Abstract
Objectives
MRI remains the preferred imaging investigation for glioblastoma. Appropriate and timely neuroimaging in the follow-up period is considered to be important in making management decisions. There is a paucity of evidence-based information in current UK, European and international guidelines regarding the optimal timing and type of neuroimaging following initial neurosurgical treatment. This study assessed the current imaging practices amongst UK neuro-oncology centres, thus providing baseline data and informing future practice.
Methods
The lead neuro-oncologist, neuroradiologist and neurosurgeon from every UK neuro-oncology centre were invited to complete an online survey. Participants were asked about current and ideal imaging practices following initial treatment.
Results
Ninety-two participants from all 31 neuro-oncology centres completed the survey (100% response rate). Most centres routinely performed an early post-operative MRI (87%, 27/31), whereas only a third performed a pre-radiotherapy MRI (32%, 10/31). The number and timing of scans routinely performed during adjuvant TMZ treatment varied widely between centres. At the end of the adjuvant period, most centres performed an MRI (71%, 22/31), followed by monitoring scans at 3 monthly intervals (81%, 25/31). Additional short-interval imaging was carried out in cases of possible pseudoprogression in most centres (71%, 22/31). Routine use of advanced imaging was infrequent; however, the addition of advanced sequences was the most popular suggestion for ideal imaging practice, followed by changes in the timing of EPMRI.
Conclusion
Variations in neuroimaging practices exist after initial glioblastoma treatment within the UK. Multicentre, longitudinal, prospective trials are needed to define the optimal imaging schedule for assessment.
Key Points
• Variations in imaging practices exist in the frequency, timing and type of interval neuroimaging after initial treatment of glioblastoma within the UK.
• Large, multicentre, longitudinal, prospective trials are needed to define the optimal imaging schedule for assessment.
Supplementary Information
The online version of this article (10.1007/s00330-020-07387-3) contains supplementary material, which is available to authorized users.
Keywords: Glioblastoma, Neuroimaging, Guideline, Survey
Introduction
Glioblastoma is the most common and aggressive primary malignant brain tumour in adults. It carries an annual incidence of 4.64 per 100,000 in England, with a peak between 65 and 75 years of age [1]. The current standard of care for newly diagnosed patients is maximal safe resection, followed by radiotherapy with concomitant and adjuvant temozolomide (TMZ) [2]. Despite this regimen, glioblastoma almost always recurs; the median overall survival is 14.6 months, whilst 5-year survival is below 10% [3].
Clinical review and serial neuroimaging remain the primary monitoring tools used to evaluate disease status and assess treatment response. However, on review of current UK, European and international guidelines, there is considerable variation and a paucity of evidence-based information regarding the optimal frequency, timing and type of neuroimaging following initial neurosurgical intervention [4–10]. Nevertheless, pragmatic neuroimaging time points are typically used in routine clinical practice which include an early post-operative MRI (EPMRI), a pre-radiotherapy MRI (PRMRI) and time points assessing the response of chemoradiotherapy both during and following completion of adjuvant TMZ [4–10].
EPMRI is frequently performed primarily to determine the extent of resection and to assess residual disease [11, 12]. There are recommendations that the EPMRI should be performed within 48–72 h due to the confounding effects of surgically induced contrast enhancement [11, 13]. The primary purpose for PRMRI is to delineate target volumes for radiotherapy planning, although this can also be achieved using fusion of the EPMRI with computed tomography (CT) in many cases [14].
Following completion of radiotherapy and concomitant TMZ, the first MRI examination is recommended to be performed 4–12 weeks subsequently [4, 7]. At this time point, approximately 20–30% of patients demonstrate a treatment-related effect, termed ‘pseudoprogression’ [15]. Pseudoprogression manifests as a transient increase in contrast enhancement and remains stable or eventually subsides without any change in treatment. Pseudoprogression appears to be the imaging manifestation of a subacute treatment-related tissue reaction which comprises inflammation, oedema, and increased permeability of the blood-brain barrier. The precise pathophysiological mechanism is still poorly understood, but histologic features typically associated with treatment effects such as bland necrosis with prominent vascular fibrinoid necrosis, reactive gliosis, oedema, demyelination and vascular hyalinisation are seen in those with pseudoprogression [16]. Pseudoprogression appears within 6 months of radiotherapy completion [17, 18], which is earlier than radiation necrosis [15, 19], another post-treatment-related effect (PTRE). Pseudoprogression appears to be more frequent in patients with a methylated MGMT gene promoter [15, 20].
Differentiating pseudoprogression from true progression has important implications in glioblastoma management but remains a major challenge as no standardised imaging biomarker has been definitively proven to be reliable [13]. In cases of suspected pseudoprogression, short-interval confirmatory MRI is recommended within 4–6 weeks whilst adjuvant TMZ treatment is continued [4, 9]. However, conventional structural imaging alone is often insufficient and unreliable. Advanced MR imaging techniques, such as perfusion imaging (dynamic susceptibility contrast-enhanced, DSC), permeability imaging (dynamic contrast-enhanced, DCE), 1H-magnetic resonance spectroscopy (MRS) and position emission tomography (PET) using radiolabelled amino acid tracers (l-[methyl-11C]methionine [MET], 18F-fluoroethyl-tyrosine [FET], 18F-fluoro-l-dihydroxy-phenylalanine [FDOPA] and 11C-alpha-methyl-l-tryptophan [AMT]), can provide additional physiological and metabolic information which may be helpful in distinguishing tumour progression from pseudoprogression [21–41].
