Abstract
In the past two decades, three highly pathogenic human coronaviruses severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus, and, recently, SARS-CoV-2, have caused pandemics of severe acute respiratory diseases with alarming morbidity and mortality. Due to the lack of specific anti-CoV therapies, the ongoing pandemic of coronavirus disease 2019 (COVID-19) poses a great challenge to clinical management and highlights an urgent need for effective interventions. Drug repurposing is a rapid and feasible strategy to identify effective drugs for combating this deadly infection. In this review, we summarize the therapeutic CoV targets, focus on the existing small molecule drugs that have the potential to be repurposed for existing and emerging CoV infections of the future, and discuss the clinical progress of developing small molecule drugs for COVID-19.
Keywords: anti-CoV, coronavirus, COVID-19, drug repurposing, drug targets, MERS-CoV, SARS-CoV, SARS-CoV-2, small molecule drugs
1 |. INTRODUCTION
Coronaviruses (CoVs) are a large family of enveloped and nonsegmented positive-sense RNA viruses which can infect a wide range of hosts, including human and animals.1 CoVs belong to the family Coronaviridae of the order Nidovirales and can be classified into four genera (alpha, beta, gamma, and delta).2 Human coronaviruses (HCoVs) were first discovered in the 1960s and since, there have been seven identified HCoVs, including two α-CoVs, HCoV-229E and HCoV-NL63, as well as five β-CoVs HCoV-OC43, HCoV-HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2.1–4 All HCoVs are believed to cross species barriers and emerge originally as zoonoses.4–7 Human strains HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1 usually cause mild symptoms of common cold.8 However, in the past two decades, three highly pathogenic HCoVs, SARS-CoV, MERS-CoV, and SARS-CoV-2, have emerged with human-to-human transmission, causing severe acute respiratory diseases with alarming morbidity and mortality.2,9
SARS-CoV emerged initially in southern China in November 2002 and spread to 29 countries and regions through international air travel, resulting in 8098 cases and 774 deaths with a fatality rate of 9.6% by the end of the pandemic in July 2003.10 MERS-CoV was first identified in Saudi Arabia in 2012. Dromedary camels are the major reservoir host for MERS-CoV which are involved in direct or indirect transmission to humans.1,11–14 At the end of January 2020, there were a total of 2519 laboratory-confirmed cases including 866 associated deaths (34.3% of cases) reported in 27 countries, the majority of which were reported from Saudi Arabia (2106 cases including 783 associated deaths).15 At the end of 2019, the third highly pathogenic HCoV, named SARS-CoV-2 (2019-nCoV), was reported in Wuhan, China, as the cause of coronavirus disease 2019 (COVID-19) outbreak.16 Human-to-human transmission of SARS-CoV-2 was confirmed, mainly through respiratory droplets and indirect contact via contaminated surfaces.17–19 Most people with COVID-19 suffer mild to moderate respiratory illness and recover without special treatment; however, older people and those with comorbidities such as cardiovascular disease and diabetes are more likely to develop severe disease with high mortality.20 Although SARS-CoV-2 possesses a relatively lower case-mortality rate as compared with SARS-CoV and MERS-CoV, it can be transmitted more efficiently, even by infected people in mild condition or asymptomatic carriers, making it challenging to control.21,22 The World Health Organization declared the COVID-19 outbreak a public health emergency of international concern on January 30 and a pandemic on March 11, 2020, successively. As of November 29, 2020, there are more than 61 million reported cases of COVID-19, including more than 1,448,990 deaths in over 180 countries and regions.23 Despite the significant clinical impact and the availability of very recently FDA-approved Veklury (remdesivir), there remains an urgent need for more approved antiviral therapeutics effective for CoV infections.
Developing new highly effective anti-CoV drugs may require several years of drug development efforts. However, facing the urgency of the ongoing pandemic COVID-19, screening the existing broad-spectrum antiviral drugs or other drugs targeting viral or host proteins involved in the virus life cycle may serve as a fast and efficient approach for combating this deadly infection.1 This repurposing strategy offers diverse advantages over de novo drug discovery including a less time-consuming development process, reduced costs and risks, as well as available pharmacokinetic (PK) and safety profiles.24 In this review, we discuss the potential drugs and drug targets against CoV, focusing on the existing small molecule drugs that may be repurposed for existing and emerging CoV infections of the future, and highlight the clinical progress in developing small molecule drugs for the ongoing pandemic of COVID-19.
2 |. CORONAVIRUS TARGETS FOR DRUG DEVELOPMENT
2.1 |. Coronavirus genomes and structures
Coronaviruses possess a positive-sense, single-stranded RNA genome, and a helically symmetrical nucleocapsid. The RNA genome contains a 5ʹ-methylated cap and a 3ʹ-polyadenylated tail ranging from 26.4 to 31.7 kb in size.25,26 CoVs share similar genome organization of 5ʹ-leader-UTR-replicase (ORF1a/b)-spike (S)-envelop (E)-membrane (M)-nucleocapsid (N)-3ʹ-UTR-poly(A) tail (Figure 1).1,27,28 The open reading frames (ORFs) 1a and 1b take up two-thirds of the genome and encode two large replicase-transcriptase polyproteins (pp1a and pp1ab).26 Self-cleavage of pp1a and pp1ab produces 16 nonstructural proteins (nsp1–16), including two viral cysteine proteases, nsp3 (papain-like protease [PLpro]) and nsp5 (3C-like or main protease [3CLpro]), nsp12 (RNA-dependent RNA polymerase [RdRp]), nsp13 (helicase) and other nsps with known or unknown functions which are likely involved in viral transcription and replication.26,29–31 The later ORFs encode four main structural proteins: S, E, M, and N proteins and accessory proteins, the number and function of which may vary depending on specific CoV.26 The S protein is a class I fusion protein that comprises two subunits, the amino-terminal receptor-binding S1 and carboxy-terminal membrane fusion S2.32 It forms homotrimers which make up the spike structure on the viral surface and mediates host attachment and membrane fusion during entry, determining host range and cell tropism.33 The E and M proteins play an important role in forming the viral envelope and maintaining its structural shape, whereas the E protein also has ion channel activity required for pathogenesis.26,34 The N protein is the only protein that exists in the nucleocapsid. It contains two separate domains, an N-terminal domain (NTD) and a C-terminal domain (CTD), and both domains can bind to RNA by using different mechanisms. The N protein was found to be involved in processes associated with viral genome and replication cycle as well as host cellular response to viral infections.35,36
FIGURE 1.
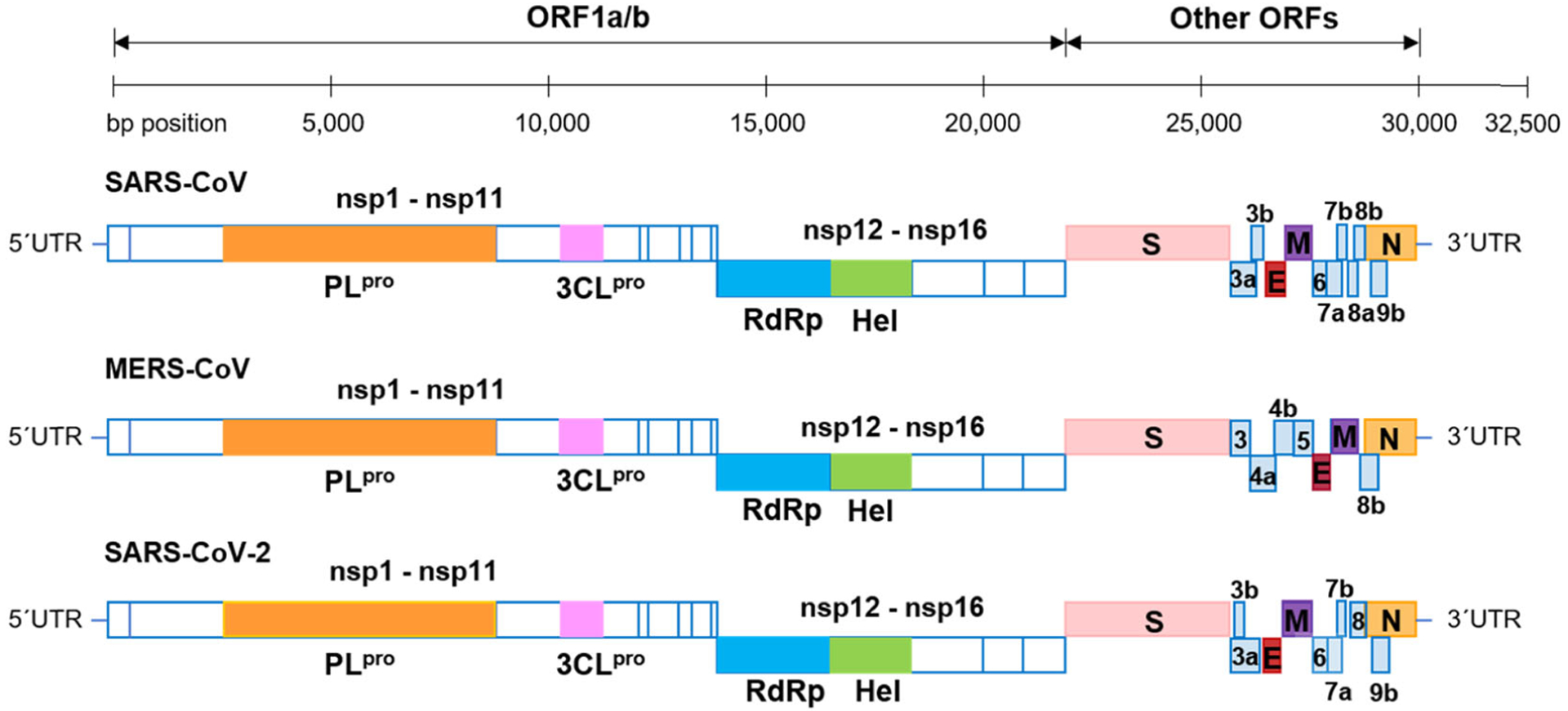
The genome organization of SARS-CoV, MERS-CoV, and SARS-CoV-2. The ORFs 1a/b encode 16 nsps. Other ORFs encode structural proteins including S, E, M, and N proteins as well as accessory proteins. E, envelop; M, membrane; MERS-CoV, Middle East respiratory syndrome coronavirus; N, nucleocapsid; nsp, nonstructural protein; ORF, open reading frame; S, spike; SARS-CoV, severe acute respiratory syndrome coronavirus
2.2 |. Life cycle and key targets
The CoV life cycle includes several essential steps, which can be targeted for the development of anti-CoV therapeutics. The first step is viral entry that is initiated by the binding of the surface S1 unit of the S protein to a cellular receptor (Figure 2).26,28,37 The S1 subunit consists of two independent domains, NTD and CTD. Most CoVs, such as SARS-CoV and MERS-CoV, use CTD as the receptor binding domain (RBD).38 Many CoVs recognize peptidases as their cellular receptors and cell entry even occurs without the enzymatic domain of these proteins; however, the molecular mechanism of virus entry remains elusive.39 SARS-CoV and SARS-CoV-2 utilize angiotensin-converting enzyme 2 (ACE2) as their cellular receptors,40,41 whereas MERS-CoV binds to dipeptidyl peptidase 4 (DPP4) for virus entry.42 Once RBD of the S1 subunit binds to the host receptor, it will induce conformational changes in the S2 subunit (the stalk region of S), approximate viral and cell membrane via inserting the fusion peptide of S2 into target cell membrane, and finally enable fusion.1,43 This process requires two proteolytic cleavages by host proteases, including priming cleavage at the S1/S2 junction site for separating the RBD and fusion domains of the S1/S2 proteins and activating cleavage at the S2ʹ site for exposing the fusion peptide.38,44,45 These host receptors (ACE2 and DPP4), RBD of the S1 subunit and the S2 subunit can serve as potential anti-CoV targets, but anti-CoV therapeutics such as monoclonal antibodies (mAbs) and antiviral agents targeting these proteins should avoid inducing immunopathological effects and antibody-dependent enhancement.46
FIGURE 2.
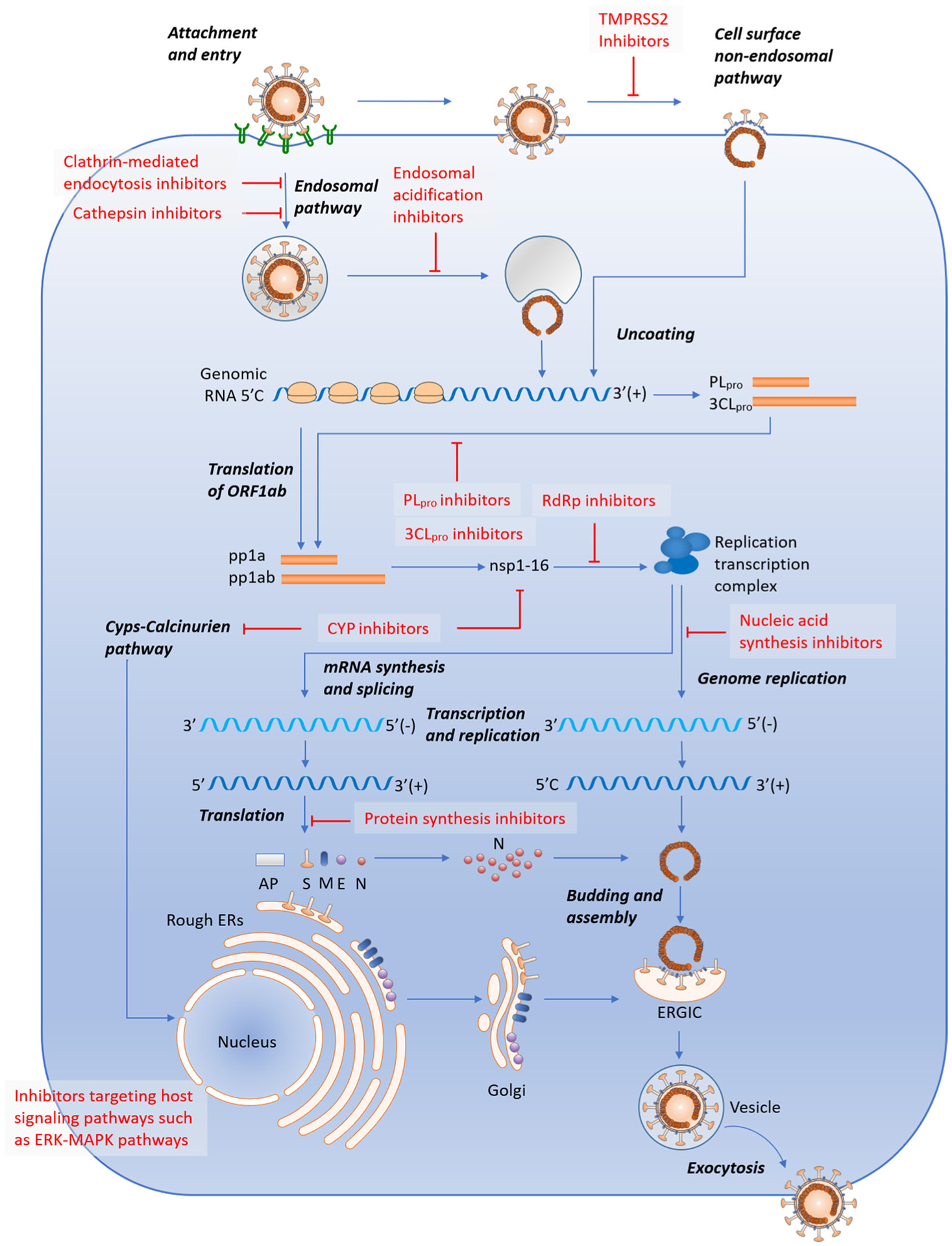
Candidate drugs for CoV infections targeting different processes of the CoV life cycle. Adapted with permission from Ref. 28, American Society for Microbiology. AKT, protein kinase B; AP, accessory protein; 3CLpro, 3C-like protease; Cyps, cyclophilins; E, envelope; ER, endoplasmic reticulum; ERGIC, endoplasmic reticulum-Golgi intermediate compartment; ERK, extracellular signal-regulated kinases; M, membrane; MAPK, mitogen-activated protein kinases; mTOR, mammalian target of rapamycin; N, nucleocapsid; NFAT, nuclear factor of activated T cells; nsp, nonstructural protein; ORF, open reading frame; PI3K, phosphoinositol 3-kinase; PLpro, papain-like protease; RdRp, RNA-dependent RNA polymerase; S, spike; TMPRSS2, transmembrane protease serine 2
CoVs were found to utilize the endosomal pathway and/or the cell surface nonendosomal pathway for host cell entry.1 In the endosomal pathway, the pH-dependent endosomal cysteine protease cathepsins mediate the proteolytic processing that, together with low pH, overcomes the energetic barrier for fusion and facilitates CoV cell entry.47–49 In addition, other host protease, such as transmembrane protease serine 2 (TMPRSS2) and TMPRSS11D (also known as airway trypsin-like protease), were reported to activate S protein for cell surface nonendosomal virus entry at the plasma membrane via cleaving S into the S1 and S2 subunits.50 Accumulated studies showed CoVs enter the cell directly from the cell surface in the presence of protease such as TMPRSS2 and trypsin.41,51,52 Inhibitors targeting these host proteases such as cathepsins and TMPRSS2 are also potential anti-CoV agents and their combination use is a rational strategy to fully block the entry of CoVs by inhibiting both endosomal and nonendosomal entry pathways.41,53
After cell entry, CoVs disassemble and release the nucleocapsid and viral RNA into the cytoplasm followed by translation of ORF1a/b into viral pp1a and pp1ab.26 The polyproteins pp1a and pp1ab are self-cleaved by PLpro and 3CLpro which are encoded within nsp3 and nsp5, respectively, and subsequently produce nsp1 to nsp16.1,26 Many of these nsps form replicase-transcriptase complex (RTC) for viral RNA synthesis of which the core component is the catalytic subunit of RdRp (nsp12).54 RTC transcribes the full-length positive genomic RNA to form a full-length negative-strand template and overlapping subgenomic negative-strand templates for synthesis of genomic and subgenomic RNAs, respectively.1,26 Positive-sense subgenomic RNAs are subsequently translated to afford structural and accessory proteins. Structural proteins S, E, and M are then moved to endoplasmic reticulum-Golgi intermediate compartment (ERGIC) and interact with the helical nucleocapsid which is originally produced by the assembly of the N protein with genomic RNA in the cytoplasm, finally resulting in the form of mature virions.28 The viral life cycle is completed once the assembled virions are transported to the cell surface and released through exocytosis.28
These nsps and structural proteins, E, M, and N, are also potential targets for anti-CoV drug discovery.55 3CLpro and RdRp are of particular interest and substantial efforts have been made towards these two targets.56,57 3CLpro is conserved among CoVs and has no human homolog, making it an ideal anti-CoV target.58 Currently, the crystal structures of SARS-CoV-2 3CLpro with peptide-aldehyde inhibitors (PDB: 6LZE and 6M0K) or peptide with a Michael receptor N3 (PDB: 2H2Z) have been solved which are anticipated to facilitate the design and development of other 3CLpro inhibitors through molecular docking studies (Figure 3A).58,59 RdRp plays an essential role in viral replication and transcription and is a major target of many existing drugs of the nucleotide class.57 The cryo-EM structures of SARS-CoV-2 RdRp either in the apo form (PDB: 7BV1 and EMDB: EMD-30209) or in complex with a template-primer RNA and remdesivir (PDB: 7BV2 and EMDB: EMD-302010) have also been determined, providing excellent models to elucidate how RdRp inhibitors work and also solid templates for modeling and optimizing the existing nucleotide drugs (Figure 3B).60
FIGURE 3.
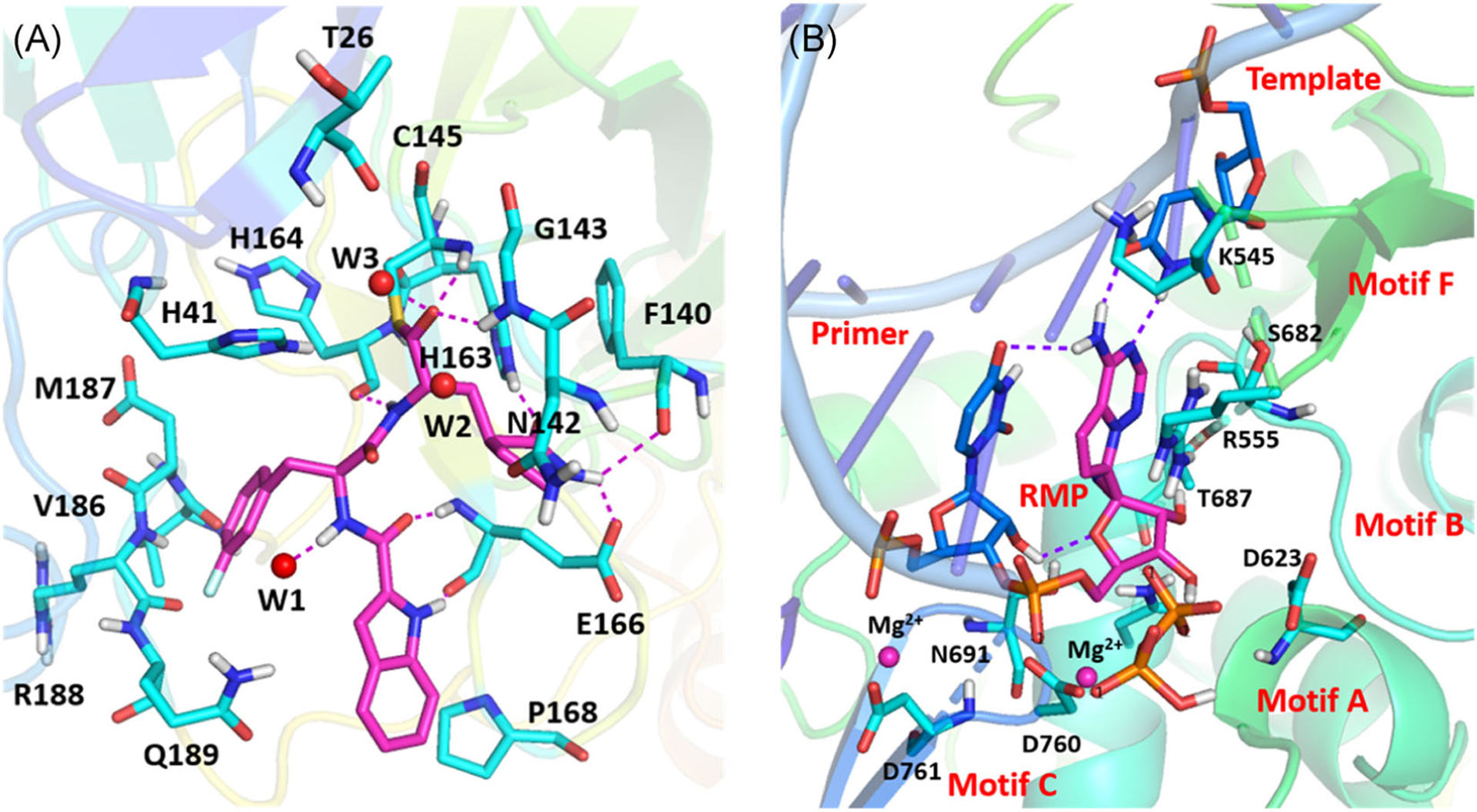
The determination of SARS-CoV-2 3CLpro and RdRp crystal structures facilitates the design and development of SARS-CoV-2 inhibitors. (A) The crystal structure of SARS-CoV-2 3CLpro in complex with a peptide-aldehyde inhibitor (PDB: 6M0K). (B) The cryo-EM structure of SARS-CoV-2 RdRp in complex with a template-primer RNA and remdesivir (PDB: 7BV2). 3CLpro, 3C-like protease; cryo-EM, cryogenic electron microscopy; RdRp, RNA-dependent RNA polymerase; SARS-CoV, severe acute respiratory syndrome coronavirus
3 |. VIRUS-BASED SMALL MOLECULE DRUGS FOR CORONAVIRUS
3.1 |. Protease inhibitors
Lopinavir (1; Figure 4) and ritonavir (2) are antiretroviral drugs of the protease inhibitor class and widely used as a fixed dose combination medication named Kaletra to treat and prevent human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS).61 Typically, ritonavir is used at a lower dose to inhibit the enzyme cytochrome P450–3A4 (CYP3A4) and decrease the metabolism of other combined protease inhibitors including lopinavir. Studies have shown that lopinavir inhibits the replication of SARS-CoV and MERS-CoV with single-digit micromolar EC50 values (Table 1) which are in the range of the plasma concentration (8–24 μM) of lopinavir observed in AIDS patients.62–64 Lopinavir and ritonavir were postulated to inhibit SARS-CoV 3CLpro and treatment of lopinavir/ritonavir (LPV/r) alone or combined with ribavirin was associated with improved clinical outcomes in nonrandomized trials of SARS patients.65–67 LPV/r administration also improved outcome of MERS infection in a nonhuman primate model68 and a randomized controlled trial of LPV/r and interferon β1b (IFN-β1b; interferons are a group of signaling proteins which can be released by a virus-infected cell and help nearby cells to strengthen their antiviral defenses) for MERS treatment has been underway in Saudi Arabia since November 2016.69 Recently, lopinavir was reported to inhibit SARS-CoV-2 induced cytopathic effect (CPE) with an half-maximal inhibitory concentration (IC50) value of 9.12 μM70 and multiple clinical trials of LPV/r treatment for COVID-19 have been initiated. However, a retrospective study that has enrolled 199 adult patients with severe COVID-19 revealed that there was no significant difference between LPV/r-treated group (n = 99) and control group with standard care (n = 100) in clinical improvement, mortality, and reducing viral loads.71 Delayed treatment initiation may partially account for the ineffectiveness of LPV/r for COVID-19 treatment. Thus, more clinical data are still needed to confirm or exclude the possibility of a treatment benefit of LPV/r for COVID-19 patients.
