Abstract
Summary
Both sarcopenia and low bone mineral density (BMD) have become public health concerns. We found that presarcopenic and/or sarcopenic individuals were more likely to have lower BMD. And this relationship has race and sex-specific discrepancy.
Purpose
The purpose of the study was to investigate the racial and gender differences in the relationship between sarcopenia and BMD among older adults.
Methods
Totally, 5476 subjects (mean age = 65.7 ± 6.4) of non-Hispanic White (n = 3297), non-Hispanic Black (n = 1265), and non-Hispanic Asian (n = 914) were analyzed. Sarcopenia was defined according to the revised European consensus on definition and diagnosis of sarcopenia (EWGSOP2). General linear model and multivariable linear regression model were used to examine the relationship between sarcopenia and regional/whole body BMD stratified by race and sex. Adjustments were conducted for physiological, behavioral, and disease factors.
Results
Comparing with normal older participants, presarcopenic and sarcopenic elderly were more likely to have lower BMD. Although the difference was not statistically significant in a few sub-groups, among the three racial groups, the strongest association between sarcopenia and BMD was found in non-Hispanic Black people, followed by non-Hispanic White people and non-Hispanic Asian people. In addition, significant differences of BMD across sarcopenia stages were found in more sub-groups in women than in men after adjusting for covariates.
Conclusions
In this older cohort, sarcopenia is significantly related to low regional/whole-body BMD, and these associations vary by race and sex. Consideration in race and sex is warranted when developing strategies to maintain or minimize BMD loss.
Keywords: Bone mineral density, Older adults, Race, Sarcopenia, Sex
Introduction
Initially, the term sarcopenia was used to describe the decrease in muscle mass with aging [1]. More recently, however, it has been defined as a progressive and generalized skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality [2]. A recent systematic review and meta-analysis of the general population, including 35 articles and a total of 58,404 individuals, reported that the overall estimate of sarcopenia prevalence was 10% among adults aged 60 years and older [3]. In addition, sarcopenia may cause a considerable economic burden for those involved and for the healthcare system. For example, a recent study aimed at estimating the cost of sarcopenia in the USA showed that the total estimated annual cost of hospitalizations in sarcopenic individuals was $40.4 billion [4].
Meanwhile, in the aging population, fractures can dramatically increase functional disability, affect life quality, and lead to a higher mortality rate [5, 6]. Several risk factors have been revealed to predispose older adults to fractures. These include but not limited to osteoporosis, old age, female sex, low body mass index, previous fragility fracture, current smoking, and alcohol intake of 3 or more units daily [7, 8]. Among them, osteoporosis, characterized by low bone mineral density (BMD) and micro-architectural deterioration of bone tissue [9, 10], is a well-known main risk factor for fractures. Osteoporosis affects more than 200 million people worldwide [11]. Thirty percent of postmenopausal women in the USA have osteoporosis, and 40% of them develop fragility fractures [12]. And the cost of treatment for osteoporotic fractures was estimated at $17 billion in 2005 and is expected to increase to $25.3 billion by 2025 [13].
As the population ages rapidly worldwide, both sarcopenia and osteoporosis have emerged as serious public health concerns. Both conditions are likely to become increasingly prevalent in the future, increasing the incidence of fragility fractures and leading to greater morbidity, mortality, and socioeconomic costs [14, 15]. Previous studies have reported that the prevalence of both sarcopenia and osteoporosis exhibit race and sex differences [16, 17]; however, the reported relationship between sarcopenia and osteoporosis/BMD in currently available literature remains controversial. For instance, a European study demonstrated that sarcopenia is associated with low BMD and osteoporosis in middle-aged and elderly men [18]. In a female American cohort, muscle strength was strongly associated with BMD at the femoral neck site but not at the lumbar spine site for women aged 60 years and over [19]. Some studies identified no independent effect of sarcopenia on total hip BMD [20, 21]. In addition, although race is an important factor when examining health disparities in the USA, the relationships between sarcopenia and BMD by race and sex have not been evaluated thoroughly, especially for minority populations. We hypothesize the inconsistent relationships might be partly explained by racial and gender differences. If differences do exist by race and/or sex, then strategies to maintain bone mass or minimize bone loss may need to be modified accordingly. Therefore, the aim of this study was to obtain epidemiological insights into the older population by looking for associations between sarcopenia and site-specific BMD (whole body, lumbar spine [L1-L4], femoral neck, and total radius) across racial groups (non-Hispanic White people, non-Hispanic Black people, and non-Hispanic Asians), and sex.
Methods
Subjects
The study population consisted of three large cohorts. The first cohort, collected in the southern (New Orleans and Baton Rouge, LA) and the Midwestern USA (Omaha, NE and Kansas City, MO), consisted of 3297 unrelated healthy non-Hispanic White people (935 males, 2362 females). The second cohort was composed of 1265 healthy unrelated non-Hispanic Black people (689 males, 576 females) from two cities (New Orleans and Baton Rouge, LA) in the southern USA. The third cohort consisted of 914 unrelated healthy non-Hispanic Asian people (311 males, 603 females), recruited from two cities (Changsha, Hunan, and Xi’an, Shanxi) in the central of People’s Republic of China.
Through the Kansas City Osteoporosis Study (KCOS), Louisiana Osteoporosis Study (LOS), and Chinese Osteoporosis Study (COS), we are building a large cohort and database for human complex disease studies. The above three studies used the same inclusion and exclusion criteria [20, 21]. Specifically, the inclusion criteria were (1) willing to participate in the study, blood drawn and have a bone densitometry exam and (2) is at least 18 years of age. The exclusion criteria were the following:
Female subjects who have had bilateral oophorectomy
Female subjects who are or could be pregnant
Diabetes mellitus, except for those controlled under medication
Serious residuals from cerebral vascular disease
Chronic liver failure
Chronic renal failure
Significant chronic lung disease
Chronic obstructive pulmonary disease (COPD)
Alcohol abuse refers to people who cannot limit drinking, regularly become intoxicated, and cannot perform major responsibilities at school, work, or home
Treatment with anticonvulsant therapy for more than 6 months duration
Corticosteroid therapy at pharmacologic levels over a period of 6 months
Evidence of other metabolic or inherited bone diseases, e.g., hyper- or hypoparathyroidism, osteomalacia, Paget’ s disease, osteogenesis imperfecta, or others
Collagen diseases (i.e., hypochondrogenesis and osteogenesis imperfecta)
Rheumatoid arthritis (except for those minor cases that involve only hand joint and wrist)
Chronic gastrointestinal diseases, e.g., celiac disease, postgastrectomy, ulcerative colitis, Crohn’s disease, liver transplant, and cirrhosis
Briefly, none of the participants had chronic diseases, medications, or treatments which might affect bone and/or soft tissue metabolism. Those who did not meet the above criteria were not included in the sample population. Therefore, the quality of the sample population was guaranteed. In total, 15,032 subjects who met the above criteria were recruited. In the current study, we only included older adults aged 60 years and over. Therefore, the data of 5476 older adults were analyzed. The study was approved by the institutional review boards of all involved institutions. Consent forms were obtained by each participant before data collection.
