Abstract
Introduction:
Low Vitamin D levels have been associated with Chronic Obstructive Pulmonary Disease (COPD) and acute exacerbations.
Objectives:
There is paucity of data on vitamin D and COPD, its severity and exacerbations in populations that are exposed to sunlight regularly with high levels of physical activity most of their lives.
Methods:
Serum levels of 25-OH-Vitamin-D was assessed in 100 COPD subjects and 100 age- and gender-matched controls from the rural community based MUDHRA cohort in South India. Levels of <20ng/ml was defined as Vitamin D deficiency. Smoking habits, occupation, Charlson co-morbidity index, Standard of living index(SLI), body mass index(BMI), 6-minute walking distance were examined for associations with by logistic regression between controls and COPD subjects. Unconditional logistic regression was used to examine the association with exacerbation of COPD.
Results:
Vitamin D deficiency was observed in 64.5%(95%CI 57.7–70.8) of the subjects in spite of regular exposure to sunlight. Subjects with COPD had higher risk of Vitamin D deficiency (Adjusted OR: 5.05; 95%CI 1.4–17.8) as compared to controls. Among subjects with COPD, Vitamin D deficient subjects were 3 times more likely to have exacerbations in the previous year (Adjusted OR:3.51; 95%CI 1.27–9.67) as compared to COPD subjects without Vitamin D deficiency. Levels of Vitamin D below 20.81 ng/ml and below 18.45 ng/ml had the highest levels of combined sensitivity and specificity for COPD and exacerbations respectively.
Conclusion:
In a rural population exposed to sunlight many hours in a day throughout their lives, low Vitamin D levels were associated with COPD and exacerbations of COPD
Keywords: COPD, Vitamin D, Acute exacerbation of COPD, spirometry, six minute walk distance
Introduction
Chronic obstructive pulmonary disease (COPD) is a common cause of mortality and morbidity globally1,2. COPD exacerbations are associated with significant morbidity, mortality, impaired quality of life and costs1,2. There is interest on protective factors with a potential for attenuating and ameliorating the frequency and severity of COPD exacerbations.Vitamin D is of particular interest due to its various effects on lungs, tissue remodeling, reduction of pro-inflammatory cytokines and beneficial modulation of both innate and adaptive immune systems3–5, regulating more than 1000 genes andbeing critical for normal human physiology beyond the skeletal system6.
Majority of studies exploring relationship of vitamin D with COPD, its severity and exacerbations are conducted in areas with sub-optimal sunlight and lack of sunlight throughout the year or in cities where most residents spend time indoors (homes or offices)7–9. There are limited data on the association of vitamin D with severity of COPD and its exacerbation in populations that are both adequately exposed to sunlight and maintain higher levels of physical activity through the lifecourse. The Mysuru stUdies of Determinants of Health in Rural Adults (MUDHRA) cohort in rural areas ofMysore district, South India, where sunlight is abundant and members the cohort are mostly farmers and or manual laborers, was set up in 2009.The cohort profile has been previously published10.
This study aimed to evaluate whether vitamin D levels were associated with lower lung functions in non-COPDindividuals and COPD patients. Furthermore, we examined if lower vitamin D levels were associated with COPD severity and acute exacerbation of COPD (AECOPD) in the previous year and during 6 months of prospective follow-up.
Methods
Ethics Statement:
Study was approved by Institutional Ethical Committeeat JSS Medical College, Mysuru, India and Institutional Review Board at Florida International University(FIU), USA. Subjects who provided written informed consent were enrolled in the study.
Study Population
Subjects in the MUDHRA Cohort in 2009 were recruited usinga two stage sampling10. In the first, two talukas (administrative sub-districts) were selected out of seven, and in the second, 16 villages were randomly selected. All men and women aged 30 years and above were invited to participate in a baseline study to establish the prevalence of COPD and its risk factors according to the protocol from Burden of Obstructed Lung Disease (BOLD) Study, where 8457 subjects were recruited11,12. Of these, 1692 underwent spirometry and 1085 subjects provided acceptable spirometry readings13, and constituted the MUDHRA cohort.
Of the original 1085 members, 869 were retraced between 2014–15, and baseline assessments were repeated with a comprehensive questionnaire14, spirometry for lung function estimation, 6-minute walk test15 andCharlson’s co-morbidity index16,17. Current smoker was defined as a person who was smoking during the time of study18. Biomass fuel smoke exposure index was calculated by multiplication of average hours of exposure with number of years of exposure19. Occupation was categorised as heavy work and non-heavy work based on guidelines from Occupational Safety and Health Administration, USA20.
Among 869 subjects, 108 subjects were diagnosed as having COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (age above 40 years and no history of asthma, post bronchodilator FEV1/FVC ratio <0.70 in spirometry). Subjects with COPD were categorized on severity of airflow limitation in spirometry and multi-dimensional assessment of COPD (GOLD ABCD). Exacerbation of COPD was defined as change in symptoms more than expected diurnal variation which necessitated additional clinical intervention including prescription of additional medications which includes antibiotics or systemic steroids or both21. The subjects with COPD who were stable and able to perform spirometry and six-minute walk test were enrolled according to GOLD criteria.From 147 subjects who had normal lung functions, 108 age and gender matched subjects were included as controls by stratified random sampling method using a computer system.
Serumvitamin D(25-OH-Vitamin D). levels from these subjects were carried outby chemi-immuno-luminescence assay (CLIA) method [Roche, Basel, Switzerland]. Vitamin D levels of <20ng/ml was defined as vitamin D deficiency according to US Endocrine Society Guidelines22. Of this subset, 100 of the 108 subjects with COPD, and 100 of the 108 controls were prospectivelyfollowed up and 6 monthslater had repeat spirometry and assays for vitamin D. All participants with COPD were clinically monitored for any exacerbations.
Statistical Analysis:
Statistical analysis was performed using Epi info v7 (CDC, Atlanta, USA) and SPSS v20 (IBM Corp, Armonk, USA). Descriptive statistics included mean and standard deviation for continuous variables and frequencies for categorical/nominal variables. T-test was used for comparing means between continuous variables and Chi-square was used forcomparing frequencies/percentages between categorical/nominal variables. Pearson’s correlation was conducted to examine correlations between vitamin D levels and select variables. Conditional logistic regression was used to examine independent association of vitamin D levels with COPD. A sub-group analysis was conducted to identify variables independently associated with COPD exacerbations by logistic regression. The p-value of <0.05 was considered statistically significant.R software v3.6.3 was used forinterpolated contour plot.
