Abstract
Parietal cortex activity contributes to higher-level cognitive processes, including endogenous visual attention and saccade planning. While visual attention is the process of selecting pertinent information from the environment, saccade planning may involve motor attention in the planning of a specific movement, including the process of selecting the correct path. We isolated areas in parietal cortex involved in saccade planning, while controlling visual attention, to understand the relationship between the two processes. Using our novel stimulus, participants performed a delayed saccade task and an endogenous covert visuospatial attention task with peripheral targets in identical locations. We compared multiple target locations across the two domains at the level of the individual to better understand variability in the relationship between these two maps. The anterior-posterior organization of saccade planning and visual attention maps varied among, but not within, participants, and 14 – 29% of the maps for each task overlapped one another across hemispheres. Interestingly, within the region of co-activation, over 67% of the voxels responded to the same location for both tasks. These cortical areas of overlap may represent regions of the brain specifically involved in the transfer of information from vision to action along the visuomotor pathway. These results further establish the relationship between maps associated with saccade planning and visual attention at the individual level, indicating the lack of a single saliency map in parietal cortex.
Keywords: functional magnetic resonance imaging, human
1. INTRODUCTION
A skilled magician will get the audience to attend to one aspect of the performance so spectators will miss the sleight of hand. It doesn’t take much; a sudden movement in a person’s peripheral vision or instruction to carefully watch a particular activity is enough to cause the audience to move their eyes elsewhere. These magic tricks require shifts of visual attention with and without concomitant eye movements. This observation illustrates the longstanding debate regarding the level of connectedness between the neural mechanisms underlying visuospatial attention and eye movements. If one can attend to a location without moving the eyes or plan to move the eyes in one direction while attending to another, are endogenous covert visual and saccade planning attentional mechanisms separate?
While activity in topographically organized regions, or maps, of parietal cortex contribute to both endogenous visuospatial attention (Brefczynski & DeYoe, 1999; Silver et al., 2005; Szczepanski et al., 2013) and saccade planning (Schluppeck et al., 2006; Sereno et al., 2001), continued controversy exists regarding the role of this brain region (See Freedman and Ibos (2018) for a thorough examination of the ongoing debate) in the necessary selection and integration of both visual and motor information to successfully execute goal-directed movements (Gold & Shadlen, 2000). Parietal cortex activity has previously been labeled as primarily involved in the sensory aspects of a task (Bisley & Goldberg, 2010), or only in the context of the upcoming movement (Quian Quiroga et al., 2006), or both (Freedman & Ibos, 2018). Complicating this issue, evidence from non-human primates suggests that neurons in portions of parietal cortex demonstrate different coding mechanisms in the same population of neurons for sensory and motor components, depending on task demands (Bennur & Gold, 2011).
Consistent with the notion that the same regions of parietal cortex contribute to both the sensory and motor components of a task, some have hypothesized that spatial attention is a singular neurophysiological process across modalities involving the same set of neurons via a salience map (Bisley & Goldberg, 2003; Purcell et al., 2012). Evidence in support of this theory includes the significant overlap of visual attention and saccade planning-related activation in humans (Astafiev et al., 2003), and an improvement in attention-mediated behavioral performance when visual and motor targets overlap (Abrams et al., 1998; Carbone & Schneider, 2010; Macaluso et al., 2003). However, we and others have demonstrated that endogenous covert visual attention and saccade planning, and/or their associated regions of activation in posterior parietal cortex, can be dissociated (Casteau & Smith, 2020; Hu et al., 2009; Khan et al., 2009; Konen et al., 2007; Puckett et al., 2017; Quian Quiroga et al., 2006; Snyder et al., 1998), with attention-mediated task performance primarily being influenced by changes in domain-specific visual or motor task demands (Huddleston et al., 2013).
A tangle of meso-scale human functional neuroimaging studies performed at the group level and using different experimental approaches add to this debate regarding the organization of attention maps in parietal cortex. Evaluating group-level activation data in the same participants for both endogenous visual attention and saccade planning activation, but not actual topography, leads to significant overlap across tasks within parietal cortex (Astafiev et al., 2003; Corbetta et al., 1998). This group level analysis may obliterate any individual variation in parietal cortex organization, a brain region known for dynamic organization (Corbetta et al., 1990; Greenberg et al., 2010; Li et al., 2007) and greater variability among participants (Frost & Goebel, 2012; Scolari et al., 2015). Individual differences, measured by extent of activation or fMRI BOLD signal quality, in topographic maps correlate with behavioral performance on a number of tasks including endogenous covert visuospatial attention (Huddleston & DeYoe, 2008; Szczepanski et al., 2010), working memory (Hampson et al., 2006), and delayed saccades (Kunowski & Huddleston, 2011). Thus, individual differences must be recognized when comparing cortical patterns of activation during both saccade planning and endogenous covert visual attention tasks in this particular brain region.
In addition to group level analysis challenges, different paradigms have been used to study saccade planning. A potential confound of previous saccade planning topography studies is the rapid onsets of peripheral visual targets, which may induce a spatially-specific response co-localizing the focus of saccade planning and involuntary capture of visuospatial attention. This stimulus feature may influence the overall allocation of attention during the task (Hopfinger & West, 2006). Evaluating visual attention and saccade planning through mapping of pro- and anti-saccades addresses this issue by spatially separating the visual stimulus and the movement target. However, these actions also require an inhibition of a pro-saccade to the visual target, creating increased cortical activation (Connolly et al., 2000; D’eSouza et al., 2002) and potentially compromising any interpretation of activation patterns. Additionally, mapping of anti-saccades has been limited to left / right hemifields at the group level (Medendorp et al., 2005), limiting the ability to address potential topography. To address these methodological concerns, we developed a saccade planning task in which the central focus of visual attention did not have a spatial component during saccade planning, allowing us to isolate the spatially-specific saccade planning signal in parietal cortex.
To our knowledge, the spatial selectivity of saccade planning and endogenous visual attention maps across multiple targets within each hemisphere and within participants has not been previously reported. We specifically addressed two components of these maps including spatial specificity and individual variability. The overall objective of the current study was to resolve the extent to which saccade planning and endogenous covert visual attention maps exist in the same geographical regions of parietal cortex as a first step to determine if a single spatial saliency map exists in humans versus the presence of separate maps. Secondarily, we quantified the level of spatially-specific congruency in regions of map overlap as a possible location for information transfer from sensory to motor aspects of the task. We hypothesized that cortical activation occurs in different parietal regions based on task demands. We further hypothesized that the organization of these parietal maps would vary among individuals due to the variability in the organization of parietal cortex (Frost & Goebel, 2012), and the flexibility of this area to encode task-specific parameters (Harvey et al., 2013).
2. METHODS
No part of the study procedures or analyses were pre-registered prior to conducting the research. We report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, all manipulations, and all measures in the study. All inclusion and exclusion criteria were established prior to the start of the study. All fMRI analyses were performed using publicly available AFNI software (R. W. Cox, 1996). Raw functional neuroimaging data (.AFNI BRIK and HEAD files) for each of the Experiments and Tasks, de-skulled anatomical images (AFNI BRIK and HEAD files), participant demographics file (PDF), and a stimulus timing file (PDF) for each run are freely available on the University of Wisconsin – Milwaukee Digital Commons (URL: https://dc.uwm.edu/kinesioloqy_facdata/1/; (Huddleston et al., 2019)). Stimulus presentation files (Presentation, Neurobehavioral Systems) and raw eye tracking data can also be found at the same site.