Measurement
BMD (g/cm2) of the whole body, lumbar spine (L1–L4), femoral neck, and total radius were measured with Hologic QDR-4500 DXA scanners at all sites. DXA instruments were calibrated daily by measuring spine phantoms and operated by trained and certified investigators. Long-term precision in our hands, the coefficient of variation (CV), was less than 1.6% for BMD measurement across the study centers. Identical scan protocols were conducted for all the participants. DXA can also be used to measure limb lean tissue mass (appendicular), which can be considered as a good proxy for muscle mass [22]. Appendicular skeletal muscle mass was calculated as the sum of muscle mass in the arms and legs. Skeletal muscle mass index (SMI) was calculated as appendicular skeletal muscle mass divided by height squared.
Height and weight were measured in light indoor clothing and without shoes using a calibrated stadiometer and a calibrated balance beam scale, respectively. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Grip strength of both hands was measured in duplicate by a handheld dynamometer (TEC Inc., Clifton, NJ) with the maximum value recorded in kilograms.
Diagnosis of sarcopenia
The definition of sarcopenia in the current study was based on the revised European consensus on definition and diagnosis of sarcopenia (EWGSOP2) [2]. EWGSOP2 proposed that muscle strength is the most reliable measure of muscle function; thus, low muscle strength was recommended as the primary parameter of sarcopenia. Specifically, presarcopenia is identified by low muscle strength. Sarcopenia is diagnosed with the presence of low muscle strength and low muscle quantity/quality. Severe sarcopenia is defined by low muscle strength, low muscle quantity/quality, plus low physical performance. The current objective cutoff points for low muscle quantity/quality in men and women are SMI ≤ 7.0 kg/m2 and SMI ≤ 5.5 kg/m2, respectively. The cutoff points for low muscle strength in men and women were grip strength < 27 kg and < 16 kg, respectively. However, physical performance was not available in the current study. Therefore, participants were categorized into three stages: the normal stage, the presarcopenia stage (low muscle strength), and sarcopenia stage (low muscle strength and low muscle quantity/quality).
Demographic, physiological, and behavioral and disease factors
Self-reported questionnaires were used to collect information including demographics (race, sex), physiological factors (age, BMI), and behavioral and disease factors (menopausal hormone therapy, smoking, alcohol use, regular exercise, milk consumption, minutes of sun exposure per day, hypertension history, and fracture history). Specifically, race included non-Hispanic White people, non-Hispanic Black people, and non-Hispanic Asian people. Menopausal hormone therapy was defined by the response to the question “Do you routinely taken estrogen and/or progesterone since menopause?” Subjects were defined as smokers if they reported being a former or current smoker. Participants were considered to have alcohol use disorder if they have a history of alcoholism or reported a drinking problem. Regular exercise was defined as at least one of any type of exercise per week. Milk consumption was assessed by the question “Do you drink milk (including almond milk, fortified soy, rice, etc.)?” Sun exposure was determined by the response to “Do you receive at least 15 min of exposure to sun per day on the average?”
Statistical analysis
Categorical and continuous variables were reported as frequencies and means. Analysis of variance (ANOVA) was performed to test the association of sarcopenia with physiological factors by race and sex. And the subsequent comparisons were performed by Student-Newman-Keuls (SNK) to locate the source of significances. Chi-square/Fisher’s exact test were performed to test the associations of sarcopenia with behavioral and disease factors by race and sex. The interactions were examined by multi-factor analysis of variance. Statistically significant interactions were found between race and sex, race and sarcopenia stages, and gender and sarcopenia stages for BMD in this study (Table 1). Thus, we did multiple comparisons of BMD across sarcopenia stages by race and gender. General linear models were used to determine whether the means of BMD of the three groups (normal, presarcopenia, sarcopenia) differ. The flexibility of general linear models to incorporate both factors and covariates is a good fit for this study to explore BMD difference across different sarcopenia stages while considering covariates [23]. Therefore, general linear models were used to assess the least squares means of BMD across sarcopenia stages by race and sex, as well as the p value for linear trends across sarcopenia stages, adjusting for age and BMI.
Table 1.
The interactions between race and gender, race and sarcopenia stages, and gender and sarcopenia stages for BMD
|
P values |
||||
|---|---|---|---|---|
| WB BMD | LS BMD | FN BMD | TR BMD | |
| Race × sex | 0.010 | 0.016 | 0.008 | 0.003 |
| Race × sarcopenia status | 0.007 | 0.039 | 0.016 | 0.003 |
| Sex × sarcopenia status | 0.001 | 0.003 | 0.012 | 0.001 |
The EWGSOP2 definition of sarcopenia is being used
WB BMD whole-body bone mineral density, LS BMD lumbar spine bone mineral density, FN BMD femoral neck bone mineral density, TR BMD total radius bone mineral density
The preliminary results suggested that the presarcopenic and/or sarcopenic older adults tended to have lower BMD compared with the normal participants. Therefore, multivariable linear regression models were adopted to further examine significant differences in the association of sarcopenia stages with BMD by race and sex. Normal participants were used as reference groups in model 1 and model 2. Model 1 adjusted for age and BMI. Model 2 adjusted for variables in model 1, plus exercise, smoking, milk consumption, alcohol use disorder, 15 min of sun exposure per day, hypertension history, and fracture history, plus menopausal hormone therapy in women. All statistical analyses were performed with SPSS 20.0 and stratified by race and sex. P < 0.05 was considered statistically significant.
Results
Descriptive statistics
In this study sample, 64.7% of the respondents were women. Approximately 60.2% were non-Hispanic White people (71.6% were women), 23.1% were non-Hispanic Black people (45.5% were women), and 16.7% were non-Hispanic Asian people (66.0% were women). Among the participants, 77.5% of them were normal, 18.9% had presarcopenia, and 3.6% had sarcopenia. Regardless of race or sex, participants with sarcopenia tended to be older and generally had lower BMI values (see Table 1).
