Abstract
Background and Objectives:
Several studies have focused on the alterations of hematological parameters for a better understanding of the COVID-19 pathogenesis and also their potential for predicting disease prognosis and severity. Although some evidence has indicated the prognostic values of thrombocytopenia, neutrophilia, and lymphopenia, there are conflicting results concerning the leukocyte and monocyte count.
Materials and Methods:
In this retrospective Double Centre study, we reviewed the results of WBC and monocyte counts of 1320 COVID-19 patients (243 of whom (18.4%) had severe disease) both on admission and within a 7-day follow-up.
Results:
We found that both the number of monocytes and the percentage of monocytosis were higher in the severe group; however, it was not statistically significant. On the other hand, we found that not only the mean number of WBCs was significantly higher in the severe cases also leukocytosis was a common finding in this group; indicating that an increased number of WBC may probably predict a poor prognosis. Also, the monocyte count was not affected by age; however, univariate analysis showed that the percentage of leukocytosis was significantly greater in the older group (>50) with an odds ratio of 1.71 (P: 0.003).
Conclusion:
Alteration of monocytes either on admission or within hospitalization would not provide valuable data about the prediction of COVID-19 prognosis. Although the rapidly evolving nature of COVID-19 is the major limitation of the present study, further investigations in the field of laboratory biomarkers will pave the way to manage patients with severe disease better.
Keywords: COVID-19, Follow-up studies, Prognosis, Monocyte, Leukocytosis
INTRODUCTION
December 2019 will never be forgotten in the history of medicine when an outbreak of pneumonia of unknown etiology in Wuhan sooner or later prompted the World Health Organization (WHO) to issue a public health warning emergency (1). This is not the first nor will it be the last time that a member of β-coronaviruses (CoVs), as mysterious enveloped positive-strand RNA pathogens (2), is waging a full-scale war against human health. Although the disease course is non-severe in most COVID-19 patients and the number of recovered cases is promising (3), the number of deaths is still alarming (4). The respiratory tract involvement was mostly noted at the beginning of the spread but soon several studies indicated the hyper-inflammatory nature of the disease (5, 6), which may lead to the injury of non-pulmonary organs (7–9).
Several studies have focused on alterations in hematological parameters to better understand the disease pathogenesis and their potential for predicting prognosis and severity (10). Several studies are reporting that severe COVID-19 is associated with an increased number of white blood cell count. However, one in four COVID–19 positive patients, on the other hand, may experience some form of leukopenia, with the majority (63.0%) exhibiting lymphopenia (11). Although some evidence has indicated the prognostic values of thrombocytopenia (12, 13), neutrophilia, and lymphopenia (14–16) in SARS-CoV-2 infection, there are conflicting results concerning monocyte count in this infection. While some studies have reported an increased monocyte count (17, 18), others showed no significant changes (19) and a few reported a decrease in the number of monocytes (20). This controversy shows that the monocytes’ alteration is still open to debate and this issue remains to be extensively discovered. In a case report study of a 55-year-old woman, Singh et al. indicated that during the hospital stay the initial monocytopenia was subsequently accompanied by monocytosis. By the analysis of peripheral blood film, they also reported the presence of activated monocytes which showed marked anisocytosis with prominent cytoplasmic vacuolization and few granules; indicating that both the increased number and activation of monocytes may be associated with a favorable outcome (21). Notably, several primary examinations in addition to previous data from epidemic SARS-CoV-1 propose that monocytes can play an early and fundamental role in the progression of the coronavirus disease to the severe condition by stimulating cytokine storm, acute respiratory distress syndrome (ARDS), and dispersed peripheral tissue injury (22). Also, it has been indicated that angiotensin-converting enzyme type 1 and 2 (ACE1/2) which serve as host cell receptor for human pathogenic coronaviruses are presented on the human monocyte subsets and can be involved in the vascular homeostasis regulation. Consequently, SARS-CoV-2-activated monocytes might be seriously involved in the pathogenic outcome of the disease via disturbance of ACE2 function (23). In the present study, we targeted a group of 1320 confirmed COVID-19 patients to evaluate the monocyte profile and its correlation with the alteration of white blood cells (WBC) during a 7-day follow-up. Also, we aimed to investigate if there is any correlation between the monocyte counts and disease severity; hoping that the results of our study will shed light on the prognostic value of monocytes in COVID-19.
