark of the plant.
Determination of the minimum inhibitory concentration (MIC).
Using the solvent DMSO 5%, stocks of the extract were prepared with different concentrations (0.3 mg/ml, 0.03 mg/ml, and 0.003 mg/ml). In the further stage, the minimum concentration that inhibits the growth (MIC) of Streptococcus mutans was determined using standard protocol for dilution antimicrobial susceptibility (13). To determine the MIC, Muller Hinton Broth (MHB) was prepared. One ml of MHB medium was distributed into each test tubes and then autoclaved. Using a sterile loop, a standard strain of S. mutans (ATCC 35668) was inoculated into each of the test tubes, and the bacterial concentrations adjusted to 0.5 McFarland (1.5 × 108 CFU/ml) absorbance. From the stoke extracts prepared in the previous step, 1 ml was added to the tube containing 1 ml of the bacterial suspension using the broth dilution method. After adding the different concentration of the Magnolia extract to the test tubes, and incubating at 37°C for 24 h, MIC of the extract was determined for S. mutans. Since the observation of turbidity in the tubes means bacterial growth, the concentration of the first transparent tube without turbidity was considered as MIC (The experiment was repeated three times). Following the MIC, a Magnolia extract concentration of 3% was created as a mouthwash. Using distilled water and permitted food colors, a placebo mouthwash with the same color of Magnolia’s was made to be used as a negative control. The mouthwash was approved by the Ethics Committee of Shahed University (Letter NO. 41/191668).
Trial design.
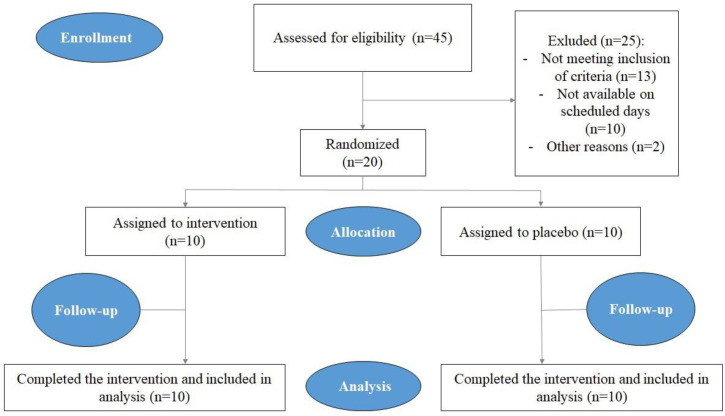
This crossover, single-blinded, placebo-controlled, randomized study performed on 20 volunteers in a range of 18 to 35 years old. The sample size was estimated to be 39 individuals on the basis of a qualitative assessment in the P= 0.35, d = 0.15, and Alpha= 0.05. The research was conducted on 20 people but considering cross over method, a total of 40 samples were evaluated. Before starting the research, one member of the research team explained different stages of the study to all participants and asked them to read and sign the informed consent form if they accept it. The inclusion criteria were 1) the absence of systemic disease, 2) no consumption of antibiotics from one month prior to until the end of the project, 3) not using any common mouthwash (Fig. 1). The exclusion criteria were 1) having any history of food allergy, 2) no agreement for signing the informed consent form. All participants were asked not to change their usual methods for their oral and dental health.
Fig. 1.
CONSORT flow chart of the study.
With regards to their oral health status, all participants were classified into two groups of moderate health (A) and poor health (B) based on the standard table for initial assessment of caries risk (14) and the plaque index (15). Samples of plaque and the plaque index were evaluated in four phases (Before starting the project, after administration of the first dose of mouthwash, after a period of rest and after administration of the second dose of mouthwash) in both groups with poor health and moderate health status. Each group of A and B were divided into two 5-person groups (A1, A2) and (B1, B2) respectively. Initially, the Magnolia mouthwash was administered to 5-person group A1 and 5-person group B1, while a placebo mouthwash was administered to 5-person group A2 and 5-person group B2 at the same time for one week. Then, both groups A and B stopped using mouthwash for a week. In the next step, the Magnolia mouthwash was administered to the 5-person groups A1 and B1 while the placebo was administered to the 5-person groups A2 and B2 all for a week. Sampling of microbial plaque (aiming specific culture and evaluating the number of Streptococcus mutans) was performed in four sets. All samples were taken at 6 am before using mouth wash, brushing, and having breakfast. Protocols in this study approved by the Ethical Committee of Shahed University of Medical Sciences (Code No: 41/191668) in accordance with the World Medical Association Declaration of Helsinki. Also, the study was registered in IRCT system (Code: IRCT2014071518487N1).