Among non-Hispanic White men, factors significantly associated with sarcopenia included regular exercise, milk consumption, current or past smokers, and fracture. For non-Hispanic White women, sun exposure, regular exercise, alcohol use disorder, postmenopausal hormone therapy, hypertension, and fracture were significantly associated with sarcopenia. Among non-Hispanic Black men, factors significantly associated with sarcopenia were regular exercise, current or past smokers, and hypertension. For non-Hispanic Black women, sun exposure, regular exercise, and fracture were significantly associated with sarcopenia. Among non-Hispanic Asian men, factors significantly associated with sarcopenia were regular exercise and fracture. For non-Hispanic Asian women, sun exposure and regular exercise were significantly associated with sarcopenia (P < 0.05). Overall, the effects of behavioral and disease factors on sarcopenia varied by race and sex. Regular exercise was significantly associated with sarcopenia, regardless of race or sex (P < 0.05) (see Tables 2 and 3).
Table 2.
Physiological characteristics of the study population across sarcopenia status by race and sex (N= 5476)
| Normal (1) 4245(77.5%) | Presarcopenia (2) 1036(18.9%) | Sarcopenia (3) 195(3.6%) | P value | Comparison* | |
|---|---|---|---|---|---|
| NHW | |||||
| Men (N = 935) | |||||
| Age | 66.3 ± 6.4 | 67.7 ± 8.6 | 69.4 ± 10.4 | 0.016* | 2, 3 > 1 |
| BMI | 28.3 ± 4.5 | 26.8 ± 5.3 | 21.3 ± 2.8 | < 0.001*** | 1, 2 > 3 |
| Women (N = 2362) | |||||
| Age | 65.5 ± 5.6 | 69.5 ± 9.2 | 70.2 ± 8.4 | < 0.001*** | 3, 2 > 1 |
| BMI | 27.6 ± 6.0 | 26.5 ± 6.5 | 20.6 ± 2.4 | < 0.001*** | 1, 2 > 3 |
| NHB | |||||
| Men (N = 689) | |||||
| Age | 62.4 ± 3.4 | 63.7 ± 5.7 | 65.4 ± 2.7 | 0.002** | 2, 3 > 1 |
| BMI | 27.4 ± 5.1 | 25.6 ± 4.9 | 20.1 ± 2.6 | < 0.001*** | 1, 2 > 3 |
| Women (N = 576) | |||||
| Age | 63.9 ± 4.9 | 65.5 ± 7.5 | 67.3 ± 6.9 | 0.012* | 2, 3 > 1 |
| BMI | 32.2 ± 7.3 | 31.2 ± 7.3 | 21.4 ± 2.3 | 0.006** | 1, 2 > 3 |
| NHA | |||||
| Men (N = 311) | |||||
| Age | 66.3 ± 5.3 | 70.5 ± 6.9 | 73.1 ± 8.1 | < 0.001*** | 3 > 2 > 1 |
| BMI | 25.1 ± 2.9 | 24.0 ± 3.3 | 20.9 ± 2.2 | < 0.001*** | 1 > 2 > 3 |
| Women (N = 603) | |||||
| Age | 64.3 ± 4.5 | 67.8 ± 7.0 | 68.2 ± 7.4 | < 0.001*** | 2, 3 > 1 |
| BMI | 25.1 ± 3.5 | 23.0 ± 5.7 | 20.0 ± 1.9 | < 0.001*** | 1 > 2 > 3 |
The EWGSOP2 definition of sarcopenia is being used
NHW non-Hispanic White people, NHB non-Hispanic Black people, NHA non-Hispanic Asian people, BMI body mass index
P < 0.05
P < 0.01
P < 0.001
Table 3.
Behavioral and disease characteristics of the study population across sarcopenia status by race and sex
| Normal 4245(77.5%) | Presarcopenia 1036(18.9%) | Sarcopenia 195(3.6%) | P value | |
|---|---|---|---|---|
| NHW | ||||
| Men (N = 935) | ||||
| Sun exposure 15 min per day (yes, %) | 87.1 | 87.2 | 86.7 | 0.998 |
| Exercise (yes, %) | 75.7 | 67.1 | 53.3 | 0.018* |
| Milk (yes, %) | 79.6 | 84.1 | 86.7 | 0.047* |
| Smoker (yes, %) | 58.6 | 60.4 | 80.0 | 0.025* |
| Alcohol (yes, %) | 50.3 | 70.7 | 86.7 | 0.024* |
| Hypertension (yes, %) | 39.7 | 44.5 | 46.7 | 0.465 |
| Fracture (yes, %) | 32.7 | 36.0 | 40.0 | 0.028* |
| Women (N = 2362) | ||||
| Sun exposure 15 min per day (yes, %) | 72.6 | 69.8 | 60.7 | 0.039* |
| Exercise (yes, %) | 69.8 | 60.3 | 52.6 | 0.030* |
| Milk (yes, %) | 72.9 | 76.4 | 72.6 | 0.326 |
| Smoker (yes, %) | 41.6 | 39.4 | 40.5 | 0.699 |
| Alcohol (yes, %) | 74.6 | 76.7 | 64.3 | 0.038* |
| Postmenopausal hormone therapy (yes, %) | 17.1 | 7.6 | 13.1 | <0.001*** |
| Hypertension (yes, %) | 33.8 | 39.4 | 45.5 | 0.002** |
| Fracture (yes, %) | 28.5 | 34.1 | 39.6 | 0.022* |
| NHB | ||||
| Men (N = 689) | ||||
| Sun exposure 15 min per day (yes, %) | 89.2 | 86.1 | 84.6 | 0.547 |
| Exercise (yes, %) | 69.5 | 63.8 | 46.2 | 0.003** |
| Milk (yes, %) | 77.9 | 83.3 | 76.9 | 0.368 |
| Smoker (yes, %) | 73.4 | 78.3 | 92.3 | 0.017* |
| Alcohol (yes, %) | 68.8 | 68.1 | 76.9 | 0.807 |
| Hypertension (yes, %) | 47.2 | 66.4 | 70.8 | 0.016* |
| Fracture (yes, %) | 23.8 | 20.3 | 23.1 | 0.684 |
