Summary Statement:
Near-infrared spectroscopy monitoring provides a practical method to follow trends in superficial cerebral cortex oxygenation during and after cardiovascular surgery. Determination of the limits of cerebral pressure-flow autoregulation is now possible using processed oximetry signals in relation to arterial pressure.
Clinicians have sought methods to monitor tissue oxygenation for over a century.1 These efforts culminated in the development of the ear oximeter in the 1940s and then pulse oximetry in the 1970s. The observation that tissue including bone is transparent to light in the near-infrared spectrum (700–1300 nm) led to the introduction of cerebral oximetry.2,3 A growing understanding of the importance of neurological complications for patient outcomes after cardiac surgery has provided a strong impetus for implementing cerebral oximetry into clinical practice albeit adoption in the US remains low.4–7 Other applications continue to evolve including monitoring during non-cardiac surgery such as shoulder surgery in the beach chair position or during one-lung ventilation for thoracic surgery.8,9 The purpose of this clinically focused review is to summarize the applications of cerebral oximetry monitoring for adult patients undergoing cardiovascular surgery.
Principles of NIRS Monitoring
In the US there are seven FDA approved cerebral oximetry monitoring systems including Invos™ 5100C and 7100 (Medtronic/Covidien, Boulder, CO); ForeSight Elite™ (Casmed/Edwards Lifesciences, Irvine, CA); Equanox™ 3- and 4-wavelength versions (Nonin Medical, Plymouth, MN); O3 Regional Oximetry™ (Massimo, Irvine, CA), and NIRO-200 NX™ system (Hamamatsu Photonics, Hamamatsu City, Japan). All approved monitors use similar technology that is susceptible to artifacts and limitations as previously reviewed (figure 1).2
Figure.
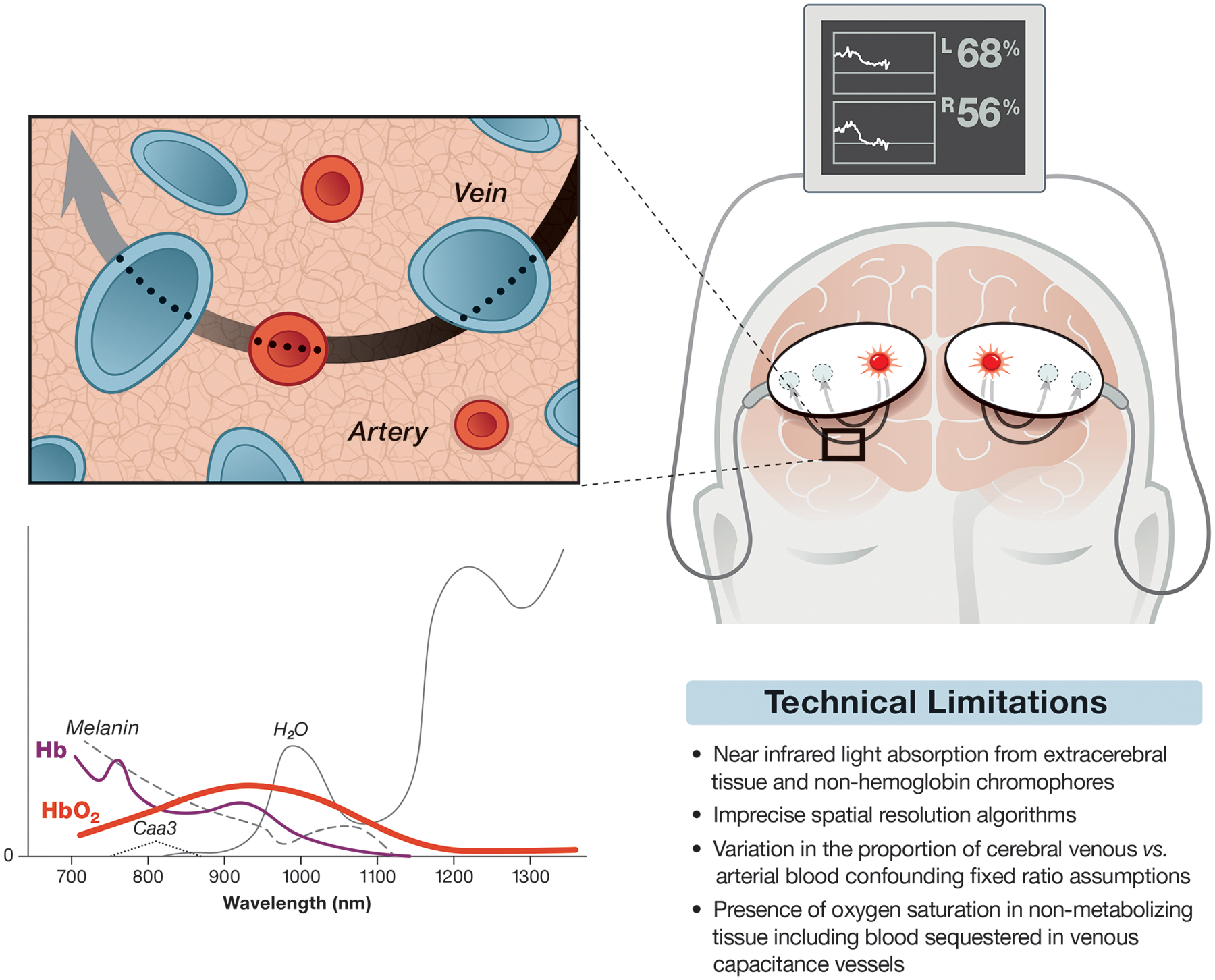
Schematic representation of cerebral oximetry monitoring depicting adhesive light emitter/sensors placed on a hypothetical patient’s forehead. The sensors are connected via cables to a monitor providing regional cerebral oxygen saturation for the right and left superficial frontal lobes. The path of light from the light source to the sensors is elliptical as suggested by computer modeling. The inset is meant to represent the microcirculation with a proposed 70:30 ratio of veins and arteries. Also shown is a sketch of near infrared light spectra versus hypothetical absorption (y-axis). Deoxyhemoglobin (Hb) and oxyhemoglobin (HbO2) are highlighted as thin and thick colored lines, respectively, while absorption spectrum for other light absorbing substances are shown including cytochrome aa2 (Caa3). Commercial cerebral oximeters focus on those wavelengths below the peak absorption of H2O but still in the range of light absorption from melanin. Note the 810 nm isobestic wavelength where peak absorption of Hb and HbO2 occurs. The absorption of near infrared light at this wavelength is used for measuring total hemoglobin for calculating relative cerebral oxygen saturation as well as for estimating cerebral blood volume for monitoring cerebral vasoreactivity (see text). A table summarizing some limitations of cerebral oximetry is further shown.
