Abstract
BACKGROUND:
To fully characterize the risk for dementia associated with cigarette smoking, studies must consider competing risks that hinder the observation of dementia or modify the chance that dementia occurs (i.e., death). Extant research examining the competing risks fails to account for the occurrence of death following dementia, limiting our understanding of the relation between smoking and dementia.
OBJECTIVE:
Examine the impact of smoking status, lifetime smoking exposure, and duration of abstinence on incident dementia, death following dementia, and death without dementia.
METHODS:
Multi-state models estimated hazard ratios (HR) for 95% confidence interval (CI) of 10,681 cognitively healthy adults for transition from baseline to dementia, baseline to death, and dementia to death based on smoking status, lifetime cigarette exposure, and abstinence duration.
RESULTS:
Compared to never smokers, current smokers had increased risk of dementia (HR=1.66; 95%CI 1.18-2.32; p=.004), and death from baseline (HR=2.98; 95%CI 2.24-3.98; p<.001) and incident dementia (HR=1.88; 95%CI 1.08-3.27; p=.03). Pack years increased risk of death from baseline (HR=1.01; 95%CI 1.00-1.01; p<.001), but not dementia risk (HR=1.00; 95%CI 1.00-1.00; p=.78) or death following dementia (HR=1.01; 95%CI 1.00-1.01; p=.05). Recent quitters (quit <10 years), compared to never smokers, had increased risk of death after baseline (HR=2.31; 95%CI 1.55-3.43; p<.001), but not dementia (HR=1.17; 95%CI 0.73-1.88; p=.52) or death following dementia (HR=1.01; 95%CI 0.42-2.41; p=.99).
CONCLUSIONS:
Current smoking increases the risk for dementia and death, but dementia is better attributed to smoking recency than lifetime exposure. Smoking cessation at any age might reduce these risks for cognitively healthy individuals.
Keywords: cigarette smoking, dementia, mortality, statistics, smoking cessation
1. Introduction
Cigarette smoking is the leading preventable cause of death and disability in the United States (U.S.), accounting for 480,000 deaths and over $300 billion in medical costs annually [1, 2]. Smoking harms nearly every organ in the human body, including the brain [2]. Although the link between smoking and various forms of dementia is not singular, smoking is thought to contribute to dementia risk, either directly or indirectly (e.g., via cardiovascular health [3]). Cigarette smoking prospectively increases the risk of Alzheimer’s disease onset by 70%, accounting for 13.9% of individuals with Alzheimer’s disease and related dementias (ADRD) [4-6]. Currently, 5.8 million adults have ADRD, resulting in annual medical costs of approximately $290 billion [7]. Moreover, the incidence of dementia is expected to triple by 2050 [7]. Smoking and ADRD represent two of the most devastating public health crises that face the U.S. today [1, 2, 7].
Unfortunately, the smoking-dementia relationship is continually questioned [8, 9], possibly due to early studies suggesting that smoking protected against dementia onset [10, 11]. However, a large 2010 meta-analysis demonstrated that tobacco industry affiliation moderated the effects of smoking on dementia; studies affiliated with the tobacco industry showed favorable or null results, while the majority not affiliated with the industry demonstrated consistent negative impact of smoking on dementia [12].
Competing risks (i.e., events of similar clinical importance that prevent or alter the likelihood of the main outcome of interest such as dementia [13]) complicate studies of geriatric populations because increasing age (the strongest risk factor for ADRD [7]) is associated with risk for medical co-morbidities [14] and they may further underestimate the relationship between smoking and dementia. The multitude of negative health effects of cigarette smoking and known impact on mortality [1, 2] may result in a smoker not developing dementia, or being excluded from studies examining risk of dementia, due to earlier mortality and subsequent ineligibility for epidemiological studies [15]. Therefore, case control studies (vs. cohort studies) may underestimate the risk of cigarette smoking on dementia due to the competing smoking-related risks of death and disease (i.e., cardiovascular, lung, cancer) [16].
Recent work by Abner and colleagues [17] utilized Fine and Grey’s competing risk model [18] to address competing risks and found that smoking was associated with an increased risk of death, but not dementia. This competing risk model breaks down the composite outcome of death or dementia (Fig 1A) into subdistributed hazards to more accurately determine the impact of smoking on competing outcomes (Fig 1B). The Fine and Grey method [18], however, does not allow for transition from dementia to death. A multi-state model (Fig 1C), that accounts for the informative eventuality of death and allows for transition from baseline to dementia, and also from dementia to death, would provide the most comprehensive estimate of the effect of smoking on dementia [19].
Figure 1.