FIGURE 4.
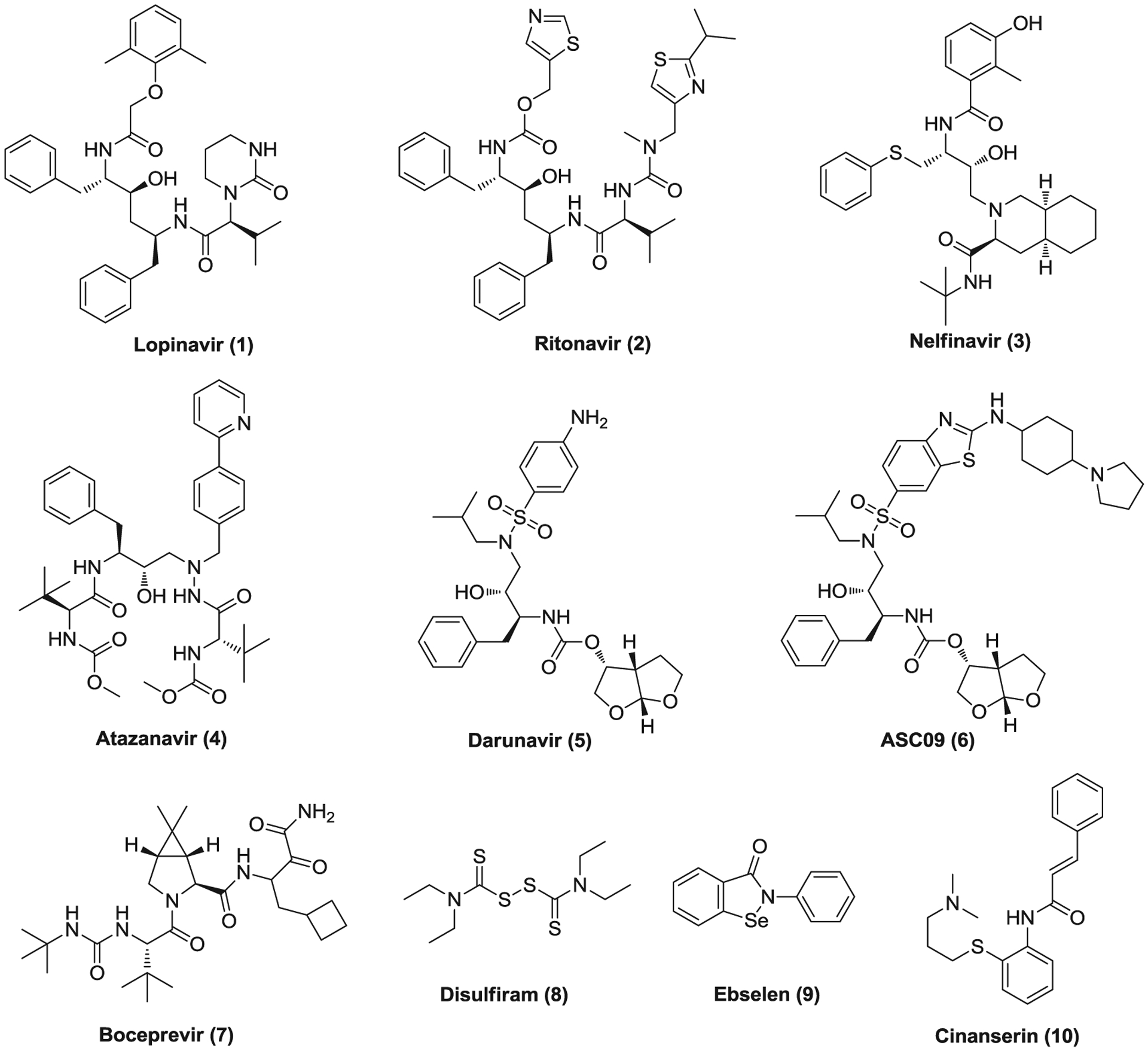
The potential drugs targeting coronavirus proteases
TABLE 1.
The virus-based small molecule drugs with therapeutic potentials for CoVs
| Antiviral agent | Drug class and/or targets | Activity against coronaviruses (cells/virus strain) | Ref. |
|---|---|---|---|
| Lopinavir (1) | 3CLpro inhibitor | SARS-CoV: EC50 = 17.1 μM, CC50 > 32 μM (Vero E6/Frankfurt-1) EC50 at 48 h = 4–8 μg/ml (Vero E6/HKU39849) MERS-CoV: EC50 = 8.0 μM, CC50 = 24.4 μM (Huh7/EMC/2012) SARS-CoV-2: EC50 = 9.12 μM, CC50 > 50 μM (Vero) |
62,63,70 |
| Nelfinavir (3) | 3CLpro inhibitor | SARS-CoV: EC50 = 0.048 μM, CC50 = 14.75 μM (Vero E6/FFM-1) SARS-CoV-2: EC50 = 9.12 μM, CC50 = 51.75 μM (Vero E6) |
72,73 |
| Atazanavir (4) | 3CLpro inhibitor | SARS-CoV-2: EC50 = 2.0 μM, CC50 = 312 μM (Vero) EC50 = 0.22 μM (A549) |
75 |
| darunavir (5) | 3CLpro inhibitor | Not reported | |
| ASC09 (6) | 3CLpro inhibitor | Not reported | |
| Boceprevir (7) | 3CLpro inhibitor | SARS-CoV-2: EC50 = 1.9 μM, CC50 > 100 μM (Vero 76) | 79 |
| Disulfiram (8) | PLpro inhibitor | SARS-CoV-2 3CLpro: IC50 = 9.35 μM | 59 |
| Ebselen (9) | 3CLpro inhibitor | SARS-CoV-2: EC50 = 4.6 μM (Vero E6) | 59 |
| Cinanserin (10) | 3CLpro inhibitor | SARS-CoV: decreased viral replication by more than 1000-fold at 50 μg/ml with no toxic effect SARS-CoV-2: EC50 = 20.61 μM, CC50 > 200 μM (Vero E6) |
59,88 |
| Ribavirin (11) | RdRp inhibitor | SARS-CoV: EC50 = 80 μg/ml, CC50 > 200 μg/ml (Vero E6/HKU39849) MERS-CoV: EC50 = 41.4 μg/ml (Vero RML6/hCoV-EMC/2012) SARS-CoV-2: EC50 = 109.5 μM, CC50 > 400 μM (Vero E6) |
100,101,110 |
| Remdesivir (12) | RdRp inhibitor | SARS-CoV: EC50 = 0.069 μM, CC50 > 10 μM (HAE) MERS-CoV: EC50 = 0.074 μM, CC50 > 10 μM (HAE) SARS-CoV-2: EC50 = 0.77 μM, CC50 > 100 μM (Vero E6) Reduced lung viral load and improved clinical signs and respiratory functions in a mouse (or rhesus macaque) models of SARS-CoV or MERS-CoV infection |
110,112,114,118,119 |
| EIDD-1931 (13) | RdRp inhibitor | SARS-CoV: EC50 = 0.14 μM (HAE) MERS-CoV: EC50 = 0.024 μM (HAE); EC50 = 0.15 μM, CC50 > 10 μM (Calu-3) SARS-CoV-2: EC50 = 0.30 μM, CC50 > 10 μM (Vero E6); EC50 = 0.08 μM (Calu-3) Reduced viral load and improved pulmonary function in a mouse model of SARS-CoV or MERS-CoV infection |
129 |
| Favipiravir (15) | RdRp inhibitor | SARS-CoV-2: EC50 = 22.50 μM, CC50 > 100 μM (Vero E6) | 110 |
| Galidesivir (16) | RdRp inhibitor | SARS-CoV: EC50 = 57.7 μM, CC50 > 296 μM (Vero 76/Urbani) MERS-CoV: EC50 = 68.4 μM, CC50 > 100 μM (Vero E6/Jordan N3) |
141 |
| Penciclovir (17) | RdRp inhibitor | SARS-CoV-2: EC50 = 95.96 μM, CC50 > 400 μM (Vero E6) | 110 |
| Mycophenolic acid (18) | IMPDH inhibitor; noncompetitive PLpro inhibitor | MERS-CoV: EC50 = 0.17 μg/ml, CC50 > 32 μg/ml (Vero); EC50 = 2.87 μM, CC50 > 70 μM (Vero E6) Its prodrug MMF (19) treatment resulted in a worse outcome in a marmoset model of MERS-CoV infection. SARS-CoV-2: EC50 = 0.87 μM, CC50 > 128 μM (Vero E6/Wk521) |
159–161,165 |
| Merimepodib (20) | Noncompetitive IMPDH inhibitor | SARS-CoV-2: decreased viral titers by over 10-fold at 3.3 μM | 171 |
| Mizoribine (21) | IMPDH and GMP-synthetase inhibitor | SARS-CoV: EC50 = 3.5 μg/ml, CC50 > 200 μg/ml (Vero E6/Frankfurt-1); EC50 = 16 μg/ml (Vero E6/HKU39849) | 100 |
| Gemcitabine hydrochloride (22) | Nucleic acid synthesis inhibitors | SARS-CoV: EC50 = 4.96 μM, CC50 > 10 μM (Vero E6/MA15) MERS-CoV: EC50 = 1.22 μM, CC50 > 10 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 1.24 μM, CC50 >40 μM (Vero E6) |
178,179 |
| Oseltamivir (23) | Neuraminidase inhibitor | Not reported | |
| Umifenovir (24) | S protein/ACE2, membrane fusion inhibitor | Inhibited SARS-CoV-2 replication at 10–30 μM | 192 |
| Rimantadine (25) | Influenza M2 protein inhibitor | SARS-CoV: EC50 at 48 h = 8–16 μg/ml (Vero E6/HKU39849); EC50 at 48 h = 8–16 μg/ml (fRhK4 cells/10 strains), CC50 = 64 μg/ml | 63 |
| Resveratrol (26) | Nucleocapsid protein | Significantly reduced MERS-CoV titers at 150 μM at 48 h postinfection SARS-CoV-2: EC50 = ~66 μM, CC50 > 250 μM (Vero E6) |
204,205 |
Abbreviations: 3CLpro, 3C-like protease; ACE2, angiotensin-converting enzyme 2; CC50, cytotoxic concentration 50%; EC50, half-maximal effective concentration; GMP, guanosine-5′-monophosphate; IMPDH, inosine-5′-monophosphate dehydrogenase; MERS-CoV, Middle East respiratory syndrome coronavirus; PLpro, papain-like protease; RdRp, RNA-dependent RNA polymerase; SARS-CoV, severe acute respiratory syndrome coronavirus.
Compounds 3–6 are also antiretroviral medications that are used to treat HIV infections as protease inhibitors. Nelfinavir (3) was found to inhibit the replication of SARS-CoV and SARS-CoV-2 in Vero E6 cells with EC50 values of 0.048 and 2.89 μM, respectively.72,73 When treating patients with nelfinavir at an oral dose of 1875 mg BID, high peak and trough serum concentrations (13.3 and ~5.5 μM, respectively) were observed,74 higher than its in vitro EC50 values against SARS-CoV-2, indicating its therapeutic potential to combat COVID-19. Recently, atazanavir (4) was reported to inhibit SARS-CoV-2 replication in Vero and A549 cells with EC50 values of 2.0 and 0.22 μM, respectively. It also suppresses cell death and proinflammatory cytokine production in SARS-CoV-2-infected monocytes.75 Whereas, to date, no in vitro antiviral activities against SARS-COV-2 were reported for darunavir (5) and ASC09 (6), several clinical trials have been launched to evaluate the efficacy of darunavir/cobicistat, ASC09/ritonavir (ASC09F), and ASC09F/oseltamivir for treatment of COVID-19. Recently, a single-center, randomized, and open-label trial involving 30 patients with mild COVID-19 revealed that a 5-day treatment of darunavir/cobicistat did not increase the proportion of negative conversion at Day 7 compared with standard care alone.76 It should be noted that HIV protease belongs to the aspartic protease family and its inhibitors were designed to fit its C2 symmetric catalytic site that is lacked in the cysteine proteases, CoV 3CLpro and PLpro.77 It remains questionable whether HIV protease inhibitors could effectively inhibit 3CLpro or PLpro of SARS-CoV-2.
Boceprevir (7) is an NS3 serine protease inhibitor of hepatitis C virus (HCV) which was used to treat hepatitis caused by HCV genotype 1.78 It inhibits SARS-COV-2 3CLpro with an IC50 of 4.13 μM but was not active against the enterovirus A71 (EV-A71) 2A and 3C cysteine proteases (IC50 > 20 μM).79 Boceprevir showed potent antiviral activity against SARS-CoV-2 (EC50 = 1.9 μM) in a CPE assay meanwhile displaying low cytotoxicity (CC50 > 100 μM), indicating its great potential in COVID-19 treatment. Disulfiram (8) is an Food and Drug Administration (FDA)-approved drug used as a second-line treatment of alcohol dependence.80 It irreversibly inhibits acetaldehyde dehydrogenase (ALDH1A1) by covalently modifying the cysteine residue of the active site and causes uncomfortable hangover symptoms after alcohol consumption.81 Recent studies showed disulfiram inhibits PLpro of SARS-CoV and MERS-CoV with micromolar IC50 values, acting as a competitive (or mixed) inhibitor and an allosteric inhibitor, respectively.82 Disulfiram exhibited synergistic inhibition with 6-thioguanine or mycophenolic acid (MPA) against MERS-CoV PLpro. Interestingly, disulfiram was also reported to inhibit SARS-CoV-2 3CLpro activity with an IC50 value of 9.35 μM.59 However, the in vitro anti-CoV activity of disulfiram remains to be demonstrated. Ebselen (9) is a seleno-organic drug, which can mimic glutathione peroxidase activities and react with peroxynitrite. It possesses anti-inflammatory, antioxidant, antifungal, and cytoprotective properties83,84 and has been investigated to treat various human conditions such as reperfusion injury85 and hearing loss.86 Recently, ebselen was screened out as a potent SARS-CoV-2 3CLpro Mpro inhibitor (IC50 = 0.67 μM) and displayed inhibition against SARS-CoV-2 with an EC50 value of 4.67 μM in Vero E6 cells using a plaque reduction assay.59 Cinanserin (10) is an antagonist of 5-HT2A and 5-HT2C receptors discovered in the 1960s.87 It was found to inhibit SARS 3CLpro (IC50 = 5 μM) and treatment of Vero cells with cinanserin (50 μg/ml) resulted in more than 3log reduction in SARS-CoV RNA production with nontoxic effect.88 Cinanserin also displays moderate inhibition against SARS-CoV-2 with an EC50 of 20.61 μM.59 Nevertheless, long-term treatment of cinanserin at a high dose (120 mg/kg daily for 59–81 weeks) in rats led to malignant hepatoma.89 Thus, this molecule may serve as a lead compound for the development of highly effective CoV 3CLpro inhibitors with reduced toxicity and antiserotonin activity.
3.2 |. Nucleic acid synthesis inhibitors
3.2.1 |. RdRp inhibitors
Ribavirin (11; Figure 5) is a guanosine nucleoside analog which displays antiviral activity against a wide range of both DNA and RNA viruses in vitro due to its multiple mechanisms of action.90,91 It has been used to treat respiratory syncytial virus (RSV) infection,92 HCV infection,93,94 and some viral hemorrhagic fevers.95–97 Ribavirin is a prodrug and its metabolized form mimics purine RNA nucleotides and interferes with RNA metabolism required for viral replication by inhibiting messenger RNA (mRNA) capping and viral RNA polymerase and inducing mutations via incorporation into RNA.91,98,99 Owing to its broad antiviral properties, ribavirin was widely investigated during the SARS and MERS outbreaks. It inhibits the replication of SARS-CoV and MERS-CoV in Vero cells with EC50 values of 80 μg/ml (HKU39489) and 41.4 μg/ml (hCoV-EMC/2012), respectively.100,101 High-dose treatment of ribavirin as a monotherapy or in combination with corticosteroid or LPV/r was used for SARS patients,102 but a retrospective, uncontrolled cohort analysis involving 229 cases in Singapore indicated that use of ribavirin did not appear to confer any benefit for SARS patients. Therefore, its clinical benefit remained uncertain.102–105 Cotreatment of ribavirin and IFN-α2b improves outcome in MERS-CoV-infected rhesus macaques,106 but no obvious survival benefit was observed in small cohorts of MERS patients.107–109 Ribavirin was also reported to inhibit the replication of SARS-CoV-2 at high concentration, with an EC50 of 109.5 μM.110 One clinical trial of ribavirin, LPV/r and IFN-β2b combination for COVID-19 treatment has been completed with no reported results yet. However, high-dose ribavirin treatment was associated with significant toxicity such as hemolysis and hemoglobin decrease which hampers its wide clinical application in severe CoV-infected patients.105
FIGURE 5.
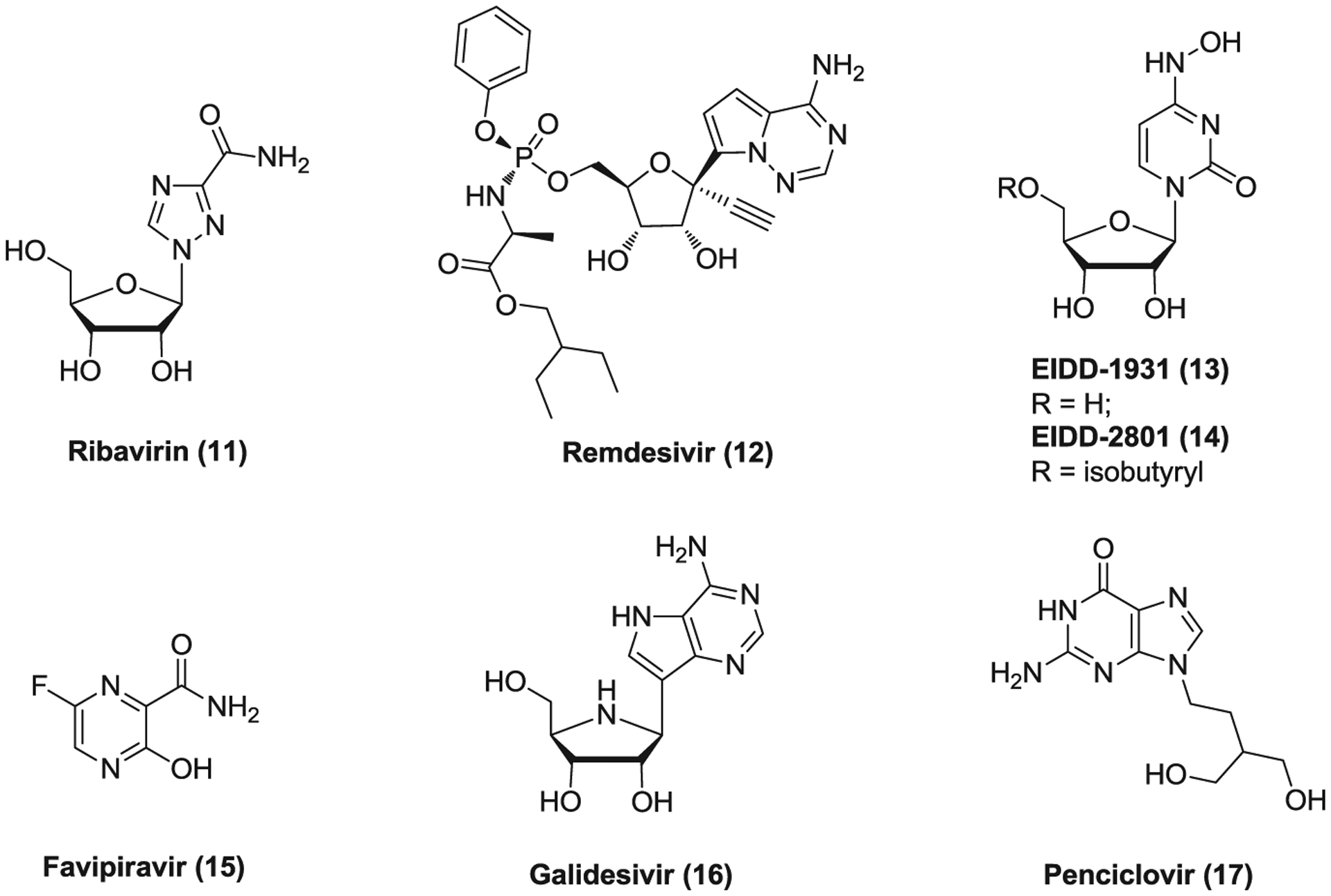
The potential RNA-dependent RNA polymerase inhibitors against coronaviruses
Remdesivir (12, GS-5734) is a broad-spectrum antiviral agent of an adenosine analogue that is highly effective against filoviruses, paramyxoviruses, RSV, and pathogenic CoVs.111,112 It is a phosphoramidate prodrug and metabolized into the active triphosphate form (GS-441524) that inhibits viral RNA polymerase and causes delayed chain termination of nascent viral RNA.113,114 Remdesivir was originally developed by Gilead Science to combat Ebola virus (EBOV) infection. It inhibits EBOV replication in multiple relevant human cell types with nanomolar to submicromolar EC50 values. Although its use achieved significant survival benefit in a rhesus monkey model of EBOV disease (EVD),115,116 a retrospective analysis involving 673 patients with EVD revealed that the groups with mAb REGN-EB3 or MAb114 administration showed better survival rates than the groups treating with ZMapp or remdesivir.117 Remdesivir effectively inhibits a wide range of human and zoonotic CoV replication in human airway epithelial (HAE) cells and displays EC50s of 0.069 μM for SARS-CoV and 0.074 μM for MERS-CoV.114 Both prophylactic and early therapeutic administration of remdesivir reduced lung viral load and improved clinical signs of disease as well as respiratory functions in a SARS-CoV infected mouse model.112 Similar efficacy was also observed for prophylactic and therapeutic remdesivir treatment in a mouse model and a nonhuman primate (rhesus macaque) model of MERS-CoV infection, respectively.118,119 As a broad anti-CoV agent, remdesivir was found to inhibit SARS-COV-2 replication in Vero E6 cells with an EC50 of 0.77 μM, acting as an RdRp inhibitor as well.110,120,121 Due to its available PK and safety profiles as well as potent in vitro antiviral activity against SARS-CoV-2, remdesivir was fast advanced into human clinical trials in several countries such as China and the United States to treat COVID-19. Recently, a retrospective study showed compassionate-use of remdesivir was associated with clinical improvement in 36 of 53 patients with COVID-19.122 However, another randomized, double-blind, placebo-controlled, multicenter study which enrolled 237 adult patients with severe COVID-19 revealed that remdesivir treatment was not associated with significant clinical benefits.123 Contrarily, according to the final report of a double-blind, randomized, placebo-controlled trial involving 1063 patients hospitalized with COVID-19, patients (538/1063) receiving remdesivir showed a shortened recovery time of 11 days as compared with 15 days of the control group (521/1063) receiving placebo.124 A press release from Gilead reported that, in a comparative analysis of the phase 3 SIMPLE-Severe trial and a real-world retrospective cohort of patients with severe COVID-19, treatment of remdesivir resulted in an improvement in clinical recovery and a 62% reduction in mortality compared with standard of care.125 Very recently, a randomized clinical trial involving 596 patients with moderate COVID-19 showed that patients receiving 5-day treatment course of remdesivir had significantly higher odds of a better clinical status distribution on Day 11 than those receiving standard care, but no statistically significant difference in clinical status on Day 11 was observed between the group receiving 10-day course of remdesivir and the control receiving standard care.126 Thus, the clinical benefit of remdesivir for COVID-19 remains to be validated by more data from the ongoing human phase 3 randomized, double-blind, placebo-controlled clinical trials.