| Women (N = 576) | ||||
| Sun exposure 15 min per day (yes, %) | 72.9 | 82.7 | 100.0 | 0.042* |
| Exercise (yes, %) | 74.5 | 69.1 | 50.0 | 0.009** |
| Milk (yes, %) | 75.8 | 71.8 | 75.0 | 0.692 |
| Smoker (yes, %) | 42.4 | 53.6 | 25.0 | 0.077 |
| Alcohol (yes, %) | 49.1 | 48.2 | 25.0 | 0.624 |
| Postmenopausal hormone therapy (yes, %) | 3.0 | 1.8 | — | 0.741 |
| Hypertension (yes, %) | 49.4 | 54.5 | 50.0 | 0.619 |
| Fracture (yes, %) | 10.9 | 17.3 | 25.0 | 0.031* |
| NHA | ||||
| Men (N = 311) | ||||
| Sun exposure 15 min per day (yes, %) | 86.7 | 87.0 | 84.6 | 0.976 |
| Exercise (yes, %) | 74.4 | 65.7 | 61.1 | 0.003** |
| Milk (yes, %) | 61.9 | 67.2 | 78.9 | 0.116 |
| Smoker (yes, %) | 30.2 | 27.6 | 21.1 | 0.504 |
| Alcohol (yes, %) | 37.2 | 37.9 | 31.6 | 0.782 |
| Hypertension (yes, %) | 34.0 | 36.2 | 39.1 | 0.245 |
| Fracture (yes, %) | 9.1 | 10.6 | 14.6 | 0.006** |
| Women (N = 603) | ||||
| Sun exposure 15 min per day (yes, %) | 67.7 | 80.4 | 47.1 | 0.035* |
| Exercise (yes, %) | 67.4 | 65.9 | 58.3 | 0.036* |
| Milk (yes, %) | 61.9 | 62.0 | 68.3 | 0.718 |
| Smoker (yes, %) | 1.4 | 4.7 | 2.4 | 0.080 |
| Alcohol (yes, %) | 8.1 | 10.1 | 4.9 | 0.550 |
| Postmenopausal hormone therapy (yes, %) | 2.5 | 0.8 | 7.3 | 0.064 |
| Hypertension (yes, %) | 23.0 | 27.1 | 36.8 | 0.364 |
| Fracture (yes, %) | 24.5 | 17.8 | 24.4 | 0.283 |
The EWGSOP2 definition of sarcopenia is being used
NHW non-Hispanic White people, NHB non-Hispanic Black people, NHA non-Hispanic Asian people, Exercise self-reported regular exercise, Milk self-reported milk consumption, Smoker self-reported current or past smokers, Alcohol self-reported alcohol use disorder
P < 0.05
P < 0.01
P < 0.001
Association of sarcopenia and BMD
Figure 1 and Table 4 display the adjusted mean distributions of regional/whole body BMD across sarcopenia stages by race and sex. After adjusting for age and BMI, a negative linear relationship was found between sarcopenia and regional/whole body BMD in non-Hispanic White people, non-Hispanic Black people, and non-Hispanic Asian men (P < 0.05). However, no clear directionality was found to the associations between sarcopenia stages and regional/whole body BMD in non-Hispanic Asian women (P > 0.05).
Fig. 1.
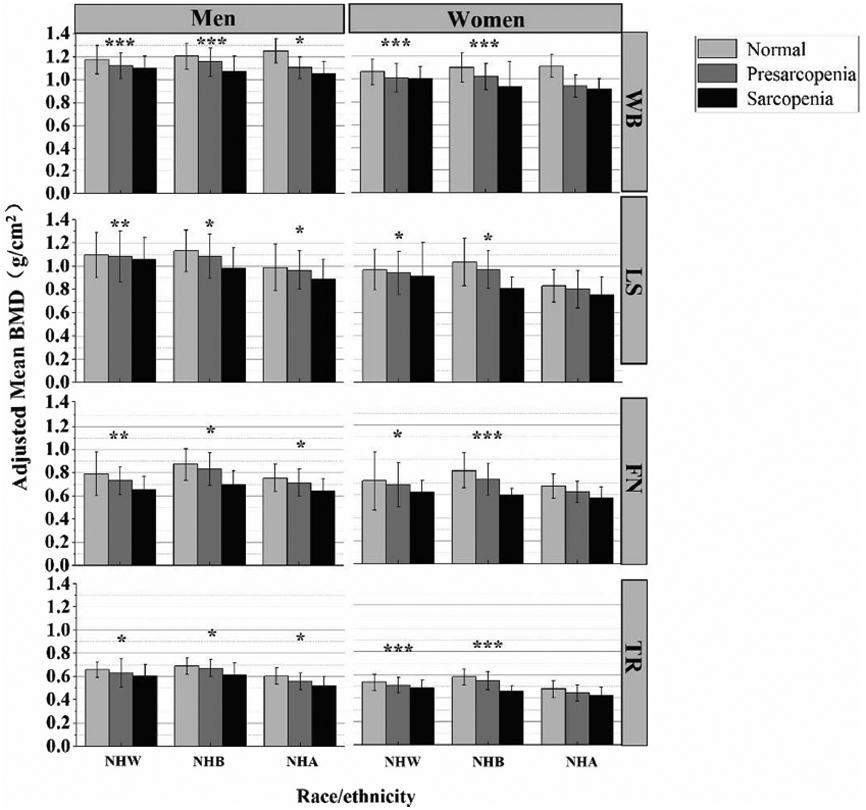
Adjusted mean regional/whole body bone mineral density (BMD) across sarcopenia stages by race and sex, adjusted for age and BMI. The P values were for linear-trend analysis across the three sarcopenia categories. WB, whole-body bone mineral density, LS lumbar spine bone mineral density, FN femoral neck bone mineral density; TR, total radius bone mineral density; NHW, non-Hispanic White people; NHB, non-Hispanic Black people, NHA non-Hispanic Asian people The EWGSOP2 definition of sarcopenia is being used. *P < 0.05; **P < 0.01; ***P < 0.001
Using the normal participants as the reference group, the relationship between sarcopenia stages and BMD is shown in Fig. 2 and Table 5. After controlling for physiological factors (model 1), non-Hispanic White men with presarcopenia or sarcopenia had significantly lower whole-body BMD compared with the reference group (r = − 0.049, 95% CI = [− 0.07, − 0.029], P < 0.001; r = − 0.056, 95% CI = [− 0.119, − 0.007], P = 0.041). While holding physiological factors in model 1 constant, the mean whole-body BMD of non-Hispanic White men with presarcopenia was 0.049 g/cm2 lower than their normal counterparts, and non-Hispanic White men with sarcopenia was 0.056 g/cm2 lower than their normal counterparts. Similarly, non-Hispanic Black men with presarcopenia and sarcopenia had significantly lower whole-body BMD compared with the reference group (r = − 0.043, 95% CI = [− 0.067, − 0.020], P < 0.001; r = − 0.097,95% CI = [− 0.167, − 0.028], P = 0.006). When controlling for physiological factors, and behavioral and disease factors (model 2), the regression coefficients decreased slightly in non-Hispanic White men with presarcopenia and sarcopenia but increased slightly in non-Hispanic Black men with presarcopenia and sarcopenia.
Fig. 2.
