Abstract
Metacognition (monitoring) of emotion recognition is fundamental for social interactions. Correct recognition of and confidence in the emotional meaning inferred from others’ faces are fundamental for guiding and adjusting interpersonal behavior. Yet, although emotion recognition impairments are well documented across neurodegenerative diseases, the role of metacognition in this domain remains poorly understood. Here, we evaluate multimodal neurocognitive markers of metacognition in 83 subjects, encompassing patients with behavioral variant frontotemporal dementia [bvFTD, n = 18], Alzheimer’s disease [AD, n = 27]), and demographically-matched controls (n = 38). Participants performed a classical facial emotion recognition task and, after each trial, they rated their confidence in their performance. We examined two measures of metacognition: (i) calibration: how well confidence tracks accuracy; and (ii) a metacognitive index (MI) capturing the magnitude of the difference between confidence and accuracy. Then, whole-brain grey matter volume and fMRI-derived resting-state functional connectivity were analyzed to track associations with metacognition. Results showed that metacognition deficits were linked to basic emotion recognition. Metacognition of negative emotions was compromised in patients, especially disgust in bvFTD as well as sadness in AD. Metacognition impairments were associated with reduced volume of fronto-temporo-insular and subcortical areas in bvFTD and fronto-parietal regions in AD. Metacognition deficits were associated with disconnection of large-scale fronto-posterior networks for both groups. This study reveals a link between emotion recognition and metacognition in neurodegenerative diseases. The characterization of metacognitive impairments in bvFTD and AD would be relevant for understanding patients’ daily life changes in social behavior.
Keywords: Metacognition, Emotion recognition, Neurodegenerative diseases, VBM, Functional connectivity
1. Introduction
Metacognition refers to the cognitive control, monitoring, and regulation over cognitive processes that guide adaptive behavior (D. Fernandez-Duque, Baird, & Posner, 2000; Fleming & Dolan, 2012; D. C. Mograbi & Morris, 2018). In particular, metacognition of emotion recognition is fundamental for establishing and maintaining social interactions (Begue et al., 2019). To a large extent, adjustments of social behavior rely on accurate recognition of facial emotions and our beliefs (confidence) about whether we trust the meanings inferred therefrom (Begue et al., 2019; Kelly & Metcalfe, 2011). Some authors suggested that social interactions might not be possible without metacognition, as individuals infer others’ emotional states based on current observations, past experiences, and metaknowledge (Adolphs, 2009; Kelly & Metcalfe, 2011; D. C. Mograbi & Morris, 2013). Previous studies show that metacognition is supported by the prefrontal cortex (Eslinger, Moore, Anderson, & Grossman, 2011; Rosen et al., 2014) as well as fronto-posterior networks (Baird, Smallwood, Gorgolewski, & Margulies, 2013; Fleming & Dolan, 2012; Garrison, 2014; Vaccaro & Fleming, 2018). Neurodegenerative diseases, such as behavioral variant frontotemporal dementia (bvFTD) and Alzheimer’s disease (AD), provide an outstanding opportunity to study metacognitive impairments, as they present deficits in self-awareness (Rosen, 2011; Rosen et al., 2014; Shany-Ur et al., 2014) and atrophy across areas subserving metacognition. Yet, although deficits in social cognition (M. Bertoux et al., 2012; Fittipaldi et al., 2019; Gregory et al., 2002; A. Ibanez & Manes, 2012; Piguet, Hornberger, Mioshi, & Hodges, 2011; Torralva et al., 2007) and particularly in emotion recognition (M. Bertoux et al., 2015; Bora, Velakoulis, & Walterfang, 2016; F. Kumfor & Piguet, 2012) are well documented in dementia patients, relevant metacognitive processes remain poorly understood. Against this background, the present study aims to characterize the metacognitive processing of emotion recognition and its neural correlates in bvFTD and AD patients.
Emotion recognition of facial expressions is impaired in different neurodegenerative diseases (Bora et al., 2016; F. Kumfor & Piguet, 2012). Meta-analytical results in patients with bvFTD show deficits in negative facial emotions recognition, with greater differences for anger and disgust (Bora et al., 2016). However, inconsistent patterns have been reported in the recognition of separate emotions (e.g.: fear, sadness, and surprise) across studies, with patients performing either worse than or similar to controls (for a review, see F. Kumfor and Piguet (2012)). AD patients display a generalized deficit in the recognition of all facial emotions, with greater impairments for sadness and anger (for a review, see Torres Mendonça De Melo Fádel, Santos De Carvalho, Belfort Almeida Dos Santos, and Dourado (2019)). Finally, recognition of happiness is usually preserved in patient populations (F. Kumfor & Piguet, 2012). Regarding neuroanatomical substrates, general deficits in negative emotion recognition have been associated with damage in the amygdala (Fiona Kumfor, Irish, Hodges, & Piguet, 2013) as well as the orbitofrontal (Couto et al., 2013; Kamminga et al., 2015; Fiona Kumfor et al., 2013), dorsal prefrontal (M. Bertoux et al., 2014; M. Bertoux et al., 2012), and temporal (Rosen et al., 2006; Werner et al., 2007) cortices. In particular, recognition of anger and disgust is related with the integrity of the orbitofrontal and insular cortices (Craig, 2002; A. Ibanez, Gleichgerrcht, & Manes, 2010; Omar, Rohrer, Hailstone, & Warren, 2011), while sadness is differentially underpinned by limbic and temporal regions (Arias et al., 2020; Blair, Morris, Frith, Perrett, & Dolan, 1999).
In neurodegenerative diseases, metacognitive mechanisms have been mainly examined in relation to cognitive domains such as memory (Cosentino et al., 2015; D. Mograbi, Brown, Landeira-Fernandez, & Morris, 2014; D. C. Mograbi, Brown, Salas, & Morris, 2012; Rosen et al., 2014; Thomas, Lee, & Balota, 2013), attention (Diego Fernandez-Duque & Black, 2007; D. C. Mograbi et al., 2012), and perception (Diego Fernandez-Duque & Black, 2007; Garcia-Cordero et al., 2019), as well as anosognosia – lack of awareness of having a disorder or disability (D. C. Mograbi & Morris, 2018). In particular, bvFTD patients seem to present more metacognitive compromise than AD, which shows milder but constant insight loss (Hornberger et al., 2014). Neuroanatomical findings show that metacognitive processes rely principally on the prefrontal (ventromedial and orbitofrontal) regions (Eslinger et al., 2011; Rosen et al., 2014) and other subsidiary areas, such as the insula, the hippocampus, the cingulate cortex, the basal ganglia, and the thalamus (Amanzio et al., 2011; O’Keeffe et al., 2007; Perrotin et al., 2015; Shany-Ur et al., 2014), in addition to fronto-posterior functional networks (Baird et al., 2013; Fleming & Dolan, 2012; Garrison, 2014; Vaccaro & Fleming, 2018). In both bvFTD and AD, atrophy of the ventromedial and frontopolar prefrontal cortices correlated with general lack of insight. Conversely, the temporal cortex and amygdala were associated with social and emotional unawareness in both conditions (Hornberger et al., 2014). Yet, except for a behavioral study showing reduced emotion discrimination confidence in Parkinson’s disease (Mattavelli et al., 2020), no study has assessed the metacognition of emotion recognition in neurodegenerative conditions, let alone integrating multimodal brain signatures. This is an important gap to fill, as reduced metacognition of emotion recognition could worsen social interaction by amplifying the patients’ social cognition deficits (O’Keeffe et al., 2007). Finally, there is an unresolved debate on whether metacognition is a global phenomenon (Shimamura, 2000; Vaccaro & Fleming, 2018) or a domain-specific process supported by distinct components (Baird et al., 2013; Fleming, Ryu, Golfinos, & Blackmon, 2014; McCurdy et al., 2013). The assessment of the neural substrates of both emotion recognition and its related metacognition may help to clarify this debate. Briefly, then, although emotion recognition difficulties and metacognitive deficits in cognitive domains have been widely studied in neurodegenerative diseases, evidence of metacognition of emotion recognition is scarce.
Here, we assessed metacognition of emotion recognition in bvFTD and AD patients, including analyses of its neuroanatomical and functional connectivity correlates. First, using a facial emotion recognition task, we examined two metacognitive measures, namely: calibration, i.e., how well confidence tracks accuracy (Begue et al., 2019; Fleming & Lau, 2014); and a metacognitive index (MI), i.e., the magnitude of the difference between confidence and accuracy (Begue et al., 2019). Second, we tested the association between the MI and both structural and resting-state functional neuroimaging outcomes. We expected more profound deficits in the recognition and metacognition for negative emotions across the neurodegenerative diseases. In addition, we hypothesized that there would be an association between emotion recognition and limbic regions (e.g., amygdala, hippocampus, insula), and between metacognition and fronto-subcortical hubs (e.g., prefrontal cortex, cingulate cortex, insula). For bvFTD, we predicted that emotion recognition and metacognition would be associated with fronto-temporo-insular areas. For AD, we expected emotion recognition to be related with posterior areas, and metacognition, with fronto-parietal regions. With regards to functional connectivity, we anticipated that a disconnection between frontal and posterior hubs would be associated with impaired metacognition. In short, this study seeks to offer novel evidence on the behavioral and neural signatures of the metacognition of emotion recognition in dementia patients.
2. Materials and methods
We report how we determined our sample size (participants sections), all data exclusions (section imaging acquisition and analysis), all inclusion/exclusion criteria (participants‘ section), all manipulations, and all measures in the study (section 2.2). We confirm that all inclusion/exclusion criteria were established before data analysis.”
2.1. Participants
The study encompassed 83 participants from two clinical centers located in Argentina and Chile. Eighteen patients with probable bvFTD and 27 with AD were recruited. Diagnosis was initially made by a group of experts in dementia. We used the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat) standardized diagnostic assessment to align the local sites’ procedures. This brief tool, completed in full for every participant, incorporates impressions from the physician or other significant examiners, with inputs from the evaluating neuropsychologist. In addition, we followed formal criteria for diagnosing AD and bvFTD, as done in previous multicentric sites (Donnelly-Kehoe et al., 2019; Moguilner et al., 2018; Moguilner et al., 2020; Sedeno et al., 2017). The staff was trained in these consensus diagnosis methods. Moreover, we have implemented a control strategy based on the use of a common training manual for clinical and cognitive assessment in all clinics, as well as a quality assurance checklist. All patients completed a harmonized extensive battery of neurological, neuropsychiatric, and neuropsychological assessments. BvFTD subjects were included following the revised criteria of probable bvFTD (Rascovsky et al., 2011). These patients presented social and behavioral impairments confirmed by their caregivers and fronto-temporal atrophy confirmed by MRI or frontal hypoperfusion assessed with PET. AD patients were diagnosed following NINCDS-ADRDA criteria (McKhann et al., 1984) and exhibited a temporo-posterior and more distributed atrophy –see Figure 1 (top panel) and Table A.1 and A.2 for details. Patients were at different stages of disease –see Table A.3 for cognitive and functional assessment data. We also recruited 38 healthy subjects with no history of psychiatric or neurological diseases. All groups were matched in age [F(2,79) = 1.79; p = 0.17], years of education [F(2,79) = 2.22; p = 0.12] and gender [χ² (2, N = 83) = 4.40; p = 0.11] –see Table A.3 for demographic details. All participants provided signed informed consent in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Institution. No part of the study procedures and analyses were pre-registered prior to the research being conducted. Sample size was determined by availability and convention.
