Abstract
Granulocyte colony-stimulating factor (G-CSF) is a cytokine most well-known for maturation and mobilization of bone marrow neutrophils. Although it is used therapeutically to treat chemotherapy induced neutropenia, it is also highly expressed in some tumors. Case reports suggest that tumors expressing high levels of G-CSF are aggressive, more difficult to treat, and present with poor prognosis and high mortality rates. Research on this topic has suggested that G-CSF has tumor-promoting effects on both tumor cells and the tumor microenvironment. G-CSF has a direct effect on tumor cells to promote tumor stem cell longevity, and overall tumor cell proliferation and migration. Additionally, it may promote pro-tumorigenic immune cell phenotypes such as M2 macrophages, myeloid-derived suppressor cells, and regulatory T cells. Overall, the literature suggests a plethora of pro-tumorigenic activity that should be balanced with the therapeutic use. In this review, we present an overview of the multiple complex roles of G-CSF and G-CSFR in tumors and their microenvironment and discuss how clinical advances and strategies may open new therapeutic avenues.
Keywords: G-CSF, G-CSF receptor, tumor microenvironment, G-CSF producing tumors, colorectal cancer, macrophages, T cells, STAT3
Introduction
Granulocyte colony-stimulating factor (G-CSF), also known as colony-stimulating factor 3 (CSF3), is a glycoprotein that stimulates granulopoiesis and leads to proliferation, maturation and mobilization of neutrophils. Initially, it was believed that G-CSF and its receptor (G-CSFR) were mainly expressed by myeloid cells, but there have also been reports of expression in fibroblasts, endothelial cells, and bone marrow stromal cells (1). More recent studies have shown that G-CSF is also expressed in other tissues as well as the placenta, adult neural stem cells, B-cells and cardiomyocytes (2, 3). Despite the production of G-CSF from immune cells, of particular interest is the growing body of evidence that some tumor cells might produce G-CSF.
G-CSF is most well-known for its function in maturation and mobilization of neutrophils from the bone marrow. Thus, it is used as an adjuvant treatment in severe cases of chemotherapy-induced neutropenia (4). In contrast to the short-term clinical administration of G-CSF, evidence points to chronic production by tumors and potentially important effects on immune cells. G-CSF is highly produced in several cancers, such as colon (5), breast (6), thyroid (7–9), pancreatic (10–12), bladder (13), lung (14) and liver cancer (15). These studies and others reviewed here have shown that the presence of G-CSF within the tumor microenvironment may promote malignancy progression, metastasis, poor prognosis and decreased overall patient survival. In this review, we have gathered available data to decipher the multi-faceted role of G-CSF in tumor growth and possible implications of the pro-tumorigenic effects in the tumor microenvironment.
The biology of G-CSF signaling pathways
In the hemopoietic system, G-CSF facilitates proliferation and differentiation of myeloid progenitors into neutrophils and their mobilization into the peripheral blood (16). G-CSF is a glycoprotein consisting of an immunoglobulin (Ig)-like domain, a cytokine receptor-homologous (CRH) domain, three fibronectin type III (FNIII) domains in the extracellular region, a transmembrane domain and a cytoplasmic domain. Structural analysis of G-CSFR has shown that its cytoplasmic domain consists of four tyrosine residues (Y704, Y729, Y744, Y764), serving as phospho-acceptor docking sites for either Src homology type 2 (SH2) or phosphotyrosine binding (PTB) domains of signaling proteins (17, 18). Human G-CSF (molecular weight ∼30,000) consists of 174 amino acids, while murine G-CSF (molecular weight ∼25,000) consists of 178 amino acids. G-CSF receptor (G-CSFR also known as CD114 or CSF3R) (molecular weight ∼100,000–130,000) consist of 812 and 813 amino acids in human and mouse, respectively (19). The crystallographic analysis of the human (determined to 2.8 Å resolution) and murine G-CSF/G-CSFR complex structure revealed a 2:2 stoichiometry by means of a cross-over interaction between the Ig-like domains of GCSF-R and GCSF. This conformation is quite different from the murine G-CSF/G-CSFR complex, but shows a similar structure to that of the IL-6/gp130 signaling complex (20, 21), implying the importance of those small structural changes when designing G-CSF used for therapeutic use.
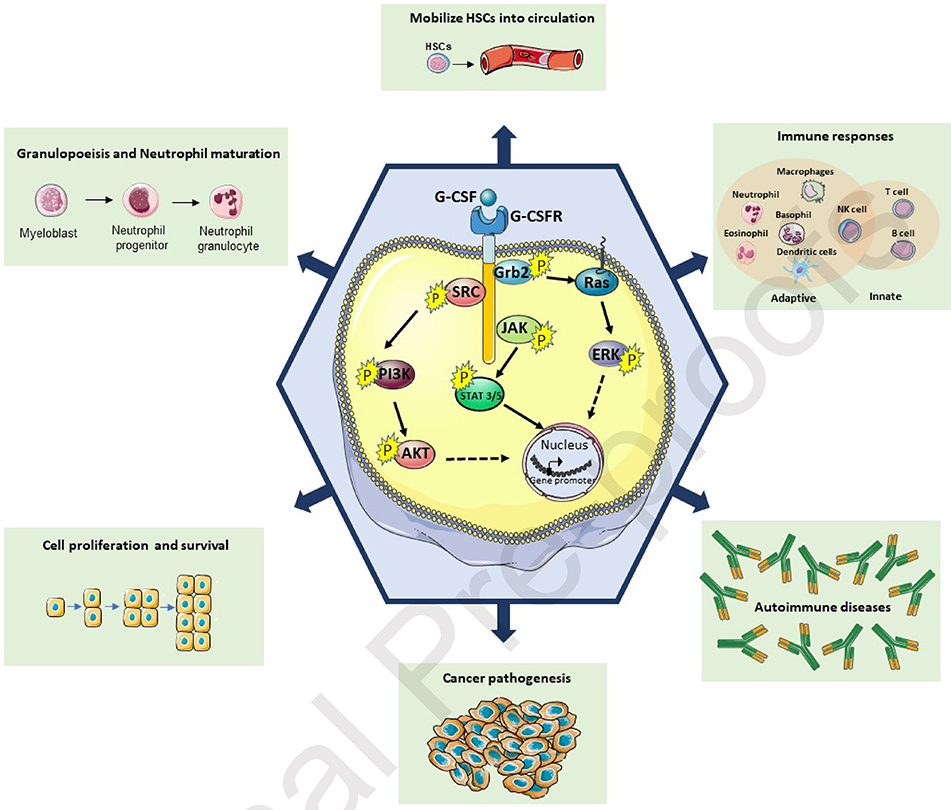
G-CSF expression can be triggered by inflammatory signals including LPS, TNF-a and IL-1β (22). When binding to its receptor, G-CSF triggers signal transduction molecules such as transcription factors (STAT 1, 3 and 5) and activates three main signaling pathways; JAK/STAT, PI3K/AKT, and MAPK/ERK (Figure 1). Upon initial activation, G-CSFR dimerizes, which leads to a phosphorylation cascade of the JAK/STAT pathway. Phosphorylated JAK2 phosphorylates the STAT (STAT3 or STAT5) proteins, which upon dimerization migrate into the nucleus to activate the transcription of target genes. The second signaling pathway that is activated upon G-CSF binding is the PI3K pathway. Although the molecular mechanism of the PI3K activation is not completely understood, studies have suggested that the activation of this pathway is SRC kinase-mediated (23, 24). The last documented signaling pathway of the G-CSFR is the MAPK/ERK mediated by the p21/Ras pathway (Shc and Grb2 proteins) (25). G-CSF/G-CSFR complex formation furthermore activates Lyn, a Src kinase in myeloid cells, via Gab2-mediated recruitment of Shp2 (26).
Figure 1: Schematic illustration of intracellular G-CSF/G-CSFR complex signaling pathways.
Under physiological conditions, upon G-CSF binding to G-CSFR, three major downstream pathways are activated. JAK/STAT, PI3K/AKT, and MAPK/ERK as shown here. After cascading events of kinase-substrate phosphorylations, those pathways promote gene expression and lead to a multitude of effects such as, Neutrophil maturation and HSC mobilization.
The above signaling pathway leads to activation of NF-κB and C/EBPβ transcription factors and their subsequent binding to regulatory elements of the G-CSF promoter leading to activation of neutrophils in the bone marrow (27, 28). Under normal conditions, activation of the aforementioned pathways leads to neutrophil maturation and mobilization to the bloodstream. Aberrant activation of the signaling pathways or mutations of the G-CSFR pathway affect the myeloid lineage and have been directly linked with malignances. Mutations that affect the Ig-like, CRH and fibronectin domains have been identified in patients with severe neutrophilic leukemia (SNL), while mutations of the transmembrane and cytoplasmic domains have been related to chronic neutrophilic leukemia (CNL), myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) (29).
The role of G-CSF in the immune system
In addition to controlling granulopoeisis through stimulating hematopoietic mobilization of stem cells and the production of neutrophils, G-CSF has also been reported to regulate inflammation and presents immunomodulatory effects by mediating innate and adaptive immune responses as described below (30–32).
i. G-CSF regulation of innate immune cells
In the innate immune system, the most well-known function of G-CSF is in maturation and trafficking of neutrophils from the blood to the periphery (33). One study suggested that macrophages also produce very high levels of G-CSF in response to bacteria that may also modulate dendritic cell responses, thus suggesting a role in the interaction between innate and adaptive immune cells (34). Binding of G-CSF to its receptor leads to phosphorylation of JAK kinases and subsequent recruitment of STAT5 transcription and has been reported to be involved in the maturation of granulocyte/macrophage precursors of activated G-/GM-CSFR (35). In in vitro studies using bone marrow cell culture models, G-CSF was found to play important role in generation of Gr-1high/F4/80 M2-like macrophages present in the intestine after the adoptive transfer (36). Furthermore, our group recently reported that G-CSFR−/− bone marrow-derived macrophages (BMDMs) produced decreased IL-10 and increased IL-12 in colon tumors suggesting that G-CSF promotes a pro-tumorigenic macrophage phenotype (37). Other groups have suggested that G-CSF promotes development of myeloid-derived suppressor cells (MDSCs) in a STAT3 dependent manner that promote tumor progression (38). In agreement with these studies, another group showed the G-CSF promoting MDSC activity led to chemoresistance in uterine cancer (39). G-CSF promotion of IL-10 production by macrophages and the role in generation of MDSCs suggest important regulatory functions that may be critical in inflammatory diseases and cancer.