In cases where tumour progression is confirmed with neuroimaging, management typically consists of second-line chemotherapy including the combination of procarbazine, lomustine and vincristine (PCV) [9, 42, 43], TMZ re-challenge [44, 45] or supportive care. There is less evidence to support re-irradiation and second surgery [9, 46, 47]. The specific strategy used depends on factors including performance status, risk of disability and prior treatment. In the absence of any tumour progression, a further MRI examination is generally recommended at the end of adjuvant TMZ [4], which serves as a new baseline for subsequent MRI examinations at 3–4 monthly intervals [9].
As the majority of the above recommendations were motivated by a need for reference standards in neuro-oncology clinical trials [48], we suspected that real-world neuro-oncology follow-up imaging practices vary considerably between UK centres. Given the lack of reliable and detailed data on current practice, we have surveyed all UK neuro-oncology centres to determine how neuroimaging is currently being used in the management of glioblastoma. Importantly, the survey will provide baseline data which is required to inform the design of studies aimed to optimise follow-up MRI imaging practice.
Methods
Participants
The UK Research Ethics Service (RES) provided written confirmation that ethical approval was not necessary for this study. Eligible participants were neuro-oncologists, neuroradiologists and neurosurgeons from all thirty-one neuro-oncology centres within the UK (public National Health Service academic centres). The participants were specialty leads or joint leads for their respective neuro-oncological service. As no national database of these neuro-oncology experts exists, we therefore identified potential participants’ contact details through institution websites and by liaison with relevant learned societies and known experts.
Survey design
The survey featured forty-three questions, divided into single choice, multiple choice and free text questions (Appendix 1). Participants were asked specialty-specific and cross-specialty questions regarding the current imaging practices following initial treatment for glioblastoma at their institution, as well as their opinion on ideal practice, which was defined as practice without time or cost constraint.
We used tailored design and bimodal methodology [49] to increase response rates and obtain high-quality feedback. The questionnaire was designed with feedback from a neuro-oncology charity (a member of UK’s James Lind Alliance Priority Setting Partnership who wish to establish the value and benefit of neuro-oncological interval imaging) [50] and improved thereafter following pilot testing by neuro-oncologists, neuroradiologists and neurosurgeons from two centres who were specialty leads for their respective neuro-oncological service. There were no concerns regarding recall bias; nonetheless, two elements of the design reduced this risk. First, every individual neuro-oncologist, neuroradiologist and neurosurgeon who was the specialty lead at their respective neuro-oncological service was asked to answer the questionnaire to give a collective centre response for the core questions. Second, only the specialty leads were selected to complete the questionnaire because typically they are involved in designing imaging protocols and are aware of annual audit findings.
An online survey tool, Survey Monkey (www.surveymonkey.com), was used for data collection. Each participant was invited to complete the online survey via e-mail. Multiple individualised follow-up emails were sent to ensure completion of the survey. Where we received data from both joint leads, the questionnaire which contained more detailed responses was used for final analysis.
Statistical analysis
We compiled data from the completed surveys in Microsoft Excel (Microsoft Corp). SPSS (IBM Corp) was used for descriptive statistical analysis and to make comparisons between groups using the chi-squared test (Yates corrected). For smaller sample sizes, Fisher exact test was used. Statistical significance was set at p < 0.05.
Results
Subject characteristics
We identified 124 eligible participants from 31 centres; of these, 109 completed the survey. One duplicate response was removed. In twelve centres where joint leads from the same specialty responded, the questionnaire which contained the more detailed response was used for final analysis. There were no discrepancies in any of the joint responses. The final analysis comprised 92 respondents across 31 centres, including one neuro-oncologist who covered two centres. Thus, 100% centre and specialty lead response rates were achieved. The characteristics of survey respondents are summarised in Table 1.
Table 1.
Background characteristics of 92 respondents from 31 neuro-oncology centres included for final analysis. All respondents were leads or joint leads for their respective neuro-oncological service. All respondents were UK consultant grade (i.e. independent practitioner)
| Characteristic | Value, n (%) |
|---|---|
| Speciality of respondents | |
| Neuroradiologist | 31 (34%) |
| Neurosurgeon | 31 (34%) |
| Neuro-oncologist | 30* (33%) |
| Location of respondents | |
| East Midlands | 3 (3%) |
| East of England | 3 (3%) |
| South-East | 9 (10%) |
| London | 18 (20%) |
| North-East England | 6 (7%) |
| North-West England | 11 (12%) |
| Northern Ireland | 3 (3%) |
| Scotland | 12 (13%) |
| South-West | 6 (7%) |
| Wales | 3 (3%) |
| West Midlands | 9 (10%) |
| Yorkshire | 9 (10%) |
| Number of newly diagnosed glioblastoma cases per centre per year | |
| 0 < 50 | 3 (10%) |
| 50 < 100 | 7 (22%) |
| 100 < 150 | 13 (42%) |
| 150 < 200 | 8 (26%) |
| > 200 | 0 (0%) |
| Time as consultant | |
| Years (median; range) | 11; 1–29 |
*A single neuro-oncologist covered two centres
EPMRI
Most centres (87%, 27/31) reported that they routinely perform EPMRI (Fig. 1). The main reasons given for performing EPMRI were to quantify residual tumour volume and establish a baseline for subsequent examinations (Table 2). There were no significant differences in the rationale for EPMRI between specialties (p = 0.59).
Fig. 1.