Figure 1. Patients’ atrophy patterns and data collection pipeline.
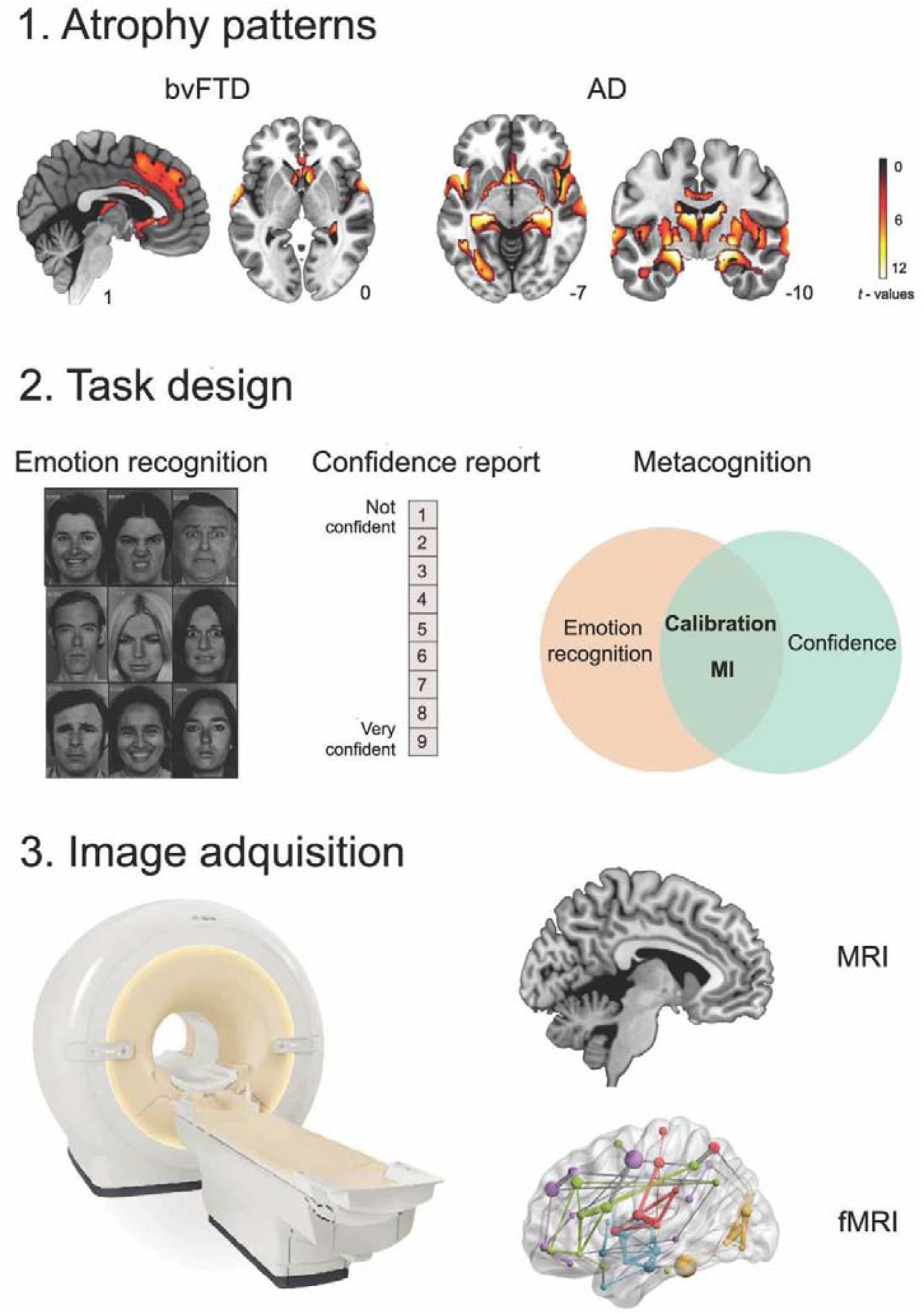
1. Atrophy of bvFTD and AD patients compared to matched controls. Neurodegeneration was observed in frontal and temporal areas for bvFTD and in posterior (temporal) regions for AD (p < 0.001, extent threshold = 50 voxels). 2. Both patient and control groups performed the emotion recognition task (Ekman’s faces) and reported their confidence about their performance. Scores of emotion recognition (accuracy) and confidence were obtained. Schematic representation of metacognition and its components: calibration (how well confidence tracks accuracy) and the metacognitive index (MI), indexing how large the difference between confidence and accuracy is. 3. Structural and functional MRI were acquired to explore the structural and functional brain correlates of emotion recognition and the MI in each patient group.
2.1. Behavioral task
Participants performed a task including an emotion recognition and a metacognition stage. First, they were presented with the Pictures of Facial Affect test version included in the mini-SEA (M. Bertoux, Funkiewiez, O’Callaghan, Dubois, & Hornberger, 2013; M. Bertoux et al., 2014). Participants were asked to identify the emotions expressed by faces in different pictures and to select the answer between the options displayed on the screen (anger, disgust, fear, sadness, happiness, surprise, and neutral). The task comprised 35 faces, with five trials per emotion. After each trial, participants provided a confidence judgment about their performance on a rating scale ranging from 1 (not confident) to 9 (very confident). The emotion recognition and confidence scores were registered by hand by the evaluator –see Figure 1 (middle panel) for details. Emotion recognition (accuracy) and confidence scores were calculated as proportions, considering the score in each emotion. Thereupon, we obtained a global score for total and negative (the sum of fear, disgust, sadness, and anger) emotions.
2.2. Statistical analyses
First, the proportion of emotion recognition (accuracy) was compared among groups via ANCOVA and Tukey’s HSD test for post hoc comparisons, using country as a covariate. Then, we analyzed two dimensions of metacognitive performance: calibration and the MI. As calibration is determined by how well confidence tracks accuracy (Begue et al., 2019; Fleming & Lau, 2014), we assessed whether the proportions of recognition (accuracy) and confidence were similar. Related-samples Wilcoxon sign tests were performed within the bvFTD and AD groups, in order to assess whether both proportions had similar distribution. Calibration is deemed good when the proportions of emotion recognition and confidence are similar. Bonferroni correction was applied to adjust p-values for multiple comparisons across the total of 10 tests (Tusche, Bockler, Kanske, Trautwein, & Singer, 2016) (each emotion and their combinations) and the statistical threshold was set at p < 0.05.
On the other hand, the MI is the magnitude of the difference between confidence and accuracy (Begue et al., 2019), and it was calculated as follows:
considering both performance on each emotion type and associated confidence ratings. The MI has continuous values (from 0 to 1, with values closer to zero indicating accurate metacognition) and was compared between bvFTD, AD, and controls, via ANCOVA and Tukey’s HSD tests for post hoc comparisons, using country as covariate. Receiver operating characteristics (ROC) curve analyses were performed to evaluate the discriminating power of the MI and recognition for each emotion. For more details, see Appendix, section 1.3. Then, as the MI reflects the magnitude of metacognitive impairment, we used this index in the neuroimaging analyses to identify associations with brain structure and connectivity (see sections 2.5.1 and 2.5.2).
2.3. Image acquisition
Structural T1 scans from Center 1 were acquired in a Philips Ingenia 3.0 T with a standard head coil. The parameters were: matrix size= 224 × 224 × 160, 1 mm isotropic, TR = 8.3 s, TE = 3.8 s and flip angle = 8°. Functional spin echo volumes, parallel to the anterior-posterior commissures, covering the whole brain, were sequentially and ascendingly acquired with the following parameters: matrix size = 80 × 80 × 49, 3 mm isotropic, TR = 2.64 s, TE = 0.03 s, flip angle = 90°, number of volumes = 220.
Participants from Center 2 were scanned with a Siemens Skyra 3.0 T with a standard head coil, but with different parameters: (a) Recordings a: T1 parameters: matrix size= 224 × 224 × 208, 1 mm isotropic, TR = 1.71 s, TE = 2.25 s and flip angle = 8°. Functional EP2D-BOLD pulse sequences, parallel to the anterior-posterior commissures, covering the whole brain, were acquired sequentially intercalating pair-ascending first. fMRI parameters: matrix size: 76 × 76 × 46, 3 mm isotropic, TR = 2.66 s, TE = 0.03 s, flip angle = 90°, number of volumes = 300. (b) Recordings b: T1 parameters: matrix size= 256 × 256 × 192, 1 mm isotropic, TR = 2.4 s, TE = 2 s and flip angle = 8°. Functional EP2D-BOLD pulse sequences, parallel to the anterior-posterior commissures, covering the whole brain, were acquired sequentially intercalating pair-ascending first. fMRI parameters: matrix size: 76 × 76 × 46, 3 mm isotropic, TR = 2.66 s, TE = 0.03 s, flip angle = 90°, number of volumes = 240.
During the functional MRI resting-state session, participants were instructed not to think about anything in particular, to keep their eyes closed, and avoid moving and falling asleep. All subjects were scanned, except for one bvFTD patient that was not able to perform the functional MRI session (structural MRI: n = 83; functional MRI: n = 82). See Figure 1 for more information about the data collection pipeline.
2.4. Imaging analysis
2.4.1. Structural image preprocessing and analysis
A voxel-based morphometry (VBM) analysis was performed on the structural images. T1-weighted images in native space were first segmented using the default parameters of SPM12 (bias regularization was set to 0.001 and bias FWHM was set to 60-mm cut-off) into white matter (WM), grey matter (GM), and cerebrospinal fluid (CFS). Then, a template was generated with the ‘DARTEL (create template)’ module to increase the accuracy of inter-subject alignment (Ashburner, 2007) from the complete data set using the GM and WM segmented images (default parameters indicated by SPM12). Next, we ran the ‘Normalize to MNI space’ module from DARTEL Tools to affine register the last template from the previous step and all GM segmented scans into MNI space. Subsequently, all images were modulated to correct volume changes by Jacobian determinants, and avoid a bias in the intensity of an area due to its expansion during warping. Finally, in line with previous recommendations (Good et al., 2001), an isotropic Gaussian kernel of 12-mm full width at half maximum was applied to all images. These final maps were entered in the second-level analysis to perform the correspondent statistic.