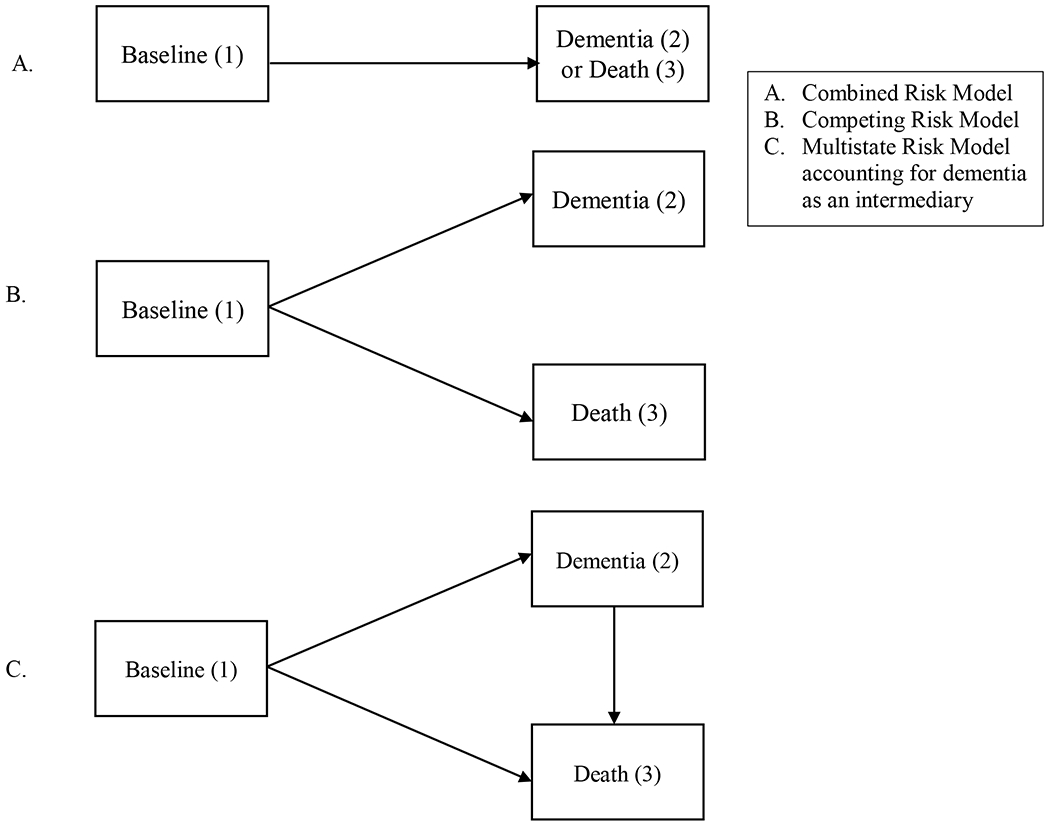
Benefit of multistate models in dementia risk (adapted from [41]).
Inclusion of potential proximal variables for dementia, like nursing home placement, would also allow for a more comprehensive assessment of the impact of smoking on dementia. More than two-thirds of nursing home residents have dementia [20], making it one of the primary reasons for nursing home placement [21]. Therefore, nursing home placement may be a proxy for undiagnosed dementia. Although the literature is limited, findings generally support that smoking is linked to increased risk for nursing home placement [22].
Granular analyses of the impact of various smoking-related variables (e.g., lifetime smoking, current smoking, duration of abstinence) on dementia risk is necessary, especially in models considering risks for dementia, death, and death following dementia. These variables provide insight into possible biological mechanisms related to the damage caused by smoking (e.g., dose effect) and whether longer abstinence can ameliorate this risk. Although research suggests that former smokers are at decreased risk for dementia onset compared to current smokers [6], it remains unclear how long it takes for smoking-related risk to abate. Research suggests greater lifetime tobacco exposure is related to increased risk of various forms of dementia, but results are mixed [17, 23, 24]. These studies do not include nursing home placement as potential indicator of dementia or account for competing smoking-related risks (i.e., death), perhaps underestimating the impact of smoking on incident dementia. The lone study utilizing competing risk analyses [18] does not examine the transition from dementia to death, and uses a relatively small sample (N=531) from one, racially homogenous, U.S. location [17].
The current study examined the impact of smoking status, lifetime smoking exposure, and duration of abstinence on the key outcomes of incident dementia diagnosis (including dementia indicated by a composite of nursing home placement and a cognitive rating scale), death, and the transition from dementia to death among a large, racially diverse sample of baseline cognitively healthy adults to provide insight into the impact and potential mechanisms of action of this modifiable risk factor. Using data from the National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set (UDS) [25], we tested four hypotheses: 1) compared to never smokers, current smokers will be at elevated risk for subsequent dementia onset as well as death directly following baseline and after dementia (Model 1); 2) former smokers (those who quit smoking prior to baseline) will be comparable to never smokers in terms of subsequent dementia onset (Model 1); 3) among all participants, greater lifetime tobacco exposure will be associated with earlier onset of dementia as well as death (Model 2); and 4) the longer participants are abstinent, the more comparable they will be in terms of dementia risk compared to never smokers (Model 3).
2.0. Methods
2.1. Study Design and Participants
The NACC UDS [25] collects data from multiple U.S.-based Alzheimer’s Disease Centers (ADCs). Approximately 725 variables including, but not limited to, sociodemographic, medical history, current medications, family history, neurological examination findings, functional status, neuropsychological test results, clinical diagnoses, imaging, and genetic information were collected (but not required) for each participant. Clinicians determined clinical diagnoses (e.g., normal cognition, dementia) at the initial visit and follow-up visits using clinical data collected at the research visit (Appendix). Data were collected from 33,444 participants between September 2005 to December 2018. Inclusion criteria were completion of baseline and at least one follow-up visit, baseline age ≥ 45 years old, and baseline cognitively healthy status. To examine the impact of smoking variables on subsequent dementia diagnosis, participants with a baseline diagnosis of dementia or mild cognitive impairment (MCI) were excluded, as well as those predisposed to or diagnosed with strongly heritable forms of dementia (Down syndrome, dominantly inherited Alzheimer’s disease (AD) mutation, hereditary Frontotemporal Lobar Degeneration (FTLD) mutation, or hereditary mutation other than AD or FTLD). Further exclusion criteria by model are described in the analysis section (Fig 2). Participants included in these analyses (N=10,578) were, on average, 72 (SD 9.5) years old at baseline.
Figure 2.
