Abstract
Objective:
Childhood physical and sexual abuse are stressful experiences that may alter the emotional response to future stressors. Stress-related emotional function is supported by brain regions that include the prefrontal cortex (PFC), hippocampus, and amygdala. The present study investigated whether childhood physical and sexual abuse are associated with stress-elicited brain activity in young adulthood.
Methods:
Participants (N=300; Mage=20.0; 151 female) completed a psychosocial stress task during functional magnetic resonance imaging (fMRI). Measures of physical and sexual abuse were included in a linear mixed effects model to estimate the unique relationship each type of childhood abuse had with stress-elicited brain activity.
Results:
Stress-elicited dorsolateral PFC, ventromedial PFC, and hippocampal activity decreased as the frequency of childhood sexual abuse increased. There were no regions in which stress-elicited activation varied with physical abuse.
Conclusions:
The present findings suggest there is a unique relationship between childhood sexual abuse and the stress-elicited PFC and hippocampal activity of young adults that is not observed following childhood physical abuse.
Significance:
These findings may have important implications for understanding the mechanisms by which childhood sexual abuse impacts the development of future psychopathology.
Keywords: fMRI, childhood trauma questionnaire, psychosocial stress, hippocampus, prefrontal cortex
1. Introduction
Childhood physical and sexual abuse are prevalent issues (lifetime prevalence of physical abuse is 28% and lifetime prevalence of sexual abuse is 21%) in the United States and are associated with increased risk of future psychopathology (Dong et al., 2003; Felitti et al., 1998; Gilbert et al., 2009). Specifically, abuse during childhood is linked to emotional dysfunction (e.g., symptoms of anxiety, depression, and posttraumatic stress disorder; PTSD) as well as changes in the psychophysiological response to stress (e.g., blunted cortisol response to stress) that often persists into adulthood (Anda et al., 2006; Chen et al., 2010; Gilbert et al., 2009; Linares et al., 2013; Pierrehumbert et al., 2009; Trickett et al., 2014). The impact of childhood abuse is significant; these experiences are linked to approximately 45% of childhood-onset psychiatric disorders (e.g., mood, anxiety, substance use, and disruptive behavior disorders) and 80% of childhood and adolescent suicide attempts (Dube et al., 2001; Green et al., 2010). Further, stress has been linked with negative physical and psychological health outcomes (McEwen, 1998; McEwen & Gianaros, 2010, 2011). Given that the psychological impact of childhood physical and sexual abuse often persists into adulthood (Anda et al., 2006; Chen et al., 2010; Gilbert et al., 2009), it is important to understand the mechanisms by which these types of experiences lead to changes in stress reactivity.
Prior work has established that childhood abuse disrupts the function of the prefrontal cortex (PFC), hippocampus, and amygdala (Boccia et al., 2016; Elsey et al., 2015; Fonzo et al., 2013; McLaughlin et al., 2015; Skokauskas et al., 2015). These brain regions constitute a neural circuit that supports the expression and regulation of emotion (Goodman et al., 2018; Hartley & Phelps, 2010; Ochsner & Gross, 2005; Orem et al., 2019; Wood et al., 2012, 2015). The amygdala plays an important role in many emotion-related functions. For example, it has been linked to fear learning and memory, emotional expression and reactivity, as well as anxiety and depression (Büchel et al., 1998; Casey et al., 2017; Knight et al., 2003; LaBar et al., 1998; Pattwell & Bath, 2017; Rauch et al., 2006). The amygdala receives top-down projections from prefrontal brain regions, including the ventromedial and dorsomedial PFC, which support emotion regulation processes (Casey et al., 2017; Gee et al., 2013; Silvers et al., 2016). Lateral regions of the PFC (i.e., dorsolateral and ventrolateral PFC) are also responsible for important emotion processes, including the reappraisal of emotion and the redirection of attention (Buhle et al., 2014; Casey et al., 2017; Wager et al., 2008). In addition to modulatory inputs from the PFC, the amygdala also receives projections from the hippocampus, which provide contextual information for emotional processes (Bannerman et al., 2004; Hartley & Phelps, 2010; Pattwell & Bath, 2017). Disruption of this circuit increases the risk of developing the types of psychopathology that are associated with emotion dysfunction, including anxiety, depression, and PTSD (PTSD; Burrus, 2013; Cisler et al., 2015; Dretsch et al., 2019; Elsey et al., 2015; Gee et al., 2013; Grant et al., 2011, 2014; Harnett et al., 2018). Taken together, the PFC, amygdala, and hippocampus form a neural circuit that underlies important emotional processes. The dysfunction of this circuitry has been implicated in a variety of psychiatric disorders.
Prior research on childhood abuse has largely focused on distinct types of abuse in isolation (e.g., sexual abuse only), without taking other types of adversity (e.g., physical abuse and other types of violence exposure) into account. While this approach has been informative, evidence indicates that different types of abuse and violence exposure often co-occur (Dong et al., 2003, 2004). Therefore, a comprehensive understanding of the impact of childhood abuse requires the concurrent evaluation of a broader range of events. For example, a recent meta-analysis examined brain activity associated with a range of traumatic experiences (i.e., physical or sexual abuse, combat-related trauma, and natural disasters) associated with PTSD (Boccia et al., 2016). Distinct patterns of activation emerged for each type of precipitating event. Specifically, anterior cingulate and dorsolateral PFC activation was associated with physical or sexual abuse (Boccia et al., 2016), whereas ventromedial PFC, insula, and hippocampal activation was linked to combat-related trauma (Boccia et al., 2016). Finally, dorsolateral PFC and parahippocampal cortex activation was associated with natural disaster (Boccia et al., 2016). Similarly, behavioral investigations have demonstrated that different types of childhood abuse are associated with unique health outcomes (Archer et al., 2017; Westermair et al., 2018). For example, sexual abuse has been linked to increased risk of suicide attempts, eating disorders, and borderline personality disorder, when controlling for exposure to other types of abuse (Brewerton, 2007; Fergusson et al., 2008; Johnson et al., 1999; Yates et al., 2008). In contrast, physical abuse has been linked with antisocial and depressive personality disorders when controlling for other types of abuse (Johnson et al., 1999). Further, sexual abuse, but not physical abuse, has been linked to higher risk of developing anxiety, depression, and PTSD symptoms, as well as greater severity of these symptoms (Adams et al., 2018; Chen et al., 2010). In light of evidence that suggests distinct types of childhood abuse are associated with different psychological outcomes (Adams et al., 2018; Archer et al., 2017; Boccia et al., 2016; Brewerton, 2007; Chen et al., 2010; Fergusson et al., 2008; Johnson et al., 1999; Westermair et al., 2018; Yates et al., 2008), it is important to understand whether brain function also varies with these distinct types of childhood abuse.
There has been limited neuroimaging research that has concurrently evaluated childhood physical and sexual abuse in an attempt to differentiate the neural outcomes. Instead, the majority of prior neuroimaging studies have either examined individual types of abuse in isolation or combined physical and sexual abuse into a single variable (Boccia et al., 2016; Cisler et al., 2015; Elsey et al., 2015; Fonzo et al., 2013; Lanius et al., 2002, 2005; Raine et al., 2001; Shin et al., 1999; Tomoda et al., 2009). Thus, prior research has generally not attempted to disentangle the distinct impact of physical and sexual abuse on the brain. The limited research that has attempted to separate these relationships is nascent and inconclusive. Thus, the extant literature has yet to converge on solid conclusions regarding the independent effects of physical and sexual abuse on brain function. For example, prior research found that greater amygdala reactivity during negative mood induction was associated with sexual abuse (Yamamoto et al., 2017), whereas other work found no relationship between sexual abuse and brain activity elicited by a social exclusion task (van Harmelen et al., 2014). Similarly, prior research found that dorsolateral PFC activity to emotional faces varied with physical abuse (Nicol et al., 2015), whereas other research found no relationship between physical abuse and brain function elicited by social exclusion (van Harmelen et al., 2014; Yamamoto et al., 2017). Differences in the tasks used to elicit neural responses likely contribute to the heterogeneity of results in the literature. For example, only one of these prior studies (van Harmelen et al., 2014) is based on a task (i.e., the social exclusion task) with components (i.e., motivated performance, uncontrollability of outcomes, and social/evaluative threat) that reliably elicit a stress response (Dedovic et al., 2009; Dickerson & Kemeny, 2004). Thus, the emergent nature of research on this topic, in combination with the variability in the tasks prior studies have used to study brain function, has few definitive conclusions. Therefore, the unique relationships between brain function and distinct types of childhood abuse requires further study to better understand the neural outcomes of childhood physical and sexual abuse. This line of research may elucidate the neural mechanisms by which childhood abuse leads to future psychopathology.
Much of the prior research on childhood abuse has focused on individuals who have developed psychopathology and were recruited from clinical settings (Ahn et al., 2016; Bremner, 1999; Bremner et al., 1997; Cisler et al., 2015; Fonzo et al., 2013; Grant et al., 2011, 2014; Jaworska et al., 2014; Kitayama et al., 2007; Nicol et al., 2015; Rinne-Albers et al., 2016; Skokauskas et al., 2015; Souza-Queiroz et al., 2016; van den Bulk et al., 2016). While it is important to understand the effects of childhood violence and abuse within clinical populations, prior findings from patient groups may not generalize to the broader population and can be difficult to interpret outside the specific settings sampled. In contrast, community samples (i.e., individuals recruited from the community, rather than clinical settings) often more accurately represent the community from which they are drawn, thereby increasing the generalizability of the findings. The present study utilized data from a large community sample to investigate the relationship between childhood physical and sexual abuse and the brain activity elicited by a psychosocial stress task (i.e., the Montreal Imaging Stress Task [MIST]; Dedovic et al., 2005). The MIST (described in more detail in Section 2.4) incorporates motivated task performance, uncontrollable outcomes, and social/evaluative threat. Prior work has demonstrated that the MIST reliably elicits a stress response (e.g., cortisol, skin conductance, heart rate) and is associated with neural activity within the PFC, hippocampus, amygdala, and inferior parietal lobule (Dedovic et al., 2005, 2009; Dickerson & Kemeny, 2004; Orem et al., 2019; Pruessner et al., 2008; Wheelock et al., 2016). Given that research attempting to separate the impact of physical and sexual abuse on neural activity is still emerging, the present study provides novel insight into the distinct impact physical and sexual abuse have on neural reactivity to stress.