Results:
The flow diagram for patient inclusion and exclusion is presented in Figure 1 and demographic variables in Table 1. In general vitamin D levels were lower than normal among both subjects with COPD and non-COPD controls with a range(4.73 – 29.4 ng/ml). Seventy-one (35.5%; 95%CI 29.2–42.4) subjects hadvitamin D insufficiency (defined as >20ng/ml and <30ng/ml22); 129 (64.5%; 95%CI 57.7–70.8) were vitamin D deficient (<20ng/ml); and 12(6%) had severe vitamin D deficiency (<10ng/ml). There was no significant difference between controlsrandomly selected to participate in this study and those not selected in relation toage and lung function(data not shown). Pack-years of smoking amongCOPD subjects (mean 36.6±10.9) were significantly higher (p<0.001) than healthy controls who smoked but did not develop COPD (mean 27.1±7.1). Most subjects were manual laborers (83%) involved in heavy work throughout their careers. Charlson’s co-morbidity scores were low in cases and controls, without significant difference.Nearly half the subjects in this study had standard of living index (SLI) of <20 indicating their lower socio-economic status. COPD subjects had significantly lower BMI and 6-minute walk distance than controls. COPD subjects had significantly lower levels of Vitamin D levels at baseline and at 6 months follow-up. There were no significant differences between COPD subjects with and without exacerbations except for lower Vitamin D levels and 6-minute walk distance (Table 1) and higher dyspnea and CAT scores (Table 2).
Figure 1:
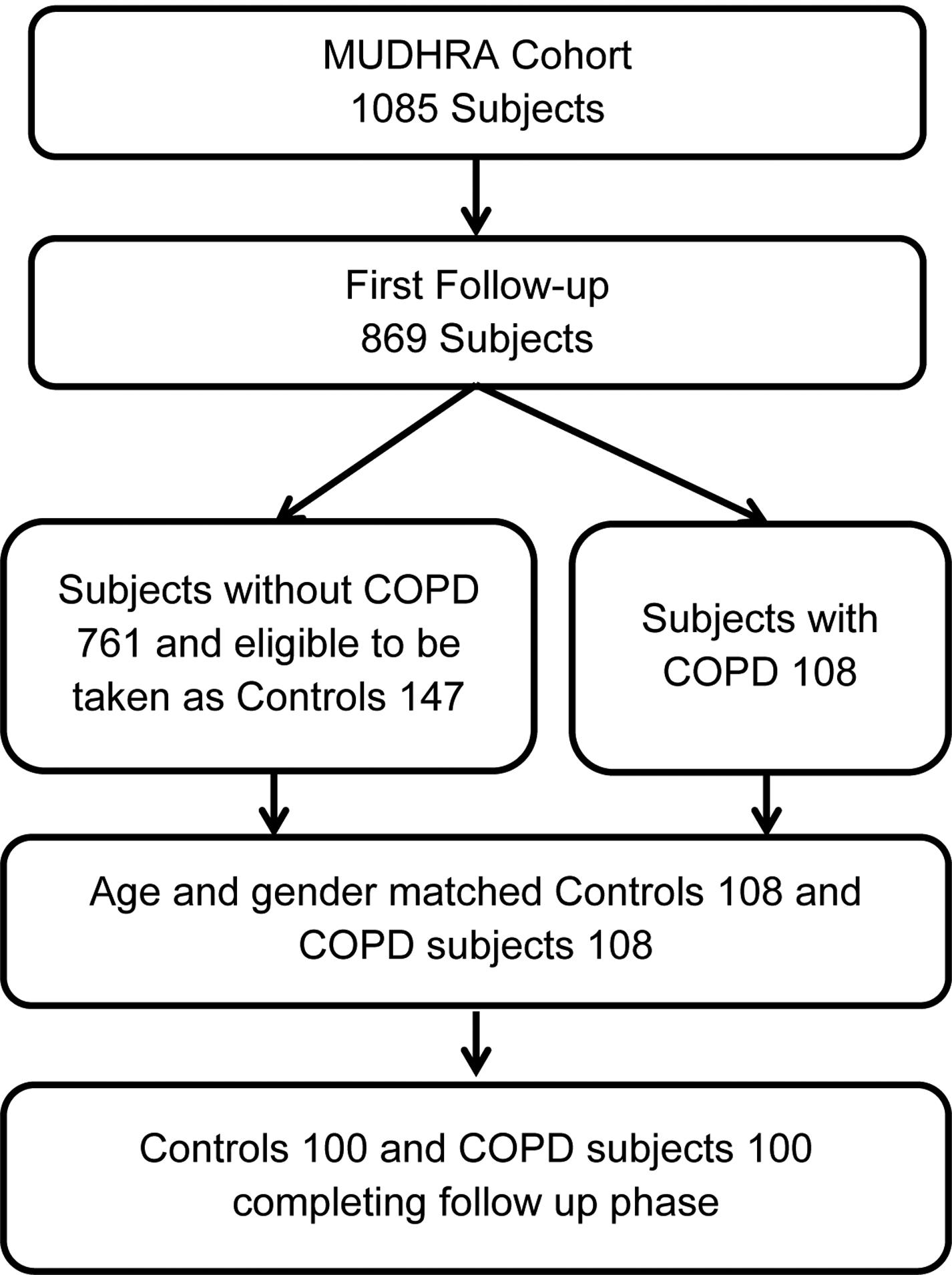
Flow chart showing the subject enrolment to the study.
Table 1:
Demographic characteristics of study population and comparison of Control and COPD groups; and among COPD subjects, between subjects without exacerbation and subjects with exacerbation.Frequencies shown as n (%).