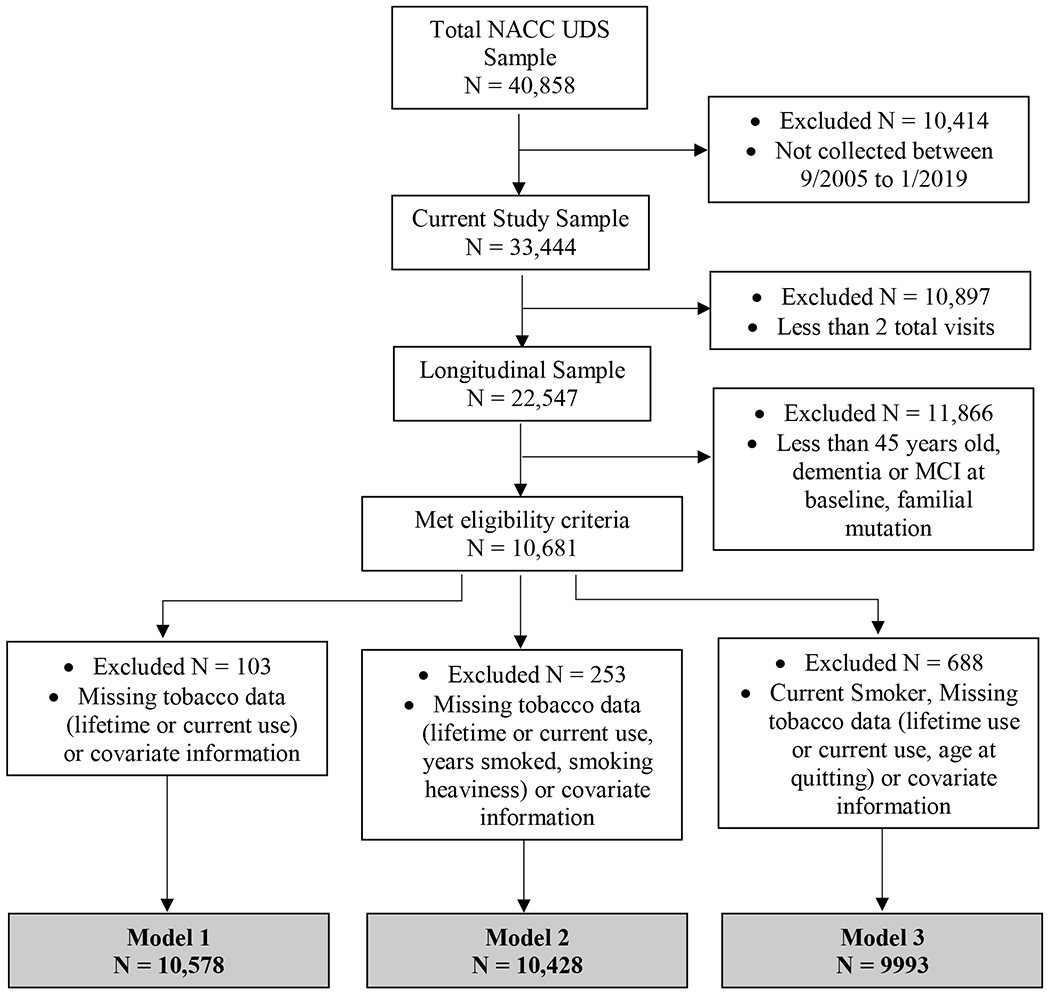
Consortium Diagram
2.2. Measures
Tobacco-related information was consistently collected at baseline only; follow-up appointments completed after March 2015 did not assess smoking information. Smoking status was coded as follows. Current smokers reported smoking > 100 cigarettes in their lifetime and smoking within the past 30 days. Former smokers reported smoking > 100 cigarettes in their lifetime but denied smoking in the past 30 days. Never smokers reported smoking ≤ 100 cigarettes in their lifetime. Lifetime exposure was defined as pack years, calculated by multiplying years smoked by average number of packs smoked per day. Average cigarettes smoked per day were codified in the original dataset. To obtain an exposure amount, the median of ranges of packs per day used (e.g., 1-1.5 pack range became 1.25 packs). Duration of abstinence was calculated by subtracting reported age of quitting from baseline age. To determine the relationship between duration of abstinence and risk of dementia, while permitting interpretation of non-linear behavior over the abstinence time period, abstinence duration was categorized by decades of abstinence (i.e., abstinent for < 10 years, abstinent between 10 to < 20 years, abstinent between 20 to < 30 years, abstinent for ≥ 30 years).
Participants with normal cognition or cognitively impaired but not MCI at baseline were categorized as Cognitively Healthy (see Appendix for grouping rationale). Dementia diagnosis at follow-up was identified one of two ways: 1) via clinical evaluation at follow-up, or 2) if placed in a nursing home and CDR® Dementia Staging Instrument (CDR [26, 27]) global score ≥ 1. Follow-up contact occurred approximately annually. Nursing home placement (i.e., participant permanently moved to a nursing home) and death were reported by a designated informant at follow-up. Participants who were discontinued or lost to follow-up could not report these statuses. Age-at-event was determined by death date for death events, and follow-up visits date for incident dementia and nursing home placement events.
Additional baseline measures included patient-reported presence of recent/active diabetes or recent/active hypertension and Mini-Mental State Exam score (MMSE) [28]. Demographic variables included race, education, gender, and age at baseline (Table 1). “Unknown” responses of covariates were included as their own category for analyses.
Table 1.