Through a dual-pathogen high-throughput screening campaign, EIDD-1931 (13, NHC), a pyrimidine ribonucleoside analogue, was identified as a potent inhibitor of RSV, influenza A viruses of human, avian and swine origins, and influenza B viruses.127 Its active triphosphate form is incorporated into nascent RNA instead of cytidine triphosphate, increasing the chance of viral mutagenesis. This incorporation may also reduce viral RNA polymerase processivity and/or increase the frequency of delayed chain termination. EIDD-2801 (14) was developed as an isopropylester prodrug of EIDD-1931 that was orally bioavailable and showed broad ant-influenza virus activity in cultured cells and good in vivo efficacy in the ferret model of influenza infection with high resistance barrier.128 EIDD-1931 is also highly effective against multiple zoonotic CoVs in HAE cell cultures associated with increased viral mutation rates and shows EC50s of 0.3 μM against SARS-CoV-2 in Vero E6 cells and 0.08 μM against MERS-CoV in Calu-3 cells respectively.129 Both prophylactic and therapeutic administration of its prodrug EIDD-2801 improved pulmonary function and reduced virus titer and body weight loss in a mouse model of SARS-CoV or MERS-CoV infection. Moreover, EIDD-1931 is active against remdesivir-resistant CoVs as well. These findings together suggested that EIDD-1931 and its prodrug EIDD-2801 have great potential to be developed as a highly effective antiviral to treat MERS, COVID-19 and emerging CoV infections of the future. Currently, two human phase 2 clinical trials with this prodrug EIDD-2801 are ongoing to evaluate its safety, tolerability, and antiviral activity in patients with COVID-19.
Favipiravir (15, T-705) is an antiviral drug of a pyrazinecarboxamide derivative which was approved to treat influenza in Japan.130,131 It is a prodrug which is metabolized to a triphosphate form via intracellular phosphoribosylation targeting viral RNA polymerase. This active form was recognized as an efficient purine nucleoside analogue for incorporation to the RNA, which can lead to lethal mutagenesis. Two consecutive incorporation events efficiently block RNA synthesis.132–134 Favipiravir does not strongly affect cellular transcription and has a high resistance barrier to influenza virus.135 However, favipiravir was found inactive against influenza virus A (WSN) in primary human bronchial tracheal epithelial cells, posing a doubt on its efficacy in influenza treatment.127 In addition to influenza virus, favipiravir was also effective against a wide range of RNA viruses including EBOV and SARS-CoV-2.110,136–138 It inhibits SARS-CoV-2 replication with an EC50 of 61.88 μM in Vero E6 cells.110 Despite its relatively low in vitro activity against SARS-CoV-2, favipiravir has entered several clinical trials to evaluate its efficacy in COVID-19 treatment. In an open-controlled study, favipiravir/IFN-α treatment group (n = 35) showed better therapeutic effects on COVID-19 in terms of disease progression and viral clearance compared to the control group (n = 45) treated with LPV/r plus IFN-α.139 In another randomized, controlled, multicenter study involving 240 patients with COVID-19 infection, compared to arbidol (an antiviral medication used to treat influenza infections, see Section 3.3, 24) group (n = 120), favipiravir treatment (n = 116) did not significantly improve the clinically recovery rate at Day 7, but it shortened the latency to relief for pyrexia and cough and only caused mild and manageable adverse effects.140 These data support further investigation of the clinical potential of favipiravir for COVID-19 treatment.
Galidesivir (16, BCX4430), a novel synthetic adenosine analog, is an antiviral agent, which was developed as a potential treatment for EBOV and Marburg virus (MARV) infection. Its active triphosphate form suppresses viral RNA polymerase function, acting as an RNA chain terminator. Postexposure intramuscular administration of BCX4430 led to significant protection against EBOV and MARV disease in rodent models.141 Galidesivir displays broad-spectrum antiviral activities against a wide range of viruses including flaviviruses, bunyaviruses, arenaviruses, paramyxoviruses, and CoVs.141–143 It inhibits SARS-CoV and MERS-CoV replication with EC50 values of 57.7 and 68.4 μM, respectively.141 Additionally, galidesivir was found to bind to SARS-CoV-2 RdRp tightly via molecular docking.144 Although no in vitro activity against SARS-CoV-2 was reported, it has been advanced into a human phase 1 clinical trial to evaluate its safety, PKs and antiviral effects in COVID-19 treatment.
Penciclovir (17) is an antiviral medication of a guanosine analogue, which is used to treat various herpesvirus infections.145–147 Penciclovir is first mono-phosphorylated by viral thymidine kinase which is a rate-limiting step in its activation. Further phosphorylation by cellular kinase yields the active triphosphate form, thereby inhibiting viral DNA polymerase and leading to chain termination, with less influence on the normal cellular processes.148 Penciclovir has low toxicity and good selectivity and is often used as a topical treatment due to its poor oral absorption. Through screening existing antiviral drugs, penciclovir was also found to inhibit SARS-CoV-2 replication with an EC50 of 95.96 μM in Vero E6 cells.110
3.2.2 |. Other nucleic acid synthesis inhibitors
MPA (18, Figure 6), also called mycophenolate, is an immunosuppressant drug, which is used to prevent draft rejection in organ transplantation and treat Crohn’s disease.149,150 It is commonly administered as the mycophenolate sodium salt form or the prodrug mycophenolate mofetil (19, MMF). MPA is a potent, noncompetitive inhibitor of the enzyme inosine-5′-monophosphate dehydrogenase (IMPDH) that catalyzes the de novo synthesis of guanosine-5′-monophosphate (GMP) from inosine-5′-monophosphate (IMP).151 It inhibits the proliferation of T and B lymphocytes, and antibody formation as well as the glycosylation, expression, and function of adhesion molecules.150,151 MPA exhibits broad antiviral activities against different viruses such as flavivirus,152–155 Chikungunya,156 and HCV.157,158 MPA was reported to significantly inhibit MERS-CoV replication with EC50 values of 0.17 μg/ml and 2.87 μM in different assays.159,160 However, in a common marmoset model of MERS-CoV infection, MME treatment resulted in a worse outcome with more severe disease and higher viral loads compared to the untreated group.161 MPA was also found to be inactive against SARS-CoV up to 30 μM in vitro and in a mouse model.162 In addition, cases were reported that renal transplant recipients developed severe or fatal MERS when receiving immunosuppressant medication MMF.163,164 Recently, MPA was reported to be effective against SARS-CoV-2 with an EC50 of 0.87 μM.165 However, it remains to be validated whether treatment with the IMPDH inhibitor MPA really works for CoV infections.
FIGURE 6.
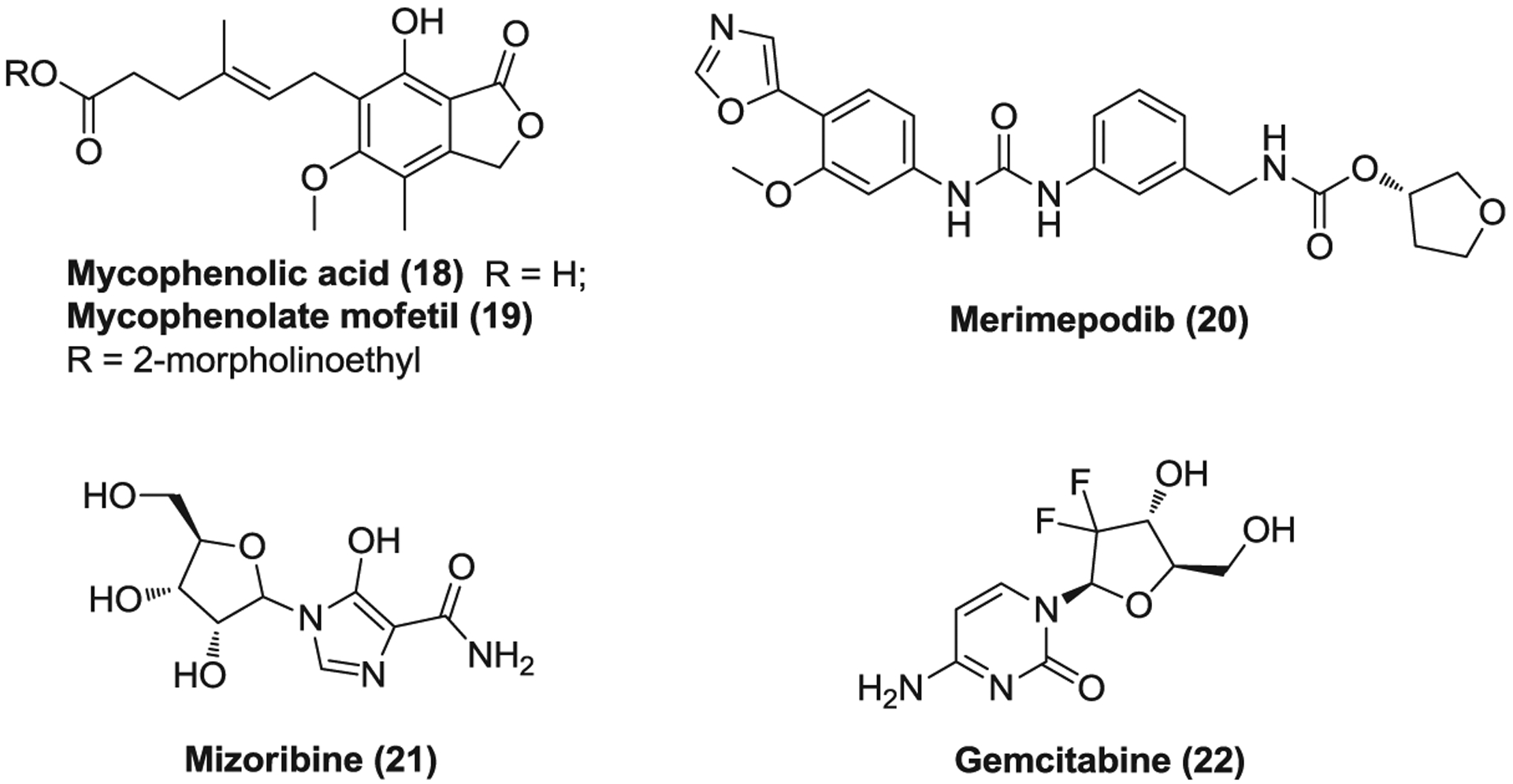
The inhibitors against the nucleic acid synthesis of coronaviruses
Merimepodib (20, VX-497) is another novel, specific, reverse, and noncompetitive IMPDH inhibitor which selectively suppresses lymphocyte proliferation and immunoglobulin production.166 Merimepodib possesses broad-spectrum antiviral activities,167–169 highly effective against HCV, hepatitis B viru (HBV), human cytomegalovirus (HCMV), encephalomyocarditis virus (EMCV), and RSV with EC50s ranging from 0.38 to 1.14 μM.167,170 A phase 2 clinical trial has been completed to evaluate its efficacy in combination with PEG-IFN-β2a and ribavirin for the treatment of chronic hepatitis C. Intriguingly, merimepodib inhibited SARS-CoV-2 replication in vitro in a dose-dependent manner in Vero cells, and pretreatment of merimepodib significantly reduced viral titers (over 1 log) at a concentration of 3.3 μM, offering the potential to treat COVID-19.171
Mizoribine (21, MZB) is an immunosuppressive drug of an imidazole nucleoside that has been used in renal transplantation, lupus nephritis, and rheumatoid arthritis (RA).172 MZB is a prodrug which is phosphorylated by adenosine kinase in cells into mizoribine 5′-monophosphate. This active monophosphate form blocks the de novo synthesis of GMP from IMP via inhibiting both IMPDH and GMP-synthetase.173 It arrests DNA synthesis in the S stage of the cell cycle without incorporation into nucleotides and suppresses lymphocyte proliferation.174 MZB inhibits SARS-CoV replication in a plaque assay with EC50 values of 3.5 μg/ml for strain Frankfurt-1 and 16 μg/ml for strain HKU39849. Meanwhile, it reduces the infectious SARS-CoV titers to one-tenth or less at the concentration of 10 μg/ml in a reduction assay.100
Gemcitabine (22, dFdC) is a cytosine arabinoside analogue which was used as a first-line treatment in various types of solid tumor such as pancreatic cancer and non-small-cell lung cancer.175 Gemcitabine is absorbed via nucleoside transporters and first phosphorylated intracellularly by deoxycytidine kinase to yield gemcitabine monophosphate (dFdCMP) as a rate-limiting step.176 The monophosphate form is then converted to active gemcitabine diphosphate (dFdCDP) and triphosphate (dFdCTP) catalyzed by other kinases. dFdCTP is a DNA polymerase inhibitor and can be incorporated into DNA, resulting in masked chain termination while dFdCDP inhibits ribonucleoside reductase and depletes the deoxyribonucleotide pools necessary for DNA synthesis, subsequently potentiating the effects of dFdCTP.177 Gemcitabine hydrochloride inhibits SARS-CoV, MERS-CoV, and SARS-CoV-2 replication with low toxicity and EC50s of 4.96, 1.22, and 1.24 μM, respectively.178,179 Notably, dFdCTP could also be incorporated into RNA.180 These findings together suggest gemcitabine has therapeutic potential to combat COVID-19.
3.3 |. Other virus-based inhibitors
Oseltamivir (23, Figure 7), brand name Tamiflu, is an orally administered antiviral medication that was used to treat and prevent influenza A and influenza B. It inhibits influenza’s neuraminidase enzyme with high selectivity and prevents the release of progeny virions from the infected host cells.181 Oseltamivir is administered in a prodrug form of oseltamivir phosphate that is quickly metabolized into the active oseltamivir carboxylate with high bioavailability.181 Oseltamivir can reduce the severity and duration of the symptoms of influenza and the risk of associated complications when administered within 48 h of the onset of infection.182–184 A case was reported that a 52-year-old woman with SARS-CoV-2 infection and a history of type 2 diabetes in Taiwan began to receive supportive therapy with oseltamivir and levofloxacin (a broad-spectrum antibiotic of fluoroquinolones) on Day 3 of hospitalization, and on Day 15 her vital signs were stable without oxygen therapy need.185 Despite the lack of in vitro and in vivo data, several clinical trials have been launched to evaluate the efficacy of oseltamivir as a monotherapy or in combination with other antivirals such as chloroquine and ASC09F for the treatment of COVID-19.
FIGURE 7.
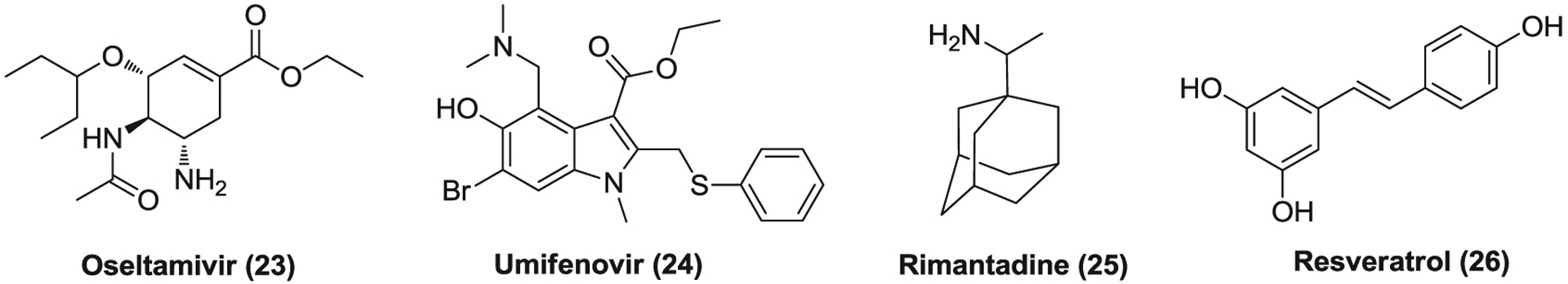
Other virus-based drugs effective against coronaviruses
Umifenovir (24), brand name Arbidol, is an antiviral drug, which was approved in Russia and China to treat influenza infections.186,187 It inhibits membrane fusion between virus and targets host cells, blocking viral entry.188 Arbidol is effective against a wide range of pH-dependent viruses such as EBOV, RSV, and HCV.189–191 It was reported that arbidol can efficiently inhibit SARS-CoV-2 replication at a concentration of 10–30 μM.192 Currently, arbidol is undergoing several clinical trials to evaluate its efficacy for COVID-19 treatment. A retrospective cohort study revealed that the combination group (n = 16) treated with arbidol and LPV/r showed more favorable clinical response compared to the control group (n = 17) with only LPV/r treatment.193 Another retrospective cohort analysis showed that patients with COVID-19 in the arbidol group (n = 16) had a short duration of positive RNA test in comparison with those in the LPV/r group (n = 34).194 In addition, as mentioned in favipiravir section (Section 3.2), in a randomized clinical trial, no significant difference was observed in clinical recovery rate of Day 7 between arbidol-treated group (62/120) and favipiravir-treated group (71/116).140 However, a retrospective study including 81 patients with mild COVID-19 indicated that treatment of arbidol (n = 45) did not improve the prognosis or accelerate the clearance of SARS-COV-2 compared to the control with standard care (n = 36).195
Rimantadine (25) is an orally available antiviral medication of a cyclic primary amine that is used to treat influenza A infection.196,197 It suppresses the activity of influenza A/M2 ion channel and blocks viral entry, resulting in the inhibition of viral replication.198–200 Rimantadine also has some NMDA antagonistic activities like amantadine, possessing therapeutic potential to treat Parkinson’s disease.201 Rimantadine inhibits SARS-CoV (HKU39849) replication (EC50 = 8–16 μg/ml) in Vero E6 cells,63 but has no documented in vitro activity against other CoVs.
Resveratrol (26) is a natural polyphenol whose food sources mainly include the skin of grapes, blueberries, raspberries, and mulberries.202 It is a phytoalexin produced by several plants in response to environmental stress such as injury and pathogen infections.202 Resveratrol displays various pharmacological and physiological properties including anticancer, anti-inflammation, antioxidant, antiviral, and so forth.203,204 Currently, numerous clinical trials have been conducted to evaluate its efficacy to treat different human conditions. Resveratrol was found to significantly inhibit MER-CoV infection, prolong cellular survival after virus infection, and decrease the expression of nucleocapsid protein.204 Recently, resveratrol was also reported to inhibit SARS-CoV-2 infection in Vero E6 cells with an EC50 of ~66 μM.205
4 |. HOST-BASED SMALL MOLECULE DRUGS FOR CORONAVIRUS
4.1 |. Protein synthesis inhibitors
Compound 27–29 (Figure 8) are protein synthesis inhibitors that target the eukaryotic ribosome. These compounds were found associated with mRNA and transfer RNA (tRNA) binding sits; anisomycin (27) and homoharringtonine (29) bind to A-site of the peptidyl transferase center while emetine (28) interacts with the ribosomal E-site.206–209 Anisomycin is an antibiotic isolated from cultures of various Streptomyces which prevents the release of nascent peptide from the polyribosome, without affecting the formation of aminoacyl transfer ribonucleic acid. Partial inhibition of DNA synthesis is also observed at the presence of anisomycin, likely due to the inhibitory effect on essential protein for DNA synthesis.210 At low concentration, anisomycin can activate p38-MAPK and c-Jun N-terminal kinase (JNK) signaling pathways.211 Anisomycin effectively inhibits SARS-CoV and MERS-CoV infection with EC50s of 0.191 and 0.003 μM, respectively.178
FIGURE 8.
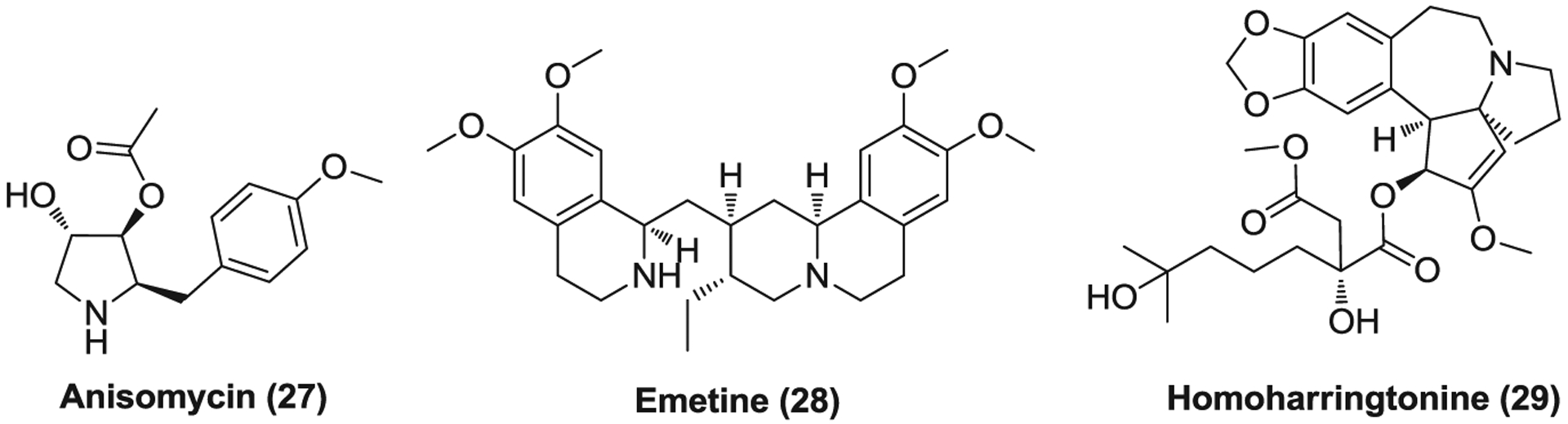
The inhibitors against protein synthesis of coronaviruses
Emetine is antiprotozoal drug of a natural alkaloid that is also used to induce vomiting.212 Emetine displays broad-spectrum antiviral activities, effective against Zika virus (ZIKV), EBOV, CoV, HIV-1, and so forth.213 Emetine significantly inhibits SARS-CoV, MERS-CoV, and SARS-CoV-2 infection with EC50s of 0.051, 0.014, and 0.46 μM, respectively.178,214 However, its potential cardiotoxicity may hamper its further clinical use in the treatment of CoV infections.215 Homoharringtonine is a natural plant alkaloid derived from Cephalotaxus fortunei which was approved by FDA to treat chronic myeloid leukemia (CML).216 It inhibits the first cycle of the elongation phase of eukaryotic translation via blocking aminoacyl-tRNA binding and peptide bond formation.217 Homoharringtonine, like other protein synthesis inhibitors, showed potency against CoVs as well, with EC50s of 0.072 μM for MERS-CoV and 2.55 μM for SARS-CoV-2.178,214
4.2 |. Drugs targeting host signaling pathways
4.2.1 |. Cyclophilin inhibitors
Cyclosporine (30, cyclosporin A, CsA, Figure 9) is a natural product used as an immunosuppressive drug to prevent rejection in organ transplants and treat various immune-related diseases.218 CsA first forms complex with cyclophilins of lymphocytes, especially of T cells, and then binds to calcineurin to inhibit its activity. Calcineurin is a calcium-calmodulin-activated serine/threonine-specific phosphatase which activates nuclear factor of activated T cells (NFAT) via dephosphorylation. Inhibition of calcineurin function blocks the translocation of NFAT from the cytosol into the nucleus, subsequently suppressing the transcription of genes for interleukin 2 (IL-2) and other related cytokines.219,220 SARS-CoV nsp1 and full replicating SARS-CoV were found to indirectly activate the calcineurin/NFAT pathway and enhance the induction of IL-2, which is likely to play an important role in virus replication. Nsp1 significantly increases the stimulatory effect of phorbol 12-myristate 13-acetate and ionomycin on NFAT activation, whereas CsA can block the increase of NFAT activity.221,222 CsA inhibits the replication of SARS-CoV (EC50 = 3.3 μM), HCoV-NL63 (EC50 = 2.3 μM), and HCoV-229E (EC50 = 2.3 μM), possibly acting on genome replication and/or transcription.221,223 In addition, CsA suppresses MERS-CoV-induced CPE in Vero cells at the concentration of 9 μM while treatment with a combination of CsA and IFN-α was more effective than either agent used alone against MERS-CoV replication.224 Recently, CsA was found to inhibit SARS-CoV-2 replication with an EC50 of 5.82 μM as well.70
FIGURE 9.