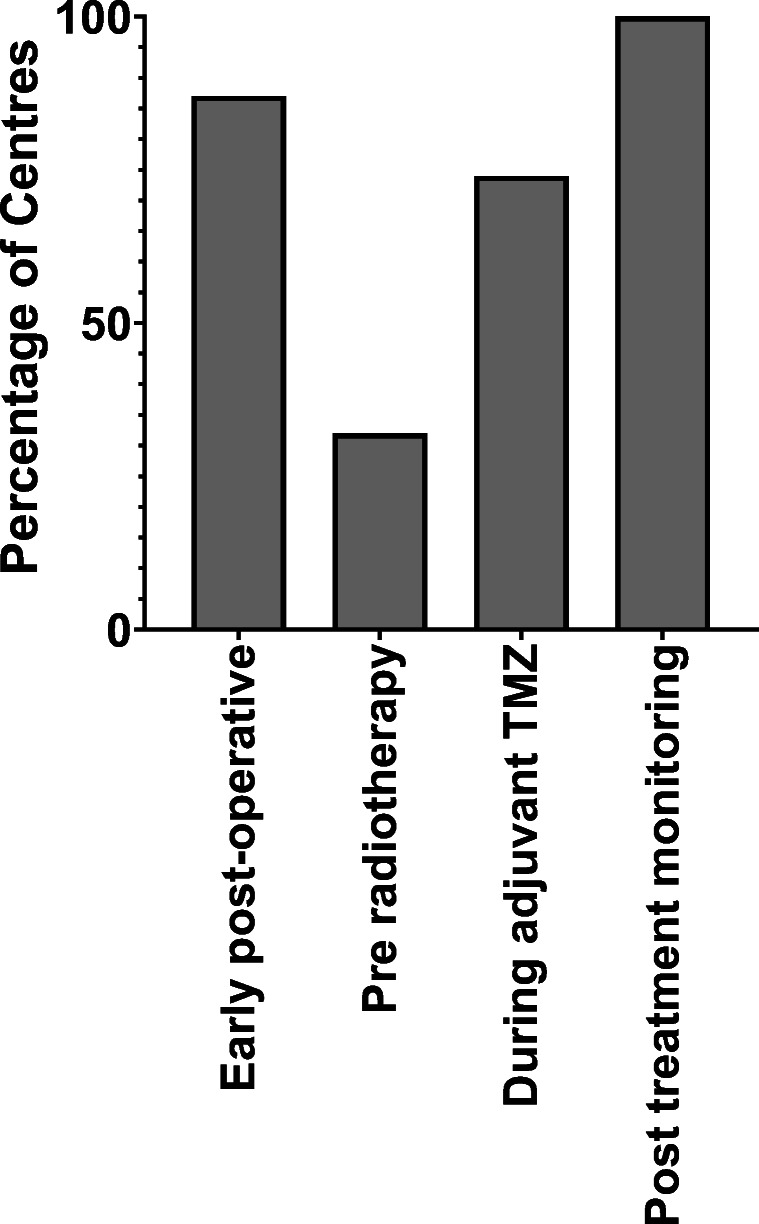
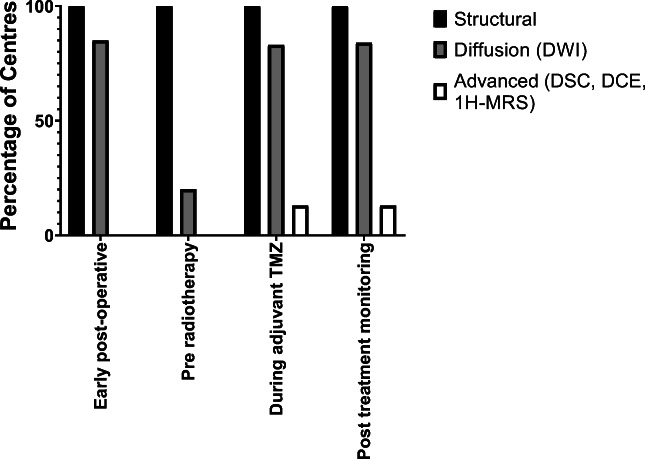
Percentage of centres that perform MRI at the different time points. All centres performed MRI after adjuvant TMZ, most centres performed MRI early post-operatively and during adjuvant TMZ treatment, whereas only 32% of centres routinely performed pre-radiotherapy MRI. TMZ = temozolomide
Table 2.
The rationale given for performing the early post-operative MRI (EPMRI) and pre-radiotherapy MRI (PRMRI)
| Early post-operative MRI | Neuroradiologist | Neurosurgeon | Neuro-oncologist | Total |
|---|---|---|---|---|
| Purpose | ||||
| Establish amount of residual tumour | 27 | 29 | 27 | 83 (90%) |
| Baseline to allow later assessment of treatment response | 25 | 24 | 22 | 71 (77%) |
| Plan radiotherapy | 12 | 14 | 21 | 47 (51%) |
| Differentiate between residual tumour and haemorrhage | 16 | 13 | 14 | 43 (47%) |
| Differentiate between residual tumour and post-operative enhancement | 15 | 11 | 14 | 40 (43%) |
| Differentiate between residual tumour and post-operative ischaemia | 14 | 13 | 10 | 37 (40%) |
| Establish amount of residual tumour suitable for further resection | 12 | 14 | 10 |
36 (39%) p = 0.59 |
| Speciality specific | ||||
| EPMRI used to assess treatment response on later MRI | 22 (71%) | – | – | |
| EPMRI used to decide on further debulking surgery | – | 11 (35%) | – | |
| EPMRI used in the management of chemoradiotherapy | – | – | 21 (70%) | |
| Pre-radiotherapy MRI | ||||
| Purpose | ||||
| Plan radiotherapy | 9 | 6 | 10 | 25 (83%) |
| Baseline to allow later assessment of treatment response | 7 | 2 | 3 | 12 (40%) |
| Establish amount of residual tumour | 4 | 2 | 2 | 8 (27%) |
| Establish amount of residual tumour suitable for further resection | 1 | 0 | 1 |
2 (7%) p = 0.74 |
In response to specialty-specific questions, EPMRI was reported to be used frequently by neuroradiologists and neuro-oncologists for purposes of treatment assessment and chemoradiotherapy planning respectively (71%, 22/31; 70%, 21/30). However, only a third of neurosurgeons (35%, 11/31) stated that the EPMRI formed an integral part of their decision-making process for further debulking surgery. It was reported that further debulking surgery was ultimately undertaken in only a small percentage (5–10%) of patients.