Demographic information for participants by baseline smoking status.
| Demographics | |||
|---|---|---|---|
| Baseline Smoking Status | Never Smoker | Former Smoker | Current Smoker |
| N | 5827 | 4425 | 429 |
| Age at baseline – Mean (SD)*** | 71.7 (9.7) | 72.8 (8.7) | 68.8 (9.2) |
| Females (N; %) *** | 3980; 68.3% | 2650; 59.9% | 270; 62.9% |
| Race (N; %) *** | |||
| White | 4693; 80.5% | 3707; 83.8% | 277; 64.6% |
| African American | 858; 14.7% | 589; 13.3% | 141; 32.9% |
| American Indian or Alaskan Native | 27; 0.5% | 18; 0.4% | 1; 0.2% |
| Native Hawaiian or Pacific Islander | 4; 0.1% | 3; 0.1% | 0; 0.0% |
| Asian | 193; 3.3% | 63; 1.4% | 0; 0.0% |
| Other | 40; 0.7% | 36; 0.8% | 10; 2.3% |
| Unknown | 12; 0.2% | 9; 0.2% | 0; 0.0% |
| Education (N; %) *** | |||
| Less than high school | 273; 4.7% | 181; 4.1% | 35; 8.2% |
| High School | 825; 14.2 | 634; 14.4% | 89; 20.3% |
| 13-15 Years (Some College) | 1008; 17.4% | 930; 21.1% | 112; 26.1% |
| 16 Years (Bachelor’s Degree) | 1333; 23.0% | 1076; 24.4% | 84; 19.6% |
| >16 Years (some graduate) | 2367; 40.8% | 1584; 36.0% | 109; 25.4% |
| MMSE Score Baseline – Mean (SD) *** | 29.81 (13.01) | 29.23 (11.28) | 27.86 (10.10) |
| Recent History of Cardiovascular/ Cerebrovascular Risk (N; %) | |||
| Diabetes*** | 582; 10.0% | 547; 12.4% | 61; 14.3% |
| Hypertension** | 2690; 46.3% | 2173; 49.2% | 220; 51.5% |
2.3. Analyses
Chi-square tests of independence and Kruskal-Wallis tests were used to examine differences between baseline smoking status (never, former, and current) in demographics, cardiovascular/cerebrovascular risk, and MMSE at baseline. Multi-state models used Cox proportional hazards to estimate hazard ratios (HR) and 95% confidence intervals (CI) for smoking predictors and were setup to mimic an illness-death model [19] (Fig 1C), where there are three states a subject can occupy (healthy, dementia, or death), and subjects can transition from healthy to dementia or healthy to death, and those who transitioned to dementia can then transition to death. Each transition used age as the time scale, with a delayed-entry setup to account for varying age of study entry and varying age of dementia diagnosis. In addition to the smoking predictor, each model and transition included baseline reported education, gender, recent/active hypertension, and recent/active diabetes as a priori covariates due to their known impact on dementia risk [7]. “Unknown” responses for main predictor variables were considered to be missing data and were excluded from analyses.
Multi-state time-to-event regression and diagnostics were performed using R 3.6.1, with mstate [19, 29] package for data formatting, and survival [30, 31] package for fitting. IBM SPSS Statistics V25 Premium was used for all other analyses. No major concerns for non-proportionality of covariates were found.
Model 1 examined the differences between never, former, and current smokers on age to dementia onset. Model 2 examined the role of lifetime smoking exposure in pack years (measured as a continuous variable) on age to dementia onset. Model 3 examined duration of abstinence among former and never smokers on age to dementia onset, using never smokers as the reference category.
Due to the three transitions in each model, and the smoking predictor being included as a covariate in each transition direction within a model, each model has multiple p-values associated with the smoking outcome (i.e., primary covariate). Benjamini-Hochberg correction to these p-values were performed, controlling false discovery rate (FDR) at 5% for the smoking-related covariates within each model [32, 33].
2.3.1. Sensitivity Analyses.
Pack-years was added as a covariate in Model 3 to determine if level of smoking exposure affected results. Due to collinearity between pack years and duration of abstinence (i.e., “never smokers” pack year value is always 0), the effect of pack years could only be estimated for the baseline to dementia transition. To ensure findings related to conversion from dementia to death were not solely due to the known impact of smoking on cardiovascular risk factors (e.g., stroke) [2], we descriptively examined duration of time in each state prior to transition and presence of stroke by smoking status.
3. Results
Current smokers were younger, more likely to be African American, less educated, and had lower baseline MMSE scores than never and former smokers. Never smokers had lower rates of diabetes and hypertension and were more likely to be male than current smokers or former smokers (Table 1).
3.1. Smoking status
Current smokers were at increased risk for dementia, and conversion to death from both baseline and incident dementia, compared to never smokers (Model 1 in Table 2; Fig 3). Former smokers were at decreased risk for dementia onset compared to never smokers, but did not statistically differ from never smokers in terms of risk for death from either baseline or dementia diagnosis. Findings remained substantively unchanged after correcting for FDR.
Table 2.