Next, multiple regression analyses were performed to assess the relation between emotion recognition (accuracy) and the MI and grey matter volume in each patient group (bvFTD and AD) in tandem with controls. This procedure was carried out to increase behavioral variance and statistical power (O’Callaghan et al., 2015; Sollberger et al., 2009). For both analyses, total grey volume (obtained using VBM8 toolbox for SPM12), scanner type, and age were used as covariates (whole-brain analysis, p < 0.001 (Garcia-Cordero et al., 2019; García-Cordero et al., 2016; Irish, Piguet, Hodges, & Hornberger, 2014), extent threshold = 50 voxels).
2.4.2. Functional image preprocessing and analysis
Functional MRI images were pre-processed using the Data Processing Assistant for Resting-State fMRI (DPARSF V2.3; http://rfmri.org/DPARSF). To ensure that magnetization achieved a steady state, the first five volumes of each subject’s resting-state sequence were eliminated. As in previous studies (Fittipaldi et al., 2020; García-Cordero et al., 2016; Salamone et al., 2018; Yoris et al., 2018), pre-processing steps included slice-timing correction (using middle slice of each volume as the reference scan), realignment to the first scan of the session in order to correct head movement, normalization to the MNI space using the Echo-Planar Imaging (EPI) template provided by SPM, bandpass filtering (0.01–0.1 Hz), and smoothing using a 8-mm full-width-at-half-maximum isotropic Gaussian kernel. Six motion parameters, cerebrospinal fluid, white matter, and global signals were regressed in order to reduce the effect of motion and physiological artefacts (e.g., cardiac and respiration effects). Cerebrospinal fluid and white matter masks were obtained from the tissue segmentation of each subject’s T1 scan in native space with SPM12 (after co-registration of each subject’s structural image with the functional image). Motion parameters (average translation and rotation) were estimated during realignment step and were matched between groups. For more information of this analysis, see Appendix, section 1.2 and Table A.4.
Then, we explored associations between the resting-state functional connectivity data and the MI. First, for each subject, we extracted the mean time course of the BOLD signal of each region of the Automated Anatomical Labelling Atlas (Tzourio-Mazoyer et al., 2002). A connectivity matrix was obtained for each subject through Person’s correlations and a Fisher z-transformation was applied. To discard an effect of the scanner type, the data was also z-scored based on the mean and standard deviation of the control group of the corresponding center. The z-scored connectivity was used to perform Spearman’s correlations with the MI for bvFTD-controls and AD-controls and for each emotion (statistical threshold set at p < 0.001; only negative correlations were considered). Both patients and controls were included in each analysis to increase behavioral variance and statistical power (O’Callaghan et al., 2015; Sollberger et al., 2009).
3. Results
3.1. Behavioral results
3.1.1. Emotion recognition
BvFTD patients presented emotion recognition impairments in total and negative emotions, and in anger, disgust, and surprise, in comparison to controls. AD patients showed emotion recognition deficits against the control group, in total and negative emotions, and in anger, sadness, surprise, and neutral faces. No differences were found for fear and happiness (see Table A.5 for statistical details).
3.1.2. Metacognition of emotion recognition
3.1.2.1. Calibration
Calibration deficits of total score and negative emotions were found for both patient groups. For bvFTD patients, poor calibration was obtained in anger, disgust, and fear. AD patients showed impairments in anger, disgust (borderline), fear, sadness, surprise, and neutral faces. Calibration of happiness showed no differences in any group. All significant effects had mainly large or medium sizes (see Figure 2 and Table A.6 and A.7 for statistical details).
Figure 2.
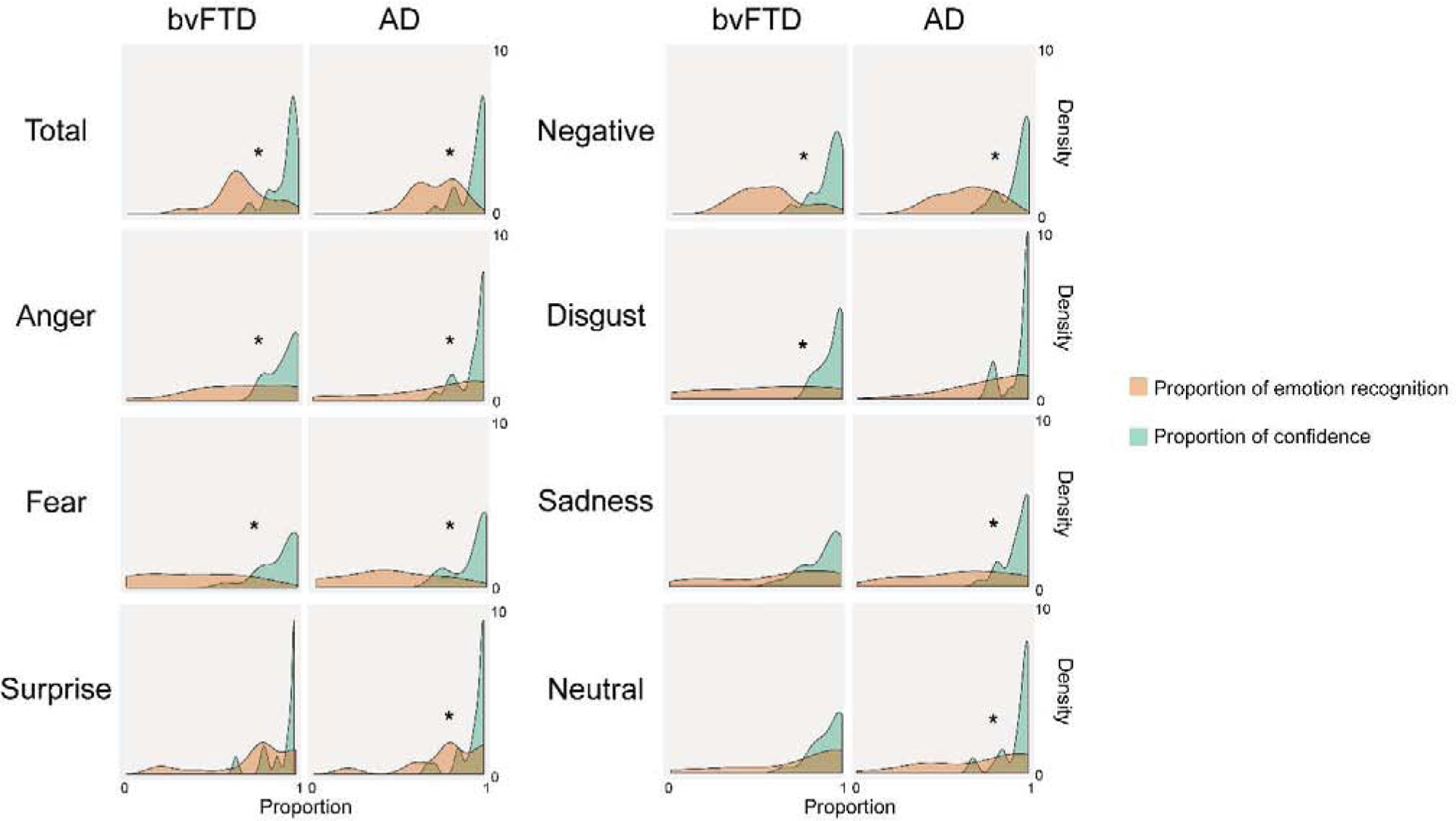
Density plots (smoothed histogram) of the proportion of emotion recognition and confidence for each patient group. Asterisks indicate significant differences between proportions (i.e., poor calibration) after Bonferroni correction (p < 0.05).
3.1.2.2. Metacognitive index
Relative to controls, bvFTD patients presented higher MI (worse performance) in total and negative emotions, especially in anger and disgust, in surprise and in happiness. AD patients also showed higher MI for total and negative emotions, as well as specific emotions (anger, sadness, surprise, and neutral faces). For fear, no significant differences were found. All effect sizes of significant differences were mainly large or medium (see Figure 3 and Table A.8 for statistical details).
Figure 3.
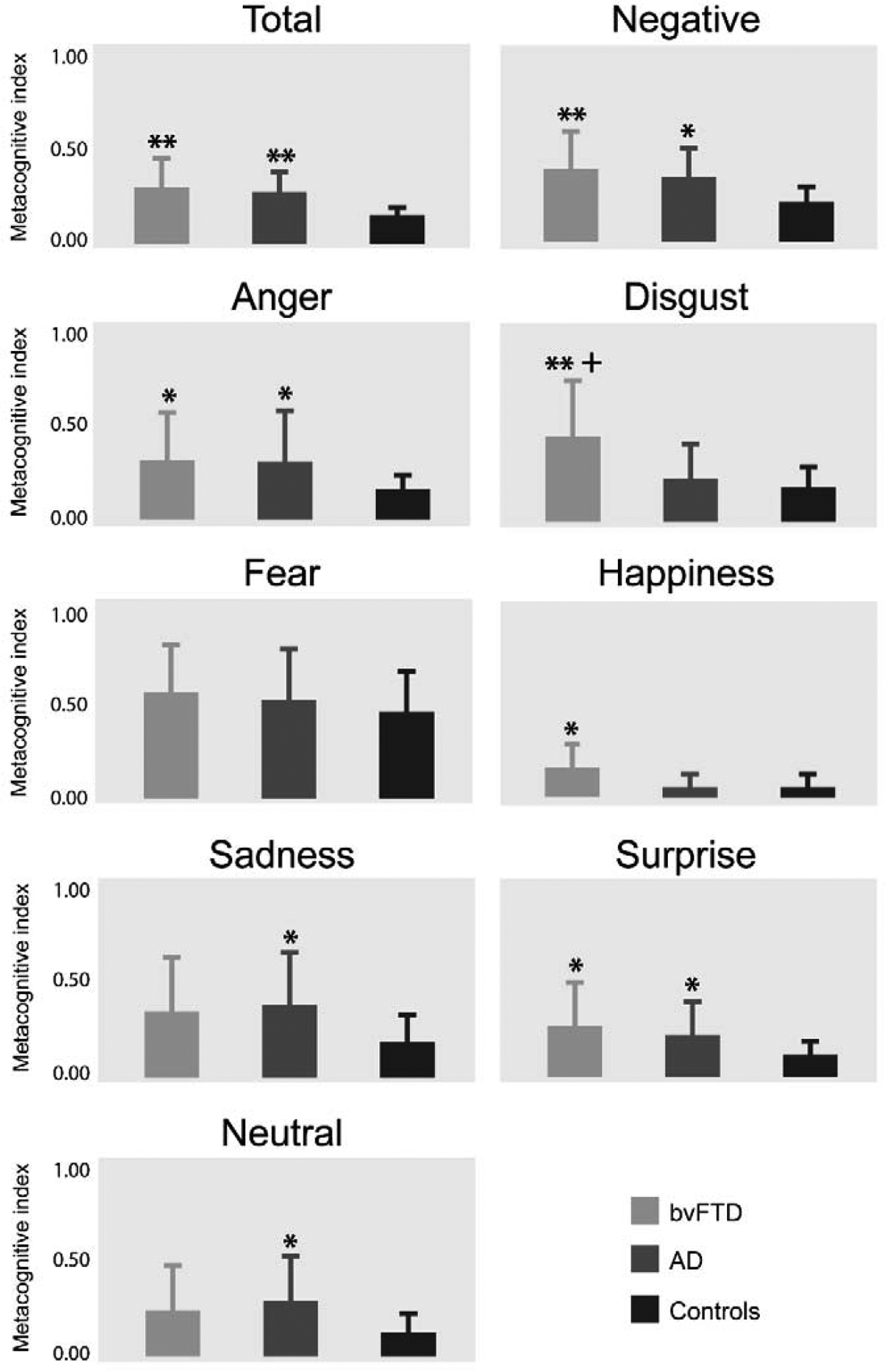
Metacognitive performance of bvFTD, AD, and controls for each emotion. Metacognitive index (MI) nearer zero represents better performance. ** p < 0.001 against controls; * p < 0.05 against controls; + p < 0.05 against AD.