2.1. Subjects
Nine participants completed Experiment 1 (21 – 41 years, 6 females, 1 left-handed) and ten completed Experiment 2 (18 – 43 years, 6 females, 1 left-handed, 2 participants completed both Experiments 1 and 2). Calculating sample sizes for neuroimaging data when performing individual analysis at the voxel level is challenging due to the high number of voxels included in the analysis. We included ten participants in Experiment 2 for the comparison between saccade planning and endogenous covert attention tasks. Effect size is reported for all significant findings. All participants were free of neurological deficits, college educated, reported to have normal or corrected-to-normal vision, and provided written informed consent as approved by the Institutional Review Board of the Medical College of Wisconsin. All were screened per the standard MRI safety protocol by the MRI technician and study staff separately to ensure safety while in the MR scanner. All methods were performed in accordance with the guidelines and regulations set forth by the Institutional Review Board of the Medical College of Wisconsin.
2.2. Stimulus and task
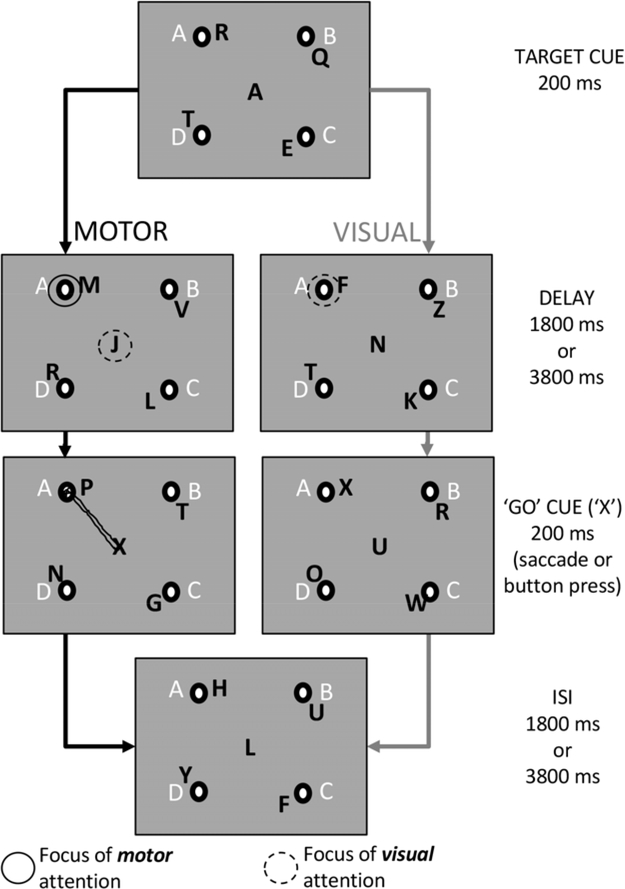
In Experiment 1, participants completed a delayed saccade task, while controlling the focus of visual attention, allowing us to map saccade planning in parietal cortex. In Experiment 2, participants completed the delayed saccade task and a second endogenous covert spatial attention task to directly compare saccade planning and spatial attention maps in parietal cortex in the same individuals. The stimulus (Presentation, Neurobehavioral Systems, Albany, CA) for both experiments is illustrated in Figure 1. In the saccade planning tasks of both Experiments 1 and 2 (left column of Fig. 1), participants performed a delayed saccade task in which the target location cue and the ‘go’ cue were embedded in the central Rapid Serial Visual Presentation (RSVP) letter stream (200 ms letter presentation with no gaps). Participants visually attended to the central stream of letters to identify these cues. Participants shifted their motor planning focus to the cued peripheral target location when a target letter was presented. For example, when a target cue was given (‘A’ in Fig. 1), the subject selected the correct response trajectory to accurately saccade to the intended target location (solid circle in top panel of left column in Fig. 1) while continuing to visually attend to the center letter stream awaiting the ‘go’ saccade cue (dashed circle in top panel of left column in Fig. 1). A ‘Go’ cue (‘X’) was presented after a variable delay of 1800 or 3800 ms (Second panel of left column in Fig. 1). Once the ‘X’ was presented, the participant made a saccade as quickly and as accurately as possible to the previously cued target location before returning gaze to the central letter stream. An auditory tone indicated the end of a trial. The auditory cue prevented participants from continuing to plan a saccade to the previously cued location if they missed the ‘go’ cue. The order of target cues was counterbalanced within each run and participants completed four trials to each peripheral location, for a total of 16 trials each run (186 seconds, 7–10 runs per task). Participants practiced the task outside the scanner prior to data collection. Practice terminated when subjects could identify 75% of the target letters and go cues within the central letter stream. No participant required more than 16 trials of practice.
Figure 1. Trial time series for the Saccade Planning and Endogenous Covert Visual Attention Tasks.
Black letters by the four targets represent the peripheral RSVP letter streams at each target location. White letters indicated the cue letter for the target (A, B, C, and D) which were static and always present. Circles show the location (and spatial dissociation) of saccade planning and endogenous covert visual attention foci over the delay period for the two tasks. The Experiment 1 stimulus was identical to the Saccade Planning Task of Experiment 2 without the peripheral letter streams (the visual attention task was not completed as a part of Experiment 1). Through our experimental design we removed visual transients from the stimulus and provided target cues centrally to maintain endogenous covert visual attention away from the peripheral targets during the delayed saccade task. In this way, we separated the foci of visual and saccade planning, and were able to identify specific patterns of activation during separate task conditions and across participants.
Once we were able to establish our ability to map saccade planning related activity in parietal cortex while controlling visual attention in Experiment 1, we moved on to Experiment 2. In Experiment 2, the stimulus was intentionally identical for both endogenous covert visual attention and saccade planning tasks with the addition of RSVP letter streams in the periphery required for the endogenous covert visual attention task (black letters in Fig. 2). The Saccade Planning Task of Experiment 2 was identical to that of Experiment 1 with the exception that non-informative peripheral letters streams at the target locations were added to the visual display to maintain stimulus consistency across the two tasks of Experiment 2. The advantage of our stimulus across the two tasks in Experiment 2 was that the domain of interest (visual attention or saccade planning) always had a spatial component while the domain of no interest did not. This stimulus design allowed us to investigate task-specific topography in parietal cortex.
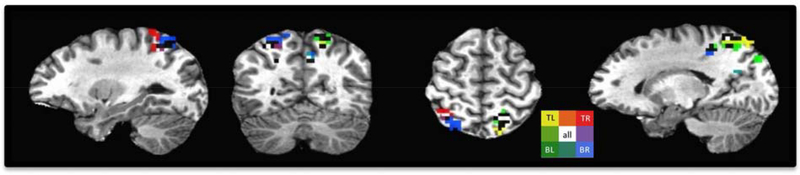
Figure 2. Topographic parietal activation during the delay activity preceding a saccade from one participant during the Saccade Planning Task.