Cox-regression proportional hazard models for Models 1, 2, and 3 for baseline cognitively healthy individuals
| Model 1 (Smoking Status) | |||
|---|---|---|---|
| Baseline to Incident Dementia (1→2) | Baseline to Death (1→3) | Incident Dementia to Death (2→3) | |
| Hazard Ratio (95% confidence interval); p value; Absolute number of events | Hazard Ratio (95% confidence interval); p value; Absolute number of events | Hazard Ratio (95% confidence interval); p value; Absolute number of events | |
| Never Smoker | 1.00 (Reference); 465 | 1.00 (Reference); 479 | 1.00 (Reference); 165 |
| Former Smoker | .845 (.730 – .978); p = .024*; 304 | 1.124 (.983 – 1.285); p = .087; 412 | 1.087 (.843 – 1.402); p = .521; 102 |
| Current Smoker | 1.656 (1.181 – 2.321); p = .004**; 37 | 2.981 (2.235 – 3.976); p < .001***; 53 | 1.879 (1.081 – 3.266); p = .025*; 17 |
| Model 2 (Smoking Exposure) | |||
| Baseline to Incident Dementia (1→2) | Baseline to Death (1→3) | Incident Dementia to Death (2→3) | |
| Hazard Ratio (95% confidence interval); p value; Absolute number of events | Hazard Ratio (95% confidence interval); p value; Absolute number of events | Hazard Ratio (95% confidence interval); p value; Absolute number of events | |
| Pack Years | 1.001 (.997 – 1.004); p = .780; 787 | 1.008 (1.005 – 1.010); p < .001***; 928 | 1.006 (1.000 – 1.013); p = .054; 278 |
| Model 3† (Abstinence Duration) | |||
| Baseline to Incident Dementia (1→2) | Baseline to Death (1→3) | Incident Dementia to Death (2→3) | |
| Hazard Ratio (95% confidence interval); p value | Hazard Ratio (95% confidence interval); p value | Hazard Ratio (95% confidence interval); p value | |
| Never Smoker | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Quit < than 10 years | 1.171 (.728 – 1.885); p = .516 | 2.308 (1.553 – 3.429); p < .001*** | 1.007 (.420 – 2.413); p = .988 |
| Quit 10 to < than 20 years | 1.206 (.883 – 1.646); p = .239 | 1.361 (.997 – 1.858); p = .053 | 1.578 (.896 – 2.780); p = .114 |
| Quit 20 to < than 30 years | .790 (.597 – 1.046); p = .100 | 1.279 (1.014 – 1.611); p = .037*‡ | 1.055 (.631 – 1.764); p = .837 |
| Quit 30 or more years | .780 (.655 – .930); p = .006** | .993 (.848 – 1.162); p = .927 | 1.064 (.789 – 1.435); p = .684 |
p<.05
p<.01
p<.001
Note. Covariates for adjusted models included gender, education, diabetes status, and hypertension status. Presented p-values are prior to adjusting for false discovery rates.
Findings from Model 3 remained substantively unchanged after adding smoking exposure (pack years) to the model as a sensitivity analysis.
Findings were no longer significant after adjusting for false discovery rates.
Figure 3.
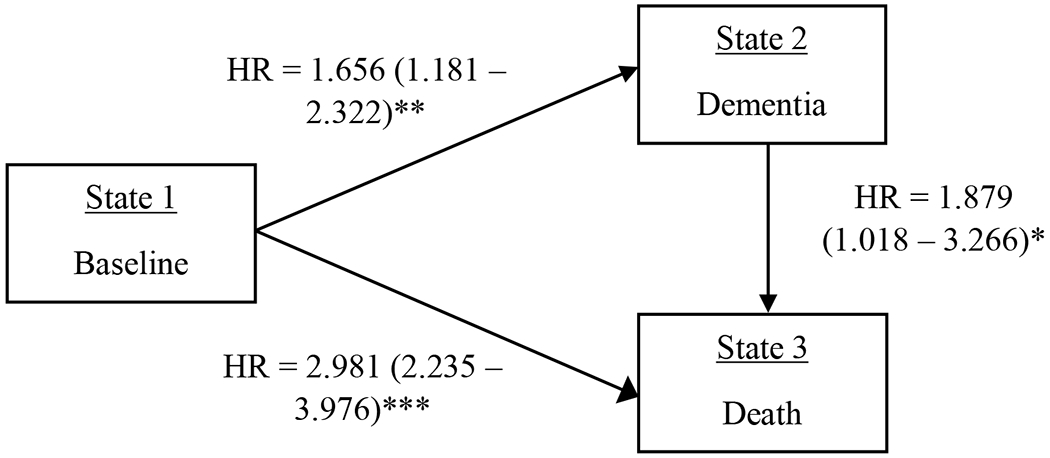
Multi-state hazard ratios, confidence intervals, and p-values for current smokers compared to never smokers.
Note. *p < .05; **p < .01; ***p < .001.
3.2. Lifetime exposure
Results revealed no significant association of smoking exposure (in pack years) on incident dementia diagnosis. Greater lifetime cigarette exposure was related to increased risk of death from baseline, but not from dementia diagnosis (Model 2 in Table 2). Results remained substantively unchanged after correcting for FDR.
3.3. Abstinence duration
Former smokers with up to 30 years of abstinence did not show statistically significant differences in risk of dementia compared to never smokers (Model 3 in Table 2), although a linear trend for reduced risk over time was observed. Interestingly, former smokers with more than 30 years of abstinence were less likely to convert to dementia compared to never smokers. When converting from baseline to death, participants abstinent for < 10 years and between 20 to < 30 years were at increased risk of conversion, all other results were not statistically different from never smokers. The relation between smokers quit for 20 to < 30 years was no longer significant when controlling for FDR; all other results remained substantively unchanged. Former smokers did not significantly differ from never smokers in terms of risk of transition from incident dementia to death, regardless of abstinence duration.