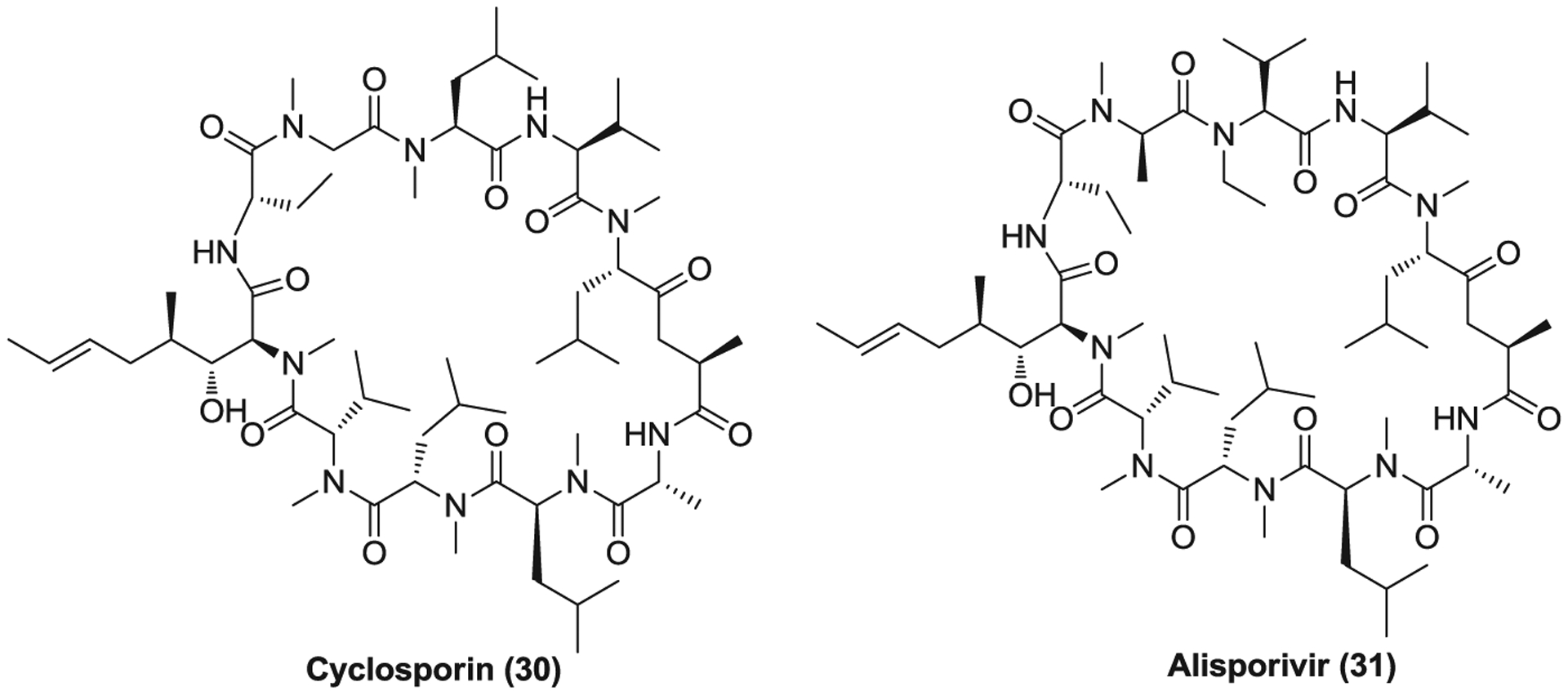
The cyclophilin inhibitors
Alisporivir (31, Debio 025) is a synthetic cyclophilin inhibitor with no immunosuppressive activity derived from the parent compound CsA.225 It has been widely investigated for its therapeutic potential to treat HCV infections.226,227 The structural changes of alisporivir, compared with CsA, enhanced the binding affinity with cyclophilins and abolished the binding of the formed alisporivir-cyclophilin complex to calcineurin, thus decreasing its immunosuppressive activity.228 Alisporivir inhibits the replication of SARS-CoV and MERS-CoV with low micromolar EC50 values (Table 2).229 Treatment with alisporivir plus ribavirin primarily showed an additive effect on in vitro antiviral activity; however, this combination treatment was not found to improve the outcome in a mouse model of SARS-CoV infection.229 Recently, alisporivir was found to inhibit SARS-CoV-2 replication in Vero E6 cells (EC50 = 0.46 μM), likely suppressing a postentry step of the SARS-CoV-2 life cycle.230 Considering the nonimmunosuppressive property of alisporivir and their similar EC50 values against CoVs, CsA and alisporivir may exert their anti-CoV activities via preventing those cyclophilin functions essential for viral replication, independent from the calcineurin/NFAT pathway.
TABLE 2.
The host-based small molecule drugs with therapeutic potentials for CoVs
| Antiviral agent | Drug class and/or targets | Activity against coronaviruses | Ref. |
|---|---|---|---|
| Anisomycin (27) | Protein synthesis inhibitor | SARS-CoV: EC50 = 0.191 μM (Vero E6/MA15) MERS-CoV: EC50 = 0.003 μM (Vero E6/Jordan N3) |
178 |
| Emetine hydrochloride (28) | Protein synthesis inhibitor | SARS-CoV: EC50 = 0.051 μM (Vero E6/MA15) MERS-CoV: EC50 = 0.014 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 0.46 μM, CC50 = 56.46 μM (Vero E6) |
178,214 |
| Homoharringtonine (29) | Protein synthesis inhibitor | MERS-CoV: EC50 = 0.072 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 2.55 μM, CC50 = 59.75 μM (Vero E6) |
178,214 |
| Cyclosporine (30) | Inhibitor of cyclophilins and their interaction with nsp1 | SARS-CoV: EC50 = 3.2 μM, CC50 > 17 μM (Vero E6/Frankfurt-1) MERS-CoV: suppressed induced cytopathic effect at 9 μM in Vero cells with no toxic effect SARS-CoV-2: EC50 = 5.8 μM, CC50 > 50 μM (Vero) |
70,221,224 |
| Alisporivir (31) | Cyclophilin inhibitor | SARS-CoV: EC50 = 8.3 μM, CC50 > 50 μM (Vero E6/Frankfurt-1); EC50 = 1.3 μM (Vero E6/MA15) MERS-CoV: EC50 = 3.6 μM, CC50 = 26.4 μM (Vero/EMC/2012); EC50 = 3.0 μM (Vero/Jordan N3) SARS-CoV-2: EC50 = 0.46 μM, CC50 > 20 μM (Vero E6) |
229,230 |
| Imatinib mesylate (32) | Abl kinase inhibitor | SARS-CoV: EC50 = 9.82 μM, CC50 > 100 μM (Vero E6/MA15) MERS-CoV: EC50 = 17.69 μM, CC50 > 100 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 3.24 μM (MOI = 0.004) and 5.32 μM (MOI = 0.01), CC50 > 30.86 μM (Vero E6) |
178,232 |
| Dasatinib (33) | Abl kinase/Src inhibitor | SARS-CoV: EC50 = 2.10 μM, CC50 > 10 μM (Vero E6/MA15) MERS-CoV: EC50 = 5.47 μM, CC50 > 10 μM (Vero E6/Jordan N3) |
178 |
| Saracatinib (34) | Abl kinase/Src inhibitor | MERS-CoV: EC50 = 2.9 μM, CC50 = 57 μM (Huh-7) | 241 |
| Rapamycin (35) | mTOR inhibitor | Inhibited MERS-CoV infection by ~60% at 10 μM via both pre- and postinfection treatment | 246 |
| Selumetinib (36) | MEK1/2 inhibitors | Inhibited MERS-CoV infection by ~70% at 1 μM via preinfection treatment and by ~90% at 10 μM via postinfection treatment | 246 |
| Trametinib (37) | MEK1/2 inhibitors | Inhibited MERS-CoV infection by ~90% at 0.1 μM via preinfection treatment and by ~70% at 1 μM via postinfection treatment | 246 |
| Baricitinib (38) | JAK and AAK1 inhibitor | Not reported | |
| Abemaciclib (41) | CDK4/6 inhibitor | SARS-CoV-2: EC50 = 6.62 μM, CC50 > 50 μM (Vero) | 70 |
| Gilteritinib (42) | FLT3 and AXL inhibitor | SARS-CoV-2: EC50 = 6.76 μM, CC50 = 37.16 μM (Vero) | 70 |
| K11777 (43) | Cathepsin inhibitor | SARS-CoV: IC50 < 0.05 μM, IC90 = 0.35 μM, CC50 > 105.6 μM (Vero 76/Urbani); IC50 < 0.05 μM, IC90 = 1.04 μM, CC50 = 85.2 μM (Vero 76/Toronto-2); its derivative SMDC256160 showed no in vivo efficacy in a mouse model of SARS-CoV infection | 255 |
| E-64-d (44) | Cathepsin inhibitor | SARS-CoV: EC50 = 0.76 μM (Vero E6/MA15) MERS-CoV: EC50 = 1.275 μM (Vero E6/Jordan N3) Inhibited SARS-CoV-2 entry |
38,178 |
| Camostat (46) | TMPRSS2 inhibitor | Reduced the infection of SARS-CoV, MERS-CoV, and SARS-CoV-2 in Calu-3 cells; showed in vivo efficacy in a mouse model of SARS-CoV infection (30 mg/kg, oral, BID, 9 days) | 41,50,53,255 |
| Nafamostat (47) | TMPRSS2 inhibitor | MERS-CoV: reduced the entry and replication at 0.1 μM in Calu-3 cells SARS-CoV-2: EC50 = 22.50 μM, CC50 > 100 μM (Vero E6) |
110,270 |
| Chloroquine hydrochloride (48) | Antiparasitic/inhibit host receptor glycosylation and endosomal acidification | SARS-CoV: EC50 = 6.54 μM (Vero E6/MA15); EC50 = 4.0 μM, CC50 > 128 μM (Vero E6/Frankfurt-1) MERS-CoV: EC50 = 6.28 μM (Vero E6/Jordan N3); EC50 = 3.0 μM, CC50 = 58.1 μM (Huh-7/EMC/2012) SARS-CoV-2: EC50 = 1.13 μM, CC50 > 100 μM (Vero E6) |
62,110,178 |
| Hydroxychloroquine sulfate (49) | SARS-CoV: EC50 = 6.54 μM (Vero E6/MA15) MERS-CoV: EC50 = 6.28 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 4.51 μM (MOI = 0.01) and 12.96 μM (MOI = 0.8), CC50 = 249.5 μM (Vero E6); EC50 at 48 h = 0.72 μM (MOI = 0.01) in Vero cells |
178,281,282 | |
| Mefloquine (50) | SARS-CoV: EC50 = 15.55 μM (Vero E6/MA15) MERS-CoV: EC50 = 7.42 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 4.33 μM, CC50 = 13.97 μM (Vero) |
70,178 | |
| Amodiaquine (51) | SARS-CoV: EC50 = 1.27 μM (Vero E6/MA15) MERS-CoV: EC50 = 6.21 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 5.15 μM, CC50 > 50 μM (Vero) |
70,178 | |
| Chlorpromazine hydrochloride (52) | Antipsychotic or antihistamine or antiemetic/phenothiazine/inhibit clathrin-mediated endocytosis | SARS-CoV: EC50 = 12.97 μM, CC50 > 100 μM (Vero E6/MA15); EC50 = 8.8 μM, CC50 = 24.3 μM (Vero E6/Frankfurt-1) MERS-CoV: EC50 = 9.51 μM (Vero E6/Jordan N3); EC50 = 4.9 μM, CC50 = 21.3 μM (Huh-7/EMC/2012) SARS-CoV-2: EC50 = 3.14 μM (MOI = 0.004) and 4.03 μM (MOI = 0.01), CC50 = 11.88 μM (Vero E6) |
62,178,232 |
| Triflupromazine hydrochloride (53) | SARS-CoV: EC50 = 6.40 μM, CC50 > 10 μM (Vero E6/MA15) MERS-CoV: EC50 = 5.76 μM (Vero E6/Jordan N3) |
178 | |
| Fluphenazine hydrochloride (54) | SARS-CoV: EC50 = 21.43 μM (Vero E6/MA15) MERS-CoV: EC50 = 5.87 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 6.36 μM (MOI = 0.004) and 8.98 μM (MOI = 0.01), CC50 = 20.02 μM (Vero E6) |
178,232 | |
| Thiothixene (55) | SARS-CoV: EC50 = 5.32 μM (Vero E6/MA15) MERS-CoV: EC50 = 9.30 μM (Vero E6/Jordan N3) |
178 | |
| Promethazine hydrochloride (56) | SARS-CoV: EC50 = 7.55 μM (Vero E6/MA15) MERS-CoV: EC50 = 11.80 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 9.21 μM (MOI = 0.004) and 10.44 μM (MOI = 0.01), CC50 = 42.59 μM (Vero E6) |
178,232 | |
| Thiethylperazine maleate (57) | MERS-CoV: EC50 = 7.87 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 7.09 μM (MOI = 0.004) and 8.02 μM (MOI = 0.01), CC50 = 18.37 μM (Vero E6) |
178,232 | |
| Ouabain (58) | Cardiotonic steroids/target ATP1A1 and inhibit CoV entry | Inhibited MERS-CoV infection at 50 nM via preinfection SARS-CoV-2: EC50 < 0.097 μM, CC50 > 50 μM (Vero) |
70,302 |
| Bufalin (59) | Inhibited MERS-CoV infection at 10–15 nM via preinfection | 302 | |
| Proscillaridin (60) | SARS-CoV-2: EC50 = 2.04 μM, CC50 > 50 μM (Vero) | 70 | |
| Digoxin (61) | SARS-CoV-2: EC50 = 0.19 μM, CC50 > 50 μM (Vero) | 70 | |
| Digitoxin (62) | SARS-CoV-2: EC50 = 0.23 μM, CC50 > 50 μM (Vero) | 70 | |
| Clomipramine hydrochloride (63) | Tricyclic antidepressant/inhibit clathrin-dependent entry | SARS-CoV: EC50 = 13.24 μM (Vero E6/MA15) MERS-CoV: EC50 = 9.33 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 5.63 μM (MOI = 0.004) and 7.59 μM (MOI = 0.01), CC50 > 29.68 μM (Vero E6) |
178,232 |
| Tamoxifen citrate (64) | Estrogen receptor modulator/inhibit CoV entry | SARS-CoV: EC50 = 92.89 μM (Vero E6/MA15) MERS-CoV: EC50 = 10.12 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 34.12 μM (MOI = 0.004) and 8.98 μM (MOI = 0.01), CC50 = 37.96 μM (Vero E6) |
178,232 |
| Toremifene citrate (65) | SARS-CoV: EC50 = 11.97 μM, CC50 > 100 μM (Vero E6/MA15) MERS-CoV: EC50 = 12.92 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 4.77 μM (MOI = 0.004) and 11.30 μM (MOI = 0.01), CC50 = 20.51 μM (Vero E6) |
178,232 | |
| Astemizole (66) | Antihistamine and anticholinergic/inhibit CoV entry | SARS-CoV: EC50 = 5.59 μM (Vero E6/MA15) MERS-CoV: EC50 = 4.88 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = ~1.1 μM (Vero E6) |
178,304 |
| Chlorphenoxamine (67) | SARS-CoV: EC50 = 20.03 μM (Vero E6/MA15) MERS-CoV: EC50 = 12.65 μM (Vero E6/Jordan N3) |
178 | |
| Niclosamide (68) | Anthelmintic drug/broad antiviral agent | SARS-CoV: EC50 < 0.1 μM, CC50 = 22.1 μM (Vero E6) SARS-CoV-2: EC50 = 0.28 μM, CC50 > 50 μM (Vero) Suppressed MERS-CoV infection by ~1000-fold at 48 h at 10 μM |
70,308,309 |
| Nitazoxanide (69) | Broad antiparasitic and antiviral drug/induce the host innate immune response | SARS-CoV-2: EC50 = 2.12 μM, CC50 = 35.53 μM (Vero E6) | 110,312 |
| Hexachlorophene (71) | Disinfectant | SARS-CoV-2: EC50 = 0.90 μM, CC50 = 19.3 μM (Vero) | 70 |
| Tilorone (72) | Antiviral drug/interferon inducer | MERS-CoV: EC50 = 10.56, CC50 > 20 μM (Vero E6) SARS-CoV-2: EC50 = 4.09 μM, CC50 = 19.67 μM (Vero) |
70,322 |
| Terconazole (73) | Antifungal drug | SARS-CoV: EC50 = 15.33 μM (Vero E6/MA15) MERS-CoV: EC50 = 12.20 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 11.92 μM (MOI = 0.004) and 16.14 μM (MOI = 0.01), CC50 = 41.46 μM (Vero E6) |
178,232 |
| Azithromycin (74) | Antibiotic/macrolide | Not reported | |
| Salinomycin sodium (75) | Polyether ionophore antibiotic | MERS-CoV: EC50 = 5.49, CC50 = 3.84 μM (Vero E6) SARS-CoV-2: EC50 = 0.24 μM, CC50 > 50 μM (Vero) |
70,322 |
| Ivermectin (76) | Antiparasitic/broad antiviral agent | SARS-CoV-2: EC50 = ~2.0 μM (Vero hSLAM/Australia/VIC01/2020) | 336 |
| Anidulafungin (77) | Antifungal/semisynthetic echinocandin | SARS-CoV-2: EC50 = 4.64 μM, CC50 > 50 μM (Vero) | 70 |
| Benztropine (78) | Anticholinergic | SARS-CoV: EC50 = 21.61 μM (Vero E6/MA15) MERS-CoV: EC50 = 16.63 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 13.8 μM (MOI = 0.004) and 17.79 μM (MOI = 0.01), CC50 » 50 μM (Vero E6) |
178,232 |
| Fluspirilene (79) | Antipsychotic (diphenylbutylpiperidine) | SARS-CoV: EC50 = 5.96 μM (Vero E6/MA15) MERS-CoV: EC50 = 7.48 μM (Vero E6/Jordan N3) SARS-CoV-2: EC50 = 3.16 μM (MOI = 0.004) and 5.32 μM (MOI = 0.01), CC50 = 30.33 μM (Vero E6) |
178,232 |
| Bazedoxifene (80) | Estrogen receptor modulator | SARS-CoV-2: EC50 = 3.44 μM, CC50 = 14.97 μM (Vero) | 70 |
| Loperamide (81) | Antidiarrheals/μ-opioid receptor agonist | SARS-CoV: EC50 = 5.9 μM, CC50 = 53.8 μM (Vero E6/Frankfurt-1) MERS-CoV: EC50 = 4.8 μM, CC50 = 15.5 μM (Huh-7/EMC/2012) SARS-CoV-2: EC50 = 9.27 μM, CC50 = 29.26 μM (Vero) |
62,70 |
| Cepharanthine (82) | Bis-benzylisoquinoline alkaloids/anti-inflammatory | SARS-CoV-2: EC50 = 4.47 μM, CC50 > 50 μM (Vero) | 70 |
| Berbamine (83) | MERS-CoV: EC50 = 13.14, CC50 > 20 μM (Vero E6) SARS-CoV-2: EC50 = 7.87 μM, CC50 > 50 μM (Vero) |
70,322 | |
| Tetrandrine (84) | MERS-CoV: EC50 = 12.68, CC50 > 20 μM (Vero E6) SARS-CoV-2: EC50 = 3.00 μM, CC50 = 14.92 μM (Vero) |
70,322 | |
| Reserpine (85) | Antihypertensive | SARS-CoV: EC50 = 3.4 μM, CC50 = 25 μM (Vero E6/H.K. strain) | 348 |
| Ivacaftor (86) | Potentiator of CFTR/treat cystic fibrosis | SARS-CoV-2: EC50 = 6.57 μM, CC50 = 12.47 μM (Vero) | 70 |
| ESI-09 (87) | EPAC inhibitor | Resulted in about 2log and 4log reduction in MERS-CoV and SARS-CoV titer in Vero E6 cells, respectively, at 10 μM | 350 |
| Eltrombopag (88) | Thrombopoietin (c-mpl) receptor agonist | SARS-CoV-2: EC50 = 8.27 μM, CC50 > 50 μM (Vero) | 70 |
| Hydroxyprogesterone caproate (89) | Progesterone receptor agonist | SARS-CoV-2: EC50 = 6.30 μM, CC50 > 50 μM (Vero) | 70 |
| Ciclesonide (90) | Corticosteroid/anti-inflammatory/target nsp15 | SARS-CoV-2: EC50 = 4.33 μM, CC50 > 50 μM (Vero) | 70 |
Abbreviations: AAK1, AP2-associated protein kinase 1; CC50, cytotoxic concentration 50%; EC50, half-maximal effective concentration; EPAC, exchange protein directly activated by cAMP; JAK, Janus kinase; MERS-CoV, Middle East respiratory syndrome coronavirus; MOI, multiplicity of infection; mTOR, mammalian target of rapamycin; SARS-CoV, severe acute respiratory syndrome coronavirus; TMPRSS2, transmembrane protease serine 2.
4.2.2 |. Inhibitors targeting kinase signaling pathways
Imatinib (32, Figure 10) is a small-molecule Abl kinase inhibitor which is highly effective to treat early-phase CML.231 Imatinib mesylate inhibits the replication of SARS-CoV, MERS-CoV, and SARS-CoV-2 with EC50s of 9.82, 17.69, and 5.32 μM, respectively.178,232 Imatinib was found to target Abelson tyrosine-protein kinase 2 (Abl2) that was required for efficient SARS-CoV and MERS-CoV replication. Imatinib specifically blocks viral fusion with the endosomal membrane and cell-cell fusion via inhibiting Abl kinase activity.233,234 Currently, several clinical trials are ongoing to evaluate the efficacy of imatinib in COVID-19 treatment. Dasatinib (33) and saracatinib (34, AZD-0530) are dual Abl kinase and Src inhibitors.235,236 Dasatinib was approved to treat CML and acute lymphoblastic leukemia237 while saracatinib, due to the insufficient efficacy in cancer patients, has subsequently been investigated to treat other human conditions such as Parkinson’s disease and pulmonary fibrosis.238–240 Dasatinib was also found to be effective against SARS-CoV (EC50 = 2.10 μM) and MERS-CoV (EC50 = 5.47 μM).178 Saracatinib significantly inhibits MERS-CoV, HCoV-229E, and HCoV-OC43 at the early stages of the viral life cycle in Huh-7 cells with EC50s of 2.9, 2.4, and 5.1 μM, respectively.241 Moreover, treatment of saracatinib in combination with gemcitabine exhibited a synergistic inhibitory effect against MERS-CoV infection with minimal cytotoxic effect in Huh-7 cells. It was demonstrated that multiple Src kinases, often together with Abl kinase, play an important role in the life cycle of various viruses.242–245 Knockdown of Src kinases, Fyn or Lyn, led to an obvious reduction in MERS-CoV titers.241 Thus, these two dual inhibitors, dasatinib and saracatinib, may exert their anti-CoV activities via inhibition of both multiple members of Src family and Abl kinase.
FIGURE 10.
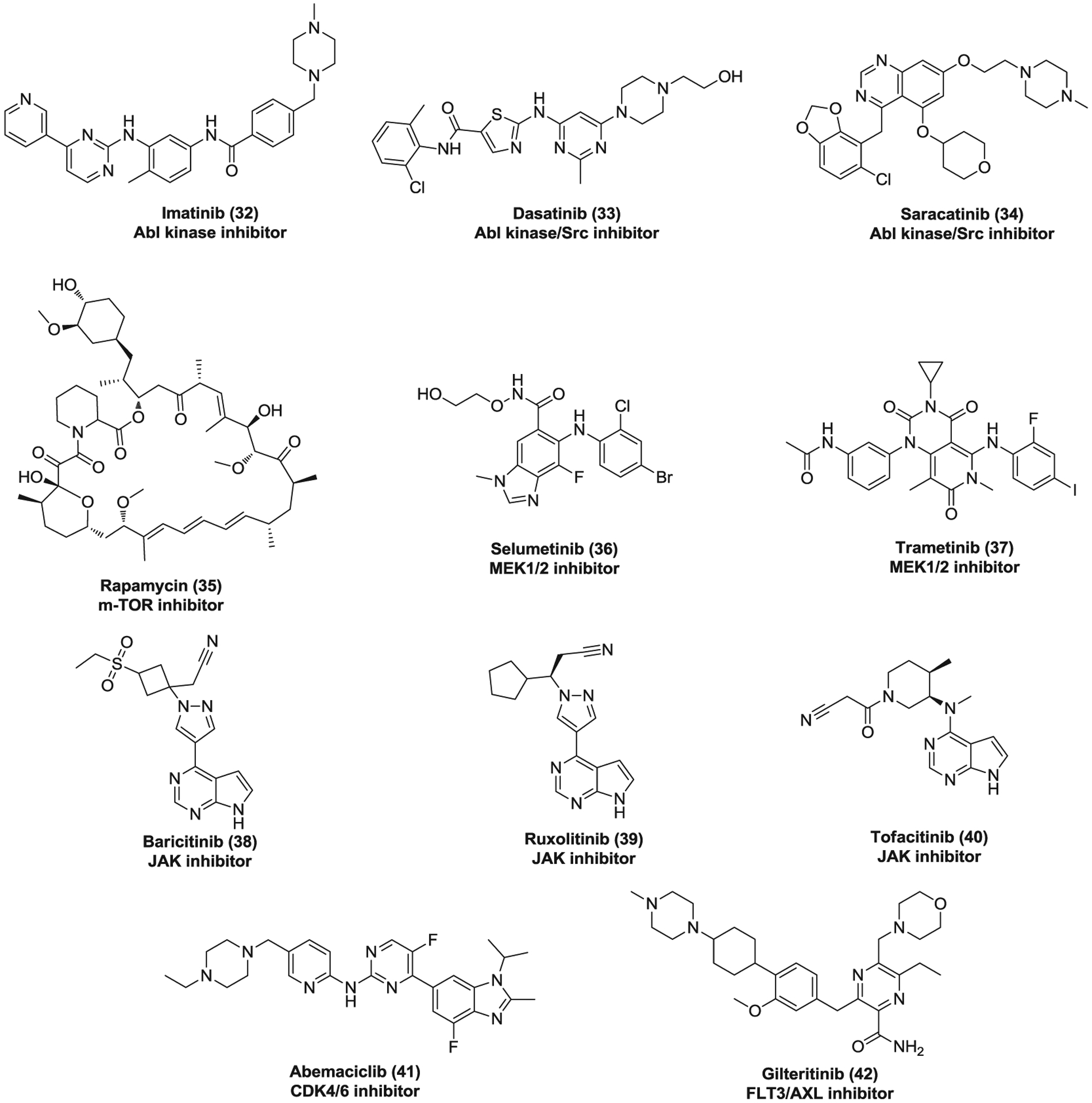
The inhibitors targeting kinase signaling pathways against coronaviruses
A kinome analysis of human hepatocytes infected with MERS-CoV suggested that extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) and phosphoinositol 3-kinase (PI3K)/serine-threonine kinase (AKT)/mammalian target of rapamycin (mTOR) signaling responses are selectively modulated in the host, which are essential for MERS-CoV infection.246 Rapamycin (35, Sirolimus), an mTOR inhibitor, suppressed MERS-CoV infection via both pre- and postinfection treatment with inhibition of ~60% at 10 μM.246 Selumetinib (36) and trametinib (37) are MEK1/2 inhibitors which affect the ERK/MAPK pathway by inhibiting the activity of MAPK kinase.247 Selumetinib showed potent antiviral activity against MERS-CoV with inhibition of ~70% at 1 μM when added before infection.246 Pretreatment with trametinib significantly inhibited MERS-CoV replication with a percentage of ~90% at 0.1 μM. More importantly, trametinib also showed potency against MERS-CoV with inhibition of ~70% at 1 μM via postinfection treatment, indicating its great therapeutic potential for MERS.246 These data together provide strong evidence for critical roles of mTOR and MEK1/2 in MERS-CoV infection.