The statistical threshold for this participant was F = 15.22, p = .0001, FDR q = .0065. Legend indicates the four target location colors (top left = yellow, top right = red, bottom right = blue, bottom left = green). Voxels active for two adjacent locations indicated with intermediate colors with voxels active for all locations represented in white. Voxels showing significant activation for three locations or for two non-adjacent locations are represented in black.
In the Endogenous Covert Visual Attention Task of Experiment 2, the target location cue was presented in the central RSVP identical to the Saccade Planning Task, however the ‘X’ cue was now presented in the cued peripheral letter stream. Rather than maintaining a central focus of visual attention, participants now voluntarily shifted their covert visual attention to the cued peripheral target letter stream over the delay period while maintaining central gaze (dashed circle in the top panel pf the right column in Fig. 1). Participants pressed a button with the dominant hand when the ‘X’ was identified in the cued stream. The auditory tone cued the subject to shift visual attention back to the central letter stream in preparation for the next trial. In this condition, all spatial components of the motor response were removed by requiring only a single button press at the time of the ‘X’ cue presentation, regardless of cued location. The target cue was 100% predictive of the letter stream that contained the ‘X’ cue in the periphery, although not all trials contained an ‘X’. These trials served as ‘catch’ trials (1–2 per run). Participants completed four trials to each peripheral location plus the catch trials, for a total of 18 trials each run (198 seconds, 7–10 runs per task). The order of target cues was counterbalanced within each run. Participants completed all 7–10 runs for a single task on separate scan days, with the order of task completion randomized across days and participants. We randomized the order of task completion for the two participants who completed both tasks on the same session/day.
2.3. Magnetic resonance imaging parameters
Functional magnetic resonance imaging (fMRI) was performed on a 3T GE Signa MR scanner (General Electric, Waukesha, WI) using standard echo-planar imaging (EPI) with a standard 8-channel head coil for all experiments. All anatomical data were collected using an SPGR sequence (TR 9.6 s, TE 3.9 ms, flip angle 12°, voxel size 0.97 × 0.97 × 1.1 mm3). Participants viewed a back-projected image behind their head with the use of prisms for task-related scans. The participant’s right eye movements were recorded during scanning using a remote optic bright-eye eye tracker system (ASL Remote Optics Eye Tracking System, Bedford, MA). Eye movement data were collected at 120 Hz. Event-related fMRI scan parameters included a field of view (FOV) of 240 × 240 mm, TE of 25 ms, repetition time TR of 2 s, flip angle of 77°, 4 mm axial slices and 2.73 × 2.73 mm in-plane resolution for 37 slices for whole-brain imaging. Functional scans were registered to the anatomical images for comparison within each subject for subsequent display and analysis. Statistical maps were not collapsed across subjects as significant inter-subject variability in cortical landmarks exists in parietal cortex (Scheperjans et al., 2008), and data averaging might blur the within-subject topography of the regions of interest. Rather, we completed all brain analyses separately for each participant, and results defining the brain maps were pooled for group analysis.
2.4. Data analysis
An event-related design was used for all neuroimaging experiments and all scans collected within one experimental session were concatenated for deconvolution in AFNI (R. Cox, 1996; R. W. Cox, 1996). We used an anatomical region of interest (ROI) in bilateral parietal cortex, analyzing data posterior to the central sulcus, anterior to the parietal-occipital sulcus and superior to the lateral sulcus due to the number of areas within parietal cortex known to be involved in motor planning (Gallivan & Culham, 2015). Within these bilateral ROIs, presumably multiple spatial maps exist (Hagler et al., 2007; Schluppeck et al., 2005). However, we did not attempt to differentiate among separate maps of space, but rather compared all regions within parietal cortex responsive to either the saccade planning or endogenous covert visual attention tasks.
Saccade activity recorded inside the MR scanner was used to create individual reference functions for each target location (plus an additional reference function for errors), which were then convolved with a gamma variate function to simulate the hemodynamic delay of the fMRI BOLD signal. The statistical model accounted for the delay activity in Experiment 1 and the two tasks of Experiment 2 separately. 3dDeconvolve in AFNI v. 16.0.15 (R. W. Cox, 1996) was used to perform a linear regression for each voxel using ordinary least squares drift polynomial fit. Significant controversy exists regarding the correct methodology for thresholding fMRI data (Cox et al., 2017; Yeung, 2018). We minimized the effects of thresholding by using identical thresholds across tasks and performing individual rather than group analysis. We set the statistical threshold to maximize activation while keeping the False Discovery Rate q (as output in AFNI) less than .01 and maintaining a corrected p-value of p < .001 for clusters larger than 3 neighboring voxels with adjacent sides. The FDR q-value represents the fraction of false positives expected at a selected threshold and accounts for the multiple statistical tests performed in neuroimaging analysis.
In Experiment 1, and the Saccade Planning Task of Experiment 2, the subject visually attended to a centrally located stream of letters continuously over the delay, thus cortical activity related to visual attention contributed only to the residual component of the model. Conversely, in the Endogenous Covert Visual Attention Task of Experiment 2, visual attention was spatially distributed to the cued peripheral letter stream while motor response did not require spatial planning, and thus cortical activity related to the button press contributed to the residual component of the model for this condition. The spatial location for the focus of saccade planning in Experiment 1, the Saccade Planning Task of Experiment 2, and the focus of endogenous covert visual attention in the Visuospatial Attention Task in Experiment 2 were all in the same location. In this way topographic maps could be directly compared.
We specifically chose not to use a temporal phase mapping approach to evaluate topography within our regions of activation as the spatial resolution of our voxels makes it likely that neurons encoding different targets co-localized to a single voxel. When using temporal phase mapping, a winner-take-all approach assigns a preferred single target location to each voxel. While this leads to smoother topographical representations, it may not fully capture the underlying topography, as evidenced in early visual regions (Hansen et al., 2004; Janik, 2011). Thus, each voxel was labeled as ‘active’ relative to a specific peripheral target if the significance of the regressor for that target location surpassed the corrected statistical threshold. In this way, each voxel demonstrating significant activation could be assigned up to four preferred target locations if it was active when planning saccades (or covertly attending) to all four locations. For example, if the pattern of activity in a voxel only demonstrated a significant correlation with the top left target location regressor, it was labeled a “top left voxel”. Conversely, a voxel was labeled as an ‘all’ voxel if the activity pattern correlated with all four target location regressors. In this way, we were able to most directly compare activation related to all preferred target locations between the two tasks across all spatial representations of the region. To create the topographic maps, we created masks based on the regions of significant activation within our parietal ROI for each target location and then added the masks together using AFNI 3dCalc (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dcalc.html). Our desire to assess the spatial specificity of activation for each voxel precluded us from doing a direct General Linear Test subtraction between the two tasks for Experiment 2 and limited us to doing voxel-wise comparisons with the statistical maps created using our approach.
To align the topographic maps for the two tasks for eight of the participants in which data were collected across days, we aligned the functional covert visual attention scans to the anatomical image collected on the day of the saccade planning task using AFNI align_epi_anat.py (https://afni.nimh.nih.gov/pub/dist/doc/proqram_help/align_epi_anat.py.html). We then resampled the endogenous covert attention data to match that of the saccade planning data matrix using AFNI 3dAllineate (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dAllineate.html). AFNI 3dCalc was used to add the two maps together to evaluate common target locations across the two tasks.