3.4. Sensitivity analyses
Sensitivity analyses for abstinence duration, which controlled for pack years in addition to previously listed covariates, showed similar results to those corrected for FDR (Model 3 in Table 2). Sensitivity analyses examining the potential impact of stroke on findings looked at prevalence of stroke in current versus never smokers after baseline, and duration in each state prior to transition (i.e., time before transitioning to dementia following baseline assessment, death following baseline assessment, or death following dementia diagnosis). Participants who switched states spent approximately 4-5 years in cognitive status established at baseline (i.e., in a cognitively healthy status) prior to dementia diagnosis, 5-6 years in cognitive status established at baseline prior to death, and 2-3 years following dementia diagnosis before dying. Current smokers reported fewer strokes following baseline than never smokers (50.0% versus 56.3%).
4. Discussion
This analysis is the first to comprehensively examine the role of cigarette smoking, a modifiable risk factor for ADRD, on the risk of death, dementia, or death following dementia. Moreover, this is the first study to include nursing home placement (if cognitive status was impaired as estimated by an elevated CDR [26] score), a likely proxy for ADRD diagnosis [21, 22]. Current smokers were 1.7 times more likely to convert to dementia, almost three times more likely to convert to death from baseline, and almost twice as likely to convert to death following a dementia diagnosis compared to never-smokers, supporting our first hypothesis.
These results are contrary to recently publicized work examining the competing risks of dementia and death in smoking [17] and suggest that examination of outcomes following dementia onset coupled with a more comprehensive assessment of dementia (i.e., dementia and/or nursing home placement with cognitive impairment) results in more robust impact of smoking status on ADRD-related outcomes. Notably, the NACC UDS includes data used in the aforementioned manuscript [17] as well as data collected from other ADCs. These results are consistent with existing literature demonstrating that cigarette smoking negatively impacts ADRD onset [6] as well as mortality [1, 2]. Sensitivity analyses also suggest that conversion to dementia was not solely due to cardiovascular events (i.e., stroke) for current or former smokers and remained irrespective of pack years smoked. Together, findings paint a picture that smoking not only results in death, but can also lead to suffering through lost cognitive functioning and independence.
Former smokers were significantly less likely to convert from baseline to dementia than never smokers. This unexpected finding was inconsistent with our hypothesis. Findings from Model 3 coupled with the majority (56%) of former smoker reporting abstinence for ≥ 30 years highlight a potential cohort effect of those who quit long ago. A broader shift towards healthy lifestyle changes among former smokers may have contributed to decreased risk of dementia. Notably, this change in smoking behavior did not result in decreased risk of death (from baseline or following dementia) compared to never smokers.
Contrary to our hypothesis and research demonstrating a dose-response relationship between smoking and ADRD onset [23, 24, 34-37], there was no association between lifetime smoking exposure and incident dementia onset. However, greater smoking exposure was associated with increased risk for death from baseline suggesting that the impact of lifetime smoking exposure is more related to death than other ADRD-associated outcomes (i.e., dementia onset or conversion from dementia to death) [17]. This is consistent with research highlighting the dose-response of cigarette smoking exposure on mortality [2, 38], and reinforces the critical impact of current smoking, rather than the cumulative impact of smoking, on ADRD onset.
We also explored whether there were any recency effects. Our hypothesis that, compared to never smokers, longer abstinence from smoking would be associated with lowered risk of ADRD was only partially supported by the data. Recent (<10 years) and intermediate (10 to <20 years) quitters showed no significant differences in risk for dementia compared to never smokers. However, we must be cautious in assuming that a lack of statistical significant difference infers equality; a larger sample size of recently quit smokers (< 10 years) may find a difference in terms of dementia risk. Given the trend of hazard ratio decreasing by time of abstinence (i.e., the longer someone has quit the lower their risk for incident dementia) coupled with the decreased standard errors for longer duration of abstinence, we can surmise that greater duration of abstinence is related to decreased risk of ADRD. Abstinence from smoking ≥ 30 years had significantly lower hazard-rates of developing dementia than never smokers, suggesting that early cessation is associated with improved health. This effect may be related to a larger sample size of those quit for 30 or more years, an overall change in health behaviors (e.g., greater health awareness), or a cohort effect; the underlying causes of this effect cannot be assessed in the current data.
In comparison, it took 10 years for former smokers to appear not statistically different to never smokers in terms of death risk. These findings suggest that smoking has a more robust relationship with death than with dementia onset, and consequently, the risk for dementia may be abated quicker (as the data from this study would support). Alternatively, smoking can cause a variety of negative health outcomes which may result in death, but smoking may impact fewer biological processes that influence cognitive functioning. A larger sample of recent quitters is needed to determine with more precision the duration of abstinence required for the risk of dementia to abate.
Certain study limitations exist. First, smoking data were self-reported during baseline interviews only due to only a portion of the sample having updated smoking status at follow-up. Baseline smoking status may have changed throughout the 14 years of data collection, which could have had considerable undetected impact on clinical outcomes. Future work would benefit from biochemical verification of smoking status at follow-up interviews. Reasons for smoking cessation were not collected, and may have provided information on cessation motivation as it relates to cognition or other health conditions (e.g., individuals may have quit smoking because their cognition was declining or after suffering a significant health event like a stroke or heart attack). This study is subject to healthy survivor bias [39] in that all participants must have been healthy enough to survive to the age of entry in this study (45 years minimum; 72 years on average); smokers may not have not survived to this age due to health issues potentially biasing groups. Future research would benefit from larger samples of recent quitters to compare to never smokers, as well as longer duration of follow-up, preferably starting at a younger age and followed prospectively. This cohort was highly educated and many individuals specifically wanted to be in the NACC UDS to advance science specific to dementia, limiting generalizability. Further, recruitment for inclusion in the NACC UDS is geared towards Alzheimer’s dementia, although recruitment methods differ by specific ADC and all ADCs recruit other forms of dementia (e.g., Lewy body dementia, frontotemporal dementia). While Alzheimer’s dementia is the leading type of dementia, accounting for 60 to 80 percent of current dementia diagnoses [7], future studies focused on all forms of dementia are warranted to ensure findings are replicated. Although this sample had significant racial diversity, increasing the generalizability of findings, there were significant differences in smoking status by race. Due to recent evidence suggesting an impact of race-based selection bias on subsequent conversion to dementia [40], which could influence results due to the known association between smoking and race [2], we did not use race as a covariate.