Although, prior research attempting to separate the neural impact of physical and sexual abuse has used a variety of tasks (e.g., social exclusion, negative mood induction, and emotional faces), each of these studies employed emotion-based tasks to elicit brain activity. Therefore, similar brain regions may be activated by other emotion-based tasks (e.g., the MIST). Based on the limited prior work that has assessed physical and sexual abuse (Boccia et al., 2016; Nicol et al., 2015; van Harmelen et al., 2014; Yamamoto et al., 2017), we expected that stress-related amygdala activity would vary with sexual abuse and that stress-elicited dorsolateral PFC activity would vary with physical abuse. Further, prior research has found that there is a stronger relationship between PTSD and sexual abuse than PTSD and physical abuse, which includes a higher risk of developing PTSD and greater severity of PTSD symptoms following sexual abuse (Adams et al., 2018; Chen et al., 2010). PTSD is commonly associated with changes within the hippocampus (Bremner, 1999; Bremner et al., 1997, 2003; Cassiers et al., 2018; Dark et al., in press; Hart & Rubia, 2012). Therefore, we expected stress-related hippocampal activity would vary with sexual abuse. Finally, prior work has linked physical abuse to the risk of developing antisocial personality disorder (Johnson et al., 1999). Given that antisocial personality disorder is associated with alterations within the ventromedial PFC (Raine et al., 2001, 2011), we expected stress-elicited ventromedial PFC activity would vary with physical abuse.
The present study aimed to identify the distinct relationships that physical and sexual abuse have with neural reactivity to stress. Although, the relationship abuse and brain activity have with psychopathology were not assessed in the present study, prior work indicates physical and sexual abuse increase the risk for internalizing and externalizing disorder (Anda et al., 2006; Chen et al., 2010; Dong et al., 2003; Felitti et al., 1998; Gilbert et al., 2009; Linares et al., 2013). Further, prior research suggests that changes in the function of the PFC, amygdala, and hippocampus may underlie the development of psychopathology (Bremner, 1999; Bremner et al., 1997, 2003; Cisler et al., 2015; Grant et al., 2011, 2014; Rauch et al., 2006; Souza-Queiroz et al., 2016; van den Bulk et al., 2016). Thus, alterations in the activity of these brain regions may represent a mechanism by which abuse may increase risk for future psychopathology. Therefore, determining the relationship specific types of childhood abuse have with stress-elicited neural activity may provide novel insights into neurobiological processes that may ultimately have important implications for future treatment efforts.
2. Method
2.1. Participants
All participants were recruited from a larger Birmingham-Metropolitan area cohort (n = 1,594) of the Healthy Passages study, a multi-site longitudinal study of adolescent health (Schuster et al., 2012; Windle et al., 2004). The original Healthy Passages study was approved by the Centers for Disease Control and Prevention and the Institutional Review Boards at the original study sites. Participants were initially recruited from 5th grade classrooms in local public schools, and were interviewed at four time points (Wave 1 Mage = 11.2, SD = 0.5; Wave 2 Mage = 13.1, SD = 0.5; Wave 3 Mage = 16.2, SD = 0.5; Wave 4 Mage = 19.2, SD = 1.2). Following completion of the initial portion of the study, participants were invited to return for a single functional magnetic resonance imaging (fMRI) study during which they completed a variation of the MIST (Dedovic et al., 2005; Goodman et al., 2016; Wheelock et al., 2016). Sample size was determined based on a power analysis. Exclusion criteria included: standard fMRI contraindications, left-handedness, history of blood or circulation disorders (e.g., anemia or sickle-cell), diabetes, brain or spinal abnormalities, pregnancy, previous or current head injury (e.g., traumatic brain injury), and history of psychosis. Three hundred fifty participants were recruited for the neuroimaging session. Fifty participants were excluded from analyses due to excessive motion, poor data quality, imaging artifacts, and participant dropout. The final sample included in the present MRI analyses consisted of three hundred right-handed young adults (Mscan age = 20.0, SD = 1.5, range = 17 – 23 years) from the Birmingham-Metropolitan area (149 males, 151 females; 200 Black, 100 White). Additional demographic information can be found in Table 1. No parts of the study procedures or analyses were pre-registered prior to the research being conducted.
Table 1.
Descriptive information
| N (%) | ||
|---|---|---|
| Female participants | 151 (50%) | |
| Male participants | 149 (50%) | |
| Black participants | 200 (67%) | |
| White participants | 100 (33%) | |
| M (SEM) | Range | |
| Violence exposure | 0.85 (0.04) | 0 – 3.81 |
| Physical abuse | 0.88 (0.07) | 0 – 7 |
| Sexual abuse | 0.47 (0.09) | 0 – 10 |
Note: Descriptive information for the present sample. M = mean, SEM = standard error of the mean.
Given that only a small portion of the initial cohort completed neuroimaging (approximately 22% of the initial Healthy Passages cohort from the University of Alabama at Birmingham site), we assessed demographic differences between groups who did versus did not complete the neuroimaging session. These two groups did not differ by sex [χ2(1) = 0.041, p > 0.05], exposure to violence at Wave 1 [t(1483) = 1.30, p > 0.05], severity of physical abuse [t(1196) = 0.17, p > 0.05], and severity of sexual abuse [t(1196) = −1.02, p > 0.05]. However, a greater proportion of Black participants completed neuroimaging than did not (Wave 1: 57% vs MRI: 68%) and a smaller proportion of White participants completed neuroimaging than did not (Wave 1: 43% vs MRI: 32%; χ2(1) = 12.20, p < 0.001). All participants in the present study provided written informed consent and all study procedures were approved by the University of Alabama at Birmingham Institutional Review Board.
2.2. Physical and Sexual Abuse
Participants completed the physical, sexual, and emotional abuse subscales of the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003) during the Wave 4 assessment. The present study focused on questions related to a specific gap in the literature pertaining to abuse that has the potential to harm personal physical integrity. More specifically, these analyses were designed to disentangle neural outcomes associated with physical abuse from those associated with sexual abuse. Therefore, the emotional abuse subscale was not included in the present analyses. The physical and sexual abuse subscales consist of five questions that assess physical abuse and five questions that assess sexual abuse that occurred before the age of 18. Participants responded on a three-point scale (0 = never, 1 = sometimes, 2 = often). Responses to questions on the physical and sexual abuse scales were summed and separately entered into subsequent neuroimaging analyses as continuous regressors. Thus, participants who did not endorse any abuse received a value of zero.
2.3. Violence Exposure
Participants were interviewed at four waves (described in Section 2.1 Participants) and were asked how frequently they had witnessed violence in the last twelve months, including 1) threats of violence, 2) physical violence, and 3) threat or physical violence involving a weapon. Participants were also asked how frequently they were victimized in the last twelve months, including 1) threat of violence, 2) physical violence, 3) threat or physical violence involving a weapon, and 4) victimization requiring medical treatment. Responses were given on a 4-point scale ranging from 0 – 3 (0 = never, 1 = one time, 2 = a few times, 3 = many times). Responses were averaged across witnessing and victimization questions separately, resulting in a “witnessing” score and a “victimization” score for each wave of data collection. Witnessing and victimization scores were summed at each wave of data collection, resulting in a cumulative violence exposure score for each of the 4 waves. These cumulative violence exposure scores were then averaged across the 4 waves, yielding the violence exposure variable utilized in the present study. Ninety five percent of participants in the current investigation reported violence exposure across the 4 waves of data collection (Table 1). Therefore, violence exposure was included as a covariate of no interest in the neuroimaging analyses to isolate the neural effects of physical and sexual abuse from other types of violence exposure.
The victim’s relationship to the perpetrator of abuse/violence is an important distinction between the physical abuse and violence exposure measures that were used in this study. Specifically, physical abuse questions asked about instances of abuse perpetrated by family members. In contrast, violence exposure questions asked about the participant’s general violence exposure more broadly and did not specify a perpetrator. Nevertheless, some types of abuse/violence could conceptually fall under both physical abuse and violence exposure. However, only a small/weak (r = 0.25; Cohen, 1977, pp. 77–81) relationship was observed in a bivariate correlation analysis that compared physical abuse and violence exposure. Thus, it appears that participants generally conceptualized physical abuse and violence exposure as separate types of experiences.
2.4. Task Design
Participants completed a modified version of the MIST, a challenging mental arithmetic task optimized for administration during fMRI (Dedovic et al., 2005; Goodman et al., 2016; Wheelock et al., 2016). This study used a fast event-related design consisting of two scans. Each scan was 7 min and 54 sec in duration and contained 54 trials. Each trial was separated by a 1–3 sec inter-trial interval (fixation cross) and lasted 6 sec (Supplementary Figure S1). Trials consisted of a unique math problem with a response window (0.5–5 sec duration), followed by a fixation cross (0.5 – 5 sec duration). The fixation cross was followed by 0.5 sec of visual feedback (“Right,” “Wrong,” or “Timeout”). Prior to the scanning session, participants completed a set of practice math problems outside of the MRI scanner to familiarize themselves with the task and determine the difficulty level of problems to be presented during scanning. Participants answered math problems ranging in difficulty level from easy (addition or subtraction of two single-digit integers), easy-medium (addition or subtraction of three single-digit integers), medium-hard (addition or subtraction of four single- or double-digit integers), and hard (addition, subtraction, and multiplication of four single- or double-digit integers) problems during practice. No participants were able to reliably complete medium-hard or hard problems in the allotted time (i.e., less than 5 sec) during practice. Therefore, all participants received either easy or easy-medium math problems in the scanner based on their response time during the practice session. The difficulty level of the math problems remained constant across Control and Stress conditions. Prior to the Control scan, investigators attempted to lower participant stress levels by telling them “It is OK if you do not answer all of the math problems correctly.” During the Control scan, participants were given 5 sec to respond to each math problem. Further, participants were given previously recorded positive auditory feedback independent of their actual performance on the task. For example, phrases such as “Good job, you’re doing just fine. Keep up the good work.” and “Looks like everything is going well. You’re doing just what we wanted, so keep it up.” were presented. Prior to the Stress scan, investigators informed the participants they must answer the questions correctly and warned that if they did not perform as well as others in the study, their data would not be used. In addition, participants were told that prior participants answered more than 80% of the answers correctly and if they did not answer at least 80% correct, their data would not be used. Further, during the Stress scan, the participants were given previously recorded negative auditory feedback. For example, phrases such as “You are not answering enough of these correctly. Please try as hard as you can to get these right.” and “You are not doing as well as we had hoped. Please do your best to answer these correctly.” were presented. All auditory feedback was recorded by the senior author (DCK), who is male. Failure was ensured by modulating the time in which the participant could respond in a stair-step manner, such that, on average, participants answered approximately 50% of the problems correctly. The stair-step procedure was implemented by decreasing the available response time by 0.5 sec for each correct answer, and increasing the response time by 0.5 sec for each incorrect answer. Thus, the time available to answer math problems could vary between 0.5 sec (minimum) and 5.0 sec (maximum) in 0.5 sec increments. Once a math response was selected (or response time expired), a fixation cross (0.5 – 5 sec duration) appeared on the screen until the trial ended with 0.5 sec of visual feedback (“Right,” “Wrong,” or “Time out”). Participants were debriefed following the completion of the MIST. They were told the Stress condition had been designed to limit success on the task and that the assessment was not an index of their mathematical abilities.
