Abstract
Up to 90% of postural tachycardia syndrome (PoTS) patients report headaches, and comorbid migraine headaches are common. Given this, pathophysiological interaction is possible, which may reveal key aspects of disease expression and treatment opportunities. We hypothesized that PoTS subjects – both with and without migraine – would show features of central sensitization, including allodynia and photophobia.
Eighty participants were evaluated – 30 PoTS, 30 chronic migraine (CM), and 20 non-headache healthy controls (NH) – using tilt table testing, psychophysical assessment of sensory sensitivity thresholds, and an online questionnaire to assess measures of headache burden and associated symptoms. Clinical characteristics and sensory thresholds were compared between disease groups and controls, as well as subgroup analysis within the PoTS group, based on headache phenotype.
Sensory sensitivity thresholds were significantly lower, and symptoms scores were higher in both PoTS and CM groups compared to controls. However, the patterns of expression differed between PoTS and CM, with pain threshold reductions in the forearm only of PoTS subjects (non-trigeminal sensory sensitization), compared to both periorbital and forearm sites in CM. Unexpectedly, light sensitivity thresholds were significantly lower in PoTS compared to both CM and NH.
These findings reveal an under-appreciated aspect of disease burden in PoTS, and suggests network sensitization similar to, but separable from that of migraine. The presence of both photophobia and allodynia in PoTS is reflective of exteroceptive, rather than strictly interoceptive disruption, and expands our fundamental understanding of the disorder.
Keywords: Dysautonomia, Photophobia, Allodynia, Orthostatic Headache, Light sensitivity
Introduction
Migraine and postural tachycardia syndrome (PoTS) frequently present together in practice [1–3]. Up to 90% of PoTS patients endorse headaches, with both orthostatic and non-orthostatic manifestations [2, 4]. Migrainous headache is the most widely recognized primary headache in PoTS, though rates vary (28–96%) [1–4]. Even at the lower end of this range, rates of migraine among PoTS patients clearly exceed the estimated prevalence in the population (18% women, 7% men in the U.S.) [5].
While migraine is not classically considered an autonomic disorder, autonomic and sensory dysfunction are core features of the migraine attack [6]. Hypersensitivity to benign touch and environmental light, termed allodynia and photophobia respectively, are well-documented manifestations of altered sensory function in migraine and can also be present outside the headache (ictal) phase [7]. Allodynia and photophobia appear to scale with migraine severity and may represent risk factors for headache chronification [8–11]. As such, both have been proposed as clinical markers of central sensitization [12, 13], and cited as justification for early initiation of headache treatment [14, 15]. While prior work has demonstrated small fiber nerve abnormalities on intraepidermal nerve fiber density in a subset of neuropathic POTS patients [16], to our knowledge, no prior publications directly examine quantitative sensory thresholds in PoTS patients. The objective of this study was to prospectively characterize headache subtypes and examine sensory function using psychophysical assessments in PoTS, compared to migraine and healthy, non-headache controls.
Methods:
Participants
Eighty subjects (11 male, 69 female) aged 15–72 years were recruited into PoTS, chronic migraine (CM), and non-headache (NH) control groups. CM was selected as a well-defined, clinically severe headache comparison group, known to have prominent autonomic symptomatology and reduced sensory thresholds [7, 11, 17, 18]. CM and NH subjects were recruited June 2014 - January 2018; PoTS subjects were recruited January 2015 - October 2019. Recruitment occurred via clinic screening, as well as written advertisement, and included participants from the local community and University of Utah Neurology clinics. Institutional Review Board approval and informed consent were obtained. Subjects were excluded if they had comorbid medical, ocular, or other neurological disorders known to directly affect autonomic function, sensation, or light sensitivity (e.g. prior eye injury, idiopathic blepharospasm, optic nerve disorder, or sensory or pan-autonomic neuropathy otherwise explained by another disorder) [11].
PoTS diagnosis was based on current consensus criteria [19, 20]. Screening for secondary causes of orthostasis and/or headaches was performed outside of the context of the research protocol, during the course of usual clinical care; clinical and laboratory-based assessment for PoTS, including a 10-minute head-up tilt (HUT) test, was conducted by a board-certified neurologist with specialty certification in autonomic disorders [MMC]. PoTS group exclusions: orthostasis/headaches secondary to iron deficiency, endocrine disorders, hyperventilation, panic disorder, cardiac rhythm abnormalities, volume depletion, drug abuse, medication side effect, Chiari malformation, or suspected cerebrospinal fluid leak. A total of 151 patients referred for orthostatic intolerance were screened for purposes of this study; of these, 40 met inclusion criteria, including a complete clinical evaluation for potential secondary causes of orthostasis (exclusions), and consented to participate in research. Recruitment was inclusive of PoTS subjects with phenotypic characteristics consistent with previously described neuropathic and hyperadrenergic subtypes[21], and without regard for the presence/absence of a past-medical history of headaches.
Headache diagnosis was ascertained via standardized questionnaire [11, 22] (Structured Migraine Interview; SMI) developed to capture International Classification of Headache Disorders III (ICHD-III) criteria [23]. PoTS subjects were further subdivided into PoTS with (PoTS-Mig) and without migraine (PoTS-NonMig) for subgroup analysis. Control subjects reported no history of recurrent or disabling headaches, or other neurological disorder, and were otherwise healthy. All subjects were instructed to avoid alcohol, caffeine and nicotine the day of the study; had not used opiate medication or headache-specific abortive medications within 48 hours of testing; and did not take medications that could affect autonomic function, including psychotropics, antihistamines, and benzodiazepines. Finally, PoTS and CM participants were tested after being migraine attack-free for at least 48 hours, though testing during non-acute, chronic daily headaches was permitted. Subjects had not used opiate medication or migraine-specific abortive medications during the 48 hours prior to testing. Control subjects were studied in their usual state of health.