4.1. Conclusions
Among cognitively healthy individuals, current cigarette smoking at baseline was associated with an increased the risk of incident dementia, death, and death following dementia. Pack-years were not significantly associated with dementia risk, but were significantly associated with increased risk of death following dementia. Lack of significant differences between never and former smokers (with < 30 years of abstinence) coupled with the trend of decreased risk by duration of abstinence suggests that risk of incident dementia is better attributed to recency, rather than overall exposure, of cigarette use. However, former smokers were only comparable to never smokers after 10 years of abstinence for the competing risk of death. These findings highlight the benefit of smoking cessation at any age for cognitively healthy individuals.
Acknowledgements
Data were obtained from the National Alzheimer’s Coordinating Center Uniform Data Set. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428-01 (PI James Leverenz, MD) P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421-01 (PI Bradley Hyman, MD, PhD), P30 AG062422-01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429-01(PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715-01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). This work was supported by the Veterans Affairs Advanced Fellowship in Women’s Health (Johnson) and R01 AG054059 (Gleason, PI). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Content does not represent views of the US Department of Veterans Affairs or the US Government.
Appendix
The NACC longitudinal UDS is compiled from individuals enrolled in data from all US-based Alzheimer’s Disease Centers (ADCs) Clinical Cores. For the most part, Clinical Core’s recruit from academic medical centers dementia diagnostic clinics, most of which are based in Departments of Neurology, Geriatrics or Psychiatry. While there is no uniform diagnostic process, evaluations typically consist of physical and neurological examinations, neuropsychological testing, review of medications and medical and psychiatric histories, and an interview with a study partner. Final diagnosis (i.e., normal cognition, impaired-not-MCI, MCI, or dementia/dementia syndrome) is determined by one of two methods 1) a single clinician, or 2) a consensus team (i.e., more than one clinician). Once enrolled, participants are re-evaluated either annually or biannually.
If the clinician(s) determines that the participant is impaired, yet does not believe the presentation is consistent with the diagnostic syndrome of MCI, they can designate the case as Cognitively Impaired, not MCI. A typical case for which this selection would occur is when clinicians observe cognitive impairment, but not believe what was observed is consistent with a neurodegenerative process. Examples include: 1) clinicians believe the impairment is caused by a reversible condition such as depression, anxiety, untreated sleep apnea, 2) the impairment reflects longstanding weakness, or 3) the assessment’s accuracy is in question. In other words, the pattern suggests that the case is more likely non-demented and not a prodromal case of dementia. Due to this distinction, and clear differentiation from MCI, authors opted to include the participants (N = 1,034) with baseline diagnosis of “impaired-not-MCI” in the cognitively healthy group. Importantly, results remain substantively unchanged when participants with the above diagnosis were excluded from analyses.
Footnotes
Conflicts of Interest/Disclosure Statement
The authors have no conflicts of interest to report.
References
- [1].Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ (2018) Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 67, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].U.S. Department of Health and Human Services (2014) Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- [3].Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M (2019) Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. Journal of the American College of Cardiology 73, 3326–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2017) Dementia prevention, intervention, and care. Lancet 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- [5].Durazzo TC, Mattsson N, Weiner MW, Alzheimer’s Disease Neuroimaging I (2014) Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement 10, S122–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anstey KJ, von Sanden C, Salim A, O’Kearney R (2007) Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 166, 367–378. [DOI] [PubMed] [Google Scholar]
- [7].Alzheimer’s Association (2019) Alzheimer’s disease facts and figures. Alzheimer’s Dement 15, 321–387. [Google Scholar]
- [8].Jordan H, Hidajat M, Payne N, Adams J, White M, Ben-Shlomo Y (2017) What are older smokers’ attitudes to quitting and how are they managed in primary care? An analysis of the cross-sectional English Smoking Toolkit Study. BMJ Open 7, e018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huddlestone L, Walker GM, Hussain-Mills R, Ratschen E (2015) Treating tobacco dependence in older adults: a survey of primary care clinicians’ knowledge, attitudes, and practice. BMC Fam Pract 16, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Van Duijn CM, Clayton DG, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Mortimer JA, Rocca WA, Shalat SL, Soininen H, Hofman A, Group ERFR, et al. (1994) Interaction between genetic and environmental risk factors for Alzheimer’s disease: a reanalysis of case-control studies. Genet Epidemiol 11, 539–551. [DOI] [PubMed] [Google Scholar]
- [11].Fratiglioni L, Wang HX (2000) Smoking and Parkinson’s and Alzheimer’s disease: review of the epidemiological studies. Behav Brain Res 113, 117–120. [DOI] [PubMed] [Google Scholar]