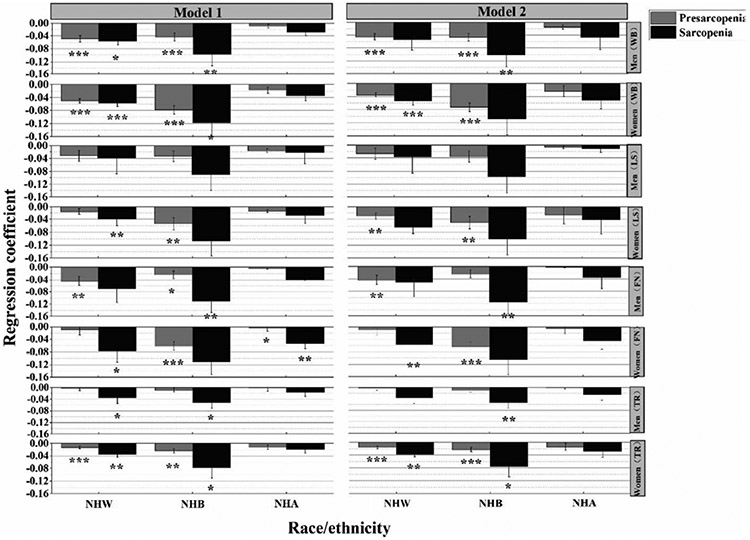
Adjusted regression coefficient of sarcopenia stages and regional/whole body BMD by race and sex. Model 1 adjusted for age and BMI. Model 2 adjusted for variables in model 1, plus exercise, smoking, milk consumption, alcohol use disorder, 15 min of sun exposure per day, hypertension history, fracture history, and menopausal hormone therapy in women. Normal participants were used for the reference group. WB, whole-body bone mineral density; LS, lumbar spine bone mineral density; FN, femoral neck bone mineral density; TR, total radius bone mineral density. NHW, non-Hispanic White people; NHB, non-Hispanic Black people; NHA, non-Hispanic Asian people. The EWGSOP2 definition of sarcopenia is being used. *P < 0.05; **P < 0.01; ***P < 0.001
The association between sarcopenia and whole-body BMD was strongest among non-Hispanic Black women (r = − 0.118, 95% CI = [− 0.239, − 0.002], P = 0.04) in model 1. But the difference disappeared after additional adjusting for behavioral and disease factors (model 2). The association between sarcopenia and whole-body BMD was strongest among non-Hispanic Black men (r = − 0.115, 95% CI = [− 0.189, − 0.041], P = 0.002) in model 2.
Overall, presarcopenic and/or sarcopenic individuals were more likely to have lower BMD compared with normal counterparts, regardless of in model 1 or model 2. Although the difference was not statistically significant in a few sub-groups. Among the three racial groups, non-Hispanic Black people had the strongest association between sarcopenia and BMD, followed by non-Hispanic White people and non-Hispanic Asian people. Among the four BMD sites in the study, the association between sarcopenia and BMD was relatively weak in lumbar spine, especially in men. In addition, significant differences of BMD across sarcopenia stages were found in more sub-groups in women than in men after adjusting for covariates.
Discussion
This study explored the relationship between sarcopenia stages and BMD across non-Hispanic White people, non-Hispanic Black people, and non-Hispanic Asian people. Overall, we conclude a generalizable negative association between sarcopenia stages and regional/whole-body BMD, although BMD of a few sites displayed no statistical difference among the three sarcopenia stages. Presarcopenic and/or sarcopenic individuals had lower BMD than their normal counterparts after controlling for physiological, behavioral, and disease factors, and the association was racial and sex-specific. These results revealed that sarcopenia should be regarded as a significant risk factor of low BMD. In addition, our results show a site-specific effect of sarcopenia stages on BMD, and significant differences of BMD across sarcopenia stages were found in more sub-groups in women than in men after adjusting for covariates. The role of racial and sex differences, as well as the site-specific effect in the relationship of sarcopenia stages and BMD, should be taken into account when developing strategies to maintain or minimize BMD loss. This study may provide particularly important information for preventing and managing sarcopenia and osteoporosis. It also provides some implications for practitioners and research scholars in this field.
When adjusting for age and BMI, a negative linear relationship was found between sarcopenia stages and regional/whole body BMD in non-Hispanic White people, non-Hispanic Black people, and non-Hispanic Asian men. A similar result was found in previous study, where sarcopenia was associated with decreased BMD and higher odds of osteoporosis [24, 25]. However, for non-Hispanic Asian women, no clear directionality association was found. That is, sarcopenia stages probably more relevant to BMD in non-Hispanic White people, non-Hispanic Black people, and non-Hispanic Asian men than non-Hispanic Asian women. Even the relationship between sarcopenia stages and BMD in non-Hispanic Asian women differed from non-Hispanic White people, non-Hispanic Black people, and non-Hispanic Asian men as identified in this study, there is not enough information to explain such a difference. Most studies have been restricted to White and Black participants only [17, 26]. The available evidence is quite limited regarding the relationship between sarcopenia stages and BMD for Asian population. Future studies examining the causes for the discrepancy is warrant.
By using the normal participants as the reference group, the relationship between sarcopenia stages and BMD showed racial and sex-specific. Among the three racial groups, non-Hispanic Black people had the strongest association between sarcopenia and BMD, followed by non-Hispanic White people and non-Hispanic Asian people. Previous studies confirmed that the prevalence of sarcopenia and osteoporosis was significantly varied among different racial groups, and more than 60.0% of the dependency in BMD could be attributed to genetic factors [27]. Although an earlier study indicated that difference in body composition, diet, and sociodemographic factors could explain most of the interracial differences in BMD [28], genomic studies suggest that the differences in BMD between different races may have more to do with genetic differences [29]. However, it is still unclear how genetic traits are involved in the interaction between sarcopenia and BMD.
The current study indicated that the relationship between sarcopenia and BMD was site-specific. Previous studies exploring the relationship between SMI and BMD suggested that the relationship was site-specific. Significant relationship was found between SMI and whole-body BMD, femoral neck BMD, and hip BMD, but not in lumbar spine BMD [30-32]. Regardless of model 1 and model 2, no significant difference was found between sarcopenia stages and lumbar spine BMD among male individuals. That is, among different sites in the study, the relationship between sarcopenia stages and lumbar spine BMD was relatively weak. Muscle stimulates the lumbar spine mainly through axial compression, while other sites are mainly through compression, bending and twisting [33]. Therefore, differences in muscle load patterns between the lumbar spine and other sites may contribute to explain this site-specific difference.
Overall, this study indicated that significant differences of BMD across sarcopenia stages were found in more sub-groups in women than in men after adjusting for covariates, which was similar to previous studies [34, 35]. We can conclude that for BMD outcomes, sarcopenia matters in women more than men. Physical activity, nutritional status, sex hormones, and growth hormones are important factors affecting muscle quality/quantity. The difference of the age-related changes of these factors between women and men may contribute to the gender difference in muscle and bone tissues [36]. However, the biological mechanisms that may account for the sex differences are not well understood and needs further study.