PRMRI
Ten centres (32%, 10/31) reported that MRI was performed routinely prior to the commencement of radiotherapy (Fig. 1). A further eight centres (26%, 8/31) reported that co-registered CT was performed instead. In the remaining fourteen centres (45%, 14/31), it was unclear whether imaging was consistently performed at this time point. The most frequently reported reason for performing the PRMRI was radiotherapy planning (83%, 25/30). There were no significant differences in the rationale for PRMRI between specialties (Table 2; p = 0.74).
MRI performed during adjuvant TMZ treatment
Twenty-three centres (74%, 23/31) reported that they had a standardised protocol for the adjuvant TMZ period (Fig. 1); three centres (10%, 3/31) avoided MRI during this period altogether; the remaining five centres did not provide a clear standardised protocol (16%, 5/31). The number and timing of scans routinely performed during adjuvant TMZ treatment varied widely (Table 3). Similarly, the standardised protocols implemented when there was suspected progression or new clinical symptoms also varied (Table 3). Most centres performed an MRI at the end of the adjuvant period (71%, 22/31).
Table 3.
Distribution of the number and timing of scans routinely performed during adjuvant temozolomide (TMZ). Also shown are the standardised protocols implemented when there is suspected progression or new clinical symptoms
| Number of scans during adjuvant period (not including post-cycle 6 TMZ) | Number of centres, n (%) |
| 0 | 3 (10%) |
| 1 | 7 (23%) |
| 2 | 12 (39%) |
| 3 | 4 (13%) |
| No standardised protocol | 5 (16%) |
| Timing of first post-chemoradiotherapy scan | Number of centres, n (%) |
| 4 weeks | 12 (39%) |
| 8 weeks | 1 (3%) |
| 12 weeks | 10 (32%) |
| End of TMZ cycle 6 only | 3 (10%) |
| No standardised protocol | 5 (16%) |
| Protocol in suspected progression | Number of centres, n (%) |
| Additional imaging at 4–6 weeks | 14 (45%) |
| Additional imaging over 4–12 weeks | 8 (26%) |
| No standardised protocol | 6 (19%) |
| No routine scans performed during adjuvant period | 3 (10%) |
| Protocol if symptomatic during this period | Number of centres, n (%) |
| Initial CT, followed by MRI | 11 (35%) |
| MRI | 20 (65%) |
MRI performed after adjuvant TMZ treatment
All centres reported to have a standardised protocol for disease monitoring following the completion of adjuvant TMZ treatment (100%, 31/31; Fig. 1). Most centres performed MRI scans at 3 monthly intervals (81%, 25/31), although the length of follow-up was more variable (Table 4). In comparison to the adjuvant TMZ period, a significantly smaller proportion of centres reported having a standardised protocol to implement following suspected progression (11/31 vs 22/31, p = 0.019), opting instead for a more case-based approach (45%, 14/31).
Table 4.
Distribution of the imaging intervals and duration of imaging after completion of adjuvant temozolomide (TMZ). Also shown is the standardised protocol implemented where there is suspected progression
| Scan interval and duration | No. of centres, n (%) |
| 3 monthly intervals for 6 months | 3 (10%) |
| 3 monthly intervals for 12 months | 4 (13%) |
| 3 monthly intervals for 18 months | 1 (3%) |
| 3 monthly intervals for 24 months | 9 (29%) |
| 3 monthly intervals for life | 8 (26%) |
| 4 monthly intervals for 18 months | 2 (6%) |
| 6 monthly intervals for life | 4 (13%) |
| Protocol in suspected progression | No. of centres, n (%) |
| Additional imaging at 4–6 weeks | 7 (23%) |
| Additional imaging at 6–12 weeks | 4 (13%) |
| No standardised protocol | 14 (45%) |
| No additional confirmatory scan | 6 (19%) |
Assessing treatment response
Only 23% (7/31) of centres assessed treatment response based on the RANO criteria [13], whilst the MacDonald criteria [51] were not used at all (Table 5).
Table 5.
Assessment of treatment response during and after adjuvant temozolomide (TMZ)
| Assessment of treatment response | No. of centres, n (%) |
|---|---|
| MacDonald criteria based | 0 (0%) |
| RANO criteria based | 7 (23%) |
| Standardised protocol not based on either MacDonald or RANO criteria | 12 (39%) |
| No standardised protocol | 12 (39%) |
Imaging sequences and technique
Structural sequences (pre- and post-contrast T1-weighted, T2-weighted, FLAIR) were used in all cases where the standardised protocol was described (Fig. 2). Diffusion weighted imaging (DWI) was used in the majority of EPMRI (85%, 23/27), during adjuvant TMZ (83%, 19/23) and after adjuvant TMZ (84%, 26/31; Fig. 2). Routine use of advanced imaging (DSC, DCE and/or MRS) on all patients was infrequent and limited to adjuvant TMZ and post-adjuvant TMZ periods (Fig. 2). Neuroradiologists from eleven centres (35%, 11/31), however, reported that advanced imaging was performed in selective cases to help differentiate between pseudoprogression and true progression during the adjuvant period ((p < 0.001). A further inconsistency between the specialties was seen during the EPMRI time point, whereby neuroradiologists reported more frequent use of volumetric imaging (p = 0.004).
Fig. 2.