In contrast to its stimulatory effects on neutrophils, G-CSF has been also shown to downregulate pro-inflammatory responses by decreasing proinflammatory cytokine release in activated monocytes and macrophages (30, 40, 41). In another function of the innate immune system, G-CSF has been shown to decrease NK cytotoxic function (42). This last observation comes from graft vs host disease studies, suggesting that besides mobilization of neutrophils, G-CSF induces inhibitory innate immune cells responses. However, our group has also shown that in a colitis-associated cancer model, neutralization of G-CSF may lead to increased NK cell influx into mouse colons and decreased tumor growth (43), suggesting an inhibitory role for G-CSF in mobilization and activity of NK cells.
ii. G-CSF regulation of adaptive immune cells
In vitro and in vivo studies have shown that G-CSF is also expressed in T lymphocytes (including, CD4, CD8, T regulatory cells (Tregs), and T helper cells (TH)) and affects T cell proliferation, cytokine production and peripheral T cell tolerance (44, 45) in regards to graft vs host disease. Human bone marrow transplant studies have shown that T cells from G-CSF-mobilized peripheral blood stem cell donors have an immunotolerant profile with upregulation of genes related to TH2 and Treg cells, downregulation of genes associated with TH1 cells, cytotoxicity, and decreased antigen presentation and graft-versus-host disease (46). Moreover, overexpression of negative regulators of TH17 differentiation was observed in donors during G-CSF treatment with increased levels of CD4+CD25highCD45RO+ Treg cells (46). Recently, it was shown that in vitro activation of human T cells led to expression of G-CSFR, enhanced the proportion of CD38+ cells, downregulated IFN-γ production and upregulated the IL-4 production implying a mechanism of constrained T cell driven pro-inflammatory responses that prevent damage and inflammatory reactivity (47). Our group recently confirmed the role of G-CSF in regulating T cell responses, particularly in promoting Tregs and inhibiting TH1 responses in the context of tumors (48). Moreover, overexpression of negative regulators of TH17 differentiation were observed in donors during G-CSF treatment with increased levels of CD4+CD25highCD45RO+ Treg cells (46). G-CSF was also shown to inhibit T cell proliferation in response to both mitogens and alloantigens and reduce their cytotoxic activity (49). In a mouse model of acute graft vs. host disease (GVHD), when donor mice received G-CSF pre-treatment, it reduced the severity of GVHD and enhanced the survival of recipient mice (49). In addition to TH cells, G-CSF has been shown to also have a TH2 polarizing effect of NKT cells (50), which may also be protective in graft vs host disease. Thus, G-CSF may be an attractive candidate for specific immune modulation in transplantation and particularly acute GVHD as well as in disorders associated with TH1/TH2 imbalance, such as TH1-mediated autoimmune diseases.
Autocrine/paracrine effect of tumor secreted G-CSF in the myeloid progenitors and cancer stem cells
While the potential sources of G-CSF in mouse models are numerous, including myeloid cells and stromal cells, under pathological conditions G-CSF tumor producing cells have also been reported (48). Tumor secreted G-CSF promotes tumor growth and metastasis by facilitating angiogenesis and enhancing mobilization of immature granulocytes (51, 52). These cells were shown to be of the MDSC phenotype that can affect tumor progression by suppressing T cell function (53). Studies have shown that in cervical cancer, G-CSF is associated with increased frequency of MDSC and resistance to chemotherapy (54). Tumor-secreted G-CSF and the MDSC-mediated pre-metastatic niche were reported to be responsible for the highly metastatic nature of other malignancies as well (55, 56). In vivo studies suggested that by recruiting MDSCs, G-CSF induces VEGF-independent angiogenesis leading to increased resistance to anti-VEGF drugs (57, 58). Also of importance, tumors expressing G-CSF with simultaneous infiltration of Gr1+CD11b+ MDSCs were shown to be refractory to the subsequent antiangiogenic treatments (58). As shown by in vitro studies in mouse colon cancer, accumulation of MDSCs has also been reported, suggesting that G-CSF can promote MDSC survival and activation via the STAT signaling pathway. In the same study, it was shown that anti-GCSF treatment led to decreased infiltration of MDSCs and tumor growth (59).
Mesenchymal stromal cells (MSCs) are also of interest in regards to G-CSF production and tumor progression. Stimulation of the STAT pathways suggests that G-CSF or GM-CSF may regulate epithelial to mesenchymal transition (EMT), a critical event in malignant transformation (60, 61). Our group also observed that stromal cells are a major source of G-CSF production in gastric and colon cancers, which can feedback on tumors to promote tumor growth and tumor cell migration (43). Additionally, another study revealed elevated levels of cytokines associated with angiogenesis (interleukin- 6 and VEGF) and EMT (transforming growth factor -β1 and -β2) in mice treated with G-CSF. These observations indicate that administration of exogenous G-CSF promoted tumor growth via cell proliferation, angiogenesis, recruitment of M2 macrophages and enhancement of EMT through the modulation of the tumor microenvironment (62).
In addition to EMT and migration, there is further evidence that G-CSF supports a pool of cancer cells expressing stem markers. Studies have shown that G-CSF could sustain a pool of stem cells via c-jun-dependent by activating SLUG, SNAIL1, or TWIST-1 transcription factors (63). In vitro and in vivo influence of G-CSFR in a cancer stem cell-like (CSC) subpopulation in neuroblastoma demonstrated that G-CSFR promoted selective activation of STAT3 in the CSCs subpopulation. This study reported an expansion of CSCs, combined with an increasing tumor growth and metastasis in human xenograft and murine neuroblastoma tumor models (64). Additionally, the authors demonstrated that STAT3 acts in a feed-forward loop to transcriptionally activate the G-CSFR and sustain neuroblastoma cancer stem cells in response to G-CSF. Depletion of the cancer stem cell subpopulation within tumors along with a decrease of tumor growth, metastasis and increased chemosensitivity were observed after inhibition of the G-CSF-STAT3 signaling loop with either anti-G-CSF antibody or STAT3 inhibitor (64).
Cancers that produce G-CSF
The proliferation promoting activity of G-CSF has suggested implications of the G-CSF in cancer growth and tumor microenvironment. Various case report studies have correlated high G-CSF plasma levels derived from G-CSF producing tumors patient with aberrant tumor progression and poor outcome. The first report of G-CSF associated tumors was in the 1970s with plasma G-CSF increased in nude mice transplanted with human lung cancer cells (65). Clinically, the diagnostic criteria for G-CSF producing tumors are extreme leukocytosis and reduction in the white blood cell count (WBC) after tumor resection (65). Histologically, G-CSF is secreted into extracellular spaces while its positive staining is presented in the cytoplasm of tumor cells at the immunohistochemical examination (11, 15, 66). There is an increasing number of reports showing a high autocrine G-CSF production in a variety of cancers, including colon (5), breast (6), pancreas (11) and urinary tract (13, 67) among others. Given the pro-tumorigenic activities mentioned above, it may be important to gain an increased understanding of the tumors that produce high levels of G-CSF and the impact of this cytokine on those tumors.
G-CSF is highly produced in gastrointestinal tract tumors
The cytokine milieu in the tumor microenvironment is an important factor that can control cell proliferation, differentiation, migration and apoptosis. A plethora of clinical reports and animal cancer models have reported the increased expression of G-CSF and its receptor in several malignancies such as GI tumors (including esophagus, colon and gastric cancers) (5, 68), pancreatic and hepatocellular carcinoma (11, 15, 69–71). The elevated G-CSF plasma levels (normal < 18.1 pg/mL) in GI tumor patients are linked with strikingly poor prognosis and few groups have tried to elucidate the tumor microenvironment and signaling pathways that are implicated in cancer development and progression (72). G-CSF may act directly on tumors that express G-CSFR and promote tumor cell proliferation (68). However, the complete mechanism by which G-CSF promotes immunosuppressive immune cell phenotypes remains unclear. Accumulating data from the past years have also suggested a molecular mechanism of G-CSF facilitating metastasis through tumor promoting neutrophils. A possible tumor promoting mechanism might be the secreted G-CSF from the primary tumor that could drive the release of neutrophil extracellular traps (NETs) which are capable of capturing tumor cells within vessels and facilitate their migration into secondary organs leading to metastases (73). All the above observations highlight the importance of G-CSF in GI cancer physiology.
i). CRC:
The first study that reported the high expression of G-CSFR in CRC patients was published in 2005 and showed that 31 out of 42 human colorectal cancers showed an up-regulation of G-CSFR when compared with normal mucosa based on gene expression and immunochemistry experiments (5). A few years before, both in vitro and in vivo murine studies documented the role of G-CSF in cancer development by promoting tumor growth and neo-angiogenesis (74). Using a tumor implantation model, upon injection of murine syngeneic rectal cancer cells followed by treatment with G-CSF, the authors reported a promotion of tumor growth when compared with the control group. The tumor promoting ability of G-CSF and its receptor has also been reported in GI epithelial cells, gastric and colon cancer, where a tumor stage-dependent increase of G-CSF expression was observed as confirmed by flow cytometry and gene expression experiments (68). The in vitro treatment of gastric carcinoma cells and colon carcinoma cells with increasing doses of recombinant G-CSF led to an increase in cell proliferation and migration, while the opposite result were observed upon the addition of monoclonal G-CSF neutralizing antibody.