| Variables | Groups | Among COPD, Exacerbation in previous year | ||||
|---|---|---|---|---|---|---|
| Control n=100 | COPD n=100 | P-value | No n=58 | Yes n=42 | P-value | |
| Age in years | ||||||
| Mean±SD | 56.9±9.7 | 57.8±10.4 | 0.49 | 56.8±9.2 | 59.2±11.9 | 0.15 |
| <55 | 34 (34) | 34 (34) | 21 (36.2) | 13 (31) | ||
| 56–60 | 30 (30) | 30 (30) | 21 (36.2) | 9 (21.4) | ||
| >60 | 36 (36) | 36 (36) | 16 (27.6) | 20 (47.6) | ||
| Gender | ||||||
| females | 10 (10) | 10 (10) | 4 (6.9) | 6 (14.3) | ||
| males | 90 (90) | 90 (90) | 54 (93.1) | 36 (85.7) | ||
| Smoking Habits (180 males) | ||||||
| Never smoker | 12 (13.3) | 3 (3.33) | 2 (3.7) | 1 (2.8) | ||
| Ever smoker | 78 (86.7) | 87 (96.7) | 52 (96.3) | 35 (97.2) | ||
| Ex-smoker | 17 (21.8) | 34 (39.1) | 22 (42.3) | 12 (34.3) | ||
| Current smoker | 61 (78.2) | 53 (60.9) | 30 (57.7) | 23 (65.7) | ||
| Pack years, Mean±SD | ||||||
| Ever smoker | 27.1±7.1 | 36.6±10.9 | <0.001 | 36.6±10.9 | 37.6±11.3 | 0.49 |
| Ex-smoker | 25.8±9.5 | 40.7±12.4 | <0.001 | 40.7±12.4 | 40.9±10.7 | 0.9 |
| Current smoker | 27.5±6.4 | 33.9±8.9 | <0.001 | 32.2±9.1 | 36.2±8.4 | 0.12 |
| Occupation | ||||||
| Heavy Work | 82 (82) | 84 (84) | 51 (87.9) | 33 (78.6) | ||
| Non Heavy | 18 (18) | 16 (16) | 7 (12.1) | 9 (21.4) | ||
| Charlson’s comorbidity score | ||||||
| Mean±SD | 1.33±1.03 | 1.48±1.2 | 0.37$ | 1.33±1.1 | 1.69±1.3 | 1.6$ |
| 0–1 | 56 (56) | 48 (48) | 31 (53.5) | 17 (40.5) | ||
| 2–4 | 44 (44) | 52 (52) | 27 (46.6) | 25 (59.5) | ||
| Standard of living index (SLI) | ||||||
| Mean±SD | 19.2±3 | 19.7±2.9 | 0.2 | 19.3±2.6 | 20.3±2.5 | 0.063 |
| >=20 | 45 (45) | 54 (54) | 27 (46.55) | 27 (64.3) | ||
| <20 | 55 (55) | 46 (46) | 31 (53.45) | 15 (35.7) | ||
| Body Mass Index (BMI) | ||||||
| Mean±SD | 21.8±2.2 | 20±3.2 | <0.001 | 20±3.2 | 20±3.3 | 0.94 |
| <18.5 (underweight) | 8 (8) | 35 (35) | 22 (37.95) | 13 (31) | ||
| 18.5–22.9 (normal) | 67 (67) | 47 (47) | 27 (46.55) | 20 (47.6) | ||
| 23–24.9 (overweight) | 20 (20) | 11 (11) | 5 (8.6) | 6 (14.3) | ||
| 25–30 (pre-obese) | 5 (5) | 7 (7) | 4 (6.9) | 3 (7.1) | ||
| Vitamin D levels ng/mL: (baseline) | ||||||
| Mean±SD | 20.2±5.1 | 16.3±5.4 | <0.001 | 17.8±4.9 | 14.4±5.5 | 0.002 |
| >20 | 48 (48) | 23 (23) | 16 (27.6) | 7 (16.7) | ||
| <=20 (deficiency) | 52 (52) | 77 (77) | 42 (72.4) | 35 (83.3) | ||
| Vitamin D levels ng/mL: (follow-up) | ||||||
| Mean±SD | 19.5±3.4 | 15.0±5.1 | <0.001 | 16.8±4.8 | 12.5±4.4 | <0.001 |
| >20 | 40 (40) | 21 (21) | 18 (31) | 3 (7.1) | ||
| <=20 (deficiency) | 60 (60) | 79 (79) | 40 (69) | 39 (92.9) | ||
| Six Minute Walk Test (% predicted) | ||||||
| Mean±SD | 80.5±4.1 | 55.5±1.8 | <0.001 | 59±15 | 50.74±10 | 0.003 |
| >= 80% | 53 (53) | 6 (6) | 6 (10.3) | 0 (0) | ||
| < 80 | 47 (47) | 94 (94) | 52 (89.7) | 42 (100) | ||
| Spirometry post bronchodilator Mean±SD | ||||||
| FVC % predicted | 90.5±6.0 | 62.6±17.8 | <0.001 | 66.3±18.5 | 57.5±15.6 | 0.014 |
| FEV1 % predicted | 94.4±3.6 | 49.2±16.6 | <0.001 | 53.8±16.2 | 42.9±15.1 | 0.001 |
| FEV1/FVC | 80.9±2.7 | 62.7±7.9 | <0.001 | 65.1±5.2 | 59.3±9.71 | 0.001 |
Non parametric
Table 2:
Grading of obstruction and symptoms among subjects with COPD.Frequencies shown as n (%).
| Variables | COPD (N=100) | Exacerbation during previous year | |
|---|---|---|---|
| No (n=58) | Yes (n=42) | ||
| GOLD Obstruction grading | |||
| Mild (>80) | 5 (5) | 4 (6.9) | 1 (2.4) |
| Moderate (50–80) | 40 (40) | 28 (48.3) | 12 (28.6) |
| Severe (30–50) | 40 (40) | 22 (37.9) | 18 (42.8) |
| Very severe (<30) | 15 (15) | 4 (6.9) | 11 (26.2) |
| Dyspnoea grading (MMRC) | |||
| 0 | 41 (41) | 39 (67.2) | 2 (4.8) |
| 1 | 42 (42) | 17 (29.3) | 25 (59.5) |
| 2 | 13 (13) | 2 (3.5) | 11 (26.2) |
| 3 | 4 (04) | 0 (0) | 4 (9.5) |
| CAT Score mean±SD (p 0.0000) | 17.9±5.6 | 15.7±5.5 | 21.0±4.1 |
| <=20 | 60 (60) | 44 (75.9) | 16 (38.1) |
| >20 | 40 (40) | 14 (24.1) | 26 (61.9) |
Among COPD subjects, a direct and positive correlation between Vitamin D levels was observed only for 6-minute walk test, but not for indicators of lung function (Figure 2). Among healthy controls, levels of vitamin D, indicators of lung function and the distance on 6-minute walk test were not significantly correlated.Lower Vitamin D levels among COPD subjects was significantly associated with MMRC dyspnea gradingand number of exacerbations last yearbut not with GOLD grading of airflow limitation and SLI (Figure 3). In the Interpolated contour plot (Figure 4), least number of exacerbations and the lower mMRC grades were observed in subjects with higher Vitamin D levels. All the subjects with 3 or more exacerbations had Vitamin D levels below 15ng/ml.