Questionnaires
Headache assessment: SMI, Migraine Disability (MIDAS) [24], Headache Impact (HIT-6) [25]. Sensory symptoms: Short-Form Photophobia Questionnaire (SF-PhotoQ) [26], Allodynia Symptom Checklist (ASC-12) [27, 28]. Systemic autonomic symptoms: COMPASS-31[29]. Headache-associated craniofacial autonomic symptoms (CAS) based on ICHD-III definition [30], reported number of 8 possible: conjunctival injection/lacrimation, nasal congestion/rhinorrhea, eyelid swelling, forehead/facial sweating, forehead/facial flushing, pupil changes, ptosis, ear fullness.
Sensory testing
Photophobia is a well-recognized component of migraine attacks, and many definitions use the term to refer specifically to triggering and/or exacerbation of headache-specific pain by light and/or glare. Importantly, discomfort to light can also be measured outside of the headache attack (ictal) phase [11, 31]. For the purposes of this report, we used the term to refer to discomfort or pain induced by light (light sensitivity) outside of the acute headache attack (interictal) phase, and do not specifically limit this definition to the context of the ocular or head pain itself. Psychophysical methods have been previously used to quantify light sensitivity in an array of light-sensitive disorders[32], and were performed in this study using our published methods [11]. In brief, subjects were seated facing two white-light 500W halogen lamps (SL-1002; Bayco, Wylie, TX). Following a 3-minute dark-adaption, light intensity was increased in a stepwise fashion from 0.1 lux to photophobia threshold, or a maximum of 16,450 Lux, using a rheostat calibrated by a luxmeter (LX1010B Luxmeter). During testing, subjects were asked to look at a fixed point between the two light sources and to say, “stop” when the intensity of light became “uncomfortable” (defined as the level of stimulus that made the subject want to blink or turn away from the light source, and confirmed by visualization of grimace). Testing was repeated three times with a three-minute dark adaptation between each trial; light intensity of the final position on the rheostat was considered the photophobia threshold for that trial and was reported in log(lux).
Psychophysical assessment of mechanical touch-induced pain thresholds was tested using published methods [9, 33]. In brief, calibrated von Frey Hair (VFH) Filaments (Stoelting Co., Wood Dale, Ill., USA) were applied in ascending order over the periorbital and forearm regions. The smallest VFH number capable of inducing pain in 2/3 trials was considered the threshold. Pain thresholds were expressed in VFH units from 11 to 20, representing the respective ascending forces of each numbered filament (4, 6, 8, 10, 15, 26, 60, 100, 180, and 300 grams respectively). Because a linear relationship exists between the log force and ranked number, VFH thresholds are expressed and analyzed as VFH numbers, rather than their forces [9, 33, 34].
Orthostatic testing
Orthostatic testing was completed after a minimum of 15 minutes quiet supine rest, following sensory testing detailed above. Tilt table testing was performed using a modified autonomic testing protocol [35]; blood pressure (BP) and heart rate (HR) were measured via a continuous beat-to-beat BP device (BMEYE Nexfin; Amsterdam, The Netherlands) and 3-lead ECG, respectively. Baseline recordings were obtained for 5 minutes. The subject was then passively tilted to a 70° angle for a period of 5 minutes, followed by a return to supine for 5 minutes. The postural HR increment was calculated by subtracting the average supine HR from the maximal sustained upright HR. Recordings were acquired in TestWorks™ software (WR Medical Electronics Co., Stillwater, MN), exported, and analyzed offline.
Analysis and Statistical methods
Mechanical sensitivity thresholds were compared across groups [9, 33]; allodynia was defined as a pain threshold one standard deviation (SD) below the control group’s mean thresholds (SD 1.3 VFH, rounded to a conservative −2 VFH ≤ the NH mean of 19 VFH) [34]. Thus, individual patients were identified as allodynic when their mean threshold was ≤ 17 VFH (≤ 100 grams of pressure). Light sensitivity thresholds were compared across groups as a continuous variable. Photophobia was defined as a perceived painful response to light (during the inter-ictal phase for headache subjects) as described above [11]; given a larger range of pain thresholds in the normal control group, we used a cutoff of light sensitivity thresholds at or below 1.5 SD of the control group’s mean (SD 0.7 logLux below the control group mean of 2.8 logLux).
Group size power calculations were based on previously published data examining light [11] and mechanical sensitivity [9] in CM and controls; we determined that a sample size of 23 and 12 respectively would generate 80% power to detect a significant difference between groups (alpha 0.05); we selected a conservative n=30 for disease groups and n=20 for controls. Data distributions were visually inspected and tested for normality using the Shapiro-Wilk test. HR increment on HUT and HIT-6 were normally distributed, whereas the remaining clinical parameters were not. Normally distributed data were analyzed using one-way ANOVA and post-hoc two-sided independent t-tests. Nonparametric data utilized the Kruskal-Wallis rank sum test for across-group comparisons, with post-hoc pair-wise two-sided Wilcoxon rank sum testing. Results were considered significant for p-values < .05, except where multiple comparisons occurred, in which case Bonferroni correction was applied. Statistical analyses were performed in R for Windows (Version 3.5.1; R Core Team, Vienna, Austria.)