Calibration and MI results showed a greater compromise in total and negative emotions for bvFTD and AD. Indeed, impairments in calibration and MI were observed for disgust in bvFTD patients, and for sadness and neutral faces in AD patients. Metacognition of anger was impaired in the two metacognitive measures and groups, and surprise was more compromised in AD. Regarding fear, calibration deficits, but no differences in the MI were observed. In contrast, for bvFTD, happiness presented good calibration and impaired MI.
Classification results showed that the MI of total and negative emotions discriminated between both groups of patients and controls. Specially, the MI of anger and disgust distinguished bvFTD from controls; and the MI of sadness discriminated between patients and controls. In bvFTD, the MI and emotion recognition presented differential discrimination power. Interestingly, only the MI of disgust, but not emotion recognition, distinguished between bvFTD and AD (all classification rates ≥ 70%). For more details, see Table A.9, A.10 and Figure A.1.
Summarizing, we observed partial circumscribed metacognitive impairments for bvFTD and a more generalized metacognitive deficit in AD. The only emotion that distinguished between groups of patients was disgust, as metacognition was selectively compromised in bvFTD, but spared in AD.
3.2. Imaging results
3.2.1. Structural associations with emotion recognition and the metacognitive index
Main results are presented in Figure 4 (also Tables A.11 to A.14 for clusters’ peaks). For bvFTD, total and negative emotions were associated with fronto-temporo-insular and subcortical areas. Emotion recognition deficits involved atrophy in areas belonging to the limbic system (amygdala, hippocampus, insula, among others) and subcortical areas, such as the basal ganglia and the thalamus. Metacognitive impairments were also related with different subcortical and cortical areas, including the frontal and temporal lobe, the insula, and the cingulate cortex. In contrast, AD presented a more cortical pattern related with emotion recognition and metacognition. Temporal and parietal lobes’ atrophy was associated with emotion recognition, and parietal and frontal regions with metacognition. Interestingly, in both groups, recognition and metacognition shared several structural substrates in areas related with emotion recognition (amygdala, insula, frontal and temporal regions), but metacognition extended to regions such as the orbitofrontal, the insular, and the cingulate cortex –which are involved in general metacognitive processes.
Figure 4. Structural associations with emotion recognition and the metacognitive index.
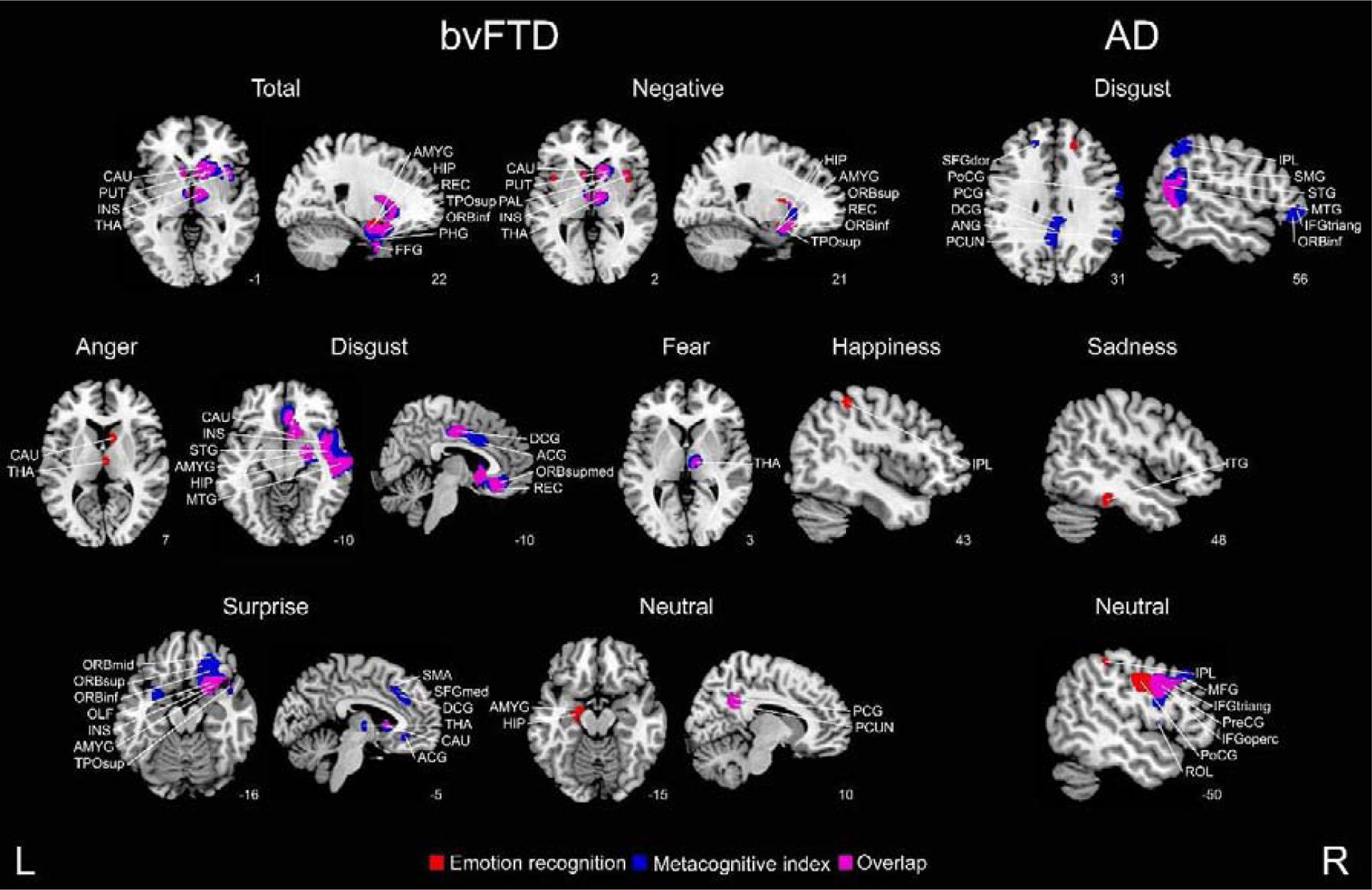
Regression analyses considering bvFTD-controls and AD-controls were conducted to identify regions specific to each patient group and emotion (p < 0 .001, extent threshold = 50 voxels). ACG = anterior cingulate gyrus; AMYG = amygdala; ANG = angular gyrus; CAU = caudate nucleus; DCG = dorsal cingulate gyrus; FFG = fusiform gyrus; HIP = hippocampus; IFGoperc = inferior frontal gyrus (pars opercularis); INS = insula; IPL = inferior parietal gyrus; ITG = inferior temporal gyrus; MFG = middle frontal gyrus; OLF = olfactory cortex; ORBinf = inferior frontal gyrus; ORBmid = middle frontal gyrus; ORBsup = superior frontal gyrus; ORBsupmed = orbitofrontal gyrus; PAL = pallidum; PCG = posterior cingulate gyrus; PCUN = precuneus; PHG = parahipocampal gyrus; PoCG = postcentral gyrus; PreCG = precentral gyrus; PUT = putamen; REC = gyrus rectus; ROL = Rolandic operculum; SFGdor = dorsolateral superior frontal gyrus; SFGmed = medial superior frontal gyrus; SMA = supplementary motor area; SMG = supramarginal gyrus; STG = superior temporal gyrus; THA = thalamus; TPOsup = superior temporal pole; L = left, R = right.
3.2.2. Functional associations with the metacognitive index
A summary of the results can be found in Figure 5, and in Tables A.15 and A.16 (clusters’ peaks). Metacognitive impairments for both groups involved disconnection between frontal and posterior regions (temporal and parietal lobes). For total and negative emotions, associations between functional connectivity and the MI included mostly fronto-temporal regions for both bvFTD and AD. In bvFTD, higher MI was associated with reduced connectivity of fronto-tempo-parietal areas for anger, and frontal-basal ganglia for disgust. For fear, connectivity between posterior regions was related with the MI in AD. For sadness, higher MI was associated with reduced connectivity between fronto-parietal for bvFTD; and between fronto-parietal, temporo-parietal regions and parietal-basal ganglia for AD. For surprise, connectivity of fronto-posterior areas and within the cingulate cortex was associated with metacognition for bvFTD and AD, respectively. Finally, metacognition of neutral faces negatively correlated with the connectivity of frontal-posterior regions only for AD.
Figure 5. Functional associations with emotion metacognition.