Figure 2:
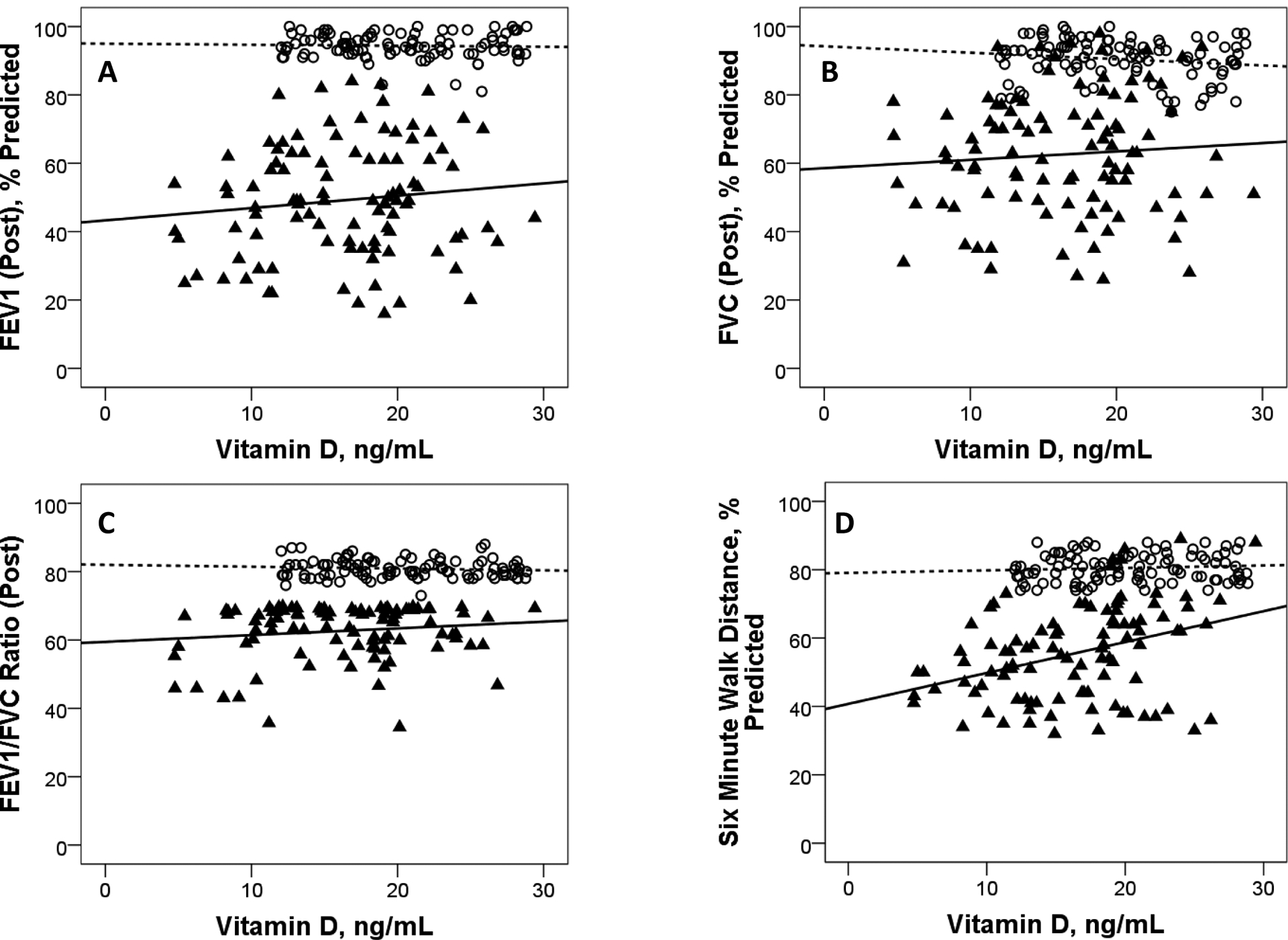
Scatter plots showing (A) Forced Expiratory Volume in 1 second % Predicted (FEV1 %), (B)Forced Vital Capacity % Predicted (FVC %), (C) FEV1/FVC Ratio and (D) six minute walk distance % Predicted (SMWD%), plottedagainst levels of vitamin D for Non COPDand COPD subjects.
(Non COPDsubjects are denoted by⃝ symbol and dotted trend line. COPD subjects are denoted by▴symboland solid trend line. PlotA: Non COPD r-0.04, p0.68, COPDr0.12, p0.24; PlotB: Non COPD r-0.16, p0.12, COPDr0.8, p0.46; PlotC: Non COPD r-0.1, p0.32, COPDr0.14 p0.18; PlotD: Non COPD r0.09, p0.38, COPD r0.36, p<0.001).
Figure 3:
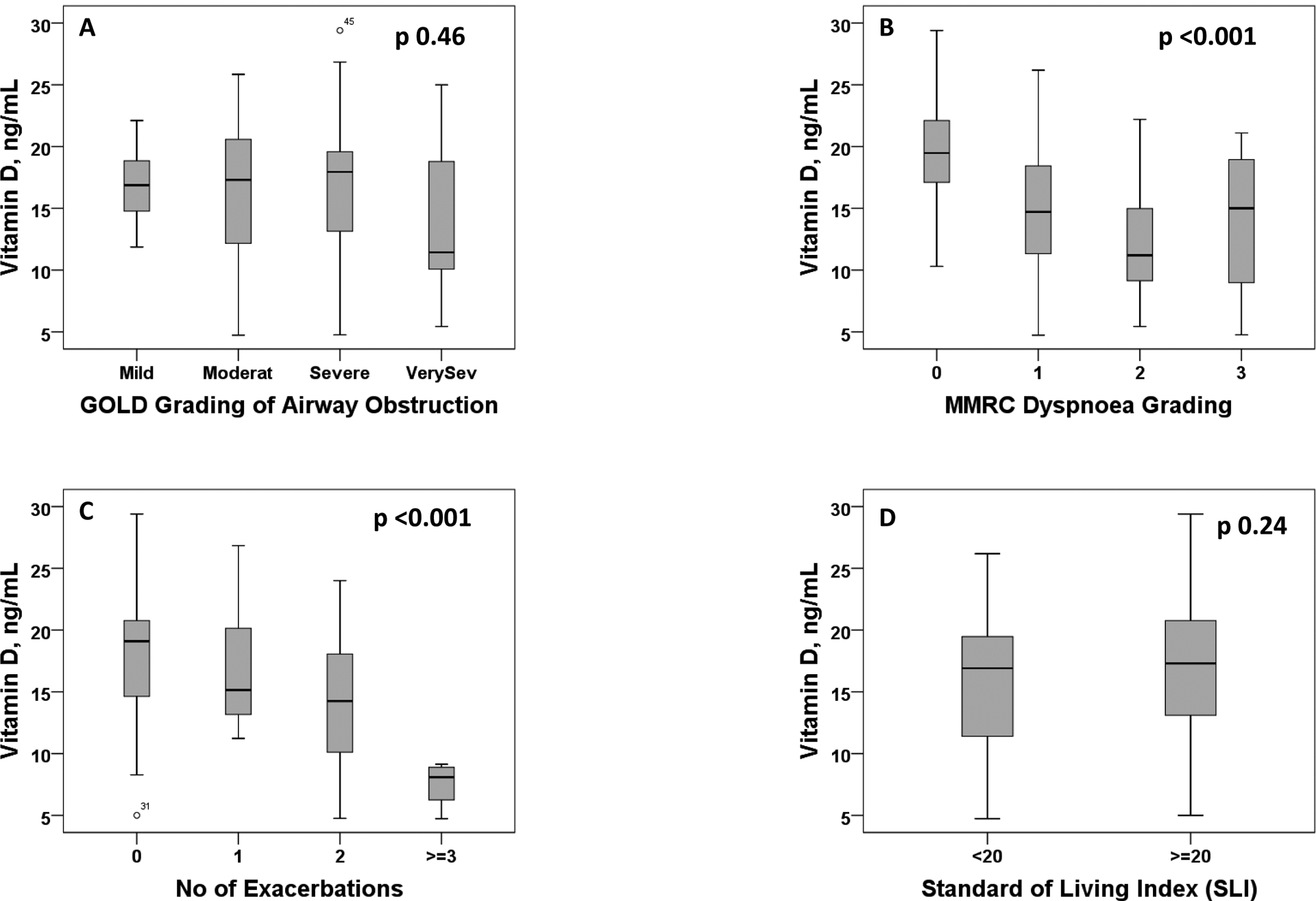
Box plot showing the levels of vitamin D among COPD subjects grouped according to (A) GOLD grading, (B) MMRC dyspnoea grading, (C) number of exacerbations and (D) Standard of living index (SLI)
Figure 4:
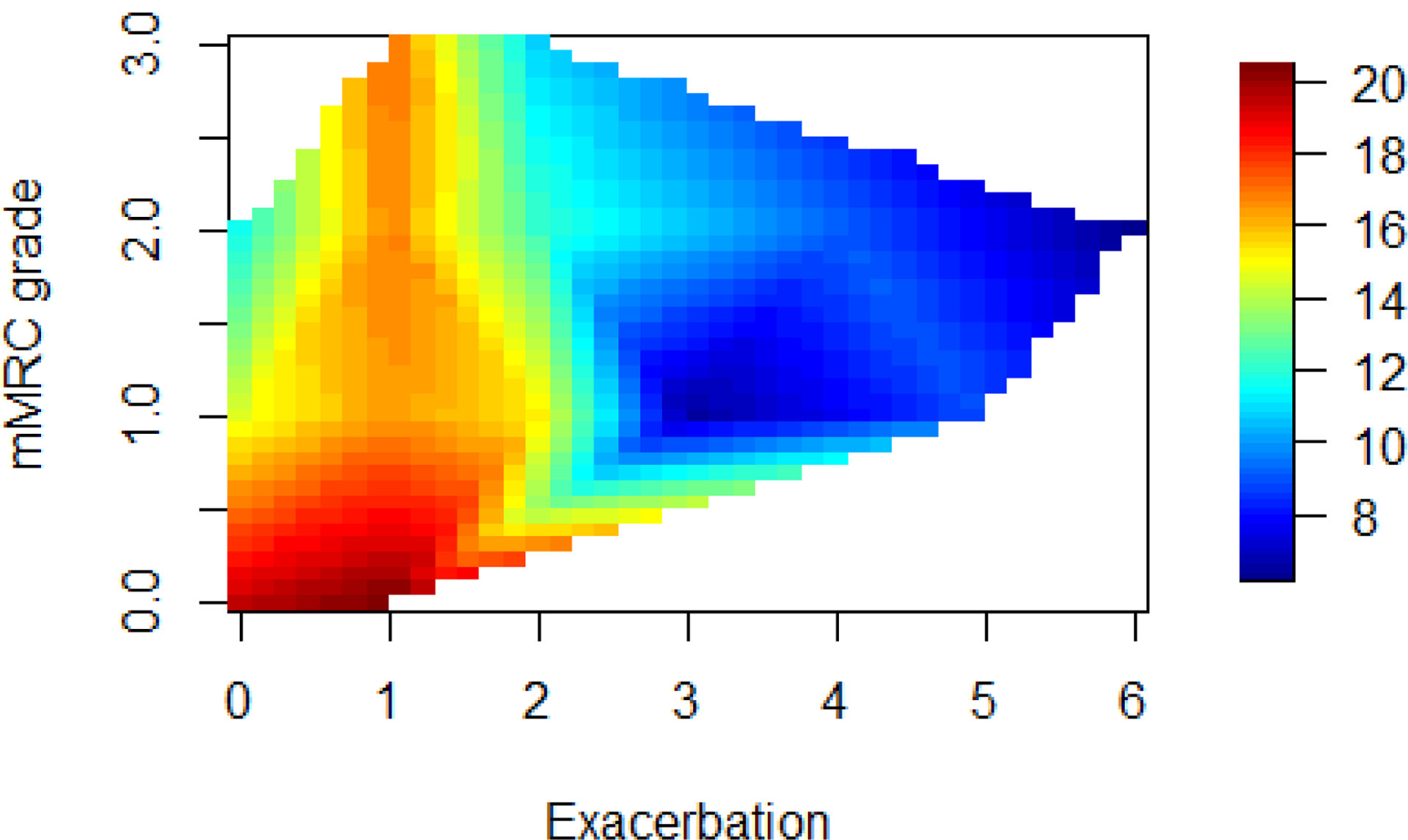
Contour Plot: The axes are those used in the allocation rule: Vitamin D levels, mMRC and COPD exacerbations. The colour in the right side bar in the graph indicates Vitamin D levels. All the subjects with 3 or more exacerbations had Vitamin D levels below 15ng/ml.
Conditional logistic regression to assess independent risk factors for COPD observed significant association withsmoking, lower Vitamin D levels and lower 6-minute walk distance. Unconditional logistic regression to assess independent risk factors for COPD exacerbations in the previous year observed lower Vitamin D levels were significantly associated with exacerbations (Table 3). In ROC analysis, Vitamin D cut-off level below 20.81ng/ml had the highest combined sensitivity and specificity for COPD, cut-off level below 18.45ng/ml had the highest combined sensitivity and specificity for COPD exacerbations.