Results
Clinical characteristics
See Table 1 for summary. There were no significant differences in age or sex across groups. As expected based on diagnostic criteria, HR increment was significantly higher in PoTS compared to NH and CM. Twenty percent of CM patients met HR criteria for PoTS, though they did not otherwise meet clinical (symptomatic) criteria for the syndrome [19–21].
Table 1.
Clinical Characteristics
| Measure | NH controls | CM | PoTS-ALL | p-value | ||
|---|---|---|---|---|---|---|
| CM vs NH | PoTS vs NH | CM vs PoTS | ||||
| N | 20 | 30 | 30 | |||
| Sex (F/M, %F) | 17/3, 85% | 25/5, 80% | 27/3, 90% | .87 Δ | .59 Δ | .45 Δ |
| Age, years (median, range) | 28.5; 17–57 | 30.5; 15–72 | 26.0; 15–59 | .21 ¥ | .79 ¥ | .09 ¥ |
| Heart rate increment, head-up tilt table, bpm (mean; SD) | 16; 11 | 21; 12 | 39; 10 | .50 ¥ | <.00001 ¥ | .0001 ¥ |
| COMPASS-31, total score (median; IQR) | 4; 14 | 34; 21 | 52; 14 | <.00001 ¥ | <.00001 ¥ | .0005 ¥ |
| Domain scores: | ||||||
| Pupillomotor | 0; 1 | 3; 2 | 3; 2 | * | * | |
| Gastrointestinal | 1; 2 | 7; 6 | 10; 7 | * | * | |
| Orthostatic | 0; 3 | 20; 12 | 28; 8 | * | * | * |
| Bladder | 0; 0 | 0; 1 | 1; 2 | * | ||
| Vasomotor | 0; 0 | 2; 6 | 6; 4 | * | ||
| Secretomotor | 0; 0 | 0; 2 | 2; 3 | * | ||
bpm, beats per minute; CM, chronic migraine; F, female; IQR, inter-quartile range; M, male; NH, non-headache; PoTS, postural tachycardia syndrome; SD, standard deviation.
chi-square statistic
two-tailed Mann-Whitney U test
significant following correction for multiple comparisons across domains (Bonferroni threshold p<.007)
Headache characteristics summarized in Table 2. Of the 30 PoTS subjects, only 3 (15%) denied recurrent, problematic headaches; 9 (30%) had ICHD-3 defined CM, 8 (27%) episodic migraine (EM), 7 (23%) recurrent headache not meeting criteria for definite migraine, and 3 (10%) persistent post-traumatic headache (2 with CM clinical phenotype, 1 with EM). Headache days/month were higher in CM (p=.03). HIT-6 and MIDAS scores were significantly different across CM and PoTS subgroups (p=.0002 and .05 respectively), with the highest scores in PoTS-Mig and CM.
Table 2.
Headache Related Measures
| Questionnaire | CM | PoTS-ALL | CM vs POTS-ALL p-value | PoTS-Mig | PoTS-NonMig | CM vs PoTS-Mig vs PoTS-NonMig p-value |
|---|---|---|---|---|---|---|
| N | 30 | 30 | 20 | 10 | ||
| Age of headache onset, years (median; IQR) | 15.5; 10 | 16.0; 13 | 0.42 ¥ | 14.5; 13 | 23.0; 9 | 0.66 † |
| Headache days per month (median; IQR) | 20; 15 | 14; 16 | 0.03 ¥ | 18; 17 | 10; 10 | 0.05† |
| Headache with activity, triggering or worsening (%) | 46% | 82% | 0.005 Δ | 93% | 44% | 0.01 ǂ |
| Orthostatic headache, headache induced by tilt table testing (%) | 17% | 48% | 0.02 Δ | 44% | 57% | 0.16 ǂ |
| HIT-6 Score, headache impact (mean; SD) | 63; 6 | 62; 12 | 0.23 ¥ | 63; 6 | 51; 12 | 0.0002† |
| MIDAS Score, headache related disability (median; IQR) | 41; 44 | 26; 42 | 0.13 ¥ | 47; 42 | 11; 22 | 0.05† |
| SF-PhotoQ, headache-associated photophobia symptoms (median; IQR) | 4; 1 | 4; 1 | 0.96 ¥ | 4; 1 | 3; 2 | 0.23† |
| ASC-12, headache-associated allodynia symptoms (median; IQR) | 5; 8 | 3.5; 8 | 0.90 ¥ | 7; 6 | 0; 3 | 0.04† |
| Headache associated craniofacial autonomic symptoms, # out of 8 (median; IQR) | 2; 3 | 1; 2 | 0.30 ¥ | 2; 3 | 0; 1 | 0.06† |
CM, chronic migraine; IQR, inter-quartile range; Mig, migraine; NonMig, non-migraine; PoTS, postural tachycardia syndrome; SD, standard deviation.
Wilcoxon rank sum test (PoTS-All vs. CM comparison)
chi-square statistic (PoTS-All vs. CM comparison)
Kruskal-Wallis rank sum test (PoTS-Mig vs. PoTS-NonMig vs. CM comparison)
chi-square statistic (PoTS-Mig vs. PoTS-NonMig. vs. CM comparison)
Triggering/exacerbation of headache by activity and orthostatic headache were significantly more frequent in PoTS-ALL compared to CM (p=.005 and .02 respectively), with 82% of PoTS-ALL subjects reporting triggering/exacerbation of headaches with activity, compared to 46% of CM subjects, and 48% of PoTS-ALL reporting orthostatic headache (headache triggering by HUT testing) compared to 17% of CM subjects.