2.5. Task Presentation
Presentation software (Neurobehavioral Systems, Inc.; Albany, CA, USA) was used to present auditory and visual stimuli. Visual stimuli were presented via an MRI-compatible video system. Participants viewed the video screen through a mirror attached to the radio frequency coil. Participants used an MRI compatible joystick (Current Designs; Philadelphia, PA, USA) to highlight their math answer in yellow (Supplementary Figure S1) and a button on the joystick to make their answer selection. Participants’ responses to the math problems were used to provide corresponding real-time visual feedback on task performance (i.e., “Right,” “Wrong,” or “Timeout”). Prerecorded auditory feedback was presented at four fixed points (i.e., after the first four sets of nine trials) during each scan through MR-compatible headphones.
2.6. Self-Reported Stress
An eight-question measure of self-reported stress level was used as a manipulation check of participant’s emotional response to Control and Stress conditions of the MIST (Wheelock et al., 2016). Participants rated each statement’s applicability on a five-point scale (1 = not at all, 3 = moderately, 5 = extremely). Four statements were phrased to convey positive experiences (e.g., “I felt calm”), while four statements were phrased to convey negative experiences (e.g., “I felt stressed”). Participant responses to positive statements were reverse coded, then the eight questions were summed. The total possible score on the scale could range from a minimum self-reported stress score of 8 to a maximum score of 40. Self-reported stress was not collected for the first 13 participants. Therefore, 287 participants were included in analyses involving self-reported stress.
2.7. Heart Rate
Heart rate was monitored during the scanning session to assess the emotional response to the Control and Stress conditions of the MIST. Heart rate was measured from the distal phalanx of the left index finger using an MRI compatible photoplethysmograph (Siemens; Munich, Germany). Cardiac data were sampled at a rate of 50 Hz and were processed using QRSTool software. Due to equipment malfunction, heart rate could not be collected from 82 participants. Additionally, heart rate data from 25 participants were excluded from further analysis due to poor signal-to-noise. Therefore, heart rate data were analyzed for a total of 193 participants.
2.8. Skin Conductance
Skin conductance response (SCR) was collected as a measure of the peripheral emotional response to psychosocial stress. SCR was measured from the thenar and hypothenar eminence of the left hand using MRI-compatible physiological monitoring equipment (Biopac Systems; Goleta, CA). A detailed description of acquisition and processing methods has been published previously (Orem et al., 2019; Wheelock et al., 2016). Briefly, SCR data were sampled at a rate of 10 kHz using a pair of radio-translucent electrodes, which were 1cm in diameter (Biopac Systems; Goleta, CA). Acqknowledge software (version 4.1) was used to collect and process SCR data. SCR data were exported to SCRalyze toolbox (version b2.1.8; Bach et al., 2009) for additional analysis. A general linear model (GLM) was performed in SCRalyze, which included math problem presentation as a regressor in the prediction of SCR. The GLM used an assumed SCR function and did not include a time or dispersion derivative (Bach et al., 2009, 2013). Beta coefficients from this 1st level GLM were then exported to SPSS software to conduct group-level analyses. As a result of equipment malfunction, SCR could not be collected from 7 participants. An additional 60 participants were excluded because they had no measurable SCR (i.e., those participants had no SCR values above 0.05 μSiemens). SCR data from 233 participants were included in analyses.
2.9. Anatomical and Functional MRI
MRI data were acquired on 3T Siemens Allegra and Prisma Scanners. In both cases, high resolution anatomical images (MPRAGE) were acquired to serve as an anatomical reference [Allegra: (TR = 2300 ms, TE = 3.90 ms, flip angle = 12°, FOV = 25.6 cm, matrix = 256 × 256, slice thickness = 1 mm, 0.5mm gap); Prisma: (TR = 2300 ms, TE = 2.98 ms, flip angle = 9°, FOV = 25.6 cm, matrix 256 × 256, slice thickness = 1 mm, 0.5mm gap)]. Blood oxygen level dependent (BOLD) fMRI data were acquired using a gradient echo-planar pulse sequence (TR = 2000 ms, TE = 30 ms, FOV = 24 cm, matrix = 64 × 64, slice thickness = 4 mm) during two scans of stimulus presentations. MRI data were preprocessed using the AFNI software package (Cox, 1996). Functional MRI data were slice time corrected, spatially blurred using a 4 mm full-width-at-half-maximum Gaussian kernel, and coregistered to the MPRAGE. Functional MRI data were corrected for motion by volume registration, censoring high-motion TRs, and inclusion of the six motion parameters in the first level model. High motion TRs were defined as volumes in which greater than three percent of voxels deviated by more than five times the median absolute deviation of the time series. Participants with less than 80% usable TRs were excluded from data analysis. The 1st level model included a regressor of interest for math problems, as well as regressors of no interest for auditory feedback events, visual feedback events, joystick movement, button presses, and the six head motion parameters. Functional MRI data were regressed against events of interest using the gamma variate hemodynamic response function. Visual feedback events were modeled using an instantaneous response function while math task and audio feedback events were modulated using the duration of the response time (math problem onset to answer selection or time out) and the duration of the audio recording, respectively. Functional MRI data from math events were resampled to 1mm3 resolution and normalized to the MNI152 template.
Group-level analyses were completed using linear mixed effects models (3dLME in AFNI). Physical and sexual abuse were included as separate factors in one model to determine whether task-elicited brain activity (Stress vs. Control) varied uniquely with either type of abuse (i.e., while accounting for the other type of abuse). Approximately 95% of the sample reported some exposure to violence in their lifetime. Therefore, violence exposure was included as a covariate in the model to ensure that the unique impact of physical or sexual abuse could be examined. Scanner type (Allegra, Prisma), race, and sex were also included as covariates in the model. Thus, the final group-analytic model included the following variables: condition (i.e., Stress or Control), physical abuse, sexual abuse, violence exposure, scanner type, race, and sex. Additionally, one random effect of subject was included to allow each participant to deviate from the group effect. Finally, exploratory sensitivity analyses were conducted to better understand how physical and sexual abuse are associated with stress-elicited neural activity in isolation. Two additional 3dLME models were run (i.e., one model for physical abuse, race, sex, and scanner and a second model for sexual abuse, race, sex, and scanner). Analyses were restricted to a whole-brain (i.e., cortical, subcortical, and cerebellar) gray matter mask to reduce the number of voxel-wise comparisons by excluding white matter and cerebrospinal fluid. The gray matter mask was created from the average of the anatomical images of the participants included in the current study (3dMean in AFNI). This averaged image was segmented into gray matter, white matter, and cerebrospinal fluid (3dSeg in AFNI). The segmented gray matter was used to mask (1,130,974 1 mm3 voxels) data in subsequent analyses. A family-wise error (FWE) corrected, voxel-wise threshold of p < 0.05 was used to reduce Type I error. The corrected significance threshold was determined by Monte Carlo simulation (AFNI: 3dClustSim [version compiled July 9, 2016] with -acf option) using an uncorrected significance threshold of p < 0.01. Smoothness was averaged across subjects based on spherical autocorrelation function (ACF) parameters (3dFWHMx) derived from residual volumes from the first level analysis. The results of this simulation yielded a critical cluster extent volume threshold of 628 mm3 for the whole brain. Two additional Monte Carlo simulations were carried out, one for the hippocampus/parahippocampal gyrus and the other for the amygdala using a small volume correction, based on a priori hypotheses for these regions (bilateral hippocampus: 232 mm3; bilateral amygdala: 111 mm3; Allendorfer et al., 2019; Orem et al., 2019). For all three Monte Carlo simulations, results corresponding to AFNI clusterize options for nearest neighbor 1 and two-sided criteria were used to determine cluster volume thresholds for each simulation.
3. Results
3.1. Behavioral Data
Paired samples t-tests were used to compare self-reported stress, heart rate, and SCR between the Stress and Control conditions of the MIST to confirm that participants found the Stress condition stressful. Stress ratings [t(287) = 22.02, p < 0.001], heart rate [t(193) = 9.48, p < 0.001], and SCR [t(230) = 10.31, p < 0.001] were higher during the Stress than Control condition of the MIST (Table 2). These results confirmed the validity of the stress manipulation.
Table 2.
Emotional response to Stress and Control conditions of the MIST
| Measures | Mean | Std. Dev. | SEM | Sig | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|
| Stress | Control | Stress | Control | Stress | Control | |||
| Self-reported stress | 25.76 | 15.22 | 6.79 | 5.86 | 0.40 | 0.35 | p < 0.001 | 1.01 |
| Heart rate | 73.30 | 68.22 | 13.23 | 10.84 | 1.00 | 0.82 | p < 0.001 | 16.28 |
| SCR | 0.81 | 0.39 | 0.69 | 0.51 | 0.05 | 0.03 | p < 0.001 | 10.48 |
Note: MIST = Montreal Imaging Stress Task; SCR = skin conductance response. Heart rate is listed in beats per minute, SCR values are represented as beta coefficients. Std. Dev. = standard deviation; SEM = standard error of the mean.
Partial correlations were used to examine the relationships between physical abuse, sexual abuse, and violence exposure and the 3 measures of the emotional response (i.e., self-reported stress, heart rate, and SCR), while controlling for race and sex. The analysis revealed that physical and sexual abuse were correlated with each other (r = 0.20, p = 0.024). Physical and sexual abuse were not correlated with any of the measures of the emotional response (i.e., self-reported stress, heart rate, and SCR). Violence exposure was correlated with sexual abuse (r = 0.30, p = 0.001), self-reported stress (r = −0.20, p = 0.024), and SCR (r = −0.23, p = 0.008), but not with physical abuse or heart rate (both ps > 0.05). Additionally, differential (Stress-Control) self-reported stress was correlated with heart rate (r = 0.21, p = 0.016) and SCR (r = 0.26, p = 0.003). Finally, heart rate was correlated with SCR (r = 0.21, p = 0.018) (Table 3).
Table 3.
Partial correlations for adverse experiences and emotional response
| 1 | 2 | 3 | 4 | 5 | 6. | |
|---|---|---|---|---|---|---|
| 1.00 | — | — | — | — | — | |
| 2. Sexual abuse | 0.20* | 1.00 | — | — | — | — |
| 3. Violence exposure | 0.11 | 0.30** | 1.00 | |||
| 4. Self-reported stress | 0.15 | 0.04 | −0.20* | 1.00 | — | |
| 5. Heart rate | 0.00 | 0.01 | −0.03 | 0.21* | 1.00 | |
| 6. SCR | −0.12 | −0.09 | −0.23** | 0.26** | 0.21* | 1.00 |
Note: SCR = skin conductance response. Self-reported stress, heart rate, and SCR reflect the differential values calculated by subtracting responses during the Control condition from responses during the Stress condition of the Montreal Imaging Stress Task. Only participants with data on all measures were included in partial correlations (N = 131).
p < 0.05;
p < 0.01.