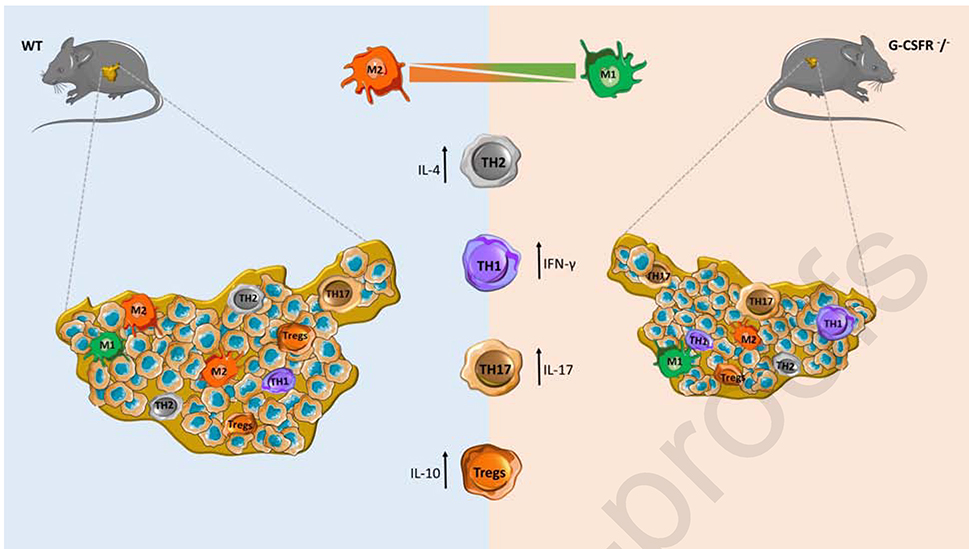
The implication of G-CSF in immune cells has also been described from our group, in an azoxymethane/dextran sodium sulfate (AOM/DSS) mouse model of colitis-associated cancer, suggesting that a tumor protective reaction is caused when anti-G-CSF treatment is used (43). The treatment resulted in colon neoplasm shrinkage and macrophages with decreased IL-10 levels and increased expression of IL-12, implying a polarization of these cells with pro-tumorigenic and anti-tumorigenic characteristics respectively (Figure 2). The balance of IL-2, IFNγ and IL-10 secreted cytokines was also disturbed in the mice tumors that were treated with anti-G-CSF compared to isotype control, leading to increase number of other immune cells types such as NK, CD4 and CD8. In order to further understand the tumor microenvironment and identify the immune cells implicated, we recently reported the impact of G-CSF in tumor-associated macrophages (TAMs) and T subpopulations cells in mouse models of colorectal cancer (48). We showed that injection of G-CSFR−/− mice with MC38 colon cancer cells resulted in decreased tumor growth and an elevated cytokine profile with decreased levels of IL-10 and increased levels of IFNγ and IL-17A along with an increase in a cytotoxic immune response (Figure 2). Similar results were observed upon adoptive transfer of G-CSFR−/− CD4+ or CD8+ T cells into the peritumoral region of WT or Rag2−/− mice, suggesting a pro-tumorigenic role for G-CSF in gastrointestinal tumors through inhibiting CD4+ and CD8+ T cell responses by promoting IL-10 secretion and reducing cytotoxic responses. In order to characterize the macrophage phenotype and their role in tumor progression, adoptive transfer of WT or G-CSFR−/− macrophages was performed, in order to monitor the development of colon and pancreatic tumor (37). Both in vitro and in vivo experiments showed that G-CSFR genetic ablation led to increased levels of IL-12, NOS2 expression and NO production in concert with decreased levels of IL-10, enhancing the important role of G-CSF blockade in anti-tumorigenic adaptive immune responses mediated through macrophage related tumor cytotoxicity. Another study reported the role of G-CSF in controlling non-coding genes (75). In vitro experiments showed that treatment of the HCT8 colon cancer cell line with G-CSF led to upregulation of miR-125b, which in turn promoted tumor cell migration and invasion. Additionally, other studies have also tried to elucidate the impact and the survival outcomes of the G-CSF treatment in metastatic colon cancer, where neutropenic patients received G-CSF (filgrastim or pegfilgrastim) as a maintenance therapy during chemotherapy (known as secondary prophylaxis) (76, 77). Although the treatment of G-CSF reduced the grades 3 and 4 of neutropenia and febrile neutropenia, no statistically significant benefit on the survival rate was observed. All the above data provide more details on the role and the complexity of G-CSF in tumor development and progression through possible activation of different molecules and signaling pathways in different tumor cells.
Figure 2. Role of G-CSF in moderating the tumor microenvironment of colon cancer.
In a mouse models of colon cancer, G-CSF affects the polarization of tumor associated macrophages (TAMs) and T cells. As shown here, G-CSFR−/− mice had significantly decreased tumor growth of MC38 colon cancer cells. Mouse tumor size, cytokine expression, T cell phenotype, and cytotoxic activity were analyzed and alterations were observed between WT and G-CSFR−/− mice. Furthermore, the G-CSF/G-CSFR stimulated IL-10-producing, FoxP3-expressing CD4+ T cells, whereas G-CSFR−/− T cells exhibited increased IFNγ and IL-17A production, leading to increased cytotoxic activity in the tumor microenvironment. G-CSFR−/− TAMs were characterized by higher levels of NOS2 expression and NO production, which led to greater tumor related cytotoxicity both in vitro and in vivo.
ii). Upper GI Tract:
G-CSF-producing cancers from the upper gastrointestinal tract including esophagus and esophagogastric junction may be rare, with only few cases being reported thus far (78–80). Statistics have shown that almost 90% of the patients presenting G-CSF-secreting esophagus cancer were males, and so far, this sex related tendency remains unanswered. The main symptoms that were found in these patients were leukocytosis, persistent fever and elevation of the C-reacting proteins (CRP) with few cases showing also neutrophil infiltration in the tumor (81). In 2011, another case study reported a G-CSF producing carcinosarcoma where the immunohistochemical analysis showed positive staining for G-CSF and interleukin-6 (IL-6) in the spindle/pleomorphic cells (82). More recently, another G-CSF carcinosarcoma producing tumor was reported in the esophagus with diffuse neutrophil infiltration and G-CSF immunostaining in the sarcoma component (83). Although rare, G-CSF producing tumors of the upper GI tract are associated with aggressive tumor types.
iii). Gastric Cancer:
Likewise, high levels of G-CSF and its receptor have been reported in gastric cancers, particularly in aggressive tumors (84–86). In 2014, a case was reported for a G-CSF secreting gastric cancer with the patient presenting an immunohistochemical G-CSF and HER-2 positive staining (87). In the same year, our group also documented high expression of the G-CSF and G-CSFR in gastric cancer patient samples (68). In this study, a series of gastric and colon cancer samples showed that both stromal cells and carcinoma cells produce G-CSF which may act on G-CSFR expressing tumor cells to promote tumor cell proliferation and migration. Another case report in 2017, described a gastric adenosquamous carcinoma linked with severe anemia and leukocytosis. The histopathologic evaluation showed that the tumor consisted of areas of both squamous cell carcinoma and adenocarcinoma while the immunohistochemical analysis revealed a positive anti-G-CSF antibody staining (85). High levels of G-CSF and its receptor were also reported in a clinical study of 40 gastric cancer patients when compared to normal mucosa tissues (88). Overexpression of G-CSF and G-CSFR were associated with high cancer antigen 72–4 (CA 72–4) and VEGF-A levels and associated with later tumor stages, metastases and poor survival. Recently, intercorrelation analysis work conducted in our lab to decipher the cytokine network in gastric cancers demonstrated that G-CSF is linked with the Map kinase-activated protein kinase 2 (MK2) and its downstream mediators that were also found to be upregulated in these tumors and correlated with metastases (89).
iv). Pancreatic cancer:
Pancreatic cancer is another G-CSF-producing tumor type where plasma G-CSF detection provides an important evaluation of early prognosis and tumor stage (90). Thus far, there are some pancreatic cancer reports indicating high G-CSF production and the majority were reported in Japan (91, 92). The first reported case of G-CSF secreting pancreatic cancer was reported in 1989 when a 71-year-old male patient was diagnosed with pancreatic carcinoma, based on a high G-CSF serum levels (93). Another case was described for a ductal adenocarcinoma producing G-CSF as diagnosed by immunohistochemical evaluation (94). In 1996, a rare case of anaplastic carcinoma (identified as pancreatic cystadenocarcinoma) producing G-CSF was also described. The patient presented high G-CSF concentration in the aspirated tumor fluid (mucin) at its early stage without leukocytosis, presenting G-CSF concentration higher than 2400 pg/mL (11). Most of the pancreas cancers producing G-CSF refer to anaplastic carcinoma of the pancreas. Patients were diagnosed upon showing fever, leukocytosis, weight loss and abdominal pain, with all cases showing overexpression of the G-CSF in the tumors, detected through histological examination and positive immunohistochemical staining (69, 70, 95–97).
v). Liver cancer
The first case of a rapidly growing and poorly differentiated hepatocellular carcinoma (HCC) producing G-CSF was reported in 1999. The patient presented with elevated levels of G-CSF and the immunohistochemical staining confirmed the presence of secreted G-CSF in the cytoplasm of HCC cells (98). Several more cases of HCC secreting G-CSF have been reported worldwide with most of them being diagnosed as poorly/moderately differentiated HCCs as previously reviewed (15, 71). The case presented by Kohono, et al, showed a HCC case that was reported with the G-CSF producing tumor composed of sarcomatous spindle-shaped malignant cells, pleomorphic cells with intense infiltration of neutrophils and macrophages, while the immunohistochemical staining detected the presence of G-CSFR. High serum levels of G-CSF and parathyroid hormone-related protein (PTHrP) have also been reported in patients with cholangiocellular carcinoma (99–101). Recently, another case of HCC producing G-CSF was reported, with the patient presenting a continuous fever, granulocytosis, elevated levels of G-CSF and a huge liver mass in the right lobe. Again, in this case, the immunohistochemical staining confirmed the presence of G-CSF in the cytoplasm of poorly differentiate HCC (102). A liver tumor that had metastasized from esophagogastric junction cancer has also been reported to produce G-CSF (81). This case reported progressive leukocytosis accompanied by high serum levels of G-CSF. Upon hepatectomy, there was decreased serum levels of G-CSF. Taken together, the cases mentioned above suggest that G-CSF production by tumors of the gastrointestinal tract is prevalent with esophagus, stomach, pancreas, liver, and particularly colon tumors. These tumors were usually reported with an aggressive phenotype and metastasis suggesting that perhaps G-CSF may be a possible biomarker for outcome on GI tumors.
G-CSF in breast cancer
In addition to colon cancer, breast cancer is the second most common cancer associated with G-CSF production. A study involving a large group of human samples revealed that triple-negative breast cancer exhibited higher G-CSF expression associated with higher amounts of CD163+ macrophages (tolerant macrophage, M2 phenotype) and a poorer overall survival rate when compared to other breast cancers of different histology (103). These studies suggest that higher G-CSF expression in this breast cancer subtype may facilitate cancer cell migration. Additionally, other studies have confirmed the expression of G-CSF in breast cancer tissues and its association with invasiveness (103–105). In line with these findings, breast cancer invasive MDA-MB-231 cells exhibit higher G-CSF expression compared to noninvasive breast cancer cells (T47D and MCF-7 cells) (106), suggesting an association between G-CSF expression and malignancy. Other studies have also reported that G-CSF plasma levels are significantly higher in breast cancer patients compared with healthy controls. After tumor resection, the plasma level of G-CSF decreased significantly, as opposed to M-CSF, suggesting that this cytokine may be helpful in the diagnosis and stage of breast cancer, as its level increased in patients with clinical stage III and IV and patients with N3 tumor when compared to patients with benign breast cancer and healthy controls (105).
Some studies in breast cancer have also focused on the effects of G-CSF on immune cells. One study examining a luminal A histological subtype with increased motility displayed high G-CSF expression and neutrophil aggregation associated to neutrophil extracellular trap (NETs) formation (107). Additionally, findings from another group showed that blocking G-CSFR with neutralizing antibodies inhibited metastasis in a neutrophil dependent manner (108). Another report suggested that G-CSF regulates macrophage phenotype and is linked with poor outcome in triple negative breast cancer (103). In addition to neutrophils and macrophages, G-CSF has been shown to promote MDSC cells, enhancing breast cancer growth in both human tissues and mouse models and has been suggested as a target to inhibit MDSCs activity (109, 110).