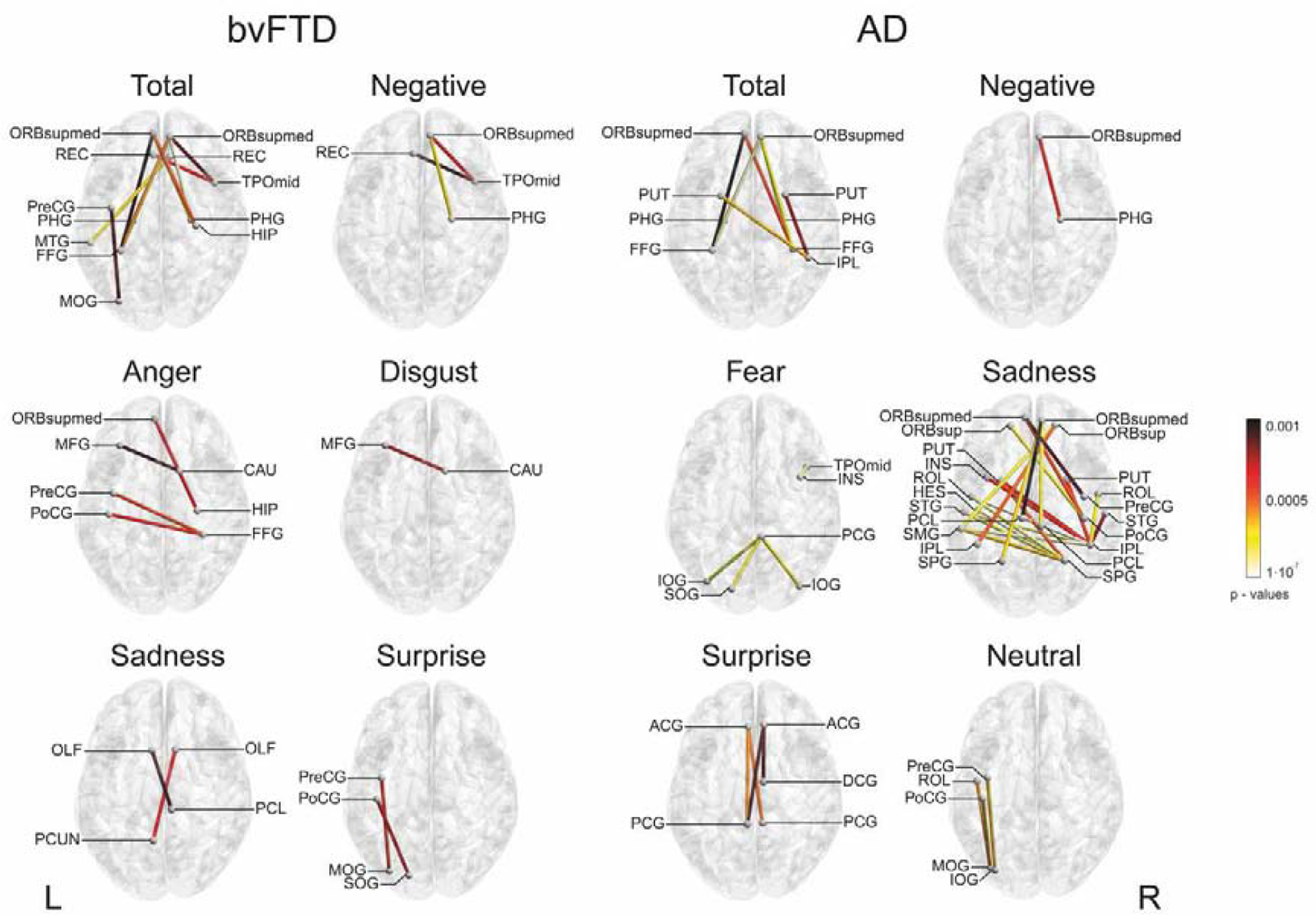
Spearman’s correlations were performed between resting-state functional connectivity of bvFTD-controls and AD-controls and the MI in each patient group and emotion (p < 0.001). Only negative correlations were considered. ACG = anterior cingulate gyrus; CAU = caudate nucleus; DCG = dorsal cingulate gyrus; FFG fusiform gyrus; HES = Heschl’s gyrus; HIP = hippocampus; INS = insula; IOG = inferior occipital gyrus; IPL = inferior parietal gyrus; MFG = middle frontal gyrus; MOG = middle occipital gyrus; MTG = middle temporal gyrus; OLF = olfactory cortex; ORBsup = superior frontal gyrus; ORBsupmed = orbitofrontal gyrus; PCG = posterior cingulate gyrus; PCL = paracentral lobule; PCUN = precuneus; PHG = parahipocampal gyrus; PoCG = postcentral gyrus; PreCG = precentral gyrus; PUT = putamen; REC = gyrus rectus; ROL = Rolandic operculum; SMG = supramarginal gyrus; SOG = superior occipital gyrus; SPG = superior parietal gyrus; STG = superior temporal gyrus; TPOmid =middle temporal pole; L = left, R = right.
4. Discussion
To our knowledge, this is the first study assessing metacognition of emotion recognition across different neurodegenerative diseases and including anatomo-functional correlates of the metacognitive performance. Metacognition of negative emotions was specially affected in patients. Metacognition impairments were associated with less grey matter volume of fronto-temporo-insular and subcortical areas in bvFTD and of fronto-parietal regions in AD; and with disconnection of fronto-posterior functional networks for both groups. The relevance of this study lies in the link between emotion recognition and metacognition in neurodegenerative diseases. Our results suggest that emotion recognition and metacognition deficits share cerebral substrates and are associated with specific brain damage and network dysfunction according the dementia type.
Relative to controls, neurodegenerative diseases presented deficits of metacognition in total and negative emotions. BvFTD patients showed impairments in metacognition of disgust, whereas those with AD exhibited a more generalized deficit in all emotions, especially sadness and neutral faces. Our results are in line with the consistent loss of insight found in AD, despite not revealing more impaired metacognition in bvFTD (Hornberger et al., 2014).
For sadness, bvFTD patients presented adequate calibration and null MI differences regarding controls, indicating accurate metacognitive performance. Previous studies have found normal sadness recognition (Kessels et al., 2007; Lavenu, Pasquier, Lebert, Petit, & Van der Linden, 1999) and related emotional reactivity (Werner et al., 2007) in FTD. Moreover, a recent study showed diminished subjective experience of sadness in bvFTD, together with spared facial mimicry of sadness in response to a sad film, suggesting that some level of awareness may be preserved in these patients (Hua et al., 2020). The bvFTD patients also presented preserved recognition and metacognition of neutral faces, probably as a result of the context-independent task. In more complex scenarios, patients misunderstand the situations and rate neutral situations as morally wrong (Baez, Manes, et al., 2014) or judge neutral intentions more severely (Baez, Couto, et al., 2014). Thus, bvFTD patients’ performance on neutral conditions should depend on the context and available explicit information (Garcia-Cordero et al., 2019; A. Ibanez & Manes, 2012).
Based on the conscious awareness model (CAM) (D. C. Mograbi & Morris, 2013), we suggest that the prevalent metacognitive impairments in AD might be related to initial damage of memory deficits (McKhann et al., 1984; D. C. Mograbi & Morris, 2013), a domain partially preserved in bvFTD (Rascovsky et al., 2011). The model includes a central processor that receives and compares information about the performance (effective or failed) with long-term memories and experiences from the self. When memory is compromised, the comparison process will not function adequately (i.e., limited conscious experience of performance). AD patients are impaired at integrating information from online performance and memory representations of the self, resulting in limited conscious experience of performance and impaired self-evaluation (Lenzoni, Morris, & Mograbi, 2020). On the other hand, the specific deterioration in bvFTD can be interpreted by other particular mechanisms beyond the CAM model. Patients with frontal lobe damage can perform well in traditional tests (Burgess, Alderman, Volle, Benoit, & Gilbert, 2009; Mesulam, 1986) and exhibit normal outcomes in multiple cognitive and social domains when explicit information is provided (A. Ibanez & Manes, 2012). In our experiment, bvFTD patients presented variable performance, with both preserved and affected metacognition according to the emotion evaluated. Finally, we found type-specific deficits in emotion recognition and metacognition, implying that patients who were not able to correctly monitor their performance also presented impaired recognition. According to the CAM model, recognition and metacognition are part of the same feedback system, in which poor metacognitive abilities would lead to inaccurate emotion recognition and no behavior correction (D. C. Mograbi & Morris, 2013). This lack of improvement in emotion recognition mechanisms would affect the patients’ social interactions and worsen their ability to infer others’ emotional states. Despite the impact of metacognition on emotion recognition, only one recent study (Mattavelli et al., 2020) investigated this process through an emotional discrimination task and posterior confidence report in Parkinson’s disease. However, the authors analyzed only behavioral measures of metacognition and did not consider brain correlates. Importantly, as in other reports (D. Fernandez-Duque & Black, 2005; F. Kumfor et al., 2011; F. Kumfor & Piguet, 2012; Park et al., 2017; Rosen et al., 2004), the task could induce bias triggered by the imbalance between positive, negative, and neutral stimuli. In this context, happiness seems to be easier to recognize than negative emotions. Future studies should use more balanced categories, as recently reported (Maxime Bertoux et al., 2021).
Regarding anatomical results, emotion recognition involved fronto-temporo-insular and subcortical areas for bvFTD and temporo-parietal regions for AD. Metacognitive deficits correlated with atrophy in fronto-temporo-insular and subcortical areas for bvFTD, and with fronto-parietal areas for AD. These results suggest a shared anatomical structure for recognition and metacognition in both groups. Previous research indicates that metacognition is supported by the prefrontal cortex (D. Fernandez-Duque et al., 2000; Fleming & Dolan, 2012), a region involved in updating schema-information coming from posterior regions (D. Fernandez-Duque et al., 2000; Fleming & Dolan, 2012; Garcia-Cordero et al., 2019; Hebscher & Gilboa, 2016) and in generating error signals when there is a mismatch between performance and expectations (Rosen, 2011). In particular, the ventromedial prefrontal cortex is associated with monitoring of emotional inputs, and in cognitive generation and regulation of emotion (Rosen, 2011; Rosen et al., 2014). Likewise, the insular and the cingulate cortices are related with autonomic activation in monitoring process and error perceiving (Craig, 2009; Critchley et al., 2003; García-Cordero et al., 2016; Shany-Ur et al., 2014; Ullsperger, Harsay, Wessel, & Ridderinkhof, 2010), while the temporal lobes underlie memory abilities and autobiographical information (Dickerson & Sperling, 2008; Eslinger et al., 2005; D. C. Mograbi & Morris, 2013). In agreement with this evidence, our results showed that reduced volume of the prefrontal, insular, cingulate, and temporal cortices was related with metacognitive impairments.
On the other hand, the association we found between connectivity of fronto-posterior hubs and metacognition replicates previous research (Baird et al., 2013; Fleming & Dolan, 2012; Garrison, 2014; Vaccaro & Fleming, 2018). Considering the atrophy and functional findings, our results suggest that bvFTD metacognition impairments may result from disruptions along two networks: the fronto-temporo-insular network (Baez, García, & Ibanez, 2017; A. Ibanez, 2018; A. Ibanez et al., 2017; A. Ibanez & Manes, 2012; Agustin Ibanez & Schulte, 2021; Ibáñez, 2019), involved in contextual modulation of socioemotional cognition; and fronto-subcortical networks (O’Keeffe et al., 2007), related with emotion and executive control. We suggest that subcortical areas would not be able to coordinate multimodal internal and external information coming from the cortex with the metaknowledge to improve social conduct. On the other hand, we propose that an interruption of parietal/temporal-limbic-prefrontal networks (Abu-Akel, 2003) would be related with AD’s metacognitive impairments. This network facilitates a posterior-anterior integration of information. Specially, the frontal and temporal hubs are related with deficient self-knowledge update and limited access to memory of self-evaluation processes (Lenzoni et al., 2020). In short, we found specific metacognitive impairments for bvFTD and AD associated with particular damage of hubs and networks disconnection, related with the atrophy patterns and physiopathological process underlying these diseases.