MATERIALS AND METHODS
Patients and procedures.
To investigate the alteration of the white blood cell (WBC) and monocyte (Mon) counts in patients infected with SARS-CoV-2, we conducted this retrospective Double-Centre study that was approved by the Shahid Beheshti University of Medical Sciences Ethics Committee. Patients recruited at Taleghani and Shohadae Tajrish Hospitals in Tehran, Iran, from February 20 to May 20, 2020, were inspected using chest CT and PCR analysis. An expert radiologist reviewed all imaging features, including pure consolidation, pure ground-glass opacity (GGO), mixed consolidation/GGO, crazy-paving, reversed halo, intralesional traction bronchiectasis, intralesional vascular enlargement, linear opacities, pleural effusion, and pericardial effusion. We assigned a thin-section CT involvement score based on the involvement extent of abnormal areas. The presence of SARS-CoV2 in pharyngeal swab specimens was also detected by RT-PCR analysis. Conditions for the amplifications were 50°C for 15 min, 95°C for 3 min, followed by 45 cycles of 95°C for 15 s and 60°C for 30 s. We retrospectively extracted data on the WBC and monocyte counts from patients’ electronic medical records both on admission and at discharge. We also reviewed the alteration of these parameters in hospitalized patients within a 7-day follow-up. The percentage of leukocytosis (WBC >11 × 109/L) and monocytosis (monocyte >0.7 × 109/L) was also calculated in the studied population. Either admission to the intensive care unit (ICU) or death was defined as criteria for the severity of the disease.
Statistical analysis.
The continuous variables were examined to determine the normality of the distribution using histograms, measures of skewness and kurtosis, and Kolmogorov–Smirnov test. The normally distributed variables were described as the means ± standard deviation (SD) and the skewed distributed variables were expressed as the median and interquartile range (25–75%). Categorical variables were summarized as frequencies (percentages). The normally distributed continuous variables were compared between non-severe and severe groups using the two independent sample t-test and non-normally distributed variables with the Mann–Whitney U test. Logistic regression models were applied to assess the associations of age group, sex, and their combinations with leukocytosis and monocytosis. For each model, the odds ratio (OR) and the 95% confidence interval (CI) were calculated. All tests were two-sided, and a P value of less than 0.05 was considered to indicate a statistically significant difference. All the statistical analyses were performed using the IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
Role of the funding source.
The funder of the study had no role in study design, data collection, data analysis, and interpretation, or writing of the manuscript. The corresponding authors had full access to all the data in this study and had final responsibility for the decision to submit for publication.
RESULTS
Baseline characteristics and the association of age and sex with the WBC and monocyte counts in COVID-19 patients.
Of 1320 COVID-19 patients, 243 (18.4%) patients were defined as the severe group; those who were either admitted to ICU or experienced death. Of 243 severe cases, 59 (24.3%) were admitted to ICU and cured with intensive care services. On the other hand, the remaining 184 cases (75.7%) who died of the disease were defined as the “death” group. While the mean age of the patients was 52.15 (±19.22), patients with the severe disease were significantly older than those with the non-severe disease (mean age of 48.94 vs 66.47; P: <0.001). Among patients with severe disease, we also found an older age range in patients who died compared to the ICU group (mean age of 69.8 vs 56.0; P: <0.001). As represented in Table 1, while the mean number of white blood cells (WBC) was 7.96 (±6.50) in all COVID-19 patients, it was significantly higher in the severe cases compared to those with the non-severe disease (9.74 vs 7.47; P: <0.001). Accordingly, we found that the severe cases displayed a greater percentage of leukocytosis (WBC >11 × 109/L). Of particular interest, the same finding was found concerning the number of monocytes in these patients. As illustrated in Table 1, both the number of monocytes and the percentage of monocytosis (monocyte >0.7 × 109/L) were higher in the severe group; however, it was not statistically significant. Values greater than 11 × 109/L and 0.7 × 109/L for WBC and monocytes were defined as leukocytosis and monocytosis, respectively (24).
Table 1.