Autonomic and Sensory Symptoms
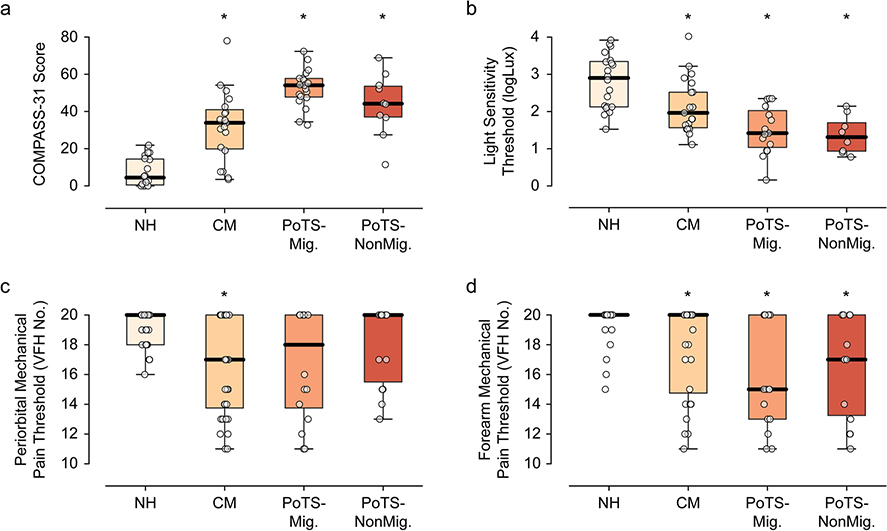
COMPASS-31 scores in PoTS and controls were similar to published scores [36]; COMPASS-31 scores were significantly higher in both PoTS and CM compared to controls (pair-wise testing p<.0001 for both; Fig. 1a), as well as PoTS-ALL compared to CM (p=.0005; Table 1). Symptom domain scores were significantly higher in CM compared to NH in pupillomotor, gastrointestinal, and orthostatic domains; and in POTS compared to NH for all six domains. Finally, the only domain where CM and POTS significantly differed was that of orthostatic symptoms. One or more headache-associated CAS was present in 67% of PoTS and 70% of CM. Of PoTS subjects with 1 ≥ CAS, most had migraine, though 3 with non-migraine headaches also reported CAS. The number (out of a total of 8 possible) of headache-associated CAS was greatest in the CM and PoTS-Mig groups (p=.06; Table 2).
Fig. 1.
Autonomic and sensory function for chronic migraine (CM), PoTS with and without migraine headache, and non-headache (NH) controls. a) COMPASS-31 assesses systemic autonomic symptoms, which are elevated in CM, and highest in the PoTS groups. b) Light sensitivity thresholds are decreased across all groups compared to controls (not shown), and are the lowest in the PoTS-Mig group. c - d) Mechanical sensory thresholds are lowest in the periorbital region of CM and PoTS-Mig subjects, whereas forearm thresholds are lowest in the PoTS-Mig and PoTS-NonMig group.
Headache-associated photophobia (SF-PhotoQ) did not differ between CM and PoTS-ALL (p=.23; Table 2), though allodynia scores (ASC-12) were significantly different across groups (p=.04; Table 2), with the highest scores in the PoTS-Mig and CM groups.
Sensory Thresholds
Median light sensitivity thresholds were significantly lower in both PoTS-ALL and CM compared to NH (p=.009 and <.0001 respectively; Fig. 1b). Interestingly, light sensitivity thresholds were significantly lower in both PoTS-Mig and POTS-NonMig compared to CM (p=.007). PoTS subjects overall (PoTS-ALL) were significantly more likely than CM to have light sensitivity: 65% PoTS, 38% CM, 5% NH (as defined by −1.5 SD below the NH mean threshold, see Methods).
Median mechanical pain thresholds were significantly lower in PoTS-ALL subjects at the forearm compared to controls (p=.0003), whereas in CM, thresholds were significantly reduced at both forearm and periorbital sites (p=.004 and <.0001 respectively; Fig. 1c–d). PoTS subjects were just as likely as CM to have allodynia, as defined by VFH testing at either location: 53% of PoTS and 57% CM, compared to 10% in controls. Overall, PoTS subjects exhibited forearm sensitivity (47% of the PoTS group vs. 30% CM and 5% NH) more frequently than periorbital sensitivity (41% PoTS, 30% CM, and 5% NH).
Discussion
Migraine headache is highly comorbid in PoTS [1–4]; likewise, autonomic symptoms may be underappreciated in chronic migraine [37]. We hypothesize that overlapping, and perhaps synergistic, pathophysiology impacts the clinical expression of both disorders and reveals underappreciated treatment opportunities [38]. By assessing clinical, physiologic, and psychophysical measures of autonomic and sensory function in PoTS patients, and comparing these to CM and NH control groups, our chief finding was of lower light and mechanical pain thresholds (forearm < periorbital region) in PoTS subjects both with and without migraine compared to CM and controls (Fig. 1; Table 3). Further, we find evidence of significant interictal autonomic symptom burden in CM, which corresponds with lingering autonomic symptomatology outside of the migraine attack itself.
Table 3.