Cytokine storm (CS) involves excessive and uncontrolled release of inflammatory cytokines which is comparatively common in severe cases of COVID-19.248,249 It has become a major cause of lung damage and often leads to the aggravation, even mortality. Thus, combining antiviral and anti-inflammatory treatments may help to prevent further injury.250 Compounds 38–40 are Janus kinase (JAK) inhibitors that can suppress JAK-mediated cytokine release.251 Clinical trials have been initiated to evaluate the safety and efficacy of these JAK inhibitors combined with antivirals in COVID-19. Among them, baricitinib (38) is particularly attractive. Besides its anti-inflammatory property, baricitinib binds with high affinity and inhibits the AP2-associated protein kinase 1 (AAK1) which is an important modulator of clathrin-mediated endocytosis for SARS-CoV-2.250 Moreover, the plasma concentration of baricitinib is sufficient to inhibit AAK1 when dosing 2 or 4 mg once daily, indicating its therapeutic potential for COVID-19.252 However, it is worth noting that anti-inflammatory therapy may delay the clearance of virus and increase the chance of secondary infection. Besides, JAK inhibitors also inhibits IFN-α production which is important in eliminating virus. Thus, it remains a critical concern as how to balance the risk and benefit ratio of anti-inflammatory therapy for COVID-19.
Abemaciclib (41) is a selective CDK4/6 inhibitor used to treat advanced or metastatic breast cancers253 while gilteritinib (42) is a dual FLT3 and AXL inhibitor which was approved by FDA for treatment of patients with relapsed or refractory acute myeloid leukemia.254 These two drugs were found to inhibit SARS-CoV-2 replication with a similar EC50 value of ~6.7 μM,70 although their exact mechanisms of antiviral action require further investigations.
4.3 |. Inhibitors targeting host proteases
4.3.1 |. The endosomal protease inhibitors
The pH-sensitive endosomal proteases cathepsins involve endosomal viral entry and activate CoV membrane fusion via proteolysis of viral S glycoprotein following receptor binding and induced conformational changes in S glycoprotein.48,49 This proteolytic activation can be blocked by cathepsin inhibitors such as K11777 (43, Figure 11) and E-64-d (44, EST).38,255,256 K11777 is an irreversible cysteine protease inhibitor and potently inhibits SARS-CoV replication with IC50 of <0.05 μM for strains Urbani and Toronto-2 in Vero 76 cells in a CPE assay. Meanwhile, it displays low IC90s of 0.35 and 1.04 μM against strains Urbani and Toronto-2, respectively, in a virus reduction assay.255 K11777 also possesses acceptable safety and PK profiles in rodent, dogs, and primates, offering the potential to treat CoV infections including COVID-19.257
FIGURE 11.
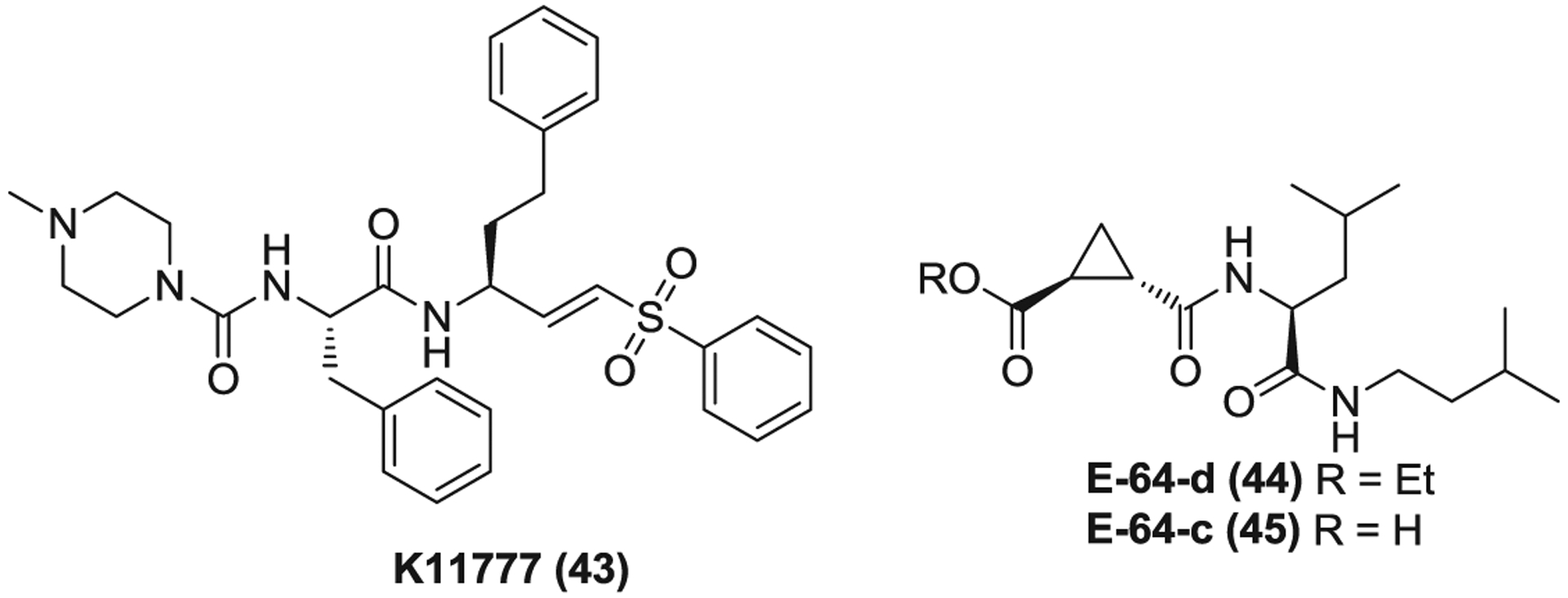
The endosomal protease (cathepsin) inhibitors
E-64-d is an ester prodrug of an epoxide which is rapidly hydrolyzed in the gut to afford the acid form E-64-c (45).258 This series of compounds inhibit cysteine proteases by covalently modifying the cysteine residue of its active site.259 E-64-d was originally developed to treat muscular dystrophy in the 1980s and failed in phase 3 clinical trials due to the lack of sufficient efficacy.260 However, these trials have established its safety and PK profiles.261–263 E-64-d significantly inhibits SARS-CoV and MERS-CoV replication with EC50s of 0.76 and 1.28 μM, respectively. Recently, E-64-d was also reported to inhibit SARS-CoV-2 entry via blocking cathepsin L-mediated S glycoprotein activation.38 These data together suggest cathepsin inhibitor, E-64-d is also a promising candidate to be developed for COVID-19 treatment.
4.3.2 |. The surface protease inhibitors
The surface serine protease TMPRSS2 mediates cell surface nonendosomal virus entry at the plasma membrane via cleavages and activation of the S protein.51,52,264 Camostat (46, Figure 12) is a serine protease inhibitor which was approved in Japan to treat chronic pancreatitis.265,266 Camostat, as a TMPRSS2 inhibitor, partially blocks the entry of SARS-CoV, MERS-CoV, and SARS-CoV-2 into TMPRSS2-expressing cells while simultaneous treatment of camostat and E-64-d can completely inhibit viral entry, mainly due to the dual blockade of the cell surface and endosomal entry pathways.41,50,53 Camostat also completely blocks syncytium formation and reduces the infection of SARS-CoV, MERS-CoV, and SARS-CoV-2 in the lung cell line Calu-3.41,50,53 In addition, camostat treatment effectively protected mice against death induced by SARS-CoV infection when dosed orally at 30 mg/kg twice daily for 9 days.255
FIGURE 12.
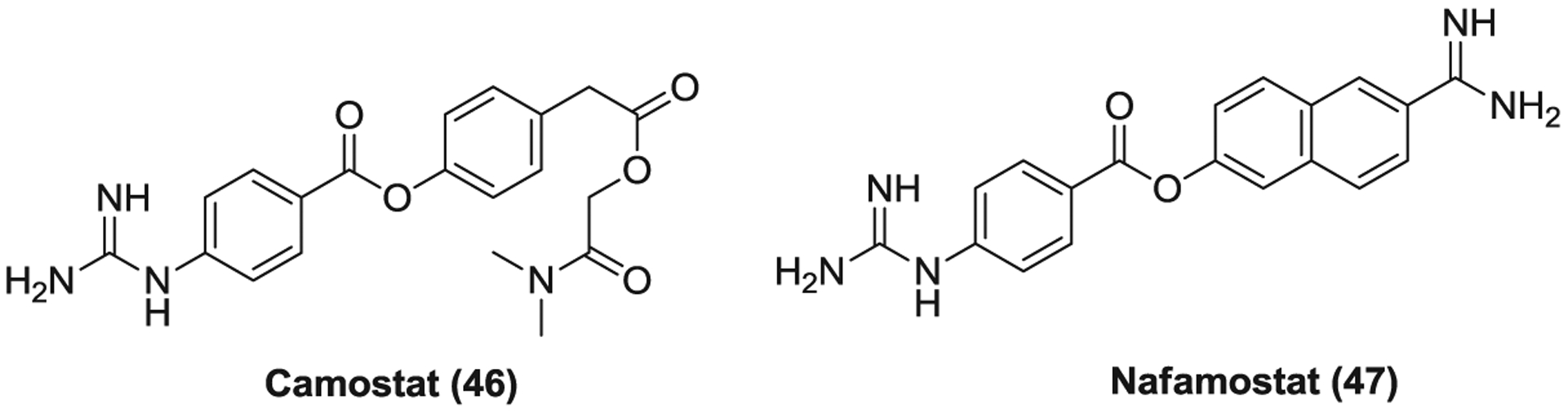
The surface protease (TMPRSS2) inhibitors. TMPRSS2, transmembrane protease serine 2
Nafamostat (47) is a broad-spectrum serine protease inhibitor that is mainly used to treat pancreatitis and disseminated intravascular coagulation.267,268 Nafamostat was screened out as a potent inhibitor of S-mediated membrane fusion, possibly through the inhibition of TMPRSS2 like camostat.269 Nafamostat is more effective than camostat in reducing the entry and replication of MERS-CoV in Calu-3 cells.270 It was also found to inhibit SARS-CoV-2 replication with an EC50 of 22.5 μM.110 Currently, several clinical trials are underway to evaluate the efficacy of both camostat and nafamostat as a monotherapy or combined with hydroxychloroquine in COVID-19 treatment. Three cases were reported that treatment with nafamostat improved COVID-19 associated pneumonia of elderly patients who were receiving antiviral drugs and supplementary oxygen therapy.271 While accumulated studies showed the TMPRSS2-mediated cell surface route is essential for viral entry into primary target cells and viral spread in the infected host,41,53,255,272 it was also found that simultaneous treatment with camostat and E-64-d displays more potent inhibitory activity against SARS-CoV infection in Calu-3 cells compared to either agent used alone.53 Thus, combination use of cathepsin and TMPRSS2 inhibitors may be a promising strategy to efficiently block CoV infections.
4.4 |. Inhibitors targeting CoV entry into host cell
4.4.1 |. The quinoline derivatives targeting endosomal acidification
Chloroquine (48, Figure 13) is a synthetic medication of a 4-aminoquinoline derivative which was discovered in 1934 and specifically used as an antimalarial agent.273 Besides its antimalarial effect, chloroquine was subsequently found to possess anti-inflammatory and immunomodulatory properties which have encouraged its new uses to treat autoimmune diseases such as RA and systemic lupus erythematosus (SLE).274 In addition, chloroquine is also effective against a series of pH-dependent viruses, including HIV, dengue virus (DENV), HCV, influenza A virus, EBOV, CoV, and so forth.275 It was reported to block viral entry mainly by suppressing glycosylation of host receptors and endosomal acidification.274–276 Due to its anti-inflammatory property, chloroquine inhibits the production of various proinflammatory cytokines and the activation of macrophages induced by viral infection and may improve the clinical symptoms of infected patients.273,275 Chloroquine inhibits the replication of SARS-CoV, MERS-CoV and SARS-CoV-2 with EC50s of 6.54, 6.28, and 1.13 μM, respectively.110,178 Different research groups have reported that chloroquine functions at both entry and postentry stages of SARS-CoV and SARS-CoV-2 infections110,276,277 whereas it inhibits an early step in the replicative cycle of MERS-CoV.62 However, chloroquine was ineffective to reduce lung virus titers when administered by the intraperitoneal (IP) route in a mouse model of SARS-CoV infection, likely due to the insufficient blockade of viral entry pathways.278
FIGURE 13.
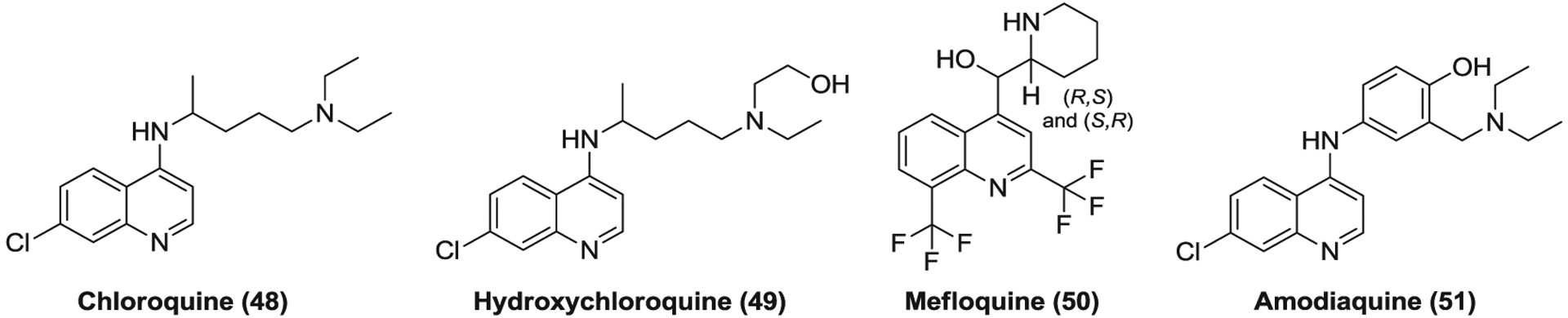
The quinoline derivatives targeting endosomal acidification
Hydroxychloroquine (49) is a chloroquine derivative which shares high similarities with chloroquine in chemical structure, mechanisms of action, and therapeutic applications.274 Hydroxychloroquine has an N-hydroxyethyl side chain and thus less tissue accumulation and toxicity than chloroquine.279,280 As expected, hydroxychloroquine displays antiviral activities against SARS-CoV, MERS-CoV, and SARS-CoV-2 with similar EC50 values to those of chloroquine.178,281,282 Considering its in vitro anti-CoV and anti-inflammatory properties, established clinical safety and PK profiles, and the low cost, chloroquine, and hydroxychloroquine have been fast advanced into numerous clinical trials to evaluate their efficacy in COVID-19 treatment.
According to the early clinical results from more than 100 inpatients with COVID-19 in China, chloroquine phosphate treatment might be associated with improved radiological findings, enhanced viral elimination and delayed disease progression.283 Two French studies reported that hydroxychloroquine could reduce viral load in patients with COVID-19, especially used in combination with azithromycin (an antibiotic of a macrolide used to treat diverse bacterial infections, see Section 4.5, 75).284,285 However, the result from another study revealed that no clinical benefit was observed for combination treatment of hydroxychloroquine and azithromycin in critically ill patients with COVID-19.286 In addition, a randomized parallel-group study enrolling 62 patients with mild COVID-19 indicated that hydroxychloroquine treatment was associated with shortened clinical recovery time (temperature and cough) and improved pneumonia compared to placebo.287 In a multicenter retrospective observational study involving 2541 patients with COVID-19, treatment with hydroxychloroquine alone (162/1202, 12.5%) and in combination with azithromycin (157/783, 20.1%) was associated with reduction in in-hospital mortality while groups treating with azithromycin alone or neither drug showed death rates of 22.4% (33/147) and 26.4% (108/409), respectively.288 However, these clinical data on chloroquine and hydroxychloroquine are far from convincing and several limitations exist such as a small cohort, absence of randomization, and no control arm.289,290 Very recently, a retrospective multicenter cohort study involving 1438 patients revealed that treatment with hydroxychloroquine (54/271), azithromycin (21/211), or both (189/735), compared with neither treatment (28/221), was not associated with significant differences in in-hospital fatality.291 Another randomized clinical trial indicated that the group (n = 1542) treating with hydroxychloroquine showed no statistically significant difference in 28-day mortality (26.8% vs. 25.0%) compared to the control receiving usual care (n = 3132).292 Moreover, although chloroquine and hydroxychloroquine were demonstrated to be relatively well tolerated in patients with malaria and SLE, they can cause QTc prolongation and arrhythmia, with an increased risk especially used in combination with other medications such as azithromycin known to prolong QT interval.274,293
Mefloquine (50) and amodiaquine (51) are antimalarial medications of the quinoline class that exhibit similar anti-CoV activities to chloroquine. Mefloquine displays EC50s of 15.5, 7.42, and 4.33 μM for SARS-CoV, MERS-CoV, and SARS-CoV-2, respectively, while amodiaquine effectively inhibits the replication of these three CoVs with EC50 values of 1.27–6.21 μM.70,178 Like chloroquine, amodiaquine failed to reduce lung virus titers via IP administration in a SARS-CoV-infected mouse model.278
4.4.2 |. The phenothiazine derivatives targeting clathrin-mediated endocytosis
Compounds 52–57 (Figure 14) are phenothiazine derivatives which act as antagonists on different postsynaptic and presynaptic receptors such as dopamine receptors, serotonin receptors and histamine receptors. Compounds 52–55 are antipsychotic medications while promethazine (56) and thiethylperazine (57) are used as an antihistamine and an antiemetic, respectively. These medications were found to inhibit SARS-CoV, MERS-CoV, and SARS-CoV-2 replication with EC50 values ranging from 4.03 to 21.4 μM.178,232 They block clathrin-dependent entry (IC50 = 3.23–7.48 μM) via preventing the assembly of clathrin-coated pits at the plasma membrane.294–296 In addition, chlorpromazine (52) was reported to suppress MERS-CoV replication at both an early and a postentry stage, suggesting that it has other antiviral mechanism beside blocking clathrin-mediated endocytosis.62 Chlorpromazine was also reported to block the entry of HCV,297 alphaviruses,298 infectious bronchitis virus,299 and mouse hepatitis virus type 2 (MHV-2) by targeting clathrin-mediated endocytosis.300 Based on its in vitro anti-SARS-CoV-2 activity (EC50 = 4.03 μM),232 two clinical trials have been initiated to evaluate the efficacy of chlorpromazine in COVID-19 treatment.
FIGURE 14.
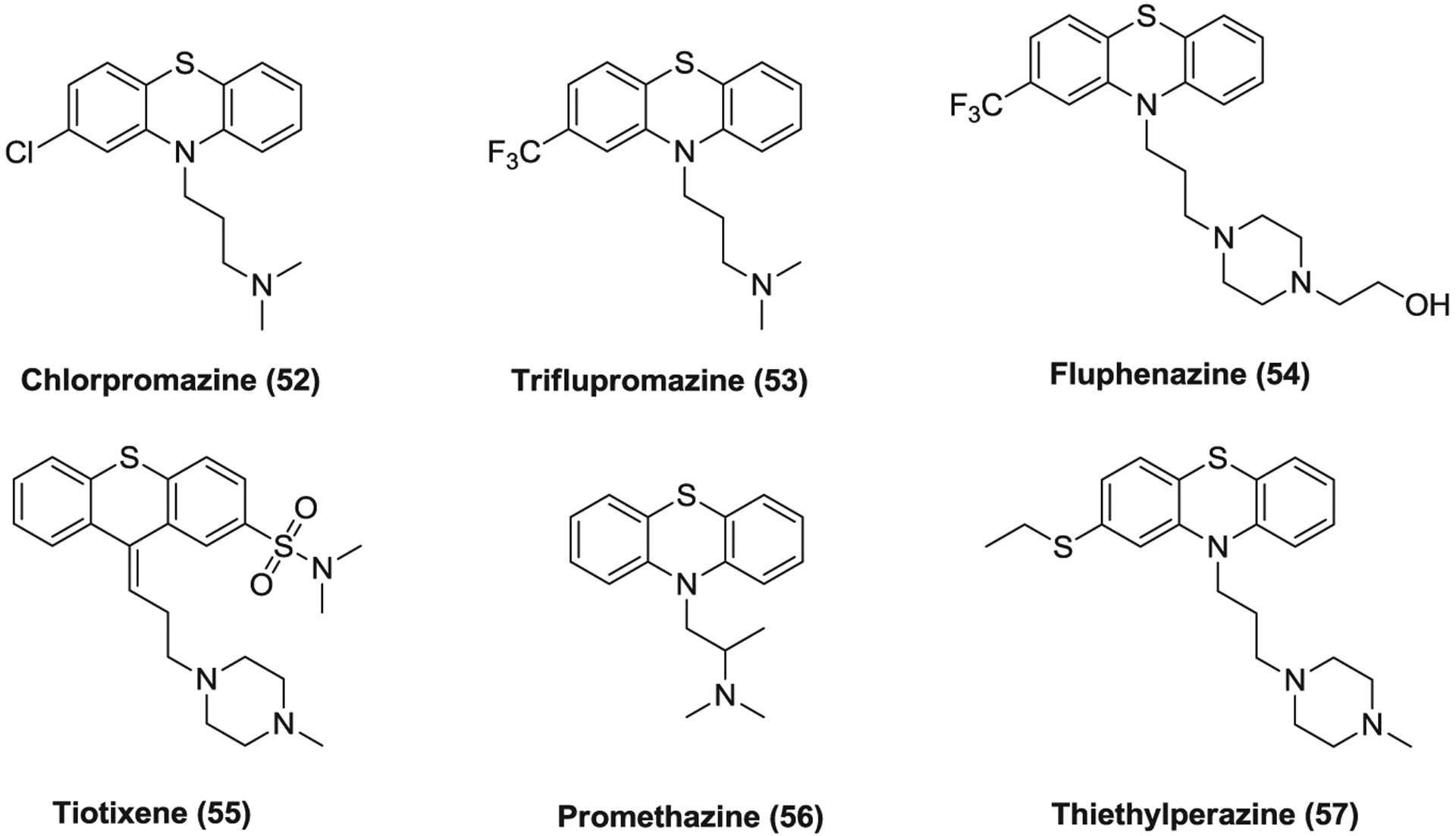
The phenothiazine derivatives targeting clathrin-mediated endocytosis
4.4.3 |. The cardiotonic steroids that inhibit CoV entry into host cell via targeting ATP1A1-mediated Src signaling
Compounds 58–61 (Figure 15) are medications of cardiotonic steroids that can increase the force of myocardium contraction and cardiac output by inhibiting the Na/K-ATPase, also known as the sodium-potassium ion pump.301 The ATP1A1 α subunit was found to be critical for CoV infection, and cardiotonic steroids, ouabain (58) and bufalin (59), inhibit CoV infection at low concentrations by targeting ATP1A1 without affecting the transport function of Na+/K+-ATPase.302 However, these antiviral effects can be relieved by different Src kinase inhibitors, indicating the crucial role of ATP1A1-mediated Src signaling in the inhibition of CoV infection. Ouabain blocks viral entry at an early stage before the formation of early endosomes, but it remains to be elucidated how ATP1A1-mediated Src signaling could affect clathrin-mediated entry.302 Ouabain significantly inhibit SARS-CoV-2 infection (EC50 < 0.097 μM) while proscillaridin (60), digoxin (61), and digitoxin (62) show potency against SARS-CoV-2 with EC50s of 2.01, 0.19, and 0.23 μM, respectively.70 However, the potential cardiotoxicity of cardiotonic steroids may hamper their further clinical use in patients with CoV infections.