Table 3:
Univariate and Logistic Regression analysis. Conditional logistic regression was done for COPD and Control groups, which was matched for age and gender. Unconditional Logistic Regression was done for risk of exacerbation in subjects with COPD.
| COPD (Control vs COPD) | Exacerbation of COPD in previous year (No vs Yes) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Conditional Logistic Regression | Univariate | Unconditional Logistic Regression | |||||
| Variable | OR (95% CI) | P-value | Adj OR (95% CI) | P-value | OR (95% CI) | P-value | Adj OR (95% CI) | P-value |
| Smoking Habits (180 males): | Ref Non-smokers | |||||||
| Ex-smokers | 8 (1.7–48.4) | 0.003# | 59.1 (2.8–1260) | 0.01 | 1.1 (0.05–70) | 0.999# | 8×10^9$ | 0.999 |
| Current smokers | 3.48 (0.86–20.1) | 0.058# | 16.5 (1.1–248) | 0.043 | 1.53 (0.07–94) | 0.999# | 18×10^8$ | 0.999 |
| Occupation: | Ref Non Heavy Work | |||||||
| Heavy Work | 1.15 (0.5–2.41) | 0.86 | 4.26 (0.53–34) | 0.17 | 1.98 (0.67–5.9) | 0.3 | 2.87 (0.73–11.22) | 0.131 |
| Charlson’s comorbidity index: | Ref Score 0–1 | |||||||
| Score 2–4 | 1.38 (0.79–2.41) | 0.32 | 3.8 (0.5–28) | 0.19 | 1.69 (0.75–3.78) | 0.21 | 1.49 (0.6–3.8) | 0.41 |
| Standard of living index (SLI) | Ref >=20 | |||||||
| <20 | 0.7 (0.39–1.22) | 0.26 | 0.5 (0.15–28) | 0.3 | 0.48 (0.21–1.1) | 0.08 | 0.51 (0.21–1.27) | 0.15 |
| Body Mass Index (BMI): | Ref Normal | |||||||
| Underweight | 6.23 (2.65–14.65) | <0.001 | 2.71 (0.65–11.2) | 0.17 | 0.8 (0.32–1.96) | 0.7 | 0.68 (0.23–1.95) | 0.47 |
| Overweight | 1.02 (0.5–2.09) | 0.95 | 0.39 (0.08–1.7)) | 0.22 | 1.35 (0.45–4.1) | 0.6 | 1.44 (0.43–4.82) | 0.56 |
| Vitamin D levels: | Ref >20 ng/ml | Ref >=19 ng/mL | ||||||
| <=20 ng/mL (deficiency) | 3.09 (1.68–5.68) | <0.001 | 5.05 (1.4–17.8) | 0.012 | 5.38 (2.06–14.1) | <0.001 | 3.51 (1.27–9.67) | 0.016 |
| Six Minute Walk Distance, % predicted | ref ≥80% | |||||||
| <80 | 17.67 (7.1–44.1) | <0.001 | 17.12 (3.8–76.8) | <0.001 | undefined | 0.039 | 350000 (0–10^12)$ | 0.97 |
Fisher exacts values.
the values are >100,000
Discussion
This study is unique as it evaluated Vitamin D levels in COPD subjects and healthy controls fromgeneral population, most of whom (83%) have been physically active with hard manual labor most of their life and exposed to sunlight for many hours in a day in a tropical country. It was surprising that even the healthy population from these rural areas who had spent most of their life exposed to sunlight working in the fields would have such low vitamin D levels, which was reconfirmed at six months follow-up(Table 1).The subjects from MUDHRA cohort are young compared to other populations. Possible explanation could be due to the fact that they have multiple risk factors for developing COPD; Tobacco Smoke, Biomass smoke exposure since childhood is coupled with occupational dust exposure (agriculture) and poor nutrition. They also regularly use pesticides in the fields.We observed a significant association of low Vitamin D levels with presence of COPD, severity of COPD, acute exacerbations of COPD, mMRC dyspnea scores and 6-minute walk distance, but not with lung function variables such as FEV1, FVC, FEV1/FVC ratios. Among healthy subjects, there was no correlation between Vitamin D levels and any of the lung function variables. The main associations with COPD observed in this study were smoking (positive), Vitamin D levels (negative) and for AECOPD was Vitamin D (negative).
Low Vitamin D levels were significantly associated with COPD in our study. Several studies have confirmed the association of COPD with low Vitamin D levels23. A meta-analysis comparing Vitamin D levels in 3224 COPD patients and 6699 controls, from 12 studies, most of which were from high income countries reported inverse and significant relationship between Vitamin D deficiency andprevalent COPD (77% higher)8. The NHANES-III observed an association between FEV1, FVC and lower Vitamin D levels3.For FEV1, there was a 126 ml difference between the highest and the lowest quintiles of Vitamin D23.
It is suspected that lower Vitamin D in African-Americans increase their susceptibility to COPD3. In India, Vitamin D deficiency is prevalent and may be related to the dark skin color and poor nutrition23.Patients with COPD are at very high risk of being Vitamin D deficient due to a variety of reasons; aging skin is less effective in producing Vitamin D, poor nutrition and outdoor activities, increased catabolism of Vitamin D by steroids and lower storage capacity3. Indirect evidence explains association between Vitamin D and COPD. The normal functioning of both innate and adaptive immunity are dependent on Vitamin D such as maturation of dendritic cells, negative regulation of pro-inflammatory cytokines and chemokines, maturation and development of T cells, especially Th1 cells. Vitamin D is also linked to apoptosis and intercellular adhesion8. Increased expression of anti-microbial peptides such as cathelicidin and beta-defensins are important functions of Vitamin D to maintain homeostasis24,25. Immune cells express both Vitamin D receptor (VDR) and hydroxylase enzymeand can potentially reduce the pathogenic load of micro-organisms25. Vitamin D in airway epithelium helps to kill pathogens via a TLR and CD14 dependent mechanisms26. Low Vitamin D levels can increase susceptibility to recurrent infections, higher airway microbial colonisation, and lead to an accelerated decline in lung functions8,26. An increased airway smooth muscle and structural alterations in the lungs including airway remodeling due to increased MMP’s, increased collagen deposition have been reported in Vitamin D deficiency8. Vitamin D receptors(VDR) are observed to be low in COPD lungs27. VDR is an important nuclear hormone receptor and animal studies that have knocked out VDR have observed lung changes similar to COPD; increased inflammation, up-regulation of various MMP’s, early onset emphysema and decline in lung functions27. Polymorphisms in the VDR has been shown to affect susceptibility to COPD acute exacerbations25. Clinical trials with Vitamin D supplementation have shown to reduce the risk of moderate and severe acute COPD exacerbations25.
Our study observed lower 6-minute walking distance among COPD subjects with Vitamin D deficiency. This could be related to the effect of Vitamin D on muscles; proximal myopathy leading to muscle weakness, gradual atrophy and loss of muscle mass has been reported in Vitamin D deficiency28. Studies in VDR knockout mice have confirmed the role of Vitamin D in maintaining normal muscle fiber size29. This observation has been seen in human clinical studies as well and is probably related to the effect of Vitamin D on myostatin, which negatively regulates muscle mass28. VDR polymorphisms BsmI(rs1544410) and FokI(rs2228570), are associated with muscle strength in COPD subjects. Higher Vitamin D levels are associated with a higher number of type II muscle fibers, muscle function and physical performance, especially in the elderly23. Higher exercise capacity and carbon-moxide transfer capacity were associated with higher Vitamin D levels25. An improvement in muscle mass and lower limb function was seen following Vitamin D supplementation28,30,31, but more studies are needed for a conclusive evidence23.