Baseline characteristics and hematologic findings of patients with COVID-19.
| All Patients N (%); Mean (±SD) | Non-Severe N (%); Mean (±SD) | Severe (ICU & Death) N (%); Mean (±SD) | P Value | |
|---|---|---|---|---|
| Sample size | 1320 (100%) | 1077 (81.6%) | 243 (18.4%) | |
| Age (Year) | 52.15 (±19.22) | 48.92 (±18.04) | 66.47 (±17.70) | <0.001 |
| Sex | 516 (39.1%) | 414 (38.4%) | 102 (42%) | 0.308 |
| Female | 804 (60.9%) | 663 (61.6%) | 141 (58%) | |
| Male | 7.96 (±6.50) | 7.47 (±6.20) | 9.74 (±6.23) | <0.001 |
| WBC | 16.3% | 11.4% | 34.1% | <0.001 |
| > 11 | 0.20 (0.13–0.30) | 0.19 (0.12–0.29) | 0.24 (0.13–0.42) | 0.116 |
| Monocyte | 5.8% | 5.1% | 8.8% | 0.281 |
| > 0.7 |
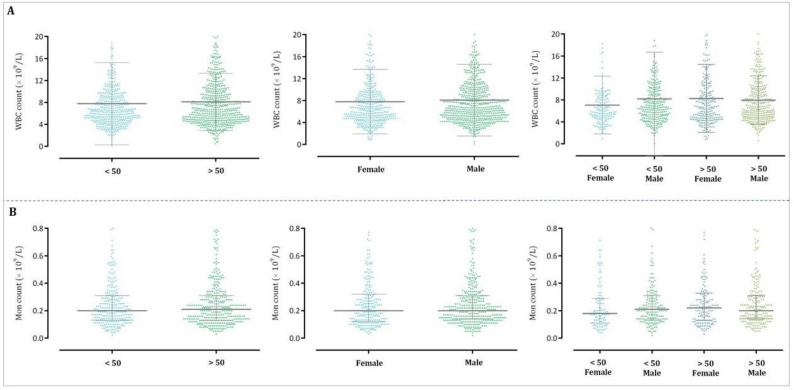
Univariate analysis showed that the WBC count differed between age categories, representing that the number of WBCs was higher in patients aged more than 50 as compared to those who were less than 50 years old (7.77 vs 8.10; P: 0.402). As represented in Table 2, the percentage of leukocytosis was also significantly greater in the older group with an odds ratio (OR) of 1.71 (95% CI: 1.20–2.43). Noteworthy, when we analyzed the confounding effect of age and sex combination on the WBC count, we found that the cases older than 50 years and female sex have the higher WBC count with a greater percentage of leukocytosis as compared with the other groups (OR:3.27; 95% CI:1.64–6.52). Although in agreement with the number of WBC count female patients aged more than 50 years experienced a greater extent of monocytosis (OR: 2.78; 95% CI: 0.75–10.23), it was not statistically significant (Table 2). The distribution patterns of the WBC and monocyte counts concerning age, sex, and their combinations were represented in Fig. 1.
Table 2.
Univariate analysis of the admission WBC and monocyte counts between age categories, sex, and their combinations.