FIGURE 15.
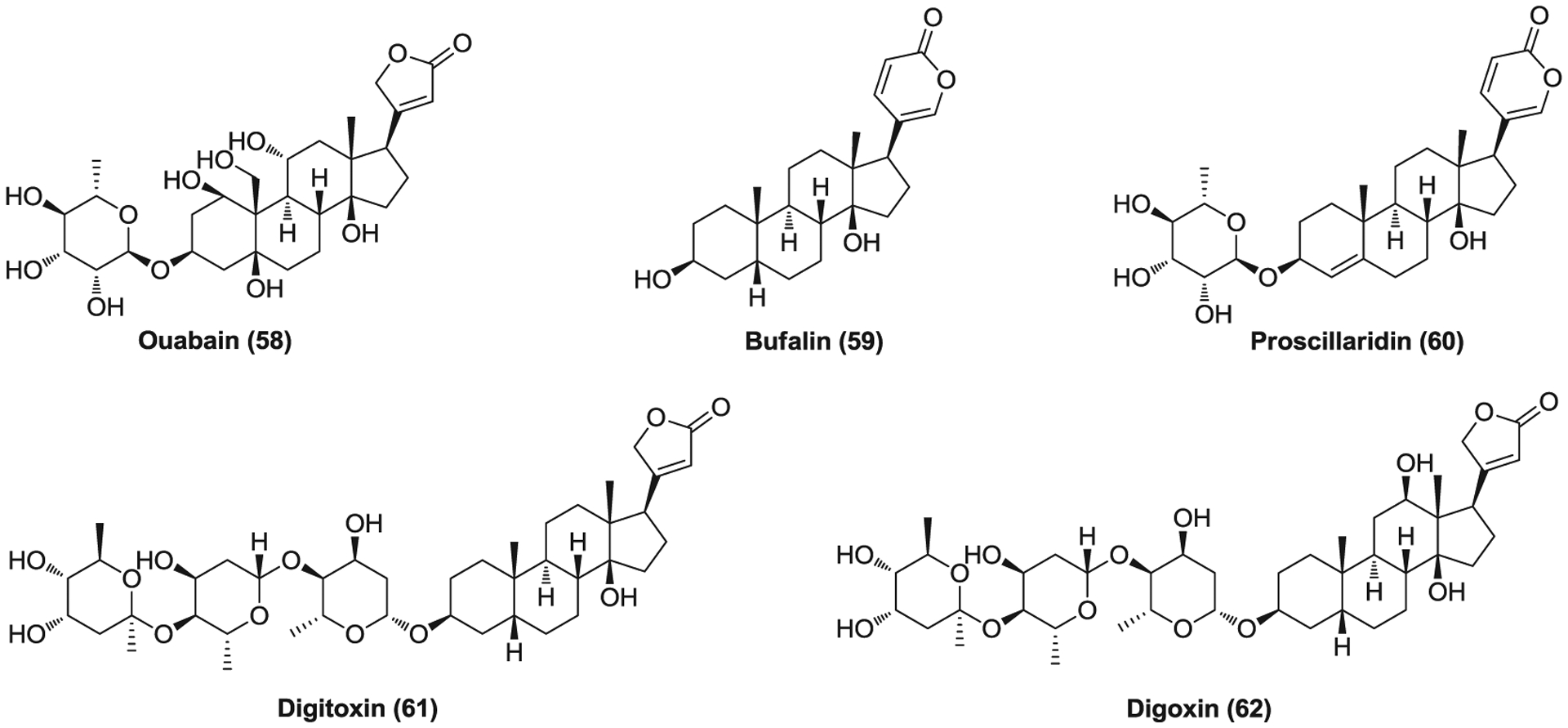
The cardiotonic steroids that inhibit coronavirus entry into host cell via targeting ATP1A1-mediated Src signaling
4.4.4 |. Other drugs that inhibit CoV entry into host cell
Clomipramine (63, Figure 16) is a tricyclic antidepressant which blocks the reuptake of serotonin and norepinephrine back into neurons, resulting in increased serotonergic and noradrenergic neurotransmission.303 Its hydrochloride was reported to inhibit SARS-CoV (EC50 = 13.24 μM), MERS-CoV (EC50 = 9.33 μM), and SARS-CoV-2 (EC50 = 7.59 μM) infections while it blocks MERS-CoV entry with an IC50 of 8.79 μM.178,232,294 Tamoxifen (64) and toremifene (65) are selective estrogen receptor modulators which are used to treat breast cancer. Tamoxifen citrate and toremifene citrate exhibit similar potency against MERS-CoV (EC50 = 10.12 and 12.92 μM, respectively) and SARS-CoV-2 (EC50 = 8.98 and 11.30 μM, respectively), and varied antiviral activities against SARS-CoV (EC50 = 92.89 and 11.97 μM, respectively).178,232 Astemizole (66) and chlorphenoxamine (67) are antihistamine and anticholinergic medications. Astemizole significantly inhibits SARS-CoV, MERS-CoV, and SARS-CoV-2 infections with EC50s of 5.59, 4.88, and ~1.1 μM, respectively, while chlorphenoxamine displays relatively low potency against SARS-CoV (EC50 = 20.03 μM) and MERS-CoV (EC50 = 12.65 μM).178,304 Tamoxifen and astemizole suppress clathrin-dependent entry with IC50s of 7.46 and 3.48 μM, respectively, and the anti-CoV activities of toremifene and chlorphenoxamine possibly attribute to this mechanism as well due to their highly similar structures.294
FIGURE 16.
Other drugs that inhibit coronavirus entry into host cell
4.5 |. Other Host-based inhibitors
Niclosamide (68, Figure 17) is an FDA-approved anthelmintic drug that has been widely used in human to treat tapeworm infections by inhibiting oxidative phosphorylation.305 It was found to regulate multiple signaling pathways and biological processes and effective against various viral infections such as CoVs, ZIKV, and EBOV.9,306 Niclosamide inhibits SARS-CoV and SARS-CoV-2 replication with EC50s of <0.1 and 0.28 μM, respectively.70,307,308 It also suppresses MERS-CoV infection by up to 1000-fold at 48 h post infection (PI) at a concentration of 10 μM likely through S-phase kinase-associated protein 2 (SKP2) inhibition.309 These data suggest that niclosamide, an inexpensive and well-tolerated drug, has great potential being repurposed to treat CoV infections. However, it should be mentioned that niclosamide has limited aqueous solubility and relatively low oral bioavailability and developing nano-based formulations of niclosamide or new optimized analogues may be a fast and useful approach to improving its PK properties and maximizing its therapeutic potential.9 More comprehensive understanding of niclosamide as a broad spectrum antiviral agent and its therapeutic potential for COVID-19 was recently reviewed by us,9 and more effective analogues with lower cytotoxic effects were also reported.310,311
FIGURE 17.
Other host-based inhibitors (68–73) against coronaviruses
Nitazoxanide (69) is a thiazolide medication which was initially developed as an oral antiparasitic agent and approved by FDA to treat diarrhea caused by Cryptosporidium parvum and Giardia intestinalis in adults and children at least 12 months old.312 Nitazoxanide was subsequently found as a broad antiviral agent and numerous clinical trials have been conducted to evaluate its efficacy for the treatment of influenza, viral gastroenteritis caused by rotavirus and norovirus, HBV, HCV, and HIV infections.312 Nitazoxanide was reported to induce the host innate immune response via enhancing the production of type 1 IFN-α and IFN-β, activating protein kinase R, and so forth.312–315 Nitazoxanide is rapidly metabolized into its active circulating form tizoxanide (70) in plasma and peak and trough serum concentrations of tizoxanide were 17.3 μM (4.6 μg/ml) and 3.0 μM (0.8 μg/ml), respectively, when treating patients with nitazoxanide controlled release tablets twice daily in a phase 2b/3 clinical trial.316 Moreover, the maximum plasma concentration of tizoxanide can reach as high as 37 μM (10 μg/ml) after oral administration of one 500 mg nitazoxanide tablet with food.317,318 Nitazoxanide significantly inhibits SARS-CoV-2 replication with an EC50 of 2.12 μM, which is far below its maximum serum concentration, indicating its great potential to treat SARS-CoV-2 infection.110 Given its in vitro evidence and immunomodulatory effect as well as the favorable in vivo PK and safety profiles, several clinical trials have been initiated to investigate the efficacy of nitazoxanide in COVID-19 treatment.
Hexachlorophene (71) is an organochlorine compound that is often used in soaps and toothpaste as an anti-infective and antibacterial agent. It strongly inhibits MHV and SARS-CoV-2 replication with EC50s of 1.2 and 0.9 μM, respectively.70,319 Tilorone (72) is a synthetic, orally bioavailable small molecule which displays antiviral activities associated with inducing IFN.320 It has been approved in Russia for the treatment of several viral infections such as influenza and acute respiratory viral infection.321 Tilorone is effective against a broad range of CoVs, displaying EC50s of 10.56 μM for MERS-CoV and 4.09 μM for SARS-CoV-2, respectively.70,322 Terconazole (73) is a broad antifungal drug of a triazole derivative which is often used to treat vaginal yeast infection as a lotion or a suppository.323 It binds to the cytochrome P450 enzyme of fungi and inhibits 14α-desmethyl sterol synthesis, consequently resulting in the accumulation of 14-methylsterols in the membrane.324,325 Terconazole suppresses SARS-CoV, MERS-CoV and SARS-CoV-2 infection with EC50s ranging from 11.92 to 16.14 μM.178,232
Azithromycin (74, Figure 18) is a macrolide that is effective against a broad range of Gram-positive and Gram-negative bacteria and used to treat diverse bacterial infections such as middle ear infections and pneumonia.326 It inhibits bacterial protein synthesis via binding to its ribosome and thus preventing mRNA translation.327 Azithromycin possesses high tissue penetration and anti-inflammatory properties, unrelated to its antimicrobial activity.328,329 Due to these properties, azithromycin is also used to treat many chronic lung diseases including chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, and so forth.330–332 Considering its clinical benefit in lung disease, numerous clinical trials have been initiated to test the efficacy of azithromycin in conjunction with hydroxychloroquine in COVID-19 patients. However, as mentioned in the discussion of hydroxychloroquine (see Section 4.4.1), the clinical benefit of azithromycin for COVID-19 patients remains controversial, and the combination use of azithromycin and hydroxychloroquine may cause additive cardiac toxicity, especially to those who have cardiac-related comorbidities.
FIGURE 18.
Other host-based inhibitors (74–77) against coronaviruses
Salinomycin (75) is a polyether ionophore antibiotic isolated from Streptomyces albus.333 It is highly effective against Gram-positive bacteria and used as a coccidiostat for poultry. It disturbs the natural Na+/K+ cations balance, changes the intracellular pH, and finally results in cell death.334 In addition, salinomycin was shown to inhibit cancer stem cells in different types of human cancers.335 Salinomycin sodium potently inhibits SARS-CoV-2 and MERS-CoV infection with EC50s of 0.24 and 5.49 μM, respectively.70,322 Nevertheless, the severe toxicity of salinomycin may limit its potential use as an antiviral agent. Ivermectin (76) is an FDA-approved antiparasitic drug that also displays broad antiviral activities, effective against DENV, HIV, chikungunya virus (CHIKV), and so forth.336–338 Ivermectin inhibits SARS-CoV-2 replication with an EC50 of ~2.0 μM and results in a ~5000-fold reduction in viral RNA at 48 h when treated 2 h PI at a concentration of 5 μM, warranting further investigation for possible benefits in humans.336 Anidulafungin (77, LY303366) is a semisynthetic echinocandin which is used to treat fungal infections.339 It inhibits the synthesis of 1,3-β-d-glucan, a major fungal cell wall component.340 Anidulafungin undergoes slow chemical degradation to its inactive forms under physiological conditions, not through hepatic enzymatic metabolism or renal elimination.341 Anidulafungin was found to suppress SARS-CoV-2 infection with an EC50 of 4.64 μM.70
Benztropine (78, Figure 19) is an anticholinergic drug which blocks the activity of the muscarinic acetylcholine receptor. Its mesylate inhibits SARS-CoV, MERS-CoV, and SARS-CoV-2 with micromolar EC50 values ranging from 13.8 to 21.6 μM.178,232 Fluspirilene (79) is an antipsychotic drug of the diphenylbutylpiperidine class used to treat schizophrenia.342 It was found effective against SARS-CoV and MERS-CoV (EC50 = 5.96 and 7.48 μM, respectively).178 Bazedoxifene (80) is a third-generation selective estrogen receptor modulator that is used to treat postmenopausal osteoporosis.343 Bazedoxifene suppresses SARS-CoV-2 infection with an EC50 of 3.44 μM.70 Loperamide (81) is a medication used to treat diarrhea. As an opioid-receptor agonist, it targets μ-opioid receptors in the myenteric plexus and decreases its activity.344 Loperamide inhibits SARS-CoV, MERS-CoV, and SARS-CoV-2 infection with EC50s of 8.8, 4.9, and 9.27 μM, respectively.62,70 It possibly acts at an early step in viral replicative cycle.62 Compounds 82–84 are bis-benzylisoquinoline alkaloids that possess anti-inflammatory effect.345–347 Cepharanthine (82), berbamine (83), and tetrandrine (84) show potency against SARS-CoV-2 with EC50s of 4.47, 7.87, and 3.00 μM, respectively.70 Reserpine (85), a well-known antihypertensive drug, was found to inhibit SARS-CoV replication with an EC50 of 3.4 μM.348
FIGURE 19.
Other host-based inhibitors (78–85) against coronaviruses
Ivacaftor (86, Figure 20), a drug used to treat cystic fibrosis, displays antiviral activity against SARS-CoV-2 (EC50 = 6.57 μM).70,349 ESI-09 (87) is an exchange protein directly activated by cAMP (EPAC) inhibitor, and ESI-09 treatment was reported effective in protecting cell cultures against MERS-CoV and SARS-CoV at the concentration of 10 μM, resulting in about 2log and 4log reduction in virus titer, respectively.350 Eltrombopag (88) is a small molecule agonist of the thrombopoietin (c-mpl) receptor that was developed for certain conditions associated with thrombocytopenia.351 Eltrombopag inhibits SARS-CoV-2 replication with an EC50 of 8.27 μM.70 In addition, platelets were reported to play an important role in innate immunology in the lung such as the defense against various respiratory viral infections.352 These findings together indicate that eltrombopag may have potential to combat the severe COVID-19. Hydroxyprogesterone caproate (89, OHPC) is an agonist of the progesterone receptor which is used to prevent preterm birth in pregnant women and to treat gynecological disorders.353 OHPC shows micromolar potency against SARS-CoV-2 (EC50 = 6.30 μM).70 Ciclesonide (90) is an inhaled corticosteroid used to treat asthma and allergic rhinitis.354 It inhibits SARS-CoV-2 replication (EC50 = 4.33 μM), and mutants in nsp3 and nsp4 of SARS-CoV-2 showed resistance against ciclesonide.70,355 Cases were also reported that ciclesonide inhalation treatment was associated with clinical improvement in three COVID-19 patients.356 These data, together with its antiviral and anti-inflammatory properties, warrant further studies for the clinical benefit of ciclesonide in COVID-19 treatment.
FIGURE 20.
Other host-based inhibitors (86–90) against coronaviruses
5 |. CONCLUSIONS AND FUTURE DIRECTIONS
Given the urgent unmet medical need in combating the COVID-19 pandemic, numerous human clinical trials have currently been launched to evaluate potential treatments for COVID-19 including biologicals such as vaccines, convalescent plasma, mAb, antiviral agents, immunomodulatory agents (supporting agents), and other miscellaneous agents with known or unknown mechanism of CoV inhibition. The representative small molecule compounds, which are undergoing clinical trials for COVID-19 with recruiting participants, were summarized in Table 3. Most of them are repurposed agents previously designed for other human conditions. Hydroxychloroquine and chloroquine were once granted emergency authorization by FDA for use in COVID-19 treatment; however, their clinical efficacy remains controversial and their use may be associated with potential severe side effects for some patients.357 Very recently, FDA revoked the Emergency Use Authorization of hydroxychloroquine and chloroquine for emergency use to treat COVID-19.358 Among these molecules, remdesivir, an RdRp inhibitor, seems to be most promising candidate which was reported to reduce the duration of recovery in a human phase 3 trial and has been authorized for emergency use in the U.S.124,359 On October 22, 2020, FDA approved Veklury (remdesivir), the first antiviral drug approved to treat COVID-19, for use in adult and pediatric patients 12 years of age and older and weighing at least 40 kg (about 88 pounds) requiring hospitalization.360 As inflammatory CS is comparatively common in COVID-19 patients,250 a large number of adjunctive therapies have also been investigated in clinical trials including corticosteroids and immunomodulatory agents such as JAK inhibitors and fingolimod. A controlled, open-label trial revealed that the use of dexamethasone (a common corticosteroid medication) resulted in lower 28-day mortality among hospitalized patients with COVID-19 who were receiving respiratory support, but no benefit was observed for those with less severe symptoms.361 Although use of ACE inhibitor (ACEI)/angiotensin receptor blocker (ARB) was reported to lower all-cause mortality compared with ACEI/ARB nonusers among COVID-19 inpatients with hypertension, the rationality of ACEI/ARB for treating COVID-19 remains disputed.362,363
TABLE 3.
Representative small molecule compounds for COVID-19 in human clinical trials with recruiting participantsa
| Agents | Drug class and/or targets | Phase |
|---|---|---|
| Lopinavir/ritonavir | HIV protease inhibitor/CYP3A4 inhibitor | Phase 4 |
| Darunavir/cobicistat | HIV protease inhibitor/CYP3A inhibitor | Phase 3 |
| ASC09/ritonavir | HIV protease inhibitor/CYP3A4 inhibitor | Phase 3 |
| Atazanavir | HIV protease inhibitor | Phase 2/3 |
| Ribavirin | Broad antiviral agent/RdRp inhibitor | Phase 2 (completed) |
| Remdesivir | RdRp inhibitor | Approved |
| EIDD-2801 | RdRp inhibitor | Phase 1/2 |
| Favipiravir | RdRp inhibitor | Phase 2/3 |
| Galidesivir | RdRp inhibitor | Phase 1 |
| Oseltamivir | Influenza neuraminidase inhibitor | Phase 4 |
| Arbidol | Broad antiviral agent/inhibit membrane fusion | N/A |
| Danoprevir | HCV NS3/4A protease inhibitor | Phase 4 |
| Clevudine | Anti-HBV/nucleoside analog | Phase 2 |
| Emtricitabine | HIV nucleoside reverse-transcriptase inhibitor | Phase 3 |
| Tenofovir disoproxil | HIV nucleoside reverse-transcriptase inhibitor | Phase 3 |
| Merimepodib | Noncompetitive IMPDH inhibitor | Phase 2 |
| Cyclosporine | Immunosuppressive/cyclophilin inhibitors | Phase 4 |
| Imatinib | Abl kinase inhibitor | Phase 2 |
| Sirolimus | mTOR inhibitor | Phase 1/2 |
| Acalabrutinib | Bruton′s tyrosine kinase inhibitor | Phase 2 |
| Tacrolimus | Immunosuppressive | Phase 3 |
| Baricitinib | JAK inhibitor | Phase 2/3 |
| Ruxolitinib | JAK inhibitor | Phase 2/3 |
| Tofacitinib | JAK inhibitor | Phase 2 |
| TD-0903 | JAK inhibitor | Phase 1/2 |
| Camostat | Serine protease (TMPRSS2) inhibitor | Phase 2/3 |
| Nafamostat | Serine protease (TMPRSS2) inhibitor | Phase 2/3 |
| Chloroquine | Antimalarial/immunomodulatory | Phase 1–4 |
| Hydroxychloroquine | Antimalarial/immunomodulatory | Phase 1–4 |
| Amodiaquine | Antimalarial/immunomodulatory | Phase 2 |
| GNS651 | Chloroquine analog | Phase 2 |
| Atovaquone | Antipneumocystis/antimalarial | Phase 2 |
| Fingolimod | Immunomodulatory | Phase 2 |
| Colchicine | Immunomodulatory | Phase 2 |
| CM-4620 | Inhibitor of CRAC channels/immunomodulatory | Phase 2 |
| Azithromycin | Macrolide antibiotic/immunomodulatory | Phase 1–4 |
| Methylprednisolone | Corticosteroid | Phase 3 |
| Dexamethasone | Corticosteroid | Phase 3/4 |
| Hydrocortisone | Corticosteroid | Phase 3/4 |
| Budesonide | Corticosteroid | Phase 2–4 |
| Prednisone | Corticosteroid | Phase 2 |
| Ciclesonide | Corticosteroid | Phase 3 |
| Aescin | Mixture of saponins/anti-inflammatory | Phase 2/3 |
| N-Acetylcysteine | Replenish glutathione stores/anti-inflammatory | Phase 2 |
| Aspirin | Inhibit cyclooxygenase (COX) enzyme | N/A |
| Ibuprofen | Nonsteroidal anti-inflammatory drug | Phase 4 |
| Naproxen | Nonsteroidal anti-inflammatory drug | N/A |
| BLD-2260 | Inhibitor of calpain 1, 2, and 9 | Phase 2 |
| Levamisole | Anthelmintic | Phase 2 |
| Nitazoxanide | Antiprotozoal/broad antiviral agent | Phase 4 |
| Niclosamide | Anthelmintic/broad antiviral agent | Phase 2/3 |
| Ivermectin | Anthelmintic | Phase 2/3 |
| Icosapent ethyl | Prodrug of eicosapentaenoic acid | Phase 2/4 |
| Selinexor | Exportin 1 inhibitor | Phase 2 |
| Spironolactone | Potassium-sparing diuretics | Phase 3 |
| Triiodothyronine | A thyroid hormone | N/A |
| Tranexamic acid | Antifibrinolytic/lysine analog | Phase 2 |
| Verapamil | Calcium channel blockers | Phase 2/3 |
| Amiodarone | Class III antiarrhythmic agent | Phase 2/3 |
| Valsartan | Angiotensin receptor blocker/antihypertensive | Phase 4 |
| Losartan | Angiotensin receptor blocker/antihypertensive | Phase 2–4 |
| Candesartan | Angiotensin receptor blocker/antihypertensive | Phase 2/3 |
| Linagliptin | Inhibitor of dipeptidyl peptidase 4 | Phase 3 |
| Sildenafil | Inhibitor of PDE5 | Phase 3 |
| Simvastatin | HMG-CoA reductase inhibitor | Phase 2 |
| Dapagliflozin | SGLT2 inhibitor/antidiabetic | Phase 3 |
| Rivaroxaban | Factor Xa inhibitor | N/A |
| Clopidogrel | Antiplatelet/inhibit ADP P2Y12 receptor | N/A |
| Fluvoxamine | Serotonin reuptake inhibitor | Phase 2 |
| Deferoxamine | Iron and aluminum chelator | Phase 1/2 |
| Formoterol | β2 agonist/bronchodilator | Phase 2/3 |
Abbreviations: CRAC, Ca2+ release-activated Ca2+; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IMPDH, inosine-5′-monophosphate dehydrogenase; JAK, Janus kinase; mTOR, mammalian target of rapamycin; N/A, not applicable; PDE5, cGMP-specific phosphodiesterase type 5; RdRp, RNA-dependent RNA polymerase; SGLT2, sodium-glucose transport protein 2; TMPRSS2, transmembrane protease serine 2.
Data were collected from https://clinicaltrials.gov/ when searching COVID-19 and SARS-CoV-2 for condition or disease. Access date: November 3, 2020. These compounds may be used as single treatment or combination use.
Although many virus-based and host-based small molecule drugs were reported with potent in vitro efficacy against CoVs, only a few are likely to be advanced into clinical trials to unravel their potential. Most of them possess one or several drawbacks including high EC50/Cmax values at clinically relevant dosages, severe side effects, immunosuppression, poor PK profiles or the lack of efficient drug delivery method. These limitations hamper their further clinical development as anti-CoV agents. Developing nano-based formulations and new delivery methods are rapid and promising strategies to improve the PK properties of some existing drugs and maximize their therapeutic potential for clinical applications. Another useful approach is combinational use of anti-CoV agents targeting different processes or proteins involved in virus life cycle that may slow the development of drug resistance and reduce the effective concentration of individual drugs and thus the potential side effects. Alternatively, these drugs can also serve as lead compounds to develop more effective, safer, and more specific anti-CoV drugs along the pipeline through medicinal chemistry optimization and drug development efforts. In the long run, it is imperative to develop more effective and broad-spectrum anti-CoV drugs such as RdRp inhibitors and 3CLpro inhibitors to ultimately fight the circulating and emerging CoV infections of the future.
ACKNOWLEDGEMENTS
This study was partially supported by grants AI131669, AI140726, and AI141178 from the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (Hongmin Li and Jia Zhou). Jia Zhou is also partly supported by John D. Stobo, the M.D. Distinguished Chair Endowment Fund, and the John Sealy Memorial Endowment Fund at UTMB. Hongmin Li is additionally supported by NIH grants AI133219, AI134568, AI140406, and AI140491. Pei-Yong Shi was supported by NIH grants AI142759, AI134907, and AI145617, UL1TR001439, and awards from the Sealy & Smith Foundation, Kleberg Foundation, John S. Dunn Foundation, Amon G. Carter Foundation, Gilson Longenbaugh Foundation, and Summerfield Robert Foundation.