Sample collection.
To evaluate the dental plaque index, in each round, the participants were asked to use plaque disclosing tablets in a period after waking up and before breakfast and brushing. Thereby, plaque index was calculated for each individual based on the percentage of tooth surfaces exhibiting stained plaque. Then, the plaque samples were collected from each participant before breakfast and brushing for the assessment of the levels of S. mutans. Considering two aspects which may intensely influence the accuracy of plaque sampling, dental location and the method of collecting and processing, we used Individual Sampling Method (16) to maximize the accuracy of the counts. The samples were collected by dragging from all (buccal/mesial/distal/lingual/occlusal) surfaces of individual PFM molars using a sterile swab and transferred to capped tubes containing 0.5 ml normal saline water.
Laboratory assessment.
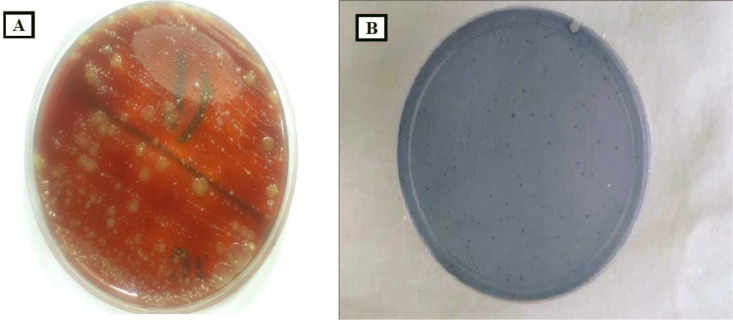
Samples were immediately put in cold chain pack (Ice-packs) upright on side of sample tubes and transported and submitted to the microbiology laboratory of Imam Reza hospital in Mashhad, Iran, and inoculated on blood agar medium using a standard loop (0.01 μl). Inoculated plates were incubated for 48 hours at 37°C in a candle jar (containing 5% carbon dioxide). At the end of the incubation period, all suspicious colonies were Gram stained and subjected to the catalase test. Colonies of alpha-hemolytic, Gram-positive, catalase-negative which revealed cocci in chain arrangement in microscopy were considered as Streptococcus (17). To differentiate S. mutans colonies from others, all colonies with the above features were transferred into Mitis Bacitracin salivarius agar (MSBA) medium and incubated for 48 hours once again. The dark blue colonies with 3–2 mm in diameter grown in this differential medium were considered as S. mutans (18). These detected colonies were counted at every sampling stage (Fig. 2).
Fig. 2.
Colony of bacteria on (A) blood agar medium and (B) Mitis Bacitracin salivarius agar medium (MSBA).
Statistical analyses.
The data were presented as means ± standard deviation (SD). To analysis any correlation between using Magnolia mouthwash with Plaque index or with S. mutans prevalence, independent-T test and paired-T test was applied using SPSS v. 16. A p-value < 0.5 was considered significant.
RESULTS
The number of S. mutans in dental plaque before starting the project and during the resting phase did not differ significantly between the Magnolia mouth-wash group and the placebo one. But after the first and second rounds of mouthwash administration, the number of S. mutans in dental plaque statistically showed a significant difference between the Magnolia mouthwash group and the placebo one. (P<0.001 and P<0.00 in the first and second rounds of mouthwash administration respectively) (Table 1).
Table 1.
Average count of S. mutans colony forming unit per plaque sample at different stages of the project based on types of mouthwash
| Project stages | Type of mouthwash | Number of samples | Average count of colonies (CFU/ml) |
|---|---|---|---|
| Before starting the project | Magnolia | 10 | 83*102 |
| Placebo | 10 | 86*102 | |
| First round of mouthwash administration | Magnolia | 10 | 43*102 |
| Placebo | 10 | 83*102 | |
| Rest stage | Magnolia | 10 | 64*102 |
| Placebo | 10 | 70*102 | |
| Second round of mouthwash administration | Magnolia | 10 | 33*102 |
| Placebo | 10 | 70*102 |
Among the Magnolia mouthwash consumers, there was no significant difference between the average number of S. mutans in plaque samples taken from the moderate and the poor oral health groups neither after the first nor the second round of mouth washing. But among the placebo mouthwash consumers, there was a significant difference between the average number of S. mutans in plaque samples taken from the individuals in medium and poor oral health groups both after the first and the second round of mouth washing (P<0.00) (Table 2).