Near-infrared spectroscopy measurement of tissue oxygen saturation is based on a modification of the Beer-Lambert law whereby the difference in the intensity of transmitted and received light is a function of the concentration of a light absorbing substance in a solution. Oxy- and deoxy-hemoglobin have distinct peak absorption wavelengths in the near-infrared range. An isobestic wavelength at 810 nm provides a measurement of total hemoglobin concentration. Tissue saturation can be measured, thus, as the derived ratio of oxyhemoglobin to total hemoglobin concentrations. Other substances absorb near infrared light including water, melanin, bilirubin, and cytochrome C each with distinct but overlapping absorption spectrum. Commercial cerebral oximetry monitors mostly restrict light to 700 to 850 nm wavelengths to focus on hemoglobin species.2
Cerebral oximetry monitoring is performed by attaching adhesive pads to the forehead that contain a near-infrared light source and sensors. Emitted photons must traverse various tissues including skin, muscle, bone, dura, and cerebral spinal fluid to reach the cerebral cortex. Although there is some scatter of photons at each tissue interface their general path has been modeled to be elliptical with the depth of their penetration a function of the distance between the light source and sensors by a factor of roughly 1/3.2 A transmitter-sensor distance of 4 cm results in light penetration of tissue of ~1.3 cm. The use of two or more sensors at different distances from the light source allows for spatial resolution of reflected photons. For example, sensors placed 3 cm and 4 cm from the light source provide an estimate of light absorbed by superficial and deeper tissue, respectively. Subtraction algorithms evaluate the difference between deeper and superficial photon absorption for measurement oxygen saturation.
It is estimated that 85% of cerebral oximetry measurements are derived from the superficial cerebrum and 15% from extra-cerebral tissue including the scalp.2,10,11 This confounding effect of scalp light absorption might explain decrements in cerebral oxygen saturation observed during general anesthesia after the IV administration of the α-adrenergic agonist phenylephrine but not after administration of the indirect acting α- and β-adrenergic agonist ephedrine.12 An opposing argument is that an imbalance of cerebral oxygen supply versus demand occurs after α-adrenergic stimulation of receptors located on contractile pericytes in the microcirculation such that cerebral oximetry measurements are accurately reduced.13 The latter possibility was examined in a study of patients with brain tumors undergoing positron emission tomography where either a continuous IV infusion of phenylephrine or ephedrine were randomly administered.14 Cerebral metabolic rate for O2, cerebral blood flow, and cerebral oxygen saturation were not decreased by phenylephrine. Ephedrine, however, increased cerebral blood flow and cerebral oxygen saturation compared with phenylephrine. Thus, cerebral oximetry decrements after phenylephrine are not due to compromised cerebral oxygenation; its effect on cerebral oxygen saturation measurements are inconsistent.