Both race and sex are demographic contributors to various health outcomes, including sarcopenia and osteoporosis/BMD [37, 38]. Race and sex add complexity to the relationship between sarcopenia stages and BMD. Therefore, we tried to minimize the confounding effects of these factors and stratified our analysis by race, as well as sex. It is known that non-Asians and women have a higher prevalence of sarcopenia than Asians and men, respectively [3, 39]. Hence, evaluating the interaction influences of race, sex, and sarcopenia on BMD is worthy of further study.
This study has several limitations. First, the causal relationship between sarcopenia stages and BMD cannot be drawn due to the cross-sectional design of the study. Second, muscle performance was not available in the current study. Consequently, the definition of sarcopenia in the current study did not reflect the complete implementation of the EWGSOP2 criteria. Third, the participants in the current study were required to travel to the research center and thus may represent a selection bias toward healthier individuals. Fourth, socioeconomic status has been reported to be associated with osteoporosis/BMD [40, 41]. However, socioeconomic status was not considered in the current study. The potential socioeconomic differences between the racial groups might exist and moderate the differences. Fifth, each racial group in this study was from a different location. Geographical variation is associated with a number of important factors for human health, such as differences in latitude, sun exposure time, and diet. All these factors may directly or indirectly lead to differences in sarcopenia and/or osteoporosis [42-44]. Therefore, these region-specific differences might impact the generalizability of the results. Sixth, the limited assessment of behavioral factors should be concerned. Although behavioral factors were covariates in the current study, all were treated as dummy variables. Some information might be missing without taking details of behavioral factors into account. For example, the assessment “do you drink milk” did not provide details on actual consumption. In addition, both current and past smokers were considered as “smoker,” and smoking amount was not included. This might not reflect the actual effects of smoke on health outcomes. Furthermore, “at least one of any type of exercise per week” might cause possible self-report bias, and the exercise frequency/type/intensity was not examined in the study. Future studies considering details on the assessment of behavioral factors when examining the associations between muscle and bone are warranted.
Conclusion
Overall, this study provides evidence that sarcopenia is significantly related to low regional or whole-body BMD and that these associations varied by race and sex. Presarcopenic and/or sarcopenic individuals had significantly lower BMD, regardless of site, than their normal counterparts for most sub-groups. Among the three racial groups, non-Hispanic Black people had the strongest association between sarcopenia and BMD, followed by non-Hispanic White people and non-Hispanic Asian people. Among the four BMD sites in the study, the association between sarcopenia and BMD is relatively weak in lumbar spine, especially in men. Furthermore, significant differences of BMD across sarcopenia stages were found in more sub-groups in women than in men after adjusting for covariates. Consideration in racial and sex-specific discrepancy when developing strategies to improve bone and muscle health in the aging process is warranted.
Supplementary Material
Appendix
Table 4.
Adjusted regression coefficient of sarcopenia stages and regional/whole body BMD by race and sex in model 1
| Race |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NHW |
NHB |
NHA |
||||||||
| R 2 | 95% CI | P value | R 2 | 95% CI | P value | R 2 | 95% CI | P value | ||
| WB BMD | ||||||||||
| Men | Presarcopenia | − 0.049 | (− 0.070, − 0.029) | < 0.001 | − 0.043 | (− 0.067, − 0.020) | < 0.001 | − 0.009 | (− 0.022, 0.041) | 0.555 |
| Sarcopenia | − 0.056 | (− 0.119, − 0.007) | 0.041 | − 0.097 | (− 0.167, − 0.028) | 0.006 | − 0.028 | (− 0.070, 0.013) | 0.182 | |
| Women | Presarcopenia | − 0.050 | (− 0.066, − 0.042) | < 0.001 | − 0.078 | (− 0.103, − 0.052) | < 0.001 | − 0.017 | (− 0.037, 0.003) | 0.095 |
| Sarcopenia | − 0.056 | (− 0.081, − 0.030) | < 0.001 | − 0.118 | (− 0.239, − 0.002) | 0.040 | − 0.034 | (− 0.057, 0.009) | 0.156 | |
| LS BMD | ||||||||||
| Men | Presarcopenia | − 0.032 | (− 0.064, 0.001) | 0.054 | − 0.033 | (− 0.066, 0.001) | 0.054 | − 0.016 | (− 0.045, 0.077) | 0.326 |
| Sarcopenia | − 0.038 | (− 0.061, 0.137) | 0.447 | − 0.089 | (− 0.188, 0.009) | 0.074 | − 0.022 | (− 0.110, 0.026) | 0.223 | |
| Women | Presarcopenia | − 0.015 | (− 0.055, 0.024) | 0.442 | − 0.054 | (− 0.091, − 0.017) | 0.005 | − 0.014 | (− 0.044, 0.016) | 0.364 |
| Sarcopenia | − 0.039 | (− 0.047, − 0.010) | 0.003 | − 0.107 | (− 0.283, 0.069) | 0.233 | − 0.029 | (− 0.088, 0.010) | 0.121 | |
| FN BMD | ||||||||||
| Men | Presarcopenia | − 0.044 | (− 0.073, − 0.014) | 0.003 | − 0.024 | (− 0.049, − 0.001) | 0.042 | − 0.005 | (− 0.028, 0.038) | 0.773 |
| Sarcopenia | − 0.069 | (− 0.159, 0.020) | 0.129 | − 0.110 | (− 0.186, − 0.039) | 0.003 | − 0.039 | (− 0.083, 0.005) | 0.084 | |
| Women | Presarcopenia | − 0.009 | (− 0.042, 0.023) | 0.579 | − 0.062 | (− 0.090, − 0.034) | < 0.001 | − 0.004 | (− 0.045, − 0.001) | 0.019 |
| Sarcopenia | − 0.077 | (− 0.126, − 0.011) | 0.049 | − 0.111 | (− 0.243, 0.021) | 0.099 | − 0.053 | (− 0.087, − 0.020) | 0.002 | |
| TR BMD | ||||||||||
| Men | Presarcopenia | − 0.004 | (− 0.035, 0.012) | 0.831 | − 0.008 | (− 0.022, 0.005) | 0.217 | − 0.002 | (− 0.016, 0.025) | 0.678 |
| Sarcopenia | − 0.034 | (− 0.076, − 0.008) | 0.012 | − 0.051 | (− 0.090, − 0.012) | 0.011 | − 0.017 | (− 0.045, 0.010) | 0.213 | |
| Women | Presarcopenia | − 0.013 | (− 0.020, − 0.006) | < 0.001 | − 0.023 | (− 0.037, − 0.010) | 0.001 | − 0.012 | (− 0.026, 0.002) | 0.096 |
| Sarcopenia | − 0.035 | (− 0.037, − 0.008) | 0.003 | − 0.077 | (− 0.141, − 0.013) | 0.018 | − 0.018 | (− 0.042, 0.005) | 0.119 | |
Model 1 adjusted for age and BMI. Normal participants were used for the reference group. The EWGSOP2 definition of sarcopenia is being used WB whole-body bone mineral density, LS lumbar spine bone mineral density, FN femoral neck bone mineral density, TR total radius bone mineral density, NHW non-Hispanic white people, NHB non-Hispanic black people, NHA non-Hispanic Asian people
Table 5.