Two MRI scanners (i.e. Siemens Allegra and Prisma) were used to collect neuroimaging data in the present study. Therefore, t-tests were completed to determine whether physical or sexual abuse differed for participants scanned on the Allegra compared to participants scanned on the Prisma. T-tests revealed no differences in physical or sexual abuse reported by participants scanned on the Allegra versus the Prisma (ps > 0.05).
3.2. Neuroimaging Data
Neuroimaging analyses revealed differences in task-elicited brain activity between the Stress and Control conditions of the MIST that varied with sexual abuse (Table 4). Specifically, differential activation (Stress vs Control) of the right dorsolateral PFC, left ventromedial PFC, and bilateral hippocampus varied with sexual abuse. There were no differences in task-elicited brain activity between the Stress and Control conditions that varied with physical abuse. For descriptive purposes, the mean BOLD signal response was extracted (using 3dROIstats in AFNI) from volumes of activation within the above regions to further characterize the relationship between sexual abuse and brain activity. Differential activation within these regions (i.e., right dorsolateral PFC, left ventromedial PFC, and bilateral hippocampus) showed a negative relationship with sexual abuse. Specifically, participants who reported higher levels of sexual abuse demonstrated lower differential activation within these brain regions (Figures 1–4). Further examination of Stress and Control conditions showed that the negative relationship between brain activity and sexual abuse was driven by neural reactivity to the Stress condition (Supplementary Figures S2–S5). Specifically, neural activity during the Control condition did not vary with sexual abuse. In contrast, task-elicited neural activity during the Stress condition was lower at higher levels of sexual abuse (Supplementary Figures S2–S5). The main effect of task (i.e., Stress and Control) on neural activity can be found in Supplementary Figure S6. Finally, results of sensitivity analyses can be found in Supplementary Table S1. Briefly, there were no regions where physical abuse was associated with neural reactivity to stress. There were several regions where sexual abuse was associated with neural reactivity to stress (i.e., ventromedial PFC and hippocampus), which were similar to results found in the main analysis.
Table 4.
Brain activity that varied with physical and sexual abuse
| Region | Hemisphere | Volume (mm3) | MNI (x,y,z) | F-Statistic |
|---|---|---|---|---|
| Physical abuse | ||||
| No significant activation | ||||
| Sexual abuse | ||||
| Ventromedial PFC | Left | 1478 | −14, 61, −7 | 17.64 |
| Dorsolateral PFC | Right | 1639 | 48, 45, 5 | 15.00 |
| Hippocampus | Right | 1568 | 30, −9, −29 | 24.44 |
| Hippocampus | Left | 272 | −25, −43, 2 | 11.15 |
Note: One full model was run that included physical and sexual abuse as predictors and violence exposure, race, sex, and scanner type as covariates. Regions, hemispheres, volumes, coordinates from Montreal Neurological Institute (MNI), and F-statistics for the peak voxel of significant clusters of activation are reported. All clusters were significant at p < 0.05 (corrected). PFC=prefrontal cortex.
Figure 1.
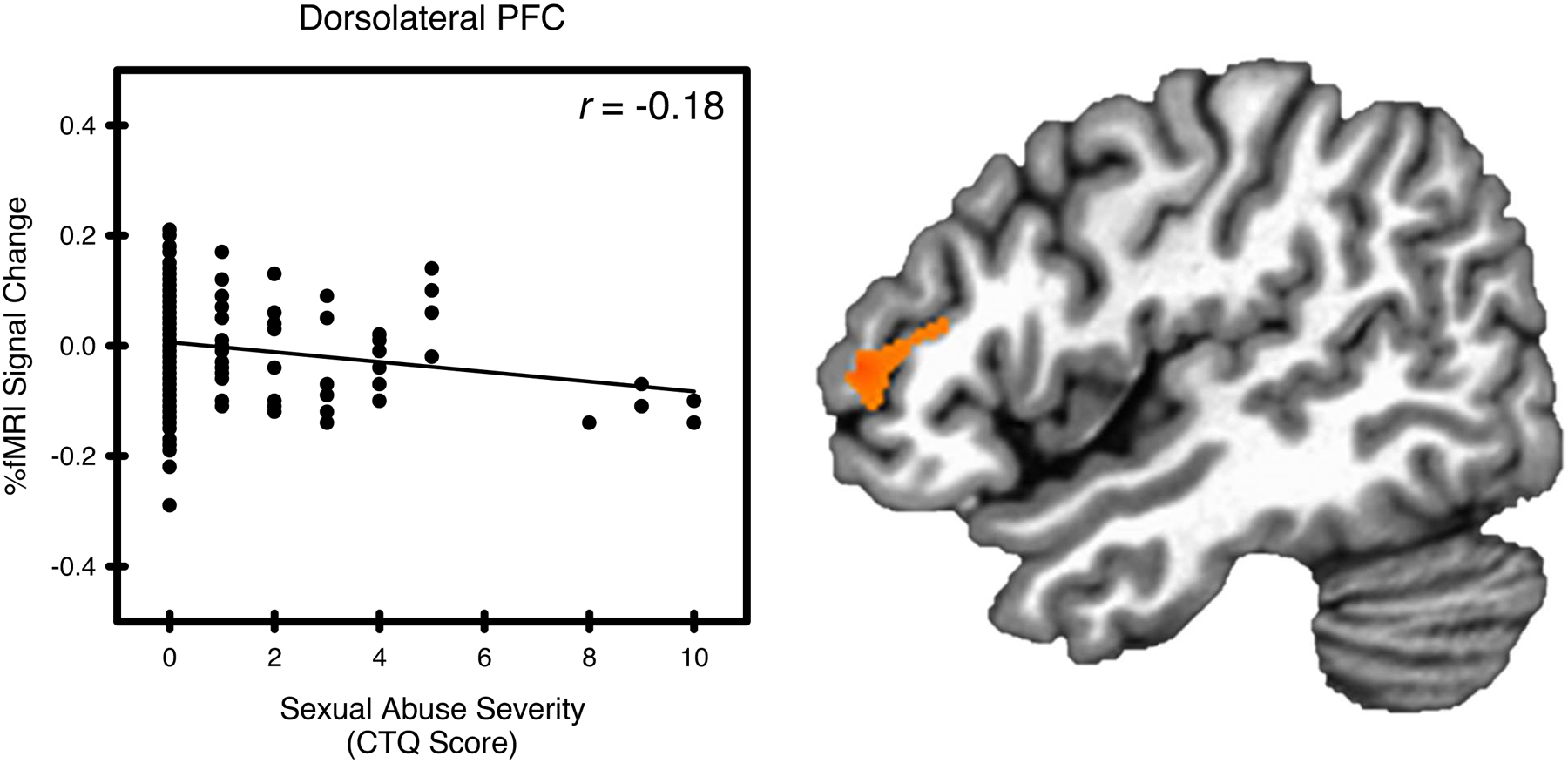
Sexual abuse and stress-elicited right dorsolateral prefrontal cortex (PFC) activity. Stress-elicited dorsolateral PFC activity showed a negative relationship with sexual abuse, as measured by the Childhood Trauma Questionnaire (CTQ).
Figure 4.
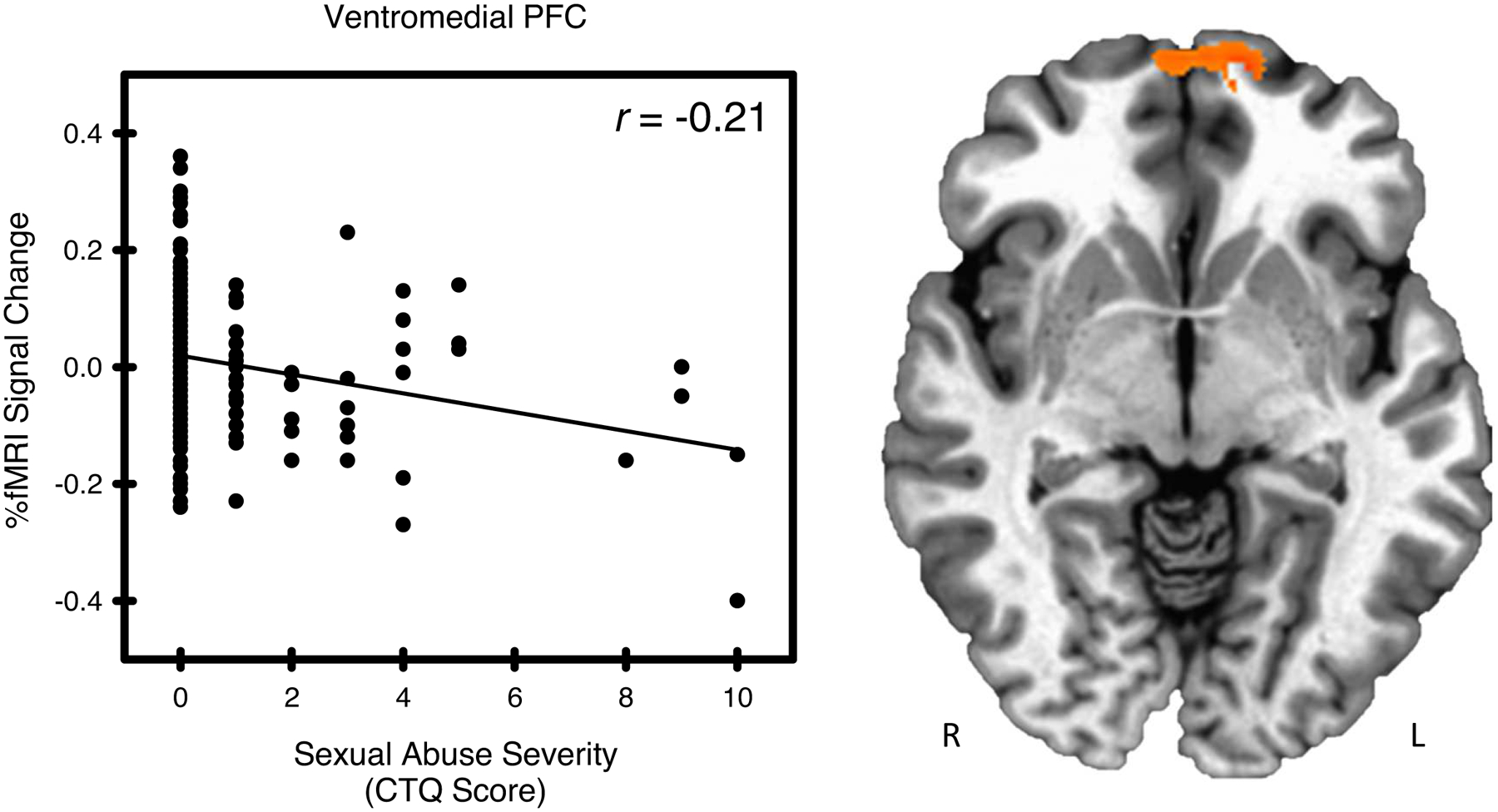
Sexual abuse and stress-elicited left ventromedial prefrontal cortex (PFC) activity. Stress-elicited ventromedial PFC activity showed a negative relationship with sexual abuse, as measured by the Childhood Trauma Questionnaire (CTQ).