Imaging protocols at the different time points. Where the standardised protocol was described, most centres used diffusion weighted imaging (DWI), apart from at the pre-radiotherapy time point. Note that the figure shows the percentage of centres that routinely used advanced imaging techniques, as opposed to centres that only used advanced imaging techniques in selected patients, Structural = pre- and post-contrast T1-weighted, T2-weighted, FLAIR; DWI = diffusion weighted imaging; DSC = dynamic susceptibility contrast-enhanced MRI (perfusion); DCE = dynamic contrast enhanced MRI (permeability); 1H-MRS = 1H-MR spectroscopy
Ideal practice
In response to questions regarding ideal practice, thirty-seven respondents (40%, 37/92) reported that they would change their imaging protocol. Thirty-five respondents (38%, 35/92) did not suggest any changes, and the remaining twenty respondents (22%, 20/92) did not give a response.
Amongst those who suggested change in their current imaging protocol, approximately half (46%, 17/37) reported that adding advanced sequences such as DSC, DCE, MRS or PET would provide meaningful benefit, for subsequent comparison and to help differentiate between tumour progression and treatment-related changes. Although responses did not differ significantly between the three specialties questioned at EPMRI, PRMRI, and during and after adjuvant TMZ with regard to changing sequences (p = 0.1, p = 0.1, p = 0.6 and p = 0.8 respectively), we made three observations. Neuroradiologists were the main advocates for proposing advanced MRI during and following the adjuvant period (100%, 14/14). On the other hand, neuro-oncologists were more likely to suggest that further evidence is needed for analytical and clinical validation of these modalities in routine clinical practice (56%, 5/9). Conversely, neurosurgeons preferred to routinely add a volumetric acquisition to the EPMRI protocol to assess residual tumour volume (43%, 6/14; p = 0.04).
In terms of timing, it was noted that a small number of respondents (14%, 5/37), predominantly neurosurgeons, stated that they would want the EPMRI to be performed earlier than 72 h, whereas a minority of neuroradiologists stated that they would delay the timing to beyond 72 h to coincide with radiotherapy planning (8%, 3/37; p < 0.001). A further ideal practice suggestion was to ensure that EPMRI scans were available to all patients undergoing debulking surgery, including out of hours (27%, 10/37). Only a few respondents, predominantly neuro-oncologists, reported that they would add PRMRI (11%, 4/37; p = 0.7).
Discussion
Summary of findings
The GIN CUP study highlighted the variation in imaging practices in the treatment and follow-up periods of glioblastoma amongst the 31 neuro-oncological units in the UK. Over 80% of centres routinely performed EPMRI whereas only 32% performed PRMRI. During adjuvant TMZ treatment, there was considerable variation in the timing and frequency of imaging. There was more consistency at the end of the adjuvant period, at which point most centres (71%) performed MRI routinely, followed by monitoring scans at 3 monthly intervals (81%). The addition of advanced sequences was the most popular suggestion for ideal imaging practice, followed by changes in the timing of EPMRI.
Comparison with other studies and study relevance
Over two decades ago, a prospective study showed that the volume of residual enhancing disease seen on EPMRI was an independent prognostic biomarker for both progression and overall survival [11]. This formed a rationale to perform EPMRI, which RANO subsequently advocated for use in trials in 2010 [13]. Compared to a 2010 survey of UK neuro-oncologists, our findings suggest an increase in the popularity of EPMRI in routine clinical practice (80% vs 50%) [4].
Our survey revealed that the most common reason for undertaking EPMRI was to determine the extent of residual tumour. However, recent studies have shown false-positive results where non-neoplastic enhancement appears within 72 h of surgery [52, 53]. Furthermore, 24% of cases appear to give false-negative results, as proven by histopathology or short-term follow-up [54]. In the recent era of TMZ chemotherapy and advanced neurosurgical techniques, a retrospective study demonstrated that EPMRI after tumour resection did not significantly affect overall survival [55]. In our study, the identification of residual disease infrequently altered subsequent management, as evidenced by neurosurgeons self-reporting that only 5–10% of patients undergo early repeat resection based on findings from EPMRI. This early repeat resection rate was 0% in a recent UK survey of 22 neurosurgical units, despite 16% (13/80) of cases being deemed operable [56]. It is possible that such evidence might explain why some centres choose not to perform EPMRI.
There is evidence that PRMRI could provide a more accurate baseline for subsequent imaging studies compared to EPMRI, due to the detection of any interval changes occurring between EPMRI and the initiation of chemoradiotherapy [57]. These changes include tumour growth [57–59] and new reactive non-neoplastic enhancement [11, 52, 53], both of which can confound treatment response assessment on subsequent imaging studies.
Pseudoprogression and radiation necrosis are two well-documented forms of PTRE. Pseudoprogression generally occurs within the six months following completion of chemoradiotherapy, and resolves or stabilises without additional treatment [15, 17–19, 60], whereas radiation necrosis generally occurs beyond 6 months, up to several years after radiotherapy, and is often more severe and progressive [15, 19]. Structural imaging alone cannot reliably discriminate between true progression and PTRE, due to the common features of contrast enhancement, perilesional oedema-like appearance and mass effect. Over the last two decades, there have been numerous promising studies to make this distinction, including the use of DSC, MRS and amino acid PET (Supplementary Table 1) [21–41, 61]. In our study, advanced techniques were used by only 10% of centres routinely and a third of centres in selected cases. This is in contrast to a survey of neuroradiologists across 220 European institutions in 2016, which reported routine use of advanced imaging for glioma follow-up (82% DSC, 80% MRS) [62]. However, the response rate for this European survey was 3%, and the results may not be representative of UK (or European) practice. The relatively low routine use of advanced imaging in our study might be related to the limited availability of expertise and software, as well as the increased operational costs, although many respondents suggested that advanced imaging techniques would improve their practice.