To directly compare the extent of activation during the Saccade Planning Task and the Endogenous Covert Visual Attention Task of Experiment 2, we superimposed the two maps and counted the number of voxels in parietal cortex active during both saccade planning and endogenous covert visual attention, as well as the number of active voxels within the ROIs unique to each using identical statistical thresholds for the two tasks within the same individual. For the subset of voxels that overlapped in activation for both tasks, we were interested to know if they showed significant activation for the same locations across saccade planning and endogenous covert visual attention. To assess this, we compared the preferred target location(s) for each voxel across the two tasks and categorized the level of similarity as exact (e.g., saccade planning = top left location; visual attention = top left location), contained the same (e.g., saccade planning = top left location; visual attention = top left and bottom left locations), contained the same hemisphere (i.e., saccade planning = top left location; visual attention = bottom left location), contained the same horizontal hemifield (saccade planning = top left location; visual attention = top right location), or none (e.g., motor saccade planning = top left location; visual attention = bottom right location).
Post-processing of eye tracking data was completed with Eyenal (Applied Science Laboratories, Bedford, MA) and visualized in Excel (Microsoft, Redmond, WA). Performance measures for the Saccade Planning Tasks in Experiments 1 and 2 included percentage of misses (participants failed to perform a saccade on a particular trial) and percentage of trials in which the participants originally performed a saccade to the incorrect target, but then corrected their movement to finally fixate on the correct target. In the Endogenous Covert Visual Attention Task of Experiment 2, the performance measures included reaction time for the button press, after the ‘X’ was displayed, and miss rate. Only correct trials were included in the creation of the reference functions for the fMRI data analysis.
3. RESULTS
Consistent with previous studies (Leone et al., 2014; Schluppeck et al., 2006; Sereno et al., 2001), we identified regions of parietal cortex responsive to spatial shifts in saccade planning (Fig. 2). Importantly, this representation occurred while visual attention was maintained in the center of the screen due to the task requirement to monitor the central RSVP for task cues and with no peripheral transients to potentially involuntarily capture visual attention. This spatially specific activation in bilateral cortex varied among individuals. Many regions of parietal cortex activated in a spatially-specific manner with our tasks, including precuneus, superior parietal lobule, multiple areas within the intraparietal sulcus (IPS), lateral parietal, and the inferior post-central sulcus (Fig. 2).
3.1. Characterization of brain activity associated with saccade planning
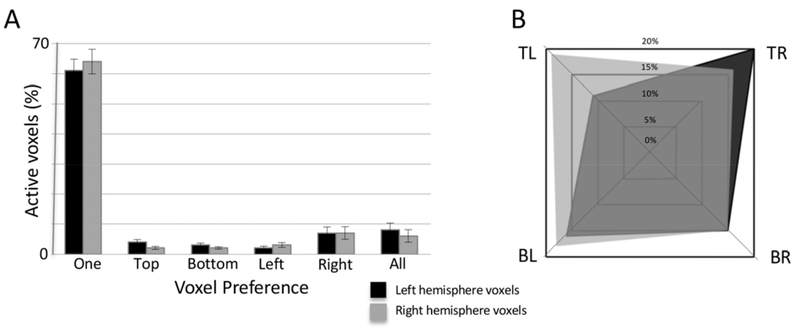
To address the organization of activation in these responsive regions, we counted the number of target locations for which each voxel in the parietal region of interest responded (which is different than the winner-take-all approach of temporal phase mapping (Hansen et al., 2004; Janik, 2011)) from both Experiment 1 and the Saccade Planning Task of Experiment 2. Over 65% of the voxels significantly responded to only one target location with less than ten percent of all significantly active voxels responding in preparation to saccade to all four target locations (Fig. 3A). The remaining voxels preferred two adjacent locations across either the horizontal (e.g., top left and bottom left locations) or vertical hemifields (e.g., top left and top right locations). To further quantify the organization of these areas of activation, we divided voxels significantly responsive to only one location by their preferred saccade target location and hemisphere. Figure 3B demonstrates the distribution of target preference within the left (black) and right (grey) hemispheres for the four target locations. While no significance was noted between hemispheres for the percentage of voxels responsive to a particular target location (F(1, 144) = 0.54, p = .464), Fig. 3B shows a higher percentage of voxels preferring contralateral targets for both hemispheres in the group averaged data for the upper two target locations. Also, no significant differences were noted in the percentage of voxels responsive to a particular target location across the hemispheres (F(3,144) = 0.56, p = .642) or between the hemispheres (F(3,144) = 1.17, p = .323). As a large swath of parietal cortex showed significant spatially specific activation outside of known regions involved in endogenous covert visual attention and saccade planning, we wanted to ensure the reliability of the activation patterns. We re-analyzed the data from two participants (first and last tested), dividing the data sets into the first and last five runs of the saccade planning experiment, applying a 6 mm Full-Width-Half-Maximum (FWHM) smoothing filter, and setting a conservative clustering minimum of 5 voxels for visualization purposes. We then mapped the activation patterns from each half of the dataset for each of the four peripheral target locations separately. The vast preponderance of voxels overlapped across datasets, while some variance did exist due to differences in performance (Supplementary Figure 1). No participants completed the same task across days for further comparison.
Figure 3. Target location specificity of the saccade planning map in parietal cortex.
The total number of active voxels varied among individuals (n = 19), thus the percentage of total voxels is presented. (A) Saccade Planning Map Specificity. The vast majority of significantly active voxels during the Saccade Planning Task were responsive to only one target location, with all other combinations of target location preferences representing less than 30% of the total number of voxels. No hemispheric differences existed. (B) Distribution of Preferred Single Target Map Locations in Saccade Planning Map. Voxels from the far left two columns in (A) were divided by preferred target location. All target locations were represented in both hemispheres.
3.2. Direct comparison of activation patterns between saccade planning and endogenous covert visual attention tasks
In Experiment 2, participants performed similarly in their accuracy between the visual and saccade planning tasks (Saccade Planning Task error rate 7.71 +/− 8.04%; Endogenous Covert Visual Attention Task error rate 10.37 +/− 5.43%; two-tailed paired t-test t(9) = 0.927, p = 0.378), thus any differences noted in the activation patterns presumably could not be attributed to differences in difficulty. When comparing the total number of active voxels, the extent of activation was significantly greater for the Endogenous Covert Visual Attention Task versus the Saccade Planning Task (F(1, 19)) = 37.74, p < .0001, partial eta squared .677, observed power 1.000), although no significant differences existed in activation patterns across cortical hemispheres (Main effect of hemisphere F(1, 19) = 0.090, p = .768, task by hemisphere interaction F(1,18) = 1.010, p = .328).