Adjusted regression coefficient of sarcopenia stages and regional/whole body BMD by race and sex in model 2
| Race |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NHW |
NHB |
NHA |
||||||||
| R 2 | 95% CI | P value | R 2 | 95% CI | P value | R 2 | 95% CI | P value | ||
| WB BMD | ||||||||||
| Men | Presarcopenia | − 0.045 | (− 0.066, − 0.025) | < 0.001 | − 0.046 | (− 0.070, − 0.022) | < 0.001 | − 0.014 | (− 0.068, 0.041) | 0.620 |
| Sarcopenia | − 0.054 | (− 0.117, 0.008) | 0.089 | − 0.101 | (− 0.171, − 0.031) | 0.005 | − 0.047 | (− 0.122, 0.029) | 0.222 | |
| Women | Presarcopenia | − 0.035 | (− 0.068, − 0.023) | < 0.001 | − 0.073 | (− 0.099, − 0.048) | < 0.001 | − 0.024 | (− 0.078, 0.009) | 0.053 |
| Sarcopenia | − 0.054 | (− 0.080, − 0.029) | < 0.001 | − 0.109 | (− 0.229, 0.011) | 0.075 | − 0.051 | (− 0.104, 0.002) | 0.062 | |
| LS BMD | ||||||||||
| Men | Presarcopenia | − 0.027 | (− 0.060, 0.005) | 0.100 | − 0.036 | (− 0.070, 0.002) | 0.540 | − 0.007 | (− 0.079, 0.094) | 0.866 |
| Sarcopenia | − 0.037 | (− 0.062, 0.135) | 0.467 | − 0.097 | (− 0.196, 0.002) | 0.055 | − 0.012 | (− 0.233, 0.008) | 0.068 | |
| Women | Presarcopenia | − 0.029 | (− 0.048, − 0.010) | 0.002 | − 0.050 | (− 0.087, − 0.012) | 0.009 | − 0.027 | (− 0.082, 0.028) | 0.338 |
| Sarcopenia | − 0.064 | (− 0.083, 0.025) | 0.477 | − 0.101 | (− 0.276, 0.074) | 0.256 | − 0.041 | (− 0.187, 0.015) | 0.052 | |
| FN BMD | ||||||||||
| Men | Presarcopenia | − 0.043 | (− 0.072, − 0.013) | 0.005 | − 0.024 | (− 0.050, 0.001) | 0.062 | − 0.002 | (− 0.051, 0.055) | 0.931 |
| Sarcopenia | − 0.051 | (− 0.161, 0.018) | 0.118 | − 0.115 | (− 0.189, − 0.041) | 0.002 | − 0.035 | (− 0.139, 0.009) | 0.083 | |
| Women | Presarcopenia | − 0.009 | (− 0.042, 0.023) | 0.573 | − 0.064 | (− 0.092, − 0.035) | < 0.001 | − 0.005 | (− 0.065, 0.002) | 0.068 |
| Sarcopenia | − 0.057 | (− 0.126, − 0.011) | 0.001 | − 0.105 | (− 0.238, 0.028) | 0.122 | − 0.045 | (− 0.098, 0.007) | 0.092 | |
| TR BMD | ||||||||||
| Men | Presarcopenia | − 0.002 | (− 0.016, 0.012) | 0.763 | − 0.010 | (− 0.023, 0.004) | 0.167 | − 0.001 | (− 0.039, 0.022) | 0.594 |
| Sarcopenia | − 0.035 | (− 0.077, 0.007) | 0.105 | − 0.053 | (− 0.092, − 0.013) | 0.009 | − 0.024 | (− 0.083, 0.002) | 0.062 | |
| Women | Presarcopenia | − 0.016 | (− 0.023, − 0.009) | < 0.001 | − 0.023 | (− 0.036, − 0.009) | < 0.001 | − 0.015 | (− 0.047, 0.003) | 0.056 |
| Sarcopenia | − 0.038 | (− 0.038, − 0.009) | 0.002 | − 0.076 | (− 0.140, − 0.012) | 0.020 | − 0.029 | (− 0.053, 0.015) | 0.264 | |
Adjusted regression coefficient of sarcopenia stages and regional/whole body BMD by race and sex. Model 2 adjusted for variables in model 1, plus exercise, smoking, milk consumption, alcohol use disorder, 15 min of sun exposure per day, hypertension history, and fracture history, plus menopausal hormone therapy in women. Normal participants were used for the reference group. The EWGSOP2 definition of sarcopenia is being used WB whole-body bone mineral density, LS lumbar spine bone mineral density, FN femoral neck bone mineral density, TR total radius bone mineral density, NHW non-Hispanic white people, NHB non-Hispanic black people, NHA non-Hispanic Asian peoples
Footnotes
Conflicts of interest None.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00198-020-05744-y.