However, most of the evidence linking Vitamin D and COPD are mostly cross-sectional association studies from high income countries7–9. A vexing and an important question for the clinician as well as an epidemiologist is to be able to predict which smoker develops COPD. Can smokers with low Vitamin D levels be at risk for COPD? Can it be used as a biomarker for predicting the development of COPD? There are no prospective community based long-term studies in young healthy smokers to evaluate whether low Vitamin D is a risk factor for developing COPD.
We observed that low Vitamin D levels were significantly and directly correlated with acute exacerbations of COPD with a dose-response gradient (Figure 3,c). Studies evaluating baseline Vitamin D levels and risk of AECOPD have reported mixed results. A large (n=973) study of mostly white subjects, 40% of whom were Vitamin D deficient and 33% had Vitamin D insufficiency did not find an association between Vitamin D levels, COPD exacerbation rates and time to first exacerbation over one year32. A non-significant trend was observed in NHANES6 and Norwegian24 studies. A meta-analysis found that subjects with AECOPD had lower levels of Vitamin D than stable COPD, but wasnot statistically significant8.
Another important question is whether a COPD subject receiving Vitamin D supplementation is protected from exacerbations? A randomized placebo-controlled trial observed no benefit of using high dose Vitamin D (1,00,000IU every 4 weeks for 1 year) to prevent COPD acute exacerbations, except in sub-group of subjects with very severe Vitamin D deficiency (<10ng/ml). Nearly two thirds of the study subjects had Vitamin D deficiency (<20ng/ml), though there was a significant improvement in serum Vitamin D levels after supplementation, there were no differences in the number of AE’s, time to first and second AE’s, hospital admissions and death between the group that received Vitamin D and the placebo groups33. The multicenter ViDiCO trial that used 1,20,000 IU every 2 months observed that there was a significant reduction in moderate to severe AE COPD only in subjects who had Vitamin D deficiency but not in those without34. A meta-analysis that included 5 studies found Vitamin Dsupplementation was beneficial7. Only one of the four studies did not show any benefit, but it had a smaller sample and had used lower doses of Vitamin D for shorter duration (60,000 IU/month) of6 weeks as compared to other studies that observed a benefit, which used 100,000 to 2,40,000 IU per month for 3–12 months. The benefits ranged from increase in inspiratory muscle strength, improved maximal oxygen uptake, reduced AE COPD and improved FEV17.
We observed significantly lower Vitamin D levels for very severe COPD as compared to other sub-groups of COPD severity (Figure 3,a). Lower levels of Vitamin D may be associated with higher COPD severity classified according to GOLD guidelines. We should also consider the possibility of reverse causality since those with severe COPD may not be physically active outdoors. A meta-analysis observed that subjects with severe and very severe COPD had lower Vitamin D levels than subjects with mild and moderate COPD8. Low Vitamin D levels can worsen COPD severity by increasing the risk for frequent respiratory infections, poor immune response to various pathogens and increasing proliferation of airway smooth muscles7. A meta-analysis of 18 studies7 observed a significantly lower levels of Vitamin D in moderate COPD compared to mild COPD (pooled RR 0.72; 95% CI:0.63–0.82) and lower in severe COPD as compared to moderate COPD (pooled RR 0.74; 95% CI: 0.56–0.98). COPD subjects had greater than two times (OR 2.32) risk of being Vitamin D deficient after adjusting for other confounding factors23. In a cohort from Norway, subjects with severe Vitamin D deficiency had a larger decline in FEV1 compared to those without severe Vitamin D deficiency. The ECLIPSE study observed correlations between Vitamin D levels and FEV1, 6-minute walk distance, bronchodilator response and emphysema score on CT scan23.
Our study had several strengths. This was a community-based study in the MUDHRA cohort that has been in follow-up for ten years. Vitamin D levels were assessed twice in six months to confirm that the Vitamin D levels remained stable over time and were reproducible. The main limitation of the study is that we have not assessed the reasons for low Vitamin D levels among people who are highly physically active and exposed to sunlight for many hours in a day for most of their lives. We speculate that poor nutrition mostly due to poverty could be a reason, but this needs to be confirmed in future studies. The other limitations include the possibilities of information bias as the exposures have not been objectively measured but obtained mostly by recall. Most of the COPD subjects were men and due to the low number of women in the study, the study findings may not be generalizable to women, and we were unable to conduct difference between sexes.
In conclusion, we observed a strong relationship of low Vitamin D levels with higher rates ofCOPD, COPD severity and acute exacerbations of COPD in this rural population exposed to sunlight throughout their lives and involved in hard labour. There is a need to perform longitudinal studies to test whether young healthy subjects exposed to risk factors such as smoking or biomass fuel exposure with low Vitamin D levels would indeed have a higher risk of developing COPD and whether Vitamin D supplementation in these subjects would prevent or at least delay the development of COPD. Only then, the issue of whether low Vitamin D levels are causative or just an association (due to poor physical activity and poor nutrition which is common in subjects with COPD) can be conclusively resolved.
Acknowledgements:
We acknowledge and thank the people of the MUDHRA Cohort, study subjects and GramaPanchayat members for their kind cooperation. We thank Mr. Sathish Chandran M, Mr. Raju M (field staff and spirometry technicians), Ms. Shobha (lab technician) and Mr. Poorvachar (senior lab technician), for their kind assistance and cooperation. We thank Dr. S Ravi, Professor, Department of Statistics, University of Mysore, for their valuable inputs. We also thank Dr. H Basavanagowdappa, The Principal, JSS Medical College, and Dr. M D Ravi, The Director, JSS Hospital, Mysuru for their support.
Funding:
This work was supported by funding from National Institutes of Health(NIH), Fogarty International Center(FIC),Global Health Equity Scholars(GHES)Grant R25 TW009338&D43 TW010540. The funding agency had no say in design of study, conduct of study, data analysis and writing the manuscript.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to declare for this study.
Ethics Statement:
The approval was granted by Institutional Ethical Committee (IEC) of JSS Medical College, Mysuru, India (letter number JSS/MC/IEC/2519/2013-14, 31 July, 2014) and Institutional Review Board (IRB) of Florida International University (FIU), USA (IRB Protocol Approval #: IRB-14-0299, 16th October 2014).