G-CSF producing tumors of the genitourinary tract
Several cases of urinary tract cancer have been reported to produce G-CSF (67, 111, 112). while the expression of G-CSF receptor has been described in a set of ovarian tumor biopsies and ovarian cancer cell lines (113). In 1988, Iwata et al. reported a case of G-CSF producing bladder cancer; the neoplasia diagnosed as sarcomatoid carcinoma with foci of transitional cell carcinoma presented marked infiltration of polymorphonuclear leukocytes (114). Later, another group reported a G-CSF producing bladder cancer in which the histological diagnosis was compatible with squamous cell carcinoma (115). In 2010, the aggressive nature of G-CSF producing cancers was also reported in four more cases of uterine cervical carcinoma. All cases showed elevated levels of G-CSF and all patients died from disease progression in less than 15 months (116). Studies in uterine cervical cancer have also demonstrated that G-CSF produced by tumor cells causes tumor related leukocytosis and that MDSCs are responsible for the rapidly growing and radio/chemo-resistant nature of these tumors (54, 117).
G-CSF producing tumors of the lung
Tumor-related leukocytosis and G-CSF- producing lung tumors have been reported to occur in the clinical course of lung cancer and this phenomenon is related mostly to non-small cell lung carcinoma (NSCLC) (118, 119). The aggressive nature of G-CSF secreting cancer was also reported in a case of lung cancer producing GCSF with rapid spread to the peritoneal cavity linked with multiple metastases in the adrenal glands, gallbladder, intestine, pancreas, liver, skin, and peritoneum (120). Elevated levels of G-CSF have been proposed as a marker of shorter survival in NSCLC patients even if a subsequent resection of a cytokine-secreting tumor has been successful. A few cases of lung cancer with simultaneous production of G-CSF and IL-6 have also been reported (119, 121), indicating how the complexity of the cytokine balance in the tumor microenvironment may affect cell proliferation and tumor progression.
G-CSF producing tumor of the head and neck
In head and neck squamous cell carcinoma, G-CSF producing tumors are prevalent, and G-CSF has been reported to be present in the primary tumor and in metastases (8, 122). In a case of thyroid tumor, high concentration of G-CSF, was not only detected in the primary tumor tissue, but also in metastatic lesions present in the lung and skin examined at autopsy (8). Another case of reported thyroid carcinoma of anaplastic type produced GM-CSF and G-CSF only after metastasizing to the lungs (123). Alternatively, G-CSF was present only in the primary tumor as seen in a hypopharyngeal squamous cell carcinoma case (122), where it was mainly expressed in the highly transformed area. The above reports indicate the broad variety of G-CSF presence in several primary or metastatic tumors such as squamous cell carcinomas or adenocarcinomas.
Therapeutic role of G-CSF
In 1986, the molecular cloning and expression of cDNA for hG-CSF was first described in E. Coli and few years later this recombinant protein was commercialized (124, 125). Since then, G-CSF is being widely used to prevent complications of severe febrile neutropenia (related to aplastic anemia, chemotherapy or leukemia) through reinforcing the production of granulocytes or antigen presenting cells (126–128). G-CSF is used to treat febrile neutropenia in patients (presenting less than 1500/μl neutrophils) with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs or in patients with chemotherapy induced neutropenia (129). The first clinical trials that tested the efficacy of the G-CSF treatment in chemotherapy caused neutropenia, were used in patients treated for small cell lung cancer with cyclophosphamide, doxorubicin, and etoposide in both the USA (1991) (127) and Europe (1993) (130). In both trials, the results showed reduction of febrile neutropenia, infection, antibiotic treatment, days of hospitalization but also a reduction in the chemotherapy dose per se. At that time, another clinical trial reported the use of G-CSF as a treatment in non-Hodgkin lymphoma patients, which were being treated with vincristine, doxorubicin, prednisolone, etoposide, cyclophosphamide, and bleomycin and led to reduction of neutropenia (131). For more than 20 years, the administration of G-CSF has been effectively used in decreasing the risk of patients developing febrile neutropenia when receiving myelosuppressive chemotherapy regimens (106, 132–134). Despite the fact that G-CSF is used as a powerful trigger for granulopoiesis in chemotherapy-induced neutropenia, concerns have been raised against the concomitant use of G-CSF and chemoradiation due to potential toxic interactions. Since 2006, the American Society of Clinical Oncology has advised avoiding the combination of G-CSF and chemoradiation (135).
Recently, clinical and pre-clinal trials have reported that the combined use of G-CSF with chemoradiation is safe (136). Moreover, another study in a preclinical mouse model in 2016, reported how the combination of radiation therapy (RT) with G-CSF treatments led to N1 (antitumor) polarization of tumor associated neutrophils (TANs) (137). In another APC approach, immunotherapy that included the vaccination of cancer patients with tumor antigen-associated peptide-pulsed dendritic cells (DCs), has been introduced in order to promote effective immune responses against cancer antigens (138). Recently, a cancer immunotherapy clinical study showed that when DC vaccines are primed with low-dose rhG-CSF 16–18 h prior to apheresis, result in 50% more harvested monocytes leading to higher levels of immunogenicity (139).
It is broadly accepted that blockade of PD-1/PDL-1 pathway is considered as one of the most applied tools of the anti-tumor immunotherapy for the past years, since the expression of these molecules has been reported in a multitude of tumors including lung, breast, ovarian, GI, urothelial cancer and melanomas (140). However, it has been reported that in several tumors, blockade of these molecules is efficient only in a reduced number of patients with the majority of tumor cells escaping treatment (141–143). As shown by our group recently in GI tumors, G-CSF inhibits the adaptive immune responses through IFNg and IL-17 dependent mechanisms (48). G-CSF reduces the infiltration of the CD4 and CD8 cells into the tumor microenvironment leading to a reduced efficacy of the PD-1/ PDL-1 blockade immunotherapy (144). Taken together the above data suggest the possibility of tumors with upregulation of G-SCF expression as being resistant to immunotherapy approaches. In the future, combination of PD-1/ PDL-1 blockade and anti-G-SCF treatment could possibly be a more efficient approach to improve the existing immunotherapy regimens and deal with immunotherapy resistant tumors.
Despite the fact that the administration of G-CSF is known to have significant clinical benefits, the tumor neo-angiogenesis caused by G-CSF leads to chemoresistance as shown in paclitaxel (PTX) chemotherapy treated breast carcinoma mice. However, correlation between high G-CSF expressing tumors and chemoresistance has also been reported in other cases such as medulloblastoma and uterine cervical cancers (54, 145). In a medulloblastoma, one study reported that G-CSF treatment following chemotherapy led to higher percentages of G-CSFR positive tumor cells that exhibited chemoresistance. This data suggests that exogenous administration of G-CSF might be responsible for the reduced tumor cell chemosensitivity, thus leading to their survival (145). An additional example of the chemoresistance in G-CSF producing tumor was also reported in uterine cancer cells. The authors demonstrated that high G-CSF expression led to resistant tumors to cisplatin-based chemotherapy regimen, through an MDSC- mediated mechanism, suggesting that depletion of these cells might contribute to a more favorable outcome. Taken together all the above data indicate the need for a combined strategy between chemotherapy and blocking possible G-CSF protomorigenic effects in anti- tumoral treatments and reducing the possibility for the occurrence of chemoresistant tumor cells.
Furthermore, additional non-tumor related therapeutic roles for G-CSF have also been documented. Studies have reported that G-CSF has been used to trigger the immune system in patients with pneumonia, HIV, severe diabetic foot infections and more recently, its neuroprotective properties were reported to treat stroke (146–149). In 2020, few cases also reported that rhG-CSF treatment for patients with COVID-19 with lymphopenia did not lead to a clear clinical improvement (150). Although the data have not shown a comprehensible correlation between the administration of prophylactic G-CSF with SARS-CoV-2 infection, autopsy from the lungs revealed neutrophil infiltration in pulmonary capillaries and mucositis, implying that G-CSF does not protect against the risk of a COVID-19 infection, but it could enhance the proinflammatory pathways leading to higher risk (151, 152). Overall, as with any manipulation of the immune system, there are pros and cons of therapeutic administration of cytokines for tumor related or non-related diseases.
Future perspectives
A plethora of studies have shown that G-CSF secreting tumors are highly aggressive and directly linked with secondary metastasis, worse prognosis and low survival rates, while other studies have suggested that within the G-CSF secreting tumor the dynamic changes caused in the tumor microenvironment can be used as markers of early disease progression and therapeutic response. Despite the fact that the widely applicable use of G-CSF as a prophylaxis in the chemotherapy-induced neutropenia has provided an important tool to overcome this pathological condition, scientists and clinicians should also take into account the pro-tumorigenic properties of G-CSF. As reported in this review, more and more studies are reporting the role of G-CSF in tumor growth and tumor microenvironment. In vivo and in vitro administration of G-CSF may lead to altered cytokine production, support inflammation and directly affect the polarization of macrophages and T helper cells, promoting tumorigenesis. Thus, understanding the complex biology of the autocrine G-CSF biology in the tumor microenvironment, might in the future provide advanced strategies onto novel therapeutic avenues for the treatment and proper clinical managements of patients with chemotherapy-induced neutropenia.