Cerebral oximetry has been validated in humans after intra-arterial injection of the near-infrared absorbing dye idocyanine green, with PET scanning, and by comparison with jugular mixed venous blood.15–17 The measurements do not require pulsatile flow as with pulse oximetry. Rather, it averages the oxygenation of venous, capillary, and arterial blood. Cerebral oximetry requires estimating the distribution of blood between the arterial and venous vasculature. Manufacturers use fixed ratio assumptions in their algorithms that range from 25% to 30% for arterial and 70% to 75% for venous cortical blood volume. These estimates, though, are not constant and vary between individuals and clinical scenarios. The accuracy of cerebral oximetry fixed arterial to venous blood ratio assumptions was investigated in 23 volunteers during isocapnic hypoxemia.18 All five cerebral oximetry monitors tested accurately detected hypoxemia induced by randomly varying the fractional inspired oxygen concentration from 100% to 70%. The average bias (difference between cerebral oxygen saturation reading and manufacturer weighted jugular venous and arterial blood oxygen saturation) with declining oxygen varied markedly between manufacturer and with arterial oxygen saturation. The ForeSight Elite™, NIRO-200NX™, and EQUANOX™−3 wavelength had greater positive mean bias at low arterial oxygen saturation. A positive bias represents cerebral oximetry overestimation of the weighted average of arterial and jugular oxygen saturation. Bias was negatively influenced by darker skin pigment and female sex. The amount of bias during hypoxia was reduced when the difference between measured arterial and jugular venous oxygen saturation was used in the calculation rather than a fixed weighted average. Thus, the proportion of arterial and venous blood in the brain is not fixed but dynamically varies.
Cerebral Oxygen Desaturation
There is no consensus on what decrement of cerebral oxygen saturation from baseline represents a clinically important threat to cerebral oxygenation. Nonetheless, a widely used paradigm for defining a “desaturation” is a > 20% oxygen saturation reduction from baseline or an absolute value of <50%.2 These definitions originate from studies of patients undergoing carotid endarterectomy where cerebral oxygen saturation was evaluated during documented episodes of cerebral ischemia.19–22 We prospectively applied this definition in a observational study of 235 patients at eight centers to determine the frequency of cerebral oxygen desaturation during cardiopulmonary bypass.23 We observed desaturations in 61% of patients. A corrective intervention algorithm was effective in restoring cerebral oxygenation in 92% of persistent episodes. In a randomized, masked study involving 201 patients undergoing cardiac surgery at eight different centers, cerebral desaturation defined as a decrement > 10% from baseline occurred in 63% of patients and 40% had decrements > 20% of baseline.24 Due to wide inter-individual steady state variability and dynamic error in the measurements, cerebral oximetry does not provide an absolute cerebral oxygen saturation measurement but, rather, should be interpreted as a trend monitor.18,25 Thus, determining an absolute threshold for defining “desaturation” difficult. As a result, the use of cerebral oxygen saturation cut-offs to define “desaturation” in clinical practice may have less relevance than in clinical research.
Clinical Applications
There is a variety of proposed applications of cerebral oximetry monitoring for patients undergoing cardiac surgery including for preoperative risk stratification.26 Case reports have described its value for detecting malposition of aortic and venous cannulae particularly during aortic arch surgery, or to confirm selective antegrade cerebral perfusion.27 The highest interest for cerebral oximetry understandably has been to reduce the risk of neurological complications. Other applications include monitoring of somatic tissue oxygenation, monitoring of patients during extracorporeal membrane oxygenation, and for cerebral blood flow autoregulation monitoring.
Avoiding Neurological Complications
Neurological complications after cardiac surgery have a spectrum of manifestations that include delirium, delayed neurocognitive recovery (i.e., postoperative cognitive dysfunction), and clinical or subclinical stroke.4–6 Of these, the mechanism(s) of stroke is best understood with the majority of episodes due cerebral embolism. Cerebral hypoperfusion, though, can directly cause ischemic injury particularly in the setting of cerebral arterial stenosis or interruption of cerebral blood flow. It may further exacerbate ischemic injury from embolism by reducing collateral flow and/or reducing microembolism washout.28 The value of cerebral oximetry for detecting cerebral hypoperfusion has been evaluated in patients undergoing carotid endarterectomy. In a study of 323 patients, cerebral oximetry had a sensitivity of 68% and specificity was 94% for detecting cerebral ischemia during carotid artery cross-clamping compared with electroencephalography or somatosensory evoked potentials.21 It must be acknowledged that the different brain regions monitored may have varying collateral blood supply.
In contrast to stroke, the etiology of neurocognitive dysfunction is unclear but it is likely the result of the interaction of patient related factors any combination of cerebral microembolism, hypoperfusion, central nervous system inflammation, or other variables.4–6 In a recent randomized trial we found a 28% relative reduction in the risk for postoperative delirium in patients having blood pressure targets during cardiopulmonary bypass set above the lower limit of cerebral autoregulation compared with usual care.29 The latter suggests that optimizing cerebral perfusion may allow for reducing the risk for postoperative delirium.