Table 2.
Average count of S. mutans colony forming units per plaque sample at different stages of the project based on oral health status
| Number of samples | Health status | Type of mouthwash | Average count of S. mutans colony forming units (CFU/ml) | |
|---|---|---|---|---|
| Mouthwash (First round) | 5 (A1) | Moderate | Magnolia | 44*102 |
| 5 (B1) | Week | 51*102 | ||
| 5 (A2) | Moderate | Placebo | 69*102 | |
| 5 (B2) | Week | 98*102 | ||
| Mouthwash (Second round) | 5 (A2) | Moderate | Magnolia | 31*102 |
| 5 (B2) | Week | 35*102 | ||
| 5 (A1) | Moderate | Placebo | 59*102 | |
| 5 (B1) | Week | 81*102 |
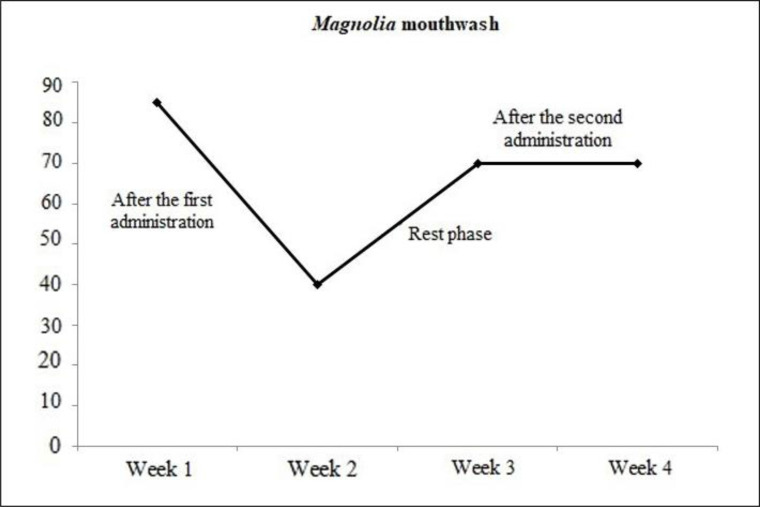
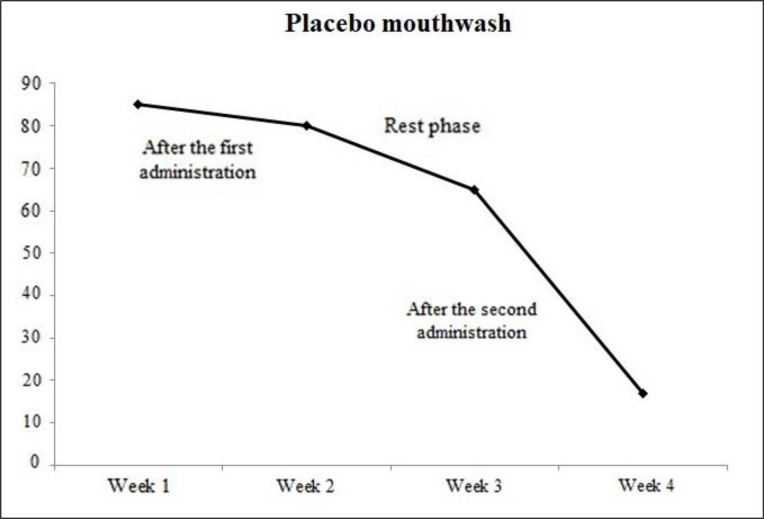
The results of this research showed that the plaque index before starting the project and during the resting phase did not differ significantly between the Magnolia mouthwash group and the placebo one. But after the first and the second rounds of mouthwash administration, the plaque index statistically showed a significant difference between the Magnolia mouthwash group and the placebo one. (P<0.00 and P<0.001 in the first and second rounds of mouthwash administration respectively) (Fig. 3).
Fig. 3.