Acknowledgments
This manuscript was supported by NCI R01CA207051 (EJB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78(11):2791–808. [PubMed] [Google Scholar]
- 2.Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schäbitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–98. Epub 2005/07/07. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimoji K, Yuasa S, Onizuka T, Hattori F, Tanaka T, Hara M, Ohno Y, Chen H, Egasgira T, Seki T, Yae K, Koshimizu U, Ogawa S, Fukuda K. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell. 2010;6(3):227–37. doi: 10.1016/j.stem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Chen L, Liu F, Zhao N, Xu L, Fu B, Li Y. Efficacy and tolerability of granulocyte colony-stimulating factors in cancer patients after chemotherapy: A systematic review and Bayesian network meta-analysis. Sci Rep. 2019;9(1):15374. Epub 2019/10/25. doi: 10.1038/s41598-019-51982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Liu F, Xu Z, Chen C, Wu X, Li G, Li J. Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad Med J. 2005;81(955):333–7. doi: 10.1136/pgmj.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukui Y, Kawashima M, Kawaguchi K, Takeuchi M, Hirata M, Kataoka TR, Sakurai T, Kataoka M, Kanao S, Nakamoto Y, Hirata K, Yoshimura M, Yoshikawa K, Ishiguro H, Toi M. Granulocyte-colony-stimulating factor-producing metaplastic carcinoma of the breast with significant elevation of serum interleukin-17 and vascular endothelial growth factor levels. Int Cancer Conf J. 2018;7(3):107–13. Epub 2018/06/01. doi: 10.1007/s13691-018-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita T, Ogasawara Y, Naito M, Doihara H, Shimizu N. Anaplastic Thyroid Carcinoma Associated with Granulocyte Colony-Stimulating Factor: Report of a Case. Surgery Today. 2005;36(1):63–7. doi: 10.1007/s00595-005-3100-x. [DOI] [PubMed] [Google Scholar]
- 8.Nakada T, Sato H, Inoue F, Mizorogi F, Nagayama K, Tanaka T. The production of colony-stimulating factors by thyroid carcinoma is associated with marked neutrophilia and eosinophilia. Internal medicine (Tokyo, Japan). 1996;35(10):815–20. doi: 10.2169/internalmedicine.35.815. [DOI] [PubMed] [Google Scholar]
- 9.Yazawa S, Toshimori H, Nakatsuru K, Katakami H, Takemura J, Matsukura S. Thyroid Anaplastic Carcinoma Producing Granulocyte-Colony-Stimulating Factor and Parathyroid Hormone-Related Protein. Internal Medicine. 1995;34(6):584–8. doi: 10.2169/internalmedicine.34.584. [DOI] [PubMed] [Google Scholar]
- 10.Kitade H, Yanagida H, Yamada M, Satoi S, Yoshioka K, Shikata N, Kon M. Granulocyte-colony stimulating factor producing anaplastic carcinoma of the pancreas treated by distal pancreatectomy and chemotherapy: report of a case. Surg Case Rep. 2015;1(1):46. Epub 2015/05/30. doi: 10.1186/s40792-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uematsu T, Tsuchie K, Ukai K, Kimoto E, Funakawa T, Mizuno R. Granulocyte-colony stimulating factor produced by pancreatic carcinoma. International journal of pancreatology: official journal of the International Association of Pancreatology. 1996;19(2):135–9. doi: 10.1007/BF02805227. [DOI] [PubMed] [Google Scholar]
- 12.Vinzens S, Zindel J, Zweifel M, Rau T, Gloor B, Wochner A. Granulocyte Colony-stimulating Factor Producing Anaplastic Carcinoma of the Pancreas: Case Report and Review of the Literature. Anticancer Res. 2017;37(1):223–8. doi: 10.21873/anticanres.11310. [DOI] [PubMed] [Google Scholar]
- 13.Satoh H, Abe Y, Katoh Y, Komine Y, Nakamura M, Tamaoki N. Bladder Carcinoma Producing Granulocyte Colony-Stimulating Factor: A Case Report. Journal of Urology. 1993;149(4):843–5. doi: 10.1016/S0022-5347(17)36229-8. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Asano S, Ueyama Y, Mori M, Okabe T, Kondo Y, Ohsawa N, Kosaka K. Granulocytosis and colony-stimulating activity (CSA) produced by a human squamous cell carcinoma. Cancer. 1979;43(2):605–10. doi: . [DOI] [PubMed] [Google Scholar]
- 15.Kohno M, Shirabe K, Mano Y, Muto J, Motomura T, Takeishi K, Toshima T, Yoshimatsu M, Ijichi H, Harada N, Aishima S, Uchiyama H, Yoshizumi T, Taketomi A, Maehara Y. Granulocyte colony-stimulating-factor-producing hepatocellular carcinoma with extensive sarcomatous changes: report of a case. Surgery Today. 2013;43(4):439–45. doi: 10.1007/s00595-012-0202-0. [DOI] [PubMed] [Google Scholar]
- 16.Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol. 2005;175(11):7085–91. doi: 10.4049/jimmunol.175.11.7085. [DOI] [PubMed] [Google Scholar]
- 17.Akbarzadeh S, Ward AC, McPhee DO, Alexander WS, Lieschke GJ, Layton JE. Tyrosine residues of the granulocyte colony-stimulating factor receptor transmit proliferation and differentiation signals in murine bone marrow cells. Blood. 2002;99(3):879–87. doi: 10.1182/blood.v99.3.879. [DOI] [PubMed] [Google Scholar]
- 18.Ward AC, Oomen SP, Smith L, Gits J, van Leeuwen D, Soede-Bobok AA, Erpelinck-Verschueren CA, Yi T, Touw IP. The SH2 domain-containing protein tyrosine phosphatase SHP-1 is induced by granulocyte colony-stimulating factor (G-CSF) and modulates signaling from the G-CSF receptor. Leukemia. 2000;14(7):1284–91. doi: 10.1038/sj.leu.2401822. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga R, Ishizaka-Ikeda E, Pan CX, Seto Y, Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991;10(10):2855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aritomi M, Kunishima N, Okamoto T, Kuroki R, Ota Y, Morikawa K. Atomic structure of the GCSF-receptor complex showing a new cytokine-receptor recognition scheme. Nature. 1999;401(6754):713–7. doi: 10.1038/44394. [DOI] [PubMed] [Google Scholar]
- 21.Tamada T, Honjo E, Maeda Y, Okamoto T, Ishibashi M, Tokunaga M, Kuroki R. Homodimeric cross-over structure of the human granulocyte colony-stimulating factor (GCSF) receptor signaling complex. Proc Natl Acad Sci U S A. 2006;103(9):3135–40. Epub 2006/02/21. doi: 10.1073/pnas.0511264103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SF, Lin SS, Yang HC, Chou YY, Gao JI, Lu SC. LPS-Induced G-CSF Expression in Macrophages Is Mediated by ERK2, but Not ERK1. PLoS One. 2015;10(6):e0129685. Epub 2015/06/26. doi: 10.1371/journal.pone.0129685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartalucci N, Tozzi L, Bogani C, Martinelli S, Rotunno G, Villeval JL, Vannucchi AM. Co-targeting the PI3K/mTOR and JAK2 signalling pathways produces synergistic activity against myeloproliferative neoplasms. J Cell Mol Med. 2013;17(11):1385–96. Epub 2013/11/17. doi: 10.1111/jcmm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood. 2006;107(5):1847–56. Epub 2005/11/10. doi: 10.1182/blood-2005-04-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dwivedi P, Greis KD. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol. 2017;46:9–20. Epub 2016/10/24. doi: 10.1016/j.exphem.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Futami M, Zhu QS, Whichard ZL, Xia L, Ke Y, Neel BG, Feng GS, Corey SJ. G-CSF receptor activation of the Src kinase Lyn is mediated by Gab2 recruitment of the Shp2 phosphatase. Blood. 2011;118(4):1077–86. Epub 2011/06/02. doi: 10.1182/blood-2009-12-261636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn SM, Coles LS, Lang RK, Gerondakis S, Vadas MA, Shannon MF. Requirement for nuclear factor (NF)-kappa B p65 and NF-interleukin-6 binding elements in the tumor necrosis factor response region of the granulocyte colony-stimulating factor promoter. Blood. 1994;83(9):2469–79. [PubMed] [Google Scholar]
- 28.Nishizawa M, Nagata S. Regulatory elements responsible for inducible expression of the granulocyte colony-stimulating factor gene in macrophages. Mol Cell Biol. 1990;10(5):2002–11. doi: 10.1128/mcb.10.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liongue C, Ward AC. Granulocyte colony-stimulating factor receptor mutations in myeloid malignancy. Front Oncol. 2014;4:93. Epub 2014/05/01. doi: 10.3389/fonc.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleetwood AJ, Cook AD, Hamilton JA. Functions of Granulocyte-Macrophage Colony-Stimulating Factor. Critical Reviews in Immunology. 2005;25(5):405–28. doi: 10.1615/CritRevImmunol.v25.i5.50. [DOI] [PubMed] [Google Scholar]
- 31.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33(5):949–55. Epub 2012/03/16. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 32.Roberts AW. G-CSF: a key regulator of neutrophil production, but thaťs not all! Growth Factors. 2005;23(1):33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 33.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17(4):413–23. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 34.Martins AJ, Spanton S, Sheikh HI, Kim SO. The anti-inflammatory role of granulocyte colony-stimulating factor in macrophage-dendritic cell crosstalk after Lactobacillus rhamnosus GR-1 exposure. Journal of Leukocyte Biology. 2011;89(6):907–15. doi: 10.1189/jlb.0810445. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nature Reviews Immunology. 2008;8(7):533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 36.Meshkibaf S, William Gower M, Dekaban GA, Ouk Kim S. G-CSF preferentially supports the generation of gut-homing Gr-1 high macrophages in M-CSF-treated bone marrow cells. Journal of Leukocyte Biology. 2014;96(4):549–61. doi: 10.1189/jlb.1A0314-172R. [DOI] [PubMed] [Google Scholar]
- 37.Karagiannidis I, de Santana Van Vilet E, Said Abu Egal E, Phinney B, Jacenik D, Prossnitz ER, Beswick EJ. G-CSF and G-CSFR Induce a Pro-Tumorigenic Macrophage Phenotype to Promote Colon and Pancreas Tumor Growth. Cancers (Basel) 2020;12(10). Epub 2020/10/06. doi: 10.3390/cancers12102868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrams SI, Waight JD. Identification of a G-CSF-Granulocytic MDSC axis that promotes tumor progression. Oncoimmunology. 2012;1(4):550–1. doi: 10.4161/onci.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawano M, Mabuchi S, Matsumoto Y, Sasano T, Takahashi R, Kuroda H, Kozasa K, Hashimoto K, Isobe A, Sawada K, Hamasaki T, Morii E, Kimura T. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci Rep. 2015;5:18217. Epub 2015/12/15. doi: 10.1038/srep18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hareng L, Hartung T. Induction and Regulation of Endogenous Granulocyte Colony-Stimulating Factor Formation. Biological Chemistry. 2002;383(10). doi: 10.1515/BC.2002.172. [DOI] [PubMed] [Google Scholar]