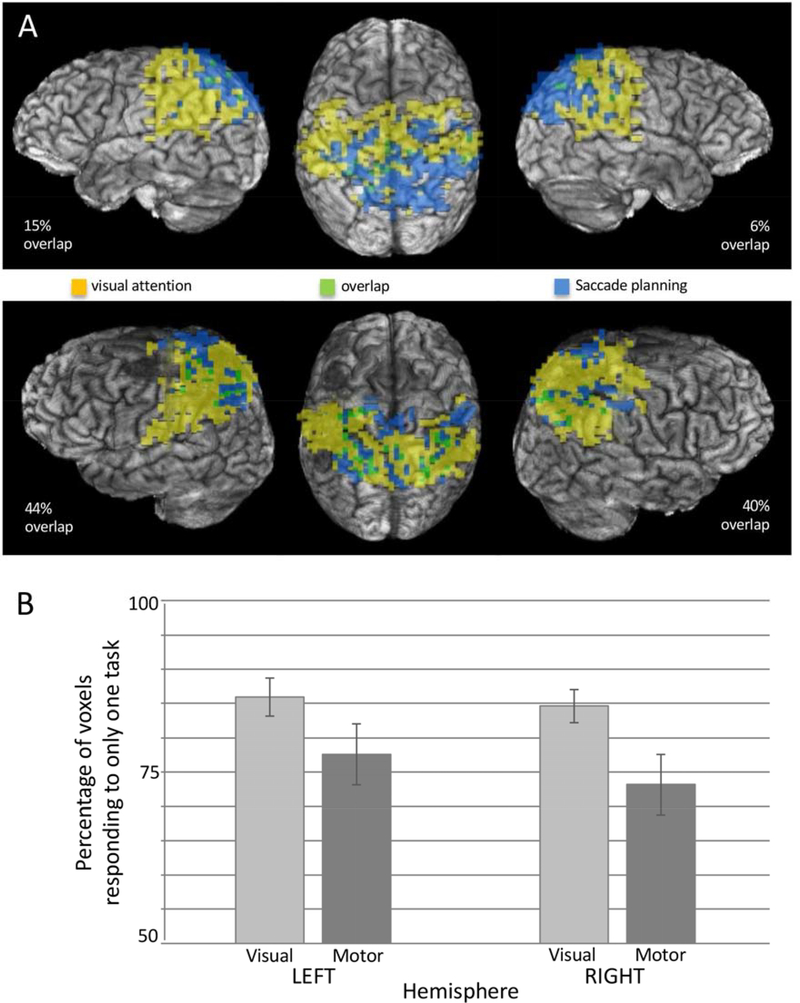
We next quantified the extent to which the activation patterns related to each task were independent of one another. As the extent of activation for the Saccade Planning Task was less than for the Endogenous Covert Visual Attention Task, we were interested to learn the extent to which the saccade planning maps were completely encompassed by the endogenous covert visual attention maps. The extent of activation for either task varied considerably among participants (Fig. 4A). Due to the participant-specific variation, we calculated the percentage of total active voxels that were uniquely responsive to only one of the two tasks. Somewhat surprisingly, the extent of parietal activation specific to each task did not overlap more than 29% of the total activation (Fig. 4B). The percentage of overlapping voxels within an activation area was task dependent (F(1,9) = 16.824, p = .003, partial eta squared = .651, observed power = .953), most likely due to the difference in the overall number of activated voxels. However, neither the main effect of hemisphere nor the interaction was significant (p > .05). Our second surprise was that while the organization of the maps relative to one another was quite consistent across the two hemispheres within each participant, the relative position of the regions of activation was not consistent across all participants. Figure 4A demonstrates the two observed spatial relationships between the endogenous covert visual attention and saccade planning regions of activation. Exactly half of participants demonstrated more posterior activation during the endogenous covert visual attention task while the other half showed more posterior activation during the saccade planning task in Experiment 2.
Figure 4. Overlapping activation in parietal cortex responsive to the Saccade Planning and Endogenous Covert Visual Attention Tasks.
(A) Images from two participants showing the greatest and the least overlap (Statistical threshold for the participant in the top panel F = 15.22; statistical threshold for the participant in the second panel F = 16.94). These two participants also show clear differences in the organization of visual attention versus saccade planning regions, yet consistency within participants across hemispheres. (B) Group data illustrating the percentage of voxels unique to a particular map. That is, the percentage of the total map that did not overlap with the map from the other modality.
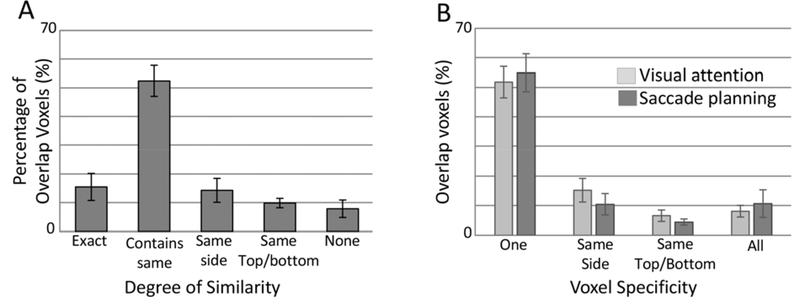
We were particularly interested in the common regions of activation between the two tasks. Were these regions responsive to the same target locations regardless of task? To answer this question, we compared the ‘preferred’ locations for each task within the same voxel and categorized based on their similarity (Fig. 5A). On average, 67% of the voxels responded to the same target location between the two tasks (15% of ‘overlap’ voxels responded to the identical single target location between the two tasks (e.g., TL for both tasks in the same voxel), and 52% of voxels responded to the same target location across tasks, but also significantly responded to an additional target location in at least one task (e.g., TL for saccade planning, and TL and BL for endogenous covert visual attention task)). Only 8% of the voxels active during both tasks did not show any similarity in preferred target locations across tasks, which fell well below chance.
Figure 5. Characterization of the overlap regions of saccade planning and endogenous covert visual attention maps.
(A) Percentage of voxels within the overlap region responding to the same or similar target locations between the two tasks. Definitions of the categories are described in the methods. (B) The percentage of voxels active during both tasks that responded to varying numbers of peripheral targets.
One potential explanation for this high percentage of voxels with significant activation for the same target locations across tasks would be that these ‘overlap’ voxels were ones that responded to multiple targets. This would then increase the likelihood that the same or similar target locations would be significant across the two tasks during this analysis. To confirm that these results were not an artifact of the number of targets these ‘overlap’ voxels responded to during the two tasks, we calculated the percentage of voxels active during both tasks that responded to 1–4 peripheral targets (Fig. 5B). In fact, over 50% of the ‘direct spatial overlap’ voxels showed significant activation for only one target location (Saccade Planning Task 55%; Endogenous Covert Visual attention task 52%) with only 11% and 9% of voxels respectively responding to all target locations for the saccade planning and endogenous covert visual attention tasks. These values align closely to the distribution of voxels within the entire saccade planning map where 62% of the overall voxels showed significant activation for a single target location and 7% responded to all target locations. Thus, while 14–29% of the overall regions of activation overlapped between the tasks (Fig 4B), approximately 90% of that overlap region responded to identical or adjacent target locations in both hemispheres.