Abbreviations:
- 3CLpro
3C-like protease
- AAK1
AP2-associated protein kinase 1
- ACE2
angiotensin-converting enzyme 2
- ACEI
ACE inhibitor
- AIDS
acquired immunodeficiency syndrome
- AKT
protein kinase B
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- ARB
angiotensin receptor blocker
- ASC09F
ASC09/ritonavir
- CC50
cytotoxic concentration 50%
- CHIKV
chikungunya virus
- Cmax
the maximum serum concentration
- CML
chronic myeloid leukemia
- COPD
chronic obstructive pulmonary disease
- CoV
coronavirus
- COVID-19
coronavirus disease 2019
- CPE
cytopathic effect
- CRAC
Ca2+ release-activated Ca2+
- CS
cytokine storm
- CSCs
cancer stem cells
- CTD
C-terminal domain
- DENV
dengue virus
- DPP4
dipeptidyl peptidase 4
- E
envelop
- EBOV
Ebola virus
- EC50
half maximal effective concentration
- EMCV
encephalomyocarditis virus
- EPAC
exchange protein directly activated by cAMP
- ER
endoplasmic reticulum
- ERGIC
endoplasmic reticulum-Golgi intermediate compartment
- ERK
extracellular signal-regulated kinase
- EUA
Emergency Use Authorization
- EVD
EBOV disease
- EV-A71
enterovirus A71
- GMP
guanosine-5′-monophosphate
- HAE
human airway epithelial
- HBV
hepatitis B virus
- HCoV
Human coronavirus
- HCV
hepatitis C virus
- HCMV
human cytomegalovirus
- HIV
human immunodeficiency virus
- HTS
high-throughput screening
- IC50
half maximal inhibitory concentration
- IFN
interferon
- IL-2
interleukin 2
- IMP
inosine-5′-monophosphate
- IMPDH
inosine-5′-monophosphate dehydrogenase
- JAK
Janus kinase
- JNKc
Jun N-terminal kinase
- LPV
rlopinavir/ritonavir
- M
membrane
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MARV
Marburg virus
- MERS-CoV
Middle East respiratory syndrome coronavirus
- MHV-2
mouse hepatitis virus type 2
- mTOR
mammalian target of rapamycin
- N
nucleocapsid
- NFAT
nuclear factor of activated T cells
- nsp
nonstructural protein
- NTD
N-terminal domain
- ORF
open reading frame
- PDE5
cGMP-specific phosphodiesterase type 5
- PI
post infection
- PI3K
phosphoinositol 3-kinase
- PK
pharmacokinetic
- PKR
protein kinase R
- PLpro
papain-like protease
- RA
rheumatoid arthritis
- RBD
receptor binding domain
- RdRp
RNA-dependent RNA polymerase
- RSV
respiratory syncytial virus
- RTC
replicase-transcriptase complex
- S
spike
- SARS-CoV
severe acute respiratory syndrome coronavirus
- SARS-CoV-2
severe acute respiratory syndrome coronavirus-2
- SGLT2
sodium-glucose transport protein 2
- SKP2
S-phase kinase-associated protein 2
- SLE
systemic lupus erythematosus
- TMPRSS2
transmembrane protease serine 2
- WHO
World Health Organization
- ZIKV
Zika virus
AUTHOR BIOGRAPHIES
Jimin Xu graduated in Basic Pharmacy from China Pharmaceutical University in 2009 and obtained his Ph.D. degree from Shanghai Institute of Materia Medica, Chinese Academy of Sciences in 2014 under the supervision of Professor Fajun Nan. During the subsequent two years, he worked as a Research Scientist in UniTris Biopharma (Shanghai) Co., Ltd and Pharmaron Beijing Co., Ltd successively. Dr. Xu is currently pursuing his postdoctoral training at the Chemical Biology Program, Department of Pharmacology and Toxicology at University of Texas Medical Branch under the supervision of Professor Jia Zhou. His research interests currently focus on the rational design and chemical synthesis of small molecules as novel pharmacological probes and therapeutics for infectious diseases and human cancers.
Yu Xue received his Ph.D. in Medicinal Chemistry from China Pharmaceutical University (CPU) in 2018 under the supervision of Professor Liping Sun at CPU and Professor Ao Zhang at Shanghai Institute of Materia Medica, Chinese Academy of Sciences. He is currently pursuing his postdoctoral training under the supervision of Professor Jia Zhou at the Chemical Biology Program, Department of Pharmacology and Toxicology at University of Texas Medical Branch. His research interests focus on design and synthesis of novel small molecules as chemical probes and drug candidates for infectious diseases, cancer and other human diseases.
Richard Zhou is an undergraduate student in the Biomedical Engineering School of University of Texas at Austin, pursuing 2020 summer college internship research studies at the Department of Pharmacology and Toxicology, University of Texas Medical Branch.
Pei-Yong Shi is I.H. Kempner Professor of Human Genetics at University of Texas Medical Branch. He received his Ph.D. in virology in 1996 from Georgia State University. After postdoctoral training at Yale University, he joined Bristol-Myers Squibb as a Principal Scientist to develop HIV and HCV therapeutics from 1998 to 2000. He then moved to the Wadsworth Center, New York State Department of Health, to study West Nile virus. From 2008 to 2015, he served as Dengue Unit Head and Executive Director to lead drug discovery at Novartis Institute for Tropical Diseases. He has a long-standing interest in virology, drug discovery, vaccine development, and infectious diagnostics. Dr. Shi has published over 280 peer-reviewed articles.
Hongmin Li received his Ph.D. in Molecular Biology from Institute of Biophysics, Chinese Academy of Sciences in 1995. After graduation, he joined as a postdoctoral affiliate in Dr. Roy Mariuzza’s group at the Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institutes. In 2000, Dr. Li was recruited as a faculty member in the Wadsworth Center, New York State Department of Health (NYSDOH). Dr. Li held a Research Scientist 6 position (professor-equivalent) at the Wadsworth Center, NYSDOH. He also had a joint Associate Professorship appointment at the Department of Biomedical Sciences, School of Public Health, University at Albany. Dr. Li was recently relocated in November, 2020, and is currently R. Ken and Donna Coit Endowed Chair Professor at the Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona. Dr. Li authored more than 90 manuscripts, several book chapters, and two patents.
Jia Zhou received his Ph.D. in organic chemistry from Nankai University, China in 1997. Then he joined the chemistry faculty in the same university and was promoted to Associate Professor there. He started his postdoctoral research in organic chemistry with Dr. Sidney M. Hecht at the University of Virginia in 1999. After further postdoctoral training in medicinal chemistry with Dr. Alan P. Kozikowski at Georgetown University Medical Center, he worked in US pharmaceutical industry at Acenta Discovery, and PsychoGenics, Inc. as a Senior Principal Scientist for 7 years. Dr. Zhou is currently a tenured Professor and also a faculty member of the Center for Addiction Research, Center for Biodefense and Emerging Infectious Diseases, Sealy Center for Structural Biology and Biophysics, and Sealy Center for Molecular Medicine at UTMB. Dr. Zhou is elected as a 2020 National Academy of Inventors (NAI) Fellow. He is an author of more than 180 peer-reviewed papers, 7 book chapters, and an inventor of 26 patents.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Zumla A, Chan JFW, Azhar EI, Hui DSC, Yuen K-Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA. 2020;323(8): 707–708. [DOI] [PubMed] [Google Scholar]
- 3.Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11 Suppl): S223–S227. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthony SJ, Gilardi K, Menachery VD, et al. Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. mBio. 2017;8(2):e00373–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B, Ge X, Wang L-F, Shi Z. Bat origin of human coronaviruses. Virol J. 2015;12(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huynh J, Li S, Yount B, et al. Evidence supporting a zoonotic origin of human coronavirus strain NL63. J Virol. 2012; 86(23):12816–12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osman EEA, Toogood PL, Neamati N. COVID-19: living through another pandemic. ACS Infect Dis. 2020; 6:1548–1552. 10.1021/acsinfecdis.0c00224 [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Shi PY, Li H, Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis. 2020;6(5):909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. December 31, 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/. Accessed May 26, 2020.
- 11.Reusken CB, Haagmans BL, Müller MA, Gutierrez C, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13(10): 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haagmans BL, Al Dhahiry SHS, Reusken CBEM, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14(2):140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370(26):2499–2505. [DOI] [PubMed] [Google Scholar]
- 14.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). January, 2020. https://www.who.int/emergencies/mers-cov/en/. Accessed May 26, 2020.
- 16.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan China: a descriptive study. Lancet 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. 2020, March 19. Getting your workplace ready for COVID-19: how COVID-19 spreadsGeneva: World Health Organization.
- 20.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 21.Hoehl S, Rabenau H, Berger A, et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382(13):1278–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gates B Responding to Covid-19—a once-in-a-century pandemic? N Engl J Med. 2020;382(18):1677–1679. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Coronavirus disease (COVID-2019) situation reports. Weekly Epidemiological and Operational updates December 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed December 2, 2020. [Google Scholar]
- 24.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 25.Woo PCY, Huang Y, Lau SKP, Yuen K-Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8): 1804–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehr AR, Perlman S. Coronaviruses: An Overview of their Replication and Pathogenesis. In: Maier HJ, Bickerton E, Britton P eds. Coronaviruses: Methods and Protocols. New York, NY: Springer; New York; 2015:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28(2):465–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020; 92(4):418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3(6):e00473–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo PC, Lau SK, Li KS, Tsang AK, Yuen KY. Genetic relatedness of the novel human group C betacoronavirus to Tylonycteris bat coronavirus HKU4 and Pipistrellus bat coronavirus HKU5. Emerg Microbes Infect. 2012;1(11):e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tortorici MA, Veesler D. Chapter Four—Structural Insights into Coronavirus Entry. In: Rey FA, ed. Advances in Virus Research. Vol 105. Academic Press; 2019:93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh Tomar PP, Arkin IT. SARS-CoV-2 E protein is a potential ion channel that can be inhibited by Gliclazide and Memantine. Biochem Biophys Res Commun. 2020;530(1):10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBride R, van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6(8): 2991–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyall J, Gross R, Kindrachuk J, et al. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77(18):1935–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu L, Liu Q, Zhu Y, et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun. 2014;5(1):3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA. 2009;106(14):5871–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peeples L News feature: avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc Natl Acad Sci USA. 2020; 117(15):8218–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gierer S, Bertram S, Kaup F, et al. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J Virol. 2013;87(10): 5502–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA. 2005;102(33):11876–11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosch BJ, Bartelink W, Rottier PJ. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol. 2008;82(17): 8887–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol. 2013;87(23):12552–12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84(24): 12658–12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85(2): 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86(12):6537–6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gil C, Ginex T, Maestro I, et al. COVID-19: drug targets and potential treatments. J Med Chem. 2020;63: 12359–12386. 10.1021/acs.jmedchem.0c00606 [DOI] [PubMed] [Google Scholar]
- 56.Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung S-H. An overview of severe acute respiratory syndrome–coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem. 2016;59(14):6595–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J, Song W, Huang H, Sun Q. Pharmacological therapeutics targeting RNA-dependent RNA polymerase, proteinase and spike protein: from mechanistic studies to clinical trials for COVID-19. J Clin Med. 2020;9(4):1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai W, Zhang B, Jiang XM, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin Z, Du X, Xu Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811): 289–293. [DOI] [PubMed] [Google Scholar]
- 60.Yin W, Mao C, Luan X, et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003; 63(8):769–802. [DOI] [PubMed] [Google Scholar]
- 62.de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58(8):4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.López Aspiroz E, Santos Buelga D, Cabrera Figueroa S, et al. Population pharmacokinetics of lopinavir/ritonavir (Kaletra) in HIV-infected patients. Ther Drug Monit. 2011;33(5):573–582. [DOI] [PubMed] [Google Scholar]
- 65.Nukoolkarn V, Lee VS, Malaisree M, Aruksakulwong O, Hannongbua S. Molecular dynamic simulations analysis of ritronavir and lopinavir as SARS-CoV 3CLpro inhibitors. J Theor Biol. 2008;254(4):861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 67.Chu CM. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004; 59(3):252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan JFW, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arabi YM, Asiri AY, Assiri AM, et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeon S, Ko M, Lee J, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64(7):e00819–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto N, Yang R, Yoshinaka Y, et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318(3):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Z, Yao H, Shen J, et al. Nelfinavir is active against SARS-CoV-2 in Vero E6 cells. ChemRxiv. 2020. 10.26434/chemrxiv.12039888.v1 [DOI] [Google Scholar]
- 74.Driessen C, Kraus M, Joerger M, et al. Treatment with the HIV protease inhibitor nelfinavir triggers the unfolded protein response and may overcome proteasome inhibitor resistance of multiple myeloma in combination with bortezomib: a phase I trial (SAKK 65/08). Haematologica. 2016;101(3):346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fintelman-Rodrigues N, Sacramento CQ, Lima CR, et al. Atazanavir inhibits SARS-CoV-2 replication and proinflammatory cytokine production. bioRxiv. 2020. 10.1101/2020.04.04.020925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J, Xia L, Liu L, et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect Dis. 2020;7(7):ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020; 19(3):149–150. [DOI] [PubMed] [Google Scholar]
- 78.Njoroge FG, Chen KX, Shih N-Y, Piwinski JJ. Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection. Acc Chem Res. 2008;41(1):50–59. [DOI] [PubMed] [Google Scholar]
- 79.Ma C, Sacco MD, Hurst B, et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. 10.1038/s41422-020-0356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krampe H, Ehrenreich H. Supervised disulfiram as adjunct to psychotherapy in alcoholism treatment. Curr Pharm Des. 2010;16(19):2076–2090. [DOI] [PubMed] [Google Scholar]
- 81.Lipsky JJ, Shen ML, Naylor S. In vivo inhibition of aldehyde dehydrogenase by disulfiram. Chem-Biol Interact. 2001: 130–132. [DOI] [PubMed] [Google Scholar]
- 82.Lin M-H, Moses DC, Hsieh C-H, et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir Res. 2018;150:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schewe T Molecular actions of Ebselen—an antiinflammatory antioxidant. Gen Pharmacol. 1995;26(6):1153–1169. [DOI] [PubMed] [Google Scholar]
- 84.Marshall AC, Kidd SE, Lamont-Friedrich SJ, et al. Structure, mechanism, and inhibition of Aspergillus fumigatus thioredoxin reductase. Antimicrob Agents Chemother. 2019;63(3):e02281–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parnham M, Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opin Invest Drugs. 2000;9(3): 607–619. [DOI] [PubMed] [Google Scholar]
- 86.Kil J, Lobarinas E, Spankovich C, et al. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390(10098):969–979. [DOI] [PubMed] [Google Scholar]
- 87.Neuman RS, Zebrowska G. Serotonin (5-HT2) receptor mediated enhancement of cortical unit activity. Can J Physiol Pharmacol. 1992;70(12):1604–1609. [DOI] [PubMed] [Google Scholar]
- 88.Chen L, Gui C, Luo X, et al. Cinanserin is an inhibitor of the 3C-Like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro. J Virol. 2005;79(11):7095–7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Itil TM, Polvan N, Holden JM. Clinical and electroencephalographic effects of cinanserin in schizophrenic and manic patients. Dis Nerv Syst. 1971;32(3):193–200. [PubMed] [Google Scholar]
- 90.Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177(4050):705–706. [DOI] [PubMed] [Google Scholar]
- 91.Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hall CB, Walsh EE, Hruska JF, Betts RF, Hall WJ. Ribavirin treatment of experimental respiratory syncytial viral infection: a controlled double-blind study in young adults. JAMA. 1983;249(19):2666–2670. [PubMed] [Google Scholar]
- 93.Reichard O, Yun Z-B, Sönnerborg A, Weiland O. Hepatitis C viral RNA titers in serum prior to, during, and after oral treatment with ribavirin for chronic hepatitis C. J Med Virol. 1993;41(2):99–102. [DOI] [PubMed] [Google Scholar]
- 94.Paeshuyse J, Dallmeier K, Neyts J. Ribavirin for the treatment of chronic hepatitis C virus infection: a review of the proposed mechanisms of action. Curr Opin Virol. 2011;1(6):590–598. [DOI] [PubMed] [Google Scholar]
- 95.Ascioglu S, Leblebicioglu H, Vahaboglu H, Chan KA. Ribavirin for patients with Crimean-Congo haemorrhagic fever: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(6):1215–1222. [DOI] [PubMed] [Google Scholar]
- 96.Bausch DG, Hadi CM, Khan SH, Lertora JJ. Review of the literature and proposed guidelines for the use of oral ribavirin as postexposure prophylaxis for Lassa fever. Clin Infect Dis. 2010;51(12):1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soares-Weiser K, Thomas S, Garner GGT. Ribavirin for for Crimean-Congo hemorrhagic fever: systematic review and meta-analysis. BMC Infect Dis. 2010;10(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eriksson B, Helgstrand E, Johansson NG, et al. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother. 1977;11(6):946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olschlager S, Neyts J, Gunther S. Depletion of GTP pool is not the predominant mechanism by which ribavirin exerts its antiviral effect on Lassa virus. Antiviral Res. 2011;91(2):89–93. [DOI] [PubMed] [Google Scholar]
- 100.Saijo M, Morikawa S, Fukushi S, et al. Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus. Antiviral Res. 2005;66(2–3):159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci Rep. 2013;3:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng VC, Chan JF, To KK, Yuen KY. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 2013;100(2):407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leong HN, Ang B, Earnest A, Teoh C, Xu W, Leo YS. Investigational use of ribavirin in the treatment of severe acute respiratory syndrome, Singapore, 2003. Trop Med Int Health. 2004;9(8):923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.So LKY, Lau AC, Yam LY, et al. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361(9369):1615–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Booth CM. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. [DOI] [PubMed] [Google Scholar]
- 106.Falzarano D, de Wit E, Rasmussen AL, et al. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19(10):1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shalhoub S, Farahat F, Al-Jiffri A, et al. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70(7): 2129–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lo MK, Jordan R, Arvey A, et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep. 2017;7(1):43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tchesnokov EP, Feng JY, Porter DP, Gotte M. Mechanism of inhibition of ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11(4):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2):e00221–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siegel D, Hui HC, Doerffler E, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f] [triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J Med Chem. 2017;60(5):1648–1661. [DOI] [PubMed] [Google Scholar]
- 117.Mulangu S, Dodd LE, Davey RT, et al. A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci USA. 2020;117(12):6771–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shannon A, Le NT-T, Selisko B, et al. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antivir Res. 2020;178:104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gordon CJ, Tchesnokov EP, Woolner E, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020; 295(20):6785–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19—final report. N Engl J Med. 2020; 383:1813–1826. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gilead. Gilead Presents Additional Data on Investigational Antiviral Remdesivir for the Treatment of COVID-19 [press release]. 2020, July 10. [Google Scholar]
- 126.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoon J-J, Toots M, Lee S, et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62(8):e00766–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Toots M, Yoon J-J, Cox RM, et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11(515):eaax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12(541):eabb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46(4):977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Curr Treat Options Infect Dis. 2016; 8(4):311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baranovich T, Wong S-S, Armstrong J, et al. T-705 (Favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vivo. J Virol. 2013;87(7):3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49(3):981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5’-triphosphate towards influenza A virus polymerase. PLOS One. 2013;8(7): e68347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goldhill DH, Te Velthuis AJW, Fletcher RA, et al. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci USA. 2018;115(45):11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Furuta Y, Takahashi K, Shiraki K, et al. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82(3):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020. 10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020. 10.1101/2020.03.17.20037432 [DOI] [Google Scholar]
- 141.Warren TK, Wells J, Panchal RG, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508(7496):402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Julander JG, Siddharthan V, Evans J, et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir Res. 2017;137:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Westover JB, Mathis A, Taylor R, et al. Galidesivir limits Rift Valley fever virus infection and disease in Syrian golden hamsters. Antivir Res. 2018;156:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shiraki K Antiviral Drugs Against Alphaherpesvirus. In: Kawaguchi Y, Mori Y, Kimura H eds., Human Herpesviruses. Singapore: Springer; 2018:103–122. [DOI] [PubMed] [Google Scholar]
- 146.Sacks SL, Wilson B. Famciclovir/penciclovir. Adv Exp Med Biol. 1999;458:135–147. [DOI] [PubMed] [Google Scholar]
- 147.Randolph B Penciclovir for the treatment of herpes simplex labialis: a review. ASDC J Dent Child. 2001;68(3): 189–190. [PubMed] [Google Scholar]
- 148.Shiraki K Antiviral drugs against alphaherpesvirus. Adv Exp Med Biol. 2018;1045:103–122. [DOI] [PubMed] [Google Scholar]
- 149.Fickert P, Hinterleitner TA, Wenzl HH, Aichbichler BW, Petritsch W. Mycophenolate mofetil in patients with Crohn’s disease. Am J Gastroenterol. 1998;93(12):2529–2532. [DOI] [PubMed] [Google Scholar]
- 150.Silverman Kitchin JE, Pomeranz MK, Pak G, Washenik K, Shupack JL. Rediscovering mycophenolic acid: a review of its mechanism, side effects, and potential uses. J Am Acad Dermatol. 1997;37(3):445–449. [DOI] [PubMed] [Google Scholar]
- 151.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3): 85–118. [DOI] [PubMed] [Google Scholar]
- 152.Morrey JD, Smee DF, Sidwell RW, Tseng C. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antivir Res. 2002;55(1):107–116. [DOI] [PubMed] [Google Scholar]
- 153.Sebastian L, Sebastian L, Madhusudana SN, Ravi V, Desai A. Mycophenolic acid inhibits replication of Japanese encephalitis virus. Chemotherapy. 2011;57(1):56–61. [DOI] [PubMed] [Google Scholar]
- 154.Leyssen P, Balzarini J, De Clercq E, Neyts J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J Virol. 2005;79(3):1943–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diamond MS, Zachariah M, Harris E. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology. 2002;304(2):211–221. [DOI] [PubMed] [Google Scholar]
- 156.Khan M, Dhanwani R, Patro IK, Rao PVL, Parida MM. Cellular IMPDH enzyme activity is a potential target for the inhibition of Chikungunya virus replication and virus induced apoptosis in cultured mammalian cells. Antivir Res. 2011;89(1):1–8. [DOI] [PubMed] [Google Scholar]
- 157.Pan Q, de Ruiter PE, Metselaar HJ, et al. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55(6):1673–1683. [DOI] [PubMed] [Google Scholar]
- 158.Ye L, Li J, Zhang T, et al. Mycophenolate mofetil inhibits hepatitis C virus replication in human hepatic cells. Virus Res. 2012;168(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chan JFW, Chan KH, Kao RYT, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67(6):606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hart BJ, Dyall J, Postnikova E, et al. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95(Pt 3):571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chan JFW, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barnard DL, Day CW, Bailey K, et al. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res. 2006;71(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Faure E, Poissy J, Goffard A, et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLOS One. 2014;9(2):e88716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15(4):1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kato F, Matsuyama S, Kawase M, Hishiki T, Katoh H, Takeda M. Antiviral activities of mycophenolic acid and IMD-0354 against SARS-CoV-2. Microbiol Immunol. 2020;64(9):635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jain J, Almquist SJ, Shlyakhter D, Harding MW. VX-497: a novel, selective IMPDH inhibitor and immunosuppressive agent. J Pharm Sci. 2001;90(5):625–637. [DOI] [PubMed] [Google Scholar]
- 167.Markland W, McQuaid TJ, Jain J, Kwong AD. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother. 2000;44(4):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tong X, Smith J, Bukreyeva N, et al. Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antiviral Res. 2018;149:34–40. [DOI] [PubMed] [Google Scholar]
- 169.Li S-F, Gong M-J, Shao J-J, Sun Y-F, Zhang Y-G, Chang H-Y. Antiviral activity of merimepodib against foot and mouth disease virus in vitro and in vivo. Mol Immunol. 2019;114:226–232. [DOI] [PubMed] [Google Scholar]
- 170.Marcellin P, Horsmans Y, Nevens F, et al. Phase 2 study of the combination of merimepodib with peginterferon-α2b, and ribavirin in nonresponders to previous therapy for chronic hepatitis C. J Hepatol. 2007;47(4):476–483. [DOI] [PubMed] [Google Scholar]
- 171.Bukreyeva N, Mantlo EK, Sattler RA, Huang C, Paessler S, Zeldis J. The IMPDH inhibitor merimepodib suppresses SARS-CoV-2 replication in vitro. bioRxiv. 2020. 10.1101/2020.04.07.028589 [DOI] [Google Scholar]
- 172.Tsuzuki K Role of mizoribine in renal transplantation. Pediatr Int. 2002;44(2):224–231. [DOI] [PubMed] [Google Scholar]
- 173.Kawasaki Y Mizoribine: a new approach in the treatment of renal disease. Clin Dev Immunol. 2009;2009:681482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yokota S Mizoribine: mode of action and effects in clinical use. Pediatr Int. 2002;44(2):196–198. [DOI] [PubMed] [Google Scholar]
- 175.Alvarellos ML, Lamba J, Sangkuhl K, et al. PharmGKB summary: gemcitabine pathway. Pharmacogenet Genom. 2014; 24(11):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wong A, Soo RA, Yong W-P, Innocenti F. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev. 2009;41(2):77–88. [DOI] [PubMed] [Google Scholar]