- 41.Schlahsa L, Jaimes Y, Blasczyk R, Figueiredo C. Granulocyte-colony-stimulatory factor: a strong inhibitor of natural killer cell function. Transfusion. 2011;51(2):293–305. Epub 2010/08/16. doi: 10.1111/j.1537-2995.2010.02820.x. [DOI] [PubMed] [Google Scholar]
- 42.Su YC, Li SC, Hsu CK, Yu CC, Lin TJ, Lee CY, Liao HF. G-CSF downregulates natural killer cell-mediated cytotoxicity in donors for hematopoietic SCT. Bone Marrow Transplant. 2012;47(1):73–81. Epub 2011/02/28. doi: 10.1038/bmt.2011.22. [DOI] [PubMed] [Google Scholar]
- 43.Morris KT, Castillo EF, Ray AL, Weston LL, Nofchissey RA, Hanson JA, Samedi VG, Pinchuk IV, Hudson LG, Beswick EJ. Anti-G-CSF treatment induces protective tumor immunity in mouse colon cancer by promoting protective NK cell, macrophage and T cell responses. Oncotarget. 2015;6(26):22338–47. doi: 10.18632/oncotarget.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franzke A, Piao W, Lauber J, Gatzlaff P, Könecke C, Hansen W, Schmitt-Thomsen A, Hertenstein B, Buer J, Ganser A. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102(2):734–9. Epub 2003/04/03. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- 45.Morikawa K, Morikawa S, Nakamura M, Miyawaki T. Characterization of granulocyte colony-stimulating factor receptor expressed on human lymphocytes. Br J Haematol. 2002;118(1):296–304. doi: 10.1046/j.1365-2141.2002.03574.x. [DOI] [PubMed] [Google Scholar]
- 46.Toh HC,Sun L, Soe Y, Wu Y, Phoon YP, Chia WK, Wu J, Wong KY, Tan P. G-CSF induces a potentially tolerant gene and immunophenotype profile in T cells in vivo. Clinical Immunology. 2009;132(1):83–92. doi: 10.1016/j.clim.2009.03.509. [DOI] [PubMed] [Google Scholar]
- 47.Malashchenko VV, Meniailo ME, Shmarov VA, Gazatova ND, Melashchenko OB, Goncharov AG, Seledtsova GV, Seledtsov VI. Direct anti-inflammatory effects of granulocyte colony-stimulating factor (G-CSF) on activation and functional properties of human T cell subpopulations in vitro. Cell Immunol. 2018;325:23–32. Epub 2018/01/19. doi: 10.1016/j.cellimm.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Karagiannidis I, Jerman SJ, Jacenik D, Phinney BB, Yao R, Prossnitz ER, Beswick EJ. G-CSF and G-CSFR Modulate CD4 and CD8 T Cell Responses to Promote Colon Tumor Growth and Are Potential Therapeutic Targets. Front Immunol. 2020;11:1885. Epub 2020/09/15. doi: 10.3389/fimmu.2020.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan L, Delmonte J, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86(12):4422–9. [PubMed] [Google Scholar]
- 50.Crough T, Nieda M, Nicol AJ. Granulocyte colony-stimulating factor modulates alpha-galactosylceramide-responsive human Valpha24+Vbeta11+NKT cells. J Immunol. 2004;173(8):4960–6. doi: 10.4049/jimmunol.173.8.4960. [DOI] [PubMed] [Google Scholar]
- 51.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107(50):21248–55. Epub 2010/11/16. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106(16):6742–7. Epub 2009/04/03. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srivastava MK, Zhu L, Harris-White M, Kar UK, Kar U, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7(7):e40677. Epub 2012/07/16. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawano M, Mabuchi S, Matsumoto Y, Sasano T, Takahashi R, Kuroda H, Kozasa K, Hashimoto K, Isobe A, Sawada K, Hamasaki T, Morii E, Kimura T. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Scientific Reports. 2015;5(1):18217–. doi: 10.1038/srep18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasano T, Mabuchi S, Kozasa K, Kuroda H, Kawano M, Takahashi R, Komura N, Yokoi E, Matsumoto Y, Hashimoto K, Sawada K, Morii E, Kimura T. The Highly Metastatic Nature of Uterine Cervical/Endometrial Cancer Displaying Tumor-Related Leukocytosis: Clinical and Preclinical Investigations. Clin Cancer Res. 2018;24(16):4018–29. Epub 2018/05/11. doi: 10.1158/1078-0432.CCR-17-2472. [DOI] [PubMed] [Google Scholar]
- 56.Yokoi E, Mabuchi S, Komura N, Shimura K, Kuroda H, Kozasa K, Takahashi R, Sasano T, Kawano M, Matsumoto Y, Kodama M, Hashimoto K, Sawada K, Kimura T. The role of myeloid-derived suppressor cells in endometrial cancer displaying systemic inflammatory response: clinical and preclinical investigations. Oncoimmunology. 2019;8(12):e1662708. Epub 2019/09/25. doi: 10.1080/2162402X.2019.1662708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phan VT, Wu X, Cheng JH, Sheng RX, Chung AS, Zhuang G, Tran C, Song Q, Kowanetz M, Sambrone A, Tan M, Meng YG, Jackson EL, Peale FV, Junttila MR, Ferrara N. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proceedings of the National Academy of Sciences. 2013;110(15):6079–84. doi: 10.1073/pnas.1303302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proceedings of the National Academy of Sciences. 2009;106(16):6742–7. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng Q, Wang Y, Yuan W, Ma J. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell. 2016;7(2):130–40. Epub 2016/01/21. doi: 10.1007/s13238-015-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valdembri D, Serini G, Vacca A, Ribatti D, Bussolino F. In vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSF. The FASEB Journal. 2002;16(2):1–19. doi: 10.1096/fj.01-0633fje. [DOI] [PubMed] [Google Scholar]
- 61.Zgheib A, Lamy S, Annabi B. Epigallocatechin Gallate Targeting of Membrane Type 1 Matrix Metalloproteinase-mediated Src and Janus Kinase/Signal Transducers and Activators of Transcription 3 Signaling Inhibits Transcription of Colony-stimulating Factors 2 and 3 in Mesenchymal Stromal. Journal of Biological Chemistry. 2013;288(19):13378–86. doi: 10.1074/jbc.M113.456533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hori S, Miyake M, Onishi S, Morizawa Y, Nakai Y, Tatsumi Y, Onishi K, Iida K, Gotoh D, Itami Y, Tanaka N, Fujimoto K. Evaluation of pro- and anti-tumor effects induced by three colony-stimulating factors, G-CSF, GM-CSF and M-CSF, in bladder cancer cells: Is G-CSF a friend of bladder cancer cells? International Journal of Oncology. 2019. doi: 10.3892/ijo.2019.4772. [DOI] [PubMed] [Google Scholar]
- 63.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-Selected Human Lung Cancer Stem Cells: Cytokine Network, Tumorigenic and Metastatic Properties. PLoS ONE. 2008;3(8):e3077–e. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwal S, Lakoma A, Chen Z, Hicks J, Metelitsa LS, Kim ES, Shohet JM. G-CSF Promotes Neuroblastoma Tumorigenicity and Metastasis via STAT3-Dependent Cancer Stem Cell Activation. Cancer Research. 2015;75(12):2566–79. doi: 10.1158/0008-5472.CAN-14-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asano S, Urabe A, Okabe T, Sato N, Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49(5):845–52. [PubMed] [Google Scholar]
- 66.Nicola NA. Hemopoietic Cell Growth Factors and Their Receptors. Annual Review of Biochemistry. 1989;58(1):45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- 67.Morinaga R, Kawahara T, Kuroda S, Inayama Y, Uemura H. Granulocyte Colony-Stimulating Factor-Producing Bladder Cancer. Case Rep Oncol. 2019;12(2):603–7. Epub 2019/08/06. doi: 10.1159/000502174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. British Journal of Cancer. 2014;110(5):1211–20. doi: 10.1038/bjc.2013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitade H, Yanagida H, Yamada M, Satoi S, Yoshioka K, Shikata N, Kon M. Granulocyte-colony stimulating factor producing anaplastic carcinoma of the pancreas treated by distal pancreatectomy and chemotherapy: report of a case. Surgical Case Reports. 2015;1(1):46–. doi: 10.1186/s40792-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinzens S, Zindel J, Zweifel M, Rau T, Gloor B, Wochner A. Granulocyte Colony-stimulating Factor Producing Anaplastic Carcinoma of the Pancreas: Case Report and Review of the Literature. Anticancer research. 2017;37(1):223–8. doi: 10.21873/anticanres.11310. [DOI] [PubMed] [Google Scholar]
- 71.Araki K, Kishihara F, Takahashi K, Matsumata T, Shimura T, Suehiro T, Kuwano H. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007;27(5):716–21. doi: 10.1111/j.1478-3231.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 72.Fan Z, Li Y, Zhao Q, Fan L, Tan B, Zuo J, Hua K, Ji Q. Highly Expressed Granulocyte Colony-Stimulating Factor (G-CSF) and Granulocyte Colony-Stimulating Factor Receptor (G-CSFR) in Human Gastric Cancer Leads to Poor Survival. Med Sci Monit. 2018;24:1701–11. Epub 2018/03/23. doi: 10.12659/msm.909128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mouchemore KA, Anderson RL, Hamilton JA. Neutrophils, G-CSF and their contribution to breast cancer metastasis. FEBS J. 2018;285(4):665–79. Epub 2017/09/21. doi: 10.1111/febs.14206. [DOI] [PubMed] [Google Scholar]
- 74.Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297(4):1058–61. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Ma X, An H, Xu C, Cao W, Yuan W, Ma J. Upregulation of microRNA-125b by G-CSF promotes metastasis in colorectal cancer. Oncotarget. 2017;8(31):50642–54. Epub 2017/04/06. doi: 10.18632/oncotarget.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amadio A, Burkes R, Bailie T, McLean M, Coleman B. Impact of granulocyte colony-stimulating factors in metastatic colorectal cancer patients. Curr Oncol. 2014;21(1):e52–61. doi: 10.3747/co.21.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hecht JR, Pillai M, Gollard R, Heim W, Swan F, Patel R, Dreiling L, Mo M, Malik I. A randomized, placebo-controlled phase ii study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer. 2010;9(2):95–101. doi: 10.3816/CCC.2010.n.013. [DOI] [PubMed] [Google Scholar]