4. DISCUSSION
The concept of ‘motor attention’ has several definitions including decision making through attentional selection among motor plans (Goldberg & Segraves, 1987; Toni et al., 2001), selecting an action (Pashler, 1991), covert planning of movement (Rushworth et al., 1997; Rushworth et al., 2001), preparing and maintaining a motor plan (Symes et al., 2010), and attending to predicted proprioceptive sensations for movement and effector selection (Brown et al., 2011). For the purposes of this study, we consider ‘motor attention’ as a fundamental component of selecting a motor plan (saccade trajectory) from a pool of possible movement alternatives. The selection of relevant saccade trajectories may require a mechanism, similar to that described for visuospatial attention, acted upon topographic representations of attended space (Buschman & Kastner, 2015; Lauritzen et al., 2009). The possibility of a common selection mechanism for both sensory and motor modalities led to the design of the current study.
Our approach of controlling visual attention centrally during a delayed saccade task allowed us to unequivocally identify saccade-planning-specific regions of activation in human parietal cortex, which involved a greater extent than previously described using a group analysis (Schluppeck et al., 2005). Saccade planning maps showed considerable specificity with 85% of voxels in the left hemisphere and 84% of voxels in the right hemisphere (Fig. 3A) demonstrating a preference for a single target or adjacent targets (with no apparent preference for adjacent vertical or horizontal hemifield targets). While there tended to be greater representation for contralateral targets in each hemisphere, particularly for the upper visual field (Fig. 3B), no statistical difference was found. This potential difference in upper versus lower visual field representations is consistent with the reaching literature in which the lower visual fields are represented much more robustly (Pitzalis et al., 2013; Rossit et al., 2013). This result may be unique to movement planning as mapping endogenous visuospatial attention (Fig. 4 in (Huddleston & DeYoe, 2008) or passive viewing (Rossit et al., 2013) does not show such an effect.
The debate continues regarding the strength of contralateral target representation in both non-human (Christopoulos et al., 2018; Wardak et al., 2002) or human primates. For example, Leone et al. (2014) reported strong contralateral target representations in humans, whereas others have found much less contralateral preference for saccade planning when compared to non-human primates (Kagan et al., 2010). This more equal distribution of signals in both hemispheres for saccade planning found in the present study may be related to the saccade motor circuitry in which signals from both hemispheres are sent to the superior colliculus (Freedman & Ibos, 2018). It also may be related to the inclusion of the entire parietal region in our study instead of only the intraparietal sulcus or superior parietal lobule where stronger contralateral preference has previously been found (Schluppeck et al., 2005; Sereno et al., 2001). One last possible explanation for these results is that regions of parietal cortex might be more involved in the value of the response than on direct control of the saccade (Freedman & Ibos, 2018; Toni et al., 2001), which may necessitate bilateral representation to some extent. Similarly, others have suggested a ‘push/pull’ mechanism between hemispheres (Pinsk et al., 2004; Szczepanski et al., 2010), which could also lead to bilateral representations of all targets regardless of hemi-field.
Interestingly, the relationship of the activation areas for visual and motor tasks showed two distinct patterns across participants (Fig. 4A) with saccade planning activation occurring anterior to the endogenous covert visual attention maps in some individuals and the opposite pattern in others. That said, the pattern was consistent across left and right hemispheres within each participant. It is difficult to compare the present results with others as we chose to evaluate activation patterns within individuals rather than as a group analysis. For example, Astafiev, et al.(2003) did not find significant activation pattern differences between endogenous covert visual attention and saccadic eye movements in their group analysis. This could be due to an artifact of averaging across individuals. If heterogeneity in activation patterns, as seen in the present study, also existed in their data set, the group analysis would have washed out any differences in regions of activation across tasks. Comparing the spatially-specific activation patterns between endogenous covert visual attention and saccade planning tasks within an individual provides preliminary evidence of variability in the organization of this brain region in humans, and suggests more work is needed at the individual level to better understand the role of the various regions of parietal cortex in attention-mediated visually-guided tasks.
While some might find it unsettling to have two predominant orientations between activation patterns for endogenous covert visual attention and saccade planning in parietal cortex, it is important to note the significant variability in this region (Caminiti et al., 2015), especially when compared to the relative stability of several specific retinotopic maps along visual cortex. Regions of human parietal cortex, serving as a critical association area, have more functional flexibility than earlier visual areas, serving as a dynamic network nimble enough to code for a wide variety of task demands (Caminiti et al., 2017; Hadjidimitrakis et al., 2019). For example, some regions of parietal cortex can categorize task options beyond that of spatial representations, including arbitrary categories (Freedman & Assad, 2006) and cognitive set (Stoet & Snyder, 2004) in non-human primates and features such as motion (Corbetta et al., 1995), numerosity (Harvey et al., 2013) and color (Greenberg et al., 2010) in humans.
The parietal regions responsive to the selection and attention to saccade targets/trajectories appear to be largely independent of regions responsive during an endogenous covert attention task, with 22–29% of active voxels during saccade planning overlapping with the visual-specific-attention maps (Fig. 4B). This small overlap occurred even with identically positioned peripheral targets and identical within-participant imaging thresholds. This result is consistent with behavioral findings demonstrating dissociation in saccadic precision and endogenous covert visual attention processes (Greenwood et al., 2017; Huddleston et al., 2013). The relatively small area of co-activation in parietal cortex between the two tasks suggests that different populations of neurons were modulated by the selection of either salient visual or motor information, contrary to the concept of a single attentional map acting on both sensory and motor information.
The small overlap regions did demonstrate a high level of spatial congruency between the tasks, perhaps demonstrating sub-areas of parietal cortex involved in the integration of sensory and motor selection and may represent regions of the brain specifically involved in the transfer of information from one modality to another in the visuomotor pathway (Andersen & Cui, 2009). This transfer of information may occur via an integrative comparative network (Freedman & Ibos, 2018) or a similar information integration scheme in parietal cortex (Jerde et al., 2012; Scolari et al., 2015; Serences & Yantis, 2007). Another possible alternative to explain the differences in spatial preference among voxels would be a partial volume effect, due to our voxel size, in which separate populations of neurons preferring the different target locations are captured within a single voxel. However, we do not believe partial volume effects explain the common response to specific target locations between the two maps, as over 50% of the ‘overlap’ voxels responded significantly to only one target location.
5. CONCLUSIONS
Saccade planning has many components, and we consider motor attention (aka motor intention) to be one of the critical processes in that planning. Based on our current findings, and those of others, we posit that similarities exist in the potential mechanisms of the selection of salient spatial information across endogenous covert visual attention and movement planning (in this case, saccades). However, the processes, or at least the maps on which they act, are dissociable and in fact separate.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge Matt Verber for his assistance with programming the stimulus.
FUNDING: This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, UL1TR001436. The sponsor had no involvement in this study or manuscript.