In our study, the largest variation in timing of MRI scans existed during the adjuvant period, which is comparable to a previous study [4]. In cases of suspected progression, only half of the centres emulated the 4–6 week “confirmatory” scan recommendation from RANO research guidelines [13]. One potential explanation for this is that two consecutive MRI within a short interval (4–6 weeks) may offer limited diagnostic value, particularly if they are performed during the enlargement phase of pseudoprogression [17]. Other limitations with imaging research guidelines have been described which might also have reduced their influence in a clinical setting [63].
Most centres used the same sequences at each of the different time points. The sequences included pre- and post-contrast T1-weighted, T2-weighted, FLAIR and DWI, apart from the PRMRI when DWI sequences were obtained infrequently. Consensus recommendations by a group of predominantly US experts for a standardised imaging protocol in trials [48] include pre- and post-contrast volumetric T1-weighted imaging, T2-weighted, FLAIR and DWI sequences. In our study, the use of volumetric imaging appeared to be underemployed; this was highlighted by neurosurgeons, who valued volumetric images as part of their ideal practice.
Strengths and limitations
This was the first study to unequivocally capture UK neuro-oncology imaging practices by achieving a 100% response rate across three specialties from lead or joint lead consultants at all UK neuro-oncology centres. The 100% yield is important as it eliminated “nonresponse bias” due to an unrepresentative cohort response. The selection of these experts also eradicates “sampling bias” as these are the most expert clinicians for the subject matter. Our questionnaire was comprehensive and likely to capture the details of imaging practices following treatment for high-grade gliomas. There is benefit of including open-ended questions because they are not limited to a predetermined set of possible answer choices in order to extract more granular information. Indeed, after coding the granular information, a qualitative data analysis (QDA) provides useful information. However, although the questionnaire was designed with care and was improved after pilot testing, responses may reflect varying interpretation of the questions. It is also conceivable there may have been “recall bias”—after all, recall bias is almost impossible to entirely eradicate in surveys. However, the inclusion of experts who are typically involved in all aspects of neuro-oncology administration including attending weekly multi-disciplinary team meetings and planning departmental guidelines minimises recall bias. Additionally, questions regarding ideal practice only received responses from 50 to 60% of respondents distributed evenly across the three specialties. The reasons for poor response in this part of the survey are uncertain. Furthermore, although a health economic resource use analysis is beyond the remit of the study, it would have been interesting to explore resource use for each neuro-oncology centre, as it is possible this may have influenced imaging practices (Appendix 2). Our survey did not also address the emergence of novel therapies, such as immunotherapy and tumour-treating fields; however, these are not currently recommended as second-line treatment options in the UK outside of research trials.
Unanswered questions and future directions
The variations in imaging practices elucidated by this study are most likely due to a lack of consensus and high-level evidence on the optimal schedule for imaging investigations during and after glioblastoma treatment. In particular, there has been no definitive study which addresses the question of how often MRI should be obtained in the post-treatment follow-up period. It is noteworthy that establishing the value and benefit of neuro-oncological interval imaging forms the second of ten priorities proposed by the UK’s James Lind Alliance Priority Setting Partnership, an organisation which aims to raise awareness for important research questions [50]. Our study records current clinical practice and highlights that there is variation between centres. We reiterate that the study does not aim to present optimal practice. To achieve optimal practice in this heterogeneous patient pathway where there are multiple co-variates, large, multicentre, longitudinal, prospective trials, possibly informed by data-driven machine learning algorithms [64], are now needed. Such studies could define the optimal time points for assessment and determine whether neuroimaging performed at each defined time point after initial glioblastoma treatment results in a real change in management and, more importantly, results in a change in patient outcomes such as morbidity and overall survival.
It is also noteworthy that if imaging could be performed without time or cost constraint, the expert community would add advanced sequences to current protocols. Mismatch between UK experts’ existing and perceived ideal MRI follow-up imaging regimens may reflect resource constraints and concerns over non-harmonised and non-validated advanced imaging protocols. This finding might motivate further development of cost-effective, harmonised and validated advanced sequences.
Conclusion
The GIN CUP study assessed the current imaging practices amongst UK neuro-oncology centres and provided baseline data to inform future practice. We have shown definitively that variations in neuroimaging practices exist after initial glioblastoma treatment within the UK. Centre variation is unlikely to be in the best interests of all UK patients and is likely to reflect a lack of consensus and high-level evidence on the optimal schedule for imaging investigations during and after glioblastoma treatment. A validated post-operative imaging protocol with definitive evidence that outcomes are improved is now required. Multicentre, longitudinal, prospective trials interrogating protocols are recommended as is the development of efficient, harmonised and validated advanced sequences.
Electronic supplementary material
(DOCX 3079 kb)
Acknowledgements
This work was supported by Brainstrust who provided funding for the survey, by the Wellcome/EPSRC Centre for Medical Engineering (WT 203148/Z/16/Z) and by the Royal College of Radiologists.
Abbreviations
- ADC
Apparent diffusion co-efficient
- DCE
Dynamic contrast enhanced
- DSC
Dynamic susceptibility contrast enhanced
- DTI
Diffusion tensor imaging
- DWI
Diffusion weighted imaging
- EPMRI
Early post-operative MRI
- FLAIR
Fluid-attenuated inversion recovery
- MGMT
O6-Methylguanine-DNA methyltransferase
- MRS
Magnetic resonance spectroscopy
- PCV
Procarbazine, lomustine and vincristine
- PET
Positron emission tomography
- PRMRI
Pre-radiotherapy MRI
- RANO
Response Assessment in Neuro-Oncology
- SWI
Susceptibility weighted imaging
- TMZ
Temozolomide
- WHO
World Health Organization
Funding
This study has received funding by Brainstrust.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is T Booth, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because no human subjects/patients were included in this study.