Average plaque index in placebo mouthwash consumers after the first round
Among the Magnolia mouthwash consumers, there was no significant difference between the average of plaque index between the two groups with moderate and poor health status neither after the first, nor the second round of mouth washing. But among the placebo mouthwash consumers there was a significant difference between the average of plaque index in the group with moderate and the group with poor oral health status both after the first and the second round of mouth washing (P<0.003) (Fig. 4).
Fig. 4.
Average plaque index in Magnolia mouthwash consumers after the first round
The mean colony count of S. mutans in saliva before the start of the project was 80 × 102 CFU/ml in the Magnolia mouthwash group and 73 × 102 CFU/ml in placebo mouthwash group and there was no significant difference. Then, there was a significant difference between Magnolia and Placebo groups in the mean count of saliva bacterial colony counts after oral administration in the first and second time (P<0.001 and P<0.004, respectively) (Table 3).
Table 3.
Average count of S. mutans colony forming unit per saliva sample at different stages of the project based on types of mouthwash
| Project stages | Type of mouthwash | Number of samples | Average count of colonies (CFU/ml) |
|---|---|---|---|
| Before starting the project | Magnolia | 10 | 80*102 |
| Placebo | 10 | 73*102 | |
| First round of mouthwash administration | Magnolia | 10 | 48*102 |
| Placebo | 10 | 74*102 | |
| Rest stage | Magnolia | 10 | 69*102 |
| Placebo | 10 | 58*102 | |
| Second round of mouthwash administration | Magnolia | 10 | 45*102 |
| Placebo | 10 | 65*102 |
The means colony count of saliva bacteria in the two groups of moderate and poor health status for placebo mouthwash group in the first time were 54 × 102 CFU/ml and 76 × 102 CFU/ml and in the second time were 61 × 102 CFU/ml and 87 × 102 CFU/ml respectively. So there was a significant difference (P<0.00) (Table 4).
Table 4.
Average count of S. mutans colony forming units per saliva sample at different stages of the project based on oral health status
| Number of samples | Health status | Type of mouthwash | Average count of S. mutans colony forming units (CFU/ml) | |
|---|---|---|---|---|
| Mouthwash (First round) | (A1)5 | Moderate | Magnolia | 44*102 |
| (B1)5 | Week | 46*102 | ||
| (A2)5 | Moderate | Placebo | 54*102 | |
| (B2)5 | Week | 76*102 | ||
| Mouthwash (Second round) | (A2)5 | Moderate | Magnolia | 40*102 |
| (B2)5 | Week | 55*102 | ||
| (A1)5 | Moderate | Placebo | 61*102 | |
| (B1)5 | Week | 87*102 |
DISCUSSION
Many studies have evaluated the use of different mouthwashes from plants and their effect on oral health. However, to overcome the intricacy of studying oral bacteria, most of these surveys were conducted in vitro, which needs a relatively short time interval (19–21). The purpose of this study was 1) to determine the minimum inhibitory concentration of large-leaved magnolia (MIC) against S. mutans 2) to evaluate the role of Magnolia mouthwash in inhibition of S. mutans in dental plaque and 3) to evaluate the role of Magnolia mouthwash in the reduction of plaque index in the studied population.
A number of studies have shown that people with high levels of salivary S. mutans in the mouth are located in the high-risk group of tooth decay and exacerbation of periodontal disease (22, 23). One of the most effective ways to reduce the number of germs in the mouth is mouthwash along with brushing and flossing regularly. Reducing oral microbial populations consequently leads to tooth decay and gum disease prevention (24).
The findings of the present study showed that after mouthwash administrations (both the first and the second rounds), the number of S. mutans in dental plaque and the plaque index in Magnolia mouthwash consumers showed a significant reduction compared to the placebo mouthwash ones.
The findings of the present study showed that the mouthwash made from the extract of large-leaved Magnolia bark has the ability to inhibit the growth of S. mutans in two groups of individuals with moderate and poor oral health status. A comparison of the Magnolia mouthwash effect with the placebo one showed a significant difference in terms of growth inhibition of S. mutans (P<0.005). In different studies, anti-dental caries, anti-bleeding gums and anti-periodontal disease of Magnolia have been attributed to two main polyphenolic compounds found in the bark of this plant, Magnolol, and Honokiol. The findings of this investigation were in concordance with the findings of previous studies conducted on antibacterial effects of Magnolia extract on S. mutans (10–12). Some studies have shown that the polyphenols in this plant not only inhibit the growth of S. mutans but also reduce glucose transferase and amylase activities and acid production in this bacterial species. It also causes loss of adhesion of the bacterium to tooth surfaces (11). Magnolia effects have been reported not only on the bacteria, but also on other microorganisms, including fungi (25).