| N (%) | WBC (Mean; ±SD) | P Value | N (%) | Mon (Median; IQR) | P Value | |
|---|---|---|---|---|---|---|
| Age (Year) | ||||||
| < 50 | 42.3% | 7.77 (±7.48) | 0.402 | 43.8% | 0.20 (0.13–0.31) | 0.74 |
| > 50 | 57.7% | 8.10 (±5.21) | 56.2% | 0.20 (0.13–0.31) | ||
| Sex | ||||||
| Female | 39.1% | 7.79 (±5.87) | 0.495 | 38.9% | 0.20 (0.12–0.32) | 0.78 |
| Male | 60.9% | 8.07 (±6.52) | 62% | 0.21 (0.14–0.31) | ||
| Age & Sex | ||||||
| < 50, Female | 15.3% | 7.05 (±5.25) | 0.239 | 16.4% | 0.19 (0.11–0.29) | 0.16 |
| < 50, Male | 26.9% | 8.18 (±8.48) | 27.5% | 0.21 (0.14–0.31) | ||
| > 50, Female | 23.8% | 8.27 (±6.20) | 22.6% | 0.22 (0.13–0.33) | ||
| > 50, Male | 34% | 7.98 (±7.98) | 33.5% | 0.20 (0.14–0.31) | ||
| Leukocytosis (%) | % CI) | P Value | Monocytosis (%) | OR (95% CI) | P Value | |
| Age (Year) | ||||||
| < 50 | 12.2% | Reference | 0.003 | 5.6% | Reference | 0.82 |
| > 50 | 19.3% | (1.20–2.43) | 6% | 1.08 (0.55–2.09) | ||
| Sex | ||||||
| Female | 14.7% | Reference | 0.265 | 5.5% | Reference | 0.78 |
| Male | 17.3% | (0.86–1.71) | 6% | 1.10 (0.55–2.17) | ||
| Age & Sex | ||||||
| < 50, Female | 7% | Reference | 0.003 | 2.8% | Reference | 0.336 |
| < 50, Male | 15.2% | (1.19–4.82) | 7.2% | 2.69 (0.75–9.69) | ||
| > 50, Female | 19.7% | (1.64–6.52) | 7.4% | 2.78 (0.75–10.23) | ||
| > 50, Male | 19% | (1.60–6.10) | 5% | 1.83 (0.51–6.17) | ||
Fig. 1.
The distribution patterns of the WBC and monocyte counts concerning age, sex, and their combinations.
The admission and discharge WBC and monocyte counts were higher in the severe COVID-19 patients.
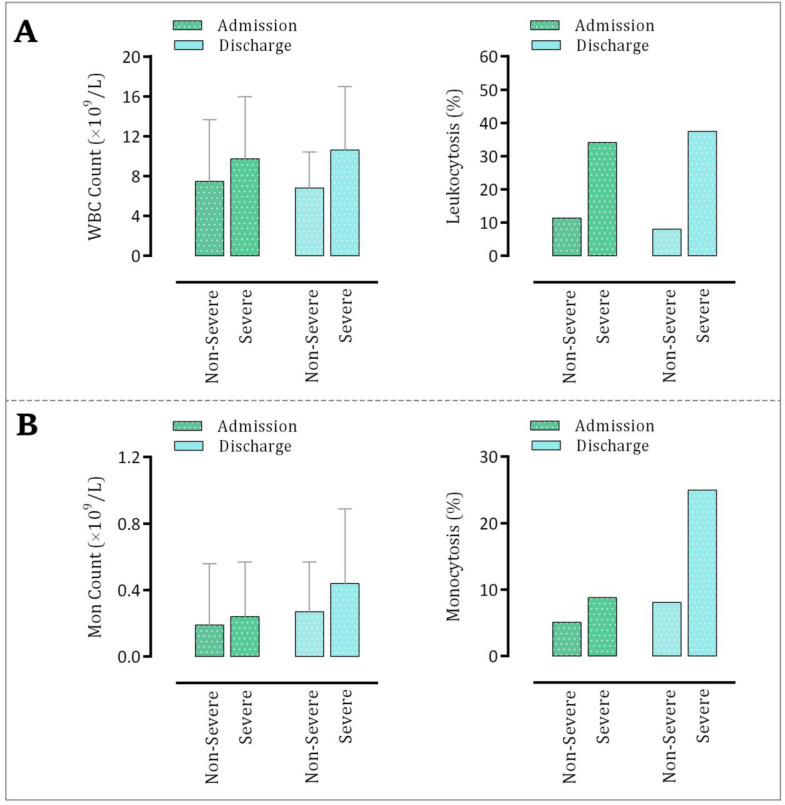
As represented in Fig. 2A, the WBC count of COVID-19 patients was significantly higher in the severe cases as compared to the non-severe group both on admission and at discharge. The percentage of leukocytosis also followed the same trend in the indicated patients. Although the mean value of WBC count in the non-severe group was lower at discharge as compared with the admission count, its count at discharge was higher in the severe group. By comparing the number of monocytes in the severe and non-severe groups we found a similar finding with WBCs, indicating that the increased level of WBCs in the severe patients may probably occur, at least partially, as a result of an increased number of monocytes. As illustrated in Fig. 2B, both the number of monocytes and the percentage of monocytosis were higher in the severe patients than their non-severe counterparts.
Fig. 2.