Quantitative Sensory Threshold Testing Results
| Testing Modality | NH | CM | PoTS-Mig | PoTS-NonMig | p-value |
|---|---|---|---|---|---|
| Light sensitivity threshold, logLux (median; IQR) | 2.90; 1.22 | 1.96; 0.95 | 1.42; 0.99 | 1.31; 0.76 | 0.007† |
| Mechanical pain thresholds, periorbital, VFH Number (median; IQR) |
20; 2 | 17; 6 | 18; 6 | 20; 5 | 0.37† |
| Mechanical pain thresholds, forearm, VFH Number (median; IQR) |
20; 0 | 20; 5 | 15; 7 | 17; 7 | 0.23† |
CM, chronic migraine; IQR, inter-quartile range; Mig, migraine; NH, non-headache; NonMig, non-migraine; PoTS, postural tachycardia syndrome; SD, standard deviation; VFH, von Frey hair.
Kruskal-Wallis rank sum test (CM vs. PoTS-Mig vs. PoTS-NonMig comparison)
Patterns of sensory sensitivity differ in PoTS compared to CM
We observed differing sensory profiles in PoTS (Mig and NonMig), compared to CM, with an overall pattern of non-trigeminal sensory sensitization (Fig. 1b–d). While cranial-predominant allodynia is expected in CM [8, 9] (Fig. 1c–D), we found significantly reduced forearm thresholds in PoTS subjects – a non-trigeminal site – as well as prominent light sensitivity in both PoTS subgroups (Fig. 1b). The latter finding supports the possibility that light sensitivity is not only independent of co-morbid migraine, but perhaps also that it reflects a more protean aversive response than traditional cephalocentric definitions of photophobia might predict [39, 40]. Taken together, the presence of non-trigeminal predominant allodynia and photophobia in PoTS subjects supports the role of a generalized pattern of sensitization in the disorder that differs phenotypically from that which is seen in migraine.
Overlapping headache and autonomic symptom characteristics in PoTS and CM groups
Eighty-five percent of our PoTS group reported recurrent, problematic headaches, with 57% meeting ICHD-III criteria for definite migraine (episodic or chronic). In our sample, headache phenotype was broadly distributed: CM (30%), EM (27%), and recurrent headache not meeting definite migraine or tension type headache criteria (23%). Only 3 PoTS subjects denied both recurrent, problematic headaches and orthostatic headache. Despite the fact that only 11 of 20 PoTS-Mig subjects had CM type headaches (the remaining 9 had EM), the CM and PoTS-Mig groups in our study had similar headache frequency and severity (HA-days/month, HIT-6 and MIDAS; Table 2). This highlights the significance of the headache burden in PoTS patients and reveals a key treatable aspect of daily symptom expression [2].
There are no prior reports of headache-associated CAS in PoTS. Published estimates of CAS in migraine ranges from 27–73% [41]. Migraine-associated CAS are thought to represent activation of the trigeminal parasympathetic reflex pathways, and are associated with more severe headache pain [42], as well as allodynia and photophobia [43]. Thus, CAS in migraine, like allodynia and photophobia, are currently viewed as markers of central sensitization [43]. Not surprisingly, we found the highest number of CAS in PoTS-Mig and CM groups, whereas CAS were only reported in 3 of 10 PoTS-NonMig subjects (all of which, on post-hoc review, met ICHD-III criteria for probable migraine) (Table 2). Thus, CAS in a PoTS patient might be viewed as an indication to screen for migraine, rather than attributable to PoTS-related autonomic symptomatology.
Pathophysiological implications of exteroceptive sensitization in PoTS
Taking into account the current findings, it is plausible that the hypothesized hyperadrenergic state and beta-receptor super-sensitivity of PoTS [44] could functionally amplify expressions of central sensitization in these patients, whether or not they have comorbid migraine. Also relevant to this discussion are emerging data that implicate pupillary dysfunction [11, 45], hyperexcitability of the visual cortex [46], and multi-sensory interactions [47] in migraine-associated photophobia – all of which could also be conceivably influenced by altered sympathetic tone. Given that signs and symptoms of pupillary dysfunction are commonly noted in the PoTS clinic [36], and the prominence of photophobia and multi-sensory hypersensitivity seen in our study, we propose that investigations of pupillary function, cortical hyperexcitability, and multi-sensory interactions serve as candidate avenues for future study of PoTS-associated sensory dysfunction, with the potential to physiologically differentiate it from migrainous phenotypes.
Migraine headache itself is a paroxysmal, multi-sensory pain disorder involving autonomic disruption, with focal presentation in craniofacial regions, and broader peripheral and central sensitization as the disease progresses [7, 48]. Beyond migraine, impaired sensory gain has also been implicated in non-migraine headache [49], as well as peripheral and visceral pain disorders [50, 51]. Interestingly, our data show a mixed pattern of altered sensory thresholds – with reduced light sensitivity and mechanical sensitivity thresholds in the forearm (> periorbital) region in PoTS subjects, regardless of clinical headache phenotype (Fig. 1c–d). While we did not assess visceral function/pain in our study, there is a well-described clinical overlap in functional abdominal/bladder syndromes in both PoTS and migraine populations [52–55]. Our findings of non-trigeminally mediated sensitization in PoTS are supportive of prior reports implicating central visceral sensitization[56]. An appealing hypothesis is that increased central sensory and autonomic network activity, in the setting of a relative hyperadrenergic state in PoTS, may favor development and/or chronification of other pain disorders (including headache), with evidence for central sensitization, as discussed above. In this view, in addition to migraine, PoTS too might be considered within the spectrum of multisensory gain disorders, each presenting with phenotypically distinct manifestations of sensory and autonomic gain dysfunction.