Footnotes
COMPETING INTERESTS: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrams RA, Oonk HM, & Pratt J (1998, February). Fixation point offsets facilitate endogenous saccades. Percept Psychophys, 60(2), 201–208. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9529904 [DOI] [PubMed] [Google Scholar]
- Andersen RA, & Cui H (2009, September 10). Intention, action planning, and decision making in parietal-frontal circuits. Neuron, 63(5), 568–583. https://doi.org/S0896-6273(09)00639-4 [pii] 10.1016/j.neuron.2009.08.028 [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, & Corbetta M (2003, June 1). Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci, 23(11), 4689–4699. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12805308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, & Gold JI (2011, January 19). Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci, 31(3), 913–921. 10.1523/JNEUROSCI.4417-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, & Goldberg ME (2003). The role of the parietal cortex in the neural processing of saccadic eye movements. Adv Neurol, 93, 141–157. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12894406 [PubMed] [Google Scholar]
- Bisley JW, & Goldberg ME (2010). Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci, 33, 1–21. 10.1146/annurev-neuro-060909-152823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski JA, & DeYoe EA (1999, April). A physiological correlate of the ‘spotlight’ of visual attention. Nat Neurosci, 2(4), 370–374. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10204545 [DOI] [PubMed] [Google Scholar]
- Brown H, Friston K, & Bestmann S (2011). Active inference, attention, and motor preparation. Front Psychol, 2, 218. 10.3389/fpsyg.2011.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman Timothy J., & Kastner S (2015, 2015/10/07/). From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron, 55(1), 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Borra E, Visco-Comandini F, Battaglia-Mayer A, Averbeck BB, & Luppino G (2017, January-February). Computational Architecture of the Parieto-Frontal Network Underlying Cognitive-Motor Control in Monkeys. eNeuro, 4(1). 10.1523/ENEURO.0306-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Innocenti GM, & Battaglia-Mayer A (2015, September). Organization and evolution of parieto-frontal processing streams in macaque monkeys and humans. Neurosci Biobehav Rev, 56, 73–96. 10.1016/j.neubiorev.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Carbone E, & Schneider WX (2010, November). The control of stimulus-driven saccades is subject not to central, but to visual attention limitations. Atten Percept Psychophys, 72(8), 2168–2175. https://doi.org/72/8Z2168 [pii] 10.3758/APP.72.8.2168 [DOI] [PubMed] [Google Scholar]
- Casteau S, & Smith DT (2020). Covert attention beyond the range of eye-movements: Evidence for a dissociation between exogenous and endogenous orienting. Cortex, 122, 170–186. 10.1016/j.cortex.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Christopoulos VN, Kagan I, & Andersen RA (2018, June 5). Lateral intraparietal area (LIP) is largely effector-specific in free-choice decisions. Sci Rep, 8(1), 8611. 10.1038/s41598-018-26366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, & Vilis T (2000, September). A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol, 84(3), 1645–1655. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10980034 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, & Shulman GL (1998, October). A common network of functional areas for attention and eye movements. Neuron, 21(4), 761–773. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9808463 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, & Petersen SE (1990, June 22). Attentional modulation of neural processing of shape, color, and velocity in humans. Science, 248(4962), 1556–1559. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2360050 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, & Petersen SE (1995, November 3). Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science, 270(5237), 802–805. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7481770 [DOI] [PubMed] [Google Scholar]
- Cox R (1996). AFNI. In [Analysis of Functional Neuroimaging (AFNI) data]. Medical College of Wisconsin. http://afni.nimh.nih.gov/afni/index.shtml [Google Scholar]
- Cox RW (1996, June). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 29(3), 162–173. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8812068 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017, April). FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect, 7(3), 152–171. 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’eSouza J, Menon R, & Everling S (2002). Prepatory set association with pro-saccades and anti-saccades in humans investigated with event-related fMRI. Journal of Neurophysiology, 89, 1016–1023. [DOI] [PubMed] [Google Scholar]
- Freedman D, & Assad J (2006). Experience-dependent representation of visual categories in parietal cortex. Nature, 443, 85–88. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, & Ibos G (2018, March 21). An Integrative Framework for Sensory, Motor, and Cognitive Functions of the Posterior Parietal Cortex. Neuron, 97(6), 1219–1234. 10.1016/j.neuron.2018.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost MA, & Goebel R (2012, January 16). Measuring structural-functional correspondence: spatial variability of specialised brain regions after macro-anatomical alignment. Neuroimage, 59(2), 1369–1381. 10.1016/j.neuroimage.2011.08.035 [DOI] [PubMed] [Google Scholar]
- Gallivan JP, & Culham JC (2015, August). Neural coding within human brain areas involved in actions. Curr Opin Neurobiol, 33, 141–149. 10.1016/j.conb.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Gold JI, & Shadlen MN (2000, March 23). Representation of a perceptual decision in developing oculomotor commands. Nature, 404(6776), 390–394. 10.1038/35006062 35006062 [pii] [DOI] [PubMed] [Google Scholar]
- Goldberg ME, & Segraves MA (1987). Visuospatial and motor attention in the monkey. Neuropsychologia, 25(1A), 107–118. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3106852 [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, & Yantis S (2010, October 27). Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci, 30(43), 14330–14339. 10.1523/JNEUROSCI.4248-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JA, Szinte M, Sayim B, & Cavanagh P (2017, April 25). Variations in crowding, saccadic precision, and spatial localization reveal the shared topology of spatial vision. Proc Natl Acad Sci U S A, 114(17), E3573–E3582. 10.1073/pnas.1615504114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjidimitrakis K, Bakola S, Wong YT, & Hagan MA (2019). Mixed Spatial and Movement Representations in the Primate Posterior Parietal Cortex. Front Neural Circuits, 13, 15. 10.3389/fncir.2019.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr., Riecke L, & Sereno MI (2007, May 01). Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage, 35(4), 1562–1577. 10.1016/j.neuroimage.2007.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, & Constable RT (2006, December 20). Brain connectivity related to working memory performance. J Neurosci, 26(51), 13338–13343. 10.1523/JNEUROSCI.3408-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KA, David SV, & Gallant JL (2004, September). Parametric reverse correlation reveals spatial linearity of retinotopic human V1 BOLD response. Neuroimage, 23(1), 233–241. 10.1016/j.neuroimage.2004.05.012 [DOI] [PubMed] [Google Scholar]
- Harvey B, Klein B, Petridou N, & Dumoulin S (2013). Topographic representation of numerosity in the human parietal cortex. Science, 341(6150), 1123–1126. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, & West VM (2006, 2006/06/01/). Interactions between endogenous and exogenous attention on cortical visual processing. Neuroimage, 31(2), 774–789. [DOI] [PubMed] [Google Scholar]
- Hu S, Bu Y, Song Y, Zhen Z, & Liu J (2009, May). Dissociation of attention and intention in human posterior parietal cortex: an fMRI study. Eur J Neurosci, 29(10), 2083–2091. https://doi.org/EJN6757 [pii] 10.1111/j.1460-9568.2009.06757.x [DOI] [PubMed] [Google Scholar]
- Huddleston WE, Aleksandrowicz MS, Yufa A, Knurr CR, Lytle JR, & Puissant MM (2013, September). Attentional resource allocation during a cued saccade task. Acta Psychol (Amst), 144(1), 112–120. 10.1016/j.actpsy.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Huddleston WE, & DeYoe EA (2008, June). The representation of spatial attention in human parietal cortex dynamically modulates with performance. Cereb Cortex, 18(6), 1272–1280. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17962221 [DOI] [PubMed] [Google Scholar]
- [Record #1956 is using a reference type undefined in this output style.]