4. Discussion
Childhood abuse is linked to negative psychological outcomes that can persist into adulthood. Determining the neural outcomes of these adverse experiences would provide new insights that could guide intervention strategies to ameliorate the negative psychological impact of these experiences. In pursuit of this goal, prior research has demonstrated a relationship between brain function and childhood abuse, primarily in clinical populations (Nicol et al., 2015; van Harmelen et al., 2014; Yamamoto et al., 2017). However, elucidating these relationships in community samples has received less attention. Further, the unique relationships that childhood physical and sexual abuse have with brain function have not been fully explored. The present study investigated the unique relationships that childhood physical and sexual abuse have with stress-related brain function in a community sample. We found that stress-elicited ventromedial PFC, dorsolateral PFC, and hippocampal activity varied with sexual abuse. Specifically, activity within these brain regions was lower among individuals who reported higher levels of sexual abuse. We found no relationship between physical abuse and brain activity. These results suggest that childhood sexual abuse has a lasting impact on neural reactivity to stress that persists into emerging adulthood. This new knowledge may have important implications for understanding the mechanisms by which adverse childhood experiences impact the development of future psychopathology and may ultimately enhance individualized treatment planning by mental health professionals.
The results of the current investigation suggest that adverse childhood experiences, specifically sexual abuse, affect neural reactivity to stress in young adulthood. More specifically, we found that stress-elicited ventromedial PFC, dorsolateral PFC, and hippocampal activity varied with childhood sexual abuse (Figures 1–4). Stress-elicited activity within these brain regions was lower in individuals who reported higher levels of sexual abuse. The present results parallel psychophysiological findings from prior work that has investigated the impact of adverse childhood experiences on stress reactivity using different methods. Specifically, prior research has demonstrated that the cortisol response to stress is attenuated in individuals who have been exposed to abuse (Lovallo et al., 2018; Trickett et al., 2014). This line of prior research has demonstrated a dose-response relationship between adverse childhood experiences (e.g., physical, sexual, and emotional abuse) and cortisol reactivity in which higher numbers of adverse childhood experiences were associated with decreased cortisol reactivity (Lovallo et al., 2018). Similar to prior work that has investigated cortisol reactivity (Lovallo et al., 2018; Trickett et al., 2014), we observed decreased neural reactivity to stress in young adults who had been previously exposed to childhood sexual abuse. Further, given that neural activity only varied with sexual abuse in the Stress, but not the Control condition, of the MIST (Supplementary Figures S2–S5), the present results do not appear to be driven by a non-specific decrease in neural reactivity in those exposed to childhood sexual abuse. Prior neuroimaging research suggests that the PFC and hippocampus influence, albeit indirectly, the physiological response to stress (Dedovic et al., 2009; Herman et al., 2005; Smith & Vale, 2006). Therefore, the present findings, when taken together with prior work, may indicate that childhood sexual abuse blunts the neurobehavioral response to stress in young adulthood.
There has been a recent shift in the conceptualization of childhood adversity. Previously, much of the literature focused on the outcomes of cumulative adversity (Felitti et al., 1998). Although studies of cumulative adversity have been informative, this approach does not differentiate the impact of distinct types of abuse. A new, dimensional framework for categorizing childhood adversity has been recently proposed, which may be crucial for differentiating the impact of distinct types of abuse. This new framework attempts to distill different types of adversity into common dimensions to characterize many types of childhood adversity in terms of deprivation and threat (McLaughlin et al., 2014; McLaughlin & Sheridan, 2016). According to this dimensional model, different experiences of adversity can be conceptualized by where they fall on both the deprivation and threat dimensions (McLaughlin et al., 2014; McLaughlin & Sheridan, 2016). For example, childhood neglect would fall high on the deprivation dimension, but relatively low on the threat dimension. In contrast, physical/sexual abuse would fall relatively low on the deprivation dimension and high on the threat dimension (McLaughlin et al., 2014; McLaughlin & Sheridan, 2016). This new framework represents an important advance toward a better understanding of how distinct types of adversity may lead to different outcomes.
Although the present study did not directly test the dimensional model, results from this and previous investigations indicate that the dimensional model may require further refinement. For example, the dimensional model groups physical and sexual abuse together. However, prior research indicates that outcomes differ following childhood physical and sexual abuse (Archer et al., 2017; Brewerton, 2007; Deblinger et al., 1989; Fergusson et al., 2008; Johnson et al., 1999; Westermair et al., 2018; Yates et al., 2008). Therefore, it is possible that physical and sexual abuse may fall along different dimensions of deprivation and threat. Indeed, the results of the present investigation are in line with prior work that has found differences in the outcomes of childhood physical and sexual abuse (Archer et al., 2017; Brewerton, 2007; Deblinger et al., 1989; Fergusson et al., 2008; Johnson et al., 1999; Westermair et al., 2018; Yates et al., 2008). Specifically, the present study found that sexual abuse was associated brain function (i.e., with stress-elicited ventromedial PFC, dorsolateral PFC, and hippocampus) in young adulthood, but physical abuse was not. Thus, it is possible that sexual abuse may fall along different dimensions of deprivation and threat than physical abuse, which may account for the differences in outcomes in the present study. Although not directly assessed by the present study, others (Davis & Siegel, 2000; Pynoos, 1994; Wolf & Pruitt, 2019) have hypothesized that there are important factors that differ between physical and sexual abuse. These factors include the grooming that is often observed in sexual abuse, which occurs when a perpetrator primes a child to be compliant with future sexual abuse. Other issues that may influence outcomes include post-event factors, such as the way parents, teachers, and other authority figures respond to the child’s disclosure, as well as the child’s preparation for criminal justice proceedings (Davis & Siegel, 2000; Pynoos, 1994; Wolf & Pruitt, 2019). These factors are not presently accounted for by the current dimensional framework, but they may contribute to the differences in outcomes between physical and sexual abuse observed in prior work (Archer et al., 2017; Brewerton, 2007; Davis & Siegel, 2000; Deblinger et al., 1989; Fergusson et al., 2008; Johnson et al., 1999; Pynoos, 1994; Westermair et al., 2018; Wolf & Pruitt, 2019; Yates et al., 2008). Therefore, studies exploring the impact of factors such as grooming and responses to disclosure may be a valuable avenue for future investigation. The introduction of a dimensional model for conceptualizing childhood adversity represents an important move toward a more nuanced understanding of the impact of such experiences. Future work should aim to more fully characterize physical and sexual abuse within this framework.
Prior work investigating the neural outcomes of physical abuse has produced inconsistent results. Specifically, while some prior work has observed a relationship between brain activity (i.e., dorsolateral PFC) and physical abuse (Nicol et al., 2015), others have not (van Harmelen et al., 2014; Yamamoto et al., 2017). The variability of results across these studies may be related to the use of different types of tasks to elicit brain activity. For example, the present results, using the MIST, are similar to those found using negative mood induction (Yamamoto et al., 2017) and social exclusion tasks (van Harmelen et al., 2014). Consistent with the present results, neither of these prior studies observed a relationship between brain activity and physical abuse. In contrast, research investigating emotional face processing has found that dorsolateral PFC activity increases as childhood physical abuse increases (Nicol et al., 2015). Tasks involving emotional faces tap into emotion perception and identification capabilities (Levine et al., 1997; Nicol et al., 2014, 2015). In contrast, the MIST is associated with stress reactivity and emotion regulation (Goodman et al., 2018; Orem et al., 2019; Pruessner et al., 2008; Wheelock et al., 2016). Finally, in the context of the dimensional model of childhood adversity (McLaughlin et al., 2014; McLaughlin & Sheridan, 2016), discussed above, physical abuse may fall differently along those dimensions compared to other types of adversity, producing variable results. Thus, while childhood physical abuse may alter neural processes associated with some types of emotional function (e.g., emotion perception and identification), its impact on stress-related neural function does not appear to persist into young adulthood.
Prior neuroimaging research suggests that the ventromedial PFC, dorsolateral PFC, and hippocampus play an important role in stress and emotion regulation processes (Casey et al., 2017; Goodman et al., 2018; Orem et al., 2019; Pattwell & Bath, 2017; Rauch et al., 2006; Wheelock et al., 2016). Further, the function of this neural circuitry has been linked to depression, anxiety, and PTSD (Burrus, 2013; Cisler et al., 2015; Dretsch et al., 2019; Elsey et al., 2015; Gee et al., 2013; Grant et al., 2011, 2014; Harnett et al., 2018). Moreover, prior behavioral research has consistently demonstrated a link between a broad range of childhood maltreatment experiences and future psychopathology, including anxiety, depression, posttraumatic stress, and personality disorders (Anda et al., 2006; Archer et al., 2017; Brewerton, 2007; Chen et al., 2010; Dube et al., 2001; Felitti et al., 1998; Fergusson et al., 2008; Gilbert et al., 2009; Green et al., 2010; Irish et al., 2010; Johnson et al., 1999; Yates et al., 2008). Thus, the alterations in stress-related PFC and hippocampal function observed in the present study may underlie the differences in emotion expression and regulation processes that have been observed in prior research (Anda et al., 2006; Brewerton, 2007; Chen et al., 2010; Dube et al., 2001; Fergusson et al., 2008; Green et al., 2010; Johnson et al., 1999; Yates et al., 2008). The present study demonstrates that activity within brain regions that regulate emotion (i.e., ventromedial PFC, dorsolateral PFC, and hippocampus) varies with prior childhood sexual abuse history, specifically under stressful conditions. Given that activity within these brain regions has been linked to mental health outcomes (Burrus, 2013; Cisler et al., 2015; Dretsch et al., 2019; Elsey et al., 2015; Gee et al., 2013; Grant et al., 2011, 2014; Harnett et al., 2018), the altered activity observed in the present study may represent a neural mechanism by which childhood sexual abuse leads to future psychopathology.
Prior neuroimaging studies that have examined a broad range of childhood adversity (i.e., physical and sexual abuse) have largely compared clinical samples (i.e., individuals recruited from clinical settings with diagnoses that include depression, posttraumatic stress, and personality disorders) to a healthy control group (Nicol et al., 2015; van Harmelen et al., 2014). In contrast, the present study utilized a community sample (i.e., participants recruited from the community who were not selected for participation based on the presence of psychopathology). These participants were recruited from 5th grade classrooms and followed until they were approximately 20 years of age. This methodology has several benefits. First, the study sample more closely approximates the demographic characteristics of the local community, which increases the generalizability of findings. Second, recruitment of community samples allows investigators to study the impact of different levels of sexual abuse as a continuous variable, rather than as discrete categories of clinical compared to non-clinical (or abused to non-abused) groups. This approach represents an important contribution to the literature, as prospective community recruitment strategies are not often utilized in neuroimaging investigations. Additionally, the use of a community sample in this investigation may play a role in the differences observed between the present results and prior neuroimaging work.