The results of the present study revealed that the minimum inhibitory concentrations (MIC) of Magnolia bark extract on S. mutans was 0.3 mg/ml. This finding is in agreement with MIC found for this plant by Babpour et al. (26).
The results of this study showed that a mouthwash made from Magnolia is able to inhibit the growth of S. mutans in dental plaque. According to the previous studies, the role of this bacterial species has been demonstrated in dental plaque formation (27, 28). Therefore, it is suggested that a mouthwash made from magnolia extract may constrain to some extent the formation of microbial plaque.
The strength of the current trial stands in the randomized, placebo-controlled design. To evaluate the effect of magnolia extract on microbial plaque in a correct way (with no bias in time durations), we considered the same duration of time for all four phases of the study. Moreover, we considered the length of rest phase for one week to make sure that there would be enough time between two phases of exposure to magnolia extraction. This span time was considered as we wanted to evaluate each phase individually and needed to avoid any probable unwanted interfere in each phase.
All participants in this study completed their treatment protocol and all attended follow-up. However, some limitations need to be thought about when interpreting the findings. Firstly, due to many difficulties in sampling and following up, the sample size of the population was considered relatively small and this was one of limitations in our study. Moreover, sampling of all volunteers in only one center may limit the generalization of results. So, it is recommended that further studies be conducted with a large sample size of population and in a multi-center across the country.
It can be concluded that Magnolia bark extract decreases the concentration of S. mutans to teeth. This may consequently reduce the volume of microbial plaque in dental surfaces and consequently reduce the plaque index.
ACKNOWLEDGEMENTS
The authors need to offer their special thanks to all respected staff of medical microbiology laboratory in Imam-Reza hospital, Mashhad for their great cooperation.
REFERENCES
- 1.Nakano K, Inaba H, Nomura R, Nemoto H, Takeda M, Yoshioka H, et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol 2006; 44: 3313–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesim S, Unalan D, Esen C, Ozturk A. The relationship between periodontal disease severity and state-trait anxiety level. J Pak Med Assoc 2012; 62: 1304–1308. [PubMed] [Google Scholar]
- 3.Kajiya M, Giro G, Taubman MA, Han X, Mayer MP, Kawai T. Role of periodontal pathogenic bacteria in RANKL-mediated bone destruction in periodontal disease. J Oral Microbiol 2010;2: 10.3402/jom.v2i0.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge Y, Caufield P, Fisch G, Li Y. Streptococcus mutans and Streptococcus sanguinis colonization correlated with caries experience in children. Caries Res 2008; 42: 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durand R, Roufegarinejad A, Chandad F, Rompré PH, Voyer R, Michalowicz BS, et al. Dental caries are positively associated with periodontal disease severity. Clin Oral Investig 2019; 23: 3811–3819. [DOI] [PubMed] [Google Scholar]
- 6.Mira A, Simon-Soro A, Curtis M. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J Clin Periodontol 2017;44 Suppl 18:S23–S38. [DOI] [PubMed] [Google Scholar]
- 7.Veitz-Keenan A, Ferraiolo DM, Keenan JR. Impact of asepsis technique on implant success. A review. Eur J Oral Implantol 2018;11 Suppl 1:S113–S121. [PubMed] [Google Scholar]
- 8.Tehrani MH, Asghari G, Hajiahmadi M. Comparing Streptococcus mutans and Lactobacillus colony count changes following green tea mouth rinse or sodium fluoride mouth rinse use in children (Randomized double-blind controlled clinical trial). Dent Res J (Isfahan) 2011;8(Suppl 1):S58–63. [PMC free article] [PubMed] [Google Scholar]