Evidence on whether interventions for cerebral oxygen desaturations mitigate risk for neurological outcomes in the past have been limited to case reports and observational studies.27 There are now several randomized, controlled algorithm-based interventional trials for cerebral oxygen desaturations in patients undergoing cardiac surgery. Two recent meta-analysis of these trials have been published and summarized in table 1.30,31 Variability between meta-analyses is likely explained by differing criteria for study inclusion and approach to data analysis. These meta-analyses demonstrate that presently there is insufficient evidence to support or refute whether interventions for cerebral oxygen desaturations during surgery improve neurological outcomes due to the many limitations of the existing studies especially the low number of studied patients. In an eight center pilot study it was estimated that 3,080 evaluable patients are likely needed to assess differences in the rate of mortality, 4,638 patients for stroke, and 1,610 for delirium. Importantly, a large percentage of strokes after cardiac surgery occur postoperatively and, thus, are not likely to be influenced by intraoperative interventions alone.32 Further, the mechanism of delayed cognitive recovery is not known and perhaps not prevented solely by avoiding compromised cerebral oxygenation only during surgery. We have found that hypotension after surgery in the ICU is common and associated with brain injury biomarker release.33 Thus, a strategy of ensuring cerebral perfusion in both the OR and in the ICU may be necessary. Finally, monitoring of any physiological parameter can only provide information to clinicians. It is the corrective interventions based on the information provided by the monitor that can potentially impact patient outcomes.34
Table 1.
Summary of data from two meta-analysis of randomized controlled studies of near infrared spectroscopy monitoring interventions in surgical patients. The data are listed as relative risk (RR, 95% confidence intervals). The number (N) of subjects evaluated for each outcome and the number (N) of randomized controlled trials evaluated are given for each publication.
| Stroke | Delirium | Delayed Neurocognitive Recovery | Acute Kidney Injury | Blood Transfusion | Length of stay in the ICU | Hospital length of stay | Mortality (within 30 days) | |
|---|---|---|---|---|---|---|---|---|
| Serraino & Murphy29 | ||||||||
| RR (95% CI) | 1.08‡ (0.40 to 2.91) | Meta-analysis not performed due to heterogeneity between trials | 0.88‡ (0.52, 1.49) | 0.93† (0.77, 1.12) | 0‡ (−0.44, 0.44) | −0.45† (−0.90, 0.01) | 0.76† (0.30, 1.96) | |
| N | 1138 | 1043 | 744 | 608 | ||||
| N of trials | 7 | 6 | 4 | 4 | ||||
| Cochrane Review30 | ||||||||
| RR (95% CI) | 0.25† (0.03, 2.20) | 0.63† (0.27,1.45) | 0.62* (0.37, 1.04) | 0.93† (0.77, 1.12) | 0.63† (0.08, 5.03) | |||
| N | 240 | 190 | 962 | 744 | 390 | |||
| N of trials | 2 | 1 | 6 | 4 | 3 | |||
Note: The strength of the evidence for each outcome was rated as *moderate; †low; or ‡very low.
Non-Cerebral Tissue Oxygenation Monitoring
Near infrared spectroscopy monitoring of somatic tissue oxygenation strongly correlates with cardiac output in experimental models of hemorrhagic shock and resuscitation.1,35 This type of monitoring has been proposed for tracking somatic tissue oxygenation in patients with extremity compartment syndromes, in patients with peripheral vascular disease undergoing revascularization, and to monitor oxygenation of free flaps.1 Case reports and observational studies have reported the use of somatic tissue oxygen saturation monitoring of the thenar eminence, forearm, and lower leg in patients undergoing cardiac surgery. A single-center observational study of 121 patients found that somatic tissue oxygen saturation (measured over the flank) < 65% or a >20% decline from baseline predicted acute kidney injury after surgery.36 A limitation of renal monitoring in adults is the variability in skin and subcutaneous tissue depth making it unknown whether the kidney or even large flank muscles are indeed interrogated with near infrared light that may penetrate only ~1.3 cm.
Mechanical Circulatory Support
The use of extracorporeal membrane oxygenation for treating cardiac and/or pulmonary failure continues to expand. Although lifesaving in many situations, its use is associated with complications including neurological complications such as stroke and seizures.37,38 Differential hypoxia syndrome (“Harlequin syndrome”) where PaO2 is less in the upper compared with the lower body can occur with peripheral venous-arterial extracorporeal membrane oxygenation. This complication occurs when left ventricular ejection of blood with a low PaO2 mixes in the aorta with blood coming from the extracorporeal membrane oxygenation circuit having a higher PaO2. Differential hypoxia syndrome is associated with acute cerebral dysfunction that can predispose to stroke or seizures. Diagnosis is challenging when critical illness or sedation renders patients incapable of participating with neurological examination. Attenuated or absent arterial pulsatility with extracorporeal membrane oxygenation may interfere with upper and lower extremity pulse oximetry that may compromise its use to detect this syndrome. For this reason some centers monitor cerebral oximetry which is not reliant on arterial pulsations. In a series of 56 patients on veno-arterial extracorporeal membrane oxygenation 32% developed acute cerebral dysfunction.37 Cerebral oxygen desaturations occurred in 74% of the patients. Cerebral oxygen desaturation and high lactate concentrations were independently associated with mortality. Early detection of differential hypoxia syndrome allows for adjustments of extracorporeal membrane oxygenation drainage/flow to reduce the content of ejected left ventricular blood with a low PaO2.