4.1. Limitations and Future Directions
The results of the current study should be interpreted within the context of their limitations. Although participants were recruited from a longitudinal study, they reported abuse only at one time point and only completed one neuroimaging session. Thus, this study is essentially cross-sectional in nature, which limits conclusions regarding causality. Longitudinal neuroimaging studies are necessary to better understand causal relationships between childhood abuse and brain function. Additionally, participants retrospectively reported their experiences of abuse. Although youth self-report does not always match other information sources (e.g., Child Protection Services records or caregiver reports), some evidence suggests that self-report may better predict future outcomes, including the development of psychopathology (McGee et al., 1995; Nofziger et al., 2019; Sierau et al., 2017; Tabone, 2019). Future work should seek to include multiple informants regarding the presence of abuse. Another issue worth considering is that emotional abuse was not included in the present analyses. Although this does not directly limit the findings of the present study, understanding the impact of childhood emotional abuse is an important avenue for future work. Nevertheless, the effort to disentangle the neural impact of physical and sexual abuse in the present study provides important foundational context for future research on this topic. Additionally, we were unable to assess the impact of the voice type (i.e., male or female) providing auditory feedback during the stress task. First, there was no variability in the gender of the voice presenting auditory feedback (i.e., auditory feedback was only presented by a male voice). Second, the details of participants’ abuse histories that would be required to answer this question (i.e., details regarding the perpetrator), were not collected as part of the present investigation. Future research might consider working to clarify the role of trauma reminders (e.g., comparing the gender of auditory feedback to the gender of the perpetrator of abuse) on neural reactivity to stress. Finally, we were unable to assess other aspects of physical and sexual abuse which may be important, including the relationship of the participant to the perpetrator, number of occurrences, developmental timing of abuse, and reactions to disclosure (Briere & Elliott, 2003; Ullman, 2007). Future work that includes these factors will help to more fully understand the outcomes of childhood physical and sexual abuse.
5. Conclusions
The present study investigated the relationship that physical and sexual abuse have with neural reactivity to psychosocial stress. Physical abuse was not associated with neural reactivity to stress in the present investigation. In contrast, sexual abuse varied with ventromedial PFC, dorsolateral PFC, and hippocampal responses to stress. These brain regions are important components of the neural circuitry that supports emotion expression and regulation processes. Thus, alterations in the stress-related function of these brain regions may represent one mechanism by which childhood sexual abuse may impact future mental health.
Supplementary Material
Figure 2.
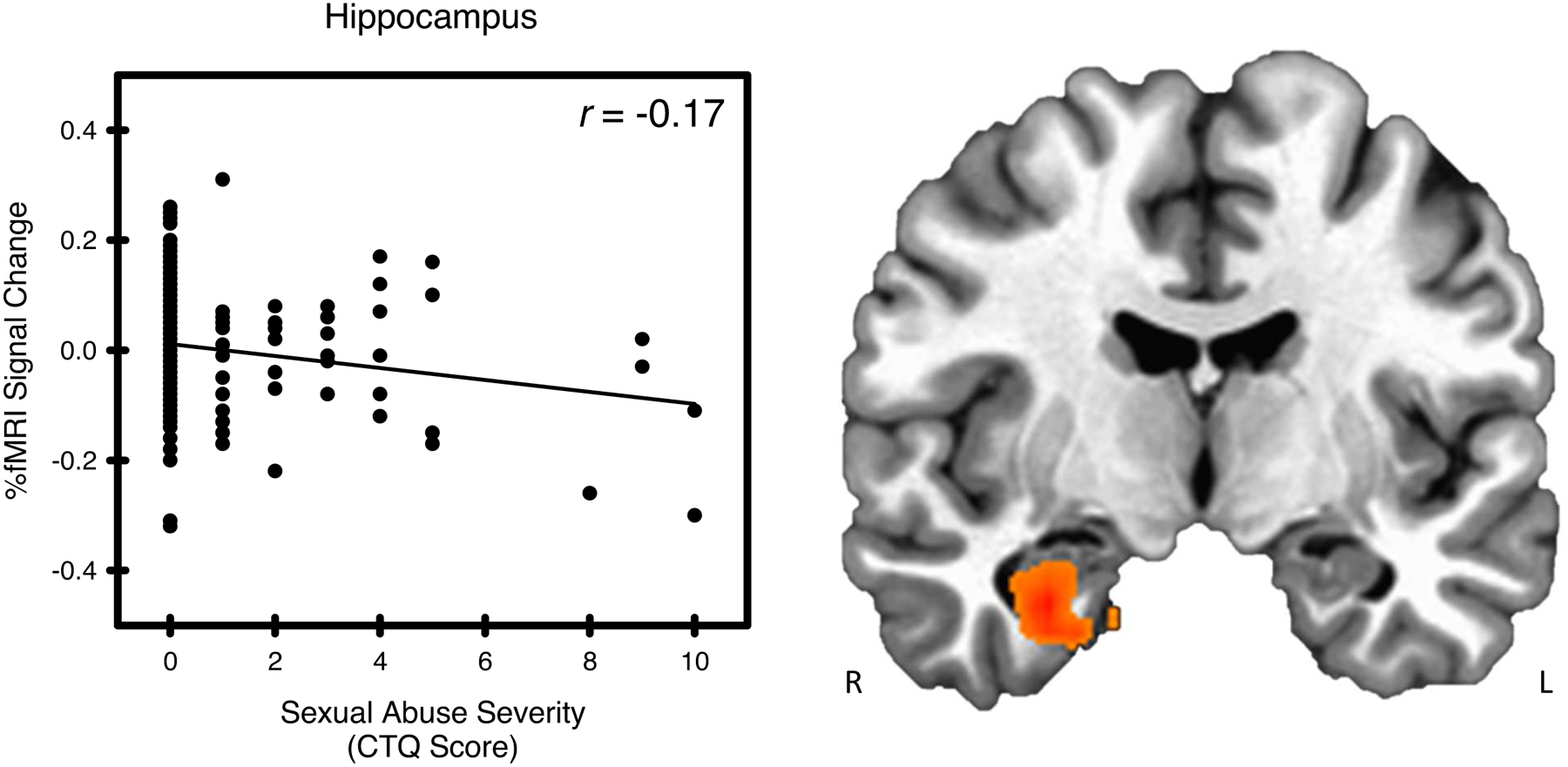
Sexual abuse and stress-elicited right hippocampal activity. Stress-elicited right hippocampal activity showed a negative relationship with sexual abuse, as assessed by the Childhood Trauma Questionnaire (CTQ).
Figure 3.
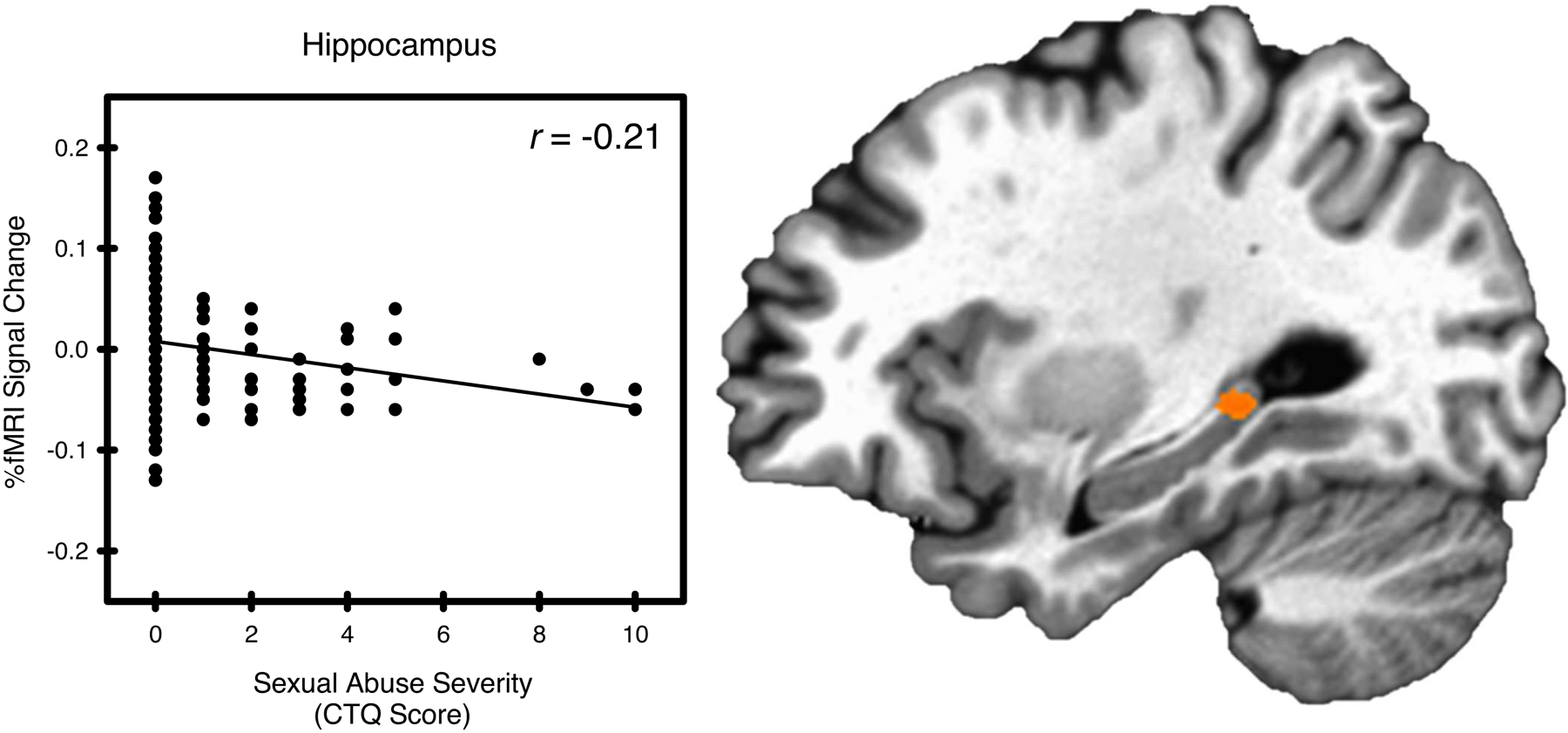
Sexual abuse and stress-elicited left hippocampal activity. Stress-elicited left hippocampal activity showed a negative relationship with sexual abuse, as assessed by the Childhood Trauma Questionnaire (CTQ).
Transparency and Openness Promotion (TOP) Guidelines.
Participants in this study did not consent to the release of their data to a third party for reuse. Therefore, we are unable to publicly archive data due to the conditions of our ethics approval. Readers seeking access to the data should contact the corresponding author (David C. Knight). Data can and will only be released to named individuals who agree to collaborate with the principal investigators (i.e., through a formal collaboration agreement). Access can be granted only in accordance with ethical procedures governing the reuse of sensitive data. No part of the study procedures and analyses were pre-registered in a time-stamped, institutional registry prior to the research being conducted. We have deposited stimulus presentation codes, audio feedback files, and data analysis codes under https://osf.io/dp3m8/. We have reported how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, all manipulations, and all measures in the study. Inclusion/exclusion criteria were established prior to data analysis.