Metacognition could be thought as a domain-general process indexed by the prefrontal cortex, a region implicated in monitoring processes (D. Fernandez-Duque et al., 2000; Fleming & Dolan, 2012). However, many studies showed dissociations between metacognition of different cognitive domains as they involved specific brain regions (Fleming et al., 2014; McCurdy et al., 2013) and functional networks (Baird et al., 2013). Our current findings support the view that metacognition of emotion recognition does not rely exclusively on the prefrontal cortex, but also on another areas (e.g., the insular and limbic system), evidenced by the disturbance and disconnection of domain-specific emotion processes. The joint participation of regions related with emotion recognition and metacognition suggest domain-specific involvement.
Some studies have considered emotion recognition as an automatic process that only requires minimal cognitive processes, contrasting with the concept of emotion schema (Izard, 2007, 2011), in which emotion processing involves interactions between emotion feelings and high-order cognition (memories, strategies, and goals). We suggest that one aspect of this higher-order cognition is the metacognitive processing that provides a continue feedback to adjust the responses to environment. As emotion schemas constitute the source of motivational processes, their disruption could impact on adaptive behavior (Izard, 2007, 2011). In line with this idea, deficits in metacognition have been related with emotional and motivational factors (Rosen, 2011) and with clinical symptoms such as anosognosia and apathy (Daniel C Mograbi & Morris, 2014; Rosen, 2011; Rosen et al., 2014). Apathetic patients are less reactive to their surroundings (S. E. Starkstein, Sabe, Chemerinski, Jason, & Leiguarda, 1996) and more prone to omit significant events (Rosen, 2011) due to lack of motivation and affective flattening. In this context, errors and their consequences are ignored or normalized, affecting monitoring processes (Daniel C Mograbi & Morris, 2014). In addition, increased apathy correlates with anosognosia (Derouesné et al., 1999) and demonstrates that omission of information may also contribute to the unawareness of having a disease. Neuroanatomical findings showed that apathy (Ott et al., 1996), anosognosia (Eslinger et al., 2005; Rosen, 2011; Wilson, Sytsma, Barnes, & Boyle, 2016), and metacognition (Eslinger et al., 2011; Fleming & Dolan, 2012; Rosen et al., 2014) are related with prefrontal function, suggesting a close link between these processes.
To our knowledge, this is the first report suggesting a link between metacognition of emotion recognition and emotional processing in neurodegeneration. Possible clinical implications of this work are related with the patients’ lack of awareness. Anosognosia and apathy might impact the relation between patient and caregiver as well as treatment response (Sergio E Starkstein, Jorge, Mizrahi, Adrian, & Robinson, 2007). This emergent evidence at the crossing of metacognition and emotion impairments may be relevant for future studies of patients’ daily living impairments, to prevent treatment drop-out and improve their quality of life.
5. Limitations
Our study presents a number of limitations. The sample size was moderate and the recruitment of participants was performed in two different clinical centers from Argentina and Chile. However, all groups were diagnosed by same consensus diagnostic criteria and were demographically matched in age, education, and gender. Moreover, country was used as a covariate in the analysis to avoid possible recruitment bias. We were not able to measure other cognitive variables (e.g., executive functions or memory). However, we consider that our results open a new agenda for the study of the relationship between metacognition of emotion recognition, general cognitive functions, social cognition, and functionality. Finally, further studies assessing other links among metacognition and emotion are needed to establish a better understanding of their relation.
6. Conclusions
The present study highlights the relevance of studying emotion recognition in relation with metacognition in neurodegenerative diseases. If a patient presents poor metacognitive abilities, emotion recognition impairments will not be corrected, potentially impacting their daily life. In addition, emotion recognition and metacognition shared brain several substrates, suggesting a close relation between both processes. As an unexplored domain in dementia, its evaluation may be relevant for the clinical and neurocognitive characterization of bvFTD and AD patients. By combining behavioral and neuroimaging measures, our work revealed that metacognition of negative emotions was mainly affected in patients and associated with specific brain structures and functional networks. These results inform neuroanatomical models of metacognition and its disruptions following brain pathology. Further assessments should evaluate the metacognition of emotion recognition across neurodegenerative diseases and in other social cognition domains. This would be especially relevant nor only for clinical outcomes and functionality, but also to promote a deeper understanding of metacognitive aspects of emotion and social behaviors.
Supplementary Material
Highlights.
Metacognition of emotion recognition is fundamental for social interactions.
Metacognition of negative emotions was affected in bvFTD and AD.
Such deficits were associated with disease-specific atrophy in each patient group.
Disconnection of fronto-posterior networks was related with metacognitive deficits.
These deficits may underlie core impairments in the daily life of patients.
8. Funding
This work was supported by CONICET; FONCYT-PICT (2017-1818, 2017-1820); CONICYT/FONDECYT Regular (1170010); FONDAP (15150012); Programa Interdisciplinario de Investigación Experimental en Comunicación y Cognición (PIIECC), Facultad de Humanidades, USACH; Sistema General de Regalías [BPIN2018000100059], Universidad del Valle [CI 5316], Alzheimer’s Association GBHI ALZ UK-20-639295; and the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat), funded by the National Institutes of Aging of the National Institutes of Health under award number R01AG057234, an Alzheimer’s Association grant (SG-20-725707-ReDLat), the Rainwater Foundation, and the Global Brain Health Institute. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, Alzheimer’s Association, Rainwater Charitable Foundation, and Global Brain Health Institute. The sponsors have no role of the in-study design, collection, analysis, interpretation, writing and submission of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
None.
Data statement
Given the patient sensitive information, the local ethical regulations, and the funding regulation for data sharing, we are unable to publicly share the databases and code at this stage. However, the datasets generated during and/or analyzed during the current study are available from the corresponding author on formal request and after IRB approval.
11. References
- 1.Abu-Akel A (2003). A neurobiological mapping of theory of mind. Brain Research Reviews, 43(1), 29e40. 10.1016/s0165-0173(03)00190-5 [DOI] [PubMed] [Google Scholar]
- 2.Adolphs R (2009). The social brain: Neural basis of social knowledge. [Research support, N.I.H., extramural research support, non-U.S. Gov’t review]. Annual Review of Psychology, 60, 693e716. 10.1146/annurev.psych.60.110707.163514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amanzio M, Torta DM, Sacco K, Cauda F, D’Agata F, Duca S, et al. (2011). Unawareness of deficits in alzheimer’s disease: Role of the cingulate cortex. Brain: A Journal of Neurology, 134(Pt 4), 1061e1076. 10.1093/brain/awr020 [DOI] [PubMed] [Google Scholar]
- 4.Arias JA, Williams C, Raghvani R, Aghajani M, Baez S, Belzung C, et al. (2020). The neuroscience of sadness: A multidisciplinary synthesis and collaborative review. Neuroscience and Biobehavioral Reviews, 111, 199e228. 10.1016/j.neubiorev.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Ashburner J (2007). A fast diffeomorphic image registration algorithm. [Evaluation Studies Research Support, Non-U.S. Gov’t]. Neuroimage, 38(1), 95e113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Baez S, Couto B, Torralva T, Sposato LA, Huepe D, Montanes P, et al. (2014). Comparing moral judgments of patients with frontotemporal dementia and frontal stroke.[Comparative Study Research Support, Non-U.S. Gov’t]. JAMA Neurology, 71(9), 1172e1176. 10.1001/jamaneurol.2014.347 [DOI] [PubMed] [Google Scholar]
- 7.Baez S, Garcıá AM, & Ibanez A (2017). The social context network model in psychiatric and neurological diseases. Current Topics in Behavioral Neurosciences, 30, 379e396. 10.1007/7854_2016_443 [DOI] [PubMed] [Google Scholar]
- 8.Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, et al. (2014). Primary empathy deficits in frontotemporal dementia. Frontiers in Aging Neuroscience, 6, 262. 10.3389/fnagi.2014.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird B, Smallwood J, Gorgolewski KJ, & Margulies DS(2013). Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. [Research Support, U.S. Gov’t, Non-P.H.S.]. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(42), 16657e16665. 10.1523/JNEUROSCI.0786-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begue I, Vaessen M, Hofmeister J, Pereira M, Schwartz S, & Vuilleumier P (2019). Confidence of emotion expression recognition recruits brain regions outside the face perception network. [Social Cognitive and Affective Neuroscience Electronic Resource], 14(1), 81e95. 10.1093/scan/nsy102 [DOI] [PMC free article] [PubMed] [Google Scholar]; Bertoux M, de Souza LC, Sarazin M, Funkiewiez A,Dubois B, & Hornberger M (2015). How preserved is emotion recognition in alzheimer disease compared with behavioral variant frontotemporal dementia? Alzheimer Disease and Associated Disorders, 29(2), 154e157. 10.1097/wad.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 11.Bertoux M, Duclos H, Caillaud M, Segobin S, Merck C, Sayette V, et al. (2021). When affect overlaps with concept: Emotion recognition in semantic variant of primary progressive aphasia. Brain: A Journal of Neurology. 10.1093/brain/awaa313 [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoux M, Funkiewiez A, O’Callaghan C, Dubois B, & Hornberger M (2013). Sensitivity and specificity of ventromedial prefrontal cortex tests in behavioral variant frontotemporal dementia. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 9(5 Suppl), S84eS94. 10.1016/j.jalz.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Bertoux M, Volle E, de Souza LC, Funkiewiez A, Dubois B, & Habert MO (2014). Neural correlates of the mini-SEA (Social cognition and Emotional Assessment) in behavioral variant frontotemporal dementia. Brain Imaging and Behavior, 8(1), 1e6. 10.1007/s11682-013-9261-0 [DOI] [PubMed] [Google Scholar]