A pitfall of peripheral veno-arterial extracorporeal membrane oxygenation is the risk for lower-limb ischemia with femoral cannulation.39 The traditional approach to diagnosis of limb ischemia is intermittent clinical exam and Doppler pulse examination that can delay detection. Somatic tissue oxygenation monitoring with near infrared spectroscopy can be used to ensure extremity perfusion distal to the site of vascular access.40 Placing sensors bilaterally on the feet or superficial lower leg muscles allows for comparing tissue oxygenation between the extremities. This method can be used as well with vascular access with large cannula as for percutaneous left ventricular assist devices. Early detection of vascular compromise allows for vascular cannula modification and/or insertion of an arterial shunt to avoid distal extremity ischemia.
Cerebral Autoregulation Monitoring
The brain has several homeostatic processes to maintain a constant supply of oxygenated blood to meet its high metabolic demand. Cerebral blood flow-pressure autoregulation is one such process whereby cerebral blood flow is maintained across a range of blood pressures ensuring a steady supply of oxygenated blood. When blood pressure is below or above the constraints of autoregulation, cerebral blood flow is pressure passive predisposing to cerebral ischemia or cerebral hyperemia, respectively. Monitoring of cerebral autoregulation at the bedside is possible using a variety of methods that mathematically model cerebral blood flow or cerebral blood volume in response to cerebral perfusion pressure (or arterial pressure in the absence of elevated intracranial pressure). Cerebral blood flow as the output signal can be monitored with middle cerebral artery transcranial Doppler blood flow velocity measurements. Mean velocity index is calculated as the Pearson correlation coefficient between low frequency (<0.05 Hz) changes in cerebral blood flow velocity and perfusion pressure. This index is near zero when cerebral blood flow is autoregulated since flow is constant despite changes in perfusion pressure. In contrast, when perfusion pressure is below or above the limits of autoregulation, mean velocity index approaches 1 since changes in pressure alter cerebral blood flow. Intracranial pressure (when clinically monitored) can serve as a surrogate for cerebral blood volume and modeled against cerebral perfusion pressure for continuous autoregulation monitoring.41 Similar to mean velocity index, pressure reactivity index is calculated as the Pearson correlation coefficient between low frequency (<0.05 Hz) changes in intracranial pressure and perfusion pressure. Arteriolar dilation or constriction in response to changes in perfusion pressure collectively effect cerebral blood volume resulting in changes in intracranial pressure. Functional autoregulation is associated with a pressure reactivity index near zero but it approaches 1 when autoregulation is impaired.
Cerebral oxygen saturation represents the balance of cerebral oxygen supply (i.e., cerebral blood flow, hemoglobin concentration, oxygen saturation) versus oxygen metabolic demand. At low frequencies in the range associated with autoregulation cerebral oxygen saturation have been shown experimentally to be an acceptable surrogate of cerebral blood flow providing a clinically feasible method for autoregulation monitoring as we have described.42 Calculating the Pearson correlation coefficient between low frequency (< 0.05 Hz) changes in cerebral oxygen saturation and perfusion pressure yields cerebral oximetry index. Similar to mean velocity index, cerebral oximetry index near zero indicates independence of cerebral blood flow from perfusion pressure while when pressure is below or above the limits of autoregulation cerebral oximetry index approaches 1.42 Our group has validated cerebral oximetry index experimentally and in adults during cardiopulmonary bypass.43
The pressure reactivity index has been used in patients with traumatic brain injury for monitoring autoregulation where it has been shown to predict poor outcome.41,44 Intracranial pressure monitoring is not typically performed in patients undergoing cardiac surgery but a similar index can be derived non-invasively from the total hemoglobin concentration measured with near infrared spectroscopy at the isobestic wavelength.45 Vasodilation and vasoconstriction respectively reciprocally increase or decrease the total hemoglobin concentration due to collective changes in cerebral vessel capacity. The variable hemoglobin volume index represents low-frequency correlation between total hemoglobin concentration and perfusion pressure (figure 1).