Acknowledgements
The original Healthy Passages Study was funded by the Centers for Disease Control and Prevention through cooperative agreements (CCU409679, CCU609653, CCU915773, U48DP000046, U48DP000057, U48DP000056, U19DP002663, U19DP002664, and U19DP002665). The present research was supported by the National Institute of Mental Health R01MH098348 and the National Institute of Alcohol Abuse and Alcoholism F31AA027137 (J.B.P).
Role of the Funding Sources
The funding sources played no role in data analysis, interpretation of results, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors declare that they have no competing financial interests or personal relationships that could have influenced the worked reported in this paper.
References
- Adams J, Mrug S, & Knight DC (2018). Characteristics of child physical and sexual abuse as predictors of psychopathology. Child Abuse & Neglect, 86, 167–177. 10.1016/j.chiabu.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Kyeong S, Suh SH, Kim J-J, Chung T-S, & Seok J-H (2016). What is the impact of child abuse on gray matter abnormalities in individuals with major depressive disorder: A case control study. BMC Psychiatry, 16(1), 397. 10.1186/s12888-016-1116-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorfer JB, Nenert R, Hernando KA, DeWolfe JL, Pati S, Thomas AE, Billeaud N, Martin RC, & Szaflarski JP (2019). FMRI response to acute psychological stress differentiates patients with psychogenic non-epileptic seizures from healthy controls—A biochemical and neuroimaging biomarker study. NeuroImage. Clinical, 24, 101967. 10.1016/j.nicl.2019.101967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield Ch., Perry BD, Dube Sh. R., & Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. 10.1007/s00406-005-0624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G, Pinto Pereira S, & Power C (2017). Child maltreatment as a predictor of adult physical functioning in a prospective British birth cohort. BMJ Open, 7(10), e017900. 10.1136/bmjopen-2017-017900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Flandin G, Friston KJ, & Dolan RJ (2009). Time-series analysis for rapid event-related skin conductance responses. Journal of Neuroscience Methods, 184(2), 224–234. 10.1016/j.jneumeth.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Friston KJ, & Dolan RJ (2013). An improved algorithm for model-based analysis of evoked skin conductance responses. Biological Psychology, 94(3), 490–497. 10.1016/j.biopsycho.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang W-N, Pothuizen HHJ, & Feldon J (2004). Regional dissociations within the hippocampus—Memory and anxiety. Neuroscience & Biobehavioral Reviews, 28(3), 273–283. 10.1016/j.neubiorev.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, & Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Abuse & Neglect Child, 27(2), 169–190. [DOI] [PubMed] [Google Scholar]
- Boccia M, D’Amico S, Bianchini F, Marano A, Giannini AM, & Piccardi L (2016). Different neural modifications underpin PTSD after different traumatic events: An fMRI meta-analytic study. Brain Imaging and Behavior, 10(1), 226–237. 10.1007/s11682-015-9387-3 [DOI] [PubMed] [Google Scholar]
- Bremner JD (1999). Alterations in brain structure and function associated with post-traumatic stress disorder. Seminars in Clinical Neuropsychiatry, 4(4), 249–255. 10.153/SCNP00400249 [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, & Charney DS (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biological Psychiatry, 41(1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, & Charney DS (2003). Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry, 53(10), 879–889. [DOI] [PubMed] [Google Scholar]
- Brewerton TD (2007). Eating Disorders, Trauma, and Comorbidity: Focus on PTSD. Eating Disorders, 15(4), 285–304. 10.1080/10640260701454311 [DOI] [PubMed] [Google Scholar]
- Briere J, & Elliott DM (2003). Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse & Neglect, 27(10), 1205–1222. 10.1016/j.chiabu.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, & Friston KJ (1998). Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron, 20(5), 947–957. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, & Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex (New York, N.Y.: 1991), 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrus C (2013). Developmental trajectories of abuse – An hypothesis for the effects of early childhood maltreatment on dorsolateral prefrontal cortical development. Medical Hypotheses, 81(5), 826–829. 10.1016/j.mehy.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Heller AS, Gee DG, & Cohen AO (2017). Development of the emotional brain. Neuroscience Letters. 10.1016/j.neulet.2017.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiers LLM, Sabbe BGC, Schmaal L, Veltman DJ, Penninx BWJH, & Van Den Eede F (2018). Structural and Functional Brain Abnormalities Associated With Exposure to Different Childhood Trauma Subtypes: A Systematic Review of Neuroimaging Findings. Frontiers in Psychiatry, 9. 10.3389/fpsyt.2018.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LP, Murad MH, Paras ML, Colbenson KM, Sattler AL, Goranson EN, Elamin MB, Seime RJ, Shinozaki G, Prokop LJ, & Zirakzadeh A (2010). Sexual abuse and lifetime diagnosis of psychiatric disorders: Systematic review and meta-analysis. Mayo Clinic Proceedings, 85(7), 618–629. 10.4065/mcp.2009.0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Sigel BA, Kramer TL, Smitherman S, Vanderzee K, Pemberton J, & Kilts CD (2015). Amygdala response predicts trajectory of symptom reduction during Trauma-Focused Cognitive-Behavioral Therapy among adolescent girls with PTSD. Journal of Psychiatric Research, 71, 33–40. 10.1016/j.jpsychires.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1977). Statistical power analysis for the behavioral sciences (2nd ed.). Academic press. [Google Scholar]
- Cox RW (1996). AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Dark HE, Harnett NG, Knight AJ, & Knight DC (in press). Hippocampal volume varies with acute posttraumatic stress symptoms following medical trauma. Behavioral Neuroscience. [DOI] [PubMed] [Google Scholar]
- Davis L, & Siegel LJ (2000). Posttraumatic stress disorder in children and adolescents: A review and analysis. Clinical Child and Family Psychology Review, 3(3), 135–154. [DOI] [PubMed] [Google Scholar]
- Deblinger E, McLeer SV, Atkins MS, Ralphe D, & Foa E (1989). Post-traumatic stress in sexually abused, physically abused, and nonabused children. Child Abuse & Neglect, 13(3), 403–408. [DOI] [PubMed] [Google Scholar]
- Dedovic K, D’Aguiar C, & Pruessner JC (2009). What stress does to your brain: A review of neuroimaging studies. Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie, 54(1), 6–15. 10.1177/070674370905400104 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, & Pruessner JC (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry & Neuroscience: JPN, 30(5), 319–325. [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Dube SR, Giles WH, & Felitti VJ (2003). The relationship of exposure to childhood sexual abuse to other forms of abuse, neglect, and household dysfunction during childhood. Child Abuse & Neglect, 27(6), 625–639. 10.1016/S0145-2134(03)00105-4 [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, & Giles WH (2004). The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect, 28(7), 771–784. 10.1016/j.chiabu.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Dretsch MN, Daniel TA, Goodman AM, Katz JS, Denney T, Deshpande G, & Robinson JL (2019). Differential neural activation when voluntarily regulating emotions in service members with chronic mild traumatic brain injury. Applied Neuropsychology: Adult, 26(1), 76–88. 10.1080/23279095.2017.1362406 [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, & Giles WH (2001). Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the Adverse Childhood Experiences Study. JAMA, 286(24), 3089–3096. [DOI] [PubMed] [Google Scholar]
- Elsey J, Coates A, Lacadie CM, McCrory EJ, Sinha R, Mayes LC, & Potenza MN (2015). Childhood trauma and neural responses to personalized stress, favorite-food and neutral-relaxing cues in adolescents. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(7), 1580–1589. 10.1038/npp.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, & Horwood LJ (2008). Exposure to childhood sexual and physical abuse and adjustment in early adulthood. Child Abuse & Neglect, 32(6), 607–619. 10.1016/j.chiabu.2006.12.018 [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Flagan TM, Sullivan S, Allard CB, Grimes EM, Simmons AN, Paulus MP, & Stein MB (2013). Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Research: Neuroimaging, 211(2), 93–103. 10.1016/j.pscychresns.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, & Tottenham N (2013). A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. Journal of Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, & Janson S (2009). Burden and consequences of child maltreatment in high-income countries. Lancet (London, England), 373(9657), 68–81. 10.1016/S0140-6736(08)61706-7 [DOI] [PubMed] [Google Scholar]
- Goodman AM, Harnett NG, Wheelock MD, Hurst DR, Orem TR, Gossett EW, Dunaway CA, Mrug S, & Knight DC (2018). Anticipatory prefrontal cortex activity underlies stress-induced changes in Pavlovian fear conditioning. NeuroImage, 174, 237–247. 10.1016/j.neuroimage.2018.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Wheelock MD, Harnett NG, Mrug S, Granger DA, & Knight DC (2016). The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience, 339, 396–401. 10.1016/j.neuroscience.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, & Shelton R (2011). Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of Psychiatric Research, 45(7), 886–895. 10.1016/j.jpsychires.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, White D, Hadley J, Hutcheson N, Shelton R, Sreenivasan K, & Deshpande G (2014). Early life trauma and directional brain connectivity within major depression. Human Brain Mapping, 35(9), 4815–4826. 10.1002/hbm.22514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett NG, Ference EW, Wood KH, Wheelock MD, Knight AJ, & Knight DC (2018). Trauma exposure acutely alters neural function during Pavlovian fear conditioning. Cortex, 109, 1–13. 10.1016/j.cortex.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, & Rubia K (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 52. 10.3389/fnhum.2012.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, & Phelps EA (2010). Changing Fear: The Neurocircuitry of Emotion Regulation. Neuropsychopharmacology, 35(1), 136–146. 10.1038/npp.2009.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, & Figueiredo H (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29(8), 1201–1213. 10.1016/j.pnpbp.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Irish L, Kobayashi I, & Delahanty DL (2010). Long-term physical health consequences of childhood sexual abuse: A meta-analytic review. Journal of Pediatric Psychology, 35(5), 450–461. 10.1093/jpepsy/jsp118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska N, MacMaster FP, Gaxiola I, Cortese F, Goodyear B, & Ramasubbu R (2014). A preliminary study of the influence of age of onset and childhood trauma on cortical thickness in major depressive disorder. BioMed Research International, 2014, 410472. 10.1155/2014/410472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Brown J, Smailes EM, & Bernstein DP (1999). Childhood maltreatment increases risk for personality disorders during early adulthood. Archives of General Psychiatry, 56(7), 600–606. 10.1001/archpsyc.56.7.600 [DOI] [PubMed] [Google Scholar]