- 177.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17(Suppl 5): v7–v12. [DOI] [PubMed] [Google Scholar]
- 178.Dyall J, Coleman CM, Hart BJ, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58(8):4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang Y-N, Zhang Q-Y, Li X-D, et al. Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg Microbes Infect. 2020;9(1):1170–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ruiz van Haperen VWT, Veerman G, Vermorken JB, Peters GJ. 2ʹ,2ʹ-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem Pharmacol. 1993;46(4):762–766. [DOI] [PubMed] [Google Scholar]
- 181.Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65(suppl_2):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heneghan CJ, Onakpoya I, Jones MA, et al. Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Health Technol Assess. 2016;20(42):1–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729–1737. [DOI] [PubMed] [Google Scholar]
- 185.Liu Y-C, Liao C-H, Chang C-F, Chou C-C, Lin Y-R. A locally transmitted case of SARS-CoV-2 infection in Taiwan. N Engl J Med. 2020;382(11):1070–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leneva IA, Russell RJ, Boriskin YS, Hay AJ. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res. 2009;81(2):132–140. [DOI] [PubMed] [Google Scholar]
- 187.Leneva IA, Burtseva EI, Yatsyshina SB, et al. Virus susceptibility and clinical effectiveness of anti-influenza drugs during the 2010–2011 influenza season in Russia. Int J Infect Dis. 2016;43:77–84. [DOI] [PubMed] [Google Scholar]
- 188.Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15(10):997–1005. [DOI] [PubMed] [Google Scholar]
- 189.Pécheur EI, Borisevich V, Halfmann P, et al. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J Virol. 2016;90(6):3086–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boriskin YS, Pécheur E-I, Polyak SJ. Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J. 2006;3(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Blaising J, Polyak SJ, Pécheur E-I. Arbidol as a broad-spectrum antiviral: an update. Antivir Res. 2014;107:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14(1): 58–60. [DOI] [PubMed] [Google Scholar]
- 193.Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020; 81(1):e21–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26(7):917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wintermeyer SM, Nahata MC. Rimantadine: a clinical perspective. Ann Pharmacother. 1995;29(3):299–310. [DOI] [PubMed] [Google Scholar]
- 197.Govorkova EA, Fang HB, Tan M, Webster RG. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob Agents Chemother. 2004;48(12):4855–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jing X, Ma C, Ohigashi Y, et al. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc Natl Acad Sci USA. 2008;105(31):10967–10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang C, Takeuchi K, Pinto LH, Lamb RA. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67(9):5585–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Belshe RB, Smith MH, Hall CB, Betts R, Hay AJ. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 1988;62(5):1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Evidente VG, Adler CH, Caviness JN, Gwinn-Hardy K. A pilot study on the motor effects of rimantadine in Parkinson’s disease. Clin Neuropharmacol. 1999;22(1):30–32. [DOI] [PubMed] [Google Scholar]
- 202.Jasiński M, Jasińska L, Ogrodowczyk M. Resveratrol in prostate diseases—a short review. Cent European J Urol. 2013;66(2):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6(6):e19881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lin S-C, Ho C-T, Chuo W-H, Li S, Wang TT, Lin C-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ellen ter BM, Dinesh Kumar N, Bouma EM, et al. Resveratrol and pterostilbene potently inhibit SARS-CoV-2 infection in vitro. bioRxiv. 2020. 10.1101/2020.09.24.285940 [DOI] [Google Scholar]
- 206.Garreau de Loubresse N, Prokhorova I, Holtkamp W, Rodnina MV, Yusupova G, Yusupov M. Structural basis for the inhibition of the eukaryotic ribosome. Nature. 2014;513(7519):517–522. [DOI] [PubMed] [Google Scholar]
- 207.Jimenez A, Carrasco L, Vazquez D. Enzymic and nonenzymic translocation by yeast polysomes. Site of action of a number of inhibitors. Biochemistry. 1977;16(21):4727–4730. [DOI] [PubMed] [Google Scholar]
- 208.Grollman AP. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci USA. 1966;56(6):1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wong W, Bai X, Brown A, et al. Cryo-EM structure of the Plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. eLife. 2014;3:e03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Grollman AP. Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J Biol Chem. 1967;242(13): 3226–3233. [PubMed] [Google Scholar]
- 211.Liu Y, Ge J, Li Q, et al. Low-dose anisomycin sensitizes glucocorticoid-resistant T-acute lymphoblastic leukemia CEM-C1 cells to dexamethasone-induced apoptosis through activation of glucocorticoid receptor and p38-MAPK/JNK. Leuk Lymphoma. 2014;55(9):2179–2188. [DOI] [PubMed] [Google Scholar]
- 212.Khandelwal N, Chander Y, Rawat KD, et al. Emetine inhibits replication of RNA and DNA viruses without generating drug-resistant virus variants. Antivir Res. 2017;144:196–204. [DOI] [PubMed] [Google Scholar]
- 213.Andersen PI, Krpina K, Ianevski A, et al. Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses. 2019;11(10):964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Choy K-T, Wong AY-L, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;178:104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ho PC, Dweik R, Cohen MC. Rapidly reversible cardiomyopathy associated with chronic ipecac ingestion. Clin Cardiol. 1998;21(10):780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gilles A, Frechin L, Natchiar K, et al. Targeting the human 80S ribosome in cancer: from structure to function and drug design for innovative adjuvant therapeutic strategies. Cells. 2020;9(3):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fresno M, Jiménez A, Vázquez D. Inhibition of translation in eukaryotic systems by harringtonine. Eur J Biochem. 1977;72(2):323–330. [DOI] [PubMed] [Google Scholar]
- 218.Fahr A Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet. 1993;24(6):472–495. [DOI] [PubMed] [Google Scholar]
- 219.Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2):119–125. [DOI] [PubMed] [Google Scholar]
- 220.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226(4674):544–547. [DOI] [PubMed] [Google Scholar]
- 221.Pfefferle S, Schöpf J, Kögl M, et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7(10):e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5(5): 1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92(Pt 11):2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de Wilde AH, Raj VS, Oudshoorn D, et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J Gen Virol. 2013;94(Pt 8):1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gallay PA, Lin K. Profile of alisporivir and its potential in the treatment of hepatitis C. Drug Des Devel Ther. 2013; 7:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Paeshuyse J, Kaul A, De Clercq E, et al. The nonimmunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology. 2006;43(4):761–770. [DOI] [PubMed] [Google Scholar]
- 227.Coelmont L, Kaptein S, Paeshuyse J, et al. Debio 025, a cyclophilin binding molecule, is highly efficient in clearing hepatitis C virus (HCV) replicon-containing cells when used alone or in combination with specifically targeted antiviral therapy for HCV (STAT-C) inhibitors. Antimicrob Agents Chemother. 2009;53(3):967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ptak RG, Gallay PA, Jochmans D, et al. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob Agents Chemother. 2008;52(4):1302–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.de Wilde AH, Falzarano D, Zevenhoven-Dobbe JC, et al. Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017;228:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Softic L, Brillet R, Berry F, et al. Inhibition of SARS-CoV-2 infection by the cyclophilin inhibitor alisporivir (Debio 025). Antimicrob Agents Chemother. 2020;64(7):e00876–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7(5):345–356. [DOI] [PubMed] [Google Scholar]
- 232.Weston S, Haupt R, Logue J, Matthews K, Frieman MB. FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro. bioRxiv. 2020. 10.1101/2020.03.25.008482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Coleman CM, Sisk JM, Mingo RM, Nelson EA, White JM, Frieman MB. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus fusion. J Virol. 2016;90(19):8924–8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sisk JM, Frieman MB, Machamer CE. Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J Gen Virol. 2018;99(5):619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Das J, Chen P, Norris D, et al. 2-Aminothiazole as a novel kinase inhibitor template. structure–activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (Dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006;49(23):6819–6832. [DOI] [PubMed] [Google Scholar]
- 236.Hennequin LF, Allen J, Breed J, et al. N-(5-Chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006;49(22):6465–6488. [DOI] [PubMed] [Google Scholar]
- 237.Keating GM. Dasatinib: a review in chronic myeloid leukaemia and Ph+acute lymphoblastic leukaemia. Drugs. 2017; 77(1):85–96. [DOI] [PubMed] [Google Scholar]
- 238.Reddy SM, Kopetz S, Morris J, et al. Phase II study of saracatinib (AZD0530) in patients with previously treated metastatic colorectal cancer. Invest New Drugs. 2015;33(4):977–984. [DOI] [PubMed] [Google Scholar]
- 239.Sanz-Blasco S, Bordone MP, Damianich A, et al. The kinase Fyn as a novel intermediate in l-DOPA-induced dyskinesia in Parkinson’s disease. Mol Neurobiol. 2018;55(6):5125–5136. [DOI] [PubMed] [Google Scholar]
- 240.Hu M, Che P, Han X, et al. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shin JS, Jung E, Kim M, Baric RS, Go YY. Saracatinib inhibits middle east respiratory syndrome-coronavirus replication in vitro. Viruses. 2018;10(6):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124(1):119–131. [DOI] [PubMed] [Google Scholar]
- 243.de Wispelaere M, LaCroix AJ, Yang PL. The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase. J Virol. 2013;87(13):7367–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kumar R, Agrawal T, Khan NA, Nakayama Y, Medigeshi GR. Identification and characterization of the role of c-terminal Src kinase in dengue virus replication. Sci Rep. 2016;6:30490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Newsome TP, Weisswange I, Frischknecht F, Way M. Abl collaborates with Src family kinases to stimulate actin-based motility of vaccinia virus. Cell Microbiol. 2006;8(2):233–241. [DOI] [PubMed] [Google Scholar]
- 246.Kindrachuk J, Ork B, Hart BJ, et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59(2):1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miller CR, Oliver KE, Farley JH. MEK1/2 inhibitors in the treatment of gynecologic malignancies. Gynecol Oncol. 2014;133(1):128–137. [DOI] [PubMed] [Google Scholar]
- 248.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020; 214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O’Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev. 2008;223(1):132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lu J Palbociclib: a first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer. J Hematol Oncol. 2015;8(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Park I-K, Mishra A, Chandler J, Whitman SP, Marcucci G, Caligiuri MA. Inhibition of the receptor tyrosine kinase Axl impedes activation of the FLT3 internal tandem duplication in human acute myeloid leukemia: implications for Axl as a potential therapeutic target. Blood. 2013;121(11):2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhou Y, Vedantham P, Lu K, et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015; 116:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Park J-E, Li K, Barlan A, et al. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc Natl Acad Sci USA. 2016;113(43):12262–12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Abdulla M-H, Lim K-C, Sajid M, McKerrow JH, Caffrey CR. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLOS Med. 2007;4(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tamai M, Matsumoto K, Omura S, Koyama I, Ozawa Y, Hanada K. In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J Pharmacobiodyn. 1986;9(8):672–677. [DOI] [PubMed] [Google Scholar]
- 259.Hook G, Hook V, Kindy M. The cysteine protease inhibitor, E64d, reduces brain amyloid-beta and improves memory deficits in Alzheimer’s disease animal models by inhibiting cathepsin B, but not BACE1, beta-secretase activity. J Alzheimers Dis. 2011;26(2):387–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Satoyoshi E Therapeutic trials on progressive muscular dystrophy. Intern Med. 1992;31(7):841–846. [DOI] [PubMed] [Google Scholar]
- 261.Imahori K, Sugita H, Miyatake T, Ishihara T, Miyatani N, Kuwabara T. Pharmacokinetics and safety of EST, a new thiol protease inhibitor in patients with muscle diseases. Rinsho yakuri/Japanese J Clin Pharmacol Ther. 1985;16(4): 749–757. [Google Scholar]
- 262.Miyahara T, Shimojo S, Toyohara K, et al. Phase I study of EST, a new thiol protease inhibitor. the 1st report: safety and pharmacokinetics at single administration. Rinsho yakuri/Japanese J Clin Pharmacol Ther. 1985;16(2):357–365. [Google Scholar]
- 263.Miyahara T, Shimojo S, Toyohara K, et al. Phase I study of EST, a new thiol protease inhibitor. the 2nd report: safety and pharmacokinetics in continuous administration. Rinsho yakuri/Japanese J Clin Pharmacol Ther. 1985;16(3): 537–546. [Google Scholar]
- 264.Glowacka I, Bertram S, Muller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23(1):34–41. [DOI] [PubMed] [Google Scholar]
- 266.Sai JK, Suyama M, Kubokawa Y, Matsumura Y, Inami K, Watanabe S. Efficacy of camostat mesilate against dyspepsia associated with non-alcoholic mild pancreatic disease. J Gastroenterol. 2010;45(3):335–341. [DOI] [PubMed] [Google Scholar]
- 267.Chen X, Xu Z, Zeng S, et al. The molecular aspect of antitumor effects of protease inhibitor nafamostat mesylate and its role in potential clinical applications. Front Oncol. 2019;9:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Al-Horani RA, Desai UR. Recent advances on plasmin inhibitors for the treatment of fibrinolysis-related disorders. Med Res Rev. 2014;34(6):1168–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64(6): e00754–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yamamoto M, Matsuyama S, Li X, et al. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60(11):6532–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jang S, Rhee J-Y. Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy. Int J Infect Dis. 2020;96:500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6): e01815–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Pastick KA, Okafor EC, Wang F, et al. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). Open Forum Infect Dis. 2020;7(4):ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5(1): e00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Keyaerts E, Vijgen L, Maes P, Neyts J, Van, Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323(1):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Barnard DL, Day CW, Bailey K, et al. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother. 2006;17(5):275–284. [DOI] [PubMed] [Google Scholar]
- 279.Shippey EA, Wagler VD, Collamer AN. Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med. 2018; 85(6):459–467. [DOI] [PubMed] [Google Scholar]
- 280.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. [DOI] [PubMed] [Google Scholar]
- 281.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732–739. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. [DOI] [PubMed] [Google Scholar]
- 284.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label nonrandomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 285.Gautret P, Lagier J-C, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50(4):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial medRxiv. 2020. 10.1101/2020.03.22.20040758 [DOI]
- 288.Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Taccone FS, Gorham J, Vincent J-L. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020;8(6):539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.White NJ, Watson JA, Hoglund RM, Chan XHS, Cheah PY, Tarning J. COVID-19 prevention and treatment: a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLOS Med. 2020;17(9): e1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323(24):2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020. [Google Scholar]
- 293.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. [DOI] [PubMed] [Google Scholar]
- 294.Liu Q, Xia S, Sun Z, et al. Testing of Middle East respiratory syndrome coronavirus replication inhibitors for the ability to block viral entry. Antimicrob Agents Chemother. 2015;59(1):742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Inoue Y, Tanaka N, Tanaka Y, et al. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81(16):8722–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123(5):1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Blanchard E, Belouzard S, Goueslain L, et al. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80(14):6964–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pohjala L, Utt A, Varjak M, et al. Inhibitors of alphavirus entry and replication identified with a stable chikungunya replicon cell line and virus-based assays. PLOS One. 2011;6(12):e28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Chu VC, McElroy LJ, Ferguson AD, Bauman BE, Whittaker GR. Avian infectious bronchitis virus enters cells via the endocytic pathway. Adv Exp Med Biol. 2006;581:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pu Y, Zhang X. Mouse hepatitis virus type 2 enters cells through a clathrin-mediated endocytic pathway independent of Eps15. J Virol. 2008;82(16):8112–8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Patel S Plant-derived cardiac glycosides: role in heart ailments and cancer management. Biomed Pharmacother. 2016;84:1036–1041. [DOI] [PubMed] [Google Scholar]
- 302.Burkard C, Verheije MH, Haagmans BL, et al. ATP1A1-mediated Src signaling inhibits coronavirus entry into host cells. J Virol. 2015;89(8):4434–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340(2):249–258. [DOI] [PubMed] [Google Scholar]
- 304.Riva L, Yuan S, Yin X, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–119. 10.1038/s41586-020-2577-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chen W, Mook RA, Premont RT, Wang J. Niclosamide: beyond an antihelminthic drug. Cell Signal. 2018;41:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Li Y, Li P-K, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett. 2014;349(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wu C-J, Jan J-T, Chen C-M, et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother. 2004;48(7):2693–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wen C-C, Kuo Y-H, Jan J-T, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50(17):4087–4095. [DOI] [PubMed] [Google Scholar]
- 309.Gassen NC, Niemeyer D, Muth D, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat Commun. 2019;10(1):5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Xu J, Berastegui-Cabrera J, Chen H, Pachón J, Zhou J, Sánchez-Céspedes J. Structure–activity relationship studies on diversified salicylamide derivatives as potent inhibitors of human adenovirus infection. J Med Chem. 2020;63(6): 3142–3160. [DOI] [PubMed] [Google Scholar]
- 311.Li Z, Xu J, Lang Y, et al. JMX0207, a niclosamide derivative with improved pharmacokinetics, suppresses Zika virus infection both in vitro and in vivo. ACS Infect Dis. ACS Infect Dis 2020;6:2616–2628. 10.1021/acsinfecdis.0c00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rossignol JF. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Clerici M, Trabattoni D, Pacei M, Biasin M, Rossignol J-F. The anti-infective nitazoxanide shows strong immumodulating effects (155.21). J Immunol. 2011;186(1 Suppl):155.21. [Google Scholar]
- 314.Elazar M, Liu M, McKenna SA, et al. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2α via protein kinase activated by double-stranded RNA activation. Gastroenterology. 2009;137(5): 1827–1835. [DOI] [PubMed] [Google Scholar]
- 315.Jasenosky LD, Cadena C, Mire CE, et al. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus. iScience. 2019;19:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Haffizulla J, Hartman A, Hoppers M, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14(7):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Galan-Herrera JF, Poo JL, Rosales-Sanchez O, et al. Bioavailability of two oral formulations of a single dose of levofloxacin 500 mg: an open-label, randomized, two-period crossover comparison in healthy Mexican volunteers. Clin Ther. 2009;31(8):1796–1803. [DOI] [PubMed] [Google Scholar]
- 318.Korba BE, Montero AB, Farrar K, et al. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77(1):56–63. [DOI] [PubMed] [Google Scholar]
- 319.Cao J, Forrest JC, Zhang X. A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs. Antiviral Res. 2015;114:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Stringfellow DA, Glasgow LA. Tilorone hydrochloride: an oral interferon-inducing agent. Antimicrob Agents Chemother. 1972;2(2):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ekins S, Lane TR, Madrid PB. Tilorone: a broad-spectrum antiviral invented in the USA and commercialized in Russia and beyond. Pharm Res. 2020;37(4):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Shen L, Niu J, Wang C, et al. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J Virol. 2019;93(12):e00023–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tolman EL, Isaacson DM, Rosenthale ME, et al. Anticandidal activities of terconazole, a broad-spectrum antimycotic. Antimicrob Agents Chemother. 1986;29(6):986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Isaacson DM, Tolman EL, Tobia AJ, et al. Selective inhibition of 14 alpha-desmethyl sterol synthesis in Candida albicans by terconazole, a new triazole antimycotic. J Antimicrob Chemother. 1988;21(3):333–343. [DOI] [PubMed] [Google Scholar]
- 325.Yoshida Y Cytochrome P450 of Fungi: Primary Target for Azole Antifungal Agents. In: McGinnis MR ed., Current Topics in Medical Mycology. New York, NY. New York: Springer; 1988:388–418. [DOI] [PubMed] [Google Scholar]
- 326.Peters DH, Friedel HA, McTavish D. Azithromycin. Drugs 1992;44(5):750–799. [DOI] [PubMed] [Google Scholar]
- 327.Parnham MJ, Haber VE, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. [DOI] [PubMed] [Google Scholar]
- 328.Steel HC, Theron AJ, Cockeran R, Anderson R, Feldman C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediat Inflamm. 2012;2012:584262–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Feola DJ, Garvy BA, Cory TJ, et al. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with pseudomonas. Antimicrob Agents Chemother. 2010;54(6):2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non–cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013; 309(12):1251–1259. [DOI] [PubMed] [Google Scholar]
- 331.Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68(5):479–503. [DOI] [PubMed] [Google Scholar]
- 332.Gotfried MH. Macrolides for the treatment of chronic sinusitis, asthma, and COPD. Chest. 2004;125(2):52–61. [DOI] [PubMed] [Google Scholar]
- 333.Antoszczak M, Huczyński A. Salinomycin and its derivatives—a new class of multiple-targeted “magic bullets”. Eur J Med Chem. 2019;176:208–227. [DOI] [PubMed] [Google Scholar]
- 334.Antoszczak M A comprehensive review of salinomycin derivatives as potent anticancer and anti-CSCs agents. Eur J Med Chem. 2019;166:48–64. [DOI] [PubMed] [Google Scholar]
- 335.Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. 2012; 2012:950658–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Varghese FS, Kaukinen P, Gläsker S, et al. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir Res. 2016;126:117–124. [DOI] [PubMed] [Google Scholar]
- 338.Tay MYF, Fraser JE, Chan WKK, et al. Nuclear localization of dengue virus (DENV) 1–4 nonstructural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir Res. 2013;99(3):301–306. [DOI] [PubMed] [Google Scholar]
- 339.Denning DW. Echinocandins: a new class of antifungal. J Antimicrob Chemother. 2002;49(6):889–891. [DOI] [PubMed] [Google Scholar]
- 340.Gobernado M, Canton E. Anidulafungin. Rev Esp Quimioter. 2008;21(2):99–114. [PubMed] [Google Scholar]
- 341.Damle BD, Dowell JA, Walsky RL, Weber GL, Stogniew M, Inskeep PB. In vitro and in vivo studies to characterize the clearance mechanism and potential cytochrome P450 interactions of anidulafungin. Antimicrob Agents Chemother. 2009;53(3):1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Abhijnhan A, Adams CE, David A, Ozbilen M. Depot fluspirilene for schizophrenia. Cochrane Database Syst Rev. 2007; (1):CD001718. 10.1002/14651858.CD001718.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yavropoulou MP, Makras P, Anastasilakis AD. Bazedoxifene for the treatment of osteoporosis. Expert Opin Pharmacother. 2019;20(10):1201–1210. [DOI] [PubMed] [Google Scholar]
- 344.Wade P, Palmer J, McKenney S, et al. Modulation of gastrointestinal function by MuDelta, a mixed μ opioid receptor agonist/μ opioid receptor antagonist. Br J Pharmacol. 2012;167(5):1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Toyama M, Hamasaki T, Uto T, et al. Synergistic inhibition of HTLV-1-infected cell proliferation by combination of cepharanthine and a tetramethylnaphthalene derivative. Anticancer Res. 2012;32(7):2639–2645. [PubMed] [Google Scholar]
- 346.Li Z, Geng Y-N, Jiang J-D, Kong W-J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014:289264–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kwan CY, Achike FI. Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action. Acta Pharmacol Sin. 2002;23(12):1057–1068. [PubMed] [Google Scholar]
- 348.Wu C-Y, Jan J-T, Ma S-H, et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci USA. 2004;101(27):10012–10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.McPhail GL, Clancy JP. Ivacaftor: the first therapy acting on the primary cause of cystic fibrosis. Drugs Today (Barc). 2013;49(4):253–260. [DOI] [PubMed] [Google Scholar]
- 350.Tao X, Mei F, Agrawal A, et al. Blocking of exchange proteins directly activated by cAMP leads to reduced replication of Middle East respiratory syndrome coronavirus. J Virol. 2014;88(7):3902–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kim TO, Despotovic J, Lambert MP. Eltrombopag for use in children with immune thrombocytopenia. Blood Adv. 2018;2(4):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Qiu J, Ma J, Zhang S, Han J, Liu S. Promoting platelets is a therapeutic option to combat severe viral infection of the lung. Blood Adv. 2020;4(8):1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Hines M, Lyseng-Williamson KA, Deeks ED. 17 α-Hydroxyprogesterone Caproate (Makena®). Clin Drug Investig. 2013;33(3):223–227. [DOI] [PubMed] [Google Scholar]
- 354.Schaffner TJ, Skoner DP. Ciclesonide: a safe and effective inhaled corticosteroid for the treatment of asthma. J Asthma Allergy. 2009;2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Matsuyama S, Kawase M, Nao N, et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral replication-transcription complex in culture cells. J Virol. 2020. 10.1128/JVI.01648-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother. 2020;26(6):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. Can Med Assoc J. 2020;192(17):E450–E453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.U.S. Food and Drug Administration (FDA). Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. June 15, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and. Accessed June 15, 2020.
- 359.U.S. Food and Drug Administration (FDA). Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. May 01, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 28, 2020.
- 360.U.S. Food and Drug Administration (FDA). FDA’s approval of Veklury (remdesivir) for the treatment of COVID-19—The Science of Safety and Effectiveness. October 22, 2020. Accessed November 2, 2020.
- 361.The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. 2020. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Resp Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020; 126(12):1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]