Our group has performed clinical studies of adult patients undergoing cardiac surgery using cerebral oximetry index. In retrospective analysis we found that the product of the magnitude and duration that mean arterial pressure was below the lower limit of autoregulation during cardiopulmonary bypass was independently associated with risk for acute kidney injury as well as for major morbidity and mortality after cardiac surgery.46,47 Importantly, simply raising mean arterial pressures targets during cardiopulmonary bypass might result in arterial pressure above the upper limit of autoregulation and risk for cerebral hyperperfusion. We have found that the product of the magnitude that mean arterial pressure is above the upper limit of autoregulation is associated with risk for delirium.48 Our group, thus, has argued in favor of personalizing blood pressure management during surgery with cerebral autoregulation monitoring.49
Conclusions
Near infrared spectroscopy provides a clinically feasible method for monitoring cerebral oxygen saturation. Limitations with the technology and inter-individual variations in these measurements must be acknowledged such that cerebral oximetry should be interpreted as a trend monitor. The insufficient evidence presently available from randomized controlled trials does not prove or disprove that optimizing cerebral oxygen saturation during cardiac surgery can improve patient outcomes. New applications of cerebral oximetry include for patients undergoing extracorporeal membrane oxygenation and as a bed-side monitor of cerebral autoregulation might expand its clinical utility.
Sources of Funding:
Funded in part by a grant from the National Heart, Lung, and Blood Institute, Rockville, MD (NIH RO1HL092259, Charles W. Hogue, MD, PI).
Footnotes
Conflicts of Interest:
Annabelle Levine, MD: None
Choy Lewis, MD: None
Charles W. Hogue, MD has received payment for advisory board membership from Medtronic, Inc (Minneapolis, MN) and Edwards Lifesciences (Irvine, CA). He serves on a Data Safety Monitoring Committee for Merck, Inc (Kenilworth, NJ)
Aaron Hudson, MD: None
References
- 1.Cohn SM: Near-infrared spectroscopy: potential clinical benefits in surgery. J Am Coll Surg 2007; 205: 322–32 [DOI] [PubMed] [Google Scholar]
- 2.Murkin JM, Arango M: Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009; 103 Suppl 1: i3–13 [DOI] [PubMed] [Google Scholar]
- 3.Ferrari M, Giannini I, Sideri G, Zanette E: Continuous non invasive monitoring of human brain by near infrared spectroscopy. Adv Exp Med Biol 1985; 191: 873–82 [DOI] [PubMed] [Google Scholar]
- 4.Hogue CW Jr., Palin CA, Arrowsmith JE: Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg 2006; 103: 21–37 [DOI] [PubMed] [Google Scholar]
- 5.Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM: Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med 2012; 366: 250–7 [DOI] [PubMed] [Google Scholar]
- 6.Gottesman RF, McKhann GM, Hogue CW: Neurological complications of cardiac surgery. Semin Neurol 2008; 28: 703–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause M, Morabito JE, Mackensen GB, Perry TE, Bartels K: Current Neurologic Assessment and Neuroprotective Strategies in Cardiac Anesthesia: A Survey to the Membership of the Society of Cardiovascular Anesthesiologists. Anesth Analg 2020; 131: 518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts ML, Lin HM, Tinuoye E, Cohen E, Flores RM, Fischer GW, Weiner MM: The Association of Cerebral Desaturation During One-Lung Ventilation and Postoperative Recovery: A Prospective Observational Cohort Study. J Cardiothorac Vasc Anesth 2020 [DOI] [PubMed] [Google Scholar]
- 9.Laflam A, Joshi B, Brady K, Yenokyan G, Brown C, Everett A, Selnes O, McFarland E, Hogue CW: Shoulder surgery in the beach chair position is associated with diminished cerebral autoregulation but no differences in postoperative cognition or brain injury biomarker levels compared with supine positioning: the anesthesia patient safety foundation beach chair study. Anesth Analg 2015; 120: 176–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smielewski P, Czosnyka M, Pickard JD, Kirkpatrick P: Clinical evaluation of near-infrared spectroscopy for testing cerebrovascular reactivity in patients with carotid artery disease. Stroke 1997; 28: 331–8 [DOI] [PubMed] [Google Scholar]
- 11.Al-Rawi PG, Smielewski P, Kirkpatrick PJ: Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke 2001; 32: 2492–500 [DOI] [PubMed] [Google Scholar]