- Kitayama N, Brummer M, Hertz L, Quinn S, Kim Y, & Bremner JD (2007). Morphologic alterations in the corpus callosum in abuse-related posttraumatic stress disorder: A preliminary study. The Journal of Nervous and Mental Disease, 195(12), 1027–1029. 10.1097/NMD.0b013e31815c044f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, & Bandettini PA (2003). Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences, 100(25), 15280–15283. 10.1073/pnas.2535780100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, & Phelps EA (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron 20(5), 937–945. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Bluhm RL, Densmore M, Boksman K, Neufeld RWJ, Gati JS, & Menon RS (2005). Functional connectivity of dissociative responses in posttraumatic stress disorder: A functional magnetic resonance imaging investigation. Biological Psychiatry, 57(8), 873–884. 10.1016/j.biopsych.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RWJ, Gati JS, & Menon RS (2002). Brain activation during script-driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biological Psychiatry, 52(4), 305–311. [DOI] [PubMed] [Google Scholar]
- Levine D, Marziali E, & Hood J (1997). Emotion processing in borderline personality disorders. The Journal of Nervous and Mental Disease, 185(4), 240–246. 10.1097/00005053-199704000-00004 [DOI] [PubMed] [Google Scholar]
- Linares LO, Shrout PE, Nucci-Sack A, & Diaz A (2013). Child maltreatment, dating perpetration of physical assault, and cortisol reactivity among disadvantaged female adolescents. Neuroendocrinology, 97(3), 252–259. 10.1159/000342958 [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Acheson A, Vincent AS, Sorocco KH, & Cohoon AJ (2018). Early life adversity diminishes the cortisol response to opioid blockade in women: Studies from the Family Health Patterns project. PloS One, 13(10), e0205723. 10.1371/journal.pone.0205723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee RA, Wolfe DA, Yuen SA, Wilson SK, & Carnochan J (1995). The measurement of maltreatment: A comparison of approaches. Child Abuse & Neglect, 19(2), 233–249. 10.1016/0145-2134(94)00119-F [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child Maltreatment and Neural Systems Underlying Emotion Regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond Cumulative Risk: A Dimensional Approach to Childhood Adversity. Current Directions in Psychological Science, 25(4), 239–245. 10.1177/0963721416655883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol K, Pope M, & Hall J (2014). Facial emotion recognition in borderline personality: An association, with childhood experience. Psychiatry Research, 218(1–2), 256–258. 10.1016/j.psychres.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Nicol K, Pope M, Romaniuk L, & Hall J (2015). Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Translational Psychiatry, 5, e559. 10.1038/tp.2015.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofziger S, Stein RE, & Rosen NL (2019). Comparing Children’s and Caseworkers’ Reports of Physical Violence. Journal of Interpersonal Violence, 34(16), 3516–3541. 10.1177/0886260516670880 [DOI] [PubMed] [Google Scholar]
- Ochsner K, & Gross J (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Orem TR, Wheelock MD, Goodman AM, Harnett NG, Wood KH, Gossett EW, Granger DA, Mrug S, & Knight DC (2019). Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to psychosocial stress. Behavioral Neuroscience, 133(2), 203–211. 10.1037/bne0000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, & Bath KG (2017). Emotional learning, stress, and development: An ever-changing landscape shaped by early-life experience. Neurobiology of Learning and Memory, 143, 36–48. 10.1016/j.nlm.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrehumbert B, Torrisi R, Glatz N, Dimitrova N, Heinrichs M, & Halfon O (2009). The influence of attachment on perceived stress and cortisol response to acute stress in women sexually abused in childhood or adolescence. Psychoneuroendocrinology, 34(6), 924–938. 10.1016/j.psyneuen.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, & Lupien S (2008). Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry, 63(2), 234–240. 10.1016/j.biopsych.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Pynoos RS (1994). Traumatic stress and developmental psycho-pathology in children and adolescents. In Pynoos RS (Ed.), Posttraumatic stress disorder: A clinical review (pp. 64–98). Sidran Press. [Google Scholar]
- Raine A, Park S, Lencz T, Bihrle S, LaCasse L, Widom CS, Al‐Dayeh L, & Singh M (2001). Reduced right hemisphere activation in severely abused violent offenders during a working memory task: An fMRI study. Aggressive Behavior: Official Journal of the International Society for Research on Aggression, 27(2), 111–129. [Google Scholar]
- Raine A, Yang Y, Narr KL, & Toga AW (2011). Sex differences in orbitofrontal gray as a partial explanation for sex differences in antisocial personality. Molecular Psychiatry, 16(2), 227–236. 10.1038/mp.2009.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, & Phelps EA (2006). Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research—Past, Present, and Future. Biological Psychiatry, 60(4), 376–382. 10.1016/j.biopsych.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Rinne-Albers MAW, van der Werff SJA, van Hoof M-J, van Lang ND, Lamers-Winkelman F, Rombouts SA, Vermeiren RRJM, & van der Wee NJA (2016). Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: A DTI study. European Child & Adolescent Psychiatry, 25(8), 869–878. 10.1007/s00787-015-0805-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster MA, Elliott MN, Kanouse DE, Wallander JL, Tortolero SR, Ratner JA, Klein DJ, Cuccaro PM, Davies SL, & Banspach SW (2012). Racial and ethnic health disparities among fifth-graders in three cities. The New England Journal of Medicine, 367(8), 735–745. 10.1056/NEJMsa1114353 [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, & Pitman RK (1999). Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. The American Journal of Psychiatry, 156(4), 575–584. 10.1176/ajp.156.4.575 [DOI] [PubMed] [Google Scholar]
- Sierau S, Brand T, Manly JT, Schlesier-Michel A, Klein AM, Andreas A, Garzón LQ, Keil J, Binser MJ, von Klitzing K, & White LO (2017). A Multisource Approach to Assessing Child Maltreatment From Records, Caregivers, and Children. Child Maltreatment, 22(1), 45–57. 10.1177/1077559516675724 [DOI] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, Weber J, Mischel W, Casey BJ, & Ochsner KN (2016). VlPFC–vmPFC–Amygdala Interactions Underlie Age-Related Differences in Cognitive Regulation of Emotion. Cerebral Cortex, bhw073. 10.1093/cercor/bhw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokauskas N, Carballedo A, Fagan A, & Frodl T (2015). The role of sexual abuse on functional neuroimaging markers associated with major depressive disorder. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 16(7), 513–520. 10.3109/15622975.2015.1048723 [DOI] [PubMed] [Google Scholar]
- Smith SM, & Vale WW (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience, 8(4), 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Queiroz J, Boisgontier J, Etain B, Poupon C, Duclap D, d’Albis M-A, Daban C, Hamdani N, Le Corvoisier P, Delavest M, Bellivier F, Guevara P, Leboyer M, Henry C, & Houenou J (2016). Childhood trauma and the limbic network: A multimodal MRI study in patients with bipolar disorder and controls. Journal of Affective Disorders, 200, 159–164. 10.1016/j.jad.2016.04.038 [DOI] [PubMed] [Google Scholar]
- Tabone JK (2019). Adolescent Maltreatment and Risk Behaviors: A Comparison of Child Protective Service Reports and Youth Self-reports. Child and Adolescent Social Work Journal. 10.1007/s10560-019-00617-8 [DOI] [Google Scholar]
- Tomoda A, Suzuki H, Rabi K, Sheu Y-S, Polcari A, & Teicher MH (2009). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage, 47 Suppl 2, T66–71. 10.1016/j.neuroimage.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, Gordis E, Peckins MK, & Susman EJ (2014). Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment, 19(1), 27–37. 10.1177/1077559513520466 [DOI] [PubMed] [Google Scholar]
- Ullman SE (2007). Relationship to perpetrator, disclosure, social reactions, and PTSD symptoms in child sexual abuse survivors. Journal of Child Sexual Abuse, 16(1), 19–36. 10.1300/J070v16n01_02 [DOI] [PubMed] [Google Scholar]
- van den Bulk BG, Somerville LH, van Hoof M-J, van Lang NDJ, van der Wee NJA, Crone EA, & Vermeiren RRJM (2016). Amygdala habituation to emotional faces in adolescents with internalizing disorders, adolescents with childhood sexual abuse related PTSD and healthy adolescents. Developmental Cognitive Neuroscience, 21, 15–25. 10.1016/j.dcn.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A-L, Hauber K, Gunther Moor B, Spinhoven P, Boon AE, Crone EA, & Elzinga BM (2014). Childhood Emotional Maltreatment Severity Is Associated with Dorsal Medial Prefrontal Cortex Responsivity to Social Exclusion in Young Adults. PLoS ONE, 9(1), e85107. 10.1371/journal.pone.0085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-Subcortical Pathways Mediating Successful Emotion Regulation. Neuron, 59(6), 1037–1050. 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermair AL, Stoll AM, Greggersen W, Kahl KG, Hüppe M, & Schweiger U (2018). All Unhappy Childhoods Are Unhappy in Their Own Way—Differential Impact of Dimensions of Adverse Childhood Experiences on Adult Mental Health and Health Behavior. Frontiers in Psychiatry, 9. 10.3389/fpsyt.2018.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Harnett NG, Wood KH, Orem TR, Granger DA, Mrug S, & Knight DC (2016). Prefrontal Cortex Activity Is Associated with Biobehavioral Components of the Stress Response. Frontiers in Human Neuroscience, 10. 10.3389/fnhum.2016.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Grunbaum JA, Elliott M, Tortolero SR, Berry S, Gilliland J, Kanouse DE, Parcel GS, Wallander J, Kelder S, Collins J, Kolbe L, & Schuster M (2004). Healthy passages. A multilevel, multimethod longitudinal study of adolescent health. American Journal of Preventive Medicine, 27 (2), 164–172. 10.1016/j.amepre.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Wolf MR, & Pruitt DK (2019). Grooming Hurts Too: The Effects of Types of Perpetrator Grooming on Trauma Symptoms in Adult Survivors of Child Sexual Abuse. Journal of Child Sexual Abuse, 28(3), 345–359. 10.1080/10538712.2019.1579292 [DOI] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, & Knight DC (2012). Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. NeuroImage, 60(1), 787–799. 10.1016/j.neuroimage.2011.12.048 [DOI] [PubMed] [Google Scholar]
- Wood KH, Wheelock MD, Shumen JR, Bowen KH, Ver Hoef LW, & Knight DC (2015). Controllability modulates the neural response to predictable but not unpredictable threat in humans. NeuroImage, 119, 371–381. 10.1016/j.neuroimage.2015.06.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Toki S, Siegle GJ, Takamura M, Takaishi Y, Yoshimura S, Okada G, Matsumoto T, Nakao T, Muranaka H, Kaseda Y, Murakami T, Okamoto Y, & Yamawaki S (2017). Increased amygdala reactivity following early life stress: A potential resilience enhancer role. BMC Psychiatry, 17(1). 10.1186/s12888-017-1201-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates TM, Carlson EA, & Egeland B (2008). A prospective study of child maltreatment and self-injurious behavior in a community sample. Development and Psychopathology, 20(2), 651–671. 10.1017/S0954579408000321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