Ethical approval
Institutional Review Board approval was not required because no human subjects/patients were included in this study.
Methodology
• Survey and literature review.
Footnotes
Thomas C. Booth and Aysha Luis are joint first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brodbelt A, Greenberg D, Winters T et al (2015) Glioblastoma in England: 2007-2011. Eur J Cancer 51(4):533–542 [DOI] [PubMed]
- 2.Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996 [DOI] [PubMed]
- 3.Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466 [DOI] [PubMed]
- 4.Sanghera P, Rampling R, Haylock B et al (2012) The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol (R Coll Radiol) 24(3):216–227 [DOI] [PubMed]
- 5.British Society of Neuroradiologists (2018) Core imaging protocol for brain tumours. British Society of Neuroradiologists, UK. Available from: https://bsnr.org.uk/_userfiles/pages/files/bsnrstandardsbraintumour.pdf. Accessed Jan 2019
- 6.The Royal College of Radiologists (2014) Tumours of the brain. Recommendations for cross-sectional imaging in cancer management, second edition. The Royal College of Radiologists, UK. Available from: https://www.rcr.ac.uk/sites/default/files/BFCR%2814%292_5_Brain.pdf. Accessed Jan 2019
- 7.National Institute for Health and Clinical Excellence (2018) NICE guideline [NG99]: brain tumours (primary) and brain metastases in adults. National Institute for Health and Clinical Excellence, UK. Available from: https://www.nice.org.uk/guidance/ng99. Accessed Jan 2019
- 8.Stupp R, Brada M, van den Bent MJ et al (2014) High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii93–ii101 [DOI] [PubMed]
- 9.Weller M, van den Bent M, Tonn JC et al (2017) European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18(6):e315–e329 [DOI] [PubMed]
- 10.National Comprehensive Cancer Network (2018) NCCN guidelines for treatment of cancer by site: central nervous system cancers. National Comprehensive Cancer Network, US. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed Jan 2019
- 11.Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34(1):45–60 discussion 60-1 [DOI] [PubMed]
- 12.Ekinci G, Akpinar IN, Baltacioğlu F (2003) Early-postoperative magnetic resonance imaging in glial tumors: prediction of tumor regrowth and recurrence. Eur J Radiol 45(2):99–107 [DOI] [PubMed]
- 13.Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972 [DOI] [PubMed]
- 14.Niyazi M, Brada M, Chalmers AJ et al (2016) ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol 118(1):35–42 [DOI] [PubMed]
- 15.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9(5):453–461 [DOI] [PubMed]
- 16.Melguizo-Gavilanes I, Bruner JM, Guha-Thakurta N, Hess KR, Puduvalli VK (2015) Characterization of pseudoprogression in patients with glioblastoma: is histology the gold standard? J Neurooncol 123(1):141–150 [DOI] [PMC free article] [PubMed]
- 17.Booth TC, Larkin TJ, Yuan Y et al (2017) Analysis of heterogeneity in T2-weighted MR images can differentiate pseudoprogression from progression in glioblastoma. PLoS One 12(5):e0176528 [DOI] [PMC free article] [PubMed]
- 18.Radbruch A, Fladt J, Kickingereder P et al (2015) Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol 17(1):151–159 [DOI] [PMC free article] [PubMed]
- 19.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- 20.Brandes AA, Franceschi E, Tosoni A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26(13):2192–2197 [DOI] [PubMed]
- 21.Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25(2):201–209 [PMC free article] [PubMed]
- 22.Sundgren PC, Fan X, Weybright P et al (2006) Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24(9):1131–1142 [DOI] [PubMed]
- 23.Lee WJ, Choi SH, Park CK et al (2012) Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol 19(11):1353–1361 [DOI] [PubMed]
- 24.Chu HH, Choi SH, Ryoo I et al (2013) Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 269(3):831–840 [DOI] [PubMed]
- 25.Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am. 2009;19(4):527–557. doi: 10.1016/j.nic.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Sugahara T, Korogi Y, Tomiguchi S et al (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 21(5):901–909 [PMC free article] [PubMed]
- 27.Hu LS, Baxter LC, Smith KA et al (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 30(3):552–558 [DOI] [PMC free article] [PubMed]
- 28.Gasparetto EL, Pawlak MA, Patel SH et al (2009) Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology 250(3):887–896 [DOI] [PubMed]
- 29.Barajas RF Jr, Chang JS, Segal MR et al (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253(2):486–496 [DOI] [PMC free article] [PubMed]
- 30.Bisdas S, Naegele T, Ritz R et al (2011) Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol 18(5):575–583 [DOI] [PubMed]
- 31.Patel P, Baradaran H, Delgado D et al (2017) MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol 19(1):118–127 [DOI] [PMC free article] [PubMed]
- 32.Zhang H, Ma L, Wang Q, Zheng X, Wu C, Xu BN (2014) Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: a systematic review and meta-analysis. Eur J Radiol 83(12):2181–2189 [DOI] [PubMed]
- 33.Wang Q, Zhang H, Zhang J et al (2016) The diagnostic performance of magnetic resonance spectroscopy in differentiating high-from low-grade gliomas: a systematic review and meta-analysis. Eur Radiol 26(8):2670–2684 [DOI] [PubMed]
- 34.Seeger A, Braun C, Skardelly M et al (2013) Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad Radiol 20(12):1557–1565 [DOI] [PubMed]