- 12.Meng L, Cannesson M, Alexander BS, Yu Z, Kain ZN, Cerussi AE, Tromberg BJ, Mantulin WW: Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. Br J Anaesth 2011; 107: 209–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV: The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 2018; 21: 1318–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch KU, Mikkelsen IK, Aanerud J, Espelund US, Tietze A, Oettingen GV, Juul N, Nikolajsen L, Ostergaard L, Rasmussen M: Ephedrine versus Phenylephrine Effect on Cerebral Blood Flow and Oxygen Consumption in Anesthetized Brain Tumor Patients: A Randomized Clinical Trial. Anesthesiology 2020; 133: 304–317 [DOI] [PubMed] [Google Scholar]
- 15.Hongo K, Kobayashi S, Okudera H, Hokama M, Nakagawa F: Noninvasive cerebral optical spectroscopy: depth-resolved measurements of cerebral haemodynamics using indocyanine green. Neurol Res 1995; 17: 89–93 [DOI] [PubMed] [Google Scholar]
- 16.Kim MB, Ward DS, Cartwright CR, Kolano J, Chlebowski S, Henson LC: Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput 2000; 16: 191–9 [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Ibaraki M, Kanno I, Fukuda H, Miura S: Changes in the arterial fraction of human cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab 2005; 25: 852–7 [DOI] [PubMed] [Google Scholar]
- 18.Bickler P, Feiner J, Rollins M: Factors affecting the performance of 5 cerebral oximeters during hypoxia in health volunteers. Anesth Analg 2013; 117: 813–823 [DOI] [PubMed] [Google Scholar]
- 19.Beese U, Langer H, Langm W, Dinkel M: Comparison of nearinfrared spectroscopy and somatosensory evoked potentials for the detection of cerebral ischemia during carotid endarterectomy. Stroke 1998; 29: 2032–2039 [DOI] [PubMed] [Google Scholar]
- 20.Grubhofer G, Plöchl W, Skolka M, Czerny M, Ehrlich M, Lassnigg A: Comparing Doppler ultrasonography and cerebral oximetry as indicators for shunting in carotid endarterectomy. Anesth Analg 2000; 91: 1339–1344 [DOI] [PubMed] [Google Scholar]
- 21.Friedell M, Clark J, Graham D, Isley M, Zhang X: Cerebral oximetry does not correlate with electroencephalography and somatosensory evoked potentials in determining the need for shunting during carotid endarterectomy. J Vasc Surg 2008; 48: 601–606 [DOI] [PubMed] [Google Scholar]
- 22.Rigamonti A, Scandroglio M, Minicucci F, Magrin S, Carozzo A, Casati A: A clinical evaluation of near-infrared cerebral oximetry in the awake patient to monitor cerebral perfusion during carotid endarterectomy. J Clin Anesth 2005; 17: 426–430 [DOI] [PubMed] [Google Scholar]
- 23.Subramanian B, Nyman C, Fritock M, Klinger R, Sniecinski R, Roman P, Huffmyer J, Parish M, Yenokyan G, Hogue C: A multicenter pilot study assessing regional cerebral oxygen desaturation frequency during cardiopulmonary bypass and responsiveness to an intervention algorithm. Anesth Analg 2016; 112: 1786–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deschamps A, Hall R, Grocott H, Mazer CD, Choi PT, Turgeon AF, de Medicis E, Bussieres JS, Hudson C, Syed S, Seal D, Herd S, Lambert J, Denault A, Deschamps A, Mutch A, Turgeon A, Denault A, Todd A, Jerath A, Fayad A, Finnegan B, Kent B, Kennedy B, Cuthbertson BH, Kavanagh B, Warriner B, MacAdams C, Lehmann C, Fudorow C, Hudson C, McCartney C, McIsaac D, Dubois D, Campbell D, Mazer D, Neilpovitz D, Rosen D, Cheng D, Drapeau D, Dillane D, Tran D, McKeen D, Wijeysundera D, Jacobsohn E, Couture E, de Medicis E, Alam F, Abdallah F, Ralley FE, Chung F, Lellouche F, Dobson G, Germain G, Djaiani G, Gilron I, Hare G, Bryson G, Clarke H, McDonald H, Roman-Smith H, Grocott H, Yang H, Douketis J, Paul J, Beaubien J, Bussieres J, Pridham J, Armstrong JN, Parlow J, Murkin J, Gamble J, Duttchen K, Karkouti K, Turner K, Baghirzada L, Szabo L, Lalu M, Wasowicz M, Bautista M, Jacka M, Murphy M, Schmidt M, Verret M, Perrault MA, Beaudet N, Buckley N, Choi P, MacDougall P, Jones P, Drolet P, Beaulieu P, Taneja R, Martin R, Hall R, George R, Chun R, McMullen S, Beattie S, Sampson S, Choi Stephen, Kowalski Stephen, McCluskey Stuart, Syed Summer, Boet Sylvain, Ramsay Tim, Saha Tarit, Mutter Thomas, Chowdhury Tumul, Uppal Vishal, Mckay William, Canadian Perioperative Anesthesia Clinical Trials Group: Cerebral Oximetry Monitoring to Maintain Normal Cerebral Oxygen Saturation during High-risk Cardiac Surgery: A Randomized Controlled Feasibility Trial. Anesthesiology 2016; 124: 826–36 [DOI] [PubMed] [Google Scholar]
- 25.Henson L, Calalang C, Temp J, Ward D: Accuracy of a cerebral oximeter in healthy volunteers under conditions of isocapnic hypoxia Anesthesiology 1998; 88: 58–65 [DOI] [PubMed] [Google Scholar]
- 26.Heringlake M, Garbers C, Kabler JH, Anderson I, Heinze H, Schon J, Berger KU, Dibbelt L, Sievers HH, Hanke T: Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology 2011; 114: 58–69 [DOI] [PubMed] [Google Scholar]
- 27.Zheng F, Sheinberg R, Yee MS, Ono M, Zheng Y, Hogue CW: Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg 2013; 116: 663–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caplan LR, Hennerici M: Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998; 55: 1475–82 [DOI] [PubMed] [Google Scholar]