Alteration in the (A) WBC and (B) monocyte counts in patients with severe and non-severe disease on admission and at discharge.
Increased number of WBCs during hospitalization of COVID-19 patients may predict a poor outcome.
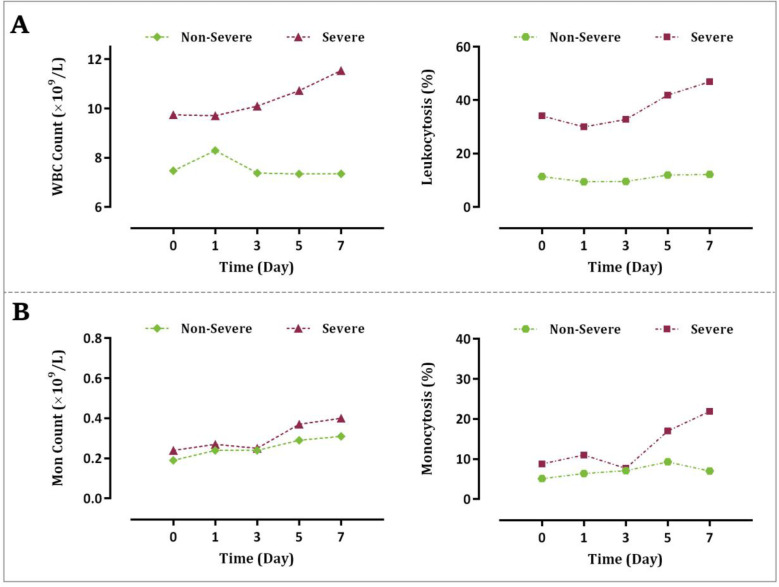
Monitoring of the WBC count and the percentage of leukocytosis within a 7-day follow-up revealed that an increasing trend in the number of WBCs may reflect the progression of the disease towards an unfavorable outcome. As illustrated in Fig. 3A, while the number of WBCs in the severe group created a curve with an increasing slope with the maximal count observed on the last day of our follow-up, their counts did not significantly alter in the non-severe group within the indicated period.
Fig. 3.
Alteration in the (A) WBC and (B) monocyte counts in patients with severe and non-severe disease within a 7-day follow-up.
Notably, the percentage of COVID-19 patients with leukocytosis was also significantly increased in the severe group as compared with the non-severe cases during hospitalization. The results of our study concerning the alteration of the monocyte count within 7-day monitoring were similar in the severe group. Although unlike WBCs the number of monocytes was increased in the non-severe COVID-19 patients, both the percentage of monocytosis and their counts were increased in the severe cases to a greater extent (Fig. 3B).
DISCUSSION
Coronavirus disease 2019 (abbreviated to COVID-19) is a kind of respiratory syndrome symptomatically spanning from healthy carriers to patients with life-threatening complications. Although most cases display no or mild-to-moderate clinical symptoms, some patients are admitted with a critical condition necessitating specialized management at intensive care units (ICU) (25). Thus far, multiple lines of studies have focused on the identification and application of novel approaches to precisely estimate COVID-19 prognosis. The results of a recent report have demonstrated that analysis of the routine laboratory parameters not only provides an appropriate diagnostic significance but the alteration of these parameters may also predict unfavorable outcomes in SARS-CoV-2 infection (9). In this retrospective DoubleCentre study reviewing the results of the WBC and monocyte counts of 1320 COVID-19 patients (243 of whom (18.4%) had severe disease), we found that both the number of monocytes and the percentage monocytosis were higher in the severe group; however, it was not statistically significant. Noteworthy, our results showed that the mean number of WBCs was significantly higher in the severe cases compared to those with the non-severe disease (9.74 vs 7.47; P: <0.001). Accordingly, we found that leukocytosis is a common finding among severe COVID-19 patients (34% vs 11%; P: <0.001); indicating that an increased number of WBC may probably predict a poor prognosis. In consistent, Huang et al. reported that the percentage of severe COVID-19 patients who had increased WBC counts was significantly higher than non-severe counterparts (54% vs 19%), further highlighting the fact that the extent of deviation from normal white blood cell counts correlates with disease severity (15). Our results were also in agreement with several studies that reported the occurrence of leukocytosis in 32% (26), 30% (15), 24% (27), 22% (28), and 21% (29) (30) of infected cases. Univariate analysis showed that the WBC count differed between age and sex categories, representing that the percentage of leukocytosis was significantly greater in the older men group (>50 years old) with an OR of 3.12 (95% CI: 1.60–6.10). Monocytosis was also higher in men and women over 50 years old with an OR of 1.83 (95% CI: 0.51–6.17) and 2.78 (0.75–10.23), respectively; however, it was not statistically significant.