- 35.van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A (2017) Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol 27(10):4129–4144 [DOI] [PMC free article] [PubMed]
- 36.Thomas AA, Arevalo-Perez J, Kaley T et al (2015) Dynamic contrast enhanced T1 MRI perfusion differentiates pseudoprogression from recurrent glioblastoma. J Neurooncol 125(1):183–190 [DOI] [PMC free article] [PubMed]
- 37.Kazda T, Bulik M, Pospisil P et al (2016) Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin 11:316–321 [DOI] [PMC free article] [PubMed]
- 38.Deuschl C, Kirchner J, Poeppel TD et al (2018) (11)C-MET PET/MRI for detection of recurrent glioma. Eur J Nucl Med Mol Imaging 45(4):593–601 [DOI] [PubMed]
- 39.Galldiks N, Dunkl V, Stoffels G et al (2015) Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging 42(5):685–695 [DOI] [PubMed]
- 40.Kebir S, Fimmers R, Galldiks N et al (2016) Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res 22(9):2190–2196 [DOI] [PubMed]
- 41.Galldiks N, Law I, Pope WB, Arbizu J, Langen KJ (2017) The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. Neuroimage Clin 13:386–394 [DOI] [PMC free article] [PubMed]
- 42.Weller M, Cloughesy T, Perry JR, Wick W (2013) Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol 15(1):4–27 [DOI] [PMC free article] [PubMed]
- 43.Parasramka S, Talari G, Rosenfeld M, Guo J, Villano JL (2017) Procarbazine, lomustine and vincristine for recurrent high-grade glioma. Cochrane Database Syst Rev 7:CD011773 [DOI] [PMC free article] [PubMed]
- 44.Perry JR, Bélanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28(12):2051–2057 [DOI] [PubMed]
- 45.Weller M, Tabatabai G, Kästner B et al (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res 21(9):2057–2064 [DOI] [PubMed]
- 46.Ryu S, Buatti JM, Morris A et al (2014) The role of radiotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 118(3):489–499 [DOI] [PubMed]
- 47.Suchorska B, Weller M, Tabatabai G et al (2016) Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol 18(4):549–556 [DOI] [PMC free article] [PubMed]
- 48.Ellingson BM, Bendszus M, Boxerman J et al (2015) Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol 17(9):1188–1198 [DOI] [PMC free article] [PubMed]
- 49.Dillman DA, Smyth JD, Christian LM. Internet, phone, mail, and mixed-mode surveys : the tailored design method. 4th edition. Hoboken: Wiley; 2014. [Google Scholar]
- 50.The James Lind Alliance, Top 10 priorities for neuro-oncology [2015]. Available from: http://www.jla.nihr.ac.uk/priority-setting-partnerships/neuro-oncology/top-10-priorities/. Accessed January 2019
- 51.Macdonald DR, Cascino TL, Schold Jr SC, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280 [DOI] [PubMed]
- 52.Bette S, Gempt J, Huber T et al (2016) Patterns and time dependence of unspecific enhancement in postoperative magnetic resonance imaging after glioblastoma resection. World Neurosurg 90:440–447 [DOI] [PubMed]
- 53.Lescher S, Schniewindt S, Jurcoane A, Senft C, Hattingen E (2014) Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg Focus 37(6):E3 [DOI] [PubMed]
- 54.Kläsner B, Buchmann N, Gempt J, Ringel F, Lapa C, Krause BJ (2015) Early [18F]FET-PET in gliomas after surgical resection: comparison with MRI and histopathology. PLoS One 10(10):e0141153 [DOI] [PMC free article] [PubMed]
- 55.Mrowczynski OD, Zammar S, Bourcier AJ et al (2018) Utility of early postoperative magnetic resonance imaging after glioblastoma resection: implications on patient survival. World Neurosurg 120:e1171–e1174 [DOI] [PubMed]
- 56.Ma R, Chari A, Brennan PM et al (2017) Residual enhancing disease after surgery for glioblastoma: evaluation of practice in the United Kingdom. Neurooncol Pract 5(Issue 2):74–81 [DOI] [PMC free article] [PubMed]
- 57.Majós C, Cos M, Castañer S et al (2016) Preradiotherapy MR imaging: a prospective pilot study of the usefulness of performing an MR examination shortly before radiation therapy in patients with glioblastoma. AJNR Am J Neuroradiol 37(12):2224–2230 [DOI] [PMC free article] [PubMed]
- 58.Pirzkall A, McGue C, Saraswathy S et al (2009) Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology 11(6):842–852 [DOI] [PMC free article] [PubMed]
- 59.Villanueva-Meyer JE, Han SJ, Cha S, Butowski NA (2017) Early tumor growth between initial resection and radiotherapy of glioblastoma: incidence and impact on clinical outcomes. J Neurooncol 134(1):213–219 [DOI] [PMC free article] [PubMed]
- 60.Booth TC, Waldman AD, Jefferies S, Jäger R (2015) Comment on “The role of imaging in the management of progressive glioblastoma. A systematic review and evidence-based clinical practice guideline” [J Neurooncol 2014; 118:435-460]. J Neurooncol 121(2):423–424 [DOI] [PubMed]
- 61.Matsusue E, Fink JR, Rockhill JK, Ogawa T, Maravilla KR (2010) Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 52(4):297–306 [DOI] [PubMed]
- 62.Thust SC, Heiland S, Falini A et al (2018) Glioma imaging in Europe: a survey of 220 centres and recommendations for best clinical practice. Eur Radiol 28(8):3306–3317 [DOI] [PMC free article] [PubMed]
- 63.Buwanabala J, Mirchandani A, Booth TC (2019) The (mis)use of imaging criteria in the assessment of glioblastoma treatment response in American Society of Neuroradiology 57th Annual Meeting. Boston, MA
- 64.Booth TC, Williams M, Luis A, Cardoso J, Ashkan K, Shuaib H (2019) Machine learning and glioma imaging biomarkers. Clin Radiol 75(1):20–32 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 3079 kb)