- Janik J (2011). Investigation of Spatio-Temporal Effects of fMRI Visual Field Mapping Techniques on V1 (Publication Number 157) Marquette University; ]. http://epublications.marquette.edu/dissertations_mu/157. [Google Scholar]
- Jerde TA, Merriam EP, Riggall AC, Hedges JH, & Curtis CE (2012, November 28). Prioritized maps of space in human frontoparietal cortex [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Neurosci, 32(48), 17382–17390. 10.1523/JNEUROSCI.3810-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan I, Iyer A, Lindner A, & Andersen RA (2010, April 27). Space representation for eye movements is more contralateral in monkeys than in humans. Proc Natl Acad Sci U S A, 107(17), 7933–7938. 10.1073/pnas.1002825107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AZ, Blangero A, Rossetti Y, Salemme R, Luaute J, Deubel H, Schneider WX, Laverdure N, Rode G, Boisson D, & Pisella L (2009, February). Parietal damage dissociates saccade planning from presaccadic perceptual facilitation. Cereb Cortex, 19(2), 383–387. https://doi.org/bhn088 [pii] 10.1093/cercor/bhn088 [DOI] [PubMed] [Google Scholar]
- Konen CS, Kleiser R, Bremmer F, & Seitz RJ (2007, September). Different cortical activations during visuospatial attention and the intention to perform a saccade. Exp Brain Res, 182(3), 333–341. 10.1007/s00221-007-0995-z [DOI] [PubMed] [Google Scholar]
- Kunowski KM, & Huddleston WE (2011, November 15). Specifying the role of posterior parietal cortex in perceptual decision making Annual Society for Neuroscience Conference Washington, D.C. [Google Scholar]
- Lauritzen TZ, D’Esposito M, Heeger DJ, & Silver MA (2009). Top-down flow of visual spatial attention signals from parietal to occipital cortex. J Vis, 9(13), 18 11–14. 10.1167/9.13.18/9/13/18/ [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone FT, Toni I, & Medendorp WP (2014, February 15). Two-dimensional spatial tuning for saccades in human parieto-frontal cortex. Neuroimage, 87, 476–489. 10.1016/j.neuroimage.2013.09.067 [DOI] [PubMed] [Google Scholar]
- Li S, Ostwald D, Giese M, & Kourtzi Z (2007, November 7). Flexible coding for categorical decisions in the human brain. J Neurosci, 27(45), 12321–12330. 10.1523/JNEUROSCI.3795-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E, Driver J, & Frith CD (2003, June 17). Multimodal spatial representations engaged in human parietal cortex during both saccadic and manual spatial orienting. Curr Biol, 13(12), 990–999. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12814544 [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, & Vilis T (2005, July). Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol, 94(1), 734–740. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15788514 [DOI] [PubMed] [Google Scholar]
- Pashler H (1991, November). Shifting visual attention and selecting motor responses: distinct attentional mechanisms. J Exp Psychol Hum Percept Perform, 17(4), 1023–1040. http://www.ncbi.nlm.nih.gov/pubmed/1837295 [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, & Kastner S (2004, July). Push-pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol, 92(1), 622–629. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14973320 [DOI] [PubMed] [Google Scholar]
- Pitzalis S, Sereno MI, Committeri G, Fattori P, Galati G, Tosoni A, & Galletti C (2013, November 15). The human homologue of macaque area V6A. Neuroimage, 82, 517–530. 10.1016/j.neuroimage.2013.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett AM, Bollmann S, Barth M, & Cunnington R (2017, November 1). Measuring the effects of attention to individual fingertips in somatosensory cortex using ultra-high field (7T) fMRI. Neuroimage, 161, 179–187. 10.1016/j.neuroimage.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Purcell BA, Schall JD, Logan GD, & Palmeri TJ (2012, March 7). From salience to saccades: multiple-alternative gated stochastic accumulator model of visual search. J Neurosci, 32(10), 3433–3446. https://doi.org/32/10/3433 [pii] 10.1523/JNEUROSCI.4622-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quian Quiroga R, Snyder LH, Batista AP, Cui H, & Andersen RA (2006, March 29). Movement intention is better predicted than attention in the posterior parietal cortex. J Neurosci, 26(13), 3615–3620. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16571770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossit S, McAdam T, McLean DA, Goodale MA, & Culham JC (2013, October). fMRI reveals a lower visual field preference for hand actions in human superior parieto-occipital cortex (SPOC) and precuneus. Cortex, 49(9), 2525–2541. 10.1016/j.cortex.2012.12.014 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Renowden S, Wade DT, & Passingham RE (1997, September). The left parietal cortex and motor attention. Neuropsychologia, 35(9), 1261–1273. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9364496 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, & Sipila PK (2001, July 15). Attention systems and the organization of the human parietal cortex. J Neurosci, 21(14), 5262–5271. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11438601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, & Zilles K (2008, September). Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Cerebral Cortex, 18(9), 2141–2157. 10.1093/cercor/bhm241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, & Heeger DJ (2006, May 10). Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci, 26(19), 5098–5108. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16687501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, & Heeger DJ (2005, August). Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol, 94(2), 1372–1384. https://doi.org/01290.2004 [pii] 10.1152/jn.01290.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari M, Seidl-Rathkopf KN, & Kastner S (2015, February). Functions of the human frontoparietal attention network: Evidence from neuroimaging. Current Opinion in Behavioral Sciences, 1, 32–39. 10.1016/j.cobeha.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, & Yantis S (2007, March 2). Spatially Selective Representations of Voluntary and Stimulus-Driven Attentional Priority in Human Occipital, Parietal, and Frontal Cortex. Cereb Cortex, 17(2), 284–293. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16514108 [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, & Martinez A (2001, November 9). Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science, 294(5545), 1350–1354. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11701930 [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, & Heeger DJ (2005, August). Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol, 94(2), 1358–1371. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15817643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, & Andersen RA (1998, May). Change in motor plan, without a change in the spatial locus of attention, modulates activity in posterior parietal cortex. J Neurophysiol, 79(5), 2814–2819. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9582248 [DOI] [PubMed] [Google Scholar]
- Stoet G, & Snyder L (2004). Single neurons in posterior parietal cortex of monkeysencode cognitive set. Neuron, 42, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Symes E, Ottoboni G, Tucker M, Ellis R, & Tessari A (2010, July). When motor attention improves selective attention: the dissociating role of saliency. Q J Exp Psychol (Hove), 63(7), 1387–1397. https://doi.org/916934104 [pii] 10.1080/17470210903380806 [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, & Kastner S (2010, January 6). Mechanisms of spatial attention control in frontal and parietal cortex [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Neurosci, 30(1), 148–160. 10.1523/JNEUROSCI.3862-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Pinsk MA, Douglas MM, Kastner S, & Saalmann YB (2013). Functional and structural architecture of the human dorsal frontoparietal attention network. Proceedings of the National Academy of Science, 110(39), 15806–15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni I, Thoenissen D, & Zilles K (2001, July). Movement preparation and motor intention. Neuroimage, 14(1 Pt 2), S110–117. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11373141 [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, & Duhamel J-R (2002). Saccadic target selection deficits after intraparietal area inactivation in monkeys. Journal of Neuroscience, 22, 9877–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung AWK (2018). An Updated Survey on Statistical Thresholding and Sample Size of fMRI Studies. Front Hum Neurosci, 12, 16. 10.3389/fnhum.2018.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.