To the best of our knowledge, while most published articles have investigated the alteration of hematological parameters only on admission, few studies have monitored their values during hospitalization (31). We found that the WBC and monocyte counts of COVID-19 patients were significantly higher in the severe cases as compared to the non-severe group at discharge. Monitoring the patients’ WBC and monocyte counts during hospitalization also disclosed a simple method of predicting COVID-19 prognosis. While the number of WBCs in the severe group created a curve with an increasing slope within a 7-day follow-up, their counts did not significantly alter in the non-severe group during hospitalization. In agreement, Wang et al. reported that COVID-19 cases who died of the disease displayed a higher number of WBCs during hospitalization than those who survived (31).
CONCLUSION
Taken together, the results of this study revealed that not only the mean number of WBCs was significantly higher in the severe cases also leukocytosis was a common finding in this group; indicating that an increased number of WBCs may probably predict a poor prognosis. In agreement, both the number of monocytes and the percentage of monocytosis were higher in the severe group; however, it was not statistically significant and its alteration either on admission or within hospitalization would not provide valuable data about the prediction of COVID-19 prognosis. Although unceasing discovery of novel data and the rapidly evolving nature of COVID-19 are major limitations at the time of writing this article, further investigations in the field of the identification and application of laboratory biomarkers that can enable to rapidly and economically predict COVID-19 prognosis will pave the way to better manage patients with severe COVID-19.
ACKNOWLEDGEMENTS
The authors thank the Shahid Beheshti University of Medical Sciences for supporting and funding this study.
REFERENCES
- 1.Lu H, Stratton CW, Tang YW. The Wuhan SARS-CoV-2–What's next for China. J Med Virol 2020;92: 546–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richman DD, Whitley RJ, Hayden FG. (2016). Clinical Virology. 4th ed. ASM press; Washington DC. [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 4.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med 2020; 201: 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Shen C, Li J, Yuan J, Yang M, Wang F, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv 2020: 10.1101/2020.03.02.20029975. [DOI] [Google Scholar]
- 6.Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 2020;9: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020;395(10228):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perisetti A, Gajendran M, Mann R, Elhanafi S, Goyal H. COVID-19 extrapulmonary illness – special gastrointestinal and hepatic considerations. Dis Mon 2020;66: 101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020;58: 1131–1134. [DOI] [PubMed] [Google Scholar]
- 11.Lu G, Wang J. Dynamic changes in routine blood parameters of a severe COVID-19 case. Clin Chim Acta 2020;508: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta 2020;506: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost 2020;18: 1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res 2020: 69:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 2020;7: 998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. J Leukoc Biol 2020; 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020;55: 102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Cerrillo I, Landete P, Aldave B, Sanchez-Alonso S, Sanchez-Azofra A, Marcos-Jimenez A, et al. Differential redistribution of activated monocyte and dendritic cell subsets to the lung associates with severity of COVID-19. medRxiv 2020: 10.1101/2020.05.13.20100925. [DOI] [Google Scholar]
- 21.Singh A, Sood N, Narang V, Goyal A. Morphology of COVID-19–affected cells in peripheral blood film. BMJ Case Rep 2020; 13(5): e236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pence BD. Severe COVID-19 and aging: are monocytes the key? GeroScience 2020; 42: 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F. Role of monocytes/macrophages in Covid-19 pathogenesis: implications for therapy. Infect Drug Resist 2020; 13: 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtman MA, Kaushansky K, Prchal JT, Levi MM, Burns LJ, Armitage J. (2017). Williams manual of hematology. 9th ed. McGraw-Hill Education/Medical; New York City. [Google Scholar]
- 25.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Liu H, Liu W, Liu J, Liu K, Shang J, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020; 43(0):E005. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]