Implications for Diagnosis and Treatment
Heyer et. al. reported that orthostatic headache was reasonably sensitive and specific for the diagnosis of PoTS in patients presenting to a specialty Headache Clinic with orthostatic intolerance [3]. However, clinically, orthostatic headache is not easily differentiated from activity-based triggering of comorbid migraine. Moreover, orthostatically-triggered headaches in PoTS may also exhibit migraine-like features [2]. In our study, we noted prominent headache triggering/exacerbation by activity in PoTS compared to CM (82% vs 46%; p=.005); similarly, PoTS subjects were more likely to report orthostatic HA than CM (48% vs 17%; p=.02). These results fit with current PoTS diagnostic criteria requiring worsening of symptoms while upright [19, 20], and are similar to previously reported rates of orthostatic headache in PoTS patients [2]. Of note, current ICHD criteria for migraine also stipulate “aggravation [of headache] by or causing avoidance of routine physical activity”[30]. As one of several optional diagnostic characteristics for migraine, “headache exacerbation by activity” represents a key area of clinical and diagnostic overlap with headache in PoTS patients. While our data suggests that “triggering/worsening of headache with activity” may be more common in PoTS than CM, this feature cannot be clearly differentiated from “headache exacerbation by routine activity,” as included in the ICHD migraine criteria. Thus, this aspect of symptom expression requires special attention during the history taking process. Future studies, ideally those utilizing a broad, representative sample, are needed to guide development of more precise definitions and diagnostic criteria for orthostatic headache and activity-triggering of migraine-type headaches, to allow for better clinical detection and distinction.
Likewise, directed evaluation for orthostatic tachycardia and autonomic symptoms are warranted in the headache clinic. CM subjects had significantly higher COMPASS-31 scores than controls, and 20% had a HR increment ≥ 30 during HUT (Table 1). It is likely that orthostatic and generalized autonomic symptoms are often overlooked – yet symptomatically relevant – aspects of disease burden in the headache clinic. In this context, the use of low-dose, non-selective beta blockers, commonly used for migraine prophylaxis, is of particular relevance therapeutically. This is also justifiable from a pathophysiological standpoint, where animal studies suggest that propranolol may impact central sensitization [57].
Limitations and future directions
While consistent with prior reports of migraine frequency in PoTS in tertiary centers (28–61%; [1–4]), our catchment likely differs from an epidemiological sample, based on our recruitment through University Neurology clinics and associated referral patterns. As a result, we are unable to meaningfully address potential differences in sensory sensitivity between clinical subgroups of PoTS (e.g. hyperadrenergic vs neuropathic), which could shed further light on the role of noradrenergic dysfunction on sensory amplification in this disorder. Despite this, we believe that our study group effectively illustrates biologically and physiologically relevant aspects of disease expression, and potentially clinically actionable commonalities and differences between PoTS and CM groups. Furthermore, our sample is likely mirroring the patient mix commonly encountered by both headache and autonomic specialists. An additional limitation of our study was the absence of comprehensive medication use histories or diaries, and thus an inability to identify CM and PoTS subjects with chronic analgesic over-use. There is an emerging body of literature to support the role of medication – in particular opioid– overuse in pain chronification and central sensitization [58–62]. This is particularly relevant if one is to attempt differentiation of migraine vs PoTS-associated sensitization patterns, and thus merits future studies designed to directly ascertain this factor.
Conclusions
We found evidence of non-trigeminal predominate sensory sensitization in PoTS subjects both with and without migraine, as well as increased upright HR and autonomic symptom scores in CM subjects. These overlapping features suggest common pathway activation in these often-comorbid conditions. However, we also observed distinguishable features in each disorder that likely reflect divergent network responses. Lower light sensitivity thresholds in PoTS without migraine are surprising, and suggest the possibility that photophobia – as a dysfunctional neuroplastic process – is a more “global” sensory-autonomic response than previously appreciated in the headache literature. Meanwhile, the divergent craniofacial/forearm allodynia thresholds between PoTS and CM show more anatomic specificity. The overall impression is of significant, and underappreciated, commonality between the sensitization wrought by these disorders, underlining the need to address both for maximum therapeutic response. Finally, the presence of both photophobia and allodynia in PoTS is reflective of exteroceptive, rather than strictly interoceptive disruption, and expands our fundamental understanding of the disorder [44]. In this context, both PoTS and migraine headache might be viewed as “sickness syndromes” with overlapping and heterogenous clinical presentations stemming from interactions between the nociceptive and autonomic systems [7]. Expanded investigation through this lens may better inform the complex pathophysiology of both disorders [11, 46, 47] and help guide new treatment approaches [38].
Acknowledgements
Jeremy Theriot for figure preparation.
Funding
NIH NIMHD LRP, American Autonomic Society Research Training Fellowship, and the American Academy of Neurology Clinical Research Scholarship (MMC); Fairclough Endowment for Headache Research (LM); NIH NINDS R01 NS 102978, 104742 (KCB).