- 78.Hoshimoto S, Hoshi N, Ozawa I, Tomikawa M, Shirakawa H, Fujita T, Wakamatsu S, Hoshi S, Hirabayashi K, Hishinuma S, Ogata Y. Rapid progression of a granulocyte colony-stimulating factor-producing liver tumor metastasized from esophagogastric junction cancer: A case report and literature review. Oncol Lett. 2018;15(5):6475–80. Epub 2018/03/01. doi: 10.3892/ol.2018.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi S, Nagata Y, Tokai H, Ito M, Fujioka H. Multidisciplinary therapy for granulocyte-colony-stimulating factor producing carcinosarcoma of the esophagus: report of a case. Clin Case Rep. 2015;3(8):681–5. Epub 2015/06/18. doi: 10.1002/ccr3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimakawa T, Asaka S, Usuda A, Yamaguchi K, Yoshimatsu K, Shiozawa S, Katsube T, Naritaka Y. Granulocyte-colony stimulating factor (G-CSF)-producing esophageal squamous cell carcinoma: a case report. Int Surg. 2014;99(3):280–5. doi: 10.9738/INTSURG-D-13-00265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoshimoto S, Hoshi N, Ozawa I, Tomikawa M, Shirakawa H, Fujita T, Wakamatsu S, Hoshi S, Hirabayashi K, Hishinuma S, Ogata Y. Rapid progression of a granulocyte colony-stimulating factor-producing liver tumor metastasized from esophagogastric junction cancer: A case report and literature review. Oncology Letters. 2018. doi: 10.3892/ol.2018.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamura K, Nakashima H, Makihara K, Ishikawa N, Cyaen T, Hachiya Y, Fukuyama T, Hamada T, Hirano Y. Granulocyte colony-stimulating factor and IL-6 producing carcinosarcoma of the esophagus manifesting as leukocytosis and pyrexia: a case report. Esophagus. 2011;8(4):295–301. doi: 10.1007/s10388-011-0293-5. [DOI] [Google Scholar]
- 83.Shioga T, Matsushima S, Yamada E, Uchiyama T, Noto H, Suzuki D, Nonaka T, Miyazawa S, Komatsu T, Yamamoto Y, Sekido H, Niino H. Esophageal Carcinosarcoma that Was Diagnosed as a Granulocyte-colony Stimulating Factor and Interleukin-6-producing Tumor with a Tumor Fever. Internal Medicine. 2018;57(19):2819–25. doi: 10.2169/internalmedicine.0677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Endo K, Kohnoe S, Okamura T, Haraguchi M, Adachi E, Toh Y, Baba H, Maehara Y. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer. 2005;8(3):173–7. doi: 10.1007/s10120-005-0330-y. [DOI] [PubMed] [Google Scholar]
- 85.Moro K, Nagahashi M, Naito T, Nagai Y, Katada T, Minagawa M, Hasegawa J, Tani T, Shimakage N, Usuda H, Gabriel E, Kawaguchi T, Takabe K, Wakai T. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor: a case of a rare malignancy. Surg Case Rep. 2017;3(1):67. Epub 2017/05/11. doi: 10.1186/s40792-017-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamano T, Morii E, Ikeda J, Aozasa K. Granulocyte colony-stimulating factor production and rapid progression of gastric cancer after histological change in the tumor. Jpn J Clin Oncol. 2007;37(10):793–6. Epub 2007/08/24. doi: 10.1093/jjco/hym094. [DOI] [PubMed] [Google Scholar]
- 87.Matsushita K, Takeno A, Tamura S, Taniguchi H, Ishida T, Sato Y, Morimoto Y, Kusama H, Hashimoto T, Kimura K, Katsura Y, Ohmura Y, Nitta K, Kagawa Y, Okisiro M, Sakisaka H, Egawa C, Takeda Y, Kato T. [A Case of Granulocyte-Colony Stimulating Factor-Producing Gastric Cancer Successfully Treated with Trastuzumab]. Gan To Kagaku Ryoho 2015;42(12):1968–70. [PubMed] [Google Scholar]
- 88.Fan Z, Li Y, Zhao Q, Fan L, Tan B, Zuo J, Hua K, Ji Q. Highly Expressed Granulocyte Colony-Stimulating Factor (G-CSF) and Granulocyte Colony-Stimulating Factor Receptor (G-CSFR) in Human Gastric Cancer Leads to Poor Survival. Medical Science Monitor. 2018;24:1701–11. doi: 10.12659/MSM.909128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qeadan F, Bansal P, Hanson JA, Beswick EJ. The MK2 pathway is linked to G-CSF, cytokine production and metastasis in gastric cancer: a novel intercorrelation analysis approach. J Transl Med. 2020;18(1):137. Epub 2020/03/26. doi: 10.1186/s12967-020-02294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Groblewska M, Mroczko B, Wereszczyńska-Siemiatkowska U, Myśliwiec P, Kedra B, Szmitkowski M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clin Chem Lab Med. 2007;45(1):30–4. doi: 10.1515/CCLM.2007.025. [DOI] [PubMed] [Google Scholar]
- 91.Akasaki T, Einama T, Tashiro K, Nagata H, Yamazaki K, Nishikawa M, Hoshikawa M, Kimura A, Noro T, Ogata S, Aosasa S, Kajiwara Y, Shinto E, Yaguchi Y, Hiraki S, Tsujimoto H, Hase K, Ueno H, Yamamoto J. Successful resection of a granulocyte colony-stimulating factor-producing carcinoma of the pancreas: A case report. Mol Clin Oncol. 2019;11(4):359–63. Epub 2019/07/22. doi: 10.3892/mco.2019.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joshita S, Nakazawa K, Sugiyama Y, Kamijo A, Matsubayashi K, Miyabayashi H, Furuta K, Kitano K, Kawa S. Granulocyte-colony stimulating factor-producing pancreatic adenosquamous carcinoma showing aggressive clinical course. Intern Med. 2009;48(9):687–91. Epub 2009/05/01. doi: 10.2169/internalmedicine.48.1900. [DOI] [PubMed] [Google Scholar]
- 93.Ohwada S, Miyamoto Y, Fujii T, Kuribara T, Teshigawara O, Oyama T, Ishii H, Joshita T, Izuo M. [Colony stimulating factor producing carcinoma of the pancreas--a case report]. Gan No Rinsho. 1989;35(4):523–7. [PubMed] [Google Scholar]
- 94.Kawakami H, Kuwatani M, Fujiya Y, Uebayashi M, Konishi K, Makiyama H, Hashino S, Kubota K, Itoh T, Asaka M. [A case of granulocyte-colony stimulating factor producing ductal adenocarcinoma of the pancreas]. Nihon Shokakibyo Gakkai Zasshi. 2007;104(2):233–8. [PubMed] [Google Scholar]
- 95.Hayashi H, Eguchi N, Sumimoto K, Matsumoto K, Azakami T, Sumida T, Tamura T, Sumii M, Uraoka N, Shimamoto F. Autopsy of anaplastic carcinoma of the pancreas producing granulocyte colony-stimulating factor. Nihon Shokakibyo Gakkai Zasshi. 2016;113(8):1408–15. doi: 10.11405/nisshoshi.113.1408. [DOI] [PubMed] [Google Scholar]
- 96.Ikeda S, Okubo K, Shibahara H, Narita M, Morita K, Takeuchi A, Kanazawa H, Ito T, Nishimura D, Katada N. [An autopsy of G-CSF-producing anaplastic carcinoma of the pancreas with impaired accumulation on FDG-PET after S-1 chemotherapy]. Gan to kagaku ryoho Cancer & chemotherapy. 2013;40(6):789–92. [PubMed] [Google Scholar]
- 97.Seki H, Yasui N, Shimada A, Matsumoto H, Domoto H. [Resection of a Granulocyte Colony-Stimulating Factor-Producing Anaplastic Carcinoma of the Pancreas, Associated with Humoral Hypercalcemia of Malignancy]. Gan To Kagaku Ryoho. 2018;45(5):859–62. [PubMed] [Google Scholar]
- 98.Nagata H, Komatsu S, Takaki W, Okayama T, Sawabe Y, Ishii M, Kishimoto M, Otsuji E, Konosu H. Granulocyte colony-stimulating factor-producing hepatocellular carcinoma with abrupt changes. World J Clin Oncol. 2016;7(5):380–6. doi: 10.5306/wjco.v7.i5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ozawa N, Doi S, Tsujikawa T, Mabuchi M, Kajiyama Y, Sato K, Kikuchi K, Takahashi M, Kawamoto M, Yasuda I. Intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor and parathyroid hormone-related protein. Nihon Shokakibyo Gakkai zasshi = The Japanese journal of gastro-enterology. 2017;114(7):1285–92. doi: 10.11405/nisshoshi.114.1285. [DOI] [PubMed] [Google Scholar]
- 100.Sohda T, Shiga H, Nakane H, Watanabe H, Takeshita M, Sakisaka S. Cholangiocellular carcinoma that produced both granulocyte-colony-stimulating factor and parathyroid hormone-related protein. International Journal of Clinical Oncology. 2006;11(3):246–9. doi: 10.1007/s10147-006-0560-y. [DOI] [PubMed] [Google Scholar]
- 101.Suzumura K, Iimuro Y, Hirano T, Asano Y, Kuroda N, Okada T, Tanaka S, Nakasho K, Fujimoto J. Granulocyte Colony-Stimulating Factor—Producing Cholangiocellular Carcinoma. International Surgery. 2015;100(1):123–7. doi: 10.9738/INTSURG-D-13-00183.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakamoto Y, Kamiyama T, Yokoo H, Shimada S, Einama T, Wakayama K, Orimo T, Kamachi H, Naka T, Mitsuhashi T, Taketomi A. Hepatocellular carcinoma producing granulocyte colony-stimulating factor: diagnosis and treatment. Int Cancer Conf J. 2019;8(1):12–6. Epub 2018/09/24. doi: 10.1007/s13691-018-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hollmén M, Karaman S, Schwager S, Lisibach A, Christiansen AJ, Maksimow M, Varga Z, Jalkanen S, Detmar M. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology. 2016;5(3):e1115177. Epub 2015/11/24. doi: 10.1080/2162402X.2015.1115177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao Y, Slaney CY, Bidwell BN, Parker BS, Johnstone CN, Rautela J, Eckhardt BL, Anderson RL. BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Res. 2014;74(18):5091–102. doi: 10.1158/0008-5472.CAN-13-3171. [DOI] [PubMed] [Google Scholar]
- 105.Liu H, Yang Z, Lu W, Chen Z, Chen L, Han S, Wu X, Cai T, Cai Y. Chemokines and chemokine receptors: A new strategy for breast cancer therapy. Cancer Med. 2020;9(11):3786–99. Epub 2020/04/06. doi: 10.1002/cam4.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee YM, Lockwood C. Prognostic factors for risk stratification of adult cancer patients with chemotherapy-induced febrile neutropenia: A systematic review and meta-analysis. International Journal of Nursing Practice. 2013;19(6):557–76. doi: 10.1111/ijn.12099. [DOI] [PubMed] [Google Scholar]
- 107.Guo B, Oliver TG. Partners in Crime: Neutrophil-CTC Collusion in Metastasis. Trends Immunol. 2019;40(7):556–9. Epub 2019/05/15. doi: 10.1016/j.it.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swierczak A, Cook AD, Lenzo JC, Restall CM, Doherty JP, Anderson RL, Hamilton JA. The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunol Res. 2014;2(8):765–76. Epub 2014/04/29. doi: 10.1158/2326-6066.CIR-13-0190. [DOI] [PubMed] [Google Scholar]
- 109.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One. 2011;6(11):e27690. Epub 2011/11/16. doi: 10.1371/journal.pone.0027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, Abrams SI. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123(10):4464–78. Epub 2013/09/16. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ito S, Iwai Y, Fujii T, Yoshida N, Hayashi S. [Two cases of bladder tumor producing granulocyte colony stimulating factor]. Hinyokika kiyo Acta urologica Japonica. 1999;45(1):57–60. [PubMed] [Google Scholar]
- 112.Tachibana M, Murai M. G-CSF production in human bladder cancer and its ability to promote autocrine growth: a review. Cytokines Cell Mol Ther. 1998;4(2):113–20. [PubMed] [Google Scholar]
- 113.Ninci EB, Brandstetter T, Meinhold-Heerlein I, Bettendorf H, Sellin D, Bauknecht T. G-CSF receptor expression in ovarian cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2000;10(1):19–26. doi: 10.1046/j.1525-1438.2000.99076.x. [DOI] [PubMed] [Google Scholar]
- 114.Iwata T, Araki H, Kushima R, Date S, Tanaka S. [A case of bladder tumor producing granulocyte colony-stimulating factor]. Hinyokika Kiyo. 1999;45(12):847–50. [PubMed] [Google Scholar]