- 9.Radvar M, Moeintaghavi A, Tafaghodi M, Ghanbari H, Fatemi K, Mokhtari MR, et al. Clinical efficacy of an herbal mouth wash composed of Salix alba, Malva sylvestrais and Althaea officinalis in chronic periodontitis patients. J Herb Med 2016; 6: 24–27. [Google Scholar]
- 10.Campus G, Cagetti MG, Cocco F, Sale S, Sacco G, Strohmenger L, et al. Effect of a sugar-free chewing gum containing magnolia bark extract on different variables related to caries and gingivitis: a randomized controlled intervention trial. Caries Res 2011; 45: 393–399. [DOI] [PubMed] [Google Scholar]
- 11.Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A. Plant polyphenols and their anti-cariogenic properties: a review. Molecules 2011; 16: 1486–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg M, Urnezis P, Tian M. Compressed mints and chewing gum containing magnolia bark extract are effective against bacteria responsible for oral malodor. J Agric Food Chem 2007; 55: 9465–9469. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. Clinical and Laboratory Standards Institute, Wayne, PA. 2006. [Google Scholar]
- 14.Roberson T, Heymann HO, Swift EJ, Jr. Sturdevant's art and science of operative dentistry. Elsevier Health Sci 2006. [Google Scholar]
- 15.Newman MG, Takei H, Klokkevold PR, Carranza FA. (2011). Carranza's clinical periodontology. Elsevier health Sci. [Google Scholar]
- 16.Hsu K-L, Osgood R, Cutter G, Childers N. Variability of two plaque sampling methods in quantitation of Streptococcus mutans. Caries Res 2010; 44: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papaioannou W, Gizani S, Nassika M, Kontou E, Nakou M. Adhesion of Streptococcus mutans to different types of brackets. Angle Orthod 2007; 77: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 18.Gamboa F, Estupiñan M, Galindo A. Presence of Streptococcus mutans in saliva and its relationship with dental caries: Antimicrobial susceptibility of the isolates. Univ Sci 2004; 9: 23–27. [Google Scholar]
- 19.Chavan SD, Shetty NL, Kanuri M. Comparative evaluation of garlic extract mouthwash and chlorhexidine mouthwash on salivary Streptococcus mutans count-an in vitro study. Oral Health Prev Dent 2010; 8: 369–374. [PubMed] [Google Scholar]
- 20.Jain I, Jain P, Bisht D, Sharma A, Srivastava B, Gupta N. Comparative evaluation of antibacterial efficacy of six Indian plant extracts against Streptococcus mutans. J Clin Diagn Res 2015;9:ZC50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malvania EA, Sharma AS, Sheth SA, Rathod S, Chovatia NR, Kachwala MS. In Vitro analysis of Licorice (Glycyrrhiza glabra) root extract activity on Streptococcus mutans in comparison to chlorhexidine and fluoride mouthwash. J Contemp Dent Pract 2019; 20: 1389–1394. [PubMed] [Google Scholar]
- 22.Choi N, Choi G, Min BS, Jang K, Choi Y, Kang M, et al. Effects of neolignans from the stem bark of Magnolia obovata on plant pathogenic fungi. J Appl Microbiol 2009; 106: 2057–2063. [DOI] [PubMed] [Google Scholar]
- 23.Dani S, Prabhu A, Chaitra K, Desai N, Patil SR, Rajeev R. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: A clinico-microbiological study. Contemp Clin Dent 2016; 7: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bescos R, Ashworth A, Cutler C, Brookes ZL, Belfield L, Rodiles A, et al. Effects of chlorhexidine mouthwash on the oral microbiome. Sci Rep 2020;10:5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Xiao J, Zhou J, Cai G, Li X, Lu J. Alternaria alternata causing leaf spot on Magnolia grandiflora in China. Plant Dis 2019;103:2672. [Google Scholar]
- 26.Babpour E, Angaji SA, Angaji SM. Antimicrobial effects of four medicinal plants on dental plaque. J Med Plant Res 2009; 3: 132–137. [Google Scholar]
- 27.Alkarimi HA, Watt RG, Pikhart H, Jawadi AH, Sheiham A, Tsakos G. Impact of treating dental caries on schoolchildren’s anthropometric, dental, satisfaction and appetite outcomes: a randomized controlled trial. BMC Public Health 2012;12:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannaa A, Carlén A, Lingström P. Dental caries and associated factors in mothers and their preschool and school children—A cross-sectional study. J Dent Sci 2013; 8: 101–108. [Google Scholar]