Footnotes
Disclosures
MMC reports consulting activities unrelated to this paper or topic of research from Eli Lilly. LM has nothing to disclose. KCB reports consulting activities unrelated to this paper or topic of research from Eli Lilly and Allergan.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Ojha A, Chelimsky TC, and Chelimsky G, Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr, 2011. 158(1): p. 20–3. [DOI] [PubMed] [Google Scholar]
- 2.Khurana RK and Eisenberg L, Orthostatic and non-orthostatic headache in postural tachycardia syndrome. Cephalalgia, 2011. 31(4): p. 409–15. [DOI] [PubMed] [Google Scholar]
- 3.Heyer GL, Fedak EM, and LeGros AL, Symptoms predictive of postural tachycardia syndrome (POTS) in the adolescent headache patient. Headache, 2013. 53(6): p. 947–53. [DOI] [PubMed] [Google Scholar]
- 4.Deb A, et al. , A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. Proc (Bayl Univ Med Cent), 2015. 28(2): p. 157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipton RB, et al. , Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache, 2001. 41(7): p. 646–57. [DOI] [PubMed] [Google Scholar]
- 6.Mosek A, et al. , Autonomic dysfunction in migraineurs. Headache, 1999. 39(2): p. 108–17. [DOI] [PubMed] [Google Scholar]
- 7.Brennan KC and Pietrobon D, A Systems Neuroscience Approach to Migraine. Neuron, 2018. 97(5): p. 1004–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigal ME, et al. , Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology, 2008. 70(17): p. 1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke L, Eliasziw M, and Becker WJ, Cutaneous allodynia in transformed migraine patients. Headache, 2007. 47(4): p. 531–9. [DOI] [PubMed] [Google Scholar]
- 10.Louter MA, et al. , Cutaneous allodynia as a predictor of migraine chronification. Brain, 2013. 136(Pt 11): p. 3489–96. [DOI] [PubMed] [Google Scholar]
- 11.Cortez MM, et al. , Altered pupillary light response scales with disease severity in migrainous photophobia. Cephalalgia, 2017. 37(8): p. 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovati C, et al. , Central sensitization in photophobic and non-photophobic migraineurs: possible role of retino nuclear way in the central sensitization process. Neurol Sci, 2013. 34 Suppl 1: p. S133–5. [DOI] [PubMed] [Google Scholar]
- 13.Noseda R and Burstein R, Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain, 2013. 154 Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landy S, Hoagland R, and Hoagland NA, Sumatriptan-naproxen migraine efficacy in allodynic patients: early intervention. Headache, 2012. 52(1): p. 133–9. [DOI] [PubMed] [Google Scholar]
- 15.Mathew NT, Pathophysiology of chronic migraine and mode of action of preventive medications. Headache, 2011. 51 Suppl 2: p. 84–92. [DOI] [PubMed] [Google Scholar]
- 16.Haensch CA, et al. , Small-fiber neuropathy with cardiac denervation in postural tachycardia syndrome. Muscle Nerve, 2014. 50(6): p. 956–61. [DOI] [PubMed] [Google Scholar]
- 17.Mathew PG, Cutrer FM, and Garza I, A touchy subject: an assessment of cutaneous allodynia in a chronic migraine population. J Pain Res, 2016. 9: p. 101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipton RB, et al. , Improving the detection of chronic migraine: Development and validation of Identify Chronic Migraine (ID-CM). Cephalalgia, 2016. 36(3): p. 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheldon RS, et al. , 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm, 2015. 12(6): p. e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman R, et al. , Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci, 2011. 161(1–2): p. 46–8. [DOI] [PubMed] [Google Scholar]
- 21.Arnold AC, Ng J, and Raj SR, Postural tachycardia syndrome - Diagnosis, physiology, and prognosis. Auton Neurosci, 2018. 215: p. 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samaan Z, et al. , Diagnosing migraine in research and clinical settings: the validation of the Structured Migraine Interview (SMI). BMC Neurol, 2010. 10: p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia, 2013. 33(9): p. 629–808. [DOI] [PubMed] [Google Scholar]
- 24.Stewart WF, et al. , Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology, 2001. 56(6 Suppl 1): p. S20–8. [DOI] [PubMed] [Google Scholar]
- 25.Rendas-Baum R, et al. , Validation of the Headache Impact Test (HIT-6) in patients with chronic migraine. Health Qual Life Outcomes, 2014. 12: p. 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JY, et al. , Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia, 2009. 29(9): p. 953–9. [DOI] [PubMed] [Google Scholar]
- 27.Lipton RB, et al. , Cutaneous allodynia in the migraine population. Ann Neurol, 2008. 63(2): p. 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakubowski M, et al. , Can allodynic migraine patients be identified interictally using a questionnaire? Neurology, 2005. 65(9): p. 1419–22. [DOI] [PubMed] [Google Scholar]
- 29.Sletten D, et al. , COMPASS 31 – A Refined and Abbreviated Composite Autonomic Symptom Score. Neurology, 2013. 80(Meeting Abstracts 1): p. P03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woehrle E, et al. , Concussion in Adolescents Impairs Heart Rate Response to Brief Handgrip Exercise. Clin J Sport Med, 2018. [DOI] [PubMed] [Google Scholar]