- [12].Cataldo JK, Prochaska JJ, Glantz SA (2010) Cigarette smoking is a risk factor for Alzheimer’s Disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis 19, 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gooley TA, Leisenring W, Crowley J, Storer BE (1999) Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 18, 695–706. [DOI] [PubMed] [Google Scholar]
- [14].Berry SD, Ngo L, Samelson EJ, Kiel DP (2010) Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 58, 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chang CC, Zhao Y, Lee CW, Ganguli M (2012) Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis Assoc Disord 26, 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Almeida OP, Hulse GK, Lawrence D, Flicker L (2002) Smoking as a risk factor for Alzheimer’s disease: contrasting evidence from a systematic review of case-control and cohort studies. Addiction 97, 15–28. [DOI] [PubMed] [Google Scholar]
- [17].Abner EL, Nelson PT, Jicha GA, Cooper GE, Fardo DW, Schmitt FA, Kryscio RJ (2019) Tobacco Smoking and Dementia in a Kentucky Cohort: A Competing Risk Analysis. J Alzheimers Dis 68, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fine JP, Gray RJ (1999) A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 94, 496–509. [Google Scholar]
- [19].Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Statistics in medicine 26, 2389–2430. [DOI] [PubMed] [Google Scholar]
- [20].Buhr GT, Kuchibhatla M, Clipp EC (2006) Caregivers’ reasons for nursing home placement: clues for improving discussions with families prior to the transition. Gerontologist 46, 52–61. [DOI] [PubMed] [Google Scholar]
- [21].Toot S, Swinson T, Devine M, Challis D, Orrell M (2017) Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr 29, 195–208. [DOI] [PubMed] [Google Scholar]
- [22].Valiyeva E, Russell LB, Miller JE, Safford MM (2006) Lifestyle-related risk factors and risk of future nursing home admission. Arch Intern Med 166, 985–990. [DOI] [PubMed] [Google Scholar]
- [23].Garcia AM, Ramon-Bou N, Porta M (2010) Isolated and joint effects of tobacco and alcohol consumption on risk of Alzheimer’s disease. J Alzheimers Dis 20, 577–586. [DOI] [PubMed] [Google Scholar]
- [24].Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y (2015) Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 10, e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, Jicha G, Carlsson C, Burns J, Quinn J, Sweet RA, Rascovsky K, Teylan M, Beekly D, Thomas G, Bollenbeck M, Monsell S, Mock C, Zhou XH, Thomas N, Robichaud E, Dean M, Hubbard J, Jacka M, Schwabe-Fry K, Wu J, Phelps C, Morris JC, Neuropsychology Work Group D, Clinical Core leaders of the National Institute on Aging-funded USAsDC (2018) Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 32, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140, 566–572. [DOI] [PubMed] [Google Scholar]
- [27].Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [28].Tombaugh TN, McDowell I, Kristjansson B, Hubley AM (1996) Mini-Mental State Examination (MMSE) and the Modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assess 8, 28–59. [Google Scholar]
- [29].de Wreede LC, Fiocco M, Putter H (2010) The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed 99, 261–274. [DOI] [PubMed] [Google Scholar]
- [30].Therneau TM, A Package for Survival Analysis in R. R package version 3., https://CRAN.R-project.org/package=survival,
- [31].Therneau TM, Grumbsch PM (2000) Modeling Survival Data: Extending the Cox Model, Springer, New York. [Google Scholar]
- [32].Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57, 289–300. [Google Scholar]
- [33].Glickman ME, Rao SR, Schultz MR (2014) False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. Journal of clinical epidemiology 67, 850–857. [DOI] [PubMed] [Google Scholar]
- [34].Harwood DG, Kalechstein A, Barker WW, Strauman S, St George-Hyslop P, Iglesias C, Loewenstein D, Duara R (2010) The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int J Geriatr Psychiatry 25, 511–518. [DOI] [PubMed] [Google Scholar]
- [35].Ferri CP, West R, Moriyama TS, Acosta D, Guerra M, Huang Y, Jacob KS, Ribeiro W, Llibre de Rodriguez JJ, Salas A, Sosa AL, Williams J, Acosta I, Liu Z, Hernandez MA, Prince MJ (2011) Tobacco use and dementia: evidence from the 1066 dementia population-based surveys in Latin America, China and India. Int J Geriatr Psychiatry 26, 1177–1185. [DOI] [PubMed] [Google Scholar]
- [36].Reitz C, den Heijer T, van Duijn C, Hofman A, Breteler MM (2007) Relation between smoking and risk of dementia and Alzheimer disease: the Rotterdam Study. Neurology 69, 998–1005. [DOI] [PubMed] [Google Scholar]
- [37].Rusanen M, Kivipelto M, Quesenberry CP Jr., Zhou J, Whitmer RA (2011) Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med 171, 333–339. [DOI] [PubMed] [Google Scholar]
- [38].Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R (2013) 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 368, 341–350. [DOI] [PubMed] [Google Scholar]
- [39].Picciotto S, Brown DM, Chevrier J, Eisen EA (2013) Healthy worker survivor bias: implications of truncating follow-up at employment termination. Occup Environ Med 70, 736–742. [DOI] [PubMed] [Google Scholar]
- [40].Gleason CE, Norton D, Zuelsdorff M, Benton SF, Wyman MF, Nystrom N, Lambrou N, Salazar H, Koscik RL, Jonaitis E, Carter F, Harris B, Gee A, Chin N, Ketchum F, Johnson SC, Edwards DF, Carlsson CM, Kukull W, Asthana SIPAsaD (in press) Enrollment factors’ influence on incident cognitive impairment in Blacks and Whites: Data from the Alzheimer’s Disease Centers. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huebner M, Wolkewitz M, Enriquez-Sarano M, Schumacher M (2017) Competing risks need to be considered in survival analysis models for cardiovascular outcomes. J Thorac Cardiovasc Surg 153, 1427–1431. [DOI] [PubMed] [Google Scholar]