- 115.Ishida K, Yuhara K, Kanimoto Y. [A case of bladder tumor producing granulocyte colony-stimulating factor]. Hinyokika kiyo Acta urologica Japonica. 2004;50(4):253–6. doi: 15188618. [PubMed] [Google Scholar]
- 116.Matsumoto Y, Mabuchi S, Muraji M, Morii E, Kimura T. Squamous cell carcinoma of the uterine cervix producing granulocyte colony-stimulating factor: a report of 4 cases and a review of the literature. Int J Gynecol Cancer. 2010;20(3):417–21. doi: 10.1111/IGC.0b013e3181d15a11. [DOI] [PubMed] [Google Scholar]
- 117.Mabuchi S, Matsumoto Y, Kawano M, Minami K, Seo Y, Sasano T, Takahashi R, Kuroda H, Hisamatsu T, Kakigano A, Hayashi M, Sawada K, Hamasaki T, Morii E, Kurachi H, Matsuura N, Kimura T. Uterine cervical cancer displaying tumor-related leukocytosis: a distinct clinical entity with radioresistant feature. J Natl Cancer Inst. 2014;106(7). Epub 2014/06/19. doi: 10.1093/jnci/dju147. [DOI] [PubMed] [Google Scholar]
- 118.Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92(9):2399–405. doi: . [DOI] [PubMed] [Google Scholar]
- 119.Sakamoto A, Katakami H, Mukae H, Taniguchi H, Maki H, Ashitani J, Ihi T, Dohtsu Y, Matsukura S. [Simultaneous production of parathyroid hormone-related protein (PTHrP) and granulocyte colony-stimulating factor (G-CSF) in lung cancer patients with hypercalcemia and leukocytosis]. Nihon Kyobu Shikkan Gakkai zasshi. 1995;33(1):34–8. [PubMed] [Google Scholar]
- 120.Kaira K, Ishizuka T, Tanaka H, Tanaka Y, Yanagitani N, Sunaga N, Hisada T, Mori M. Lung cancer producing granulocyte colony-stimulating factor and rapid spreading to peritoneal cavity. J Thorac Oncol. 2008;3(9):1054–5. doi: 10.1097/JTO.0b013e3181834f7b. [DOI] [PubMed] [Google Scholar]
- 121.Yoshida Y, Sibusa T, Ishii Y, Akino K, Kikuchi T, Mita H, Adachi Y, Nakamura M, Adachi Y, Kato Y, Endo T. Granulocyte Colony-stimulating Factor- and Interleukin-6-producing Large-cell Carcinoma of the Lung with Sarcomatoid Changes Suggestive of Epithelial-mesenchymal Transition: An Autopsy Case Report. Internal Medicine. 2019;58(22):3305–11. doi: 10.2169/internalmedicine.2819-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tamura K, Yoshinaga T, Tanino M, Kimura T, Yamada N, Nishimura M, Fukuda S, Nishihara H, Shindoh M, Tanaka S. Hypopharyngeal squamous cell carcinoma producing both granulocyte colony-stimulating factor and parathyroid hormone-related protein. Pathology International. 2008;58(10):652–6. doi: 10.1111/j.1440-1827.2008.02285.x. [DOI] [PubMed] [Google Scholar]
- 123.Hashimoto S, Kamimura R, Morimoto M, Yoshikawa K, Kakudou K, Yunhua B. A case of thyroid carcinoma that transformed to anaplastic type and produced GM-CSF and G-CSF after metastasizing to lung. The Journal of the Japanese Association for Chest Surgery. 2009;23(7):959–63. doi: 10.2995/jacsurg.23.959. [DOI] [Google Scholar]
- 124.Nagata S, Tsuchiya M, Asano S, Kaziro Y, Yamazaki T, Yamamoto O, Hirata Y, Kubota N, Oheda M, Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature. 1986;319(6052):415–8. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- 125.Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, Chazin VR, Bruszewski J, Lu H, Chen KK, Barendt J, Platzer E, Moore MAS, Mertelsmann R, Welte K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232(4746):61–5. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 126.Bonilla MA, Dale D, Zeidler C, Last L, Reiter A, Ruggeiro M, Davis M, Koci B, Hammond W, Gillio A, Welte K. Long-term safety of treatment with recombinant human granulocyte colony-stimulating factor (r-metHuG-CSF) in patients with severe congenital neutropenias. Br J Haematol. 1994;88(4):723–30. doi: 10.1111/j.1365-2141.1994.tb05110.x. [DOI] [PubMed] [Google Scholar]
- 127.Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164–70. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 128.Tichelli A, Schrezenmeier H, Socié G, Marsh J, Bacigalupo A, Dührsen U, Franzke A, Hallek M, Thiel E, Wilhelm M, Höchsmann B, Barrois A, Champion K, Passweg JR. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117(17):4434–41. Epub 2011/01/13. doi: 10.1182/blood-2010-08-304071. [DOI] [PubMed] [Google Scholar]
- 129.American Society of Clinical Oncology. Recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol. 1994;12(11):2471–508. doi: 10.1200/JCO.1994.12.11.2471. [DOI] [PubMed] [Google Scholar]
- 130.Trillet-Lenoir V, Green J, Manegold C, Von Pawel J, Gatzemeier U, Lebeau B, Depierre A, Johnson P, Decoster G, Tomita D. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer. 1993;29A(3):319–24. doi: 10.1016/0959-8049(93)90376-q. [DOI] [PubMed] [Google Scholar]
- 131.Pettengell R, Gurney H, Radford JA, Deakin DP, James R, Wilkinson PM, Kane K, Bentley J, Crowther D. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin's lymphoma: a randomized controlled trial. Blood. 1992;80(6):1430–6. [PubMed] [Google Scholar]
- 132.Aapro MS, Bohlius J, Cameron DA, Lago LD, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. European Journal of Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 133.Choi MR, Solid CA, Chia VM, Blaes AH, Page JH, Barron R, Arneson TJ. Granulocyte colony-stimulating factor (G-CSF) patterns of use in cancer patients receiving myelosuppressive chemotherapy. Supportive Care in Cancer. 2014;22(6):1619–28. doi: 10.1007/s00520-014-2121-7. [DOI] [PubMed] [Google Scholar]
- 134.Vitolo U, Angrili F, DeCosta L, Wetten S, Federico M. G-CSF use in patients receiving first-line chemotherapy for non-Hodgkin’s lymphoma (NHL) and granulocyte-colony stimulating factors (G-CSF) as observed in clinical practice in Italy. Medical Oncology. 2016;33(12):139–. doi: 10.1007/s12032-016-0850-9. [DOI] [PubMed] [Google Scholar]
- 135.2006 Update of ASCO Practice Guideline Recommendations for the Use of White Blood Cell Growth Factors: Guideline Summary. J Oncol Pract. 2006;2(4):196–201. doi: 10.1200/JOP.2006.2.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Benna M, Guy JB, Bosacki C, Jmour O, Ben Mrad M, Ogorodniitchouk O, Soltani S, Lan M, Daguenet E, Mery B, Sotton S, Magné N, Vallard A. Chemoradiation and granulocyte-colony or granulocyte macrophage-colony stimulating factors (G-CSF or GM-CSF): time to think out of the box? Br J Radiol. 2020;93(1109):20190147. Epub 2020/02/04. doi: 10.1259/bjr.20190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takeshima T, Pop LM, Laine A, Iyengar P, Vitetta ES, Hannan R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. Proc Natl Acad Sci U S A. 2016;113(40):11300–5. Epub 2016/09/20. doi: 10.1073/pnas.1613187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. Epub 2011/11/17. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 139.Shimodaira S, Yanagisawa R, Koya T, Hirabayashi K, Higuchi Y, Sakamoto T, Togi M, Kato T, Kobayashi T, Koizumi T, Koido S, Sugiyama H. In Vivo Administration of Recombinant Human Granulocyte Colony-Stimulating Factor Increases the Immune Effectiveness of Dendritic Cell-Based Cancer Vaccination. Vaccines (Basel) 2019;7(3). Epub 2019/09/19. doi: 10.3390/vaccines7030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy. J Cell Physiol. 2019;234(2):1313–25. Epub 2018/09/07. doi: 10.1002/jcp.27172. [DOI] [PubMed] [Google Scholar]
- 141.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. Epub 2012/06/02. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–62. Epub 2017/08/16. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 144.Ren D, Hua Y, Yu B, Ye X, He Z, Li C, Wang J, Mo Y, Wei X, Chen Y, Zhou Y, Liao Q, Wang H, Xiang B, Zhou M, Li X, Li G, Li Y, Zeng Z, Xiong W. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19(1):19. Epub 2020/01/30. doi: 10.1186/s12943-020-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paul MR, Huo Y, Liu A, Lesperance J, Garancher A, Wechsler-Reya RJ, Zage PE. Characterization of G-CSF receptor expression in medulloblastoma. Neurooncol Adv. 2020;2(1):vdaa062. Epub 2020/05/27. doi: 10.1093/noajnl/vdaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Babalola CP, Nightingale CH, Nicolau DP. Adjunctive efficacy of granulocyte colony-stimulating factor on treatment of Pseudomonas aeruginosa pneumonia in neutropenic and nonneutropenic hosts. J Antimicrob Chemother. 2004;53(6):1098–100. Epub 2004/05/05. doi: 10.1093/jac/dkh237. [DOI] [PubMed] [Google Scholar]
- 147.Gough A, Clapperton M, Rolando N, Foster AV, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet. 1997;350(9081):855–9. doi: 10.1016/S0140-6736(97)04495-4. [DOI] [PubMed] [Google Scholar]
- 148.Huang X, Liu Y, Bai S, Peng L, Zhang B, Lu H. Granulocyte colony stimulating factor therapy for stroke: A pairwise meta-analysis of randomized controlled trial. PLoS One. 2017;12(4):e0175774. Epub 2017/04/13. doi: 10.1371/journal.pone.0175774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuritzkes DR, Parenti D, Ward DJ, Rachlis A, Wong RJ, Mallon KP, Rich WJ, Jacobson MA. Filgrastim prevents severe neutropenia and reduces infective morbidity in patients with advanced HIV infection: results of a randomized, multicenter, controlled trial. G-CSF 930101 Study Group. AIDS. 1998;12(1):65–74. doi: 10.1097/00002030-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 150.Cheng LL, Guan WJ, Duan CY, Zhang NF, Lei CL, Hu Y, Chen AL, Li SY, Zhuo C, Deng XL, Cheng FJ, Gao Y, Zhang JH, Xie JX, Peng H, Li YX, Wu XX, Liu W, Wang J, Xiao GM, Chen PY, Wang CY, Yang ZF, Zhao JC, Zhong NS. Effect of Recombinant Human Granulocyte Colony-Stimulating Factor for Patients With Coronavirus Disease 2019 (COVID-19) and Lymphopenia: A Randomized Clinical Trial. JAMA Intern Med. 2020. Epub 2020/09/10. doi: 10.1001/jamainternmed.2020.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alkan A, Uncu A, Taşkiran I, Tanriverdi Ö. Double-edged sword: Granulocyte colony stimulating factors in cancer patients during the COVID-19 era. Clinics (Sao Paulo) 2020;75:e2033. Epub 2020/07/06. doi: 10.6061/clinics/2020/e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nawar T, Morjaria S, Kaltsas A, Patel D, Perez-Johnston R, Daniyan AF, Mailankody S, Parameswaran R. Granulocyte-colony stimulating factor in COVID-19: Is it stimulating more than just the bone marrow? Am J Hematol. 2020;95(8):E210–E3. Epub 2020/07/01. doi: 10.1002/ajh.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]