- 13.Bertoux M, Volle E, Funkiewiez A, de Souza LC, Leclercq D, & Dubois B (2012). Social cognition and emotional assessment (SEA) is a marker of medial and orbital frontal functions: A voxel-based morphometry study in behavioral variant of frontotemporal degeneration. Journal of the International Neuropsychological Society: JINS, 18(6), 972e985. 10.1017/s1355617712001300 [DOI] [PubMed] [Google Scholar]
- 14.Blair RJR, Morris JS, Frith CD, Perrett DI, & Dolan RJ(1999). Dissociable neural responses to facial expressions of sadness and anger. Brain: A Journal of Neurology, 122(5), 883e893. 10.1093/brain/122.5.883 [DOI] [PubMed] [Google Scholar]
- 15.Bora E, Velakoulis D, & Walterfang M (2016). Meta-analysis of facial emotion recognition in behavioral variantfrontotemporal dementia: Comparison with alzheimerdisease and healthy controls. Journal of Geriatric Psychiatry and Neurology, 29(4), 205e211. 10.1177/0891988716640375 [DOI] [PubMed] [Google Scholar]
- 16.Burgess PW, Alderman N, Volle E, Benoit RG, & Gilbert SJ(2009). Mesulam’s frontal lobe mystery re-examined. [Case reports research support, non-U.S. Gov’t review]. Restorative Neurology and Neuroscience, 27(5), 493e506. 10.3233/RNN-2009-0511 [DOI] [PubMed] [Google Scholar]
- 17.Cosentino S, Brickman AM, Griffith E, Habeck C, Cines S, Farrell M, et al. (2015). The right insula contributes to memory awareness in cognitively diverse older adults. Neuropsychologia, 75, 163e169. 10.1016/j.neuropsychologia.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couto B, Manes F, Montanes P, Matallana D, Reyes P, Velasquez M, et al. (2013). Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neuroscience, 7, 467. 10.3389/fnhum.2013.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. [Review]. NatureReviews. Neuroscience, 3(8), 655e666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- 20.Craig AD (2009). How do you feel–now? The anterior insula and human awareness. [Research support, non-U.S. Gov’t]. Nature Reviews. Neuroscience, 10(1), 59e70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- 21.Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, et al. (2003). Human cingulate cortex andautonomic control: Converging neuroimaging and clinicalevidence. [Research support, non-U.S. Gov’t]. Brain: A Journal of Neurology, 126(Pt 10), 2139e2152. 10.1093/brain/awg216 [DOI] [PubMed] [Google Scholar]
- 22.Derouesne C, Thibault S, Lagha-Pierucci S, Baudouin-Madec V, Ancri D, & Lacomblez L (1999). Decreased awareness of cognitive deficits in patients with mild dementia of the Alzheimer type. International Journal of Geriatric Psychiatry, 14(12), 1019e1030. doi: . [DOI] [PubMed] [Google Scholar]
- 23.Dickerson BC, & Sperling RA (2008). Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and alzheimer’s disease: Insights from functionalMRI studies. Neuropsychologia, 46(6), 1624e1635. 10.1016/j.neuropsychologia.2007.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly-Kehoe PA, Pascariello GO, Garcıá AM, Hodges JR, Miller B, Rosen H, et al. (2019). Robust automated computational approach for classifying frontotemporal neurodegeneration: Multimodal/multicenter neuroimaging. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 11(1), 588e598. 10.1016/j.dadm.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, & Grossman M (2005). Metacognitive deficits in frontotemporal dementia. Neurologia I Neurochirurgia Polska, 76(12), 1630e1635. 10.1136/jnnp.2004.053157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eslinger PJ, Moore P, Anderson C, & Grossman M (2011). Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. [Comparative Study Research Support, N.I.H., Extramural]. The Journal of Neuropsychiatry and Clinical Neurosciences, 23(1), 74e82. 10.1176/appi.neuropsych.23.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Duque D, Baird JA, & Posner MI (2000). Executive attention and metacognitive regulation. [Research Support,U.S. Gov’t, Non-P.H.S.]. Consciousness and Cognition, 9(2 Pt 1), 288e307. 10.1006/ccog.2000.0447 [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Duque D, & Black SE (2005). Impaired recognition of negative facial emotions in patients with frontotemporal dementia. Neuropsychologia, 43(11), 1673e1687. 10.1016/j.neuropsychologia.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Duque D, & Black SE (2007). Metacognitive judgment and denial of deficit: Evidence from frontotemporal dementia. Judgment and Decision Making, 2(6), 359. [Google Scholar]
- 30.Fittipaldi S, Abrevaya S, Fuente A, Pascariello GO, Hesse E, Birba A, et al. (2020). A multidimensional and multi-feature framework for cardiac interoception. Neuroimage, 212, 116677. 10.1016/j.neuroimage.2020.116677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, & Garcia AM (2019). More than words: Social cognition across variants of primary progressive aphasia. Neuroscience and Biobehavioral Reviews, 100, 263e284. [DOI] [PubMed] [Google Scholar]
- 32.Fleming SM, & Dolan RJ (2012). The neural basis of metacognitive ability. [Research Support, Non-U.S. Gov’tReview]. Philos Trans R Soc Lond B Biol Sci, 367(1594), 1338e1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming SM, & Lau HC (2014). How to measuremetacognition. [Review]. Frontiers in Human Neuroscience, 8,443. 10.3389/fnhum.2014.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]; Fleming SM, Ryu J, Golfinos JG, & Blackmon KE (2014). Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Brain: A Journal of Neurology, 137(Pt 10), 2811e2822. 10.1093/brain/awu221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Cordero I, Sedeno L, Babino A, Dottori M, Melloni M, ~ M. Martorell Caro, et al. (2019). Explicit and implicitmonitoring in neurodegeneration and stroke. Scientific Reports,9(1), 14032. 10.1038/s41598-019-50599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcıá-Cordero I, Sedeno L, de la Fuente L, Slachevsky A, ~ Forno G, Klein F, et al. (2016). Feeling, learning from andbeing aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160006. 10.1098/rstb.2016.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrison J (2014). Dissociable neural networks supporting metacognition for memory and perception. [Comment Research Support, Non-U.S. Gov’t]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 34(8), 2765e2767. 10.1523/JNEUROSCI.5232-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Good CD, Johnsrude IS, Ashburner J, Henson RN,Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. [Research Support, Non-U.S. Gov’t]. Neuroimage, 14(1 Pt1), 21e36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- 38.Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, BaronCohen S, et al. (2002). Theory of mind in patients with frontalvariant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain: A Journal of Neurology, 125(4), 752e764. [DOI] [PubMed] [Google Scholar]
- 39.Hebscher M, & Gilboa A (2016). A boost of confidence: The roleof the ventromedial prefrontal cortex in memory, decisionmaking, and schemas. Neuropsychologia, 90, 46e58. 10.1016/j.neuropsychologia.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 40.Hornberger M, Yew B, Gilardoni S, Mioshi E, Gleichgerrcht E,Manes F, et al. (2014). Ventromedial-frontopolar prefrontalcortex atrophy correlates with insight loss in frontotemporaldementia and Alzheimer’s disease. [Research Support, NonU.S. Gov’t]. Human Brain Mapping, 35(2), 616e626. 10.1002/hbm.22200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua AY, Chen K-H, Brown CL, Lwi SJ, Casey JJ,Rosen HJ, et al. (2020). Physiological, behavioral and subjective sadness reactivity in frontotemporal dementia subtypes. [Social Cognitive and Affective Neuroscience Electronic Resource], 14(12), 1453e1465. 10.1093/scan/nsaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibanez A (2018). Brain oscillations, inhibition and social inappropriateness in frontotemporal degeneration. Brain: A Journal of Neurology, 141(10), e73. 10.1093/brain/awy233Iba [DOI] [PubMed] [Google Scholar]; Cortex; A Journal Devoted To the Study of the Nervous System and Behavior, 115, 341e344. 10.1016/j.cortex.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 43.Ibanez A, Billeke P, de la Fuente L, Salamone P, Garcia AM, & Melloni M (2017). Reply: Towards a neurocomputational account of social dysfunction in neurodegenerative disease. Brain: a Journal of Neurology, 140(3), e15. 10.1093/brain/aww316 [DOI] [PubMed] [Google Scholar]
- 44.Ibanez A, Gleichgerrcht E, & Manes F (2010). Clinical effects of insular damage in humans. Brain Structure & Function, 214(5e6), 397e410. 10.1007/s00429-010-0256-y [DOI] [PubMed] [Google Scholar]
- 45.Ibanez A, & Manes F (2012). Contextual social cognition and the behavioral variant of frontotemporal dementia. [ResearchSupport, Non-U.S. Gov’t Review]. Neurology, 78(17), 1354e1362. 10.1212/WNL.0b013e3182518375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibanez A, & Schulte M (2021). Situated minds: Conceptual and emotional blending in neurodegeneration and beyond. Brain:A Journal of Neurology, 143(12), 3523e3525. 10.1093/brain/awaa392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irish M, Piguet O, Hodges JR, & Hornberger M (2014). Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer’s disease. [Comparative Study Research Support, Non-U.S. Gov’t]. Human Brain Mapping, 35(4), 1422e1435. 10.1002/hbm.22263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izard CE (2007). Basic emotions, natural kinds, emotion schemas, and a new paradigm. Perspectives on Psychological Science, 2(3), 260e280. 10.1111/j.1745-6916.2007.00044.x [DOI] [PubMed] [Google Scholar]
- 49.Izard CE (2011). Forms and functions of emotions: Matters of emotionecognition interactions. Emotion Review, 3(4), 371e378. 10.1177/1754073911410737 [DOI] [Google Scholar]
- 50.Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, & Irish M (2015). Differentiating between right-lateralisedsemantic dementia and behavioural-variant frontotemporal dementia: An examination of clinical characteristics andemotion processing. Neurologia I Neurochirurgia Polska, 86(10), 1082e1088. 10.1136/jnnp-2014-309120 [DOI] [PubMed] [Google Scholar]
- 51.Kelly KJ, & Metcalfe J (2011). Metacognition of emotional face recognition. Emotion, 11(4), 896e906. 10.1037/a0023746 [DOI] [PubMed] [Google Scholar]
- 52.Kessels RPC, Gerritsen L, Montagne B, Ackl N, Diehl J, & Danek A (2007). Recognition of facial expressions of different emotional intensities in patients with frontotemporal lobar degeneration. Behavioural neurology, 18(1), 31e36. 10.1155/2007/868431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumfor F, Irish M, Hodges JR, & Piguet O (2013). Discrete neural correlates for the recognition of negative emotions:Insights from frontotemporal dementia. Plos One, 8(6). 10.1371/journal.pone.0067457.e67457-e67457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumfor F, Miller L, Lah S, Hsieh S, Savage S, Hodges JR, et al. (2011). Are you really angry? The effect of intensity onfacial emotion recognition in frontotemporal dementia. Social Neuroscience, 6(5e6), 502e514. 10.1080/17470919.2011.620779 [DOI] [PubMed] [Google Scholar]