- 31.Perenboom MJL, et al. , Quantifying Visual Allodynia Across Migraine Subtypes: The Leiden Visual Sensitivity Scale. Pain, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams WH, et al. , The evaluation of light sensitivity in benign essential blepharospasm. Am J Ophthalmol, 2006. 142(1): p. 82–87. [DOI] [PubMed] [Google Scholar]
- 33.Burstein R, et al. , An association between migraine and cutaneous allodynia. Ann Neurol, 2000. 47(5): p. 614–24. [PubMed] [Google Scholar]
- 34.Burstein R, Cutrer MF, and Yarnitsky D, The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain, 2000. 123 ( Pt 8): p. 1703–9. [DOI] [PubMed] [Google Scholar]
- 35.Low PA, Testing the autonomic nervous system. Semin Neurol, 2003. 23(4): p. 407–21. [DOI] [PubMed] [Google Scholar]
- 36.Rea NA, Campbell CL, and Cortez MM, Quantitative assessment of autonomic symptom burden in Postural tachycardia syndrome (POTS). J Neurol Sci, 2017. 377: p. 35–41. [DOI] [PubMed] [Google Scholar]
- 37.Alstadhaug KB, Migraine and the hypothalamus. Cephalalgia, 2009. 29(8): p. 809–17. [DOI] [PubMed] [Google Scholar]
- 38.Cortelli P and Pierangeli G, Chronic pain-autonomic interactions. Neurol Sci, 2003. 24 Suppl 2: p. S68–70. [DOI] [PubMed] [Google Scholar]
- 39.Digre KB and Brennan KC, Shedding light on photophobia. J Neuroophthalmol, 2012. 32(1): p. 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn AH and Brennan KC, Unanswered questions in headache: so what is photophobia, anyway? Headache, 2013. 53(10): p. 1673–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortez MM, et al. , Craniofacial Autonomic Dysfunction in Migraine: Implications for Treatment and Prognosis. J Neuroophthalmol, 2020. 40(1): p. 67–73. [DOI] [PubMed] [Google Scholar]
- 42.Obermann M, et al. , Prevalence of trigeminal autonomic symptoms in migraine: a population-based study. Cephalalgia, 2007. 27(6): p. 504–9. [DOI] [PubMed] [Google Scholar]
- 43.Barbanti P, et al. , The phenotype of migraine with unilateral cranial autonomic symptoms documents increased peripheral and central trigeminal sensitization. A case series of 757 patients. Cephalalgia, 2016. 36(14): p. 1334–1340. [DOI] [PubMed] [Google Scholar]
- 44.Benarroch EE, Postural Tachycardia Syndrome: A Heterogeneous and Multifactorial Disorder. Mayo Clin Proc, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cambron M, et al. , Autonomic function in migraine patients: ictal and interictal pupillometry. Headache, 2014. 54(4): p. 655–62. [DOI] [PubMed] [Google Scholar]
- 46.Martín H, et al. , Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache, 2011. 51(10): p. 1520–8. [DOI] [PubMed] [Google Scholar]
- 47.Brighina F, et al. , Visual cortex hyperexcitability in migraine in response to sound-induced flash illusions. Neurology, 2015. 84(20): p. 2057–61. [DOI] [PubMed] [Google Scholar]
- 48.Demarquay G and Mauguiere F, Central Nervous System Underpinnings of Sensory Hypersensitivity in Migraine: Insights from Neuroimaging and Electrophysiological Studies. Headache, 2016. 56(9): p. 1418–1438. [DOI] [PubMed] [Google Scholar]
- 49.Bendtsen L, Central sensitization in tension-type headache--possible pathophysiological mechanisms. Cephalalgia, 2000. 20(5): p. 486–508. [DOI] [PubMed] [Google Scholar]
- 50.Woolf CJ, Central sensitization: implications for the diagnosis and treatment of pain. Pain, 2011. 152(3 Suppl): p. S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latremoliere A and Woolf CJ, Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain, 2009. 10(9): p. 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaufman MR, et al. , Overactive bladder and autonomic dysfunction: Lower urinary tract symptoms in females with postural tachycardia syndrome. Neurourol Urodyn, 2017. 36(3): p. 610–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramos ML, et al. , Comorbidity between idiopathic overactive bladder and chronic migraine. Cephalalgia, 2018. 38(3): p. 581–584. [DOI] [PubMed] [Google Scholar]
- 54.Georgescu D, et al. , Migraine in young females with irritable bowel syndrome: still a challenge. Neuropsychiatr Dis Treat, 2018. 14: p. 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Hemert S, et al. , Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front Neurol, 2014. 5: p. 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khurana RK, Visceral sensitization in postural tachycardia syndrome. Clin Auton Res, 2014. 24(2): p. 71–6. [DOI] [PubMed] [Google Scholar]
- 57.Boyer N, et al. , Propranolol treatment prevents chronic central sensitization induced by repeated dural stimulation. Pain, 2017. 158(10): p. 2025–2034. [DOI] [PubMed] [Google Scholar]
- 58.Ayzenberg I, et al. , Central sensitization of the trigeminal and somatic nociceptive systems in medication overuse headache mainly involves cerebral supraspinal structures. Cephalalgia, 2006. 26(9): p. 1106–14. [DOI] [PubMed] [Google Scholar]
- 59.Maccora S, et al. , Multisensorial Perception in Chronic Migraine and the Role of Medication Overuse. J Pain, 2020. [DOI] [PubMed] [Google Scholar]
- 60.Sandkuhler J and Gruber-Schoffnegger D, Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol, 2012. 12(1): p. 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuner R and Flor H, Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci, 2016. 18(1): p. 20–30. [DOI] [PubMed] [Google Scholar]
- 62.Kuner R, Central mechanisms of pathological pain. Nat Med, 2010. 16(11): p. 1258–66. [DOI] [PubMed] [Google Scholar]