Publisher′s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763 [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R (2017) Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord 16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL (2019) Economic impact of hospitalizations in US adults with sarcopenia. J Frailty Aging 8:93–99 [DOI] [PubMed] [Google Scholar]
- 5.Compston J (2010) Osteoporosis: social and economic impact. Radiol Clin N Am 48:477–482 [DOI] [PubMed] [Google Scholar]
- 6.Mariconda M, Costa GG, Cerbasi S, Recano P, Orabona G, Gambacorta M, Misasi M (2016) Factors predicting mobility and the change in activities of daily living after hip fracture: a 1-year prospective cohort study. J Orthop Trauma 30:71–77 [DOI] [PubMed] [Google Scholar]
- 7.Drake MT, Murad MH, Mauck KF et al. (2012) Clinical review. Risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 97:1861–1870 [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R (2008) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19:399–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JJ, Aghdassi E, Cheung AM et al. (2012) Ten-year absolute fracture risk and hip bone strength in Canadian women with systemic lupus erythematosus. J Rheumatol 39:1378–1384 [DOI] [PubMed] [Google Scholar]
- 10.Nelson HD, Haney EM, Dana T, Bougatsos C, Chou R (2010) Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann Intern Med 152(2):99–111 [DOI] [PubMed] [Google Scholar]
- 11.Schurman L, Bagur A, Claus-Hermberg H, Messina OD, Negri AL, Sánchez A, González C, Diehl M, Rey P, Gamba J, Chiarpenello J, Moggia MS, Mastaglia S (2013) Guidelines for the diagnosis, prevention and treatment of osteoporosis, 2012. Medicina 73:55–74 [PubMed] [Google Scholar]
- 12.Kanis JA (2007) WHO Technical Report. University of Sheffield, UK, p 66 [Google Scholar]
- 13.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 14.Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisani P, Renna MD, Conversano F, Casciaro E, Di Paola M, Quarta E, Muratore M, Casciaro S (2016) Major osteoporotic fragility fractures: risk factor updates and societal impact. World J Orthop 7:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis E, Litwic A, Cooper C, Dennison E (2015) Determinants of muscle and bone aging. J Cell Physiol 230:2618–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looker AC, Melton LJ, Harris T, Borrud L, Shepherd J, McGowan J (2009) Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos Int 20:1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verschueren S, Gielen E, O’Neill TW, Pye SR, Adams JE, Ward KA, Wu FC, Szulc P, Laurent M, Claessens F, Vanderschueren D, Boonen S (2013) Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int 24:87–98 [DOI] [PubMed] [Google Scholar]
- 19.Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C (2001) Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology 47:207–212 [DOI] [PubMed] [Google Scholar]
- 20.Deng HW, Shen H, Xu FH, Deng HY, Conway T, Zhang HT, Recker RR (2002) Tests of linkage and/or association of genes for vitamin D receptor, osteocalcin, and parathyroid hormone with bone mineral density. J Bone Miner Res Off J Am Soc Bone Miner Res 17(4):678–686. 10.1359/jbmr.2002.17.4.678 [DOI] [PubMed] [Google Scholar]
- 21.Du Y, Zhao LJ, Xu Q, Wu KH, Deng HW (2017) Socioeconomic status and bone mineral density in adults by race and gender: the Louisiana osteoporosis study. Osteoporos Int 28:1699–1709 [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D (2002) Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr 76:378–383 [DOI] [PubMed] [Google Scholar]
- 23.Carey G (2013) Chapter 9 The General Linear Model (GLM): A gentle introduction. In: Quantitative Methods In Neuroscience. http://psych.colorado.edu/~carey/qmin/QMIN_2013_03_17.pdf. Accessed 05 May2019 [Google Scholar]
- 24.He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW (2016) Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int 27:473–482 [DOI] [PubMed] [Google Scholar]
- 25.Cheng Q, Zhu X, Zhang X, Li H, Du Y, Hong W, Xue S, Zhu H (2014) A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass [J]. J Bone Miner Metab 32(1):78–88 [DOI] [PubMed] [Google Scholar]
- 26.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB (2001) Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res 16:1343–1352 [DOI] [PubMed] [Google Scholar]
- 27.Farber CR(2012) Systems genetics: a novel approach to dissect the genetic basis of osteoporosis [J]. Curr Osteoporos Rep 10(3):228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travison TG, Chiu GR, McKinlay JB, Araujo AB (2011) Accounting for racial/ethnic variation in bone mineral content and density: the competing influences of socioeconomic factors, body composition, health and lifestyle, and circulating androgens and estrogens [J]. Osteoporos Int 22(10):2645–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Megyesi MS, Hunt LM, Brody H (2011) A critical review of racial/ethnic variables in osteoporosis and bone density research [J]. Osteoporos Int 22(6):1669–1679 [DOI] [PubMed] [Google Scholar]
- 30.Lima RM, de Oliveira RJ, Raposo R, Neri SGR, Gadelha AB (2019) Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women [J]. Arch Osteoporos 14(1):38. [DOI] [PubMed] [Google Scholar]
- 31.Blain H, Vuillemin A, Teissier A (2001) Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over [J]. Gerontology 47(4):207–212 [DOI] [PubMed] [Google Scholar]
- 32.Kim KM, Lee EY, Lim S, Jang HC, Kim CO (2017) Favorable effects of skeletal muscle on bone are distinguished according to gender and skeletal sites [J]. Osteoporos Sarcopenia 3(1):32–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva MJ (2007) Biomechanics of osteoporotic fractures [J]. Injury 38(Suppl 3):S69–S76 [DOI] [PubMed] [Google Scholar]
- 34.Ribom E, Ljunggren O, Piehl-Aulin K, Ljunghall S, Bratteby LE, Samuelson G, Mallmin H (2004) Muscle strength correlates with total body bone mineral density in young women but not in men [J]. Scand J Med Sci Sports 14(1):24–29 [DOI] [PubMed] [Google Scholar]
- 35.Bevier WC, Wiswell RA, Pyka G, Kozak KC, Newhall KM, Marcus R (1989) Relationship of body composition, muscle strength, and aerobic capacity to bone mineral density in older men and women [J]. J Bone Miner Res 4(3):421–432 [DOI] [PubMed] [Google Scholar]
- 36.Philippou A, Maridaki M, Halapas A, Koutsilieris M (2007) The role of the insulin-like growth factor 1 (IGF-1) in skeletal muscle physiology [J]. In Vivo 21(1):45–54 [PubMed] [Google Scholar]
- 37.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine [J]. J Bone Miner Res 29(11):2520–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reginster JY, Burlet N (2006) Osteoporosis: a still increasing prevalence [J]. Bone 38(2 Suppl 1):S4–S9 [DOI] [PubMed] [Google Scholar]
- 39.Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50(5):889–896 [DOI] [PubMed] [Google Scholar]
- 40.Travison T, Chiu G, McKinlay J, Araujo A (2011) Accounting for racial/ethnic variation in bone mineral content and density: the competing influences of socioeconomic factors, body composition, health and lifestyle, and circulating androgens and estrogens. Osteoporos Int 22:2645–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro MC, Saavedra P, Jódar E, Gómez DT, Mirallave A, Sosa M (2013) Osteoporosis and metabolic syndrome according to socioeconomic status, contribution of PTH, vitamin D and bodyweight: the Canarian osteoporosis poverty study (COPS). Clin Endocrinol 78:681–686 [DOI] [PubMed] [Google Scholar]
- 42.Lips P, van Schoor NM (2011) The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab 25(4):585–591 [DOI] [PubMed] [Google Scholar]
- 43.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE (2013) The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev 34(1):33–83 [DOI] [PubMed] [Google Scholar]
- 44.Gunton JE, Girgis CM (2018) Vitamin D and muscle. Bone Rep 8:163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