Data Availability Statement
The study data are not freely available, but the MUDHRA cohort team would welcome collaborations with other researchers. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- 1.Global Initiative for Chronic Obstructive Lung Disease 2020 Report. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report).; 2020. https://goldcopd.org/gold-reports/
- 2.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D Beyond Bones in Chronic Obstructive Pulmonary Disease: Time to Act. Am J Respir Crit Care Med. 2009;179(8):630–636. doi: 10.1164/rccm.200810-1576PP [DOI] [PubMed] [Google Scholar]
- 4.Zasloff M Fighting infections with vitamin D. Nat Med. 2006;12(4):388–390. doi: 10.1038/nm0406-388 [DOI] [PubMed] [Google Scholar]
- 5.Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158(1):20–25. doi: 10.1111/j.1365-2249.2009.04001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginde AA, Mansbach JM, Camargo CA. Association Between Serum 25-Hydroxyvitamin D Level and Upper Respiratory Tract Infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384. doi: 10.1001/archinternmed.2008.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Z, Zhu B, Zhu B, Xiao C. Vitamin D deficiency is associated with the severity of COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. Published online September 2015:1907. doi: 10.2147/COPD.S89763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu M, Wang T, Wang C, Ji Y. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016; Volume 11:2597–2607. doi: 10.2147/COPD.S101382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang LL, Gong J, Liu CT. Vitamin D with asthma and COPD: not a false hope? A systematic review and meta-analysis. Genet Mol Res. 2014;13(3):7607–7616. doi: 10.4238/2014.February.13.10 [DOI] [PubMed] [Google Scholar]
- 10.Mahesh PA, Lokesh KS, Madhivanan P, Chaya SK, Jayaraj BS, Ganguly K, Krishna M. The Mysuru stUdies of Determinants of Health in Rural Adults (MUDHRA), India. Epidemiol Health. 2018;40:e2018027. doi: 10.4178/epih.e2018027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AMB, Crapo RO, Jensen RL, Burney PGJ. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2(2):277–283. [PubMed] [Google Scholar]
- 12.Jithoo A, Enright PL, Burney P, Buist AS, Bateman ED, Tan WC, Studnicka M, Mejza F, Gillespie S, Vollmer WM. Case-finding options for COPD: results from the Burden of Obstructive Lung Disease Study. Eur Respir J. 2013;41(3):548–555. doi: 10.1183/09031936.00132011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 14.International Institute for, Population Sciences (IIPS) and ORC Macro. National Family Health Survey (NFHS-2), 1998–99: India. Mumbai.; 2000. [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 17.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. doi: 10.1016/s0895-4356(02)00585-1 [DOI] [PubMed] [Google Scholar]
- 18.Mahesh PA, Jayaraj BS, Chaya SK, Lokesh KS, McKay AJ, Prabhakar AK, Pape UJ. Variation in the prevalence of chronic bronchitis among smokers: a cross-sectional study. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2014;18(7):862–869. doi: 10.5588/ijtld.13.0048 [DOI] [PubMed] [Google Scholar]
- 19.Mahesh PA, Jayaraj BS, Prabhakar AK, Chaya SK, Vijaysimha R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J Med Res. 2013;137(1):87–94. [PMC free article] [PubMed] [Google Scholar]
- 20.OSHA. Estimating Work Rates or Loads. Accessed December 26, 2019. https://www.osha.gov/SLTC/heatillness/heat_index/work_rates_loads.html [Google Scholar]
- 21.Global Initiative for Chronic Obstructive Lung Disease 2014 Report. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2014 Report).; 2014. https://goldcopd.org/gold-reports/ [Google Scholar]
- 22.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 23.Kokturk N, Baha A, Oh Y-M, Young Ju J, Jones PW. Vitamin D deficiency: What does it mean for chronic obstructive pulmonary disease (COPD)? a compherensive review for pulmonologists. Clin Respir J. 2018;12(2):382–397. doi: 10.1111/crj.12588 [DOI] [PubMed] [Google Scholar]
- 24.Persson LJP, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TML. Chronic Obstructive Pulmonary Disease Is Associated with Low Levels of Vitamin D. Hartl D, ed. PLoS ONE. 2012;7(6):e38934. doi: 10.1371/journal.pone.0038934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari R, Caram LMO, Tanni SE, Godoy I, Rupp de Paiva SA. The relationship between Vitamin D status and exacerbation in COPD patients– a literature review. Respir Med. 2018;139:34–38. doi: 10.1016/j.rmed.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 26.Sundar IK, Rahman I. Vitamin D and Susceptibility of Chronic Lung Diseases: Role of Epigenetics. Front Pharmacol. 2011;2. doi: 10.3389/fphar.2011.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundar IK, Hwang J-W, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011;406(1):127–133. doi: 10.1016/j.bbrc.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunton JE, Girgis CM. Vitamin D and muscle. Bone Rep. 2018;8:163–167. doi: 10.1016/j.bonr.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Villalta SA, Agrawal DK. FOXO1 Mediates Vitamin D Deficiency-Induced Insulin Resistance in Skeletal Muscle. J Bone Miner Res Off J Am Soc Bone Miner Res. 2016;31(3):585–595. doi: 10.1002/jbmr.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo MET, Mets T, Seal C, Wijers SL, Ceda GP, De Vito G, Donders G, Drey M, Greig C, Holmbäck U, Narici M, McPhee J, Poggiogalle E, Power D, Scafoglieri A, Schultz R, Sieber CC, Cederholm T. Effects of a Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement on Measures of Sarcopenia in Older Adults, the PROVIDE Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Med Dir Assoc. 2015;16(9):740–747. doi: 10.1016/j.jamda.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 31.Zhu K, Austin N, Devine A, Bruce D, Prince RL. A Randomized Controlled Trial of the Effects of Vitamin D on Muscle Strength and Mobility in Older Women with Vitamin D Insufficiency: EFFECTS OF VITAMIN D ON MUSCLE STRENGTH AND MOBILITY. J Am Geriatr Soc. 2010;58(11):2063–2068. doi: 10.1111/j.1532-5415.2010.03142.x [DOI] [PubMed] [Google Scholar]
- 32.Kunisaki KM, Niewoehner DE, Connett JE. Vitamin D Levels and Risk of Acute Exacerbations of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study. Am J Respir Crit Care Med. 2012;185(3):286–290. doi: 10.1164/rccm.201109-1644OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, Decallonne B, Bouillon R, Decramer M, Janssens W. High Doses of Vitamin D to Reduce Exacerbations in Chronic Obstructive Pulmonary Disease: A Randomized Trial. Ann Intern Med. 2012;156(2):105. doi: 10.7326/0003-4819-156-2-201201170-00004 [DOI] [PubMed] [Google Scholar]
- 34.Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, Islam K, McLaughlin D, Bhowmik A, Timms PM, Rajakulasingam RK, Rowe M, Venton TR, Choudhury AB, Simcock DE, Wilks M, Degun A, Sadique Z, Monteiro WR, Corrigan CJ, Hawrylowicz CM, Griffiths CJ. Vitamin D 3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120–130. doi: 10.1016/S2213-2600(14)70255-3 [DOI] [PubMed] [Google Scholar]