- 55.Kumfor F, & Piguet O (2012). Disturbance of emotion processing in frontotemporal dementia: A synthesis of cognitive and neuroimaging findings. Neuropsychology Review, 22(3), 280e297. 10.1007/s11065-012-9201-6 [DOI] [PubMed] [Google Scholar]
- 56.Lavenu I, Pasquier F, Lebert F, Petit H, & Van der Linden M (1999). Perception of emotion in frontotemporal dementia and Alzheimer disease. Alzheimer Disease and Associated Disorders, 13(2), 96e101. 10.1097/00002093-199904000-00007 [DOI] [PubMed] [Google Scholar]
- 57.Lenzoni S, Morris RG, & Mograbi DC (2020). The petrified self10 Years after: Current evidence for mnemonic anosognosia. Frontiers in Psychology, 11, 465. 10.3389/fpsyg.2020.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattavelli G, Barvas E, Longo C, Zappini F, Ottaviani D, Malaguti MC, et al. (2020). Facial expressions recognitionand discrimination in Parkinson’s disease. Journal of Neuropsychology. 10.1111/jnp.12209 [DOI] [PubMed] [Google Scholar]
- 59.McCurdy LY, Maniscalco B, Metcalfe J, Liu KY, de Lange FP, & Lau H (2013). Anatomical coupling between distinct metacognitive systems for memory and visual perception. The Journal of Neuroscience, 33(5), 1897e1906. 10.1523/jneurosci.1890-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKhann G, Drachman D, Folstein M, Katzman R, Price D, & Stadlan EM (1984). Clinical diagnosis of alzheimer’s disease:Report of the NINCDS-ADRDA work group under the auspicesof department of Health and human services task force onalzheimer’s disease. [Guideline practice guideline]. Neurology,34(7), 939e944. [DOI] [PubMed] [Google Scholar]
- 61.Mesulam MM (1986). Frontal cortex and behavior. [Case reports research support, non-U.S. Gov’t]. Annals of Neurology, 19(4), 320e325. 10.1002/ana.410190403 [DOI] [PubMed] [Google Scholar]
- 62.Mograbi D, Brown R, Landeira-Fernandez J, & Morris R (2014). Metacognition and attribution of difficulty for self and othersin Alzheimer’s disease. Psychology & Neuroscience, 7, 417e424. [Google Scholar]
- 63.Mograbi DC, Brown RG, Salas C, & Morris RG (2012). Emotional reactivity and awareness of task performance in Alzheimer’s disease. Neuropsychologia, 50(8), 2075e2084. 10.1016/j.neuropsychologia.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 64.Mograbi DC, & Morris RG (2013). Implicit awareness in anosognosia: Clinical observations, experimental evidence,and theoretical implications. [Review]. Cognitive Neuroscience, 4(3e4), 181e197. 10.1080/17588928.2013.833899 [DOI] [PubMed] [Google Scholar]
- 65.Mograbi DC, & Morris RG (2014). On the relation among mood, apathy, and anosognosia in Alzheimer’s disease. Journalof the International Neuropsychological Society: JINS, 20(1), 2e7. [DOI] [PubMed] [Google Scholar]
- 66.Mograbi DC, & Morris RG (2018). Anosognosia. Cortex, 103, 385e386. 10.1016/j.cortex.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 67.Moguilner S, Garcia AM, Mikulan E, Hesse E, GarciaCordero I, Melloni M, et al. (2018). Weighted symbolicdependence metric (wSDM) for fMRI resting-state connectivity: A multicentric validation for frontotemporal dementia. Scientific Reports, 8(1), 11181. 10.1038/s41598-018-29538-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moguilner S, Garcıá AM, Perl YS, Tagliazucchi E, Piguet O, Kumfor F, et al. (2020). Dynamic brain fluctuationsoutperform connectivity measures and mirrorpathophysiological profiles across dementia subtypes: Amulticenter study. Neuroimage, 225, 117522. 10.1016/j.neuroimage.2020.117522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Callaghan C, Bertoux M, Irish M, Shine JM, Wong S, Spiliopoulos L, et al. (2015). Fair play: Social norm compliance failures in behavioural variant frontotemporal dementia. Brain: A Journal of Neurology, 139(1), 204e216. 10.1093/brain/awv315 [DOI] [PubMed] [Google Scholar]
- 70.O’Keeffe FM, Murray B, Coen RF, Dockree PM, Bellgrove MA, Garavan H, et al. (2007). Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. [Research Support, Non-U.S. Gov’t]. Brain: A Journal of Neurology, 130(Pt 3), 753e764. 10.1093/brain/awl367 [DOI] [PubMed] [Google Scholar]
- 71.Omar R, Rohrer JD, Hailstone JC, & Warren JD (2011). Structural neuroanatomy of face processing in frontotemporal lobar degeneration. Journal of Neurology, Neurosurgery, and Psychiatry, 82(12), 1341e1343. 10.1136/jnnp.2010.227983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ott BR, Lafleche G, Whelihan WM, Buongiorno GW, Albert MS, & Fogel BS (1996). Impaired awareness of deficits in Alzheimer disease. Alzheimer Disease and Associated Disorders, 10(2), 68e76. 10.1097/00002093-199601020-00003 [DOI] [PubMed] [Google Scholar]
- 73.Park S, Kim T, Shin SA, Kim YK, Sohn BK, Park HJ, et al. (2017). Behavioral and neuroimaging evidence for facialemotion recognition in elderly Korean adults with mildcognitive impairment, alzheimer’s disease, andfrontotemporal dementia. Frontiers in Aging Neuroscience, 9,389. 10.3389/fnagi.2017.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perrotin A, Desgranges B, Landeau B, Mezenge F, La Joie R, Egret S, et al. (2015). Anosognosia in Alzheimer disease: Disconnection between memory and self-related brainnetworks. [Research Support, Non-U.S. Gov’t]. Annals of Neurology, 78(3), 477e486. 10.1002/ana.24462 [DOI] [PubMed] [Google Scholar]
- 75.Piguet O, Hornberger M, Mioshi E, & Hodges JR (2011). Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. [Research support, nonU.S. Gov’t review]. Lancet Neurology, 10(2), 162e172. 10.1016/S1474-4422(10)70299-4 [DOI] [PubMed] [Google Scholar]
- 76.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Brain: A Journal of Neurology, 134(Pt 9), 2456e2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosen HJ (2011). Anosognosia in neurodegenerative disease. [Review]. Neurocase, 17(3), 231e241. [DOI] [PubMed] [Google Scholar]
- 78.Rosen HJ, Alcantar O, Zakrzewski J, Shimamura AP, Neuhaus J, & Miller BL (2014). Metacognition in the behavioral variant of frontotemporal dementia and alzheimer’s disease. [Research support, N.I.H., extramural research support, non-U.S. Gov’t]. Neuropsychology, 28(3), 436e447. 10.1037/neu0000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosen HJ, Pace-Savitsky K, Perry RJ, Kramer JH, Miller BL, & Levenson RW (2004). Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Dementia and Geriatric Cognitive Disorders, 17(4), 277e281. 10.1159/000077154 [DOI] [PubMed] [Google Scholar]
- 80.Rosen HJ, Wilson MR, Schauer GF, Allison S, GornoTempini ML, Pace-Savitsky C, et al. (2006). Neuroanatomical correlates of impaired recognition of emotion in dementia. [Research support, N.I.H., extramural research support, non-U.S. Gov’t]. Neuropsychologia, 44(3), 365e373. 10.1016/j.neuropsychologia.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 81.Salamone PC, Esteves S, Sinay VJ, Garcıá-Cordero I, Abrevaya S, Couto B, et al. (2018). Altered neural signatures of interoception in multiple sclerosis. Human Brain Mapping, 39(12), 4743e4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sedeno L, Piguet O, Abrevaya S, Desmaras H, GarciaCordero I, Baez S, et al. (2017). Tackling variability: A multicenter study to provide a gold-standard network approach for frontotemporal dementia. Human Brain Mapping, 38(8), 3804e3822. 10.1002/hbm.23627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, & Rankin KP (2014). Self-awareness in neurodegenerativedisease relies on neural structures mediating reward-driven attention. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Brain: A Journalof Neurology, 137(Pt 8), 2368e2381. 10.1093/brain/awu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimamura AP (2000). Toward a cognitive neuroscience of metacognition. [Comment Research Support, U.S. Gov’t, P.H.S]. Consciousness and Cognition, 9(2 Pt 1), 313e323. 10.1006/ccog.2000.0450. discussion 324-316. [DOI] [PubMed] [Google Scholar]
- 85.Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, et al. (2009). Neural basis of interpersonal traits in neurodegenerative diseases. [Comment Research support, N.I.H., extramural research support, nonU.S. Gov’t]. Neuropsychologia, 47(13), 2812e2827. 10.1016/j.neuropsychologia.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Starkstein SE, Jorge R, Mizrahi R, Adrian J, & Robinson R (2007). Insight and danger in Alzheimer’s disease. European journal of neurology, 14(4), 455e460. [DOI] [PubMed] [Google Scholar]
- 87.Starkstein SE, Sabe L, Chemerinski E, Jason L, & Leiguarda R (1996). Two domains of anosognosia in Alzheimer’s disease. [Research Support, Non-U.S. Gov’t]. Neurologia I Neurochirurgia Polska, 61(5), 485e490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas AK, Lee M, & Balota DA (2013). Metacognitive monitoring and dementia: How intrinsic and extrinsic cues influence judgments of learning in people with early-stage alzheimer’s disease. Neuropsychology, 27(4), 452e463. 10.1037/a0033050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, et al. (2007). The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. [Research Support, Non-U.S. Gov’t]. Neuropsychologia, 45(2), 342e349. [DOI] [PubMed] [Google Scholar]
- 90.Torres Mendonc a De Melo Fadel B, Santos De Carvalho RL, Belfort Almeida Dos Santos TT, & M. C. N. Dourado (2019). Facial expression recognition in alzheimer’s disease: Asystematic review. Journal of Clinical and Experimental Neuropsychology, 41(2), 192e203. 10.1080/13803395.2018.1501001 [DOI] [PubMed] [Google Scholar]
- 91.Tusche A, Bockler A, Kanske P, Trautwein FM, & Singer T (2016). Decoding the charitable brain: Empathy, perspectivetaking, and attention shifts differentially predict altruisticgiving. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 36(17), 4719e4732. 10.1523/JNEUROSCI.3392-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002). Automated anatomicallabeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273e289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 93.Ullsperger M, Harsay HA, Wessel JR, & Ridderinkhof KR(2010). Conscious perception of errors and its relation to the anterior insula. [Review]. Brain Structure & Function, 214(5e6), 629e643. 10.1007/s00429-010-0261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaccaro AG, & Fleming SM (2018). Thinking about thinking: A coordinate-based meta-analysis of neuroimaging studies of metacognitive judgements. Biomolecular NMR Assignments, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Werner KH, Roberts NA, Rosen HJ, Dean DL, Kramer JH, Weiner MW, et al. (2007). Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology, 69(2), 148e155. 10.1212/01.wnl.0000265589.32060.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson RS, Sytsma J, Barnes LL, & Boyle PA (2016). Anosognosia in dementia. [Review research support, N.I.H., extramural]. Current Neurology and Neuroscience Reports, 16(9),77. 10.1007/s11910-016-0684-z [DOI] [PubMed] [Google Scholar]
- 97.Yoris A, Abrevaya S, Esteves S, Salamone P, Lori N, Martorell M, et al. (2018). Multilevel convergence of interoceptive impairments in hypertension: New evidence of disrupted body-brain interactions. Human Brain Mapping, 39(4), 1563e1581. 10.1002/hbm.23933 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