- 29.Brown CH, Neufeld KJ, Tian J, Probert J, LaFlam A, Max L, Hori D, Nomura Y, Mandal K, Brady K, Hogue CW, Cerebral Autoregulation Study G, Shah A, Zehr K, Cameron D, Conte J, Bienvenu OJ, Gottesman R, Yamaguchi A, Kraut M: Effect of Targeting Mean Arterial Pressure During Cardiopulmonary Bypass by Monitoring Cerebral Autoregulation on Postsurgical Delirium Among Older Patients: A Nested Randomized Clinical Trial. JAMA Surg 2019; 154: 819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serraino GF, Murphy GJ: Effects of cerebral near-infrared spectroscopy on the outcome of patients undergoing cardiac surgery: a systematic review of randomised trials. BMJ Open 2017; 7: e016613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y, Zhang K, Zhang L, Zong H, Meng L, Han R: Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database of Systematic Reviews 2018: Art No: CD010947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogue CW Jr., Murphy SF, Schechtman KB, Davila-Roman VG: Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100: 642–7 [DOI] [PubMed] [Google Scholar]
- 33.Hori D, Ono M, Rappold TE, Conte JV, Shah AS, Cameron DE, Adachi H, Everett AD, Hogue CW: Hypotension After Cardiac Operations Based on Autoregulation Monitoring Leads to Brain Cellular Injury. Ann Thorac Surg 2015; 100: 487–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger M, Mark JB, Kreuzer M: Of Parachutes, Speedometers, and EEG: What Evidence Do We Need to Use Devices and Monitors? Anesth Analg 2020; 130: 1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beilman GJ, Groehler KE, Lazaron V, Ortner JP: Near-infrared spectroscopy measurement of regional tissue oxyhemoglobin saturation during hemorrhagic shock. Shock 1999; 12: 196–200 [DOI] [PubMed] [Google Scholar]
- 36.Ortega-Loubon C, Fernandez-Molina M, Fierro I, Jorge-Monjas P, Carrascal Y, Gomez-Herreras JI, Tamayo E: Postoperative kidney oxygen saturation as a novel marker for acute kidney injury after adult cardiac surgery. J Thorac Cardiovasc Surg 2019; 157: 2340–2351 e3 [DOI] [PubMed] [Google Scholar]
- 37.Pozzebon S, Blandino Ortiz A, Franchi F, Cristallini S, Belliato M, Lheureux O, Brasseur A, Vincent JL, Scolletta S, Creteur J, Taccone FS: Cerebral Near-Infrared Spectroscopy in Adult Patients Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. Neurocrit Care 2018; 29: 94–104 [DOI] [PubMed] [Google Scholar]
- 38.Nasr DM, Rabinstein AA: Neurologic Complications of Extracorporeal Membrane Oxygenation. J Clin Neurol 2015; 11: 383–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonicolini E, Martucci G, Simons J, Raffa GM, Spina C, Coco VL, Arcadipane A, Pilato M, Lorusso R: Limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation: a narrative review of incidence, prevention, monitoring, and treatment. Crit Care 2019; 23: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DJ, Cho YJ, Park SH, Lim C, Park KH, Jheon S, Kim JS: Near-Infrared Spectroscopy Monitoring for Early Detection of Limb Ischemia in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation. ASAIO J 2017; 63: 613–617 [DOI] [PubMed] [Google Scholar]
- 41.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA: Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care 2009; 10: 373–86 [DOI] [PubMed] [Google Scholar]
- 42.Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, Koehler RC, Shaffner DH: Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 2007; 38: 2818–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, Hogue CW Jr.: Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010; 41: 1951–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, Hutchinson PJ, Brady KM, Menon DK, Pickard JD, Smielewski P: Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 2012; 40: 2456–63 [DOI] [PubMed] [Google Scholar]
- 45.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, Koehler RC, Shaffner DH, Brady KM: Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 2009; 40: 1820–6 [DOI] [PubMed] [Google Scholar]
- 46.Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, Brown C, Katz NM, Grams ME, Hogue CW: Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41: 464–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ono M, Brady K, Easley RB, Brown C, Kraut M, Gottesman RF, Hogue CW Jr.: Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg 2014; 147: 483–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hori D, Brown C, Ono M, Rappold T, Sieber F, Gottschalk A, Neufeld KJ, Gottesman R, Adachi H, Hogue CW: Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br J Anaesth 2014; 113: 1009–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brady KM, Hudson A, Hood R, DeCaria B, Lewis C, Hogue CW: Personalizing the Definition of Hypotension to Protect the Brain. Anesthesiology 2020; 132: 170–179 [DOI] [PubMed] [Google Scholar]


